Abstract
One of the main forms of nitrogen assimilated by microorganisms and plants is ammonium, despite its toxicity at low millimolar concentrations. Ammonium absorption has been demonstrated to be carried out by highly selective plasma membrane-located transporters of the AMT/MEP/Rh family and characterized by the presence of a well conserved hydrophobic pore through which ammonia is proposed to move. However, uncertainties exist regarding the exact chemical species transported by these membrane proteins, which can be in the form of either hydrophobic ammonia or charged ammonium. Here, we present the characterization of PvAMT1;1 from the common bean and demonstrate that it mediates the high affinity (micromolar), rapidly saturating (1 mm) electrogenic transport of ammonium. Activity of the transporter is enhanced by low extracellular pH, and associated with this acidic pH stimulation are changes in the reversal potential and cytoplasm acidification, indicating that PvAMT1;1 functions as an H+/NH4+ symporter. Mutation analysis of a unique histidine present in PvAMT1;1 (H125R) leads to the stimulation of ammonium transport by decreasing the Km value by half and by increasing the Vmax 3-fold, without affecting the pH dependence of the symporter. In contrast, mutation of the first conserved histidine within the channel modifies the properties of PvAMT1;1, increasing its Km and Vmax values and transforming it into a pH-independent mechanism.
Keywords: Ammonia, Bioenergetics, Histidine, Ion-sensitive Electrodes, Ammonium, Oocyte, Plant, Proton, Symporter
Introduction
Nitrogen is an essential nutrient for all forms of life, participating in many biological processes. Nitrogen is found in all amino acids; it appears incorporated into proteins and is present in the purine and pyrimidine bases that structure DNA and RNA. In plants, nitrogen is incorporated into chlorophyll molecules that are essential for photosynthesis, plant development, and growth. Ammonium is the preferred nitrogen source for many organisms, and its transport across cellular membranes is carried out by a family of integral membrane proteins that is conserved throughout all domains of life, the AMT/MEP/Rh family (1, 2). Members of this family of transporters share several properties like high affinity and high selectivity for ammonium, with a Km value in the micromolar range and transporting exclusively ammonium and its methylated analog, methylammonium, but no other monovalent cations. Despite these common characteristics, a controversy exists regarding the actual transport mechanism represented by these proteins, either a channel or a transporter, as well as the chemical form that is transported, ammonium or ammonia (3, 4). From the analysis of the crystal structure of AmtB from Escherichia coli (3, 4) and Amt1 from Archeoglobus fulgidus (5), it has been demonstrated that these transporters are conformed as homotrimers, with each monomer containing a central hydrophobic channel, proposed to be the pathway through which the uncharged ammonia is transported (4–6). The putative selective pore is formed by a recruitment site facing the periplasmic side, a so-called phenylalanine gate structured by the conserved Phe107 and Phe215 in EcAmtB, a central section constituted by the highly conserved His168 and His318, and a cytoplasmic vestibule. It is postulated that in the recruitment site, Ser219 and Trp148 residues in EcAmtB and conserved in the AMT/MEP/Rh family, can bind ammonium through hydrogen bonding by the former and through the π system of the latter (4), although this view is partially challenged by the results of Andrade et al. (5) who concluded that this site may be occupied by water. The conserved Phe107 and Phe215 seem to block the putative channel and may function in channel gating, although evidence for this is still lacking. Deeper into the channel of EcAmtB, the central section is partially structured by His168 and His318 and is proposed to participate in ammonium deprotonation and ammonia binding, facilitating the passage of the substrate ammonia. Finally, in the cytoplasmic vestibule and as a result of the environmental pH, ammonia is protonated, thus achieving the net ammonium transport across the membrane. However, discrepancies with this proposed mechanism exist. In the AMT4 transporters from plants, it has been demonstrated that the transport of ammonium is electrogenic, indicating a net charge movement that may correspond to either the transport of NH4+ or to the co-transport of H+/NH3 (7–9). This charge movement has been associated in a 1:1 ratio with the uptake of the labeled substrate, thus supporting the view that plant AMT transporters effect the transport of the charged species, NH4+ (10).
To obtain more information on the functioning of AMT transporters in general, and from plants in particular, we have characterized the transport properties of PvAMT1;1, a homolog from the common bean plant Phaseolus vulgaris. Our results demonstrate that PvAMT1;1 effects the electrogenic and high affinity transport of ammonium with a Km value in the micromolar range, as well as its analog methylammonium but with a lower affinity. The activity of PvAMT1;1 is pH-dependent, with an optimum pH close to 5.5 involving the transport of H+, suggesting that the bean mechanism corresponds to a H+/NH4+ symporter. Mutation analysis demonstrates that changing a His exclusively present in PvAMT1;1 (H125R) increases by 3-fold the activity of the transporter without affecting the pH dependence. However, mutation of the first conserved histidine (H211E) within the channel pore makes the transporter pH-independent, decreases its affinity for ammonium while increasing the transport capacity, and makes it impermeable to methylammonium. These results underline a central role for His211 in the symport mechanism of PvAMT1;1.
EXPERIMENTAL PROCEDURES
DNA Clones and Mutagenesis
For the cloning of PvAMT1;1 (accession number GQ377869), we employed degenerated primers designed according to sequence analysis of several plant homologs in combination with RT-PCR employing mRNA isolated from root nodules of the common bean plant P. vulgaris (Negro Jamapa). This allowed us to amplify a fragment that was used to screen a cDNA library from 15-day-old root nodules, obtaining 17 clones, 3 of which were sequenced (Instituto de Biotecnología, Universidad Nacional Autónoma de México) and showed high similarity to ammonium transporters from plants and other organisms. PvAMT1;1 was cloned into pOO2 oocyte expression vector, which contains the 5′- and the 3′-untranslated regions (UTR) of the β-globin gene from Xenopus laevis for enhanced oocyte expression. Site-specific mutagenesis was achieved with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) employing the primers shown in supplemental Table S1.
Primers were obtained from Unidad de Síntesis y Secuenciación (Instituto de Biotecnología, Universidad Nacional Autónoma de México, Cuernavaca, México) or Sigma. All clones were verified by sequencing (Instituto de Biotecnología, Universidad Nacional Autónoma de México Cuernavaca, México). Capped cRNA was transcribed in vitro by SP6 RNA polymerase using the mMessage mMachine kit (Ambion Inc., Austin, TX), after linearization of the plasmid with Bbr P1 (Roche Applied Science).
Preparation and Injection of Oocytes
The electrophysiological properties of PvAMT1;1 were obtained from its heterologous expression in X. laevis oocytes. Oocytes were obtained by surgery, manually dissected, and defolliculated with collagenase (2 mg ml−1) (Sigma). Oocytes, stages V and VI, were injected with 36 ng of PvAMT1;1 (in 50 nl), employing a Nanojector (Drummond Scientific Co., Broomall, PA). After injection, oocytes were incubated in a temperature-controlled chamber (Boekel Scientific, PA) at 16 °C in medium ND96 (in mm) as follows: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 Hepes, pH 7.4, containing gentamycin (0.1 g liter−1) and pyruvic acid (2.5 mm).
Electrophysiological Assays
The electrical activity of PvAMT1;1 was recorded 4–6 days after cRNA injection with the two-electrode voltage clamp technique, employing a GeneClamp 500B amplifier (Axon CNS, Molecular Devices, Sunnyvale, CA), connected to a PC computer (Compaq, Mexico) through the digital-analog converter Digidata 1440A (Axon CNS, Molecular Devices). Voltage protocols and data acquisition were controlled with Clampex, and data analysis was performed with Clampfit included in the pClamp 10 suite (Axon CNS, Molecular Devices). Microelectrodes were obtained from borosilicate capillaries (P1174; Sigma) and filled with 1 m KCl with a tip resistance of 0.5–1.5 megohms. Oocytes were exposed to a standard solution containing (in mm) 1 MgCl2, 1.8 CaCl2, 10 Tris/Mes, pH 7.0, with the osmolality adjusted to 240–260 mOsmol kg−1 with d-sorbitol. Bath solutions were flowed continuously at a rate of 5 ml min−1. Once the membrane potential read by the voltage and current electrodes was the same and stable for more than 2 min, the experiments were started. For all measurements, oocytes were clamped at their free running membrane potential (between −120 and −140 mV). For measurements at different pH values, the standard solution was adjusted to pH 5.5, 6.0, 7.0, or 8.0 with 10 mm Tris/Mes. Experiments were performed on more than four oocytes from more than two frogs under each condition. Results are the mean ± S.D.
pH-selective Electrodes and Recording of Intracellular pH
The pH microelectrodes were of the liquid ion exchanger type, employing the resin proton ionophore I, mixture B (Fluka, Sigma). Glass capillaries were pulled similarly to the microelectrodes used for voltage clamp and dried in an oven at 200 °C for 2 h. The electrodes were vapor silanized with bis(dimethylamino)dimethyl silane (Fluka, Sigma) at 200 °C for 4–6 h. Silanized microelectrodes were backfilled with the proton mixture and 1 m KCl. The pH electrodes were calibrated in standard solutions of pH 4–9. The average slope of the electrodes was 60 ± 3 mV before and after the recordings. If the calibration after recording did not give a slope close to 60 mV, the data were ignored.
Sequence and Data Analysis
Sequence and BLAST analysis of the PvAMT1;1 protein were done employing the CLC Genomics Workbench software (CLC Bio, Katrinebjerg, Denmark). Graphs and data analyses were drawn and performed with Origin 8. The fitted lines always showed a correlation coefficient r2 above 0.95.
RESULTS
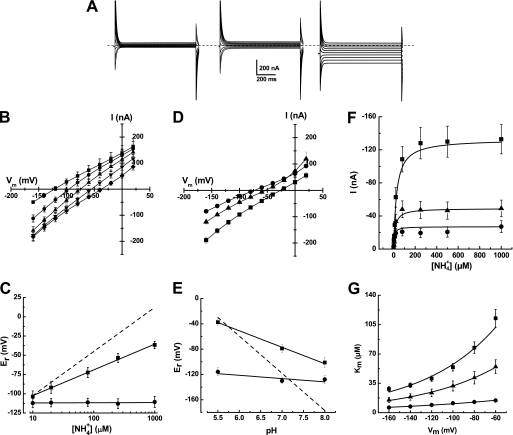
Transport properties of PvAMT1;1 were studied by its heterologous expression in X. laevis oocytes and the two-electrode voltage clamp technique. Initial studies demonstrated that exposure of oocytes injected with the cRNA from PvAMT1;1 developed inward currents when exposed to ammonium or its homolog, methylammonium, but not to any of the alkali cations, as shown in Fig. 1B. Confirmation that the inward ammonium currents were due to the expression of PvAMT1;1 was provided by the activation of smaller currents when control oocytes injected with DEPC/H2O were exposed to identical ammonium or methylammonium solutions (Fig. 1A). The results from control oocytes also demonstrated that the small inward currents activated by the alkali cations were due to endogenous mechanisms and not to the expression of PvAMT1;1 (compare Fig. 1, A and B). Fig. 1C summarizes the results from several oocytes showing that the ammonium currents were twice as large as those activated by methylammonium and more than 5-fold larger that those recorded with any of the alkali cations.
FIGURE 1.
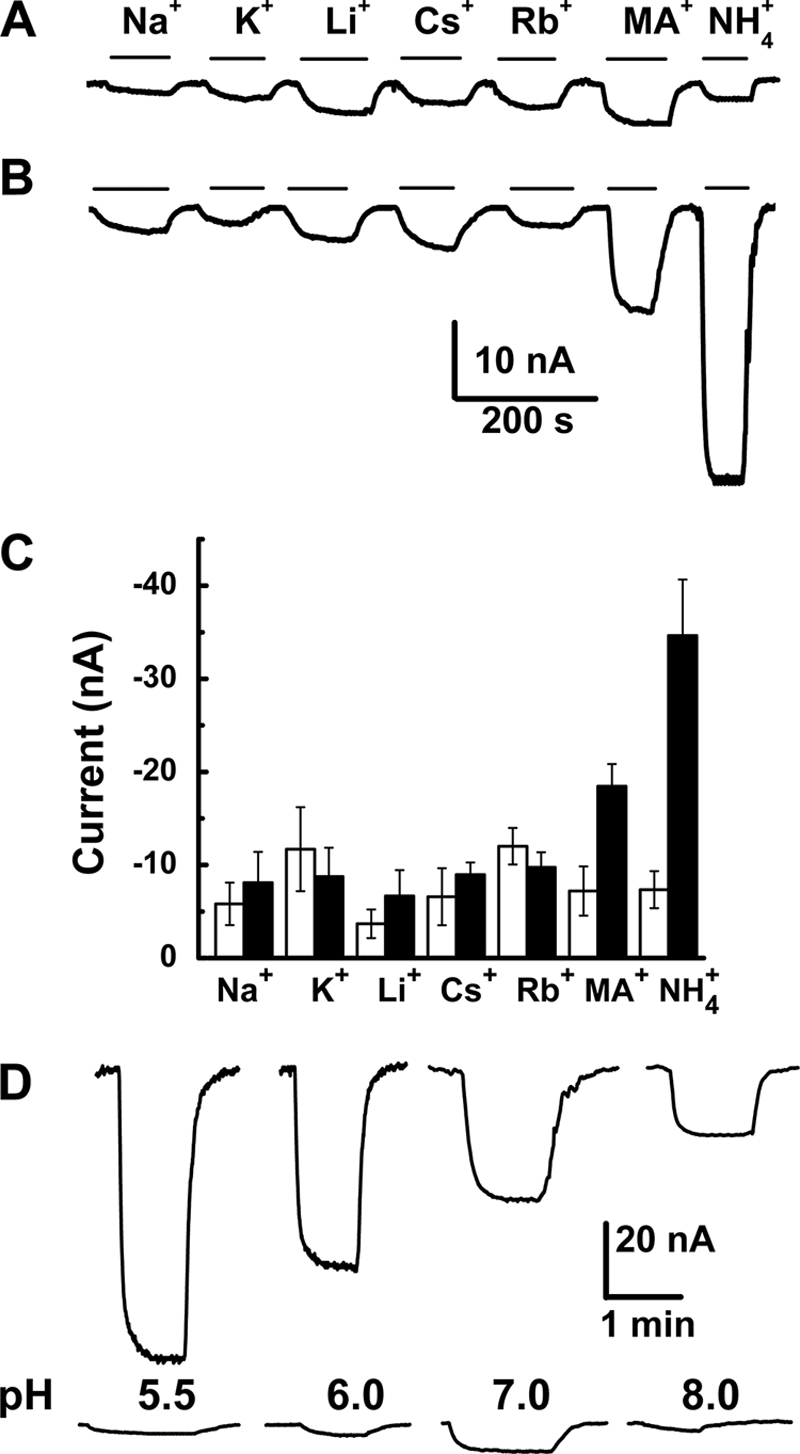
PvAMT1;1 is an ammonium transporter that depends on extracellular pH. Inward currents were activated by 1 mm chloride salts of the different monovalent cations in oocytes injected with DEPC/H2O (A) or the cRNA of PvAMT1;1 (B) at a holding potential of −150 mV. C, summary of the currents recorded from oocytes injected with DEPC/H2O (white) or cRNA of PvAMT1;1 (gray). Data are the means ± S.D. from more than 10 oocytes from more than three different frogs. D, inward currents activated by 1 mm ammonium at different extracellular pH levels and held at −150 mV from oocytes injected with the cRNA from PvAMT1;1 (top) or DEPC/H2O (bottom). Data are representative of more than 10 oocytes.
In plants, based on a membrane potential close to −150 mV (or more negative) with an external ammonium concentration at the micromolar level, accumulation of this nutrient in the low millimolar range must be active, with an H+/NH4+ symporter as the most likely mechanism (11). To prove this hypothesis, we assayed the activity of PvAMT1;1 at different external pH values, observing that as the medium became more acidic, ammonium transport increased, recording the maximal activity at pH 5.5 (Fig. 1D, top). Similar treatments to water-injected oocytes exposed to 1 mm ammonium activated small background currents with a maximum at pH 7.0, decreasing at acidic extracellular pH (Fig. 1D, bottom). These results indicated that PvAMT1;1 probably corresponded to the long sought H+/NH4+ symporter involved in ammonium uptake in plants.
Further information on the properties of PvAMT1;1 was obtained by analyzing its activity over a wide range of membrane potentials, between −160 and 20 mV. Confirming our initial observations, exposure of oocytes expressing PvAMT1;1 to 1 mm ammonium activated inward currents that were larger (Fig. 2A, right) than those recorded from the same oocytes in the absence of this cation (Fig. 2A, center) or from control water-injected oocytes exposed to 1 mm ammonium (Fig. 2A, left). The magnitude of the inward currents changed with the ammonium concentration, recording larger currents at higher concentrations, associated with changes in the reversal potential (Er) toward positive potentials (Fig. 2B). Plotting the values of Er against ammonium concentrations showed a linear relationship, with a calculated slope of 33.4 ± 3.7 mV/decade (Fig. 2C, filled squares). This value is well below the expected 59 mV/decade for a diffusion mechanism, according to the Nernst equation (Fig. 2C, dashed line). In contrast, currents recorded from control oocytes did not change in magnitude with increasing ammonium concentration (supplemental Fig. 1A), resulting in an unchanged Er (Fig. 2C, filled circles). As already shown (Fig. 1D), inward ammonium currents activated by negative potentials were larger at external acidic pH, decreasing in magnitude with alkalinization of the medium (Fig. 2, D–F). Importantly, the pH of the extracellular medium also caused changes in Er that showed a linear relationship with a slope of −26 ± 3.5 mV/pH unit (Fig. 2E), indicating that protons were also transported by the activity of PvAMT1;1. From the currents elicited by the different ammonium concentrations at variable extracellular pH and at a holding potential of −160 mV, it was possible to determine that the affinity of the transporter for ammonium increased as the pH alkalinized, obtaining values for Km of 28.2 ± 3.0 μm at pH 5.5, 15.0 ± 3.2 μm at pH 7.0, and 6.1 ± 2.1 μm at pH 8.0 (Fig. 2F). Analyses of these results confirmed the larger ammonium transport activity associated with acidic pH, obtaining values for the Vmax at −160 mV of −139.6 ± 3.3, −50.8 ± 2.0, and −23.0 ± 1.3 nA for pH 5.5, 7.0, and 8.0 respectively, according to Michaelis-Menten kinetics (Fig. 2F). The Km value was also affected by voltage and pH, observing that at acidic pH (5.5) the affinity for ammonium showed a strong voltage dependence, decreasing at more positive membrane potentials, dependence that became smaller as the pH increased to pH 7.0 and became almost voltage-independent at pH 8.0 (Fig. 2G). Assuming a single binding site for ammonium, as indicated by the Michaelis-Menten kinetics, we calculated that at acidic external pH the binding site is located at (δ) 34% within the membrane electrical field, changing to 30% at neutral pH and to 21% at pH 8.0 (supplemental material), indicating that the presence of protons influenced the effects of the electrical field across the membrane on ammonium binding (Fig. 2G, fitted lines). Changes in extracellular pH had no effect on the currents recorded from PvAMT1;1-injected oocytes in the absence of ammonium (supplemental Fig. 1B).
FIGURE 2.
Electrophysiological properties of PvAMT1;1. A, currents activated by voltage pulses between −160 and 20 mV in 20-mV steps from oocytes injected with DEPC/H2O (left) or cRNA of PvAMT1;1 in the absence (center) or presence of 1 mm NH4+ (right) at pH 5.5. B, current-voltage relationships from oocytes expressing PvAMT1;1 and exposed to different ammonium concentrations and pH 5.5 (■ = 0, ○ = 20, ▴ = 80, ▾ = 250, and ♦ = 1000 μm NH4+). C, variations in reversal potentials (Er) with extracellular ammonium concentration in oocytes expressing PvAMT1;1 (■) or DEPC/H2O-injected (●). Dashed line shows the expected slope according to the Nernst equation. D, current-voltage relationships obtained with 1 mm NH4+ at pH 5.5 (■), 7.0 (▴), and 8.0 (●). E, changes in Er caused by extracellular pH with constant 1 mm NH4+ in oocytes injected with PvAMT1;1 (■) or DEPC/H2O (●). Dashed line shows the expected slope according to the Nernst equation. F, ammonium transport kinetics for PvAMT1;1 at pH 5.5 (■), 7.0 (▴), and 8.0 (●). The lines represent the fitted Michaelis-Menten equation. G, voltage dependence of the Km at 5.5 (■), 7.0 (▴), and 8.0 (●). Data are the mean ± S.D. from at least eight oocytes from three different frogs.
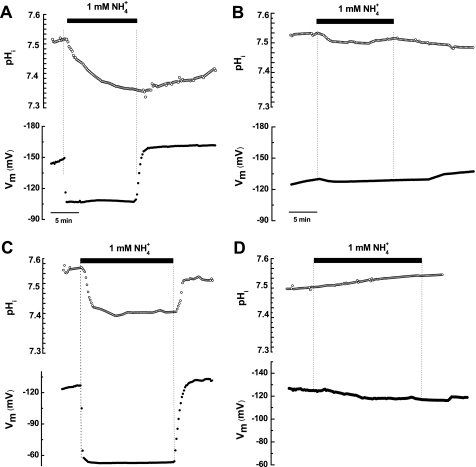
Although the results shown in Fig. 2, D–F, strongly suggested that protons were also transported by the activity of PvAMT1;1, it was necessary to obtain additional information to confirm this view. If protons were moved by the activation of PvAMT1;1, it should be expected that the intracellular pH values of the oocytes were acidified upon exposure to an ammonium solution. A prediction that was confirmed by making use of pH-selective microelectrodes in oocytes expressing PvAMT1;1 at an extracellular pH of 7.0. By doing so, we observed a clear acidification of the cytoplasm by 0.2 pH units in response to the presence of 1 mm ammonium in the medium (Fig. 3A, top, and Table 1). Parallel to this acidification was a depolarization of the membrane potential of 31 mV (Fig. 3A, bottom, and Table 1). Upon removal of extracellular ammonium, both parameters recovered their initial level, although the pH took longer to recuperate, probably because of the buffer capacity of the oocyte cytoplasm. In contrast, neither the cytoplasm pH (Fig. 3B, top, and Table 1) nor the membrane potential (Fig. 3B, bottom, and Table 1) of control oocytes were affected by the presence of 1 mm ammonium at pH 7.0 in the extracellular medium, confirming that cytoplasm acidification of PvAMT1;1-injected oocytes was a result of the activity of this protein functioning as an H+/NH4+ symporter. Similar experiments performed on oocytes exposed to an extracellular pH of 5.5 also led to the acidification of the cytoplasm by 0.12 pH units (Fig. 3C, top, and Table 1) accompanied by a depolarization of the membrane potential of 52.1 mV (Fig. 3C, bottom, and Table 1), changes that disappear upon removal of extracellular ammonium. Although the magnitude of the oocyte cytoplasm acidification was smaller at acidic extracellular pH, the changes were faster (compare Fig. 2, A and C) with a time constant (τ) = 1.3 ± 0.9 min−1 as compared with τ = 4.7 ± 1.5 min−1 at pH 7.0 (n = 4 and 5 oocytes, respectively), and this was reflected in the larger depolarization recorded at acidic pH (Table 1). Water-injected oocytes exposed to 1 mm ammonium at pH 5.5 showed a slight alkalinization (0.04 ± 0.03 pH units) without changes in membrane potential (Fig. 3D and Table 1), confirming again that the currents activated by extracellular ammonium were due to the expression of PvAMT1;1 on the plasma membrane of the oocytes. Attempts were made to record the responses from oocytes expressing PvAMT1;1 at pH 8.0; however, as the intracellular pH recordings take several minutes to perform, the development of a measurable endogenous inward current prevented us from obtaining reliable data (data not shown), as has been observed by other groups (10, 12, 13).
FIGURE 3.
Ammonium induces cytoplasm acidification in oocytes expressing PvAMT1;1. A, acidification of the cytoplasm (top) and membrane potential depolarization (bottom) caused by exposure of an oocyte expressing PvMT1;1 to 1 mm NH4+ at pH 7.0. Upon ammonium removal, both cytoplasm pH and oocyte membrane potential recovered to their initial levels, with the former taking more than 10 min. B, a control DEPC/H2O-injected oocyte exposed to the same conditions showed smaller acidification (top) and smaller change in membrane potential (bottom). C, acidification (top) and membrane depolarization (bottom) caused by 1 mm NH4+ in an oocyte expressing PvMT1;1 exposed to pH 5.5. Observe the faster changes in both parameters when compare with those recorded at pH 7.0 (see A). D, changes in cytoplasmic pH (top) and membrane potential (bottom) caused by 1 mm NH4+ in a control oocyte exposed to pH 5.5. Observe the slight alkalinization upon exposure to ammonium.
TABLE 1.
Changes in cytoplasmic pH and membrane potential from oocytes expressing PvAMT1;1 or the mutant H211E and exposed to 1 mm NH4+
Results correspond to acidification of cytoplasmic pH (ΔpH) and depolarization of membrane potential (ΔMP). Negative values for pH changes represent acidification of the cytoplasm in order to correspond to a decrease in pH. Depolarization of the membrane potential corresponds to positive values. Data are the mean ± S.D. from at least four oocytes.
| ΔpH |
ΔMP |
|||
|---|---|---|---|---|
| pH 7.0 | pH 5.5 | pH 7.0 | pH 5.5 | |
| pH units | mV | |||
| PvAMT1;1 | −0.199 ± 0.018 | −0.125 ± 0.035 | 30.9 ± 10.9 | 52.1 ± 15.5 |
| H2O | −0.043 ± 0.016 | 0.043 ± 0.028 | 2.6 ± 2.9 | −1.1 ± 1.5 |
| H211E | −0.050 ± 0.026 | −0.022 ± 0.016 | 27.4 ± 8.0 | 43.9 ± 1.3 |
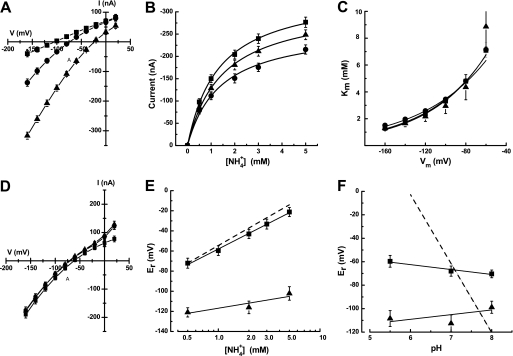
Previous reports on members of the AMT/Mep/Rh family of transporters have not shown any pH dependence (8, 9, 14), with the exception of TaAMT1;1 from wheat (15), making PvAMT1;1 only the second member where the activity of the protein is strongly affected by external pH. It is well known that histidine residues are important amino acids involved in the activity or regulation of pH-dependent transporters (16), which led us to identify histidines exclusively present in PvAMT1;1 as possible residues responsible for the activation of this transporter by acidic pH. Blast analysis demonstrated that PvAMT1;1 possesses a unique histidine at position 125 (His125) located in the extracellular loop linking transmembrane domains II and III (supplemental Fig. 2), which prompted us to modify this residue to the uncharged alanine (H125A) or the positively charged arginine (H125R). When oocytes expressing the mutant PvAMT1;1H125A were held at a membrane potential of −150 mV and exposed to 1 mm ammonium at pH 7.0, no inward currents were activated; in contrast, when the extracellular pH was decreased to 5.5, small inward currents (20.4 ± 8.0 nA) were induced in response to the presence of ammonium (Fig. 4A, H125A). Similar treatments to oocytes expressing the mutant PvAMT1;1H125R led to the recording of large inward currents at pH 7.0 (103.1 ± 31.2 nA) and even larger (176 ± 35.1 nA) when the pH was acidified to 5.5 (Fig. 4A, H125R). In control water-injected oocytes, the small inward currents recorded at pH 7.0 (7.3 ± 2.0 nA) were diminished (2.6 ± 1.8 nA) upon reducing the pH to 5.5 (Fig. 4A, H2O). Table 2 summarizes these results from more than 10 oocytes obtained from at least three frogs. PvAMT1;1H125R also showed Michaelis-Menten kinetics with a Km for ammonium equal to 16.4 ± 2.3 μm, a value similar to that obtained for the wild type (WT), but an almost 2-fold larger Vmax of 86.4 ± 5.8 nA, at pH 7.0 (n = 5 oocytes) (Fig. 4B). Acidification of the medium to pH 5.5 caused an increase in both the Km and Vmax to 37.7 ± 4.5 μm and 196.0 ± 9.4 nA, respectively, in the H125R mutant. Although these results clearly showed that the His125 residue is important in the functioning of PvAMT1;1, its dependence on external pH indicated this property resides in another amino acid.
FIGURE 4.
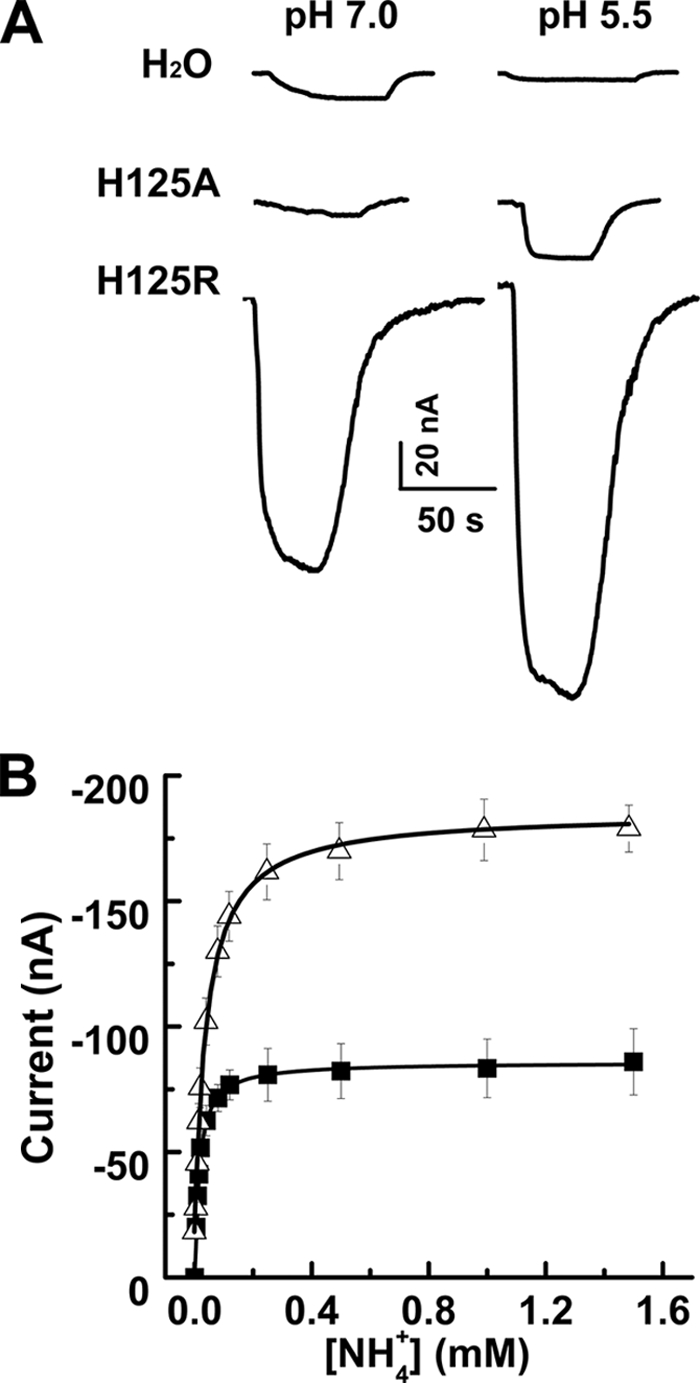
Mutations in His125 affect transport activity of PvMT1;1 without changing pH dependence. A, inward currents activated by 1 mm NH4+ in a control oocyte (H2O), an oocyte expressing the mutant H125A (H125A), and another expressing the mutant H125R (H125R) at pH 7.0 (left) or pH 5.5 (right) at a holding potential of −150 mV. B, ammonium transport kinetics at pH 5.5 from PvAMT1;1-injected oocytes (■) or the PvAMT1;1H125R mutant (Δ). The lines correspond to the fitted Michaelis-Menten equation to the experimental data (symbols). Data in B are the means ± S.D. from more than five oocytes from three different frogs.
TABLE 2.
Activity of the PvAMT1;1 His125 mutants
Values correspond to the currents activated by the various cations at 1 mm at a holding potential of −140 mV. Values are the mean ± S.D. of no less than 10 oocytes. MetAm corresponds to methylammonium.
| NaCl |
KCl |
MetAm |
Ammonium |
|||||
|---|---|---|---|---|---|---|---|---|
| pH 7.0 | pH 5.5 | pH 7.0 | pH 5.5 | pH 7.0 | pH 5.5 | pH 7.0 | pH 5.5 | |
| I (nA) | ||||||||
| H2O | 5.5 ± 2.0 | 4.7 ± 1.9 | 18.2 ± 6.8 | 5.9 ± 2.3 | 7.2 ± 2.6 | 3.1 ± 1.6 | 7.3 ± 2.0 | 2.6 ± 1.8 |
| PvAMT1;1 | 8.1 ± 3.3 | 10.1 ± 2.8 | 8.7 ± 3.9 | 5.9 ± 2.3 | 20.3 ± 6.9 | 51.2 ± 13.3 | 36.4 ± 8.2 | 78.9 ± 9.9 |
| H125R | 7.1 ± 1.4 | 7.1 ± 1.4 | 3.7 ± 2.1 | 5.2 ± 1.0 | 51.0 ± 15.2 | 74.5 ± 17.5 | 103.1 ± 31.7 | 176.8 ± 35.1 |
| H125A | 5.6 ± 0.7 | 4.6 ± 2.2 | 4.3 ± 3.1 | 1.8 ± 1.2 | 5.1 ± 2.0 | 11.7 ± 6.7 | 0.3 ± 3.0 | 20.4 ± 8.0 |
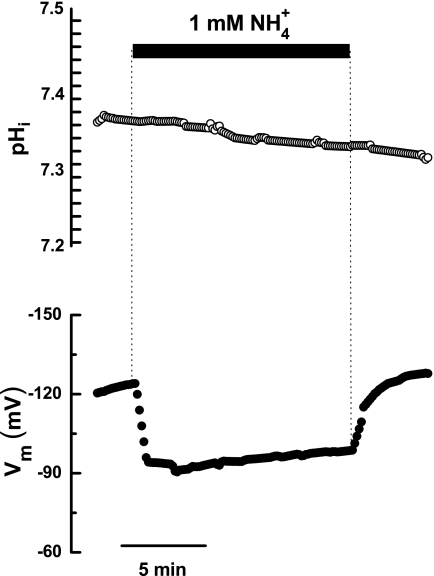
Continuing with our efforts to identify the amino acid associated with the pH dependence shown by PvAMT1;1, we proceeded to mutate the two conserved histidines that according to several authors are located in the putative conductive channel (4–6). Molecular modeling of PvAMT1;1 using the crystal structure of EcAmtB (4) and the ModWeb server showed that these two residues in particular, and the whole bean protein in general, seem to maintain a similar position/structure to that solved for the E. coli protein (supplemental Fig. 3). This observation allowed us to continue with the mutation of His211 to assess its possible role as pH sensor in PvAMT1;1. We decided to include a negative charge that could maintain the electrostatic interaction with ammonium by mutating histidine 211 to glutamic acid (H211E). Exposure of oocytes expressing the PvAMT1;1H211E mutant protein to ammonium concentrations below 50 μm failed in activating measurable inward currents, suggesting a lower affinity for ammonium. Measurable currents were only recorded with 100 μm ammonium or higher, observing no saturation up to a concentration of 5 mm (Fig. 5, A and B). Activation of an endogenous current in control oocytes at higher ammonium concentrations prevented us from reaching the saturation point. From these results, we calculated that both the Km and the Vmax increased to 1.3 mm and 300 nA, respectively, at a membrane potential of −160 mV. These changes were between 46- and 200-fold larger for the Km and 2-fold larger for the Vmax when compared with the values for the wild type. Similarly to what it was observed for PvAMT1;1, the affinity of the mutant H211E was voltage-dependent, although this response was not affected by the extracellular pH (Fig. 5C). For this mutant, it was estimated that the putative ammonium binding site was located at (δ) 0.40 inside the membrane electrical field. Current-voltage relationships showed that H211E became pH-independent, as shown in Fig. 5D. Other responses that were altered in H211E were the magnitude of the changes in Er caused by the different ammonium concentrations and by the different pH conditions, observing for both cases a linear relationship with calculated slopes of 51 and 4 mV/10-fold change in ammonium or H+ concentration, respectively (Fig. 5, E and F). These results indicated that the activity of H211E did not involve the transport of protons but only of the charged ammonium, as demonstrated by the small changes in Er caused by pH (Fig. 5F) and the almost Nernst response caused by extracellular ammonium (Fig. 5E). Furthermore, these observations suggested that ammonium should cause membrane depolarization without affecting the cytoplasmic pH. As demonstrated in Fig. 6, this was the case; exposure of an oocyte expressing H211E to 1 mm ammonium and pH 7.0 caused the depolarization of the membrane potential by 27.4 mV (Fig. 6, bottom, and Table 1) without clearly affecting the cytoplasmic pH (Fig. 6, top, and Table 1), which showed a slight and slow acidification, similar to that recorded from control oocytes. Similar results were obtained at extracellular pH 5.5, recording a depolarization that accounted for 43.9 mV, without affecting the cytoplasm pH (Table 1).
FIGURE 5.
Transport properties of the PvAMT1;1H211E mutant. A, current-voltage plots from oocytes expressing the PvAMT1;1H211E mutant exposed to zero (■), 0.5 (●), and 5.0 (▴) mm NH4+ at pH 5.5. B, ammonium transport kinetics of the PvAMT1;1H211E mutant at pH 5.5 (■), 7.0 (▴), and 8.0 (●). The lines correspond to the fitted Michaelis-Menten equation to the experimental data (symbols). C, voltage dependence of the Km at pH 5.5 (■), 7.0 (▴), and 8.0 (●). D, current-voltage plots from oocytes expressing the PvAMT1;1H211E mutant exposed to 1 mm NH4+ at pH 5.5 (■), 7.0 (▴), and 8.0 (●). E, reversal potentials recorded at different ammonium concentrations from oocytes expressing PvAMT1;1H211E (■) and control DEPC/H2O-injected oocytes (▴). F, reversal potentials recorded at three different extracellular pH values from oocytes expressing PvAMT1;1H211E in the presence (■) or in the absence (▴) of 1 mm NH4+. In E and F, the dashed lines represent the relationship according to the Nernst equation. Data are the means ± S.D. from more at least eight oocytes from three different frogs.
FIGURE 6.
In PvAMT1;1H211E, ammonium does not cause cytoplasm acidification. Changes in cytoplasmic pH (top) and membrane potential (bottom) in an oocyte expressing PvAMT1;1H211E exposed to 1 mm NH4+ at pH 7.0. Representative records are from 4 to 6 oocytes from 2 to 3 frogs.
DISCUSSION
Our results clearly demonstrate that PvAMT1;1 encodes for a highly selective ammonium transporter stimulated by an acidic environment. Expression of PvAMT1;1 in Xenopus oocytes led to the activation of inward currents upon exposure to submillimolar ammonium concentrations, showing saturation kinetics that were voltage-dependent. These currents were only activated by ammonium and its homolog, methylammonium, but not by any of the alkali cations (Fig. 1). The reversal potential of the inward currents was positively shifted by increasing ammonium concentrations, although with a sub-Nernstian response, indicating that another ion is also transported by PvAMT1;1. Acidification of the extracellular medium caused stimulation of ammonium transport and was associated with a decrease in the affinity for the substrate (Fig. 2F). Some of the properties from PvAMT1;1 are shared with other ammonium transporters (8, 9, 14, 17), with the exception of the stimulation by low pH, which has only been reported for the yeast Mep2 and the E. coli AmtB homologs (18) and for TaAMT1;1 from wheat (15). An important new finding is the acidification of the oocyte cytoplasm caused by exposure to ammonium (Fig. 3), a response that indicates PvAMT1;1 is also capable of transporting H+ together with ammonium, thus functioning as an H+/NH4+ symporter. Additional evidence that strengthens this conclusion is provided by the analyses of the responses of the membrane potential to changes in both ammonium and H+ extracellular concentrations (Fig. 2, C and E, and see the supplemental material). From these analyses, we calculated a stoichiometry of 0.8 ± 0.2 (n = 6), which indicates PvAMT1;1 does function as a symporter transporting H+ and NH4+ in a 1:1 ratio. Thus, the intracellular acidification was observed only in the presence of ammonium, and the stimulation of the inward ammonium current induced by low pH are clear indications that the movement of one ion depends on the presence of the other and that together with the 1:1 stoichiometry there is evidence clearly supporting the H+/NH4+ co-transport mechanism for PvAMT1;1. It is possible that a similar mechanism corresponds to the activity of ScMep2 that shows an optimal methylamine accumulation between pH 4 and 5, activity that was inhibited by the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) (18), an indication that this transporter may also depend on the protonmotive force (pmf) generated by the plasma membrane H+-ATPase in Saccharomyces cerevisiae. Although the activity of the wheat homolog TaAMT1;1 has been shown to be pH-dependent and higher at acidic extracellular pH, Er was not modified (15), indicating that the wheat transporter may be functioning as an H+-stimulated uniport. The fact that the activity of PvAMT1;1 is pH-dependent adds another level of regulation to this transporter, besides the likely inhibition caused by the phosphorylation of a threonine residue located in the carboxyl terminus, as reported for AtAMT1;1 (Thr460) (19, 20) and AtAMT1;2 (Thr472) (21) and as indicated by the presence of this conserved residue in PvAMT1;1 (Thr465).
The observed high affinity for ammonium and saturation of PvAMT1;1 activity at concentrations below 1 mm clearly identify this transporter with the high affinity mechanism (high affinity transport system) that has been described to be present in plants (22) and differentiate it from the low affinity transport mechanism (22) that has been associated with the activity of ion channels like KAT1 or NACC, potassium and nonselective cation channels, respectively (23, 24). Moreover, the stimulation of PvAMT1;1 by acidic pH also contrast with the higher ammonium transport observed at alkaline pH for aquaporins that have also been proposed to effect the low affinity transport NH4+/NH3 (25, 26).
Currently, among the several ammonium transporters that have been studied, it is still unclear how ammonium is transported. For the bacteria homologs, the proposed mechanism suggests that NH4+ is deprotonated to NH3, which then moves through the hydrophobic pore identified from crystal structures (4–6, 27, 28). In contrast, based on the electrogenicity of the mechanisms, it is postulated that the plant AMT homologs move either NH4+ or NH3 in symport with H+ (8, 9, 15, 29). Our results suggest that the molecular species most likely transported by the protein is NH4+, as indicated by the voltage dependence of the Km. The high values of Km at less negative membrane potentials can be interpreted as a reduction in the attraction between the protein-binding site and the charged substrate, an effect that is decreased as the potential is made more negative, resulting in lower Km values, similar to what has been reported for LeAMT1;1 (8), LeAMT1;2 (17), and TeAMT1;1 (15). It is important to emphasize that the affinity of the protein for ammonium increased almost 5-fold between pH 5.5 and 8.0 (Fig. 2D), together with a decrease in ammonium transport (Fig. 2F). These results may be explained by the interference of H+ with the ammonium-binding site and a decrease in the pmf (Fig. 2G), reinforcing the view that NH4+ is the transported species. Additionally, if ammonia were the transported species, an increase in PvAMT1;1 activity should be expected at alkaline pH, the opposite of what we observed, which is very likely due to a diminished pmf, thus supporting our conclusion that the bean transporter functions as an H+/NH4+ symporter.
In attempting to identify the amino acid responsible for the pH dependence shown by PvAMT1;1, we mutated His125, an amino acid present exclusively in PvAMT1;1. From the two mutations we studied, H125A led to the expression of a less active form of the transporter without losing the pH dependence, as indicated by the measurable activity recorded at acidic pH, as compared with the almost inactive form observed at pH 7.0 (Fig. 4). In contrast, when the positive charge linked to His125 under acidic conditions was mimicked by its substitution with Arg, H125R showed a larger activity at pH 7.0. However, in the H125R mutant the stimulatory effect of acidic pH was still observed (Fig. 4, A and B). Together, these results suggest that protonation of His125 leads to the stimulation of the transporter, a change that does not seem to affect the activation of this transporter by the plasma membrane ΔpH, indicating that other residues participate in coupling the downhill movement of H+ to the uphill uptake of ammonium. Because of the location of His125 in the first extracellular loop, between transmembrane domains II and III, it may be possible that the changes in activity observed with the H125A and H125R mutants may be through interactions with other extracellular loop regions of the protein that are longer in PvAMT1;1 than in E. coli or A. fulgidus (supplemental Fig. 3, D and E). These interactions, in turn, may be translated into conformational changes in transmembrane domain III that surrounds the putative selectivity pore (4–6), facilitating the H+/NH4+ co-transport activity of the PvAMT1;1H125R mutant.
In view of these results, we also analyzed the possible involvement of residue His211 in pH sensing, located in the putative conductive pore and highly conserved among the members of the Amt/Mep/Rh family of transporters (1). The mutation H211E caused several changes in the activity of the protein, i.e. an increase in both the Km and the Vmax values and independence of extracellular pH (Fig. 5). The changes in Km and Vmax values indicate that substitution of the imidazole ring (His) with the negatively charged carboxyl group (Glu) establishes a weaker interaction between the substrate and the protein, requiring higher concentrations of ammonium to reach half-maximal activity, while allowing higher transport rates, a phenomenon that resembles the permeation mechanism of some ion channels (30). Additionally, this substitution removed the pH dependence of the transporter and consequently did not cause the acidification of the cytoplasm (Fig. 6), making the H221E mutant partially independent of the ΔpH across the membrane and converting it into a channel-like mechanism mediating the downhill transport of ammonium. This observation also reinforces the view that the charged ammonium is the transported species instead of uncharged ammonia, as indicated by large changes in Er with extracellular ammonium (Fig. 5E), close to those predicted by the Nernst equation, and the small increase in Vmax that was observed at acidic pH (Fig. 5B). Moreover, the Km value of this mutant was still voltage-dependent (δ = 0.4), indicating that the electrical field across the membrane affects ammonium transport and by the same token that a charged particle, NH4+, is the transported species. Additionally, the increase in δ may be attributed to the observed decrease in affinity for NH4+ observed in the mutant H211E. This could imply that this mutant requires a stronger electrical field to facilitate the permeation of NH4+.
The properties of the H211E mutant are comparable with those of the yeast ammonium transporters ScMep1 and ScMep3, which naturally possess a glutamate residue in the homologous position (18, 31). These two yeast transporters are pH-independent in the pH range 5.0 to 7.5, show a lower affinity for methylamine (2 mm), and are more efficient in its transport as evinced by the toxic effects of high methylamine concentrations. In contrast, ScMep2, which possesses a histidine, shows a high affinity for ammonium and low transport capacity, and it is pH-dependent, with a maximum between pH 4 and pH 6.0 (18), similar to the PvAMT1;1 WT transporter. Another interesting observation are the properties of the E. coli homolog, EcAmtB, that as already mentioned presents the two histidines in the putative channel pore (4, 18). When expressed in the yeast triple mutant devoid of the three endogenous ammonium transporters (mep1Δ mep2Δ mep3Δ), EcAmtB caused a poor complementation of the yeast mutant showing an optimum at acidic pH (4.0). However, this complementation was better achieved by the mutant EcAmtBH168E, suggesting its transport capacity was larger than EcAmtB WT (18), and also resembled the properties of PvAMT1;1H211E. All these observations, from three different organisms, indicate that the first conserved histidine is not essential for the functioning of the transporters. On the contrary, mutation of the histidine residue to glutamate seems to confer a larger transport capacity to the three transporters together with a loss of both pH dependence and affinity for the substrate, as demonstrated here for the bean transporter (see Fig. 5), and as reported by Boeckstaens et al. (18) for the homologs from S. cerevisiae and E. coli. An additional observation with PvAMT1;1H211E was the increased selectivity observed for this mutant that was only permeable to ammonium, but not to its homolog methylamine or to any of the alkali cations (data not shown), a result that indicates that care must be taken when using [14C]methylammonium accumulation as evidence for the activity of AmtB/Mep/Rh family of transporters.
Taking into consideration the conditions present in plant cells, where intra- and extracellular ammonium concentrations may vary around 1–30 mm and 3–10 μm, respectively (11, 32), passive ammonium transport would only take place with membrane potentials more negative to −120 mV, limiting ammonium uptake upon depolarization or at lower extracellular ammonium. The functioning of PvAMT1;1 as an H+/NH4+ symporter could support the supply of this important plant nutrient, even under low extracellular ammonium because of the presence of an inwardly directed pmf established by the plasma membrane H+-ATPase (approximately −240 mV), which theoretically could help to increase the intracellular ammonium concentration up to 100-fold larger than that in the extracellular surroundings. Energization of PvAMT1;1 by the plant cell plasma membrane pmf would lead to the acquisition of ammonium under a variety of soil conditions to which plants may be exposed naturally.
One aspect that remains to be explained is the proton pathway. In the absence of a clear path for H+ transport, we would like to propose that H+ moves either by a Grotthuss mechanism or along a hydrogen-bonded chain (33), with His211 playing a central role. In the former, H+ would hop from one water molecule at one side of the membrane to the next influenced by the electrical field, with a different H+ leaving on the other side. Formation of a chain of water molecules seems to be possible in the pore of AmtB (34, 35), despite the proposal by several authors (4–6) that consider the pore of the ammonium transporters to be hydrophobic. Based on the similarity between the EcAmtB and PvAMT1;1 (supplemental Fig. 3), it is likely that a similar condition may be established in the bean transporter, where His211 would play a role in structuring the water molecules chain and thus allowing for the hopping of H+ along the channel and across the membrane. Alternatively, His211 could participate directly in the establishment of a hydrogen-bonded chain, either with the involvement of other amino acids or including water molecules, leading to the movement of H+ across the membrane. In both cases, the central role for histidine cannot be taken by glutamate.
Supplementary Material
Acknowledgment
We acknowledge Dr. Enrique Rudiño for obtaining the molecular model of PvAMT1;1.
This work was supported in part by Grants 42664 and 79191 from Consejo Nacional de Ciencia y Tecnología-México and Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México, Grant IN218308 (to O. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1, Figs. 1–3, Equations 1–4, and additional references.
- AMT
- ammonium transporter
- DEPC
- diethyl pyrocarbonate
- pmf
- protonmotive force.
REFERENCES
- 1. Wirén N. V., Merrick M. (2004) Top. Curr. Genet. 9, 1–26 [Google Scholar]
- 2. Winkler F. K. (2006) Pflügers Arch. 451, 701–707 [DOI] [PubMed] [Google Scholar]
- 3. Javelle A., Lupo D., Li X. D., Merrick M., Chami M., Ripoche P., Winkler F. K. (2007) J. Struct. Biol. 158, 472–481 [DOI] [PubMed] [Google Scholar]
- 4. Khademi S., O'Connell J., 3rd, Remis J., Robles-Colmenares Y., Miercke L. J., Stroud R. M. (2004) Science 305, 1587–1594 [DOI] [PubMed] [Google Scholar]
- 5. Andrade S. L., Dickmanns A., Ficner R., Einsle O. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 14994–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng L., Kostrewa D., Bernèche S., Winkler F. K., Li X. D. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 17090–17095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ludewig U. (2006) Transfus. Clin. Biol. 13, 111–116 [DOI] [PubMed] [Google Scholar]
- 8. Ludewig U., von Wirén N., Frommer W. B. (2002) J. Biol. Chem. 277, 13548–13555 [DOI] [PubMed] [Google Scholar]
- 9. Mayer M., Dynowski M., Ludewig U. (2006) Biochem. J. 396, 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayer M., Schaaf G., Mouro I., Lopez C., Colin Y., Neumann P., Cartron J. P., Ludewig U. (2006) J. Gen. Physiol. 127, 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M. Y., Glass A., Shaff J. E., Kochian L. V. (1994) Plant Physiol. 104, 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boldt M., Burckhardt G., Burckhardt B. C. (2003) Pflügers Arch. 446, 652–657 [DOI] [PubMed] [Google Scholar]
- 13. Bakouh N., Benjelloun F., Hulin P., Brouillard F., Edelman A., Chérif-Zahar B., Planelles G. (2004) J. Biol. Chem. 279, 15975–15983 [DOI] [PubMed] [Google Scholar]
- 14. Wood C. C., Porée F., Dreyer I., Koehler G. J., Udvardi M. K. (2006) FEBS Lett. 580, 3931–3936 [DOI] [PubMed] [Google Scholar]
- 15. Søgaard R., Alsterfjord M., Macaulay N., Zeuthen T. (2009) Pflügers Arch. 458, 733–743 [DOI] [PubMed] [Google Scholar]
- 16. Wiebe C. A., Dibattista E. R., Fliegel L. (2001) Biochem. J. 357, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludewig U., Wilken S., Wu B., Jost W., Obrdlik P., El Bakkoury M., Marini A. M., André B., Hamacher T., Boles E., von Wirén N., Frommer W. B. (2003) J. Biol. Chem. 278, 45603–45610 [DOI] [PubMed] [Google Scholar]
- 18. Boeckstaens M., André B., Marini A. M. (2008) J. Biol. Chem. 283, 21362–21370 [DOI] [PubMed] [Google Scholar]
- 19. Loqué D., Lalonde S., Looger L. L., von Wirén N., Frommer W. B. (2007) Nature 446, 195–198 [DOI] [PubMed] [Google Scholar]
- 20. Lanquar V., Loqué D., Hörmann F., Yuan L., Bohner A., Engelsberger W. R., Lalonde S., Schulze W. X., von Wirén N., Frommer W. B. (2009) Plant Cell 21, 3610–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neuhäuser B., Dynowski M., Mayer M., Ludewig U. (2007) Plant Physiol. 143, 1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang M. Y., Siddiqi M. Y., Ruth T. J., Glass A. D. (1993) Plant Physiol. 103, 1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moroni A., Bardella L., Thiel G. (1998) J. Membr. Biol. 163, 25–35 [DOI] [PubMed] [Google Scholar]
- 24. Demidchik V., Tester M. (2002) Plant Physiol. 128, 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holm L. M., Jahn T. P., Møller A. L., Schjoerring J. K., Ferri D., Klaerke D. A., Zeuthen T. (2005) Pflügers Arch. 450, 415–428 [DOI] [PubMed] [Google Scholar]
- 26. Uehlein N., Fileschi K., Eckert M., Bienert G. P., Bertl A., Kaldenhoff R. (2007) Phytochemistry 68, 122–129 [DOI] [PubMed] [Google Scholar]
- 27. Javelle A., Thomas G., Marini A. M., Krämer R., Merrick M. (2005) Biochem. J. 390, 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boogerd F. C., Ma H., Bruggeman F. J., van Heeswijk W. C., García-Contreras R., Molenaar D., Krab K., Westerhoff H. V. (2011) FEBS Lett. 585, 23–28 [DOI] [PubMed] [Google Scholar]
- 29. Guether M., Neuhäuser B., Balestrini R., Dynowski M., Ludewig U., Bonfante P. (2009) Plant Physiol. 150, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hille B. (1992) Ionic Channels of Excitable Membranes, 2nd Ed., pp. 1–814, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 31. Marini A. M., Soussi-Boudekou S., Vissers S., Andre B. (1997) Mol. Cell. Biol. 17, 4282–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kronzucker H. J., Siddiqi M. Y., Glass A. D. (1997) Nature 385, 59–61 [Google Scholar]
- 33. Decoursey T. E. (2003) Physiol. Rev. 83, 475–579 [DOI] [PubMed] [Google Scholar]
- 34. Lamoureux G., Klein M. L., Bernèche S. (2007) Biophys. J. 92, L82–L84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lamoureux G., Javelle A., Baday S., Wang S., Bernèche S. (2010) Transfus. Clin. Biol. 17, 168–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.