Abstract
Background
Hemodialysis (HD) patients are at risk for medication-related problems (MRP). The MRP number, type, and appearance rate over time in ambulatory HD patients has not been investigated.
Methods
Randomly selected HD patients were enrolled to receive monthly pharmaceutical care visits. At each visit, MRP were identified through review of the patient chart, electronic medical record, patient interview, and communications with other healthcare disciplines. All MRP were categorized by type and medication class. MRP appearance rate was determined as the number of MRP identified per month/number of months in study. The number of MRP per patient-drug exposures were determined using: {[(number of patients) × (mean number of medications)]/(number of months of study)} /number of MRP identified. Results were expressed as mean ± standard deviation or percentages.
Results
Patients were 62.6 ± 15.9 years old, had 6.4 ± 2.0 comorbid conditions, were taking 12.5 ± 4.2 medications, and 15.7 ± 7.2 doses per day at baseline. Medication-dosing problems (33.5%), adverse drug reactions (20.7%), and an indication that was not currently being treated (13.5%) were the most common MRP. 5,373 medication orders were reviewed and a MRP was identified every 15.2 medication exposures. Overall MRP appearance rate was 0.68 ± 0.46 per patient per month.
Conclusion
MRP continue to occur at a high rate in ambulatory HD patients. Healthcare providers taking care of HD patients should be aware of this problem and efforts to avoid or resolve MRP should be undertaken at all HD clinics.
Background
The Institutes of Medicine report highlighting the burden of medication errors in the United States has brought the issue of patient safety to the forefront of medical concern. [1] In the United States healthcare system, medication-related problems (MRP) cause significant patient morbidity, mortality, and cost. [2-4] MRP are implicated in 16.1% of internal medicine ward hospital admissions.[4] Surprisingly, 58.9% of admissions could definitely or possibly be avoided. Once admitted to the internal medicine ward, greater than 18% of patient deaths can be attributed to one or more drugs.[5] Adverse drug events contribute to over 100,000 deaths annually and 25% of ambulatory patients report experiencing at least one adverse drug event.[3,6] The economic burden of MRP on the healthcare system is estimated to be in excess of $177 billion.[2]
Identification and resolution of MRP can occur through provision of pharmaceutical care. Pharmaceutical care is defined as a practice in which a pharmacist takes the responsibility for the patient's drug related needs, and is held accountable for this commitment.[7] The provision of pharmaceutical care has made substantial contributions to patient morbidity and mortality in critical care and congestive heart failure patients.[8,9]
Currently, there are approximately 350,000 end-stage renal disease (ESRD) patients in the United States.[1] In addition to renal failure, these patients have a mean 5 comorbid conditions that require complex medical regimens of a median 8 medications.[10,11]
Several studies have demonstrated that ambulatory hemodialysis (HD) patients are at risk for medication-related problems. [12-17] Medication-related problems can be classified into eight general categories: untreated indications; improper drug selection; sub-therapeutic dosage; overdose; adverse drug reactions; drug interactions; failure to receive drugs; and drug use without indication.[18] Factors associated with medication-related problems in these patients include: more than three concurrent disease states present; medication regimen changed four or more times during the past 12 months; five or more medications in present drug regimen; twelve or more medication doses per day; history of noncompliance; presence of drugs that require therapeutic monitoring, and presence of diabetes.[17] Nearly all HD patients are risk due to their multiple risk factors present.
Several single-center, short-term studies in ambulatory HD patients have shown that a mean 4 – 8 MRP exists per patient.[12-14,17] Although the reports identified numerous MRP of various type and significance, results presented were obtained with average patient follow up time of 2.6 ± 1.9 months. It is unknown if the number, type, or severity of MRP continues at the same rate after a few months time. This study was conducted to determine the number, type, severity, and appearance rate of MRP, as identified through pharmaceutical care activities, in ambulatory HD patients.
Patients and methods
Over a 10 month period (August 2001 through May 2002) randomly selected patients at our freestanding, non-profit outpatient dialysis unit (Dialysis Clinic, Inc., Kansas City, MO) were evaluated. Patients were eligible for inclusion if they were greater than 18 years of age, planned to be continuously enrolled in HD therapy at the same dialysis clinic throughout the duration of the study, and agreed to participate with monthly pharmacist visits. All patients were recruited within a 2 month period and followed for 10 months.
All patients were typically hemodialyzed for three to four hours per treatment, three days per week. Patients were under the care of a private group of nephrologists. Standard practice at the HD clinic included regular patient visits by a nephrologist, averaging two to three times per week. At these encounters, the nephrologist assessed the patient and made adjustments to the HD prescription or medication list as deemed appropriate. On a monthly basis, nephrologists rotate clinic responsibilities among the group.
At each monthly visit the pharmacist reviewed the patient chart, electronic medical record including laboratory measurements, conducted a patient interview with review of all medications, and communicated with other healthcare disciplines (medicine, nursing, dietary, social work) about the health status of the patient. With the data collected, the pharmacist then evaluated appropriateness of medical therapy, identified MRP, and communicated interventions to the nephrologist via pharmacist progress note and e-mail correspondence.
Data collected included: patient demographics (age, gender, race, reason for and duration of ESRD), documented comorbid conditions, medication type and number, and number of medication doses per day. Medications were classified similar to that previously reported[11,19] as follows: anemia (erythropoietin, iron), renal bone disease (calcium or aluminum salts, sevelamer, vitamin D analogs), cardiac (any agent used for hypertension, congestive heart failure, coronary artery disease, arrhythmia), cholesterol-lowering (niacin, fibric acid agent, HMG-CoA reductase inhibitor), endocrine (any agent used for diabetes, thyroid disorders, menopause), anti-infective (including antiviral), antithrombotic (agents that may affect platelet function or prolong coagulation), psychotropic (antidepressants, antipsychotics), gastrointestinal (histamine-2 receptor antagonist [H2RA], proton pump inhibitor [PPI], promotility agents, laxatives), vitamins, analgesics, antipruritics, and other (agents with a prevalence of less than 10%).
Identified MRP were categorized as: indication without drug therapy (IWD – patient has a medical problem that requires medication therapy (an indication for medication use) but is not receiving a medication for that indication); drug use without indication (DWI – patient is taking a medication for no medically valid indication); improper drug selection (IDS – patient has a medication indication but is taking the wrong drug); sub-therapeutic dosage (UD – patient has a medical problem that is being treated with too little of the correct medication); over-dosage (OD – patient has a medical problem that is being treated with too much of the correct medication); adverse drug reaction (ADR – patient has a medical problem that is the result of a ADR or adverse effect); drug interaction (DI – patient has a medical problem that is the result of a medication-medication, medication-laboratory, or medication-food interaction); failure to receive drug (FRD – patient has a medical problem that is the result of their not receiving a medication); and other (O – all those not able to be classified as above).
Statistics
Results are expressed as mean ± standard deviation or percentages (frequency data) as appropriate. MRP appearance rate was determined as follows: the number of MRP identified per month/number of months in study. The number of MRP per patient-drug exposures were determined using the following calculation: { [(number of patients) × (mean number of medications)]/(number of months of study)} /number of MRP identified.[17]
Results
Over the ten-month period 145 patients received HD (66 pharmaceutical care group; 79 usual care group). Data from twelve patients was excluded from analysis due to patient death (n = 9), transfer (n = 2), or transplant (n = 1) during the study period. Patient demographics are shown in table 1. Overall, patients were an average of 62.6 ± 15.9 years old (range 26–92) and received dialysis for 3.1 ± 2.5 years (range 0 – 13.3). The majority of patients were male (56%) and African American (78%). Overall, diabetes mellitus was the primary cause of ESRD (47.8%), followed by hypertension (22.4%). Patients receiving pharmaceutical care had 6.4 ± 2.0 (range 3–12) comorbid conditions, were taking 12.5 ± 4.2 (range 6–21) medications, and 15.7 ± 7.2 (range 4–34) doses per day at baseline.
Table 1.
Patient Demographics
| Patient Characteristic | All (n = 133) | Pharmaceutical Care (n = 66) | Usual Care (n = 67) | p value |
| Age (years) | 62.8 ± 15.0 | 62.0 ± 14.3 | 63.5 ± 15.8 | 0.64 |
| Male gender (%) | 55.6 | 45.5 | 65.7 | 0.22 |
| Race (%) | ||||
| Black | 78.2 | 71.2 | 85.1 | 0.08 |
| Caucasian | 17.3 | 22.7 | 12.0 | 0.06 |
| Other | 4.5 | 6.1 | 3.0 | 0.66 |
| ESRD Reason (%) | ||||
| Diabetes mellitus | 48.1 | 60.6 | 35.8 | 0.007 |
| Hypertension | 21.8 | 16.7 | 26.9 | 0.23 |
| Glomerulonephritis | 14.3 | 12.1 | 16.4 | 0.84 |
| Other | 15.8 | 10.6 | 20.9 | 0.10 |
| ESRD Duration (years) | 3.1 ± 2.5 | 3.0 ± 1.9 | 3.3 ± 2.9 | 0.43 |
| # Medications | 11.4 ± 4.2 | 12.5 ± 4.2 | 10.2 ± 3.8 | 0.007 |
| # Comorbid Conditions | 5.8 ± 1.9 | 6.4 ± 2.0 | 5.1 ± 1.5 | 0.00003 |
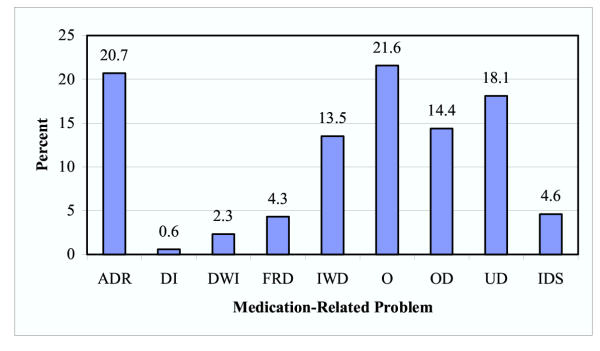
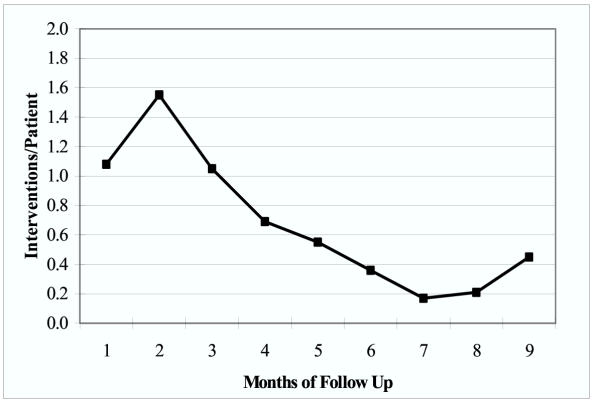
Over the 10-month period the pharmacist reviewed 5,373 medication orders and identified 354 MRP in 66 different patients. The MRP type and frequency can be seen in Figure 1. Most commonly, the pharmacist identified medication-dosing problems (33.5%) or adverse drug reactions (20.7%) of the time. An indication that was not currently being treated (IWD) was discovered 13.5% of the time. The "other" category included interventions aimed at education of the patient and/or staff, recommendations due to lack of supportive laboratory data, and recommendations due to inappropriate medication treatment duration. A MRP was identified for every 15.2 medications reviewed. Overall the MRP appearance rate was 0.68 ± 0.46 per patient per month. At the end of the study period, 0.45 MRP per patient per month were identified (Figure 2).
Figure 1.
Frequency distribution of medication-related problems in ambulatory hemodialysis patients. ADR = adverse drug reactions; DI = drug interactions; DWI = drug use without indication; FRD = failure to receive drugs; IWD = indication without drug therapy; O = other; OD = over-dosage; UD = sub-therapeutic dosage; IDS = improper drug selection.
Figure 2.
Number of medication-related problems per patient over time.
MRP were classified according to medication class involved (Table 2). Cardiovascular-related medications accounted for 29.7% of MRP: 13.3% cardiovascular medication; 8.2% cholesterol lowering medications; and 8.2% antithrombotic medications (e.g., aspirin). Endocrine medications accounted for 15.5% of identified MRP. Nephrology-specific medications (anemia and renal bone disease medications) accounted for 15% of MRP.
Table 2.
Medication Class Involvement
| Medication Class | Number (% Occurrence) |
| Endocrine | 55 (15.5) |
| Cardiac | 47 (13.3) |
| Gastrointestinal | 45 (12.7) |
| Renal bone disease | 31 (8.8) |
| Cholesterol-lowering | 29 (8.2) |
| Antithrombotic | 29 (8.2) |
| Anemia | 22 (6.2) |
| Psychotropic | 19 (5.4) |
| Anti-infective | 17 (4.8) |
| Analgesic | 15 (4.2) |
| Other | 15 (4.2) |
| Vitamins | 6 (1.7) |
| Antipruritic | 6 (1.7) |
Discussion
We determined that MRP continue to occur at a relatively high rate month after month in ambulatory HD patients. At the end of the study period, 0.45 medication-related problems per patient per month were identified. Extrapolating these findings to the 246,121 United States HD population, nearly 111,000 MRP occur each month. If the same frequency of MRP type were to occur as we found at our clinic, then each month 35,409 dosing problems occur, 21,645 adverse drug events occur, and 14,430 medical problems are not treated with appropriate medication therapy. These numbers of MRP that could occur each month appears staggering. However, these patients have multiple disease states which require multiple medications and intense follow-up. HD patients take a median 8 medications for an average 5 comorbid conditions.[10,11] Additionally, others have demonstrated that patients with chronic kidney disease are not prescribed medications, despite having a medical necessity for pharmacologic treatment.[20,21] The impact of MRP in other ESRD populations, i.e., peritoneal dialysis and transplant patients, warrants further investigation.
Patients having cardiovascular- and endocrine-related MRP are not surprising given that over 42% of patients have diabetes and 75.2% of patients have a history of hypertension.1 Cardiovascular disease (CVD) is the leading cause of mortality in ESRD patients, accounting for approximately 50% of deaths.[1] There is much opportunity to increase use of medications with known cardioprotective benefit. Unfortunately, dialysis patients are infrequently prescribed known cardioprotective medications. [20-24] The 1998 United States Renal Data System Annual Data Report (USRDS ADR) stated that 44% of patients less than 65 years of age with diabetes as the cause of ESRD were not treated with a hypoglycemic agent. This number fells 41% for patients over 65 years of age. Data from the USRDS Dialysis Morbidity and Mortality (DMMS) Wave-2 study identified 9.7% of patients treated with an HMG-CoA reductase inhibitor as of day sixty following dialysis initiation.[11] The Dialysis Outcome Practice Patterns Study (DOPPS) study identified 6.9% of United States HD patients treated with HMG-CoA reductase inhibitors.[24] This included 13.8% of patients with coronary artery disease and 16.1% of patients with a previous myocardial infarction.
Analysis of USRDS and DOPPS data also show that relatively few patients are prescribed beta-blocker therapy despite having multiple indications for therapy.[11,22] Data from the DMMS Wave-2 study showed that 18.7% of incident dialysis patients with diagnoses of coronary artery disease, chronic heart failure, and previous myocardial infarction, were prescribed a beta-blocker. This includes 25% of patients with hypertension, 24% with coronary artery disease, and 24% with a previous myocardial infarction.[22] Approximately 19% of new HD patients are given aspirin therapy.[11] The percent of point prevalent patients prescribed aspirin was higher than incident patients, suggesting greater use of aspirin with time on dialysis.[11] Finally, we also performed a medication use audit at our HD unit.[23] Over 96% of patients had at least one cardiovascular risk factor present with an average of 2.4 ± 1.3 cardiovascular risk factor per patient. Of those patients with a diagnosis of chronic heart failure, 34.4% were prescribed an ACE inhibitor and 25.0% were prescribed a beta-blocker. Patients with a previous myocardial infarction were given a lipid-lowering agent, a nitrate, a beta-blocker, and aspirin 23.1%, 23.1%, 15.4%, and 69.2% of the time, respectively. These studies highlight that medication prescribing in the treatment of cardiovascular diseases is in need of improvement in order to maximize care of the ESRD population.
We have shown that MRP continue to exist at a high frequency in these medically complex patients. To address this problem, we advocate the inclusion of pharmacists as a member of the healthcare team taking care of ambulatory HD patients. A pharmacist is specially trained to identify MRP. Pharmacist provided pharmaceutical care services to resolve MRP has shown to improve medication compliance, provide drug information, raise awareness of inappropriate medication prescribing, and improve biochemical and therapeutic responses to medication. [12-17] This will increase the number of patients achieving standard of care and improve overall patient care.[12] A recent review estimated that for every $1 spent on pharmaceutical care activities in ESRD patients, approximately $4 to the healthcare system is saved.[25]
List of Abbreviations
ADR = adverse drug reactions
DI = drug interactions
DMMS = Dialysis Morbidity and Mortality
DOPPS = Dialysis Outcome Practice Patterns Study
DWI = drug use without indication
ESRD = end-stage renal disease
FRD = failure to receive drugs
HD = hemodialysis
IDS = improper drug selection
IWD = indication without drug therapy
MRP = medication-related problem
O = other
OD = over-dosage
UD = sub-therapeutic dosage
USRDS = United States Renal Data System
Competing Interests
All authors have no competing interests with the material presented in this manuscript.
Authors' contributions
HJM and DKD mutually conceived the study, participated in its design, collected data, and prepared the manuscript. RSM contributed to the manuscript preparation an editorial comment. All authors read and approved the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
N/A
Contributor Information
Harold J Manley, Email: manleyh@umkc.edu.
Debra K Drayer, Email: debra.drayer@dciinc.org.
Richard S Muther, Email: richard.muther@dciinc.org.
References
- Kohn LT, Corrigan JM, Donaldson MS, eds . To err is human: building a safer health system. Washington, D.C.: National Academy Press; 1999. [PubMed] [Google Scholar]
- Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc(wash) 2001;41:192–9. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- Nelson KM, Talbert RL. Drug-related hospital admissions. Pharmacotherapy. 1996;16:701–707. [PubMed] [Google Scholar]
- Ebbesen J, Buajordet I, Erikssen J, Brors O, Hilberg J, Svaar H, Sandvik L. Drug-related deaths in a department of internal medicine. Arch Intern Med. 2001;161:2317–2323. doi: 10.1001/archinte.161.19.2317. [DOI] [PubMed] [Google Scholar]
- Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Segen DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- U.S. Renal Data System . USRDS 2002 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; 2002. [Google Scholar]
- Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533–543. [PubMed] [Google Scholar]
- Leape LL, Cullen DJ, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, Bates DW. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267–270. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- Gattis WA, Hasselblad V, Whellan DJ, O'Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med. 1999;159:1939–45. doi: 10.1001/archinte.159.16.1939. [DOI] [PubMed] [Google Scholar]
- United States Renal Data Systems (USRDS) 1998 Annual Data Report, National Institutes of Health, National Institute of Diabetes, Digestive, and Kidney Diseases, Bethesda, MD. 1998.
- Grabe DW, Low CL, Bailie GR, Eisele G. Evaluation of drug-related problems in an outpatient hemodialysis unit and the impact of a clinical pharmacist. Clin Nephrol. 1997;47:117–121. [PubMed] [Google Scholar]
- Kaplan B, Mason NA, Shimp LA, Ascione FJ. Chronic hemodialysis patients. Part I: Characterization and drug-related problems. Ann Pharmacother. 1994;28:316–319. doi: 10.1177/106002809402800303. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Shimp LA, Mason NA, Ascione FJ. Chronic hemodialysis patients. Part II: reducing drug-related problems through application of the focused drug therapy review program. Ann Pharmacother. 1994;28:320–4. doi: 10.1177/106002809402800304. [DOI] [PubMed] [Google Scholar]
- Stoutakis VA, Acchiardo SR, Martinez DR, Lorisch D, Wood GC. Role-effectiveness of the pharmacist in the treatment of hemodialysis patients. Am J Hosp Pharm. 1978;35:62–65. [PubMed] [Google Scholar]
- Tang I, Vrahnos D, Hatoum H, Lau A. Effectiveness of clinical pharmacist interventions in a hemodialysis unit. Clin Ther. 1993;15:459–464. [PubMed] [Google Scholar]
- Manley HJ, McClaran ML, Overbay DK, Wright MA, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS. Factors Associated with Medication-Related Problems in Ambulatory Hemodialysis Patients. Am J Kidney Dis. 2003;41:386–393. doi: 10.1053/ajkd.2003.50048. [DOI] [PubMed] [Google Scholar]
- Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP. 1990;24:1093–1097. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- Manley HJ, Bailie GR, Grabe DW. National database information: utility for an individual hemodialysis center. Am J Health-Syst Pharm. 2000;57:902–906. doi: 10.1093/ajhp/57.9.902. [DOI] [PubMed] [Google Scholar]
- McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic renal disease. Am Heart J. 2002;144:226–232. doi: 10.1067/mhj.2002.125513. [DOI] [PubMed] [Google Scholar]
- Dhingra RK, Stack AG, Wolfe RA, Yevzlin A, Port FK. Low utilization of cardiovascular drugs among incident dialysis patients with cardiac disease: A national study. J Am Soc Nephrol. 2001;12:A1035. [Google Scholar]
- Bragg JL, Mason NA, Maroni BJ, Held PJ, Young EW. Beta-adrenergic antagonist utilization among hemodialysis (HD) patients. J Am Soc Nephrol. 2001;12:A1652. [Google Scholar]
- Manley HJ, Overbay DO, Reid G, Bender W, Neufeld T, Hebbar S, Muther RS. Medical treatment of hemodialysis (HD) patients' cardiovascular risk factors (CVRF): potential for drug-related problems. J Am Soc Nephrol. 2002;13:703A. (PUB158) [Google Scholar]
- Mason NA, Bailie GR, Johnson CA, St Peter WL, Bragg JL, Young EW. Underutilization of HMG-CoA reductase inhibitors (HMGCoAIs) among hemodialysis (HD) patients: a potential drug-related problem. J Am Soc Nephrol. 2001;12:A1739. [Google Scholar]
- Manley HJ, Carroll C. The clinical and economic impact of pharmaceutical care in end-stage renal disease patients. Seminar Dial. 2002;15:45–9. doi: 10.1046/j.1525-139x.2002.00014.x. [DOI] [PubMed] [Google Scholar]