Abstract
Emerging evidence indicates that amyloid β peptide (Aβ) initially induces subtle alterations in synaptic function in Alzheimer disease. We have recently shown that Aβ binds to β2 adrenergic receptor (β2AR) and activates protein kinase A (PKA) signaling for glutamatergic regulation of synaptic activities. Here we show that in the cerebrums of mice expressing human familial mutant presenilin 1 and amyloid precursor protein genes, the levels of β2AR are drastically reduced. Moreover, Aβ induces internalization of transfected human β2AR in fibroblasts and endogenous β2AR in primary prefrontal cortical neurons. In fibroblasts, Aβ treatment also induces transportation of β2AR into lysosome, and prolonged Aβ treatment causes β2AR degradation. The Aβ-induced β2AR internalization requires the N terminus of the receptor containing the peptide binding sites and phosphorylation of β2AR by G protein-coupled receptor kinase, not by PKA. However, the G protein-coupled receptor kinase phosphorylation of β2AR and the receptor internalization are much slower than that induced by βAR agonist isoproterenol. The Aβ-induced β2AR internalization is also dependent on adaptor protein arrestin 3 and GTPase dynamin, but not arrestin 2. Functionally, pretreatment of primary prefrontal cortical neurons with Aβ induces desensitization of β2AR, which leads to attenuated response to subsequent stimulation with isoproterenol, including decreased cAMP levels, PKA activities, PKA phosphorylation of serine 845 on α-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor subunit 1 (GluR1), and AMPA receptor-mediated miniature excitatory postsynaptic currents. This study indicates that Aβ induces β2AR internalization and degradation leading to impairment of adrenergic and glutamatergic activities.
Keywords: Alzheimer Disease, Membrane Trafficking, Protein Kinase A (PKA), Receptor Desensitization, Receptor Endocytosis, AMPA Receptor, Amyloid β Peptide, β Adrenergic Receptor, mEPSC
Introduction
Alzheimer disease (AD)3 is a progressive neurodegenerative disorder characterized by extracellular amyloid plaques (1). Mounting evidence suggests that in the early phase of AD, memory loss is due to synaptic malfunction caused by diffusible amyloid β peptide (Aβ), before neuronal degeneration (2). In supporting this notion, we have recently shown that Aβ interacts with β2 adrenergic receptors (β2AR) in the central noradrenergic system to regulate synaptic functions in prefrontal cortical (PFC) neurons (3).
The central noradrenergic system is thought to determine the global orientation of the brain concerning events in the external world and within the viscera (4). It plays a critical role in arousal, which is important in regulating consciousness, attention, and information processing and is crucial for promoting certain behaviors such as mobility, learning, and pursuit of food (5). βARs, an integral part of central noradrenergic system, belong to G protein-coupled receptors (6). In the brain, βAR is widely distributed in different regions, including the frontal, parietal, piriform, and retrosplenial cortices, medial septal nuclei, olfactory tubercle, midbrain, striatum, hippocampus, and thalamic nuclei (7), regulating working memory and other basic brain functions (3, 8, 9). Recent studies have shown that polymorphism of β2AR contributes to sporadic late-onset AD (10, 11). During early AD pathogenesis, the central noradrenergic system undergoes substantial changes in response to the degeneration of its main site, the locus ceruleus (LC) (12), and decreased norepinephrine levels (13); however, the levels of adrenergic receptors are not generally increased due to the compensatory mechanism. In fact, a decrease in β2AR levels has been observed in different areas of AD brain (14), and βAR response is also decreased in fibroblasts isolated from AD patients (15).
We have recently shown that Aβ has a binding capacity to β2AR that requires the N terminus of the receptor (3). Acute treatment with Aβ initiates signaling transduction via β2AR as an allosteric partial agonist (3, 16, 17). Aβ binding does not interfere with the binding of catecholamines to the orthosteric binding sites within the receptor transmembrane helices (3). In recent years studies have shown that β2AR ligands have signaling selectivity via inducing differential conformational changes and activation of specific signaling pathways (18–23). Because of the heterogeneous binding property, it is necessary to analyze the action of Aβ on β2AR trafficking and cell function. In addition, Aβ is not catabolized as quickly as neurotransmitters at synapses, and the concentrations of the peptide are elevated in AD brains; therefore, persistent effects of Aβ on β2AR functions are of pertinence to AD pathology.
PKA, a signaling component downstream to β2AR, promotes the function of AMPA receptors by phosphorylating AMPA receptor subunit 1 (GluR1) (24–26). AMPA receptors are ligand-gated ion channels and are one of major mediators of excitatory neurotransmission. We have shown that acute treatment with Aβ increases β2AR- and PKA-dependent phosphorylation of GluR1 and frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs) mediated by AMPA receptors (3). Here we hypothesize that a persistent treatment with Aβ, in contrast to its acute effects, may impair cell function by decreasing β2AR agonist-induced phosphorylation of GluR1, which is modulated by a GluR1-β2AR complex (3, 26, 27). In this study a persistent treatment with Aβ indeed induces β2AR internalization and degradation leading to decreased mEPSCs mediated by AMPA receptors. Moreover, in AD animals, β2AR levels in cerebral tissues are significantly reduced, indicating its implication in brain adrenergic and glutamatergic dysfunction in AD.
EXPERIMENTAL PROCEDURES
Animals
Wild type, β1AR, β2AR, both β1AR and β2AR gene-knock-out (KO), and presenilin 1/amyloid precursor protein (PS1/APP) transgenic mice were used (28, 29). PS1/APP-transgenic mice overexpress both the human PS1 gene harboring two familial AD mutations, M146L and L286V, and the APP gene (695) with Swedish (K670N, M671L), Florida (I716V), and London (V717I) familial AD mutations. Wild type and PS1/APP transgenic mice (7 months old) were used for analysis of endogenous β2AR levels in the cerebrum. All animal experimental procedures were approved by the University of Illinois Animal Care and Use Committee.
Cell Culture and Aβ Treatment
Newborn WT, β1AR-KO, β2AR-KO, and β1/β2AR-KO mice were used to isolate PFC neurons under a stereomicroscope (30). Isolated neurons were plated on poly-d-lysine-coated dishes at a density of 1.0 × 105-106 cells/ml in DMEM/F-12 (1:1) medium containing 10% FBS, 1% insulin-transferin-sodium selenite supplement, 25 ng/ml nerve growth factor, 1 mm glutamine, 20 nm water-soluble progesterone, and 100 nm putrescine. The next day medium was changed to Neurobasal-A with B-27 supplement. Cytosine β-d-arabinofuranoside (2.5 μm, Sigma) was added 3 days after plating. Neurons were cultured for 2–3 weeks before experiments. HEK293 cells, wild type mouse embryonic fibroblasts (MEFs), and MEFs lacking either arrestin 2 (ARR2) or arrestin 3 (ARR3) gene were cultured in DMEM medium containing 10% FBS and antibiotics. Aβ1–42 and amino acid sequence-inverted peptide Aβ42–1 (Biopeptide) were dissolved at 10−3 m in dimethyl sulfoxide (DMSO) as previously described (31), which yields primarily monomers, dimers, and trimmers with low levels of oligomers. Cells were treated with soluble Aβ1–42 peptide as indicated. Our previous study shows that under these conditions Aβ1–42 dimers are the primary species bound to human FLAG-β2AR expressed on the plasma membrane (3).
cDNA Constructs
Human FLAG-β2AR and human FLAG-β1AR are gifts from Dr. Randy Hall (Emory University, GA). The GRK3A mutant β2AR lacking three GRK phosphorylation sites and the PKA4A mutant β2AR lacking four PKA phosphorylation sites, GFP-ARR3 and V54D dominant negative GFP-ARR3, HA-dynamin, and K44E dominant negative HA-dynamin, and the C terminus of GRK2 (also named β-adrenergic receptor kinase C-terminal (βARKct)) were constructed as described previously (32–34).
Immunofluorescence Microscopy
FLAG-tagged wild type, GRK3A mutant, or PKA4A mutant β2AR was expressed in HEK293 cells or MEFs by transfection or in freshly isolated PFC neurons by virus infection at 100 multiplicity of infection. After a 2-day expression, cells were treated with drugs for detection with anti-FLAG M1 (Sigma) or anti-β2AR antibody (Santa Cruz Biotechnology). Wild type and K44E-mutant dynamin were detected with anti-HA antibody (Covance). Lysosomes were stained by anti-Lamp I antibody (BD Pharmingen). Alexa488- and Alexa594-conjugated secondary antibodies (Invitrogen) were used to reveal the primary antibodies. Wild type and V54D-mutant ARR3 and βARKct were indicated by GFP fluorescence. Images were quantified with ImageJ-based Fiji open source image-processing package. Briefly, images were rotated to enable optimal selection of areas across the cell body. Plot profile analysis was applied to selected regions for measure of fluorescence intensity. Average of cytosolic fluorescence intensity (CFI) of β2AR was divided by that of membrane fluorescence intensity (MFI) for analysis of β2AR internalization. The ratio of CFI/MFI after stimulation was normalized against the ratio of CFI/MFI at resting state.
Receptor Degradation Assay
After serum starvation, cell surface proteins were labeled with 300 μg/ml biotin (Pierce) in PBS with calcium and magnesium containing 10 mm glucose (PBS/G) at 4 °C for 30 min. Free biotin was quenched with 100 mm glycine in PBS/G for 30 min followed by washing with ice-cold PBS/G. Cells were treated for 4 h with medicines replenished every 30 min. Cells were lysed in ice-cold lysis buffer (25 mm HEPES, pH 7.5 at 4 °C, 10% glycerol, 45 mm NaCl, 2 mm Na3VO4, 5 mm EDTA, 5 mm EGTA, 50 mm NaF, 1% Triton X-100, 8 mm NaN3, and protease and phosphatase inhibitor mixture) and homogenized using a Sonic Dismembrator 100 (Fisher). Streptavidin was added to 300-μl samples with equivalent protein concentration to precipitate biotin-labeled proteins. Eluates from beads were resolved by SDS-PAGE for detection of biotin-labeled β2AR. γ-Tubulin in supernatants was used as control.
Biotin Receptor Internalization Assay
Biotin labeling and quenching were the same as that of the degradation assay. After stimulation, residual surface biotin was stripped off with 50 mm Mesna in NT buffer (150 mm NaCl, 1 mm EDTA, 0.2% BSA, 20 mm Tris-HCl, pH 8.6) twice. The stripped cells were washed, lysed, and homogenized. Streptavidin was added to the samples to precipitate internalized biotin-labeled proteins. Eluates from beads were subjected to Western blot as mentioned above.
APP Processing Assay
Chinese hamster ovary (CHO) cells stably expressing APP cDNA containing the Val → Phe mutation at residue 717 (CHO-7PA2) is a gift from Dr. Dennis J. Selkoe (Harvard Medical School Center for Neurologic Diseases). CHO-7PA2 cells were further transfected with human β2AR cDNA in poly-d-lysine-coated dishes. Forty-eight hours after the transfection, the cells were starved for 2 h and treated for 4 h in serum-free media with Aβ (10−6 m) or together with CGP 20712A (10−5 m) and ICI 118551 (10−5 m), which were replenished every 30 min. The environment in the late endosomes and lysosomes is optimal for γ-secretase (35). For detection of APP processing to Aβ, lysosomes were prepared using the method described previously (36) with adaptation (37, 38). The lysosomes were lysed in 5 mm HEPES, 10% glycerol, 50 mm CH3COONH4, 5 mm EDTA, 5 mm EGTA, 1% Triton X-100, and protease inhibitors, pH 8.0. Aβ in the lysates were immunoprecipitated (39), and samples eluted from sepharose beads were subjected to 16% Tris-Tricine SDS-PAGE. For detection of APP, samples were subjected to 8% Laemmli SDS-PAGE.
Western Blotting
Proteins transferred to nitrocellulose membranes (Millipore) were blocked with 5% milk in blocking buffer (10 mm Tris-HCl, pH 7.4, 100 mm NaCl, 25 mm NaF, 2 mm Na3VO4, 8 mm NaN3, and 0.1% Tween 20). Membranes were incubated with primary antibodies against Aβ (Cell Signaling), APP (Sigma), β2AR, GRK-specific phospho-355/356 of β2AR (Santa Cruz Biotechnology), GluR1, and PKA-specific phospho-Ser-845 of GluR1 (Abcam) at 4 °C overnight. After washing, membranes were incubated with secondary antibodies for detection with the Li-Cor system (Li-Cor). The optical density of the bands was analyzed with Fiji image-processing package.
Fluorescence Resonance Energy Transfer (FRET) Recording
PFC neurons were infected with viruses to express protein kinase A activity reporter (AKAR2.2) at 37 °C for 24 h, then changed to virus-free medium and cultured for another 2 days. PKA activities were recorded as previously described (28, 40). Briefly, cells were rinsed and maintained in PBS/G for FRET recording. Cells were imaged on a Zeiss Axiovert 200M microscope with a 40×/1.3NA oil-immersion objective lens and a cooled CCD camera. Dual emission ratio imaging was acquired with a 420DF20 excitation filter, a 450DRLP diachronic mirror, and two emission filters (475DF40 for cyan and 535DF25 for yellow). The acquisition was set with 200-ms exposure in both channels and 20-s elapses. Images in both channels were subjected to background subtraction, and ratios of yellow-to-cyan color were calculated at different time points. In some experiments PFC neurons were pretreated with Aβ (10−6 m) for 40 min, and neurons were then washed 3 times before administration of isoproterenol (10−7 m).
Whole-cell Recording in Cultured PFC Neurons
The AMPA receptor-mediated mEPSCs in rat PFC cultures were recorded as described previously (41). The internal solution consisted of 130 mm cesium methanesulfonate, 10 mm CsCl, 4 mm NaCl, 10 mm HEPES, 1 mm MgCl2, 5 mm EGTA, 2.2 mm QX-314, 12 mm phosphocreatine, 5 mm MgATP, 0.5 mm Na2GTP, 0.1 mm leupeptin, pH 7.2–7.3, 265–270 mosm. The external solution consisted of 127 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 12 mm glucose, 10 mm HEPES, 1 μm tetrodotoxin, 5 μm bicuculline, pH 7.3–7.4, 300–305 mosm. Recording was conducted with a holding potential of −70 mV in the presence of 25 μm d-2-amino-5-phosphonovalerate to block NMDA receptor-mediated components of mEPSCs. Neurons were treated with 0.1% DMSO or Aβ1–42 (10−6 m) for 40 min followed by 3 times washing with 0.1% DMSO-containing media. Synaptic currents were analyzed with Mini Analysis Program (Synaptosoft, Leonia, NJ).
Statistical Analyses
Unpaired t tests and one- or two-way ANOVA were used to compare different groups with Prism software as indicated (Graphpad). Statistical comparisons of the amplitude and frequency of synaptic currents (mean ± S.E.) were made using the Kolmogorov-Smirnov test. A p value less than 0.05 is considered significant.
RESULTS
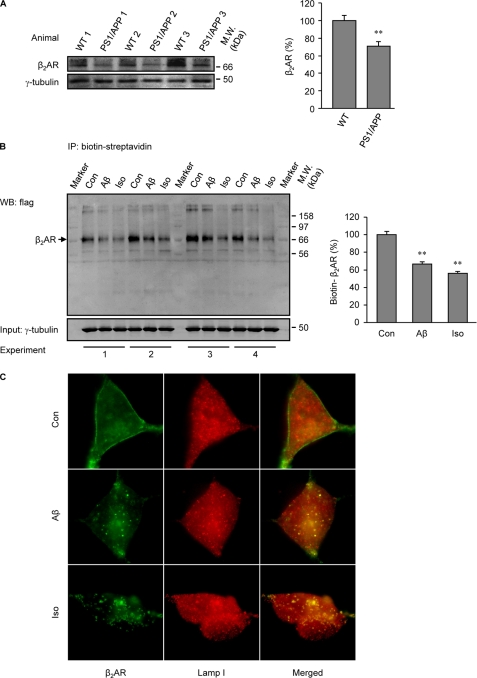
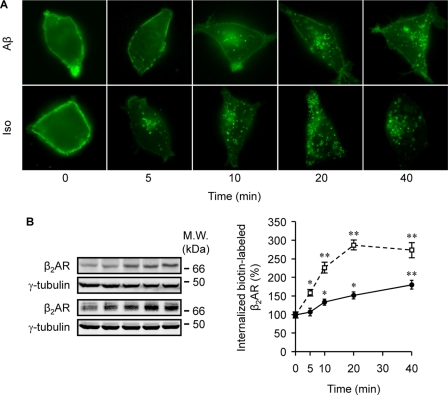
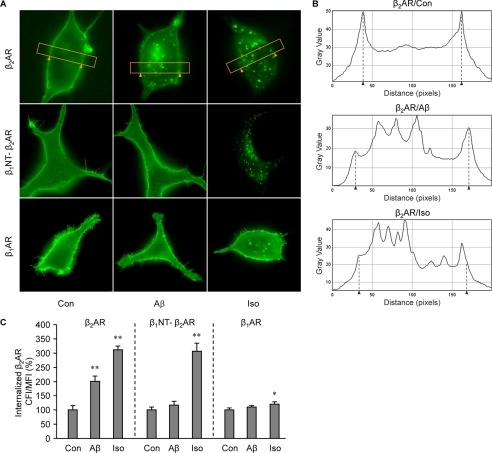
During the early development of AD, the central noradrenergic system undergoes substantial changes in response to the degeneration of its main site, the LC. Recent publications have shown that an acute treatment with Aβ initiates signal transduction via β2AR as an allosteric partial agonist (3, 16, 17). In this study we sought to understand the impact of a prolonged treatment with Aβ (10−6 m) on protein levels and function of β2AR in vivo and in vitro. We first examined β2AR protein levels in the cerebrums of 7-month-old PS1/APP double transgenic mice. The β2AR levels were significantly lower than those in wild type animals (Fig. 1A). In agreement, human β2AR expressed in HEK293 cells underwent significant degradation after 4 h of treatment with Aβ (10−6 m) (Fig. 1B). Accordingly, a 40-min stimulation of human β2AR induced colocalization of the receptor with the lysosome marker Lamp I (Fig. 1C), supporting that β2AR internalization and degradation occurs after a prolonged treatment with Aβ. We then set out to examine whether Aβ induces β2AR internalization in HEK293 cells. As we expected, the peptide induced significant receptor internalization after a minimal 10-min stimulation (Fig. 2A). The observation was further confirmed with quantitative measurement of internalized receptors labeled with biotin on the cell surface. Cells expressing human β2AR were labeled with biotin and then stimulated with Aβ or isoproterenol. Biotin remaining on the cell surface was stripped, and the internalized and biotin-labeled receptors were precipitated and blotted with anti-FLAG antibody. The Aβ-induced β2AR internalization displayed a time-dependent accumulation over 40 min of stimulation, which was slower and less robust than that induced by isoproterenol (Fig. 2B). In comparison to β2AR, neither β1AR nor the chimeric β1NT-β2AR, in which the N terminus was replaced by that of β1AR, showed internalization upon Aβ treatment (10−6 m) (Fig. 3, A and C), consistent with the fact that they do not have binding capacity to Aβ (3). As controls, both β1AR and the chimeric β1NT-β2AR displayed significant internalization upon isoproterenol stimulation (Fig. 3, A and C). Furthermore, we found that the βAR antagonist timolol (10−5 m) blocked β2AR internalization induced by either Aβ (10−6 m) or isoproterenol (10−7 m) (supplemental Fig. 1).
FIGURE 1.
Aβ induces β2AR degradation in vivo and in vitro. A, the endogenous β2AR in cerebral tissues from wild type (WT) and presenilin 1/amyloid precursor protein (PS1/APP) double transgenic mice were examined with Western blot and quantified. n = 6; **, p < 0.01, versus control by unpaired t test. IP, immunoprecipitate. B, HEK293 cells expressing FLAG-tagged human β2AR were stimulated with either Aβ (10−6 m) or isoproterenol (Iso, 10−7 m) for 4 h. The remaining biotin-labeled β2ARs were isolated and blotted, and the Western blots (WB) were quantified. n = 6; **, p < 0.01 versus control by one-way ANOVA. C, HEK293 cells expressing FLAG-β2AR were stimulated with Aβ (10−6 m) or isoproterenol (10−7 m) for 40 min, and cells were costained with anti-FLAG and anti-Lamp I antibodies.
FIGURE 2.
Aβ induces internalization of β2AR in HEK293 cells. A, effects of Aβ or isoproterenol on β2AR internalization are shown. Images show time-course effects of Aβ (10−6 m) and isoproterenol (Iso, 10−7 m) on β2AR internalization. B, levels of internalized β2AR after Aβ or isoproterenol treatment were quantified by biotinylation method. n = 8; *, p < 0.05; **, p < 0.01 versus control by two-way ANOVA followed by the modified Tukey test for multiple comparisons.
FIGURE 3.
Aβ induces internalization of β2AR but not β1AR or chimeric β1NT-β2AR. A, shown is distribution of FLAG-β2AR, FLAG-β1AR, and FLAG-β1NT-β2AR, in which the N terminus of the receptor was replaced by that of β1AR in HEK293 cells after Aβ (10−6 m) or isoproterenol (Iso, 10−7 m) treatment. B, shown are representative diagrams of fluorescence intensity in the selected regions of individual cells expressing FLAG-β2AR, indicated by the yellow frames in panel A; the cell membrane is indicated by arrows. C, Aβ-induced internalization of β2AR, β1AR, or chimeric β1NT-β2AR was quantified with Fiji image-processing package. n = 16; *, p < 0.05; **, p < 0.01 versus control by one-way ANOVA.
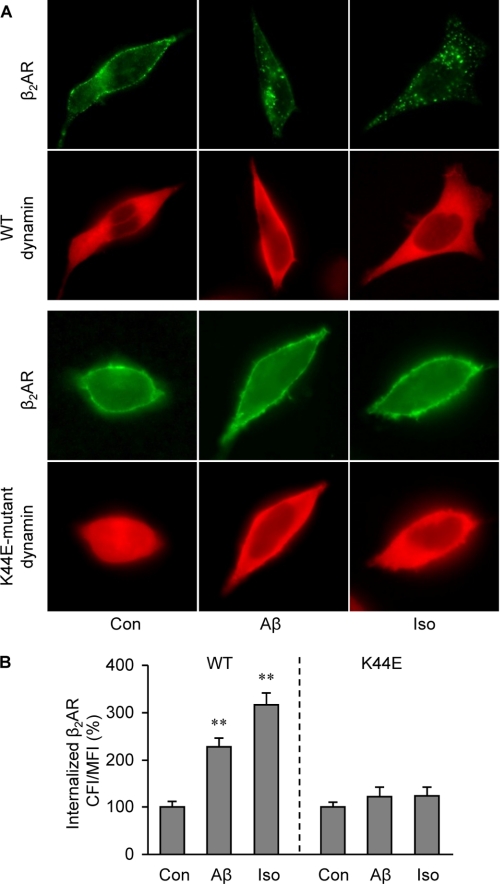
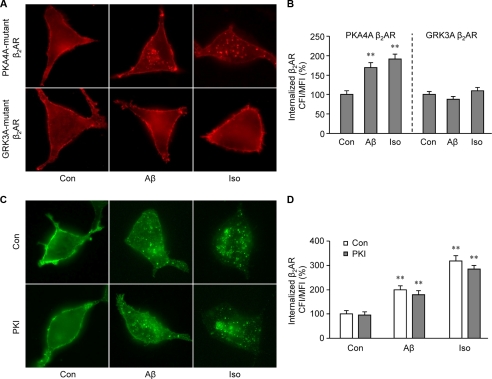
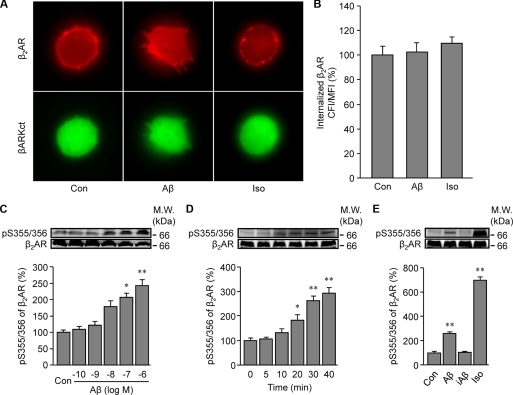
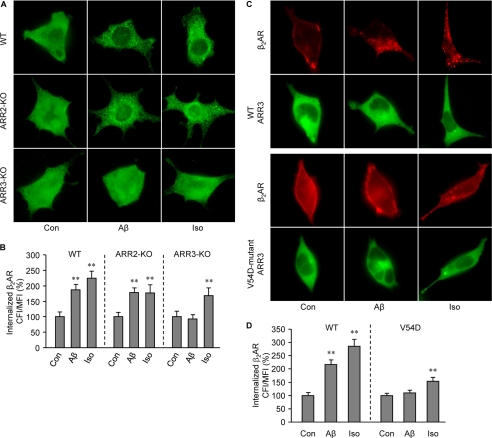
We then examined mechanisms governing Aβ-induced β2AR trafficking. First, we examined whether β2AR internalization induced by Aβ is dependent on dynamin GTPase activity. Overexpression of K44E dominant negative dynamin, but not wild type dynamin, almost completely inhibited β2AR internalization in HEK293 cells under either Aβ (10−6 m) or isoproterenol (10−7 m) stimulation (Fig. 4, A and B). This suggests that these two ligands induce receptor internalization via similar endocytosis pathways. PKA and GRK can phosphorylate β2AR at different sites, which are engaged in dynamin- and arrestin-dependent G protein-coupled receptor desensitization and trafficking. In order to know which kinase is involved in the Aβ-induced β2AR internalization, PKA and GRK phosphorylation site-mutant β2ARs, termed PKA4A β2AR and GRK3A β2AR respectively, were expressed in HEK293 cells. Immunofluorescence staining showed that mutant β2ARs lacking PKA phosphorylation sites, not GRK phosphorylation sites, were internalized upon Aβ (10−6 m) treatment (Fig. 5, A and B). Inhibition of PKA activity by a specific inhibitor, PKI (10−5 m), did not block the Aβ-induced β2AR internalization (Fig. 5, C and D). βARKct, which inhibits GRK2-mediated phosphorylation of β2AR, blocked the Aβ-induced β2AR internalization (Fig. 6, A and B). Consistently, Aβ (10−6 m), but not amino sequence-inverted Aβ (iAβ, 10−6 m), induced a dose- and time-dependent increase in GRK-dependent phosphorylation of β2AR (Fig. 6, C–E). The GRK-mediated phosphorylation of β2AR occurred at slower kinetics, consistent with the observed slower internalization rate induced by Aβ. Next, ARR2 and ARR3 are non-visual arrestins and are involved in G protein-coupled receptor endocytosis. We found that Aβ (10−6 m) and isoproterenol (10−7 m) induced β2AR internalization in wild type and ARR2-deficient MEF cells but not in ARR3-deficient cells (Fig. 7, A and B). Moreover, Aβ (10−6 m) induced β2AR internalization in HEK293 cells expressing wild type ARR3 but not V54D dominant negative ARR3 (Fig. 7, C and D). Together, these data indicate that Aβ (10−6 m) induces GRK2-, ARR3- and dynamin-dependent internalization, although the peptide effects are slower and less potent than those of isoproterenol (10−7 m). Furthermore, we found that Aβ (10−6 m) treatment increases the processing of APP to yield Aβ (supplemental Fig. 2), consistent with the recent study showing that agonist-induced β2AR endocytosis enhances γ-secretase activity for APP processing in lysosomes (35).
FIGURE 4.
Aβ-induced β2AR internalization requires GTPase dynamin. A, FLAG-β2AR was expressed together with either wild type or K44E dominant negative dynamin in HEK293 cells. Cells were stimulated with Aβ (10−6 m) or isoproterenol (Iso, 10−7 m) for 40 min, and the distribution of β2AR was examined with immunofluorescence staining. B, the images of Aβ-induced internalization of β2AR in panel A were quantified. n = 16; **, p < 0.01 versus control by one-way ANOVA.
FIGURE 5.
GRK, not PKA, phosphorylation of β2AR is required for Aβ-induced receptor internalization. A, distribution of PKA and GRK phosphorylation site-mutant β2ARs (PKA4A β2AR and GRK3A β2AR) after stimulation with Aβ (10−6 m) or isoproterenol (Iso, 10−7 m) for 40 min in HEK293 cells is shown. B, the images of Aβ-induced internalization of β2AR in panel A were quantified. n = 16; **, p < 0.01 versus control by one-way ANOVA. (C) Effect of a membrane-permeable PKA inhibitor, myristoylated-PKI, on Aβ-induced β2AR internalization. Cells were treated with Aβ (10−6 m), isoproterenol (10−7 m), and PKI (10−5 m) for 40 min, and the distribution of the receptor was examined. D, the images of Aβ-induced internalization of β2AR in panel C were quantified. n = 16; **, p < 0.01 versus control by one-way ANOVA.
FIGURE 6.
Aβ induces GRK-dependent phosphorylation of β2AR for internalization. A, shown is the effect of βARKct, a specific inhibitor of GRK2, on Aβ (10−6 m)-induced β2AR internalization. Iso, isoproterenol. B, Aβ-induced internalization of β2AR in panel A was quantified. n = 16. C and D, effects of Aβ on GRK-dependent phosphorylation of β2AR are shown. C, HEK293 cells expressing β2AR were stimulated with different doses of Aβ for 40 min. Aβ-induced GRK-dependent β2AR phosphorylation was examined and quantified. D, HEK293 cells expressing β2AR were stimulated with Aβ (10−6 m) for different times. Aβ-induced GRK-dependent β2AR phosphorylation was examined and quantified. E, HEK293 cells expressing β2AR were stimulated with Aβ (10−6 m), inverted Aβ (iAβ, 10−6 m), or isoproterenol (10−7 m). Aβ-induced GRK-dependent β2AR phosphorylation was examined and quantified. n = 6. *, p < 0.05; **, p < 0.01 versus control by one-way ANOVA.
FIGURE 7.
Aβ-induced β2AR internalization requires ARR3. A, FLAG-β2AR was expressed in wild type, ARR2-KO, or ARR3-KO MEFs. Cells were stimulated with Aβ (10−6 m) or isoproterenol (Iso, 10−7 m) for 40 min, and the distribution of β2AR was examined with immunofluorescence staining. B, the images of Aβ-induced internalization of β2AR in panel A were quantified. n = 16. **, p < 0.01 versus control by one-way ANOVA. C, FLAG-β2AR was expressed together with either wild type ARR3 or V54D dominant negative ARR3 in HEK293 cells. Cells were stimulated with Aβ (10−6 m) or isoproterenol (10−7 m) for 40 min, and the distribution of β2AR was examined with immunofluorescence staining. D, the images of Aβ-induced internalization of β2AR in panel C were quantified. n = 16. **, p < 0.01 versus control by one-way ANOVA.
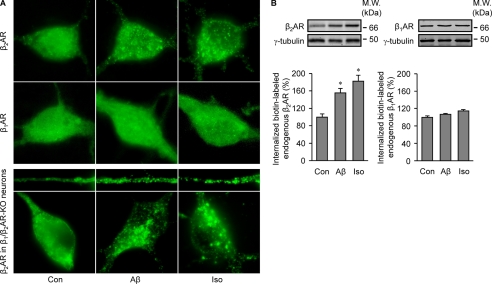
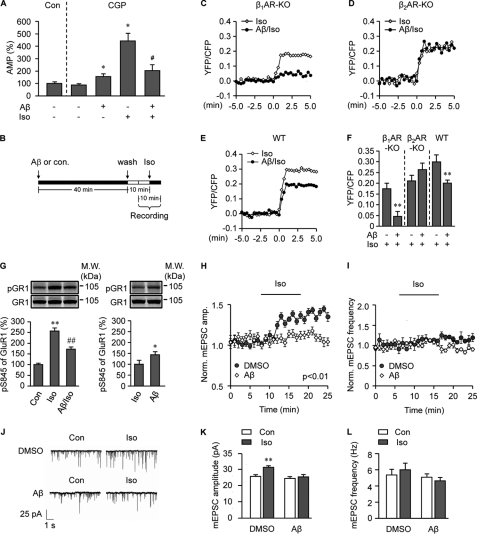
To know whether the Aβ-induced β2AR internalization impairs neuronal function, we isolated PFC neurons from neonatal mice. A 40-min treatment with Aβ (10−6 m) induced significant internalization of endogenous β2AR, not β1AR (Fig. 8A), which was quantified by the biotinylation method (Fig. 8B). Aβ (10−6 m)-induced β2AR internalization was even more evident with human β2AR overexpressed in β1AR/β2AR double-KO PFC neurons (Fig. 8A). Next, we investigated β2AR-mediated G protein-dependent signal transduction in PFC neurons. A 40-min pretreatment with Aβ (10−6 m) decreased isoproterenol (10−7 m)-induced increase in the second messenger cAMP level in the presence of β1AR antagonist CGP 20217A (10−5 m) (Fig. 9A). We also measured PKA activity induced by isoproterenol (10−7 m) in live PFC neurons with a FRET-based PKA biosensor AKAR2.2 (Fig. 9, B–F (28, 40)). Isoproterenol (10−7 m) itself induced a significant increase in PKA activity in wild type neurons (Fig. 9, C–F). However, pretreatment with Aβ (10−6 m) dramatically reduced the increase in PKA activity in response to subsequent isoproterenol stimulation. The remaining activity in wild type cells is in part due to activation of β1AR, which does not bind to Aβ (3). β1AR-KO neurons were then used to isolate the β2AR-specific effect on PKA activity. As expected, although isoproterenol (10−7 m) itself induced a significant increase in PKA activity in β1AR-KO neurons, pretreatment with Aβ (10−6 m) completely abolished the increase in PKA activity (Fig. 9, C–F). In contrast, Aβ pretreatment did not affect the isoproterenol (10−7 m)-induced increase in PKA activity in β2AR-KO neurons (Fig. 9, D and F).
FIGURE 8.
Aβ induces internalization of endogenous β2AR and transfected human β2AR, not β1AR in PFC neurons. A, shown is distribution of endogenous β2AR or β1AR in wild type PFC neurons and overexpressed FLAG-β2AR in β1/β2AR-KO PFC neurons after 40 min of treatment with Aβ (10−6 m) or isoproterenol (Iso, 10−7 m). B, Aβ-induced internalization of endogenous β2AR in PFC neurons was quantified by cell surface biotinylation. n = 6. *, p < 0.05 versus control by one-way ANOVA.
FIGURE 9.
Prolonged treatment with Aβ decreases signal transduction mediated by β2AR for AMPA receptor phosphorylation and activation in PFC neurons. A–F, prolonged treatment with Aβ decreases signal transduction mediated by β2AR. A, PFC neurons with or without 40-min pretreatment with Aβ (10−6 m) were stimulated with isoproterenol (Iso, 10−7 m) for 5 min in the presence of β1AR-specific antagonist CGP 20217A (10−5 m). The isoproterenol-induced increases in cAMP were measured. n = 12. *, p < 0.05 versus CGP 20217A control, #, p < 0.05 versus isoproterenol alone treated group by unpaired t test. B, indicated is the procedure for FRET recording to measure PKA activities induced by isoproterenol (10−7 m) after pretreatment with Aβ (10−6 m). C–F, the changes in isoproterenol-induced FRET ratios (PKA activities) after Aβ treatments are shown. The effect of a 40-min pretreatment with Aβ (10−6 m) on the intracellular PKA FRET ratio in response to isoproterenol (10−7 m) stimulation in β1AR-KO PFC neurons (C), β2AR-KO PFC neurons (D), or wild type PFC neurons (E) is shown. F, shown is a bar graph for peak PKA FRET responses in panels C, D, and E. n = 7–9. **, p < 0.01, versus isoproterenol alone treated group by unpaired t test. G–L, prolonged treatment with Aβ decreases PKA-dependent GluR1 phosphorylation and AMPA receptor-mediated mEPSCs in response to isoproterenol stimulation in PFC neurons. G, PFC neurons were treated with Aβ (10−6 m) for 40 min as indicated, and cells were then treated with isoproterenol (10−7 m) for 5 min in the presence of β1AR antagonist CGP 20217A (10−5 m) before detection of PKA phosphorylation of GluR1. n = 8–14, *, p < 0.05; **, p < 0.01 versus control; ##, p < 0.01 versus a isoproterenol alone-treated group by one-way ANOVA. GR1, AMPA receptor subunit GluR1. H and I, upon a 40-min pretreatment with Aβ (10−6 m), PFC neurons were treated with isoproterenol (10−7 m). The time course of amplitude (H) and frequency (I) of AMPA receptor-mediated mEPSCs was measured. J, traces of mEPSCs induced by isoproterenol (10−7 m) with or without 40 min of pretreatment with Aβ (10−6 m) is shown. K and L, the amplitude and frequency of AMPA receptor-mediated mEPSCs in response to isoproterenol (10−7 m) stimulation in panels H and I were plotted. n = 6. **, p < 0.01 versus control by Kolmogorov-Smirnov test for comparisons of the amplitude and frequency of synaptic currents.
Moreover, in PFC neurons cultured in the presence of β1AR antagonist CGP 20217A, pretreatment with Aβ (10−6 m) also decreased isoproterenol (10−7 m)-induced increase in PKA-mediated phosphorylation of AMPA receptor subunit GluR1 (Fig. 9G). In addition, GluR1 and β2AR form a postsynaptic protein complex in neurons (3, 26, 27), and we found that Aβ (10−6 m) also induced internalization of GluR1 in PFC neurons (supplemental Fig. 3). We also investigated isoproterenol-induced PKA-dependent mEPSCs mediated by AMPA receptors in PFC neurons after pretreatment with Aβ (Fig. 9, H–L). Isoproterenol (10−7 m) itself induced a significant increase in mEPSC amplitude (Fig. 9, H and K) and a small but not significant increase in mEPSC frequency in PFC neurons (Fig. 9, I and L). However, a 40-min pretreatment with Aβ (10−6 m) abolished isoproterenol-induced increases in AMPA receptor-mediated mEPSCs (Fig. 9, H–L).
DISCUSSION
Emerging evidence indicates that Aβ may induce subtle cellular dysfunction in synaptic plasticity contributing to early memory loss that precedes neuronal degeneration (42). Previous publications show that Aβ binds to β2AR and acutely activates the receptor-mediated signal transduction in both astrocytes and neurons (3, 16, 17). In this study we find that prolonged pretreatment with Aβ not only induces β2AR internalization and degradation but also decreases intracellular cAMP and PKA response to subsequently administered isoproterenol in primary PFC neurons. Because Aβ is not catabolized or cleared as quickly as catecholamines, it is conceivable that an elevated level of Aβ induces persistent β2AR internalization and degradation in AD. Accordingly, we found that protein levels of β2AR in the cerebrums of PS1/APP double transgenic animals are much lower than those in wild type animals. These data indicate that prolonged elevation of Aβ levels alters postsynaptic responses to neurotransmitters via decreasing β2AR levels on the plasma membrane. On the other hand, under chronic Aβ stimulation, persistent cAMP signaling leads to elevated PKA-dependent phosphorylation of its substrates such as GluR1 (3). The elevated basal phosphorylation of GluR1 would likely contribute to the hyperactivities of neurons for imbalanced neuronal network and functions (43), and it would also impair the response to further noradrenergic stimulation under physiological condition.
Consistently, a 40-min pretreatment with Aβ significantly decreases PKA-dependent phosphorylation of GluR1 at Ser-845 in response to subsequently administered isoproterenol and, as expected, decreases AMPA receptor-mediated mEPSCs in PFC neurons (Fig. 9). This is in contrast to the stimulatory effect on AMPA receptor activity by acute Aβ treatment (3, 24–26). In addition, GluR1, which exists with β2AR in a postsynaptic protein complex, is also internalized after prolonged Aβ stimulation, consistent with previous observations showing the removal of AMPA receptor from synapse (44). Therefore, this study suggests that a prolonged presence of Aβ in AD brain has dual effects on β-adrenergic and glutamatergic neurotransmission at synapses. First, the Aβ-induced β2AR signal leads to elevated basal phosphorylation of AMPA receptor (3) and contributes to hyperactivities of neurons in AD animal models (43). Second, the chronic stimulation also leads to internalization of both receptors and degradation of β2AR and impairs β-adrenergic and glutamatergic neurotransmission at synapses. This study also shows that the βAR antagonist, timolol, blocks β2AR internalization. Therefore, βAR antagonism may be beneficial in AD patients, as indicated in clinical studies (45).
In the early phase of AD, the central noradrenergic system undergoes substantial changes in response to the degeneration of its main site, the LC, as well as alteration of adrenergic receptor expression in different brain regions including cortical and limbic projection areas (14). LC degeneration results in decreased noradrenaline levels in certain brain regions of AD patients (13). The reduction of LC neurons correlates with Aβ plaques, neurofibrillary tangles, and the severity of dementia (46). However, norepinephrine levels in cerebrospinal fluids of AD patients are not generally reduced and in some cases are even increased (47, 48). This is probably due to a compensatory sprouting by surviving LC neurons (49). A chronic activation of β2AR on neurons in brain areas, including the LC, may contribute to neuronal degeneration due to its regulation on activities of L-type calcium channels and AMPA receptors (3, 26, 27). This paper, on the other hand, suggests a mechanism that Aβ impairs synaptic function in brain areas receiving noradrenergic projection via decreasing the levels of the receptors on the cell surface. This may also lead to the compensatory increase in presynaptic activities and catecholamine release of the surviving neurons in the LC. In addition, the reduction of adrenergic response in microglia may decrease norepinephrine-mediated anti-inflammatory protection, exacerbate inflammatory events, and reduce Aβ clearance, thereby exacerbating the situation in AD pathology (50, 51).
Aβ-induced β2AR internalization is both dose- and time-dependent but with a slower kinetics compared with isoproterenol. Although Aβ induces both GRK- and PKA-dependent phosphorylation of β2AR (3), Aβ-induced internalization is only dependent on GRK2-mediated phosphorylation (52, 53). The slow internalization induced by Aβ is consistent with the slow phosphorylation of β2AR by GRK, which accumulates over 40 min of stimulation. In contrast, isoproterenol induces rapid GRK phosphorylation of β2AR, which peaks at 1–2 min of stimulation followed by a gradual decrease (33, 34, 54). Moreover, Aβ-induced PKA-dependent phosphorylation of β2AR is faster compared with GRK phosphorylation, indicating biased signaling transduction associated with conformational changes of β2AR (18–23). The structural mechanisms behind these differences remain to be addressed in future. In this study Aβ-induced β2AR internalization is dependent on the non-visual arrestin 3, but not arrestin 2, which also requires dynamin GTPase activity. Together, under Aβ stimulation, β2ARs undergo endocytosis via arrestin-associated and clathrin-coated vesicles (53, 55). In addition, in agreement with the publication that the agonist-induced and clathrin-mediated endocytosis of β2AR enhances γ-secretase activity (35), we found that Aβ-induced β2AR internalization also increases the processing of APP to yield Aβ, which is of significance to AD pathology.
Together, this study reveals a noradrenergic mechanism for decreased synaptic signal transduction in AD, supporting the emerging concept that AD initially centers on soluble Aβ-induced subtle alterations in synaptic functions. Besides, β2AR and its signaling, as an integral part of the central noradrenergic system, is involved in multiple cell functions, including cell differentiation, proliferation, ion channel function, and immunoactivity of cells in the brain (27, 56–58). Moreover, β2AR and AMPA receptor are critical in normal brain functions. Thus, β2AR internalization and degradation induced by Aβ could be generally implicated in various diseases with high levels of Aβ including AD, Down syndrome, and Parkinson disease (59, 60).
Supplementary Material
Acknowledgments
We thank Dr. Dennis J. Selkoe at the Harvard Medical School Center for Neurologic Diseases for the CHO-7PA2 cell line. We also thank Mingwei Ding, Xiaoguang Gao, Ruijie Liu, and Vania De Arcangelis for technical assistance.
This work was supported, in whole or in part, by the National Institutes of Health HL082846 (to Y. X.). This work was also supported by an Alzheimer Association New Investigator Research grant (to Y. X.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- AD
- Alzheimer disease
- Aβ
- amyloid β peptide
- β2AR
- β2 adrenergic receptor
- MEF
- mouse embryonic fibroblast
- mEPSC
- miniature excitatory postsynaptic current
- AKAR2.2
- A-kinase activity reporter
- LC
- locus ceruleus
- GRK
- G-protein coupled receptor C terminal
- PFC
- prefrontal cortex
- βARKct
- β-adrenergic receptor kinase
- ARR
- arrestin
- CFI
- cytosolic fluorescence intensity
- MFI
- membrane fluorescence intensity
- PS1
- presenilin 1
- APP
- amyloid precursor protein
- Con
- control
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- ANOVA
- analysis of variance
- β1NT
- N termius of β1AR.
REFERENCES
- 1. LaFerla F. M., Oddo S. (2005) Trends Mol. Med. 11, 170–176 [DOI] [PubMed] [Google Scholar]
- 2. Selkoe D. J. (2002) Science 298, 789–791 [DOI] [PubMed] [Google Scholar]
- 3. Wang D., Govindaiah G., Liu R., De Arcangelis V., Cox C. L., Xiang Y. K. (2010) FASEB J. 24, 3511–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sara S. J. (2009) Nat. Rev. Neurosci. 10, 211–223 [DOI] [PubMed] [Google Scholar]
- 5. Csikszentmihalyi M. (1997) Finding Flow: The Psychology of Engagement with Everyday Life, pp. 1–34, BasicBooks, New York [Google Scholar]
- 6. Ramos B. P., Arnsten A. F. (2007) Pharmacol. Ther. 113, 523–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asanuma M., Ogawa N., Mizukawa K., Haba K., Hirata H., Mori A. (1991) Neurochem. Res. 16, 1253–1256 [DOI] [PubMed] [Google Scholar]
- 8. Ramos B. P., Colgan L. A., Nou E., Arnsten A. F. (2008) Neurobiol. Aging 29, 1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satler C., Garrido L. M., Sarmiento E. P., Leme S., Conde C., Tomaz C. (2007) Acta Neurol. Scand. 116, 355–360 [DOI] [PubMed] [Google Scholar]
- 10. Yu J. T., Tan L., Ou J. R., Zhu J. X., Liu K., Song J. H., Sun Y. P. (2008) Brain Res. 1210, 216–222 [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg P. B., Mielke M. M., Tschanz J., Cook L., Corcoran C., Hayden K. M., Norton M., Rabins P. V., Green R. C., Welsh-Bohmer K. A., Breitner J. C., Munger R., Lyketsos C. G. (2008) Am. J. Geriatr. Psychiatry 16, 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. German D. C., Manaye K. F., White C. L., 3rd, Woodward D. J., McIntire D. D., Smith W. K., Kalaria R. N., Mann D. M. (1992) Ann. Neurol. 32, 667–676 [DOI] [PubMed] [Google Scholar]
- 13. Matthews K. L., Chen C. P., Esiri M. M., Keene J., Minger S. L., Francis P. T. (2002) Biol. Psychiatry 51, 407–416 [DOI] [PubMed] [Google Scholar]
- 14. Westbroek W., Lambert J., Bahadoran P., Busca R., Herteleer M. C., Smit N., Mommaas M., Ballotti R., Naeyaert J. M. (2003) J. Invest. Dermatol. 120, 465–475 [DOI] [PubMed] [Google Scholar]
- 15. Huang H. M., Gibson G. E. (1993) J. Biol. Chem. 268, 14616–14621 [PubMed] [Google Scholar]
- 16. Igbavboa U., Johnson-Anuna L. N., Rossello X., Butterick T. A., Sun G. Y., Wood W. G. (2006) Neuroscience 142, 655–660 [DOI] [PubMed] [Google Scholar]
- 17. Echeverria V., Ducatenzeiler A., Chen C. H., Cuello A. C. (2005) Neuroscience 135, 1193–1202 [DOI] [PubMed] [Google Scholar]
- 18. Reiner S., Ambrosio M., Hoffmann C., Lohse M. J. (2010) J. Biol. Chem. 285, 36188–36198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Millar R. P., Newton C. L. (2010) Mol. Endocrinol. 24, 261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Violin J. D., DiPilato L. M., Yildirim N., Elston T. C., Zhang J., Lefkowitz R. J. (2008) J. Biol. Chem. 283, 2949–2961 [DOI] [PubMed] [Google Scholar]
- 21. Wisler J. W., DeWire S. M., Whalen E. J., Violin J. D., Drake M. T., Ahn S., Shenoy S. K., Lefkowitz R. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 16657–16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drake M. T., Violin J. D., Whalen E. J., Wisler J. W., Shenoy S. K., Lefkowitz R. J. (2008) J. Biol. Chem. 283, 5669–5676 [DOI] [PubMed] [Google Scholar]
- 23. Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010) Nat. Rev. Drug Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Banke T. G., Bowie D., Lee H., Huganir R. L., Schousboe A., Traynelis S. F. (2000) J. Neurosci. 20, 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu H., Real E., Takamiya K., Kang M. G., Ledoux J., Huganir R. L., Malinow R. (2007) Cell 131, 160–173 [DOI] [PubMed] [Google Scholar]
- 26. Joiner M. L., Lisé M. F., Yuen E. Y., Kam A. Y., Zhang M., Hall D. D., Malik Z. A., Qian H., Chen Y., Ulrich J. D., Burette A. C., Weinberg R. J., Law P. Y., El-Husseini A., Yan Z., Hell J. W. (2010) EMBO J. 29, 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davare M. A., Avdonin V., Hall D. D., Peden E. M., Burette A., Weinberg R. J., Horne M. C., Hoshi T., Hell J. W. (2001) Science 293, 98–101 [DOI] [PubMed] [Google Scholar]
- 28. Soto D., De Arcangelis V., Zhang J., Xiang Y. (2009) Circ. Res. 104, 770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oakley H., Cole S. L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., Vassar R. (2006) J. Neurosci. 26, 10129–10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen C. J., Liao S. L. (2003) J. Neurochem. 85, 443–453 [DOI] [PubMed] [Google Scholar]
- 31. Tong L., Thornton P. L., Balazs R., Cotman C. W. (2001) J. Biol. Chem. 276, 17301–17306 [DOI] [PubMed] [Google Scholar]
- 32. Cervantes D., Crosby C., Xiang Y. (2010) Circulation Res. 106, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu R., Ramani B., Soto D., De Arcangelis V., Xiang Y. (2009) J. Biol. Chem. 284, 32279–32287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Y., De Arcangelis V., Gao X., Ramani B., Jung Y. S., Xiang Y. (2008) J. Biol. Chem. 283, 1799–1807 [DOI] [PubMed] [Google Scholar]
- 35. Ni Y., Zhao X., Bao G., Zou L., Teng L., Wang Z., Song M., Xiong J., Bai Y., Pei G. (2006) Nat. Med. 12, 1390–1396 [DOI] [PubMed] [Google Scholar]
- 36. Docherty K., Brenchley G. V., Hales C. N. (1979) Biochem. J. 178, 361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bahadoran P., Busca R., Chiaverini C., Westbroek W., Lambert J., Bille K., Valony G., Fukuda M., Naeyaert J. M., Ortonne J. P., Ballotti R. (2003) J. Biol. Chem. 278, 11386–11392 [DOI] [PubMed] [Google Scholar]
- 38. Jadot M., Dubois F., Wattiaux-De Coninck S., Wattiaux R. (1997) Eur. J. Biochem. 249, 862–869 [DOI] [PubMed] [Google Scholar]
- 39. Klyubin I., Betts V., Welzel A. T., Blennow K., Zetterberg H., Wallin A., Lemere C. A., Cullen W. K., Peng Y., Wisniewski T., Selkoe D. J., Anwyl R., Walsh D. M., Rowan M. J. (2008) J. Neurosci. 28, 4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang J., Hupfeld C. J., Taylor S. S., Olefsky J. M., Tsien R. Y. (2005) Nature 437, 569–573 [DOI] [PubMed] [Google Scholar]
- 41. Yuen E. Y., Liu W., Kafri T., van Praag H., Yan Z. (2010) J. Physiol. 588, 2361–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Small D. H., Mok S. S., Bornstein J. C. (2001) Nat. Rev. Neurosci. 2, 595–598 [DOI] [PubMed] [Google Scholar]
- 43. Busche M. A., Eichhoff G., Adelsberger H., Abramowski D., Wiederhold K. H., Haass C., Staufenbiel M., Konnerth A., Garaschuk O. (2008) Science 321, 1686–1689 [DOI] [PubMed] [Google Scholar]
- 44. Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. (2006) Neuron 52, 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khachaturian A. S., Zandi P. P., Lyketsos C. G., Hayden K. M., Skoog I., Norton M. C., Tschanz J. T., Mayer L. S., Welsh-Bohmer K. A., Breitner J. C. (2006) Arch. Neurol. 63, 686–692 [DOI] [PubMed] [Google Scholar]
- 46. Bondareff W., Mountjoy C. Q., Roth M., Rossor M. N., Iversen L. L., Reynolds G. P., Hauser D. L. (1987) Alzheimer Dis. Assoc. Disord. 1, 256–262 [DOI] [PubMed] [Google Scholar]
- 47. Friedman J. I., Adler D. N., Davis K. L. (1999) Biol. Psychiatry 46, 1243–1252 [DOI] [PubMed] [Google Scholar]
- 48. Raskind M. A., Peskind E. R., Holmes C., Goldstein D. S. (1999) Biol. Psychiatry 46, 756–765 [DOI] [PubMed] [Google Scholar]
- 49. Szot P., White S. S., Greenup J. L., Leverenz J. B., Peskind E. R., Raskind M. A. (2006) J. Neurosci. 26, 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heneka M. T., Nadrigny F., Regen T., Martinez-Hernandez A., Dumitrescu-Ozimek L., Terwel D., Jardanhazi-Kurutz D., Walter J., Kirchhoff F., Hanisch U. K., Kummer M. P. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 6058–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heneka M. T., Ramanathan M., Jacobs A. H., Dumitrescu-Ozimek L., Bilkei-Gorzo A., Debeir T., Sastre M., Galldiks N., Zimmer A., Hoehn M., Heiss W. D., Klockgether T., Staufenbiel M. (2006) J. Neurosci. 26, 1343–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gurevich V. V., Gurevich E. V. (2004) Trends Pharmacol. Sci. 25, 105–111 [DOI] [PubMed] [Google Scholar]
- 53. Gurevich V. V., Gurevich E. V. (2006) Pharmacol. Ther. 110, 465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tran T. M., Jorgensen R., Clark R. B. (2007) Biochemistry 46, 14438–14449 [DOI] [PubMed] [Google Scholar]
- 55. Henley J. R., Cao H., McNiven M. A. (1999) FASEB J. 13, S243–S247 [DOI] [PubMed] [Google Scholar]
- 56. Hichami A., Boichot E., Germain N., Berdyshev E., Coqueret O., Lagente V. (1996) Mediators Inflamm. 5, 425–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Feinstein D. L. (1998) J. Neurochem. 70, 1484–1496 [DOI] [PubMed] [Google Scholar]
- 58. Hu X. X., Goldmuntz E. A., Brosnan C. F. (1991) J. Neuroimmunol. 31, 35–42 [DOI] [PubMed] [Google Scholar]
- 59. Englund H., Annerén G., Gustafsson J., Wester U., Wiltfang J., Lannfelt L., Blennow K., Höglund K. (2007) Dement. Geriatr. Cogn. Disord. 24, 369–374 [DOI] [PubMed] [Google Scholar]
- 60. Gallardo G., Schlüter O. M., Südhof T. C. (2008) Nat Neurosci 11, 301–308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.