Abstract
Background
The JUPITER trial demonstrated that some patients with LDL-C <130 mg/dL and hsCRP ≥2 mg/L benefit from rosuvastatin, although absolute event rates were low. We sought to determine whether coronary artery calcium (CAC) may further risk stratify a JUPITER-eligible population, and to compare hsCRP vs. CAC for risk prediction in otherwise JUPITER-eligible participants.
Methods
A total of 950 MESA participants met all JUPITER entry criteria. We compared CHD and CVD event rates and multivariable-adjusted hazard ratios after stratifying by both presence and burden of CAC (0, 1–100, >100). We also calculated 5-year number needed to treat (NNT5) by applying the benefit observed in JUPITER to the observed event rates within each CAC strata.
Findings
Median follow-up was 5.8 years. Approximately 47% of the MESA JUPITER population had CAC=0, and CHD event rates in this group were <1 per 1000 person-years. Over 2/3 of all CHD events occurred in the 25% of participants with CAC >100 (20.2 per 1000 person-years). For CHD, the predicted NNT5 for CAC 0, 1–100, and >100 was 549, 94, and 24 respectively. For CVD, the NNT5 was 124, 54, and 19. Amongst otherwise JUPITER-eligible patients, presence of CAC was associated with 4.3-fold increased CHD (95% CI 2.0 – 9.3) and 2.6-fold increased CVD (95% CI 1.5–4.5), while hsCRP was not associated with either CHD or CVD after multivariable adjustment.
Interpretation
Within MESA, approximately half of JUPITER-eligible participants had CAC=0 and experienced an extremely low 6-year event rate. Nearly all events occurred in patients with CAC. CAC appears to further risk stratify JUPITER-eligible patients and may be used to target a subgroup of patients expected to derive the most, and the least, absolute benefit from statin treatment. Focusing treatment on the subset of individuals with measurable atherosclerosis may represent a more appropriate allocation of resources.
Funding
NIH-NHLBI.
Keywords: hsCRP, CAC, and Clinical Events
INTRODUCTION
Landmark clinical trials have led to progressive liberalization of statin use in primary prevention1–3. The JUPITER trial lowered the threshold further by demonstrating that some patients with ‘normal’ LDL-C (<130 mg/dl) and hsCRP≥2 benefit from rosuvastatin4. Unfortunately, as statin trials extend to lower risk populations, even large relative risk reductions are rewarded by modest reductions in absolute risk. Thus, despite impressive relative risk reductions like those observed in JUPITER, many patients newly eligible for statins will not accrue a net benefit from treatment. There remains a need for improved personalized cardiovascular risk assessment.
Coronary artery calcification (CAC) detected by cardiac computed tomography estimates the burden of coronary atherosclerosis and is effective for further risk stratifying asymptomatic patients5. The absence of CAC in an asymptomatic adult nearly excludes clinically important coronary atherosclerosis, and is associated with a mortality rate of ~1% over 10 years6, 7. In contrast, significantly elevated CAC is associated with a nearly 10-fold increased risk of adverse coronary events after multivariable adjustment8. Further, CAC has been shown to improve classification of patients into the appropriate clinical risk groups9.
We sought to determine whether CAC testing might identify a subgroup of JUPITER-eligible patients expected to derive the most, and the least, benefit from statin treatment. Given estimates that 6.5 million individuals that would be newly eligible for statins in the United States alone based on JUPITER10, these results have important implications for guidelines and public health discussions aimed at improving the efficiency and cost-effectiveness of statin use in primary prevention.
In addition, we sought to directly compare CAC versus hsCRP as additional markers for discriminating risk in otherwise JUPITER-eligible individuals independent of hsCRP inclusion criteria. Such comparative-effectiveness analyses examining the incremental predictive value of tests in their intended target populations are critical for directing their appropriate use.
METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA)
The Multi-Ethnic Study of Atherosclerosis (MESA) is a NIH/NHLBI-funded population-based prospective cohort study aimed at describing the prevalence, progression, and significance of subclinical atherosclerosis. Details of the MESA study design have been previously published11.
Between July 2000 and September 2002, MESA enrolled 6,814 individuals at six field centers (Baltimore; Chicago; Forsyth County, North Carolina; Los Angeles; New York; and St. Paul, Minnesota). The participants were required to be age 45 to 84 and have no known clinical cardiovascular disease at the time of enrollment. Participants were recruited at each site from lists of residents, dwellings, and telephone-company customers with emphasis on ethnic diversity.
Patient Population
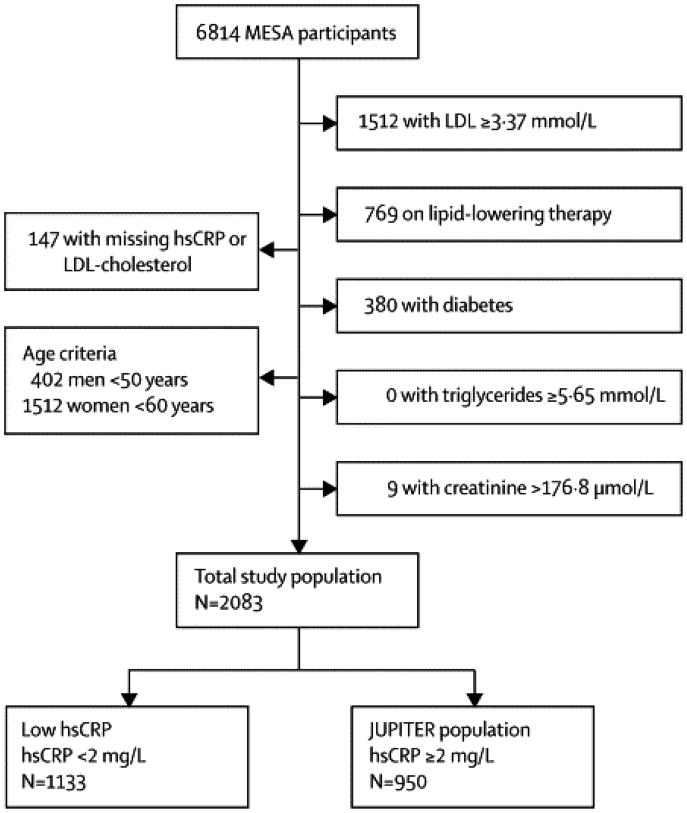
Using baseline data (MESA, 2000–2002), we identified 2,083 MESA participants (31%) who fit the following JUPITER inclusion criteria: age ≥50 for men and ≥60 for women, LDL-C <130 mg/dL, not on lipid-lowering therapy, free of diabetes, triglycerides <500 mg/dL, and creatinine ≤2 mg/dL (Figure 1). Of these 2,083 individuals comprising the “Total Study Population”, 950 (46%) had high hsCRP (hsCRP ≥2 mg/dL) and were thus eligible for the JUPITER trial (“MESA JUPITER” population, Figure 1).
Figure 1. Assembly of the Study Population.
The “MESA JUPITER” population was used to test the ability of CAC to risk stratify the JUPITER-eligible population. The “Total Study Population” was used to test the relative predictive value of CAC and hsCRP.
Cardiac CT Protocol
Cardiac CT was performed at 3 sites using a cardiac-gated electron-beam CT scanner and at 3 sites using 4-slice multidetector CT. Patients were scanned twice, with CAC (Agatston) scores averaged. Images were interpreted at the MESA CT reading center (Harbor-UCLA).
Carr et al. have reported details of the methods used by MESA for computed tomographic (CT) scanning and interpretation12. The kappa statistic for agreement on presence of CAC was 0.92, and the mean rescan percentage absolute difference in CAC was 20.1% among those with CAC>0.
hsCRP and Study Covariates
As part of the baseline examination, clinical teams at each of the six centers collected information on cardiovascular risk factors. A central laboratory (University of Vermont, Burlington) measured levels of total and HDL cholesterol, triglycerides, plasma glucose, and high-sensitivity C-reactive protein after a 12-hour fast. hsCRP was determined by BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL). The lower limit of detection was 0.17 mg/L.
Follow-up and Event Adjudication
New coronary heart disease (CHD) and cardiovascular disease (CVD) events were recorded over a median follow-up of 5.8 years. At intervals of 9 to 12 months, an interviewer contacted each participant or a family member regarding interim hospital admissions, outpatient diagnoses of CHD and CVD, and deaths. MESA was successful in obtaining medical records for approximately 98% of hospitalized events and information on 95% of outpatient cardiovascular diagnostic encounters. Follow-up telephone interviews were completed in 92% of living participants.
Two physician members of the MESA mortality and morbidity review committee independently classified events, and in the event of disagreement, the full committee adjudicated. CHD events consisted of myocardial infarction, death from coronary heart disease, definite angina, probable angina followed by coronary revascularization, or resuscitated cardiac arrest. CVD events consisted of CHD events plus stroke (not TIA), stroke death, other atherosclerotic death, and other CVD death. A detailed description of the MESA follow-up methods is available at www.mesa-nhlbi.org.
Statistical Analysis
Baseline characteristics of the 2,083 study participants were analyzed according to hsCRP status (low [<2 mg/L] or high [≥2 mg/L]). Frequencies and proportions were calculated for categorical variables, and either means with standard deviations or medians with interquartile ranges calculated for continuous variables.
We used Kaplan-Meier estimates of cumulative event-free survival to describe the occurrence of CHD and CVD events over time. To determine if CAC could further risk stratify the JUPITER population, we compared absolute CHD and CVD event rates and Cox multivariable-adjusted hazard ratios after stratifying by both presence and burden of CAC (0, 1–100, >100). Models were adjusted for age, gender, race/ethnicity, hypertension, smoking, BMI, HDL-C, antihypertensive medication use, family history of myocardial infarction, education level (a measure of socioeconomic status), and MESA site.
To compare the relative predictive power of hsCRP and CAC, we compared absolute CHD and CVD event rates stratified by hsCRP and CAC status in the total study population. In addition, we tested for interaction/effect modification between hsCRP and CAC.
Number Needed to Treat Analysis
We calculated 5-year number needed to treat (NNT5) for both CHD and CVD by applying the hazard ratio associated with rosuvastatin treatment in the JUPITER trial (0.56) to the event rates observed within each CAC strata. For this analysis, NNT were calculated directly as the reciprocal of the absolute risk difference at median follow-up of the cohort (5.8 years), based on Kaplan-Meier estimates, and then subsequently adjusted to a 5-year NNT5 according to the Altman-Anderson method13. Sensitivity analyses were conducted using the upper and lower limits of the hazard ratio observed in JUPITER.
RESULTS
Baseline Characteristics
Median age of the total study population (N=2,083) was 67 (interquartile range [IQR]) 61 – 73) years. Overall, 40% were female, with mean calculated 10-year Framingham risk of 9.7 ± 7%. Median hsCRP of the total study population was 1.8 mg/L (IQR 0.78 – 4.0). A total of 1,133 (54%) participants had hsCRP <2 mg/L and 950 (46%) had hsCRP ≥2 mg/L (MESA JUPITER population). Individuals in the MESA JUPITER subgroup were more likely to be female and either African-American or Hispanic, with more features of the metabolic syndrome (Table 1).
Table 1.
Baseline Characteristics.
| JUPITER Population | ||||
|---|---|---|---|---|
| Characteristic | Total Population (N = 2,083) | hsCRP <2 mg/L (N = 1,133) | hsCRP ≥2 mg/L (N = 950) | P |
| Age, years | 66·5 ± 9 | 66·3 ± 9 | 66·7 ± 8 | 0·30 |
| Gender, women | 40% | 31% | 51% | <0·0001 |
| Race | ||||
| ▪ Whites | 41% | 41% | 31% | 0·0001 |
| ▪ Chinese | 13% | 20% | 5% | |
| ▪ African American | 27% | 23% | 31% | |
| ▪ Hispanic | 19% | 16% | 23% | |
| BMI, kg/m2 | 27·4 ± 5 | 26·0 ± 4 | 29·1 ± 5 | <0·0001 |
| Systolic blood pressure, mmHg | 129 ± 22 | 127 ± 22 | 130 ± 21 | 0·005 |
| Diastolic blood pressure, mmHg | 72 ± 10 | 73·0 ± 10 | 71·6 ± 11 | 0·004 |
| Hypertension | 47% | 41% | 53% | <0·0001 |
| Fasting glucose | 97 ± 10 | 96 ± 9 | 97 ± 10 | 0·005 |
| Cr, mg/dL | 0·98 ± 0.2 | 0·98 ± 0.2 | 0·97 ± 0.2 | 0·17 |
| Smoking | ||||
| ▪ Former | 41% | 40% | 43% | 0·0001 |
| ▪ Current | 12% | 9% | 14% | |
| LDL, mg/dL | 102 ± 19 | 103 ± 19 | 102 ± 20 | 0·48 |
| HDL, mg/dL | 52·2 ± 16 | 52·1 ± 16 | 52·3 ± 17 | 0·77 |
| Triglycerides, mg/dL | 102 (72 – 151) | 97 (69 – 141) | 109 (77 – 160) | <0·0001 |
| Family history of heart attack | 40% | 38% | 43% | 0·03 |
| Medications for hypertension | 37% | 32% | 42% | <0·0001 |
| Education, completed HS/GED | 83% | 86% | 80% | 0·0004 |
| hsCRP, mg/L | 1·77 (0·78 – 3·99) | 0·85 (0·52 – 1·32) | 4.26 (2·96 – 7·77) | <0·0001 |
| 10-yr FRS (%) | 9·7 ± 7% | 10·1 ± 7% | 9·2 ± 7% | 0·003 |
Data presented are either mean (± standard deviation), median (interquartile range), or proportion (%).
The MESA JUPITER population closely resembled the JUPITER trial placebo group (Supplemental Figure 1). Median age of the JUPITER trial placebo group was 66 (60 – 71), mean calculated 10-year Framingham risk of 10%, and median hsCRP was 4.3 (3.0 – 7.8). The MESA JUPITER population had more females (51% vs. 38%), owing to its population-based recruitment with similar initial enrollment by gender, and to the higher hsCRP levels observed in women.
Distribution of CAC in the MESA JUPITER Population
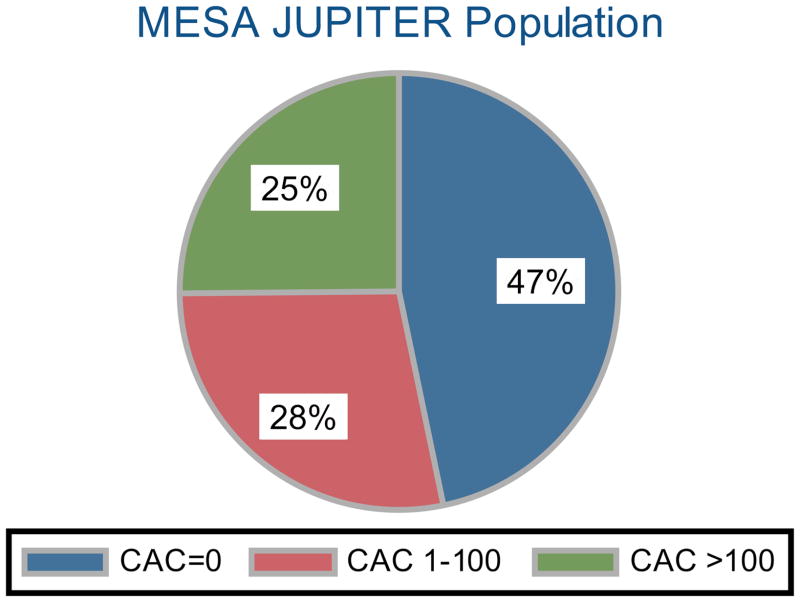
Approximately 47% of the MESA JUPITER population had a CAC score of zero. Of those with CAC, 28% had CAC scores 1–100 and 25% had CAC scores >100 (Figure 2). The prevalence of CAC and frequency of increased CAC burden was similar in the low hsCRP group (p=0.09, see Supplemental Figure 2).
Figure 2. Distribution of Coronary Artery Calcium (CAC) Burden in the MESA JUPITER population.
The number needed to scan (NNS) to identify one individual with CAC=0 is 2. The NNS to identify one individual with elevated CAC >100 is 4.
Prevalence of CAC differed according to gender. Approximately 53% of women had zero CAC, while 40% of men had a CAC score of zero. A total of 20% of women had CAC >100, while 31% of men had CAC >100.
Event Rates by Prevalence and Burden of CAC
Table 2 details the frequency of CHD and CVD events, the corresponding event rates per 1000 person-years, and the multivariable-adjusted hazard ratios associated with prevalence and burden of CAC in MESA JUPITER participants.
Table 2.
CHD and CVD Events by CAC Status in the JUPITER-eligible MESA Population
| CAC Group | CHD Events | CVD Events | |||||
|---|---|---|---|---|---|---|---|
| N (%) | CHD events (%) | Event rate (per 1000 person-years) | Hazard Ratio (95% CI) | CVD Events (%) | Event rate (per 1000 person-years) | Hazard Ratio (95% CI) | |
| Zero CAC | 444 (47%) | 2 (0.5%) | 0.8 | 1 (ref) | 9 (2.0%) | 3.7 | 1 (ref) |
| CAC present | 506 (53%) | 32 (6.3%) | 11.0 | 11.0 (2.51–48.5) | 44 (8.7%) | 16.6 | 3.20 (1.41–7.24) |
|
| |||||||
| CAC = 0 | 444 (47%) | 2 (0.5%) | 0.8 | 1 (ref) | 9 (2.0%) | 3.7 | 1 (ref) |
| CAC 1–100 | 267 (28%) | 7 (2.6%) | 4.8 | 4.91 (0.97–24.9) | 12 (4.5%) | 8.4 | 1.86 (0.73–4.76) |
| CAC >100 | 239 (25%) | 25 (10.6%) | 20.2 | 27.8 (5.97–129.8) | 32 (13.4%) | 26.4 | 6.16 (2.51–15.1) |
Adjusted for: age, gender, race, hypertension, cigarette smoking, BMI, HDL-C, anti-hypertensive medication use, family history of CHD, socioeconomic status, and MESA site
CHD and CVD event rates were low when CAC=0 (0.8 and 3.7 events per 1000 person-years, respectively). In contrast, event rates were high when CAC >100 (20.2 and 26.4 events per 1000 person years). Just 6% of all CHD events and 17% of all CVD events occurred in the 47% of individuals with CAC=0. Nearly 75% of all CHD events and approximately 60% of all CVD events occurred in the 25% of participants with CAC>100.
The presence of CAC was associated with a hazard ratio of 11.0 (95% confidence interval [CI]: 2.51 – 48.5) for CHD and 3.20 (95% CI: 1.41 – 7.24) for CVD in the MESA JUPITER population in the fully adjusted model. There was a graded increase in both CHD and CVD events with increasing burden of CAC. Participants with CAC>100 had a hazard ratio of 27.8 (95% CI: 5.97 – 129.8) for CHD and 6.16 (95% CI: 2.51 – 15.1) for CVD events compared to participants with zero CAC.
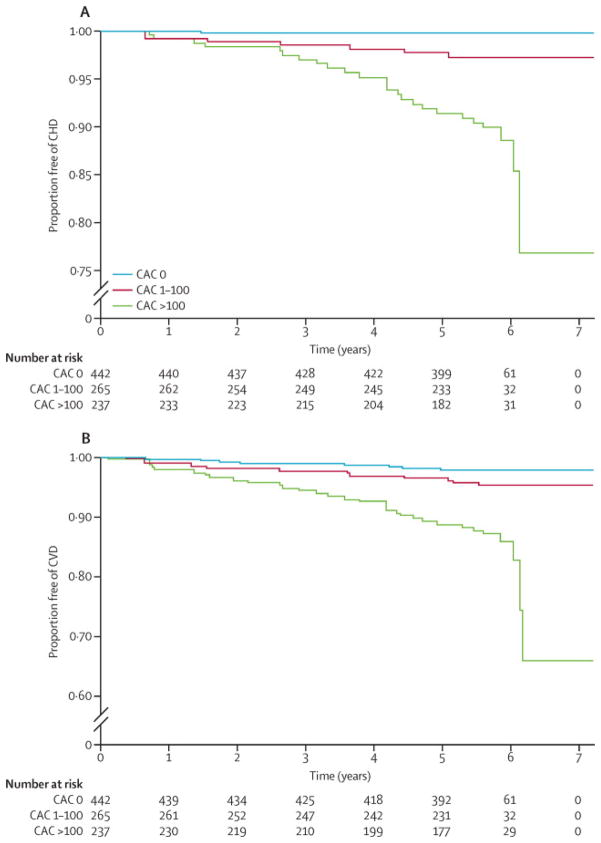
Number Needed to Treat According to CAC
Figure 3 shows Kaplan-Meier estimates of CHD and CVD event-free survival for the MESA JUPITER population by CAC burden. Table 3 shows the Kaplan-Meier failure (event) function. At median follow-up of 5.8 years, the estimated CHD event rate for CAC 0, 1–100, and >100 was 0.48%, 2.8%, and 10.8% respectively. The corresponding estimated CVD event rate was 2.1%, 4.9%, and 13.7%.
Figure 3. Kaplan-Meier Estimates of CHD and CVD Event-Free Survival by Coronary Artery Calcium (CAC) Burden in the MESA JUPITER Population.
Table 3.
Estimated 5-Year Number Needed to Treat (NNT5) to Prevent One CHD or CVD Event, by Coronary Artery Calcium (CAC) Burden
| Estimated CHD event rate at 5.8 years | Estimated CVD event rate at 5.8 years | 5-year NNT for CHD | 5-year NNT for CVD | |
|---|---|---|---|---|
| JUPITER-eligible population | ||||
| ▪ Zero CAC | 0.48% | 2.12% | 549 | 124 |
| ▪ CAC present | 6.22% | 8.87% | 42 | 30 |
|
| ||||
| ▪ CAC=0 | 0.48% | 2.12% | 549 | 124 |
| ▪ CAC 1–100 | 2.79% | 4.86% | 94 | 54 |
| ▪ CAC >100 | 10.76% | 13.65% | 24 | 19 |
NNT were calculated directly as the reciprocal of the absolute risk difference at median follow-up of the cohort (5.8 years), based on Kaplan-Meier estimates, and then subsequently adjusted to a 5-year NNT according to the Altman-Anderson method13
Using these estimates, the NNT5 to prevent a CHD event for CAC 0, 1–100, and >100 was 549, 94, and 24 respectively. The corresponding NNT5 to prevent a CVD event was 124, 54, and 19 (Table 3). The results of the sensitivity analysis are shown in Supplemental Table 1.
hsCRP vs. CAC for Risk Prediction
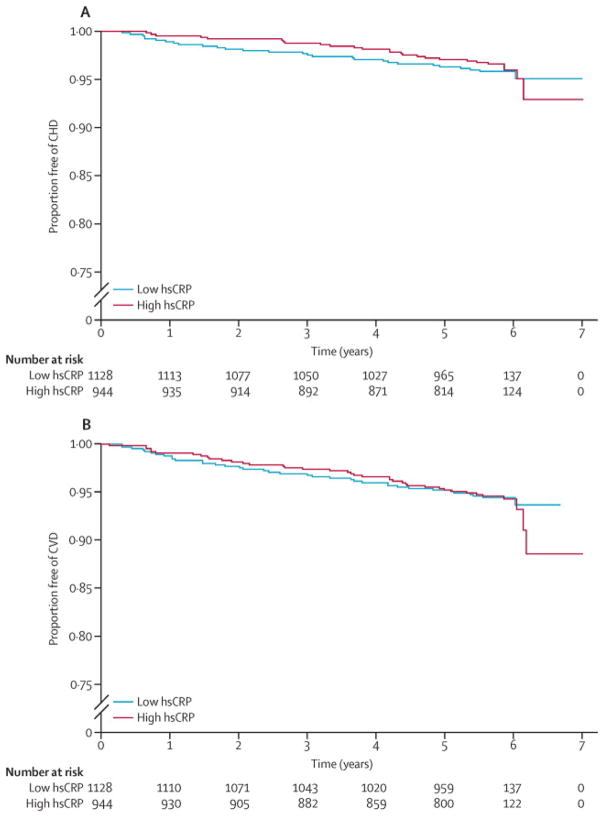
Within the total study population, overall event rates were similar in the low (<2 mg/L) and high (≥2 mg/L) hsCRP groups (7.6 vs. 6.4 CHD events [p=0.47] and 10.1 vs. 10.4 CVD events per 1000 patient-years [p=0.87]. Kaplan Meier plots stratified by hsCRP status are shown in Figure 4. hsCRP status did not predict CHD (HR: 0.98, 95% CI: 0.62 – 1.57) or CVD events (HR: 1.15, 95% CI: 0.78 – 1.68) after adjusting for age, gender, and race. In contrast, presence of CAC was a strong predictor of both CHD (HR: 6.65, 95% CI: 2.99 – 14.78) and CVD (HR: 3.06, 95% CI: 1.82 – 5.13) in similarly adjusted models. CAC prevalence, and increasing CAC burden, remained significant predictors of events after full multivariable adjustment (Table 4).
Figure 4. Kaplan-Meier Estimates of CHD and CVD Event-Free Survival by hsCRP Status in the Total Study Population.
Event-free survival did not differ by hsCRP status (Log-rank p=0.55 for CHD events, p=0.87 for CVD events).
Table 4.
Coronary Artery Calcium (CAC) vs. hsCRP for Risk Prediction in Otherwise JUPITER-Eligible Patients
| Total Population (N=2,083) | CHD Events | CVD Events | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| CRP < 2 mg/L | 1 (ref) | - | 1 (ref) | - |
| CRP ≥ 2mg/L | 0.90 (0.54–1.50) | 0.69 | 1.08 (0.71–1.64) | 0.73 |
| CAC=0 | 1 (ref) | - | 1 (ref) | - |
| CAC>0 | 4.29 (1.99–9.25) | <0.0001 | 2.57 (1.48–4.48) | 0.001 |
| CAC=0 | 1 (ref) | - | 1 (ref) | - |
| CAC 1–100 | 1.66 (0.65–4.25) | 0.29 | 1.46 (0.75–2.81) | 0.26 |
| CAC >100 | 9.35 (4.15–21.1) | <0.0001 | 4.41 (2.42–8.04) | <0.0001 |
Adjusted for age, gender, race, hypertension, cigarette smoking, BMI, HDL-C, anti-hypertensive medication use, family history of CHD, socioeconomic status, and MESA site
No change in the model (no residual confounding) when adjusted for LDL-C
CAC x hsCRP status interaction term not significant
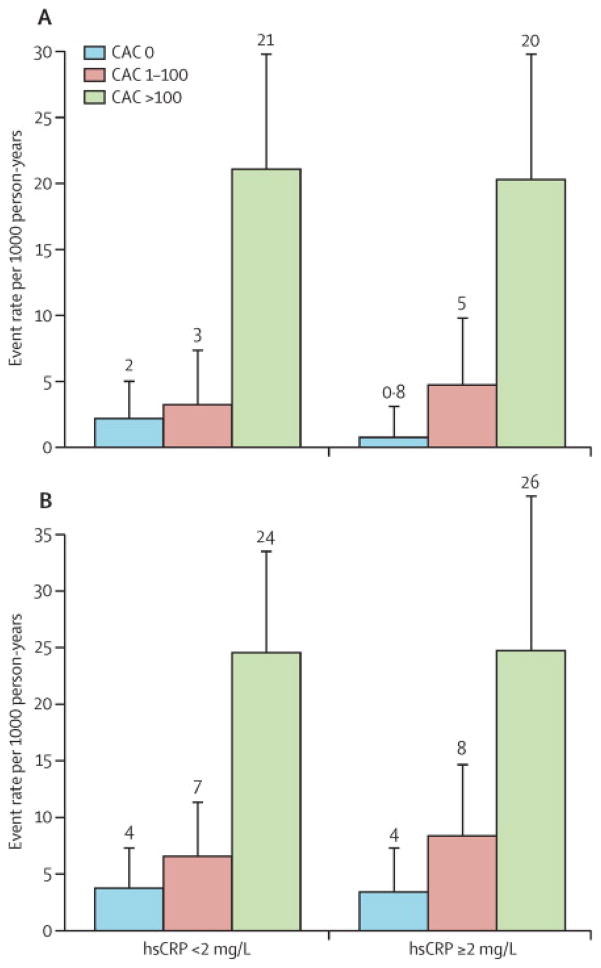
Increasing CAC burden led to similar increases in absolute CHD and CVD event rates in both the low and high hsCRP groups (Figure 5). There was no evidence of interaction between hsCRP status and CAC burden (p=0.71).
Figure 5. Absolute CHD and CVD Event Rates by Coronary Artery Calcium (CAC) Burden, Stratified by hsCRP Status.
Overall event rates were similar in the low vs. high hsCRP groups: 7.6 vs. 6.4 CHD events [p=0.47] and 10.1 vs. 10.4 CVD events per 1000 patient-years [p=0.87]. CHD and CVD event rates were higher with increasing CAC in both hsCRP groups (each p<0.0001), and the event rate distribution was not significantly different when stratified by hsCRP status (p=0.41). In age, gender, and race adjusted survival analysis, there was no interaction between CAC and hsCRP for prediction of either CHD or CVD events (p=0.7).
There was no evidence of residual confounding with hsCRP using dichotomized hsCRP status (low and high). Median hsCRP in the MESA JUPITER population with and without CVD events was 4.25 and 4.54 mg/L, respectively (p=0.61). Median hsCRP in the total study population was 1.78 mg/L in participants with CVD events, and 1.73 mg/L in those without events (p=0.67).
A total of 48 of the 71 CHD events were classified as “hard” CHD events (myocardial infarction, resuscitated cardiac arrest, or CHD Death) and 79 of 118 CVD events were classified as “hard” CVD events (hard CHD events plus stroke [not TIA] or stroke death). There were no differences in the predictive value of CAC or hsCRP when “hard” events were substituted for all CHD or CVD events.
DISCUSSION
Statin Use in Primary Prevention
As statin use is extended to lower risk populations, accurate assessment of absolute risk becomes critical for determining the net value of treatment. In this study, we show that nearly half of the MESA JUPITER population had no CAC, experienced an extremely low event rate, with an unfavorable estimated NNT5 of 549 to prevent one CHD event. In contrast, a majority of all CHD events (74%) occurred in the small (25%) group of MESA JUPITER patients with CAC>100. When CAC>100, the estimated NNT5 for CHD and CVD was small at 24 and 19, respectively. These results have important implications for future guidelines and public health discussions aimed at improving the efficiency of statin use in primary prevention.
Current primary prevention guidelines support the use of statins to treat elevated cholesterol in individuals deemed higher risk by traditional risk scoring. Future guidelines are likely to incorporate the concept of the JUPITER trial and recommend statin treatment in patients with “normal” cholesterol who are at elevated risk based on another risk factor or biomarker (like hsCRP). Based on our results, CAC should be strongly considered in these patients, substantiating the IIA recommendation for CAC screening in the updated AHA guidelines for testing in asymptomatic adults14.
CAC vs. hsCRP for Risk Prediction in Patients with Low LDL-C
Based on the inflammatory hypothesis of atherothrombosis, it has been hypothesized that elevated hsCRP may provide a mechanistic link to the individuals who will receive the greatest benefit from statins15. Without a “biomarker control group” of individuals with hsCRP <2 mg/L in JUPITER, it is impossible to determine if such low hsCRP patients would have similarly benefitted. Secondary analyses from JUPITER have shown that the relative risk reduction with rosuvastatin was remarkably consistent, and not graded, across increasing levels of hsCRP16. Secondary analysis of the Heart Protection Study found that statins achieve a similar relative risk reduction at all levels of hsCRP, including in patients with low hsCRP17. Therefore, the benefit of hsCRP testing appears to rely solely on its generally consistent association with modestly increased absolute risk, and thus anticipated higher absolute benefit from treatment16.
In this study, we found that simple presence of CAC discriminates both absolute and relative CHD risk over a much wider range than the hsCRP ≥2 mg/L (HR: 4.26 vs. 0.90 in fully adjusted multivariable models). While CAC is less strong predictor of CVD, it remains superior to hsCRP (HR: 2.57 vs. 1.08 in fully adjusted models). Our finding that hsCRP does not effectively discriminate risk has been observed in other studies18, but stands in contrast to the modest independent predictive value of hsCRP in the largest meta-analysis (age and gender-adjusted relative risk 1.63)19. Reasons for the failure of hsCRP to predict risk in MESA may include the multi-ethnic makeup of the cohort and the use of the fixed JUPITER cutpoint of 2 mg/L, which does not take into account the highly different distributions of hsCRP across gender and race in a highly diverse population.
CAC has a few advantages and a few disadvantages compared to hsCRP. CAC is a direct measure of the burden of atherosclerosis, the precursor lesion for most CHD events, and is best considered a measure of disease rather than a risk factor. Indeed, the progression of CAC over time is a strong predictor of mortality20. Another advantage of CAC is that there is little variability when the measurement is repeated21. In addition, CAC has the advantage of consistent thresholds of risk across different populations8, while hsCRP varies greatly by gender and ethnicity with limited data on varying risk thresholds22, 23.
A disadvantage of CAC is radiation exposure, although the dose using modern technology is low (0.5 – 1.5 mSv, compared to background radiation of 3 mSv/year). The average measured radiation dose was 0.89 mSv in MESA. In addition, incidental noncardiac findings such as lung nodules >4–6mm in diameter generally lead to referral for imaging follow-up at 6–12 months, despite no proven mortality benefit for following such lesions. Also, CAC is more expensive that hsCRP testing, although many metropolitan areas in the United States charge < $100. While hsCRP has possible value in monitoring the potency of the statin treatment effect24, there is no data or biologically plausible mechanism suggesting that statins lower CAC25.
Prior literature has suggested that combining hsCRP and CAC may be better than using either alone in select patients. Park et al. followed up 967 individuals without diabetes for mean 6.4 years, demonstrating that the majority of risk resided with CAC, with very high CRP (>4.05 mg/L) providing mild incremental risk improvement26. Similarly, data from the Heinz-Nixdorf Recall study, a large cohort study with a design similar to MESA, showed that improvement in coronary risk prediction and discrimination was driven predominantly by CAC, with hsCRP >3 mg/L (the JUPITER cutpoint of 2 mg/L was not studied) providing mild incremental improvement predominantly versus hsCRP <1 mg/L in persons with very low CAC scores27. Importantly, CAC and hsCRP likely identify distinct mechanisms of risk. CAC, but not hsCRP28, identifies overall burden of coronary atherosclerosis, while emerging data indicates that hsCRP may provide some insight into the stability of the coronary plaque29.
The Importance of CAC=0 and Implications for CAC Testing
We believe that our results have important public health implications. JUPITER-eligible MESA patients with no coronary calcification had a very low CHD event rate of <1 per 1000 person-years, corresponding to an ~1% 10-year event rate, consistent with prior data demonstrating excellent prognosis when CAC=06, 7. Prior reports have suggested that asymptomatic patients with zero coronary calcification can be treated to less aggressive risk factor targets, with less aggressive pharamacotherapy, emphasizing low-cost lifestyle interventions30. The NNT5 to prevent one CHD event of 549 in this study when CAC=0 appears to support a conservative strategy. Indeed, this NNT5 exceeds the 4-year number needed to treat (NNT4) of 255 for new-onset diabetes seen with statin use in a prior meta-analysis31.
Similar to many prior studies6, 8, the large majority of the events in MESA JUPITER participants occurred in the minority of individuals with elevated CAC>100. The event rate of 20–26 events per 1000 person-years in this group places them within the conventional high-risk designation of >20% 10-year risk. Based on these findings, narrowing therapy to those with CAC>100 (~1/4th of the JUPITER population) would result in treating subgroup in whom nearly 75% of all CHD events would occur. Narrowing statin therapy to those with CAC (~1/2 of the JUPITER population) would result in treating a subgroup in whom 95% of CHD events would occur over 6 years.
Comparison with JUPITER and ARIC
Event rates in MESA (Table 2) were lower than those observed in the JUPITER placebo group (13.6 CVD events per 1000 patient-years) and the ARIC JUPITER population (15.7 per 1000 patient-years)32. Despite this, the NNT5 for CVD of 19 in the MESA JUPITER population with CAC>100 is lower compared to the overall JUPITER estimate (NNT5 = 25, extrapolated from median 1.9 year follow-up)33 and overall ARIC estimate (NNT5 = 38, adjusted from mean 6.9 year follow-up)32.
Future Directions
In the near term, a cost-benefit analysis is needed to explore the potential impact of CAC-guided statin allocation in both high (JUPITER-eligible) and low hsCRP populations. A similar study for hsCRP found that hsCRP screening was not more cost-effective than traditional risk based statin allocation34. The EISNER study has previously suggested a potential cost-savings of CAC screening, with markedly reduced downstream spending in the large CAC=0 group35.
Many believe that a clinical trial is needed before CAC can be widely endorsed for risk stratifying adults in whom treatment decisions are unclear36. Such a trial could be approached in a few ways. One design would seek to demonstrate overall cost savings with non-inferior clinical outcomes (increased treatment efficiency) when CAC scoring is used to allocate statin therapy. Another approach would be to show net treatment benefit in individuals randomized to CAC screening in addition to traditional risk assessment, with those with elevated CAC receiving a multi-faceted intervention with dosed-intensity lifestyle and pharmacotherapy. However, there are multiple challenges to such a trial design, including cost and lack of knowledge regarding key assumptions (for example, will CAC testing improve adherence to therapies?)37. Another potential trial design, analogous to the JUPITER study design, would be to randomize patients with elevated CAC but Framingham 10-year CHD risk estimates of <10% to treatment or no treatment. However, such a study may be considered unethical given the strong relationship between elevated CAC and future cardiovascular events.
Limitations
The principal limitation is the uncertainty of applying the relative risk reduction observed in JUPITER to a separate population for the estimation of NNT. It is unknown, for example, if patients with elevated CAC obtain an equivalent benefit with statins compared to those with low or no CAC. The only available data is from a post-hoc analysis of the St. Francis Heart Study, which showed that atorvastatin 20mg significantly lowered events in patients with CAC>400, with non-significant event lowering in those with lower scores38. As such, our NNT results should be considered hypothesis-generating. In addition, it is unclear how the greater prevalence of women in our population (51% vs. 38%) impacts the overall results.
Conclusions
In conclusion, our study demonstrates that approximately half of the individuals meeting JUPITER entry criteria have no underlying CAC, experience a very low 6-year event rate, and may be expected to derive the least absolute benefit from statin therapy. CAC appears to further risk stratify JUPITER-eligible patients and may be used to target a subgroup of patients expected to derive the most, and the least, absolute benefit from treatment. Focusing treatment on the subset of low LDL-C individuals with measurable atherosclerosis may represent a more appropriate allocation of resources, and reduce overall health care cost, while preventing a similar number of events.
Supplementary Material
Panel: Research in context.
Systematic Review
CAC has been consistently shown to improve cardiovascular disease risk prediction beyond current global risk assessment algorithms. The absence of CAC in appropriately selected asymptomatic individuals is associated with a favorable prognosis (CHD event rate: ~1 per 1000 patient-years), as shown in a recent systematic review. While no systematic review compares CAC vs. hsCRP for cardiovascular risk prediction, the highest quality studies have suggested that CAC is a stronger predictor of adverse events as compared to hsCRP. Methodology of these studies differs sufficiently from the present report to preclude meta-analysis.
Interpretation
Our study from the Multi-Ethnic Study of Atherosclerosis (MESA), which includes baseline CAC and hsCRP measurements and 6-year follow-up, confirms the excellent prognosis associated with CAC=0 and extends this finding to the JUPITER-eligible population. In addition, our conclusion that CAC is a stronger predictor of cardiovascular events than hsCRP is consistent with prior reports, and we extend these findings to the low LDL-C (<130 mg/dL) population. Our results are consistent with the hypothesis that focusing treatment on the subset of low LDL-C individuals with measurable atherosclerosis may represent a more appropriate allocation of resources, and reduce overall health care cost, while preventing a similar number of events.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
SOURCE OF FUNDING
This project requiring no specific funding.
The MESA study which supplied the data for this analysis was supported by contracts N01-HC-95159 through N01-HC-95167 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
We had full access to all study data and take full responsibility for the decision to submit for publication.
Footnotes
CONFLICTS OF INTEREST
Dr. Matt Budoff is on the Speaker’s Bureau for GE Healthcare.
Dr. Matt Budoff runs the CT reading center for MESA in association with Harbor-UCLA.
We have no association with the JUPITER trial.
CONTRIBUTIONS
Michael Blaha, Matthew Budoff, and Khurram Nasir contributed to all portions of this work. Andrew Defilippis, Ron Blankstein, Juan Rivera, Arthur Agatson, Dan O’Leary, Joao Lima, and Roger Blumenthal were critical for: study design, data interpretation, and manuscript editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 2.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 3.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 5.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114(16):1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 6.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–88. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 9.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michos ED, Blumenthal RS. Prevalence of low low-density lipoprotein cholesterol with elevated high sensitivity C-reactive protein in the U.S.: implications of the JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) study. J Am Coll Cardiol. 2009;53:931–5. doi: 10.1016/j.jacc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 13.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:2182–99. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Ridker PM, Hansson GK Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106:204–9. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Heart Protection Study Collaborative Group. C-reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20 536 patients in the Heart Protection Study. Lancet. 2011;377:469–76. doi: 10.1016/S0140-6736(10)62174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HC, Greenland P, Rossouw JE, et al. Multimarker prediction of coronary heart disease risk: the Women’s Health Initiative. J Am Coll Cardiol. 2010;55:2080–91. doi: 10.1016/j.jacc.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–36. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Budoff MJ, Kessler P, Gao YL, Qunibi W, Moustafa M, Mao SS. The interscan variation of CT coronary artery calcification score: analysis of the Calcium Acetate Renagel Comparison (CARE)-2 study. Acad Radiol. 2008;15:58–61. doi: 10.1016/j.acra.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Lakoski SG, Cushman M, Criqui M, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–8. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Shah T, Newcombe P, Smeeth L, et al. Ancestry as a Determinant of Mean Population C-Reactive Protein Values: Implications for Cardiovascular Risk Prediction. Circ Cardiovasc Genet. 2010;3:436–44. doi: 10.1161/CIRCGENETICS.110.957431. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 25.McEvoy JW, Blaha MJ, Defilippis AP, et al. Coronary Artery Calcium Progression – An Important Clinical Measurement? A Review of Published Reports. J Am Coll Cardiol. 2010;56:1613–22. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Park R, Detrano R, Xiang M, et al. Combined use of computed tomography coronary calcium scores and C-reactive protein levels in predicting cardiovascular events in nondiabetic individuals. Circulation. 2002;106:2073–7. doi: 10.1161/01.cir.0000033819.29662.09. [DOI] [PubMed] [Google Scholar]
- 27.Möhlenkamp S, Lehmann N, Moebus S, et al. Heinz Nixdorf Recall Study Investigators. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. 2011;57:1455–64. doi: 10.1016/j.jacc.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 28.Blaha MJ, Rivera JJ, Budoff MJ, et al. Association Between Obesity, High-Sensitivity C-Reactive Protein >=2 mg/L, and Subclinical Atherosclerosis: Implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;3:1430–8. doi: 10.1161/ATVBAHA.111.223768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin J, Chang HJ, Nasir K, et al. Association between high-sensitivity C-reactive protein and coronary plaque subtypes assessed by 64-slice coronary computed tomography angiography in an asymptomatic population. Circ Cardiovasc Imaging. 2011;4:201–9. doi: 10.1161/CIRCIMAGING.109.929901. [DOI] [PubMed] [Google Scholar]
- 30.Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: a Bayesian approach. Circ Cardiovasc Qual Outcomes. 2011;4:253–6. doi: 10.1161/CIRCOUTCOMES.110.958496. [DOI] [PubMed] [Google Scholar]
- 31.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 32.Yang EY, Nambi V, Tang Z, et al. Clinical Implications of JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) in a U.S. Population – Insights From the ARIC (Atherosclerosis Risk in Communities) Study. J Am Coll Cardiol. 2009;54:2388–2395. doi: 10.1016/j.jacc.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker PM, MacFadyen JG, Fonseca FA, et al. JUPITER Study Group. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) Circ Cardiovasc Qual Outcomes. 2009;2:616–23. doi: 10.1161/CIRCOUTCOMES.109.848473. [DOI] [PubMed] [Google Scholar]
- 34.Lee KK, Cipriano LE, Owens DK, Go AS, Hlatky MA. Cost-effectiveness of using high-sensitivity C-reactive protein to identify intermediate- and low-cardiovascular-risk individuals for statin therapy. Circulation. 2010;122:1478–87. doi: 10.1161/CIRCULATIONAHA.110.947960. [DOI] [PubMed] [Google Scholar]
- 35.Shaw LJ, Min JK, Budoff M, et al. Induced cardiovascular procedural costs and resource consumption patterns after coronary artery calcium screening: results from the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) study. J Am Coll Cardiol. 2009;54:1258–67. doi: 10.1016/j.jacc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Lauer MS. Screening asymptomatic subjects for subclinical atherosclerosis: not so obvious. J Am Coll Cardiol. 2010;56:106–8. doi: 10.1016/j.jacc.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 37.Blumenthal RS, Hasan RK. Actually, it is more of a guideline than a rule. J Am Coll Cardiol. 2011;57:1601–3. doi: 10.1016/j.jacc.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 38.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–72. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.