Abstract
Context
Previous studies of testosterone supplementation in HIV-infected men failed to demonstrate improvement in muscle strength. The effects of resistance exercise combined with testosterone supplementation in HIV-infected men are unknown.
Objective
To determine the effects of testosterone replacement with and without resistance exercise on muscle strength and body composition in HIV-infected men with low testosterone levels and weight loss.
Design and Setting
Placebo-controlled, double-blind, randomized clinical trial conducted from September 1995 to July 1998 at a general clinical research center.
Participants
Sixty-one HIV-infected men aged 18 to 50 years with serum testosterone levels of less than 12.1 nmol/L (349 ng/dL) and weight loss of 5% or more in the previous 6 months, 49 of whom completed the study.
Interventions
Participants were randomly assigned to 1 of 4 groups: placebo, no exercise (n = 14); testosterone enanthate (100 mg/wk intramuscularly), no exercise (n = 17); placebo and exercise (n = 15); or testosterone and exercise (n = 15). Treatment duration was 16 weeks.
Main Outcome Measures
Changes in muscle strength, body weight, thigh muscle volume, and lean body mass compared among the 4 treatment groups.
Results
Body weight increased significantly by 2.6 kg (P<.001) in men receiving testosterone alone and by 2.2 kg (P = .02) in men who exercised alone but did not change in men receiving placebo alone (−0.5 kg; P = .55) or testosterone and exercise (0.7 kg; P = .08). Men treated with testosterone alone, exercise alone, or both experienced significant increases in maximum voluntary muscle strength in leg press (range, 22%–30%), leg curls (range, 18%–36%), bench press (range, 19%–33%), and latissimus pulls (range, 17%–33%). Gains in strength in all exercise categories were greater in men assigned to the testosterone-exercise group or to the exercise-alone group than in those assigned to the placebo-alone group. There was a greater increase in thigh muscle volume in men receiving testosterone alone (mean change, 40 cm3; P<.001 vs zero change) or exercise alone (62 cm3; P = .003) than in men receiving placebo alone (5 cm3; P = .70). Average lean body mass increased by 2.3 kg (P = .004) and 2.6 kg (P<.001), respectively, in men who received testosterone alone or testosterone and exercise but did not change in men receiving placebo alone (0.9 kg; P = .21). Hemoglobin levels increased in men receiving testosterone but not in those receiving placebo.
Conclusion
Our data suggest that testosterone and resistance exercise promote gains in body weight, muscle mass, muscle strength, and lean body mass in HIV-infected men with weight loss and low testosterone levels. Testosterone and exercise together did not produce greater gains than either intervention alone.
Weight loss during the course of human immunodeficiency virus (HIV) infection is associated with increased mortality and adverse disease outcome.1–4 Of the various therapies being considered for the treatment of HIV-associated weight loss, testosterone and exercise are attractive because they are relatively inexpensive and safe. There is a high prevalence of low testosterone levels in HIV-infected men.4–11 Serum testosterone levels are lower in those with weight loss9 and correlate with deficits in muscle mass,11 low Karnofsky scores,12 and disease progression.10,13 Replacement doses of testosterone augment lean body mass and muscle strength in healthy, hypogonadal men.14–16 These data have led to the hypothesis that testosterone replacement might also increase muscle mass and strength in HIV-infected men with low testosterone levels. Some of the studies17–29 that have examined the effects of androgen administration on weight and body composition in HIV-infected men were not placebo-controlled,23–27 and most failed to control energy intake and exercise stimulus.23–28 Two of the 4 placebo-controlled studies of testosterone supplementation of HIV-infected men17,18 reported gains in fat-free mass (FFM), while others19,20 found no change. None of the previous androgen studies in HIV-infected men has demonstrated improvements in muscle strength.17–20,23–28 The effects of resistance exercise alone and in combination with testosterone in HIV-infected men are unknown.
The objective of this study was to determine the effects of testosterone replacement, with or without a program of resistance exercise, on muscle strength and body composition in HIV-infected men with weight loss and low testosterone levels. We hypothesized that testosterone and resistance exercise would each increase muscle strength and FFM, and the 2 interventions, when administered together, would produce greater gains in these measures than either intervention alone.
METHODS
Study Design
This was a 16-week, double blind, placebo-controlled, randomized study, conducted between September 1995 and July 1998. Written informed consent, approved by the institutional review boards of Charles R. Drew University of Medicine and Science, Los Angeles, and Research and Education Institute, Torrance, Calif, was obtained from each volunteer.
The participants were HIV-infected men, between the ages of 18 and 50 years, with involuntary weight loss of at least 5% in the preceding 6 months by self-report or documented from medical records and had serum total testosterone levels less than 12.1 nmol/L (349 ng/dL). The subjects had been receiving stable antiretroviral therapy for at least 12 weeks before enrollment. We excluded patients with acute illness, prostate cancer, hypogonadism from a known cause, or diarrhea. Patients with significant cardiovascular or respiratory disease, diabetes, drug abuse, or heavy alcohol use in the last 6 months, aspartate aminotransferase or alanine aminotransferase greater than 3 times the upper limit of normal, bilirubin levels higher than 34.2 μmol/L (2 mg/dL), and prostate-specific antigen (PSA) levels higher than 4 ng/mL were excluded. The patients were excluded if they had received megestrol acetate, androgen agonists or antagonists, growth hormone, or insulin like growth factor 1 within 3 months of enrollment.
Sample Size, Subject Assignment, and Randomization
The sample size estimate was based on a previous study of testosterone replacement in healthy, hypogonadal men14 in which administration of 100 mg of testosterone enanthate weekly was associated with mean (SE) FFM increase of 5.0 (0.7) kg and 22% (3%) increase in maximum voluntary strength. Assuming that changes in HIV-infected men with low testosterone levels would be similar to those in healthy hypogonadal men, we determined that 12 subjects per group would give us 80% or more power to detect a similar change in FFM and muscle strength. We assumed a 20% to 25% dropout rate and therefore planned to enroll 60 men in the study.
Using randomization schedules, generated to create random numbers from a uniform distribution on unit interval, 61 eligible men were assigned to 1 of 4 groups. Group 1 received placebo injections (sesame oil) but did not exercise; group 2 received 100 mg/wk of testosterone enanthate but did not exercise; group 3 received placebo and participated in a resistance exercise program; and, group 4 received the testosterone treatment and participated in an exercise program. Subjects were stratified by age (19–40 years and 41–60 years). A blocking size of 16 was used.
Testosterone replacement consisted of weekly intramuscular injections of 100 mg of testosterone enanthate, administered in the General Clinical Research Center, Torrance, Calif, to ensure compliance.
The exercise intensity was standardized based on initial 1-repetition maximum (1-RM). In the first 4-weeks, the exercise regimen consisted of a high volume (3 sets of 12–15 repetitions), low intensity (60% of initial 1-RM), resistance–exercise 3 times weekly. This was followed by thrice weekly, progressive, periodized, high-intensity (90% of 1-RM on heavy days, 80% on medium days, and 70% on light days), low-volume (4 sets of 4–6 repetitions each) resistance exercise during weeks 5 through 10. During weeks 11 through 16, the loads were increased by 7% for upper body and 12% for lower body exercises, and the number of sets was increased to 5.
Outcome Measures
The primary efficacy variable was change during treatment in 1-RM strength. We also measured changes in thigh muscle volume by magnetic resonance imaging, FFM by dual-energy x-ray absorptiometry (DEXA) and deuterium oxide dilution, body weight, and health-related quality of life (HRQOL). Serum total and free testosterone, dihydrotestosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and sex hormone–binding globulin (SHBG) levels were measured on several occasions during the control and treatment periods. Absolute and percentage CD4+ and CD8+ cell counts and plasma HIV–copy number were measured at baseline and during week 16. Adverse experiences were recorded every 2 weeks.
We measured effort-dependent strength in the leg press, bench press, leg curls, latissimus pulls, and overhead press exercises using the 1-RM method. All subjects underwent an instructional period, in which lifting mechanics were demonstrated. The resistance was progressively increased until the men could not complete the lift; the maximum amount of weight that they were able to lift was recorded as the 1-RM strength. To minimize the confounding influence of the learning effect, the participants returned for reassessment of strength within 7 days of the initial assessment. The higher of the 2 1-RM values was recorded when the difference between the 2 measurements was less than 5%.
Body weight was measured every 2 weeks. Body composition was assessed by DEXA scan (Hologic 4500; Waltham, Mass), deuterium oxide dilution, and magnetic resonance imaging. For estimation of total body water, the men ingested 20 g of deuterium oxide14 and plasma samples were drawn at −15, 0, 120, 180, and 240 minutes. We measured deuterium abundance in plasma by nuclear magnetic resonance spectroscopy,30 using a correction factor of 0.985 for exchangeable hydrogen.
Energy and protein intake were standardized at 168 J/kg (40 kcal) per day and 1.5 g/kg per day. The participants were given dietary instructions 2 weeks before treatment initiation; these instructions were reinforced every 2 weeks. The nutritional intake was verified by analysis of 3-day food records during weeks 1, 8, and 16, and 24-hour food recalls every 4 weeks, by using the Minnesota Nutritional Software.
Serum total testosterone levels were measured by an immunoassay31,32 and free testosterone levels by equilibrium dialysis.32 The sensitivities of the total and free testosterone assays were 0.02 nmol/L (0.58 ng/dL) and 0.2 pmol/L (0.058 pg/mL), respectively. Intraassay and interassay coefficients of variation for the total and free testosterone assays were 8.2% and 13.2%, and 4.2%, and 12.3%, respectively. Serum LH, FSH, and SHBG levels were measured by immunofluorometric assays,31 with sensitivities of 0.05 IU/L, 0.15 IU/L, and 6.25 nmol/L, respectively. Plasma HIV RNA copy number was measured by reverse transcriptase polymerase chain reaction (Amplicor; Roche Diagnostic Systems Inc, Sommerville, NJ).
The HRQOL survey17–33 included multi-item measures of physical functioning, role limitations due to physical problems, general health perceptions, emotional well-being, role limitations due to emotional problems and social, cognitive and sexual functioning. The survey also included a 22-item symptom checklist, a disability-days item, an overall health rating item, and a self-reported time tradeoff preference assessment. Data were analyzed using linear regression. Dummy variables were used for group assignment, and baseline status and case mix variables were included in the model.
Statistical Analyses
All men who dropped out did so before week 6, and did not undergo post-treatment DEXA scan or strength measurements. The analyses of muscle strength and body composition, therefore, were performed on all randomized patients for whom these efficacy data were available. For secondary variables, the last values carried forward for withdrawn patients were analyzed. Continuous data are reported as mean (SEM) and categorical data as frequency tabulations. All variables were examined for their distribution characteristics. Variables that did not meet the assumption of a normal distribution were log-transformed and retested. If the assumption of normality could not be met by transformation, then nonparametric methods of statistical comparison were used. An analysis of variance (ANOVA) model was used to compare change from baseline between the 4 groups for muscle strength, muscle volume, body composition measures, and hormone levels. Student-Newman-Keuls method was used for pairwise comparisons following a significant ANOVA. All outcome measures were also analyzed using paired t test to detect a nonzero change from baseline at week 16 within each treatment group.
RESULTS
Patient Characteristics
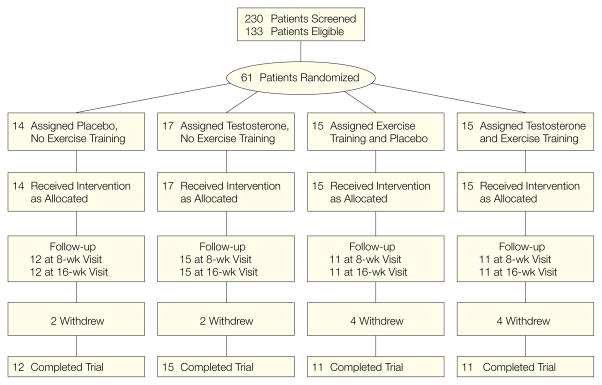
Of the 61 patients who were enrolled, 49 completed the study, 12 in group 1, 15 in group 2, 11 in group 3, and 11 in group 4. No discontinuations were attributed to adverse experiences (Figure 1). The 4 groups did not significantly differ in age, height, weight, CD4+ cell counts, prior weight loss, and baseline testosterone levels (Table 1). The percentage of men receiving anti-retroviral therapy and protease inhibitors was not significantly different between groups. The men who completed the study did not differ from those who dropped out in body weight, ethnic composition, testosterone levels, and CD4+ cell counts but were older than the latter group (35.5 vs 41.4 years, P = .01).
Figure 1.
Flow of Patients Through the Study
Table 1.
Baseline Characteristics of the Participants*
| No Exercise
|
Exercise
|
P Value | |||
|---|---|---|---|---|---|
| Placebo (n = 14) | Testosterone (n = 17) | Placebo (n = 15) | Testosterone (n = 15) | ||
| Age, y | 41.8 (2.5) | 40.8 (1.2) | 44.4 (3.0) | 40.2 (2.2) | .59 |
|
| |||||
| Height, cm | 177.8 (2.9) | 176.3 (2.5) | 176.2 (1.9) | 172.6 (2.1) | .52 |
|
| |||||
| Weight, kg | 76.7 (3.2) | 77.1 (3.5) | 71.9 (2.9) | 69.1 (3.4) | .28 |
|
| |||||
| Body mass index, kg/m2 | 24.3 (0.9) | 25.0 (1.4) | 23.2 (1.1) | 23.2 (1.1) | .66 |
|
| |||||
| Serum testosterone levels, nmol/L† | 6.1 (0.7) | 7.1 (0.8) | 7.0 (1.0) | 7.0 (1.2) | .94 |
|
| |||||
| CD4+ cell count, ×106/L | 279 (93) | 357 (85) | 229 (56) | 340 (95) | .77 |
|
| |||||
| CD8+ cell count, ×106/L | 897 (89) | 872 (92) | 842 (136) | 669 (126) | .49 |
|
| |||||
| Weight loss, kg | 6.4 (2.0) | 7.4 (1.1) | 7.1 (1.1) | 6.7 (1.1) | .97 |
|
| |||||
| Antiretroviral therapy, % | 100 | 93 | 82 | 89 | .78 |
|
| |||||
| Protease inhibitors, % | 40 | 60 | 45 | 55 | .68 |
All data are presented as mean (SEM) unless otherwise indicated.
To convert serum testosterone levels to nanograms per deciliter, multiply by 28.843.
All evaluable subjects received more than 90% of their injections. Of the 11 men in the combined testosterone and exercise group, 9 attended more than 90% and 2 attended 75% to 89% of their scheduled exercise sessions. Of the 11 men in the exercise alone group, 7 attended 90% to 100%, 3 attended 75% to 89%, and 1 attended 70% of the scheduled sessions.
Daily energy intake and percentage of energy derived from protein, carbohydrate, and fat were not significantly different between the 4 groups at baseline and did not significantly change during treatment. Specific daily energy and macronutrient intake are provided in Appendix 1 online at http://www.jama.com.
Appendix 1.
Daily Energy Macronutrient Intake*
| Variables | No Exercise
|
Exercise
|
P Value | ||
|---|---|---|---|---|---|
| Placebo | Testosterone | Placebo | Testosterone | ||
| Total energy intake, kJ/d† | 13 301.4 (120.5) | 12 364.8 (945.0) | 13 759.8 (508.2) | 11 659.2 (579.6) | .394 |
|
| |||||
| Energy intake per kg of body weight | 43 (5) | 37 (3) | 46 (3) | 41 (2) | .282 |
|
| |||||
| Total protein intake, g/d | 114 (12) | 117 (12) | 124 (5) | 110 (7) | .816 |
|
| |||||
| Protein intake per kg/d | 1.5 (0.2) | 1.5 (0.2) | 1.7 (0.1) | 1.6 (0.1) | .634 |
|
| |||||
| Energy from protein, % | 15.2 (2.1) | 15.6 (2.8) | 16.0 (2.3) | 16.0 (2.1) | .319 |
|
| |||||
| Total carbohydrate intake, g/kg per day | 374 (38) | 339 (20) | 345 (32) | 306 (13) | .388 |
|
| |||||
| Carbohydrate intake, g/kg per day | 20.6 (2.8) | 17.5 (1.1) | 19.6 (2.3) | 18.2 (1.2) | .642 |
|
| |||||
| Energy from carbohydrate, % | 48.1 (2.2) | 49.1 (2.0) | 45.3 (2.3) | 49.7 (1.9) | .459 |
|
| |||||
| Total fat intake, g | 127.7 (14.1) | 112.3 (11.2) | 121.9 (8.7) | 97.9 (7.5) | .266 |
|
| |||||
| Fat intake, g/kg per day | 1.5 (0.2) | 1.3 (0.1) | 1.6 (0.2) | 1.3 (0.1) | .299 |
|
| |||||
| Energy from fat, % | 35.8 (2.3) | 36.1 (1.8) | 36.9 (1.5) | 34.2 (1.2) | .735 |
Energy and macronutrient intake were calculated from 3-day food records (during weeks 1, 8, and 16) and 24-hour food recalls (every 4 weeks) by using the Minnesota Nutritional Software. The energy and macronutrient intake was then averaged across different days for each subject. Data are presented as mean (SEM).
To convert to kilocalories divide by 4.2.
Serum total and free testosterone levels, measured 1 week after injection, increased significantly from baseline in the testosterone groups and did not change in those receiving placebo. Serum LH, FSH, and SHBG levels decreased significantly in the testosterone-treated men but not in men treated with placebo (Table 2).
Table 2.
Serum Total and Free Testosterone, Luteinizing Hormone and Follicle-Stimulating Hormone, and Sex Hormone–Binding Globulin Levels*
| No Exercise
|
Exercise
|
|||
|---|---|---|---|---|
| Placebo | Testosterone | Placebo | Testosterone | |
| Testosterone, nmol/L†‡ | ||||
| Baseline | 6.1 (0.7) | 7.1 (0.8) | 7.0 (1.0) | 7.0 (1.2) |
|
| ||||
| Week 16 | 5.0 (0.5) | 11.7 (1.6) | 7.4 (1.2) | 10.8 (1.9) |
|
| ||||
| Change from baseline | −1.2 (0.6) | 4.5 (1.8)§ | 0.4 (1.0) | 3.8 (2.1)§ |
|
| ||||
| P value | .06 | .03 | .62 | .05 |
|
| ||||
| Free testosterone, pmol/L†‡ | ||||
| Baseline | 100.5 (13.9) | 111.0 (13.9) | 117.9 (17.3) | 93.6 (13.9) |
|
| ||||
| Week 16 | 107.5 (20.8) | 183.8 (24.2) | 121.4 (13.9) | 166.4 (20.8) |
|
| ||||
| Change from baseline | 1.0 (24.2) | 72.8 (27.7) | 3.5 (13.9) | 69.3 (20.8) |
|
| ||||
| P value | .70 | .02 | .57 | .02 |
|
| ||||
| Luteinizing hormone, U/L|| | ||||
| Baseline | 5.0 (1.0) | 3.4 (0.4) | 4.3 (0.9) | 6.6 (2.0) |
|
| ||||
| Week 16 | 3.7 (0.8) | 0.3 (0.1) | 4.6 (1.0) | 0.6 (0.3) |
|
| ||||
| Change from baseline | −1.4 (0.7) | −3.1 (0.4)§ | 0.3 (0.2) | −6.0 (1.8)§ |
|
| ||||
| P value | .17 | ,.001 | .39 | ,.001 |
|
| ||||
| Follicle-stimulating hormone, U/L|| | ||||
| Baseline | 5.8 (1.8) | 4.5 (0.5) | 7.1 (1.9) | 8.6 (2.6) |
|
| ||||
| Week 16 | 5.0 (2.0) | 0.5 (0.2) | 6.7 (1.6) | 2.0 (1.2) |
|
| ||||
| Change from baseline | −0.8 (0.8) | −4.2 (0.4)¶ | −0.4 (0.7) | −6.5 (1.6)¶ |
|
| ||||
| P value | .20 | ,.001 | .71 | ,.001 |
|
| ||||
| Sex hormone–binding globulin, nmol/L | ||||
| Baseline | 66.4 (12.1) | 48.6 (8.8) | 52.0 (12.9) | 47.5 (8.3) |
|
| ||||
| Week 16 | 63.7 (16.8) | 41.7 (7.0) | 49.1 (9.5) | 41.7 (8.9) |
|
| ||||
| Change from baseline | −2.7 (8.3) | −6.9 (3.1) | −2.9 (3.8) | −5.8 (2.4) |
|
| ||||
| P value | .75 | .05 | .46 | .05 |
The data represent the mean (SEM) of all available values on that day. However, the change represents the difference between paired values only.
Overall analysis of variance is P<.03.
To convert total testosterone levels to nanograms per deciliter, multiply by 28.843. To convert free testosterone levels to picograms per milliliter, multiply by 0.28843.
P<.05 vs placebo, no exercise.
Overall analysis of variance, P<.001.
P<.05 vs placebo, no exercise, and placebo with exercise.
Physical Outcomes
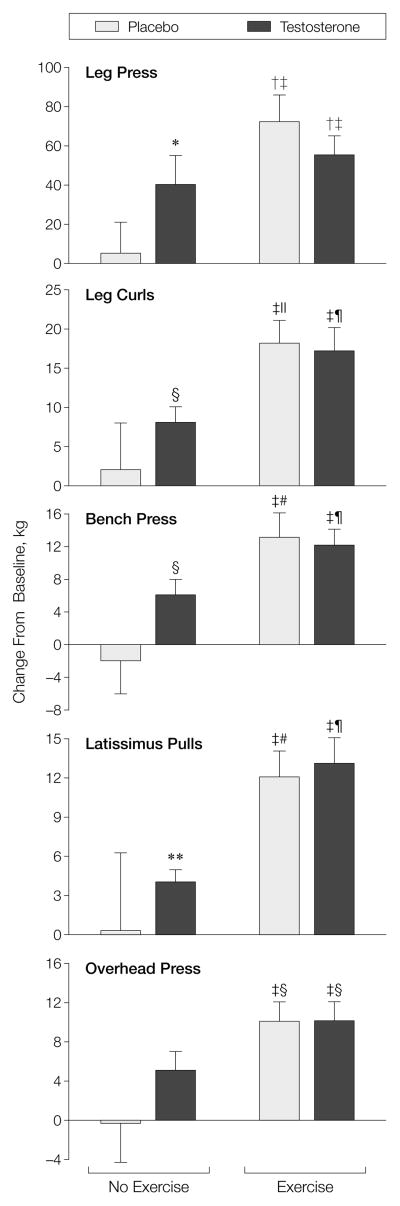
Among those who were in the placebo alone group, muscle strength did not change in any of the 5 exercises (−0.3%–−4.0%) (Table 3, RFigure 2 and Appendix 2 online at http://www.jama.com). This indicates that this strategy was effective in minimizing the influence of the learning effect. Persons in the exercise-alone group increased muscle strength in the 5 exercises by 29% to 36%, and those in the testosterone-alone group increased it by 17% to 28%. Although those in the testosterone-exercise group increased their muscle strength by 10% to 32%, it was not significantly greater than either intervention alone. The change in leg press strength correlated with change in muscle volume ( = 0.44, P = .003) and change in FFM (R = 0.55, P<.001).
Table 3.
Maximum Voluntary Muscle Strength*
| No Exercise
|
Exercise
|
|||
|---|---|---|---|---|
| Placebo | Testosterone | Placebo | Testosterone | |
| Leg press† | ||||
| Baseline | 259 (20) | 252 (31) | 260 (19) | 255 (23) |
|
| ||||
| Week 16 | 264 (33) | 292 (30) | 332 (21) | 310 (28) |
|
| ||||
| Change from baseline | 5 (16) | 40 (15) | 72 (14)‡ | 55 (10)‡ |
|
| ||||
| P value | .766 | .021 | <.001 | <.001 |
|
| ||||
| Bench press§ | ||||
| Baseline | 46 (3) | 48 (6) | 43 (4) | 45 (6) |
|
| ||||
| Week 16 | 44 (5) | 54 (5) | 56 (4) | 57 (6) |
|
| ||||
| Change from baseline | −2 (4) | 6 (2) | 13 (3)‡ | 12 (2)‡ |
|
| ||||
| P value | .682 | .001 | <.001 | <.001 |
Strength was measured, in kilograms, by the 1-repetition maximum method. The data on each day represent the mean (SEM) of all available values on that day. However, the change from baseline represents the difference between paired values only. The data on maximal voluntary strength in the overhead press, leg curl, and latissimus pull exercises are provided in Appendix 2 at http://www.jama.com.
Overall analysis of variance, P = .02.
P<.05 vs placebo, no exercise group.
Overall analysis of variance, P = .004.
Figure 2.
Mean (SEM) Change in Effort-Dependent Muscle Strength
The strength in leg press, leg curls, bench press, latissimus pulls, and overhead press exercises was measured by the 1-repetition maximum method. Asterisk indicates P = .02 vs zero change; dagger, P<.0004 vs zero change; double dagger, P<.05 vs placebo, no exercise group; section marker, P = .001 vs zero change; parallel bars, P = .0002 vs zero change; paragraph marker, P = .0001 vs zero change; pound sign, P<.0008 vs zero change; and double asterisk, P = .004, vs zero change.
Appendix 2.
Maximum Voluntary Muscle Strength*
| No Exercise
|
Exercise
|
|||
|---|---|---|---|---|
| Placebo | Testosterone | Placebo | Testosterone | |
| Leg curls† | ||||
| Baseline | 57 (4) | 62 (7) | 55 (3) | 53 (5) |
|
| ||||
| Week 16 | 57 (6) | 70 (7) | 73 (3) | 72 (6) |
|
| ||||
| Change from baseline | 2 (6) | 8 (2) | 18 (3)‡ | 17 (3)‡ |
|
| ||||
| P vs zero change | .715 | .001 | <.001 | <.001 |
|
| ||||
| Latissimus pulls§ | ||||
| Baseline | 48 (3) | 46 (6) | 42 (4) | 45 (5) |
|
| ||||
| Week 16 | 46 (6) | 50 (6) | 53 (4) | 58 (5) |
|
| ||||
| Change from baseline | 0.3 (6) | 4 (1) | 12 (2)‡ | 13 (2)‡ |
|
| ||||
| P vs zero change | .964 | .004 | <.001 | <.001 |
|
| ||||
| Overhead press|| | ||||
| Baseline | 36 (3) | 38 (4) | 38 (3) | 39 (5) |
|
| ||||
| Week 16 | 35 (4) | 43 (4) | 47 (3) | v |
|
| ||||
| Change from baseline | −0.3 (4) | 5 (2) | 10 (2) | 10 (2) |
|
| ||||
| P vs zero change | .945 | .085 | .001‡ | .001‡ |
Change in effort-dependent maximal strength. Strength was measured in kilograms by the 1-repetition maximum method in all exercises. The data on each day represent the mean (SEM) of all available values on that day. However, the change from baseline represents the difference between paired values only.
Overall analysis of variance, P =.007.
P<.05 vs placebo, no exercise group.
Overall analysis of variance, P =.018.
Overall analysis of variance, P =.04.
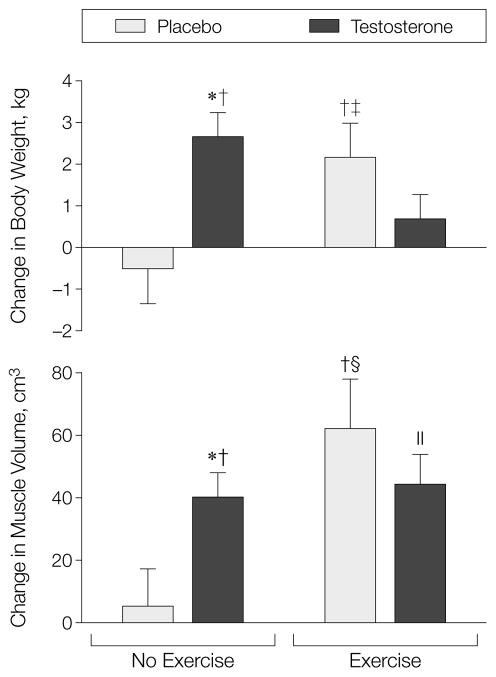
The thigh muscle volume did not significantly change in those taking placebo alone (change, 5 cm3; P = .696) but increased among those taking testosterone(change, 40 cm3; P = <.001), exercising alone (change, 62 cm3; P = .003), or taking testosterone and exercising (change 44 cm3; P = .001) (Figure 3). The exercise-alone, and testosterone-alone groups experienced greater increases in muscle volume than the placebo-alone group (P = .05). Specific tension, defined as muscle strength in the leg press exercise per unit quadriceps volume, did not change significantly in men who did not exercise regardless of whether they received placebo or testosterone but increased in both groups of men who exercised.
Figure 3.
Mean (SEM) Change in Body Weight and Thigh Muscle Volume Measured by Magnetic Resonance Imaging
Asterisk indicates P<.001 vs zero change; dagger, P<.01 compared with placebo in the no exercise group; double dagger, P = .02 vs zero change; section marker, P = .003 vs zero change; and parallel bars, P = .001.
Body weight was stable in those taking placebo alone during treatment (mean change, –0.5 kg; P = .546). Those taking testosterone alone (change, 2.6 kg; P<.001) and those who exercised alone (change, 2.2 kg, P = .023) experienced significant increases in body weight. The weight gain in these 2 groups was greater than that in the placebo-alone group (P<.05). The combination of testosterone and exercise training did not increase body weight more than testosterone alone or exercise alone. Weight gain correlated with change in FFM measured by deuterium oxide dilution (R = 0.75, P<.001) (Figure 3).
Fat-free mass, measured by deuterium oxide dilution, increased significantly from baseline in those taking testosterone alone and those exercising alone, but it did not change significantly in the placebo group (Table 4). However, the change in FFM was not significantly greater in those taking testosterone alone and exercising alone than in those taking placebo alone. The effects of 2 interventions together were not significantly greater than those produced by either intervention alone. Total body water increased significantly in those taking testosterone alone and those exercising alone, but it did not increase in those taking placebo alone. The ratio of FFM by DEXA and total body water did not significantly change in any treatment group, indicating that the apparent increases in FFM were not due to fluid retention in excess of protein accretion. Specific body composition assessed by DEXA is provided in Appendix 3 online at http://www.jama.com.
Table 4.
Body Composition Analysis by Deuterium Oxide Dilution Method*
| No Exercise
|
Exercise
|
|||
|---|---|---|---|---|
| Placebo | Testosterone | Placebo | Testosterone | |
| Fat-free mass, kg | ||||
| Baseline | 60.1 (2.0) | 62.9 (3.3) | 57.9 (2.3) | 55.7 (2.8) |
|
| ||||
| Week 16 | 59.6 (2.3) | 66.9 (2.9) | 59.9 (2.5) | 57.3 (2.9) |
|
| ||||
| Change from baseline | −0.4 (1.0) | 2.9 (1.0) | 2.0 (0.9) | 1.6 (0.8) |
|
| ||||
| P value | .67 | .05 | .04 | <.09 |
|
| ||||
| Fat mass, kg | ||||
| Baseline | 16.6 (2.4) | 14.2 (2.2) | 15.1 (3.1) | 13.3 (3.0) |
|
| ||||
| Week 16 | 16.5 (2.5) | 14.2 (2.8) | 15.7 (3.2) | 12.4 (3.0) |
|
| ||||
| Change from baseline | −0.1 (0.7) | 0.2 (0.6) | 0.6 (0.5) | 0.9 (0.7) |
|
| ||||
| P value | .89 | .81 | .24 | .19 |
|
| ||||
| Total body water, kg | ||||
| Baseline | 43.8 (1.5) | 45.9 (2.4) | 42.3 (1.7) | 40.7 (2.0) |
|
| ||||
| Week 16 | 43.5 (1.7) | 48.9 (2.1) | 43.7 (1.8) | 41.9 (2.1) |
|
| ||||
| Change from baseline | −0.3 (0.7) | 2.1 (0.7) | 1.5 (0.6) | 1.2 (0.6) |
|
| ||||
| P value | .67 | .05 | .01 | .07 |
|
| ||||
| Ratio of total body water by deuterium oxide dilution to fat-free mass by DEXA | ||||
| Baseline | 0.78 (0.02) | 0.77 (0.02) | 0.77 (0.05) | 0.79 (0.03) |
|
| ||||
| Week 16 | 0.77 (0.03) | 0.78 (0.02) | 0.75 (0.04) | 0.78 (0.02) |
|
| ||||
| Change from baseline | −0.02 (0.02) | 0.01 (0.01) | −0.01 (0.01) | −0.02 (0.02) |
|
| ||||
| P value | .23 | .71 | .35 | .35 |
The data on each day represent the mean (SEM) of all available values on that day. However, the change represents the difference between paired values only. DEXA indicates dual-energy x-ray absorbtiometry.
Appendix 3.
Body Composition Assessed by Dual-Energy X-Ray Absorptiometry Scanning*
| No Exercise
|
Exercise
|
|||
|---|---|---|---|---|
| Placebo | Testosterone | Placebo | Testosterone | |
| Fat-free mass, kg | ||||
| Baseline | 56.6 (2.1) | 58.9 (2.8) | 50.5 (5.9) | 52.1 (2.5) |
|
| ||||
| Week 16 | 56.5 (2.8) | 61.2 (2.8) | 58.4 (1.9) | 55.4 (2.7) |
|
| ||||
| Change from baseline | 0.9 (0.7) | 2.3 (0.6) | 2.4 (1.1) | 2.6 (0.4) |
|
| ||||
| P vs zero change | .219 | .004 | .059 | <.001 |
|
| ||||
| Fat mass, kg | ||||
| Baseline | 17.7 (1.9) | 15.9 (1.8) | 13.4 (2.3) | 14.6 (2.0) |
|
| ||||
| Week 16 | 15.6 (2.0) | 16.5 (1.2) | 15.6 (1.4) | 15.1 (2.1) |
|
| ||||
| Change from baseline | −0.5 (0.8) | 0.6 (1.0) | 1.1 (1.4) | −0.4 (0.9) |
|
| ||||
| P vs zero change | .516 | .580 | .364 | .712 |
|
| ||||
| Both arms, lean body mass, kg | ||||
| Baseline | 6.7 (0.3) | 7.3 (0.6) | 6.5 (0.4) | 6.3 (0.5) |
|
| ||||
| Week 16 | 6.9 (0.5) | 7.6 (0.6) | 7.0 (0.3) | 7.2 (0.5) |
|
| ||||
| Change from baseline | 0.1 (0.1) | 0.3 (0.1) | 0.5 (0.2) | 0.7 (0.1) |
|
| ||||
| P vs zero change | .200 | .04 | .04 | <.001 |
|
| ||||
| Both legs, lean body mass, kg | ||||
| Baseline | 17.3 (0.8) | 18.0 (1.2) | 17.1 (0.8) | 15.7 (0.9) |
|
| ||||
| Week 16 | 17.5 (1.1) | 18.5 (1.2) | 17.8 (0.7) | 16.6 (0.9) |
|
| ||||
| Change from baseline | 0.3 (0.3) | 0.6 (0.2) | 0.8 (0.5) | 0.5 (0.2) |
|
| ||||
| P vs zero change | .406 | .040 | .140 | .006 |
|
| ||||
| Trunk, lean body mass, kg | ||||
| Baseline | 26.3 (0.9) | 27.3 (1.1) | 25.9 (0.8) | 24.4 (1.0) |
|
| ||||
| Week 16 | 26.0 (1.2) | 28.6 (1.0) | 27.2 (0.8) | 25.7 (1.2) |
|
| ||||
| Change from baseline | 0.5 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 1.1 (0.3) |
|
| ||||
| P vs zero change | .188 | .016 | .019 | .002 |
The data on each day represent the mean (SEM) of all available values on that day. However, the change represents the difference between paired values.
Fat-free mass measured by DEXA correlated highly with total body water (R = 0.746, P<.001). Fat-free mass, measured by DEXA, did not change in the placebo-treated groups, but increased significantly in the 2 testosterone-treated groups without (change, 2.3 kg; P = .004) and with exercise (change, 2.6 kg; P<.001). The lean mass in the arms, legs, and trunk increased significantly in those who were treated with testosterone but not in those taking placebo. Truncal and whole body fat mass, and bone mineral content did not change in any group.
Health-Related Quality of Life
There was no association between the change in HRQOL measures and testosterone administration or exercise in any group.
Intent-to-Treat Analysis
We analyzed the body weight and hemoglobin data carrying the last available value forward. The mean (SE) change in body weight was significantly greater (overall ANOVA, P = .004) in men treated with testosterone alone (change, 2.9 [0.8] kg; P = .002) or exercise alone (change, 1.7 [0.6] kg; P = .02) than in those receiving placebo alone (0.7 [0.7] kg; P = .39). The mean (SE) change in hemoglobin was significantly greater in those taking testosterone alone (13.5 [5.6] g/L; P = .03) or in those taking testosterone and exercising (7.2 [2.5] g/L; P = .01) than in those in taking placebo alone (−5.6 [2.7] g/L) or those exercising alone (1.8 [5.2] g/L) (overall ANOVA, P = .032).
Adverse Experiences and Safety Measures
Hemoglobin levels increased in testosterone-treated men regardless of exercise (change, P<.05). For those who received testosterone and exercised, it increased by 6%. For those who did not, it increased by 14% (P = .04). It remained unchanged in those taking placebo. The changes in CD4+ and CD8+cell counts, and HIV RNA, bilirubin, alanine aminotransferase, aspartate aminotransferase, plasma triglycerides, low-density lipoprotein and high-density lipoprotein cholesterol, and PSA levels in testosterone-treated men were not significantly different from those in the placebo groups. One person receiving testosterone and 1 receiving placebo developed acne; 1 testosterone-treated man experienced breast enlargement.
COMMENT
In HIV-infected men with moderate weight loss and low testosterone levels, testosterone replacement and resistance exercise each was associated with significant gains in muscle strength, muscle size, and body weight. Fat-free mass did not significantly change in any group. Most of the increase in body weight in testosterone and exercise groups was due to lean mass accretion. The increase in muscle strength and FFM were correlated with increase in muscle volume. The effects of combining testosterone and exercise were not additive. We were unable to detect improvements in HRQOL; a larger sample size might be needed to detect changes in HRQOL.
There is uncertainty about what magnitude of increase in FFM is clinically significant. Testosterone and resistance exercise were each associated with gains in FFM in excess of 2 kg and significant increases in muscle strength. Therefore, we posit that these changes are clinically significant.
The testosterone regimen increased serum testosterone levels by approximately 4.3nmol/L(124ng/dL)and produced significant suppression of LH and FSH levels, providing evidence of androgenic effects. Because serum testosterone levels were measured 7 days after the previous injection, this reflects the smallest increment in testosterone levels that occurred following an injection.
There were no significant differences in the change in FFM or muscle strength during testosterone treatment between men who had baseline testosterone less than 9.5 nmol/L (<275 ng/dL) and those with baseline testosterone between 9.5 and 12.1 nmol/L (275–350 ng/dL). Baseline serum testosterone levels did not correlate with change in FFM. Further studies are needed to determine whether HIV-infected men with low normal testosterone levels respond differently to testosterone supplementation than those who are truly androgen deficient.
The treatment regimen was safe and well tolerated. Testosterone treatment was associated with significant increases in hemoglobin. Changes in serum bilirubin, alanine aminotransferase and aspartate aminotransferase, plasma lipids, and PSA levels were not significantly different from those observed among those taking placebo.
Subject compliance with the treatment regimen was high. One hundred percent of the evaluable patients received greater than 90% of their prescribed injections. The compliance with the exercise regimen was less complete; this may have affected the magnitude of strength gains achieved during exercise training.
A number of studies have examined the effects of androgen supplementation on body composition in HIV-infected men,17–29 although only some were placebo-controlled, blinded, and randomized trials. The dietary intake was not controlled in some of the studies, while others did not standardize the exercise stimulus. The methods used to assess body composition also differed among studies. Of the 4 placebo-controlled studies of testosterone,17–20 only 217,18 demonstrated significant increases in FFM. The 2 studies that did demonstrate significant increments in FFM included patients with low testosterone levels. Studies using oxandrolone and nandrolone decanoate have reported gains in FFM but not muscle strength.21,22,27–28
Our study is the first to demonstrate significant improvements in muscle strength during a pharmacological intervention in HIV-infected men. The failure to control the exercise stimulus and learning effect might have confounded the results of other studies. We minimized the confounding influence of the learning effect by using multiple training sessions during the control period. This appears to have been effective because muscle strength was unchanged among those who received placebo and did not exercise. Our findings indicate that when confounding factors such as the learning effect are minimized and the exercise stimulus and nutritional intake are standardized, testosterone replacement and resistance exercise significantly increase muscle size and strength in HIV-infected men with low testosterone levels.
Some of the initial strength gains from resistance training result from neuromuscular learning. Because we used the same equipment and weight-lifting exercises for strength testing as well as strength training, this strategy may have favored the exercise intervention over androgen treatment in terms of apparent strength gains.
Aerobic exercise improves cardiovascular fitness in HIV-infected patients34,35 but does not produce substantial skeletal muscle hypertrophy. The resistance exercise regimen used in this study resulted in significant muscle hypertrophy, lean body mass accretion, and increments in muscle strength. Similar gains in FFM and strength following resistance training were reported by Roubenoff et al,36 although that study did not have a nonexercising control group. While testosterone and exercise both induced increase in muscle volume, only men who underwent exercise experienced an increase in specific tension. These effects of strength training were achieved in the setting of a motivated patient population, through a supervised program administered by trainers in an exercise laboratory. Even in this setting, the compliance with the exercise regimen was not perfect. Similar degrees of compliance and strength gains may not be achievable with exercise in a clinical setting.
The effects of testosterone and exercise training combined were not additive in this study. The strength training has been shown to augment the anabolic effects of supraphysiologic doses of androgen in healthy, eugonadal men31 and HIV-infected men with weight loss.22,29 We do not know whether the failure to demonstrate additive effects in this study was related to testosterone dose, less than perfect compliance with the exercise regimen, or lack of prior weight-lifting experience.
In comparison with other anabolic agents, such as recombinant human growth hormone37,38 and megesterol acetate,39 testosterone and exercise are relatively inexpensive. Megesterol induces androgen deficiency and may induce a decrease in lean mass. Recombinant human growth hormone administration does not improve muscle strength in HIV-infected individuals.37,38
We conclude that testosterone induces FFM accretion and skeletal muscle hypertrophy and increases effort-dependent muscle strength in HIV-infected men with low testosterone levels and moderate weight loss. We can not extrapolate these data to HIV-infected men with normal testosterone levels or severe wasting. It would be premature to claim that testosterone administration improves muscle function because maximum voluntary strength is only one aspect of muscle function; power, endurance, and task-specific performance are other attributes that were not examined in this study. Muscle strength per unit of muscle mass (specific tension) increased in men undergoing resistance exercise, but it did not increase in those receiving testosterone alone. This implies that resistance training improves the contractile quality of skeletal muscle in HIV-infected men but that testosterone administration without exercise training does not. Further studies are needed to evaluate whether testosterone and exercise can induce clinically useful changes in muscle function and HIV-related disease outcomes.
Acknowledgments
Funding/Support: This study was supported primarily by research grant 1RO1DK49296, as well as grants 1RO1DK49393 and 1ROKDK 50473, from the National Institute of Diabetes and Digestive and Kidney Diseases; 1RO1AG14369 from the National Institute on Aging; MO-00543 from the General Clinical Research Center; P20RR11145-01 and G12RR03026 from the Research Centers in Minority Institutions Clinical Research Infrastructure Initiative Grants; and NCRR 00954 from the National Center for Resources, National Institutes of Health, Bethesda, Md; and FDA-OPD 1397 from the US Food and Drug Administration, Orphan Drug Development Program, Rockville, Md. BioTechnology General Corp, Iselin, NJ, provided testosterone enanthate and placebo injections.
Footnotes
Financial Disclosure: Dr Bhasin has received honoraria, consultant fees, and research grant support from ALZA Pharmaceuticals, Mountain View, Calif; TheraTech Inc, Salt Lake City, Utah; SmithKline Beecham Pharmaceuticals, Philadelphia, Pa; BioTechnology General, Iselin, NJ; and Unimed Pharmaceuticals Inc, Buffalo Grove, Ill.
References
- 1.Kotler DP, Tierney AR, Ferraro R, Francisco A, Wang J, Pierson RN., Jr The magnitude of body cell mass depletion determines the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Grosvenor MB, Bernhard NH, Morales LS, Bulcavage LM. Nutritional status, gastrointestinal dysfunction and survival in patients with AIDS. Am J Gastroenterol. 1989;84:1288–1293. [PubMed] [Google Scholar]
- 3.Linden CP, Allen S, Serufilira A, et al. Predictors of mortality among HIV-infected women in Kigali, Rwanda. Ann Intern Med. 1992;116:320–328. doi: 10.7326/0003-4819-116-4-320. [DOI] [PubMed] [Google Scholar]
- 4.Sellmeyer DE, Grunfeld C. 1996 Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev. 1996;17:518–52. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- 5.Dobs AS, Dempsey MA, Landeson PW, Polk BF. Endocrine disorders in men infected with human immunodeficiency virus. Am J Med. 1988;84:611–615. doi: 10.1016/0002-9343(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 6.Croxon TC, Chapman WE, Miller LK, Levitt CD, Senie R, Zumoff B. Changes in the hypothalamic pituitary-gonadal axis in human immunodeficiency virus-infected hypogonadal men. J Clin Endocrinol Metab. 1989;68:317–321. doi: 10.1210/jcem-68-2-317. [DOI] [PubMed] [Google Scholar]
- 7.Raffi F, Brisseau JM, Planchon B, Remi JP, Barrier JH, Grolleau JY. Endocrine function in 98 HIV-infected patients: a prospective study. AIDS. 1991;5:729–733. doi: 10.1097/00002030-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 8.DePaepe ME, Vuletin JC, Lee MH, Rojas-Corona RR, Waxman M. Testicular atrophy in homosexual AIDS patients. Hum Pathol. 1989;20:572–578. doi: 10.1016/0046-8177(89)90246-3. [DOI] [PubMed] [Google Scholar]
- 9.Coodley GO, Loveless MO, Nelson HD, Coodley MK. Endocrine function in HIV wasting syndrome. J Acquir Immune Defic Syndr Retrovirol. 1994;7:46–51. [PubMed] [Google Scholar]
- 10.Salehian B, Jacobson D, Grafe M, McCutchan A, Swerdloff R. Pituitary-testicular axis during HIV infection: a prospective study. Presented at: 18th Annual Meeting of the American Society of Andrology; April 15–19, 1993; Tampa, Fla. p. Abstract 9. [Google Scholar]
- 11.Grinspoon S, Corcoran C, Lee K, et al. Loss of lean body and muscle mass correlates with androgen levels in hypogondal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab. 1996;81:4051–4058. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- 12.Arver SA, Sinha-Hikim I, Beall G, Shen R, Guerrero M, Bhasin S. Serum dihydrotestosterone and testosterone levels in human immunodeficiency virus-infected men with and without weight loss. J Androl. 1999;20:611–618. [PubMed] [Google Scholar]
- 13.Dobs AS, Few WL, Blackman MR, Harman SM, Hoover DR, Graham NMH. Serum hormones in men with human immunodeficiency virus-associated wasting. J Clin Endocrinol Metab. 1996;81:4108–4112. doi: 10.1210/jcem.81.11.8923868. [DOI] [PubMed] [Google Scholar]
- 14.Bhasin S, Storer TW, Berman N, Yarasheski K, Cleveneger B, Casaburi R. A replacement dose of testosterone increase fat-free mass, and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 15.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S, Storer TW, Asbel-Sethi N, et al. Effects of testosterone replacement with a non-genital, transdermal system: Androderm, in human immunodeficiency virus-infected men with low testosterone levels. J Clin Endocinol Metab. 1998;83:3155–3162. doi: 10.1210/jcem.83.9.5079. [DOI] [PubMed] [Google Scholar]
- 18.Grinspoon S, Corcoran C, Askari H, et al. Effects of androgen adminsitration in men with the AIDS wasting syndrome: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129:18–26. doi: 10.7326/0003-4819-129-1-199807010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Coodley GO, Coodley MK. A trial of testosterone therapy for HIV-associated weight loss. AIDS. 1997;11:1347–1352. doi: 10.1097/00002030-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Dobs A, Cofrancesco J, Nolten WE, et al. The use of a transscrotal testosterone delivery system in the treatment of patients with weight loss related to human immunodeficiency virus infection. Am J Med. 1999;107:126–132. doi: 10.1016/s0002-9343(99)00193-x. [DOI] [PubMed] [Google Scholar]
- 21.Strawford A, Barbieri T, Neese R, et al. Effects of nandrolone decanoate therapy in borderline hypogonadal men with HIV-associated weight loss. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:137–146. doi: 10.1097/00042560-199902010-00005. [DOI] [PubMed] [Google Scholar]
- 22.Strawford A, Barbieri T, Van Loan M, et al. Resistance exercise and supraphysiologic androgen therapy in eugonadal men with HIV-related weight loss. JAMA. 1999;281:1282–1290. doi: 10.1001/jama.281.14.1282. [DOI] [PubMed] [Google Scholar]
- 23.Rabkin JG, Wagner GJ, Rabkin R. Testosterone therapy for human immunodeficiency virus-positive men with and without hypogonadism. J Clin Psychopharmacol. 1999;19:19–27. doi: 10.1097/00004714-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Gold J, High HA, Li Y, et al. Safety and efficacy of nandrolone decanoate for treatment of wasting in patients with HIV infection. AIDS. 1996;10:745–752. doi: 10.1097/00002030-199606001-00008. [DOI] [PubMed] [Google Scholar]
- 25.Berger JR, Pall L, Winfield D. Effect of anabolic steroids on HIV-related wasting myopathy. South Med J. 1993;86:865–866. doi: 10.1097/00007611-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Hengge UR, Baumann M, Maleba R, et al. Oxymethalone promotes weight gain in patients with advanced human immunodeficiency virus (HIV-1) infection. Br J Nutr. 1996;75:129–138. doi: 10.1079/bjn19960116. [DOI] [PubMed] [Google Scholar]
- 27.Poles MA, Meller JA, Lin A, Weiss WR, Gocke M, Dietrich DT. Oxandrolone as a treatment for AIDS-related weight loss and wasting. Presented at: the Infectious Disease Society of America Conference; September 19, 1996; New York, NY. [Google Scholar]
- 28.Bucher G, Berger DS, Fields-Gardner C, Reiter JR. A prospective study of the safety and effect of nandrolone decanoate in HIV-positive patients [abstract]. Program and abstracts of the 11th International Conference on AIDS; July 7–12, 1996; Vancouver, British Columbia. [Google Scholar]
- 29.Sattler FR, Jaque SV, Schroeder ET, et al. Effect of pharmacological doses of nandrolone decanoate and progressive resistance training in immunodeficient patients infected with the human immunodeficiency virus. J Clin Endocrinol Metab. 1999;84:1268–1276. doi: 10.1210/jcem.84.4.5610. [DOI] [PubMed] [Google Scholar]
- 30.Rebouche CJ, Pearson GA, Serfass RE, Roth CW, Finley JW. Evaluation of nuclear magentic resonance spectroscopy for determination of deuterium abundance in body fluids: application to measurement of total body water in human infants. Am J Clin Nutr. 1987;45:373–380. doi: 10.1093/ajcn/45.2.373. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–6. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 32.Sinha-Hikim I, Arver S, Beall G, et al. The use of a sensitive, equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women, and in HIV-infected women. J Clin Endocrinol Metab. 1998;83:1312–1318. doi: 10.1210/jcem.83.4.4718. [DOI] [PubMed] [Google Scholar]
- 33.Hays RD, Cunningham WE, Ettl MK, Beck CK, Shapiro MF. Health-related quality of life in HIV disease. Assessment. 1995;2:363–380. [Google Scholar]
- 34.MacArthur RD, Levine SD, Birk TJ. Supervised exercise training improves cardiovascular fitness in HIV-infected persons. Med Sci Sports Exerc. 1993;25:684–688. [PubMed] [Google Scholar]
- 35.Stringer WW, Berezovskaya M, O’Brien WA, Beck CK, Casaburi R. The effect of exercise training on aerobic fitness, immune indices, and quality of life in HIV+ patients. Med Sci Sports Exerc. 1998;30:11–16. doi: 10.1097/00005768-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Roubenoff R, McDermott A, Weiss L, et al. Short-term progressive resistance training increases strength and lean body mass in adults infected with human immunodeficiency virus. AIDS. 1999;13:231–239. doi: 10.1097/00002030-199902040-00011. [DOI] [PubMed] [Google Scholar]
- 37.Waters D, Danska J, Hardy K, et al. Recombinant human growth hormone, insulin-like growth factor I, and combination therapy in AIDS-associated wasting: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:8665–8672. doi: 10.7326/0003-4819-125-11-199612010-00001. [DOI] [PubMed] [Google Scholar]
- 38.Schambelan M, Mulligan K, Grunfeld C, et al. Recombinant human growth hormone in patients with HIV-associated wasting. Ann Intern Med. 1995;125:873–882. doi: 10.7326/0003-4819-125-11-199612010-00002. [DOI] [PubMed] [Google Scholar]
- 39.Von Roenn JH, Armstrong D, Kotler D, et al. Megestrol acetate in patients with AIDS-related cachexia. Ann Intern Med. 1994;121:393–399. doi: 10.7326/0003-4819-121-6-199409150-00001. [DOI] [PubMed] [Google Scholar]