Abstract
Background: Frequent use of personal, nonprotocol calcium supplements obscured an adverse effect of coadministered calcium and vitamin D (CaD) on cardiovascular risk in the Women's Health Initiative (WHI).
Objective: We investigated the effects of the use of personal calcium or vitamin D supplements on other outcomes in the WHI CaD Study (WHI CaD) by using the WHI limited-access clinical trials data set.
Design: The WHI CaD was a 7-y, randomized, placebo-controlled trial of CaD (1 g Ca/400 IU vitamin D daily) in 36,282 community-dwelling, postmenopausal women. The incidence of total cancer (excluding nonmelanoma skin cancers), breast and colorectal cancers, hip and total fracture, and mortality was assessed by using Cox proportional hazards models.
Results: In the WHI CaD, interactions between the use of either personal calcium or vitamin D supplements and CaD were found for total, breast, and colorectal cancers but not for fracture or mortality. In 15,646 women (43%) who were not taking personal calcium or vitamin D supplements at randomization, CaD significantly decreased the risk of total, breast, and invasive breast cancers by 14–20% and nonsignificantly reduced the risk of colorectal cancer by 17%. In women taking personal calcium or vitamin D supplements, CaD did not alter cancer risk (HR: 1.06–1.26).
Conclusions: For women in the WHI CaD who were not taking personal calcium or vitamin D supplements at randomization, CaD decreased the risk of total, breast, and colorectal cancers and did not change the risk of fractures or total mortality. The nonskeletal effects of CaD may be more important than the skeletal effects and should be considered when evaluating these supplements. The WHI CaD trial is registered at clinicaltrials.gov as NCT00000611.
INTRODUCTION
Calcium and vitamin D supplements are commonly taken either individually or in combination by older people to maintain or improve skeletal health. Recent evidence suggests that they may also have nonskeletal effects. In randomized controlled trials, CaD5 supplements administered individually or in combination decreased recurrent colorectal adenomas (1, 2), reduced the risk of cancer (3) and mortality (4), and increased the risk of MI (5, 6). The WHI previously assessed the effect of CaD supplements on skeletal (7) and nonskeletal endpoints (8–11). Postmenopausal women (n = 36,282) were randomly assigned to receive daily supplemental calcium (1 g) and vitamin D (10 μg) or matching placebos and followed for an average of 7 y. CaD had no effect of the incidence of hip or total fracture, cardiovascular outcomes, colorectal or breast cancer, or mortality (8–11).
We recently reported that calcium supplements when used without vitamin D increased the risk of MI in a meta-analysis of randomized controlled trials (6). In one of the studies in that meta-analysis (12), the risk of MI with CaD was increased by a similar amount to the risks observed with calcium alone. In contrast, there was no increased risk of MI with CaD in the WHI CaD Study (WHI CaD) (9). A noteworthy feature of WHI CaD was that more than half of the participants were taking personal, nonprotocol calcium or vitamin D supplements at randomization. Personal calcium supplements of up to 1 g/d and personal vitamin D supplements of up to 600 IU/d (and later 1000 IU/d) were permitted in WHI CaD (7). We hypothesized that the frequent use of personal calcium supplements may have obscured an adverse effect of CaD on cardiovascular endpoints in WHI CaD. Reanalysis of the publicly available limited-access WHI clinical trials database showed statistically significant interactions between personal calcium supplement use, CaD allocation, and cardiovascular events (13). In women not taking personal calcium supplements at randomization, CaD increased the risk of cardiovascular events by 13–22%, whereas CaD did not alter cardiovascular risk in women taking personal calcium supplements (13).
We therefore hypothesized that the frequent use of personal calcium and/or vitamin D supplements may have obscured the effects of CaD on other endpoints in WHI. We reanalyzed the publicly available WHI database to determine whether there were interactions between personal calcium and/or vitamin D supplement use, CaD allocation, and risk of hip or total fracture, all-cause mortality, and colorectal, breast, and total cancers and, if such interactions occurred, whether any benefits or risks of CaD on these endpoints were obscured by personal calcium or vitamin D use.
SUBJECTS AND METHODS
The design and results of the WHI CaD trial were published in full (7–11). Outcomes for hip and total fracture, mortality, and colorectal, breast, endometrial, and ovarian cancers were adjudicated centrally, whereas other cancers were adjudicated by local researchers (14). We obtained the WHI limited-access clinical trials data set from the NHLBI. A protocol was submitted to the NHLBI before any analyses were carried out. We attempted to replicate the approach of the WHI investigators where possible, carrying out prespecified analyses looking for interactions between prespecified subgroups based on the use of personal calcium or vitamin D supplements at randomization for the following endpoints: hip or total fracture, mortality, and colorectal, breast, and total cancers (total cancer excludes nonmelanoma skin cancer).
We reported the baseline characteristics at the time of randomization to CaD, whereas the WHI investigators reported these characteristics at the time of entry to the WHI program. For BMI and dietary and supplemental calcium and vitamin D intakes, we used the latest value recorded between screening and 1 mo after CaD randomization.
The effect of CaD on the time-to-first event for each endpoint was assessed by using Cox proportional hazards models stratified by age, randomization status in the WHI hormone and dietary modification trials, and relevant prevalent disease at baseline (history of breast cancer, colorectal cancer, or any cancer for breast, colorectal, and total cancer endpoints, respectively, and history of fracture for hip and total fracture) following the approach of the WHI investigators (7, 8, 10, 11). The assumption of proportional hazards was tested by performing a test for proportionality of the interaction between variables included in the model and the logarithm of time. Comparisons between subgroups were assessed by using interaction terms. All analyses were performed by using the SAS software package (version 9.1; SAS Institute). All tests were 2-tailed, and P < 0.05 was considered significant.
RESULTS
A total of 20,636 (57%) participants were taking personal calcium or vitamin D supplements at randomization, 16,100 (44%) were taking both calcium and vitamin D supplements, 3464 (10%) were taking calcium supplements alone, and 1072 (3%) were taking vitamin D supplements alone. The characteristics of the participants at randomization to the WHI CaD trial, grouped by personal use of calcium and/or vitamin D supplements, are shown in Table 1. The characteristics of the participants allocated to CaD or placebo were similar within the subgroups defined by personal calcium or vitamin D supplement use. However, participants using personal calcium or vitamin D supplements differed from those not using these supplements in several factors associated with comorbidity, such as age, BMI, blood pressure, hormone replacement therapy, smoking status, and history of MI, stroke, fracture, or diabetes (Table 1).
TABLE 1.
Characteristics of participants at randomization, by personal use of calcium and/or vitamin D supplements1
| No personal calcium or vitamin D use2 |
Personal calcium or vitamin D use2 |
|||
| CaD group(n = 7891) | Placebo group(n = 7755) | CaD group(n = 10285) | Placebo group(n = 10351) | |
| Age (y) | 62.8 ± 7.03 | 62.9 ± 7.0 | 64.0 ± 6.9 | 63.9 ± 6.8 |
| BMI (kg/m2) | 29.5 ± 5.9 | 29.4 ± 6.0 | 28.4 ± 5.7 | 28.3 ± 5.7 |
| Supplemental calcium intake (mg/d) | 0 ± 0 | 0 ± 0 | 551 ± 539 | 552 ± 522 |
| Dietary calcium intake (mg/d) | 801 ± 491 | 790 ± 470 | 828 ± 454 | 832 ± 455 |
| Supplemental vitamin D intake (μg/d) | 0 ± 0 | 0 ± 0 | 8.4 ± 5.5 | 8.4 ± 5.5 |
| Dietary vitamin D intake (μg/d) | 4.3 ± 3.2 | 4.2 ± 3.2 | 4.4 ± 3.1 | 4.4 ± 3.1 |
| Blood pressure (mm Hg) | ||||
| Systolic | 126 ± 17 | 126 ± 17 | 125 ± 17 | 125 ± 17 |
| Diastolic | 75 ± 9 | 75 ± 9 | 74 ± 9 | 74 ± 9 |
| Medical history (%)4 | ||||
| Current HRT, trials or personal | 49 | 51 | 54 | 55 |
| High cholesterol requiring medication | 12 | 12 | 13 | 12 |
| Cardiovascular disease | 14 | 15 | 14 | 15 |
| Hypertension | 33 | 35 | 33 | 32 |
| Stroke | 1.0 | 1.2 | 0.7 | 1.0 |
| Myocardial infarction | 2.2 | 2.0 | 1.6 | 1.5 |
| Any cancer | 3.8 | 4.1 | 4.4 | 3.7 |
| Breast cancer | 0.2 | 0.2 | 0.1 | 0.2 |
| Colorectal cancer | 0.1 | 0.2 | 0.2 | 0.1 |
| Any fracture since age 55 y | 14 | 14 | 16 | 16 |
| Hip fracture | 2.0 | 1.9 | 2.5 | 2.8 |
| Diabetes | 7 | 7 | 5 | 5 |
| Smoking status (%)4 | ||||
| Never | 52 | 52 | 52 | 53 |
| Past | 39 | 38 | 41 | 41 |
| Current | 9 | 9 | 7 | 6 |
CaD, calcium and vitamin D; HRT, hormone replacement therapy.
Personal calcium or vitamin D refers to use of nonprotocol calcium and/or vitamin D supplements at randomization.
Mean ± SD (all such values).
All data are from the time of randomization, except for medical history and smoking status, which are from the time at entry to the WHI clinical trials program; 91% of participants in the WHI CaD Study entered at their first annual visit and the remainder at their second annual visit. WHI, Women's Health Initiative.
A total of 24,710 (68%) participants reported taking personal calcium or vitamin D supplements on their last questionnaire (70%, year 6 visit; 19%, year 9 visit); 60% were taking both calcium and vitamin D supplements, 6% were taking calcium supplements alone, and 2% were taking vitamin D alone. A total of 17,372 (84%) participants taking personal calcium or vitamin D supplements at randomization reported taking personal calcium or vitamin D supplements on their last questionnaire; 7338 (47%) participants who were not taking personal calcium or vitamin D supplements at randomization reported taking personal calcium or vitamin D supplements on their last questionnaire.
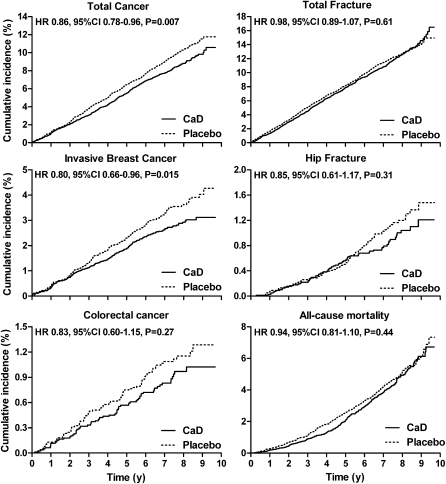
The influence of personal calcium and/or vitamin D supplement use at randomization on the effect of CaD on fractures, cancer, and mortality is shown in Table 2. Significant interactions between CaD allocation and personal calcium and/or vitamin D supplement use were found for total, breast, invasive breast, and colorectal cancers. In participants not taking personal calcium or vitamin D, CaD significantly decreased the risk of total cancer, breast cancer, and invasive breast cancer by 14–20% and nonsignificantly reduced the risk of colorectal cancer by 17%. In contrast, in women taking personal calcium or vitamin D, the HRs with CaD for these cancer endpoints ranged from 1.06 to 1.26. No significant interactions were found between CaD allocation and personal calcium or vitamin D supplement use for total or hip fracture or mortality. Kaplan-Meier estimates of the cumulative incidence for the cancer, fracture, and mortality endpoints in women not taking personal calcium or vitamin D supplements at randomization are shown in Figure 1. In women not taking personal calcium or vitamin D supplements at randomization, the number needed to treat for 5 y to prevent 1 event was 216 for invasive breast cancer, 889 for colorectal cancer, 124 for any cancer, 457 for death, 704 for any fracture, and 879 for hip fracture.
TABLE 2.
Effect of CaD supplements on health outcomes grouped by personal use of calcium and/or vitamin D supplements at randomization1
| No personal calcium or vitamin D use2 | Any personal calcium or vitamin D use2 | ||||||||
| CaD group(n = 7891) | Placebo group(n = 7755) | HR (95% CI) | P3 | CaD group(n = 10,285) | Placebo group(n = 10,351) | HR (95% CI) | P3 | P-interaction4 | |
| n (events/1000 patient-years) | n (events/1000 patient-years) | n (events/1000 patient-years) | n (events/1000 patient-years) | ||||||
| Total cancer | 633 (11.6) | 715 (13.5) | 0.86 (0.78, 0.96) | 0.007 | 946 (13.7) | 890 (12.8) | 1.06 (0.97, 1.17) | 0.20 | 0.003 |
| Total breast cancer | 261 (4.7) | 310 (5.7) | 0.82 (0.70, 0.97) | 0.021 | 412 (5.9) | 381 (5.4) | 1.08 (0.94, 1.24) | 0.27 | 0.012 |
| Invasive breast cancer | 209 (3.7) | 256 (4.7) | 0.80 (0.66, 0.96) | 0.015 | 326 (4.6) | 291 (4.1) | 1.12 (0.96, 1.31) | 0.16 | 0.005 |
| In situ breast cancer | 54 (1.0) | 58 (1.1) | 0.92 (0.63, 1.33) | 0.65 | 89 (1.2) | 91 (1.3) | 0.98 (0.73, 1.31) | 0.89 | 0.80 |
| Colorectal cancer | 67 (1.2) | 82 (1.5) | 0.83 (0.60, 1.15) | 0.27 | 102 (1.4) | 80 (1.1) | 1.26 (0.94, 1.69) | 0.12 | 0.044 |
| Total fracture | 892 (16.7) | 892 (17.1) | 0.98 (0.89, 1.07) | 0.61 | 1210 (17.9) | 1266 (18.7) | 0.96 (0.89, 1.04) | 0.32 | 0.72 |
| Hip fracture | 68 (1.2) | 82 (1.5) | 0.85 (0.61, 1.17) | 0.31 | 107 (1.5) | 117 (1.6) | 0.93 (0.71, 1.21) | 0.59 | 0.65 |
| All-cause mortality | 336 (5.9) | 349 (6.3) | 0.94 (0.81, 1.10) | 0.44 | 408 (5.7) | 458 (6.4) | 0.88 (0.77, 1.01) | 0.06 | 0.44 |
CaD, calcium and vitamin D.
Personal calcium or vitamin D refers to use of nonprotocol calcium and/or vitamin D supplements at randomization.
P values obtained from Cox proportional hazards models stratified by age, randomization status in the Women's Health Initiative hormone and dietary modification trials, and relevant prevalent disease at baseline (history of breast cancer, colorectal cancer, or any cancer for breast, colorectal, and total cancer endpoints and history of fracture for hip and total fracture).
Interaction between CaD allocation and use or nonuse of personal calcium and/or vitamin D supplements at randomization for each endpoint tested in Cox proportional hazard models.
FIGURE 1.
Cumulative incidence of cancer, fractures, and mortality in 15,646 women who were not taking personal calcium or vitamin D supplements at baseline. (Note the different range of cumulative incidence for each panel.) HRs, 95% CIs, and P values are from Cox proportional hazard models. CaD, calcium and vitamin D.
DISCUSSION
In WHI CaD, the use of personal calcium or vitamin D supplements at randomization significantly influenced the effect of CaD on the risk of cancer. In the entire WHI cohort, significant interactions were found between allocation to CaD and personal calcium and/or vitamin D supplement use for total, breast, and colorectal cancers. In the 43% of WHI CaD participants who were not taking personal calcium or vitamin D supplements at randomization, CaD decreased the risk of total, breast, and colorectal cancers by 14–20%. In contrast, in WHI CaD participants taking personal calcium or vitamin D supplements at randomization, allocation to CaD did not alter cancer risk. No significant interactions were found between CaD and personal calcium and/or vitamin D supplement use for any fracture, hip fracture, or mortality. Previously, we reported significant interactions between CaD and personal calcium supplement use at randomization for cardiovascular endpoints (13). In women not taking personal calcium supplements, CaD increased the risk of cardiovascular events by 13–22%, whereas CaD did not alter cardiovascular disease risk in women taking personal calcium supplements (13). Thus, personal calcium and/or vitamin D use in WHI CaD obscured beneficial effects of CaD on cancer incidence and adverse effects of CaD on cardiovascular events, but did not have a significant effect on the effects of CaD on fractures or mortality. On the basis of data from women not taking personal calcium or vitamin D at randomization, the treatment of 1000 women with CaD for 5 y would prevent 5 breast cancers, 1 colorectal cancer, and 8 total cancers; would cause 4 MIs or strokes; and might prevent 1 fracture and 2 deaths.
Because of the frequent use of personal calcium or vitamin D supplements, WHI CaD was, in effect, a trial that assessed the effect of adding CaD to personal calcium and/or vitamin D supplements and essentially compared higher doses of CaD with lower doses of CaD. By restricting the analyses to women not taking personal calcium or vitamin D supplements, we were able to compare the effects of CaD with those of placebo. These current analyses, together with our previous report on cardiovascular events (13), suggest that CaD has important effects on cancer and cardiovascular disease incidence. The magnitude of these effects may have been diminished by an increasing personal use of calcium and vitamin D supplements during the study. A total of 47% of the placebo group who did not take personal calcium or vitamin D supplements at randomization were taking these supplements at the end of the study, which may have further obscured the effects of CaD. In contrast, the analyses of women taking personal calcium or vitamin D supplements, which compared higher doses of CaD with lower doses of CaD, suggest that higher doses of CaD do not further decrease cancer incidence or increase cardiovascular disease incidence compared with lower doses of CaD. These results suggest that there may be a threshold effect, rather than a dose-dependent effect, of CaD on these endpoints.
The WHI trial studied CaD, so it is not possible to determine whether the observed effects on cancer and cardiovascular events were due to calcium, vitamin D, or the combination of agents. Similarly, most of the women using personal calcium or vitamin D supplements were taking both calcium and vitamin D supplements, and, again, it was not possible to determine whether the observed effects of CaD were obscured by personal calcium use, personal vitamin D use, or a combination thereof. For cardiovascular events, the risks of calcium supplements used as monotherapy are similar to those with CaD, which suggests that the risk is largely due to calcium supplements, and the increased risk of calcium is not mitigated by vitamin D use (13). It is possible that vitamin D supplements may have independent effects on cardiovascular events (15). For cancer outcomes, few data from randomized controlled trials—other than WHI—have assessed the effect of calcium or vitamin D, individually or in combination. Trivedi et al (16) reported no effects of 4 monthly doses of 100,000 IU vitamin D on cancer incidence or mortality in 2686 people followed for 5 y. We reported no effects of 1 g Ca/d (as citrate) on cancer incidence in 1471 women followed for 5 y (17). Lappe et al (3) reported a 60% reduction in cancer incidence with CaD (P = 0.01) and a 47% reduction with calcium alone (P = 0.06) in 1179 women followed for 4 y, although the apparent reductions might largely be due to an unexpectedly high incidence of cancer in the placebo group (18). Thus, data from existing randomized controlled trials do not allow a definitive answer to this issue, but raise the possibility that combination therapy is required for cancer prevention.
Preclinical studies have suggested possible roles for both calcium and vitamin D in cancer prevention. In animal models, dietary fat influences breast cancer development at several stages (19), and dietary fat and bile acids influence colorectal carcinogenesis (20, 21). Calcium binds fatty acids and bile acids in the intestinal lumen, which may prevent their absorption or direct tissue effects. Vitamin D may influence cancer development through the antiproliferative effects of 1,25-dihydroxyvitamin D shown in many tissues (22). Calcium may have a further role in the potential effects of vitamin D, because it regulates 1,25-dihydroxyvitamin D metabolism directly and indirectly through its effects on parathyroid hormone.
This analysis had several limitations. We used the publicly accessible limited-access WHI clinical trials data set for these analyses, so the analysis is limited to the information available in this data set. The hypothesis that the use of personal calcium and/or vitamin D supplements might have interacted with the CaD treatment effect in WHI CaD was based on plausibility following our findings for cardiovascular events (13) and on the evidence linking both vitamin D and calcium with cancer prevention described above. Subgroup analysis should be undertaken and interpreted with caution because of the potential for false-positive results and an overinterpretation of the findings (23, 24). To minimize these risks, we prespecified the variable of interest (personal calcium or vitamin D supplement use) before carrying out any analyses, assessed its effect by using interaction tests, and followed recommended approaches for subgroup analysis and interpretation (24, 25). We followed the approach of the WHI investigators in not adjusting P values for multiple subgroup analyses. Instead, we estimated the likelihood of false-positive tests (7)—an approved approach for addressing the multiplicity of statistical tests (26). Eight interaction tests were performed. If the effect of CaD was unrelated to personal calcium or vitamin D use, and the endpoints were independent, the probability of at least one false-positive interaction test was 33% (25).
In summary, CaD supplements decreased the risk of breast, colorectal, and total cancers in WHI CaD participants who were not taking personal calcium or vitamin D supplements at the time of randomization, but also increased the risk of cardiovascular events. The increased risk of cardiovascular events appeared largely due to calcium supplements, but it is not evident whether the beneficial effects on cancer incidence were due to calcium, vitamin D, or the combination thereof. Further studies should address this uncertainty. When the use calcium or vitamin D supplements is considered to improve skeletal health, it is important to consider the nonskeletal risks and benefits of calcium and vitamin D supplements, because these effects might outweigh the modest benefits they confer on skeletal health.
Acknowledgments
The authors’ responsibilities were as follows—MJB, AG, and IRR: designed the research; MJB and GDG: analyzed the data; MJB, AG, and IRR: wrote the manuscript; and MJB: had primary responsibility for the content. All of the authors read and approved the final manuscript. Study medications for clinical trials of calcium supplementation were supplied by Mission Pharmacal to IRR. IRR has received research support from and acted as a consultant for Fonterra. None of the other authors had any conflicts of interest to declare.
Footnotes
CaD, coadministered calcium and vitamin D; MI, myocardial infarction; NHLBI, National Heart, Lung, and Blood Institute; WHI, Women's Health Initiative.
REFERENCES
- 1.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med 1999;340:101–7 [DOI] [PubMed] [Google Scholar]
- 2.Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet 2000;356:1300–6 [DOI] [PubMed] [Google Scholar]
- 3.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85:1586–91 [DOI] [PubMed] [Google Scholar]
- 4.Autier P, Gandini S, Vitamin D. Supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007;167:1730–7 [DOI] [PubMed] [Google Scholar]
- 5.Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, Gamble GD, Grey A, Reid IR. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008;336:262–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolland MJ, Avenell A, Baron JA, Grey A, Maclennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–83 [DOI] [PubMed] [Google Scholar]
- 8.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684–96 [DOI] [PubMed] [Google Scholar]
- 9.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846–54 [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O'Sullivan MJ, Yasmeen S, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst 2008;100:1581–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaCroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, Cummings SR, Gass M, Johnson KC, Ko M, Larson J, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women's Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 2009;64:559–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 2005;365:1621–8 [DOI] [PubMed] [Google Scholar]
- 13.Bolland MJ, Grey A, Gamble GD, Reid IR. Risk of cardiovascular events with calcium/vitamin D—a re-analysis of the Women's Health Initiative. J Bone Miner Res 2011;342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol 2003;13:S122–8 [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 2010;152:315–23 [DOI] [PubMed] [Google Scholar]
- 16.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolland MJ, Reid IR. Calcium supplementation and cancer incidence. Am J Clin Nutr 2008;87:792–3 [DOI] [PubMed] [Google Scholar]
- 18.Schabas R. Artifact in the control group undermines the conclusions of a vitamin D and cancer study. Am J Clin Nutr 2008;87:792–4 [DOI] [PubMed] [Google Scholar]
- 19.Wynder EL, Cohen LA, Muscat JE, Winters B, Dwyer JT, Blackburn G. Breast cancer: weighing the evidence for a promoting role of dietary fat. J Natl Cancer Inst 1997;89:766–75 [DOI] [PubMed] [Google Scholar]
- 20.Nagengast FM, Grubben MJ, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer 1995;31A:1067–70 [DOI] [PubMed] [Google Scholar]
- 21.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst 1984;72:1323–5 [PubMed] [Google Scholar]
- 22.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health 2006;96:252–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266:93–8 [PubMed] [Google Scholar]
- 24.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064–9 [DOI] [PubMed] [Google Scholar]
- 25.Lagakos SW. The challenge of subgroup analyses—reporting without distorting. N Engl J Med 2006;354:1667–9 [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189–94 [DOI] [PubMed] [Google Scholar]