Abstract
This study aims to evaluate the potential benefit of combination therapy of 2-methoxyestradiol (2ME) and magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4) on myelodysplastic syndrome (MDS) SKM-1 cells and its underlying mechanisms. The effect of the unique properties of tetraheptylammonium-capped MNPs-Fe3O4 with 2ME on cytotoxicity was tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell-cycle distribution and apoptosis were assessed by flow cytometry. The expression of cell-cycle marker protein was measured by Western blotting. Growth inhibition rate of SKM-1 cells treated with the 2ME-loaded MNPs-Fe3O4 was enhanced when compared with 2ME alone. 2ME led to an increase of caspase-3 expression, followed by apoptosis, which was significantly increased when combined with an MNPs-Fe3O4 carrier. Moreover, the copolymer of 2ME with MNPs- Fe3O4 blocked a nearly two-fold increase in SKM-1 cells located in G2/M phase than in 2ME alone, which may be associated with an accompanying increase of p21 as well as a decrease in cyclin B1 and cdc2 expression, but there was no obvious difference between the MNPs-Fe3O4 and control group. These findings suggest that the unique properties of MNPs-Fe3O4 as a carrier for 2ME, a new anticancer agent currently in clinical trials, may be a logical strategy to enhance the therapeutic activity of MDS.
Keywords: MDS, MNPs-Fe3O4, SKM-1 cell, cell cycle
Introduction
Myelodysplastic syndromes (MDS) are a group of clonal hematopoietic stem-cell disorders characterized by ineffective hematopoiesis, peripheral blood cytopenias, and a propensity to transform to acute myeloid leukemia.1 Although new treatments for MDS are emerging, allogeneic stem-cell transplantation offers the only possible cure.2 Thus, there is an overwhelming need for new agents with more benign toxicity profiles and improved activity for the treatment of MDS.
The estrogen metabolite 2-methoxyestradiol (2ME) is a highly potent anticancer agent that effectively induces apoptosis in several cell lines, and several different proposed mechanisms have been investigated in vitro and in vivo.3–9 Notably, 2ME is not dependent on estrogen receptor binding, and has been found to be more toxic to leukemia cells than to their normal hematopoietic counterparts.10,11 However, up to now, 2ME-triggered cellular events are still not fully understood.
Nowadays, cancer therapy, particularly with respect to drug delivery, has evolved from traditional methodology.12 Nanotechnologies and nanoparticles are becoming a focus for human medical application for their unique properties.13 The diagnostic and therapeutic applications of magnetic iron oxide nanoparticles such as drug delivery, magnetic resonance imaging, hyperthermia techniques, cell separation, and tissue repair have expanded enormously in recent decades.14 The application of drug-coated polymer nanospheres and nanoparticles to inhibit the related drug resistance has attracted much attention. The authors’ earlier studies as well as the studies of others have shown that magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4), as an anticancer drug deliverer,15 could enhance the sensitivity of anticancer drugs and reverse the drug resistance of tumor cells.16 Given that 2ME targets tumor cells specifically and possesses promising clinical activity, albeit with low bio-availability, the authors of this present paper have explored a novel strategy to inhibit MDS cells by combining the unique properties of tetraheptylammonium-capped MNPs- Fe3O4 with 2ME.
Materials and methods
Main materials
Iron chloride hexagydrate, iron sulfate heptahydrate, and ammonium hydroxide were supplied by Lingfeng Chemical Reagent Co Ltd (Shanghai, China); MTT (3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium bromide) and 2ME, by Sigma (St Louis, MO); Annexin V-FITC apoptosis detection kit, by Becton Dickinson (Franklin Lakes, NJ); BCA protein assay kit, by Biosynthesis Biotechnology Co Ltd (Beijing, China); anti- cyclin B1, anti- cdc2, anti- active caspase-3, and antip21, by Cell Signaling Technology Inc (Danvers, MA); anti- β-actin, by Santa Cruz Biotechnology (Santa Cruz, CA); RPMI 1640, by Gibco BRL ( Gaithersburg, MD); and fetal bovine serum (FBS), by Sijiqing Co ( Hangzhou, China).
Preparation of 2ME-loaded MNPs-Fe3O4
MNPs-Fe3O4 was produced by electrochemical deposition under oxidizing conditions in a 0.1 M tetraheptylammonium 2-propanol solution. The deposited clusters were capped with tetraheptylammonium, which acts as a stabilizer of the colloidal nanocrystallites.17 2ME was dissolved in absolute ethanol to give a 20 mM solution, and the final concentration of ethanol in the medium of 2ME-treated cells was adjusted to 0.1% (v/v).18 Before applied in this experiment, the prepared MNPs-Fe3O4 were well distributed in RPMI 1640 medium containing 10% (v/v) heat-inactivated FBS by using ultrasound treatment in order to obtain an MNPs-Fe3O4 colloidal suspension. 2ME was conjugated with MNPs-Fe3O4 at the molar ratio of 100:1 by mechanical absorption polymerization at 4°C for 12 hours, as previously reported.16
Characteristic of MNPs-Fe3O4
To observe the particle size and morphology of MNPs-Fe3O4, the sample was dispersed in deionized water and measured using a JEM-2100 transmission electron microscope (JEOL, Tokyo, Japan).
Cell line and cell culture
SKM-1, an MDS cell line, was from the Japanese Collection of Research Bioresources Cell Bank.19 The cells were cultured in RPMI-1640 containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin in an environment of saturated humidity, 5% CO2, and 37°C. The cells in the logarithmic growth phase were used in all experiments.
Cell proliferation assay
As described previously,20 1 × 105 SKM-1 cells per well were incubated with either 2ME alone or loaded with MNPs-Fe3O4. After incubation for 12, 24, 48, and 72 hours, a 20 μL MTT solution (5 mg/mL) was added to each well at 37°C in the dark for at least 4 hours. Thereafter, the formazan crystals were solubilized in 200 μL dimethyl sulfoxide in every well, and the reduction of MTT was quantified using a Model 550 microplate reader (Bio-Rad Laboratories, Tokyo, Japan). The inhibition ratio of cells was determined as follows (1 − Atest/Acontrol) × 100%.
Annexin V-PI assays for apoptosis
Cells were stained and evaluated for apoptosis by flow cytometry. After treatment for 24 hours as described above, SKM-1 cells were collected and washed twice with PBS, suspended in 200 μL binding buffer and 10 μL Annexin V-FITC for 20 minutes in the dark, and thereafter, 300 μL binding buffer and 5 μL propidium iodide (PI) were added to each sample. The apoptotic cells were determined using a flow cytometer (Becton Dickinson) with CellQuest (Becton Dickinson) software.
Analysis of cell cycle distribution
As described before, cells were harvested, washed with ice-cold PBS, and stained with 50 μg/mL PI and 250 μg/mL RNase for 30 minutes. The percentage of cells in each phase of the cell cycle was determined with a computer-programmed ModFit LT2.0 DNA assay (Becton Dickinson) using a flow cytometer.
Western blot analysis
Western blot assay was performed as previously described.16 Briefly, total protein was isolated from the harvested cells and measured using BCA protein assay kit. Samples containing 25 μg proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked for 1 hour with 5% nonfat milk. The blots were stained with anti-p21, cyclin B1, cdc2 (1:200), or caspase-3 (1:400) antibodies overnight at 4°C, and then with a secondary horseradish peroxidase-conjugated goat antimouse antibody (1:5000) for 1 hour at room temperature. The blots were visualized by enhanced chemiluminescence (ECL system, Amersham, UK), and β-actin was used as the internal control.
Statistical analysis
All data were presented as mean ± standard deviation in triplicate, and all analyses were performed with SPSS software (v 11.5; SPSS Inc, Chicago, IL). Statistical significance and differences observed between experimental groups were determined using Student’s t-test. P < 0.05 was considered significant.
Results
Characteristics of MNPs-Fe3O4
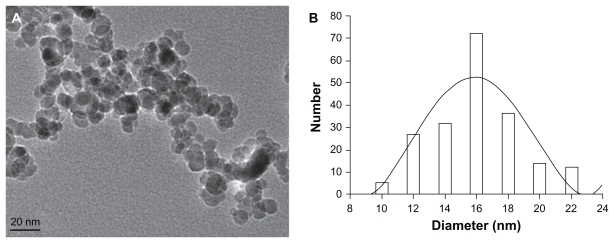
The majority of MNPs-Fe3O4 were spherical, and particle sizes were 15.9 ± 4.6 nm (Figure 1).
Figure 1.
Image (A) and mean size (B) of magnetic nanoparticles of Fe3O4.
Inhibition of cell proliferation
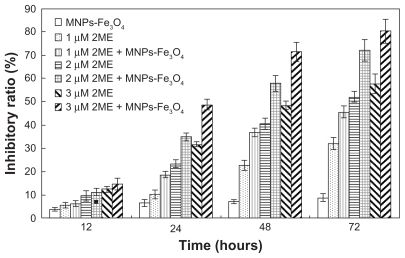
2ME significantly inhibited the growth of SKM-1 cells in a time- and dose-dependent manner (Figure 2). Notably, when 2ME was loaded with MNPs-Fe3O4, the cell inhibition rate was increased (P < 0.05); however, MNPs-Fe3O4 alone did not generate significant cytotoxicity compared with the control group.
Figure 2.
Proliferation inhibitory ratios of SKM-1 cells incubated with either 2ME alone or loaded with MNPs-Fe3O4 (100:1) for 12, 24, 48, and 72 hours.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; 2ME, 2-methoxyestradiol.
Induction of apoptosis
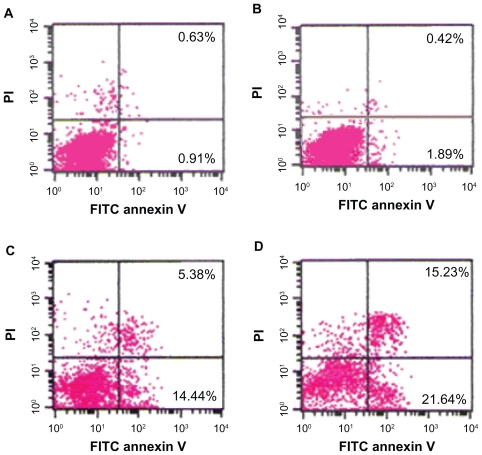
The percentage of apoptotic cells treated with 2ME alone or loaded with MNPs-Fe3O4 at 24 hours were higher than that in the control group. Notably, the apoptotic rate of the copolymer was two-fold compared with 2ME alone (P < 0.05); however, there was no significant difference between the MNPs-Fe3O4 and control groups (P > 0.05) (Figure 3).
Figure 3.
Apoptosis of SKM-1 cells treated with either 2ME alone or loaded with MNPs-Fe3O4 (100:1) for 24 hours. (A) Control. (B) MNPs-Fe3O4. (C) 2 μM 2ME. (D) Copolymer of 2 μM 2ME with MNPs-Fe3O4 (100:1).
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; 2ME, 2-methoxyestradiol; PI, propidium iodide; FITC, fluorescein isothiocyanate.
Distribution of cell cycle
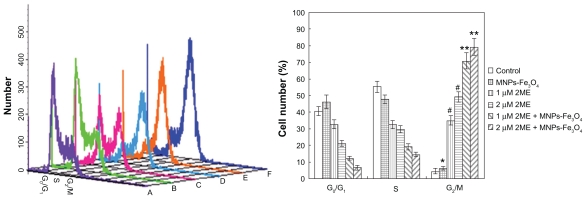
Treatment of SKM-1 cells with 2ME alone for 24 hours resulted in a shift of cell distribution into the G2/M phase compared with the control group; interestingly, when the 2ME was loaded with MNPs-Fe3O4, the number of cells in the G2/M phase was increased from 34.9% ± 2.8% and 49.3% ± 3.1% to 70.8% ± 4.8% and 79.2% ± 5.1% for 1 and 2 μM 2ME, respectively, and in the G1 phase was decreased from 32.6% ± 2.5% and 21.0% ± 1.7% to 12.2% ± 1.1% and 6.3% ± 1.5% for 1 and 2 μM 2ME, respectively, but there was no significant difference between MNPs-Fe3O4 and control group (P > 0.05; Figure 4).
Figure 4.
Cell-cycle distributions after treatment with either 2ME alone or loaded with MNPs-Fe3O4 (100:1) for 24 hours. (A) Control group. (B) MNPs-Fe3O4 alone. (C) 1 μM 2ME. (D) 2 μM 2ME. (E) Copolymer of 1 μM 2ME with MNPs-Fe3O4 (100:1). (F) Copolymer of 2 μM 2ME with MNPs-Fe3O4 (100:1).
Notes: *P > 0.05, when compared with the control group; #P < 0.05, when compared with MNPs-Fe3O4 and control groups; **P < 0.05, when compared with 2ME alone.
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; 2ME, 2-methoxyestradiol.
Expression of cell cycle proteins
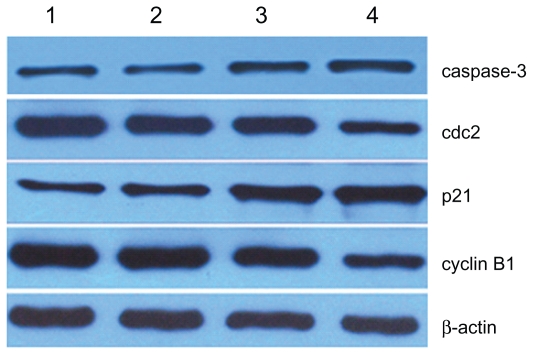
The expressions of cdc2 and cyclin B1 in SKM-1 cells treated with 2ME for 24 hours were slightly downregulated compared with the control group, and the decrease was even more apparent when combined with MNPs-Fe3O4 (P < 0.05). In addition, the level of p21 was consistently increased after treatment with 2ME, and the increase was further augmented by addition of MNPs-Fe3O4 (P < 0.05). Similar results for caspase-3 were observed; conversely, there was no obvious difference between MNPs-Fe3O4 and the control group (P > 0.05) (Figure 5).
Figure 5.
Expression of cell-cycle marker protein in SKM-1 cells treated with 2ME alone or loaded with MNPs-Fe3O4 (100:1) for 24 hours.
Notes: Column 1, control; column 2, MNPs-Fe3O4; column 3, 2 μM 2ME; column 4, the copolymer of 2 μM 2ME with MNPs-Fe3O4 (100:1).
Abbreviations: MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; 2ME, 2-methoxyestradiol.
Discussion
Recent exciting data suggest that nanomaterials have been successfully manipulated to create a new drug-delivery system that can not only solve the problem of poor water solubility of most promising currently available anticancer drugs but also reduce toxic side effects and, thereby, increase their effectiveness. MNPs-Fe3O4, one of the most promising biocompatible materials, is feasible to produce, easy to functionalize, and not only shows satisfactory water solubilization and degradation in vivo but also improves the sensitivity of anticancer drugs.16,21 In our study, the majority of synthesized MNPs-Fe3O4 were spherical and the average diameter size was 15.9 ± 4.6 nm, which is suitable for biological applications. Wang et al17 demonstrate that the unique magnetic nanoparticles have a higher surface-to-volume ratio and a relatively smaller size, which could allow faster movement and easier entry into cells. Therefore, MNPs-Fe3O4 may offer an exciting nanomaterial toward developing an effective drug-delivery system for solving the solubility of 2ME.
It is noteworthy that 2ME is considered to be a promising anticancer agent with several advantages in chemoprevention as well as therapy.9,10,22 As we know, after the adsorption of the nanoparticles on the cellular membrane, the uptake occurs via several possible mechanisms such as pinocytosis, nonspecific or receptor-mediated endocytosis, or phagocytosis. 23 In our study, 2ME inhibited cell growth significantly in a dose- and time-dependent manner, which is consistent with studies in multiple myeloma.24 Interestingly, the growth inhibition rate was augmented by the MNPs-Fe3O4, while there was no significant influence on SKM-1 cells by MNPs-Fe3O4 alone. Moreover, literature reports that the chemotherapeutic drug-coated poly (methacrylate) nanosphere composite could be internalized by an endocytotic process, and this process can cause higher intracellular drug accumulation.17 Thus, we infer that MNPs-Fe3O4 can increase the sensitivity of SKM-1 cells to 2ME via altering the pharmacokinetics of 2ME.
Cell growth is tightly regulated by cell-cycle checkpoints. It is worth noting that 2ME has been shown to block many human hematologic cell lines25 and other cells3 in the G2/M phase in vitro. In our study, 2ME resulted in a shift of cell distribution into the G2/M phase, and this shift was enhanced by MNPs-Fe3O4, while some other researchers reported that 2ME arrested cells in G1/S,9 or both G1/S and G2/M.5 These opposing results suggest that there may be fundamental differences between cell types. Our data indicated that the copolymer of 2ME with MNPs-Fe3O4 results in cell-cycle block in the G2/M phase, thereby decelerating proliferation; further studies should be investigated to establish whether the 2ME-induced accumulation of SKM-1 cells is due to a specific block in G2 or in the M phase.
Evidence in the literature suggests that the progression through the cell cycle is regulated by different types of cyclins and several different cyclin-dependent kinases (CDKs),26 and the activities of CDKs are responsible for phosphorylation of downstream targets, while the cyclin-dependent kinase inhibitors are the key players in the negative regulation of the cell cycle in response to a wide variety of antiproliferative signals. Among several CDK inhibitors, p21 is one of the important inhibitory proteins in the G2/M checkpoint, which can inactivate the cdc2–cyclinB1 complex, thus evoking cell cycle arrest.27 The results of this present study showed that 2ME decreased the expression of cyclin B1 and cdc2, whereas it induced an increase in expression of p21, and this effect was augmented by the carrier of MNPs-Fe3O4. These data and other studies incuding Barboule et al3 suggest that p21 is involved in the cyclin–cdc machinery during the G2/M-phase cell-cycle arrest in response to the copolymer of 2ME with MNPs-Fe3O4 in SKM-1 cells.
The authors of this paper are investigating the molecular mechanisms involved in mediating 2ME-induced cell apoptosis. The results showed that SKM-1 cells underwent typical apoptotic changes as indicated by flow cytometry, which is consistent with previous trials.4–8 Caspase-3 is a critical mediator of apoptosis, which is responsible for the proteolytic cleavage of many key proteins such as the nuclear enzyme poly (ADP- ribose) polymerase (PARP).28 2ME in this study increased the expression of caspase-3, and the increase was further augmented by MNPs-Fe3O4, implicating the role of active caspase-3 in SKM-1 cells. It is well established that p21 is not only involved in cell-cycle arrest, but also involved in the induction of apoptosis.3,6,29 2ME induced the increase of p21 in this study, which is inconsistent with the results of Zhou et al,4 suggesting that it depends on the cellular context whether p21 participates in regulating apoptotic function in response to 2ME. Therefore, we cannot exclude the possibility that some other mechanism participates in the 2ME-induced apoptosis. Overall, the results of this study revealed that the copolymer of 2ME with MNPs-Fe3O4 which prolongs cell-cycle arrest, can lead to cell death, mediated by the activation of the intrinsic pathway of apoptosis.
Conclusion
These results indicate that the unique properties of MNPs- Fe3O4 as a carrier for 2ME, a new anticancer agent currently in clinical trials, may be a logical strategy to enhance therapeutic activity of MDS.
Acknowledgments
This work was financially supported by National Key Basic Research Program 973 of China (No. 2010CB732404), and National Science and Technology Support Program for the “Eleventh Five-Year” Plan (No. 2008BAI61B02).
Footnotes
Disclosure
The authors have no conflicts of interest to report in this work.
References
- 1.Heaney ML, Golde DV. Myelodysplasia. N Engl J Med. 1999;340:1649–1660. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- 2.Kindwall-Keller T, Isola LM. The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant. 2009;43:597–609. doi: 10.1038/bmt.2009.28. [DOI] [PubMed] [Google Scholar]
- 3.Barboule N, Lafon C, Chadebech P, Vidal S, Valette A. Involvement of p21 in the PKC-induced regulation of the G2/M cell cycle transition. FEBS Lett. 1999;444:32–37. doi: 10.1016/s0014-5793(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhou NN, Zhu XF, Zhou JM, et al. 2-Methoxyestradiol induces cell cycle arrest and apoptosis of nasopharyngeal carcinoma cells. Acta Pharmacol Sinica. 2004;25:1515–1520. [PubMed] [Google Scholar]
- 5.Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res. 2006;99:266–274. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]
- 6.Maran A, Shogren KL, Benedikt M, Sarkar G, Turner RT, Yaszemski MJ. 2-Methoxyestradiol-induced cell death in osteosarcoma cells is preceded by cell cycle arrest. J Cell Biochem. 2008;104:1937–1945. doi: 10.1002/jcb.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao N, Rahmani M, Dent P, Grant S. 2-Methoxyestradiol-induced apoptosis in human leukemia cells proceeds through a reactive oxygen species and Akt-dependent process. Oncogene. 2005;24:3797–3809. doi: 10.1038/sj.onc.1208530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batsi C, Markopoulou S, Kontargiris E, et al. Bcl-2 blocks 2-methoxyestradiol induced leukemia cell apoptosis by a p27Kip1-dependent G1/S cell cycle arrest in conjunction with NF-κB activation. Biochem Pharmacol. 2009;78:33–34. doi: 10.1016/j.bcp.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahut WL, Lakhani NJ, Gulley JL, et al. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006;5:22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 10.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 11.Chow JM, Liu CR, Lin CP, et al. Downregulation of c-Myc determines sensitivity to 2-methoxyestradiol induced apoptosis in human acute myeloid leukemia. Exp Hematol. 2008;36:140–148. doi: 10.1016/j.exphem.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Pacheco R, Valdivia JG, Ibarra MR. Magnetic nanoparticles for local drug delivery using magnetic implants. Methods Mol Biol. 2009;544:559–569. doi: 10.1007/978-1-59745-483-4_35. [DOI] [PubMed] [Google Scholar]
- 14.Ge YQ, Zhang Y, Xia JG, et al. Effect of surface charge and agglomerate degree of magnetic iron oxidenanoparticles on KB cellular uptake in vitro. Colloids Surf B Biointerfaces. 2009;73:294–301. doi: 10.1016/j.colsurfb.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Lin BL, Shen XD, Cui S. Application of nanosized Fe3O4 in anticancer drug carriers with target-orientation and sustained-release properties. Biomed Mater. 2007;2:132–134. doi: 10.1088/1748-6041/2/2/011. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Chen BA, Xia GH, et al. The reversal effect of magnetic Fe3O4 nanoparticles loaded with cisplatin on SKOV3/DDP ovarian carcinoma cells. Int J Nanomedicine. 2009;4:107–114. doi: 10.2147/ijn.s5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XM, Zhang RY, Wu CH, et al. The application of Fe3O4 nanoparticles in cancer research: a new strategy to inhibit drug resistance. J Biomed Mater Res A. 2007;80A:852–860. doi: 10.1002/jbm.a.30901. [DOI] [PubMed] [Google Scholar]
- 18.Lambert C, Apel K, Biesalski HK, Frank J. 2-Methoxyestradiol induces caspase-independent, mitochondria-centered apoptosis in DS-sarcoma cells. Int J Cancer. 2004;108:493–501. doi: 10.1002/ijc.11579. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T, Matozaki S, Murayama T, et al. Establishment of a leukaemic cell line from a patient with acquisition of chromosomal abnormalities during disease progression in myelodysplastic syndrome. Brit J Haematol. 1993;85:469–476. doi: 10.1111/j.1365-2141.1993.tb03334.x. [DOI] [PubMed] [Google Scholar]
- 20.Marks DC, Belov L, Davey MW, Davey RA, Kidman AD. The MTT cell viability assay for cytotoxicity testing in multidrug resistant human leukemia cells. Leukemia Res. 1992;16:1165–1173. doi: 10.1016/0145-2126(92)90114-m. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder U, Sommerfeld P, Ulrich S, Sabel BA. Nanoparticle technology for delivery of drugs across the blood–brain barrier. J Pharm Sci. 1998;87:1305–1307. doi: 10.1021/js980084y. [DOI] [PubMed] [Google Scholar]
- 22.Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol. 2003;15:425–430. doi: 10.1097/00001622-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan D, Catley L, Hideshima T, et al. 2-Methoxyestradiol overcomes drug resistance in multiple myeloma cells. Blood. 2002;100:2187–2194. doi: 10.1182/blood-2002-02-0376. [DOI] [PubMed] [Google Scholar]
- 25.Dingli D, Timm M, Russell SJ, Witzig TE, Rajkumar SV. Promising preclinical activity of 2-methoxyestradiol in multiple myeloma. Clin Cancer Res. 2002;8:3948–3954. [PubMed] [Google Scholar]
- 26.Damia G, Broggini M. Cell cycle checkpoint proteins and cellular response to treatment by anticancer agents. Cell Cycle. 2004;3:46–50. [PubMed] [Google Scholar]
- 27.Hsu YL, Kuo PL, Lin LT, Lin CC. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signalregulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J Pharmacol Exp Ther. 2005;313:333–344. doi: 10.1124/jpet.104.078808. [DOI] [PubMed] [Google Scholar]
- 28.Zhang LS, Sheng R, Qin ZH. The lysosome and neurodegenerative diseases. Acta Biochem Biophys Sin. 2009;41:437–445. doi: 10.1093/abbs/gmp031. [DOI] [PubMed] [Google Scholar]
- 29.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]