Abstract
SLC6A14, also known as ATB0,+, is an amino acid transporter with unique characteristics. It transports 18 of the 20 proteinogenic amino acids. However, this transporter is expressed only at low levels in normal tissues. Here, we show that the transporter is up-regulated specifically in estrogen receptor (ER)-positive breast cancer, demonstrable with primary human breast cancer tissues and human breast cancer cell lines. SLC6A14 is an estrogen/ER target. The transport features of SLC6A14 include concentrative transport of leucine (an activator of mTOR), glutamine (an essential amino acid for nucleotide biosynthesis and substrate for glutaminolysis), and arginine (an essential amino acid for tumor cells), suggesting that ER-positive breast cancer cells up-regulate SLC6A14 to meet their increased demand for these amino acids. Consequently, treatment of ER-positive breast cancer cells in vitro with α-methyl-dl-tryptophan (α-MT), a selective blocker of SLC6A14, induces amino acid deprivation, inhibits mTOR, and activates autophagy. Prolongation of the treatment with α-MT causes apoptosis. Addition of an autophagy inhibitor (3-methyladenine) during α-MT treatment also induces apoptosis. These effects of α-MT are specific to ER-positive breast cancer cells, which express the transporter. The ability of α-MT to cause amino acid deprivation is significantly attenuated in MCF-7 cells, an ER-positive breast cancer cell line, when SLC6A14 is silenced with shRNA. In mouse xenograft studies, α-MT by itself is able to reduce the growth of the ER-positive ZR-75-1 breast cancer cells. These studies identify SLC6A14 as a novel and effective drug target for the treatment of ER-positive breast cancer.
Keywords: Amino Acid, Amino Acid Transport, Apoptosis, Autophagy, Breast Cancer, Estrogen, mTOR
Introduction
ATB0,+ (amino acid transporter responsible for the activity of system B0,+) was named “B0,+” to indicate its broad substrate selectivity (denoted by “B”), accepting neutral (denoted by “0”) and cationic (denoted by “+”) amino acids as substrates (1, 2). Its transport function is coupled to a Na+ gradient, a Cl− gradient, and membrane potential. ATB0,+ is identified as SLC6A14 according to the Human Genome Organization nomenclature. This transporter has potential for delivery of a wide variety of drugs and prodrugs into cells (3–7).
The substrate selectivity of SLC6A14 is interesting (1, 2). It transports all essential amino acids. The only excluded amino acids are glutamate and aspartate, which are nonessential. It also transports glutamine (an important precursor for nucleotide synthesis) and arginine (an amino acid essential for tumor growth). However, the transporter is expressed only at low levels in normal tissues. Tumor cells have an increased requirement for essential amino acids as well as glutamine and arginine to support their rapid growth. Essential amino acids are obligatory for protein synthesis. Leucine, an essential amino acid, is also a potent activator of mTOR (mammalian target of rapamycin) (8). Certain tumor cells metabolize glutamine at a rate far exceeding the requirement for protein and nucleotide synthesis, a phenomenon known as “glutamine addiction” (9–11). Glutamine metabolism through a set of biochemical reactions called “glutaminolysis” provides a carbon source for tumor cells, thereby sparing glucose-derived carbon for the synthesis of lipids and other essential biomolecules. Arginine is essential for several types of cancer due to lack of the arginine-synthesizing enzyme argininosuccinate synthetase (12, 13). On the basis of these findings, we hypothesized that the expression of SLC6A14 may be up-regulated in cancer to meet the increasing demand for all essential amino acids as well as glutamine and arginine. In support of this hypothesis, past studies from our laboratory have shown that SLC6A14 is up-regulated in colon cancer (14) and cervical cancer (15). The transporter is also up-regulated in breast cancer cell lines, but interestingly only in estrogen receptor (ER)2-positive cell lines (16). Furthermore, we showed that blockade of SLC6A14 in ER-positive breast cancer cells by treatment with a selective blocker (α-methyl-dl-tryptophan (α-MT)) starved the cells of glutamine, arginine, and essential amino acids, decreased cell proliferation, and caused apoptotic cell death (16). In the present study, we investigated the expression of SLC6A14 in primary breast cancer tissues, its relevance to ER status, and its potential as a drug target for in vivo breast cancer therapy.
EXPERIMENTAL PROCEDURES
Materials
l-[14C]Valine was purchased from Moravek Biochemicals (Brea, CA). Polyclonal antibody specific for human SLC6A14 has been described previously (5). All other antibodies were purchased from commercial sources. Primary tumor and normal tissues, used for RNA preparation, were obtained from the Georgia Health Sciences University Tumor Bank.
Animals
Female athymic BALB/c mice were obtained from Taconic (Germantown, NY). Frogs were obtained from Xenopus-I Inc. (Ann Arbor, MI). Use of animals in these studies adhered to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23) and was approved by the Institutional Committee for Animal Use in Research and Education of the Georgia Health Sciences University.
Construction of a Luciferase Reporter Plasmid with the SLC6A14 Promoter
Human genomic DNA was used for PCR to clone the SLC6A14 promoter using primers 5′-GTCGACCCTGCCTTCAAAGAACTGGTA-3′ and 5′-AAGCTTGCACTCTCCCCTGTTCCTA-3′. Underlined sequences represent SalI and HindIII restriction sites for subcloning the PCR product into the luciferase reporter plasmid pGL3.
Immunohistochemistry
Primary breast tissue sections (purchased from US Biomax, Inc., Rockville, MD) were subjected to immunohistochemistry to assess qualitatively the expression levels of SLC6A14 protein in normal and tumor tissues.
RT-PCR
Total RNA was isolated using TRIzolTM (Invitrogen). 2 μg of RNA was reverse-transcribed using the GeneAmp PCR system (Applied Biosystems). PCR was performed under optimal conditions specific for each of the primer pairs. GAPDH or HPRT1 (hypoxanthine-guanine phosphoribosyltransferase-1) were used as internal controls. PCR products were size-fractionated on agarose gel, stained with ethidium bromide, and imaged using an AlphaImager system. Densitometry was performed on PCR bands and normalized to the internal control. Normalized values were used to compare mRNA levels of target genes. Primers used for PCR were as follows: asparagine synthetase, 5′-GCACGCCCTCTATGACAATG-3′ (upstream) and 5′-CTCACTCTCCTCGGCTTT-3′ (downstream); CCAAT/enhancer-binding protein homologous protein (CHOP), 5′-GAGAACCAGGAAACGGAAAC-3′ (upstream) and 5′-GCAGATTCACCATTCGGTC-3′ (downstream); SLC6A14, 5′-GAAGGAGAAAGTGTCGGCTTCA-3′ (upstream) and 5′-TACCACCTTGCCAGACGATTTG-3′ (downstream); ERα, 5′-GGCTCCGCAAATGCTACGA-3′ (upstream) and 5′-CGCCAGACGAGACCAATCATC-3′ (downstream); GAPDH, 5′-TGGACCTGACCTGCCGTCTA-3′ (upstream) and 5′-AGGAGTGGGTGTCGCTGTTG-3′ (downstream); and HPRT1, 5′-GCGTCGTTAGCGATGATGAAC-3′ (upstream) and 5′-CCTCCCATCTCCTTCATGACATCT-3′ (downstream).
Analysis of SLC6A14 as an Estrogen/ER Target
The influence of estrogen on SLC6A14 expression was assessed in ER-positive breast cancer cells (ZR-75-1 and BT-474) by culturing them for several passages in the absence of phenol red and then monitoring the levels of SLC6A14 mRNA. The effect of estradiol/ER was investigated directly in BT-474 cells. These experiments were complemented with an SLC6A14 promoter activity assay in BT-474 cells using luciferase as the reporter. Cells were cotransfected with the luciferase reporter plasmid containing the SLC6A14 promoter upstream of the luciferase cDNA and a β-galactosidase expression plasmid (ratio of the two plasmid DNAs, 4:1). Luciferase activity was measured in cell lysates using luciferase assay reagent (Promega). β-Galactosidase activity in the same cell lysates was used for normalization of the promoter activity for differences in transfection efficiency.
Analysis of SLC6A14 Transport Function in Xenopus laevis Oocytes
SLC6A14-mediated transport of leucine, glutamine, and arginine and its blockade by α-MT were studied using the X. laevis oocyte heterologous expression system as described previously (4, 16). Human cloned SLC6A14 cDNA was used in these studies.
Analysis of Autophagy
Autophagy was analyzed as described previously (17, 18) by monitoring the localization pattern of constitutively expressed LC3 as well as that of ectopically expressed GFP-LC3. In the case of constitutively expressed LC3, cells were treated without or with α-MT and then used for immunocytochemical analysis of the LC3 localization pattern. For ectopically expressed LC3, cells were transfected with a GFP-LC3 expression plasmid. Briefly, cells were plated on a coverslip to reach ∼50% confluence, and transfection of these cells was done with 1 μg of the plasmid using FuGENE transfection reagent (Roche Applied Science). The cells were then maintained in culture for 24 h to reach 80–90% confluence prior to treatment with α-MT in the presence or absence of 3-methyladenine (3-MA). At the end of the treatment, cells were fixed with 4% paraformaldehyde and examined by fluorescence microscopy to count the number of cells with punctate GFP-LC3. Autophagosomal proteolysis was quantified as described previously (17). For electron microscopy, control and treated MCF-7 cells were fixed with a solution consisting of 2.5% glutaraldehyde, 1% formaldehyde, and 0.1 m cacodylate buffer (pH 7.4). The samples were then post-fixed in 1% osmium tetroxide and 0.1 m cacodylate buffer and embedded in Epon epoxy resin. Samples were cut into 0.1-μm sections, stained with uranyl acetate/lead citrate, and examined with a transmission electron microscope.
Apoptosis Assay
FACS was used to monitor cell death. MCF-7 cells were treated with or without α-MT, 3-MA, or α-MT plus 3-MA. Control and treated cells were then fixed in 50% ethanol; treated with 0.1% sodium citrate, 1 mg/ml RNase A, and 50 μg/ml propidium iodide; and subjected to FACS analysis (FACSCalibur, BD Biosciences). FITC-labeled annexin V was used to differentiate apoptotic cell death from necrotic cell death.
Migration and Invasion Assays
The influence of α-MT on cell migration and invasion was determined with ZR-75-1 cells. The migration of these cells toward fetal bovine serum was measured using the QCMTM cell migration assay kit (Millipore) according to the manufacturer's instructions. Serum-starved ZR-75-1 cells were allowed to migrate for 48 h in the presence or absence of α-MT (2.5 mm). After removing the cells on the top of the membrane filter, the inserts were stained with 0.1% crystal violet, washed, and dried. The crystal violet dye retained on the filters (representing the cells that migrated to the underside of the membrane filter) was extracted with acetic acid. The absorbance of the extract was measured at 570 nm. Cell invasion assay was performed similarly using the QCMTM cell invasion assay kit (Millipore). The inserts used in this assay were coated with ECMatrixTM, representing the extracellular matrix.
shRNA-mediated Silencing of SLC6A14
A lentivirus-based system was used for shRNA-mediated silencing of SLC6A14 in MCF-7 cells. A set of lentivirus-based shRNAs (five different shRNAs) was purchased from Open Biosystems (RHS4533-NM_007231). Recombinant lentivirus was produced in 293FT cells by cotransfection of empty vector or shRNA plasmid along with the helper vectors pLP-1, pLP-2, and pVSVG (Invitrogen). Lipofectamine 2000 was used as the transfection reagent. Lentiviral supernatant was harvested 72 h after transfection and filtered through a 0.45-μm membrane. MCF-7 cells were infected for 24 h with lentivirus in medium containing 8 μg/ml Polybrene and cultured for an additional 24 h. The expression of SLC6A14 was monitored by RT-PCR.
Xenograft in Nude Mice
Human breast cancer cells (ZR-75-1, MCF-7, and MB-231) were injected subcutaneously close to the lower mammary gland on the right side of BALB/c nude mice (10 × 106 cells/injection site). Only for MCF-7 cells, slow-releasing estrogen pellets were implanted to support the tumor growth. Implantation of estrogen pellets was done as follows. 7 days prior to injection of tumor cells, mice were anesthetized, and 3-mm pellets containing estradiol (0.18 mg/21-day release; Innovative Research of America, Sarasota, FL) were implanted subcutaneously in the animals' backs. The pellets provided a continuous release of estradiol to maintain serum concentrations of 150–250 pm, which is in the range of physiological levels seen in mice during the estrous cycle. Mice were then divided into two groups (control and treatment) with six mice in each group. The control group received water with sucrose, and the treatment group received α-MT (2 mg/ml) in water with sucrose 48 h prior to injection of tumor cells. The treatment group continued to receive α-MT (2 mg/ml) in water throughout the experimental period. Tumor size in control and treatment groups was measured periodically by caliper, and the area of tumor was calculated using the formula (width2 × length)/2.
Measurement of α-MT in Mouse Plasma
BALB/c nude mice were divided into two groups; the control group received water with sucrose, and the treatment group received α-MT (2 mg/ml) in water with sucrose for 2 weeks. Mice were then bled, and α-MT in plasma was determined by HPLC as described by Laich et al. (19).
RESULTS AND DISCUSSION
Up-regulation of SLC6A14 in ER-positive Breast Cancer
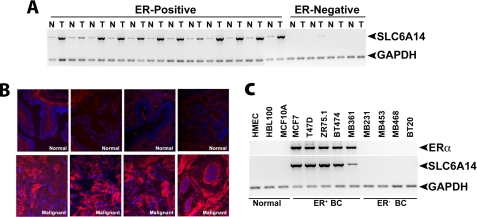
We examined the levels of SLC6A14 mRNA in primary breast cancer tissues (12 ER-positive and four ER-negative) and adjacent normal breast tissues (Fig. 1A). In each of the ER-positive breast cancer tissue specimens, SLC6A14 was up-regulated compared with the corresponding normal tissues (9.8 ± 1.0-fold). In contrast, SLC6A14 mRNA was not detectable in ER-negative breast cancer and the corresponding normal tissues. The absence of expression of SLC6A14 in normal tissues adjacent to ER-negative tumors was not surprising because ERα was absent not only in these tumor tissues but also in the corresponding adjacent normal tissues.3 The SLC6A14 protein levels were also higher in ER-positive breast cancer than in normal breast tissue (Fig. 1B). The specific up-regulation of SLC6A14 in ER-positive breast cancer was reproducible in human breast cancer cell lines (Fig. 1C). The non-malignant mammary epithelial cell lines HMEC, HBL-100, and MCF-10A as well as the ER-negative breast cancer cell lines MB-231, MB-453, MB-468, and BT-20 were negative for ERα and SLC6A14 expression. In contrast, the ER-positive breast cancer cell lines MCF-7, T47D, ZR-75-1, BT-474, and MB-361 expressed the transporter.
FIGURE 1.
Up-regulation of SLC6A14 in ER-positive breast cancer. A, RT-PCR analysis of SLC6A14 mRNA in primary human breast cancer tissues and the corresponding adjacent normal tissues. N, normal; T, tumor. B, immunohistochemical analysis of SLC6A14 protein in ER-positive breast cancer tissues and normal breast tissues. C, RT-PCR analysis of SLC6A14 and ERα mRNAs in human breast cancer cell lines. ER+ BC, ER-positive breast cancer cell lines; ER− BC, ER-negative breast cancer cell lines.
SLC6A14 as an Estrogen/ER Target
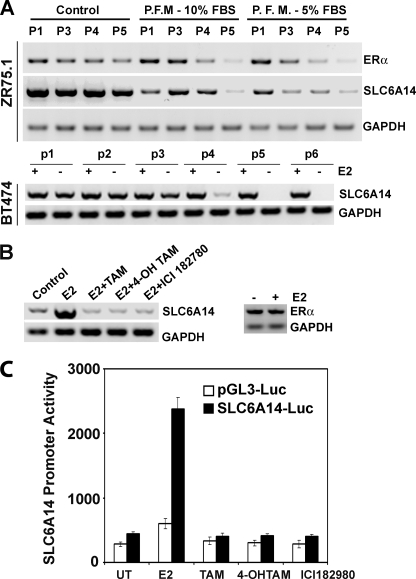
To determine whether SLC6A14 is an ER target, we first analyzed the SLC6A14 promoter sequence (∼3 kb). We found several putative ER-binding sites in the promoter. We then cultured two ER-positive breast cancer cell lines (ZR-75-1 and BT-474) for five to six passages in culture medium without phenol red and monitored the levels of SLC6A14 mRNA (Fig. 2A). Phenol red is an estrogen mimetic; therefore, if ER induces SLC6A14 expression, the absence of phenol red during the culture of ER-positive breast cancer cells is expected to reduce SLC6A14 expression. This was indeed the case. The absence of phenol red in the culture medium led to the down-regulation of ERα in both cell lines with a corresponding decrease in SLC6A14 expression. Treatment with estradiol increased SLC6A14 mRNA levels in BT-474 cells, and this effect was abolished completely in the presence of anti-estrogens (Fig. 2B). We then monitored in BT-474 cells the activity of the SLC6A14 promoter (∼3 kb) using luciferase as the reporter. Cells were cotransfected with the reporter plasmid and a β-galactosidase expression plasmid, and β-galactosidase activity was used to control for differences in transfection efficiency. Untreated cells showed significant promoter activity, and this activity was enhanced 6-fold by estrogen (Fig. 2C). Anti-estrogens blocked this effect. The stimulatory effect of estrogen on luciferase activity was not seen in cells transfected with vector alone. It has been shown by other investigators that chronic treatment (96 h) with estrogen down-regulates ERα in ER-positive breast cancer cell lines (20). In the present study, treatment of BT-474 cells with estradiol for only 24 h did not have any noticeable effect on the expression of ERα (Fig. 2B).
FIGURE 2.
Regulation of SLC6A14 expression by estrogen/ER. A, ZR-75-1 and BT-474 cells were cultured for five to six passages in control medium (10% fetal bovine serum with phenol red) and phenol red-free medium (P.F.M.; 10 or 5% charcoal-stripped fetal bovine serum), and the levels of ERα and SLC6A14 mRNAs were determined by RT-PCR. P1, P3–P5, and p1–p6 refer to passage numbers. B, BT-474 cells were treated with estradiol (E2; 10 nm) in the presence or absence of anti-estrogens (1 μm) for 24 h and then used for RT-PCR analysis of SLC6A14 and ERα mRNAs. ICI 182780 is also known as Faslodex. TAM, tamoxifen; 4-OH TAM, 4-hydroxytamoxifen. C, BT-474 cells were transfected with either the SLC6A14 promoter (∼3 kb)-luciferase (Luc) construct or empty vector and then treated with estradiol (10 nm) in the presence and absence of anti-estrogens (1 μm). Following the treatment, the reporter activity was measured. Data represent values after normalization with β-galactosidase activity for differences in transfection efficiency. UT, untreated.
Transport of Leucine, Glutamine, and Arginine via SLC6A14 and Its Blockade by α-MT
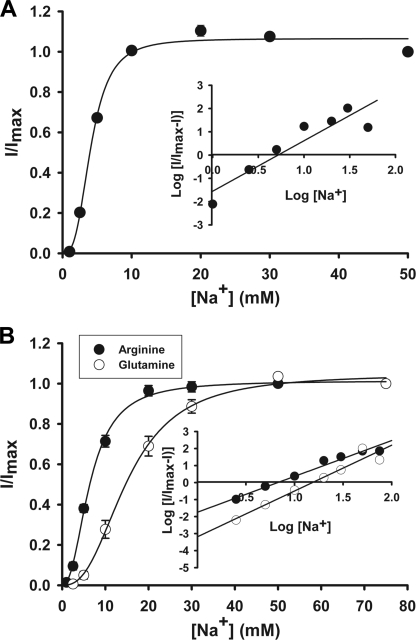
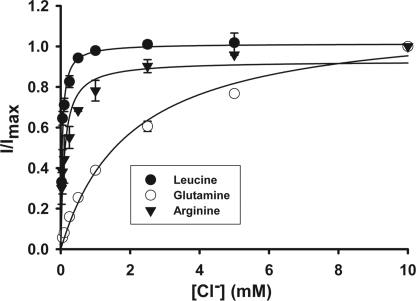
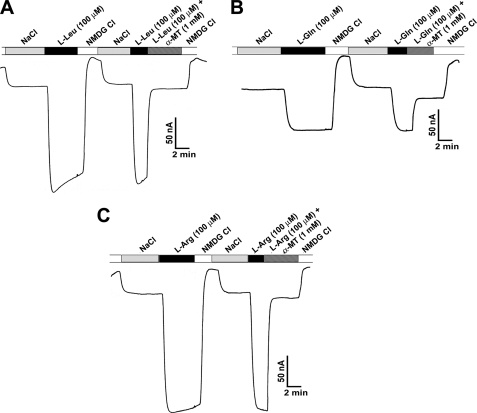
Although the characteristics of leucine transport via SLC6A14 are known with regard to Na+ and Cl− dependence and Na+:Cl−:amino acid stoichiometry (21), such information is not available for glutamine and arginine. Because of the charge differences between leucine/glutamine and arginine, the Na+ and Cl− dependence and Na+:Cl−:amino acid stoichiometry may or may not be the same for these three amino acids. Therefore, we investigated SLC6A14-mediated transport of leucine, glutamine, and arginine electrophysiologically using the oocyte expression system. Perifusion of SLC6A14-expressing oocytes with all three amino acids induced inward currents in the presence of NaCl; such currents were not detectable in the absence of either Na+ or Cl−, indicating that both ions are obligatory for transport. With each of these three amino acids, the Na+ activation kinetics exhibited a sigmoidal relationship (Fig. 3), suggesting involvement of more than one Na+ ion in the activation process. The value for the Hill coefficient (i.e. the number of Na+ ions involved in the activation process) was 2 for all three amino acids (2.2 ± 0.4 for leucine, 2.4 ± 0.3 for glutamine, and 2.1 ± 0.1 for arginine). In contrast, the Cl− activation kinetics was hyperbolic (i.e. Hill coefficient = 1) for all three amino acids, suggesting involvement of one Cl− ion in the activation process (Fig. 4). Thus, the Na+:Cl−:amino acid stoichiometry was 2:1:1 irrespective of the net charge on the amino acid substrate. We then investigated the effects of α-MT on SLC6A14-mediated transport of leucine, glutamine, and arginine. Perifusion of SLC6A14-expressing oocytes with 100 μm leucine, glutamine, or arginine induced inward currents, and these currents were blocked by 1 mm α-MT (Fig. 5).
FIGURE 3.
Na+ activation kinetics of SLC6A14-mediated transport of leucine (A) and arginine and glutamine (B). Human SLC6A14 was expressed heterologously in X. laevis oocytes by microinjection of cRNA. 4 days following injection, oocytes were used for electrophysiological studies using the two-microelectrode voltage-clamp method. Uninjected oocytes showed negligible currents when perifused with leucine, arginine, or glutamine (1 mm). cRNA-injected oocytes showed marked inward currents when perifused with these three amino acids, and the magnitude of the currents increased with increasing concentrations of Na+. (The concentration of Cl− was kept constant at 100 mm.) Because the expression levels varied from oocyte to oocyte, resulting in varying magnitudes of amino acid-induced currents, the currents were normalized by taking the magnitude of the currents induced at 100 mm Na+ as 1 in each oocyte and calculating the magnitude of the currents induced at other concentrations of Na+ as a fraction of this maximal current. The experiments were done with three different oocytes, and the results are given as means ± S.E. Insets, Hill plots.
FIGURE 4.
Cl− activation kinetics of SLC6A14-mediated transport of leucine, arginine, and glutamine. The experiments were done as described for the Na+ activation kinetics in the legend to Fig. 3. cRNA-injected oocytes showed marked inward currents when perifused with these three amino acids, and the magnitude of the currents increased with increasing concentrations of Cl−. (The concentration of Na+ was kept constant at 100 mm.) The experiments were done with three different oocytes, and the results are given as means ± S.E.
FIGURE 5.
Blockade of SLC6A14-mediated transport of leucine (A), glutamine (B), and arginine (C) by α-MT. Oocytes injected with human SLC6A14 cRNA were perifused with 100 μm leucine, glutamine, or arginine under varying experimental conditions as indicated. Perifusion of the oocytes with the amino acids induced inward currents in the presence of NaCl, and the currents disappeared when NaCl was replaced with N-methyl-d-glucamine (NMDG) chloride. Re-perifusion of the same oocytes with the amino acids again induced inward currents in the presence of NaCl, but these currents disappeared when perifusion with the same amino acids was done in the same buffer but in the presence of 1 mm α-MT.
Consequences of Blockade of SLC6A14 in Breast Cancer Cells
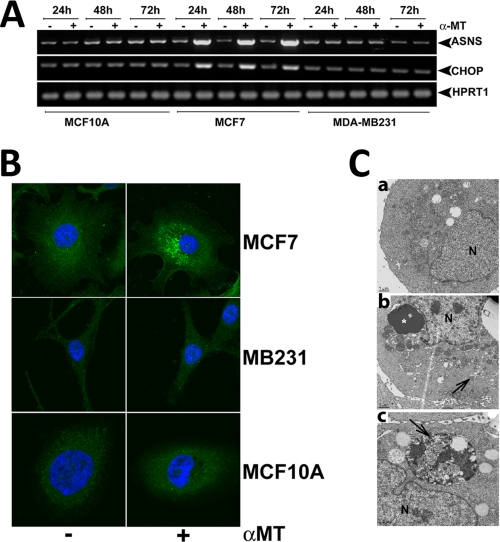
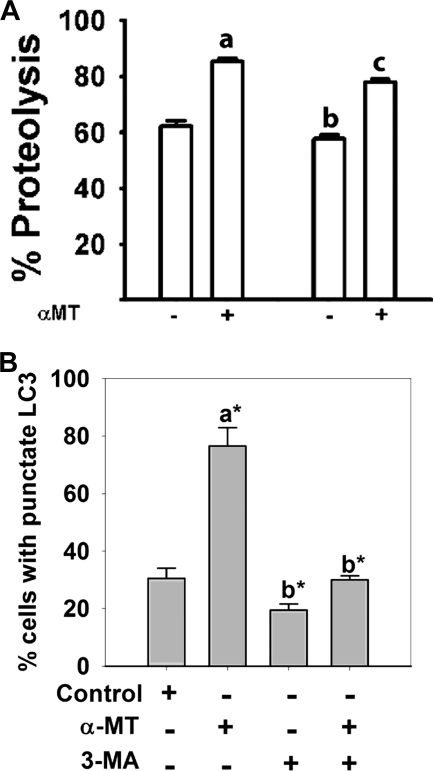
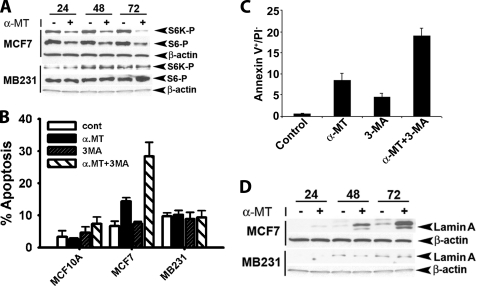
To test whether blockade of SLC6A14 causes amino acid deprivation in SLC6A14-positive breast cancer cells, we treated MCF-7 cells with α-MT (2.5 mm) for 24, 48, or 72 h and monitored the levels of asparagine synthetase and CHOP mRNAs as a readout for amino acid deprivation (22). We found that α-MT treatment increased the levels of these mRNAs in MCF-7 cells but not in SLC6A14-negative MB-231 (a cancer cell line) and MCF-10A (a non-malignant cell line) cells (Fig. 6A). Treatment of MCF-7 cells with α-MT induced autophagy as evident from the punctate localization of constitutively expressed LC3 in immunocytochemical analysis (Fig. 6B) and the presence of autophagosomes in electron microscopic analysis (Fig. 6C). Under identical conditions, MB-231 and MCF-10A cells did not undergo autophagy. The induction of autophagosomal proteolysis in MCF-7 cells by α-MT was further substantiated by increased proteolysis of 14C-labeled proteins (Fig. 7A). This α-MT-induced proteolysis was inhibited significantly by cotreatment with 3-MA, a blocker of autophagy (Fig. 7A). The inhibition of autophagy by 3-MA was more clearly evident when the punctate localization of ectopically expressed GFP-LC3 was taken as the marker of autophagy (Fig. 7B). Here, MCF-7 cells transiently transfected with GFP-LC3 were treated for 48 h with α-MT (2.5 mm), 3-MA (10 mm), or α-MT (2.5 mm) plus 3-MA (10 mm), and then the percent of cells showing punctate localization of LC3 was determined by fluorescence microscopy. 3-MA was able to block >90% of autophagy induced by α-MT.
FIGURE 6.
Effects of SLC6A14 blockade with α-MT in breast cancer cells. A, MCF-10A (a non-malignant mammary epithelial cell line), MCF-7 (an ER-positive breast cancer cell line), and MB-231 (an ER-negative breast cancer cell line) cells were treated with or without 2.5 mm α-MT for 24, 48, or 72 h and then used for analysis of asparagine synthetase (ASNS) and CHOP mRNA levels. B, MCF-7, MB-231, and MCF-10A cells were treated with or without 2.5 mm α-MT for 48 h and then used for immunocytochemical analysis of constitutively expressed LC3. C, MCF-7 cells were treated with (panels b and c) or without (panel a) α-MT (2.5 mm) for 48 h and then used for electron microscopy. Arrows indicate sequestration of the cytoplasm and organelles in pre-autophagosomes and autophagosomes, and the asterisk indicates condensed chromatin. N, nucleus.
FIGURE 7.
Induction of autophagy in MCF-7 cells by α-MT. A, autophagosomal proteolysis of 14C-labeled proteins. The concentrations of α-MT and 3-MA, when present, were 2.5 and 10 mm. The treatment time was 48 h. The extent of proteolysis was determined by the radioactivity present in the protein-free supernatant. a, significantly different from proteolysis in the absence of α-MT and 3-MA (p < 0.001); b, significantly different from proteolysis in the absence of α-MT and 3-MA (p < 0.05); c, significantly different from proteolysis in the presence of α-MT alone (p < 0.01). B, MCF-7 cells were transfected with an GFP-LC3 expression plasmid, and 24 h later, the cells were treated with or without α-MT (2.5 mm) in the presence or absence of 3-MA (10 mm) for 48 h. At the end of the treatment, cells were fixed, and the percent of cells with punctate localization of ectopically expressed GFP-LC3 was quantified. a, compared with the control; b, compared with treatment with α-MT alone; c, compared with treatment with α-MT alone; *, p < 0.001.
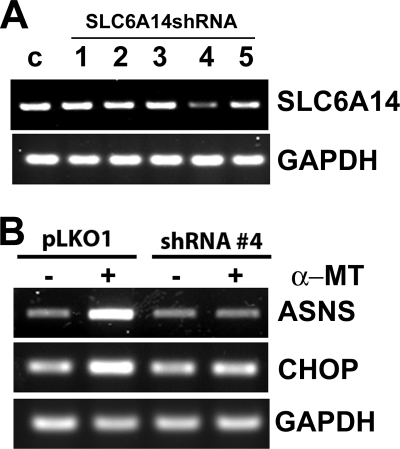
To determine whether the induction of amino acid deprivation by α-MT in ER-positive breast cancer cells was due to blockade of SLC6A14, we silenced the expression of the transporter in MCF-7 cells, an ER-positive breast cancer cell line, using shRNA. Among the five SLC6A14-selective shRNAs tested, only shRNA-4 was able to silence the transporter expression markedly (Fig. 8A). We transfected MCF-7 cells with either empty vector or vector carrying shRNA-4 and then treated the cells without or with α-MT (2.5 mm, 48 h) to monitor the expression levels of asparagine synthetase and CHOP as a readout for amino acid deprivation. In control cells transfected with empty vector, the expression levels of asparagine synthetase and CHOP were increased by α-MT treatment (Fig. 8B). This effect was markedly attenuated in cells transfected with vector carrying shRNA-4. These results show that the amino acid deprivation induced by α-MT occurs via blockade of SLC6A14.
FIGURE 8.
Influence of α-MT on asparagine synthetase and CHOP mRNA levels in control MCF-7 cells and in MCF-7 cells with shRNA-induced silencing of SLC6A14. A, five different SLC6A14-specific shRNAs were tested in MCF-7 cells using a lentivirus-based system to monitor their efficacy to silence SLC6A14. SLC6A14 mRNA levels were measured by RT-PCR with GAPDH as an internal control. c, control. B, SLC6A14 was silenced in MCF-7 cells with shRNA-4 using a lentivirus-based system and then treated without or with α-MT (2.5 mm, 48 h). RNA isolated from the cells was used for RT-PCR to monitor the levels of asparagine synthetase (ASNS) and CHOP mRNAs.
There was also evidence of mTOR inhibition in MCF-7 cells when treated with α-MT as evident from the decreased phosphorylation of S6 and S6 kinase, the downstream targets of mTOR (Fig. 9A). Again, mTOR activity in SLC6A14-negative cells was not affected by α-MT. When autophagy was blocked with 3-MA, there was a marked induction of cell death in MCF-7 cells in the presence of α-MT as monitored by cell cycle analysis with propidium iodide labeling (Fig. 9B). Treatment with α-MT alone caused significant cell death in MCF-7 cells, and this effect was further potentiated by cotreatment with 3-MA. There was very little, if any, effect on cell death in MB-231 and MCF-10A cells in response to treatment with α-MT and 3-MA, either alone or together. Treatment of MCF-7 cells with α-MT also induced apoptotic cell death as monitored by annexin V labeling (Fig. 9C). 3-MA also caused significant apoptosis, but when combined with α-MT, the effect was much greater than when the cells were treated with either agent alone. The apoptotic cell death caused in MCF-7 cells by treatment with α-MT was also demonstrable by the cleavage of lamin A (a specific substrate for activated caspase-6) (Fig. 9D). There was no evidence of apoptosis in SLC6A14-negative MB-321 cells under identical conditions.
FIGURE 9.
A, influence of α-MT on mTOR activity. MCF-7 and MB-231 cells were treated without or with α-MT (2.5 mm) for 24, 48, or 72 h, and the cell lysates were used for Western blotting. S6K-P, phosphorylated S6 kinase. B–D, influence of α-MT with or without 3-MA on cell death. B, MCF-7, MB-231, and MCF-10A cells were treated for 48 h without or with α-MT (2.5 mm), 3-MA (10 mm), or α-MT (2.5 mm) plus 3-MA (10 mm) and analyzed for cell death by propidium iodide (PI) staining and FACS-based cell cycle analysis. C, MCF-7 cells were treated for 48 h without or with α-MT (2.5 mm), 3-MA (10 mm), or α-MT (2.5 mm) plus 3-MA (10 mm) and then analyzed for apoptotic cell death by annexin V labeling. D, MCF-7 and MB-231 cells were treated without or with 2.5 mm α-MT for 24, 48, or 72 h. Cell lysates were then used for Western blot analysis of lamin A degradation product using an antibody that recognizes specifically the proteolytic cleavage product. β-Actin was used as an internal control.
The influence of α-MT (2.5 mm) on cell migration and invasion was examined not with MCF-7 cells but with ZR-75-1 cells because MCF-7 cells are non-invasive. α-MT did not have any significant influence on cell migration or invasion in ZR-75-1 cells. (Migration in α-MT-treated cells was 87 ± 7% of the control (p > 0.05); invasion in α-MT-treated cells was 95 ± 14% of the control (p > 0.05).)
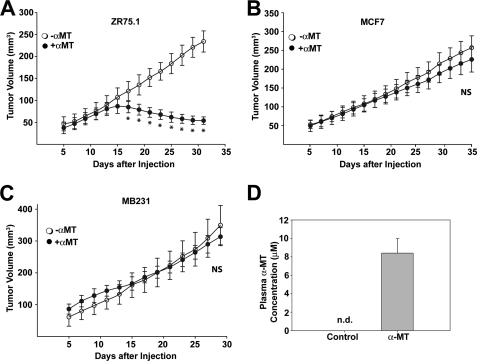
Inhibition of Tumor Growth by α-MT in Vivo
To determine the therapeutic potential of SLC6A14 as a drug target for the treatment of ER-positive breast cancer in vivo, we performed mouse xenograft studies with two ER-positive breast cancer cell lines (ZR-75-1 and MCF-7) and one ER-negative breast cancer cell line (MB-231). MCF-7 cells did not form tumors unless estrogen was administered to the mice. On the other hand, ZR-75-1 and MB-231 cells readily formed tumors without the need for estrogen administration. α-MT in drinking water (2 mg/ml) reduced the growth of ZR-75-1 cells (Fig. 10A) but did not affect the growth of MCF-7 cells (in the presence of estrogen administration) or MB-231 cells (Fig. 10, B and C). The plasma concentration of α-MT in mice after 2 weeks of administration in drinking water (2 mg/ml) was 8.5 ± 0.5 μm (Fig. 10D). α-MT was not detectable in control mice.
FIGURE 10.
In vivo efficacy of α-MT in the treatment of ER-positive breast cancer. ZR-75-1 (A), MCF-7 (B), and MB-231 (C) cells were injected subcutaneously into BALB/c nude mice (10 × 106 cells/injection site). For MCF-7 cells, slow-releasing estrogen pellets were implanted to support the tumor growth. For each cell line, the control group received water with sucrose, and the treatment group received α-MT (2 mg/ml) in water with sucrose. Tumor size was measured periodically. NS, not statistically significant (p > 0.05); *, p < 0.005 (two-tailed unpaired Student's t test). D, BALB/c nude mice were divided into two groups; the control group received water with sucrose, and the treatment group received α-MT (2 mg/ml) in water with sucrose for 2 weeks. Mice were then bled, and α-MT in plasma was determined by HPLC. n.d., not determined.
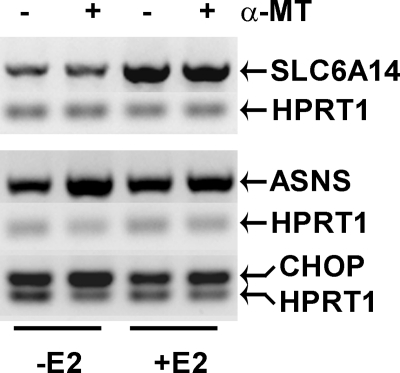
MCF-7 cells, which are SLC6A14-positive, were not affected by α-MT in vivo even though the growth of these cells was inhibited by α-MT in vitro. MCF-7 cells do not grow into a tumor in nude mice unless estrogen is administered; under these conditions, estrogen-induced up-regulation of SLC6A14 most likely compensates for the blockade of the transporter by α-MT. We confirmed this in vitro. When MCF-7 cells were grown for 24 h in phenol red-free culture medium in the absence and presence of estradiol (10 nm), the expression of SLC6A14 was higher in estradiol-treated cells (Fig. 11). In control cells cultured in the absence of estradiol, treatment with α-MT (2.5 mm, 24 h) induced the expression of asparagine synthetase and CHOP. The magnitude of this effect was significantly reduced in cells treated with estradiol (Fig. 11), showing that the elevated expression of SLC6A14 suppresses the efficacy of α-MT to block the transporter. Therefore, it may be necessary to optimize the treatment protocol (dose and treatment time) to demonstrate the efficacy of α-MT in blocking the growth of MCF-7-derived tumors in mouse xenografts in the presence of exogenous estrogen.
FIGURE 11.
Estrogen-dependent suppression of the efficacy of α-MT to induce asparagine synthetase and CHOP expression in MCF-7 cells. MCF-7 cells were cultured in phenol red-free medium in the absence or presence of estradiol (E2; 10 nm) for 24 h and then treated without or with α-MT (2.5 mm, 24 h). The cells were used for analysis of SLC6A14, asparagine synthetase (ASNS), and CHOP mRNA levels by RT-PCR. HPRT1 mRNA levels were used as an internal control.
Taken collectively, the results presented here provide evidence for SLC6A14 as an effective drug target for treatment of ER-positive breast cancer in vivo. These results can be summarized as follows. First, we have demonstrated the specific up-regulation of SLC6A14 in ER-positive primary breast cancer specimens. Second, we have shown that treatment of ER-positive breast cancer cells with α-MT led to amino acid deprivation and autophagy and that cotreatment with an autophagy inhibitor caused apoptosis and cell death. Importantly, these effects were not seen in ER-negative breast cancer cells and in non-malignant mammary epithelial cells. Third, we have shown that, in a mouse xenograft model, administration of α-MT in drinking water was effective in reducing the tumor growth of ER-positive breast cancer cells with no effect on the tumor growth of ER-negative breast cancer cells. These studies identify a novel approach for the treatment of ER-positive breast cancer by targeting, for the first time, an essential amino acid transporter. α-MT is a potent blocker of SLC6A14 in vivo, with its therapeutic efficacy evident at plasma concentrations in the micromolar range. Blockade of the transporter is unlikely to have off-target detrimental effects on normal tissues because the observed effects are specific to SLC6A14-positive tumor cells.
In conclusion, this study has provided the proof of principle that SLC6A14 is a potential drug target for the treatment of ER-positive breast cancer. α-MT in itself represents a viable drug for this purpose. It could also serve as a lead compound for the design and development of more potent blockers of SLC6A14 for future use. Therapeutic strategies are currently available for the treatment of ER-positive breast cancer using anti-estrogens such as tamoxifen, but development of resistance to this mode of treatment has become a significant problem for successful therapy by this approach. Therefore, new therapeutic strategies with molecular targets that differ mechanistically from anti-estrogens have great potential in breast cancer treatment. Our study identifies SLC6A14 as one such target that needs to be investigated in humans for its potential in the treatment of ER-positive breast cancer.
This work was supported, in whole or in part, by National Institutes of Health Grant CA152396.
S. Elangovan, V. Ganapathy, and M. Thangaraju, submitted for publication.
- ER
- estrogen receptor
- α-MT
- α-methyl-dl-tryptophan
- CHOP
- CCAAT/enhancer-binding protein homologous protein
- 3-MA
- 3-methyladenine.
REFERENCES
- 1. Ganapathy V., Inoue K., Prasad P. D., Ganapathy M. E. (2004) in Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition (Cynober L. A. ed) 2nd Ed., pp. 63–78, CRC Press, Boca Raton, FL [Google Scholar]
- 2. Ganapathy M. E., Ganapathy V. (2005) Curr. Drug Targets Immune Endocr. Metab. Disord. 5, 357–364 [DOI] [PubMed] [Google Scholar]
- 3. Nakanishi T., Hatanaka T., Huang W., Prasad P. D., Leibach F. H., Ganapathy M. E., Ganapathy V. (2001) J. Physiol. 532, 297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatanaka T., Nakanishi T., Huang W., Leibach F. H., Prasad P. D., Ganapathy V., Ganapathy M. E. (2001) J. Clin. Invest. 107, 1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatanaka T., Huang W., Nakanishi T., Bridges C. C., Smith S. B., Prasad P. D., Ganapathy M. E., Ganapathy V. (2002) Biochem. Biophys. Res. Commun. 291, 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatanaka T., Haramura M., Fei Y. J., Miyauchi S., Bridges C. C., Ganapathy P. S., Smith S. B., Ganapathy V., Ganapathy M. E. (2004) J. Pharmacol. Exp. Ther. 308, 1135–1147 [DOI] [PubMed] [Google Scholar]
- 7. Umapathy N. S., Ganapathy V., Ganapathy M. E. (2004) Pharm. Res. 21, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 8. Wang X., Proud C. G. (2009) Trends Cell Biol. 19, 260–267 [DOI] [PubMed] [Google Scholar]
- 9. DeBerardinis R. J., Lum J. J., Hatzivassiliou G., Thompson C. B. (2008) Cell Metab. 7, 11–20 [DOI] [PubMed] [Google Scholar]
- 10. Tong X., Zhao F., Thompson C. B. (2009) Curr. Opin. Genet. Dev. 19, 32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganapathy V., Thangaraju M., Prasad P. D. (2009) Pharmacol. Ther. 121, 29–40 [DOI] [PubMed] [Google Scholar]
- 12. Feun L., You M., Wu C. J., Kuo M. T., Wangpaichitr M., Spector S., Savaraj N. (2008) Curr. Pharm. Des. 14, 1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delage B., Fennell D. A., Nicholson L., McNeish I., Lemoine N. R., Crook T., Szlosarek P. W. (2010) Int. J. Cancer 126, 2762–2772 [DOI] [PubMed] [Google Scholar]
- 14. Gupta N., Miyauchi S., Martindale R. G., Herdman A. V., Podolsky R., Miyake K., Mager S., Prasad P. D., Ganapathy M. E., Ganapathy V. (2005) Biochim. Biophys. Acta 1741, 215–223 [DOI] [PubMed] [Google Scholar]
- 15. Gupta N., Prasad P. D., Ghamande S., Moore-Martin P., Herdman A. V., Martindale R. G., Podolsky R., Mager S., Ganapathy M. E., Ganapathy V. (2006) Gynecol. Oncol. 100, 8–13 [DOI] [PubMed] [Google Scholar]
- 16. Karunakaran S., Umapathy N. S., Thangaraju M., Hatanaka T., Itagaki S., Munn D. H., Prasad P. D., Ganapathy V. (2008) Biochem. J. 414, 343–355 [DOI] [PubMed] [Google Scholar]
- 17. Periyasamy-Thandavan S., Jackson W. H., Samaddar J. S., Erickson B., Barrett J. R., Raney L., Gopal E., Ganapathy V., Hill W. D., Bhalla K. N., Schoenlein P. V. (2010) Autophagy 6, 19–35 [DOI] [PubMed] [Google Scholar]
- 18. Samaddar J. S., Gaddy V. T., Duplantier J., Thandavan S. P., Shah M., Smith M. J., Browning D., Rawson J., Smith S. B., Barrett J. T., Schoenlein P. V. (2008) Mol. Cancer Ther. 7, 2977–2987 [DOI] [PubMed] [Google Scholar]
- 19. Laich A., Neurauter G., Widner B., Fuchs D. (2002) Clin. Chem. 48, 579–581 [PubMed] [Google Scholar]
- 20. Kousidou O. Ch., Berdiaki A., Kletsas D., Zafiropoulos A., Theocharis A. D., Tzanakakis G. N., Karamanos N. K. (2008) Mol. Oncol. 2, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sloan J. L., Mager S. (1999) J. Biol. Chem. 274, 23740–23745 [DOI] [PubMed] [Google Scholar]
- 22. Kilberg M. S., Shan J., Su N. (2009) Trends Endocrinol. Metab. 20, 436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]