Abstract
Estrogen receptor α (ERα) regulates gene transcription via “genomic” (binding directly or indirectly, typically via Sp1 or AP-1 sites, to target genes) and/or “nongenomic” (signaling) mechanisms. ERα activation by estrogen up-regulates the murine Ca2+-activated K+ channel α subunit gene (mSlo1) via genomic mechanisms. Here, we investigated whether ERα also drives transcription of the human (hSlo1) gene. Consistent with this view, estrogen increased hSlo1 transcript levels in primary human smooth muscle cells. Promoter studies revealed that estrogen/hERα-mediated hSlo1 transcription was nearly 6-fold more efficient than for mSlo1 (EC50, 0.07 versus 0.4 nm). Unlike the genomic transcriptional mechanism employed by mSlo1, hSlo1 exhibits a nongenomic hERα-mediated regulatory mechanism. This is supported by the following: 1) efficient hSlo1 transcription after disruption of the DNA-binding domain of hERα or knockdown of Sp1, and 2) lack of AP-1 sites in the hSlo1 promoter. Three nongenomic signaling pathways were explored: Src, Rho, and PI3K. Inhibition of Src with 10 μm PP2, and reported downstream ERK with 25 μm PD98059 did not prevent estrogen action but caused an increase in hSlo1 basal transcription; conversely, constitutively active c-Src (Y527F) decreased hSlo1 basal transcription even preventing its estrogen/hERα-mediated transcriptional activation. Rho inhibition by coexpressed Clostridium botulinum C3 transferase did not alter estrogen action. In contrast, inhibition of PI3K activity with 10 μm LY294002 decreased estrogen-stimulated hSlo1 transcription by ∼40%. These results indicate that the nongenomic PI3K signaling pathway plays a role in estrogen/hERα-stimulated hSlo1 gene expression; whereas c-Src activity leads to hSlo1 gene tonic repression independently of estrogen, likely through ERK activation.
Keywords: Estrogen, Gene Regulation, Gene Transcription, Phosphatidylinositol 3-kinase, Potassium Channels, Src, Steroid Hormone Receptor, Estrogen Receptor Alpha, MaxiK, Nongenomic
Introduction
Large conductance voltage- and Ca2+-activated potassium channel (MaxiK, BK) plays important roles in the regulation of vascular tone, neurotransmission, uresis, and other body functions (1–3). Disruption of its pore-forming α subunit gene (Slo1) results in numerous pathologies, including ataxia, hypertension, urinary bladder incontinence, and erectile dysfunction, and has been linked to generalized epilepsy and paroxysmal dyskinesia in humans (2, 4, 5). Thus, it is relevant to investigate mechanisms that control Slo1 gene expression, especially that of the human (h) gene, hSlo1, as they may result in important therapeutic venues. In this regard, we previously showed that the murine Slo1 gene (mSlo1) expression is up-regulated by estrogen via the activation of estrogen receptor alpha (ERα)4 and now examine whether its human counterpart hSlo1 responds equally to estrogen.
Activated estrogen receptors (ERs) can have nuclear and cytoplasmic roles; classically known as “genomic” and “nongenomic” pathways, respectively. In the nucleus, they bind directly to specific 13-bp palindromic DNA regulatory regions (with a 3-bp spacing of variable bases) of target genes known as estrogen response elements (EREs), thus facilitating their transcriptional activation, or may interact with other transcription factors, whereas in the cytoplasm, they can trigger signal transduction pathways. During genomic ERα-mediated transcription, estrogen binds to and activates cytoplasmic ERs, which then undergo a conformational change allowing dimerization and translocation to the nucleus where they serve as transcription factors by direct binding to promoter EREs and/or ½EREs (sites containing only half, 5 bp, of the complete palindromic ERE-binding sequence) contiguous or not with Sp1 sites, or by indirect association with target DNA via other transcription factors (tethering) most commonly, Sp1 and AP-1 (6–9). Alternatively, ligand-activated ERα has the additional ability to trigger various cytoplasmic signaling cascades, which in turn may result in transcriptional regulation of genes (nongenomic-to-genomic) or other cell functions (10–14). Described signaling cascades resulting in transcriptional activation are as follows: c-Src tyrosine kinase and downstream MAPK, PI3K, and mitogen/ERK kinase-1 (15–19). Also, monomeric G-protein Rho pathways are involved in ERα-mediated transcriptional activity (20). In the case of mSlo1, a genomic mechanism defines the up-regulation by estrogen-activated ERα, where ERα binds to quasi-perfect EREs in the mSlo1 promoter, a mechanism that may be complemented by Sp1 transcription factor (21).
Until now, only basic characteristics of the hSlo1 promoter region have been investigated classifying it as a promoter driven by a TATA-like sequence and containing several transcription factor-binding motifs such as Sp1, c/EBP, NF-κB, PU.1, PEA-3, Myo-D, and E2A (22). Notably, the hSlo1 promoter does not contain canonical or quasi-perfect ERE elements (mSlo1 contains two quasi-perfect EREs critical for ligand-dependent ERα gene regulation) or canonical AP-1 sites. Yet, the hSlo1 promoter contains ½EREs and Sp1 sites as candidate sequences for its ERα genomic regulation. Here, we explored both genomic and nongenomic/signaling mechanisms and show that unlike in the mouse, hERα promotes transcription of hSlo1 through a nongenomic signaling mechanism that utilizes PI3K. Additionally, we show that Src tyrosine kinase activation leads to repression of hSlo1 basal transcription in an estrogen-independent fashion, likely through activation of ERK. Thus, we demonstrate two signaling cascades, which result in hSlo1 gene transcriptional regulation.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
BAC clone RP11-428P16 (GenBankTM accession no. AL731556) containing hSlo1 5′-flanking sequence was used to amplify 2520-nt and 5040-nt 5′ sequences by PCR. The fragment was subcloned in pGL4.1 (Promega) in frame with hSlo1 sequences of Met-3 ATG codon plus four nt (23) and luciferase. hERα-expressing vector was provided by Dr. Bert W. O'Malley (Baylor College of Medicine). Two point mutations were introduced into hERα (NM_000125) utilizing QuikChange (Stratagene) to change the glutamic acid residue 203 and glycine residue 204 to alanine (E203A, G204A). These mutations have previously been shown to disrupt ERα DNA binding (7, 24). The mutated hERα is called here, DNA binding domain mutant of hERα (DBDM-hERα). Dr. Melanie H. Cobb (UT Southwestern) provided C3 transferase in pEF-Myc.
Estrogen Treatment of Human Vascular Smooth Muscle Cells and Real-time PCR
One lot of human coronary artery smooth muscle cells (HCASMCs) and three different lots of human aortic smooth muscle cells (HAoSMCs) were obtained. HCASMCs were from Cell Systems (catalog no. ACBRI716; lot no. 2942-RI716). These cells were isolated from an adult human with no sign of vascular, hypertensive, or diabetic disorder. Age, sex, and race of this donor were unavailable. HAoSMCs from Cell Systems (catalog no. ACBRI443; lot no. 2942-RI443) were isolated from an 8-year-old female with no sign of vascular, hypertensive, or diabetic disorder. HAoSMCs from Lifeline (catalog no. FC-0015, lot no. 01293) were isolated from an 18-year-old African American male with no sign of vascular, hypertensive, or diabetic disorder. However, another Lifeline HAoSMC lot (catalog no. FC-0015, lot no. 01127) were derived from a 46-year-old Caucasian male who died from intracerebral hemorrhage/stroke.
Human primary smooth muscle cells (P5) were grown in specialized Vasculife SMC media (Lifeline) to 50% confluency and then treated for 4 days with 100 nm β-estradiol (Sigma) or vehicle (0.0007% ethanol) in phenol red-free DMEM and 10% charcoal dextran-stripped FBS (Sigma). Total RNA was subsequently extracted from cells with TRIzol reagent (Invitrogen) followed by DNase digestion for 10 min at room temperature with RNase-free DNase Set (Qiagen) and cleaned-up with RNeasy Mini Kit (Qiagen). The quality of the RNA samples was determined by electrophoresis through agarose gels, only RNA samples with visibly 2-fold of 28 S/18 S ratio were used.
Cleaned-up total RNA (2 μg) was reverse transcribed in a final volume of 20 μl containing reverse transcriptase buffer, 0.5 mm dNTP mix, 10 units of RNasin RNase inhibitor (Promega), 4 units of Omniscript reverse transcriptase (Qiagen), and 1 μm oligo-dT primer. In parallel, the mock cDNA (reverse transcriptase-negative) was prepared in the same reaction solution lacking Omniscript reverse transcriptase. The samples were incubated at 37 °C for 60 min, and reverse transcriptase was subsequently inactivated by heating at 95 °C for 5 min.
Real-time PCR (CFX96TM manager real-time system, Bio-Rad) was used for quantification of hSlo1 cDNA using iQTM SYBR® Green Supermix (Bio-Rad). Gene-specific primers were for hSlo1 (GenBankTM NM_001014797): forward primer, 5′-GCC TCC GGA ACC TGG TGA TGC-3′ (nucleotides 2582–2602) and reverse primer 5′-CCG ATG CTG TCA TCA AAC TG-3′ (nucleotides 2875–2894); and for human GAPDH (GenBank NM_002046), forward primer, 5′-GAT GCT GGC GCT GAG TAC GTC GTG-3′ (nucleotides 367–390) and reverse primer, 5′-CCA GTA GAG GCA GGG ATG ATG TTC TG-3′ (nucleotides 712–737). The expected product sizes were 313 bp for hSlo1 and 371 bp for GAPDH. The thermal cycling conditions comprised an initial denaturation step at 95 °C for 3 min and 25 cycles at 95 °C for 45 s, 60 °C for 45 s, and 72 °C for 45 s. Controls included the mock cDNA (reverse transcriptase-negative), no-template control (H2O control), and transcript levels of the housekeeping gene, human GAPDH. Specific products were detected as clear single peaks at their melting temperature in the first derivative of fluorescence (dF/dT) versus temperature plot (melting curve).
Cell Transfection and Luciferase Assay
HeLa cells were grown in 24-well plates until 90% confluent in DMEM, 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were then transfected with promoter-luciferase constructs ± hERα or empty vector control (pcDNA3) in 8:1 (promoter constructs:hERα or pcDNA3) DNA molar ratio using Lipofectamine 2000 reagent (Invitrogen) in Opti-MEM (GIBCO). In all experiments, 5 ng of pRL-TK vector (Promega), which directs Renilla luciferase expression was cotransfected as the transfection control, and the total amount of DNA transfected (0.8 μg DNA/well) was kept constant using the pBluescript (Stratagene) vector. About 16 h after transfection, the medium was replaced with phenol red free DMEM, 10% charcoal-dextran-stripped FBS ± β-estradiol (estrogen) (Sigma) and incubated for ∼26 h before lysis. Antagonists were added 30 min before β-estradiol with the exception of ICI 182,780, which was added together with estrogen. Luciferase assay was performed using the Dual-Luciferase assay Promega kit, which also provides the passive lysis buffer. Lysis was performed with 100 μl of passive lysis buffer in the 24-well plate. Lysates from each well were aliquoted into three wells (20 μl each) of a 96-white wall plate and read using the Veritas luminometer (Turner Biosystems). Results are presented as average of at least three independent transfections.
Sp1 Gene Silencing by siRNA
Sp1 siRNA was transfected with Lipofectamine 2000. Gene silencing was confirmed by real-time PCR and at least 70% reduction in mRNA was considered successful knockdown. Primers were as follows: forward primer, 5′-GTG ATG GAA TAC ATG ATG ACA CAG CAG-3′ (nucleotides 1812–1838) and reverse primer, 5′-CGT TTC CCA CAG TAT GAC CAG GTA C-3′ (nucleotides 2071–2095) (GenBankTM NM_138473). In a typical experiment, fluorescein-labeled and scrambled siRNA were used to measure transfection efficiency and as negative control, respectively.
Statistical Analysis
All experiments were done with at least three different RNA preparations or three different batches of cells. Data are mean ± S.E. Student's t test with p < 0.05 was considered significantly different.
RESULTS
Estrogen Promotes Increased hSlo1 Transcript Expression in Human Primary Smooth Muscle Cells
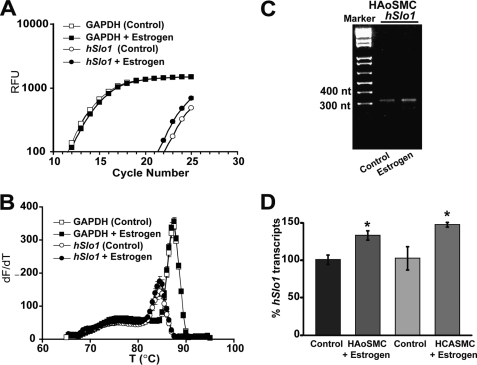
To investigate the role of estrogen in hSlo1 transcription, we first determined whether estrogen is able to regulate hSlo1 transcript expression in human vascular smooth muscle cells. To this end, we treated three lots of primary HAoSMCs and one lot of HCASMCs with 100 nm estrogen or with vehicle (control) for 4 days. Changes in hSlo1 transcript levels induced by estrogen were analyzed by real-time PCR. Estrogen increased hSlo1 transcript levels in all but one lot of HAoSMCs. Fig. 1A shows a positive example demonstrating that estrogen induced a leftward shift of the hSlo1 fluorescence curve, but not of GAPDH, toward lower threshold cycle number reflecting higher transcript levels in estrogen-treated cells with respect to control. The corresponding melting curves in Fig. 1B show a single peak for hSlo1 products (open circles, filled circles) and for GAPDH (open squares, filled squares), indicating that single specific products were amplified. For hSlo1, reactions were stopped prior saturation (at cycle 25) to allow visualization of differences in hSlo1 amplification products by agarose gel electrophoresis in control versus estrogen-treated samples (Fig. 1C). Fig. 1D shows that estrogen increased hSlo1 transcript expression by 33 ± 6.37% (n = 3, two lots) in HAoSMCs, and by 47% ± 3.15% (n = 3, one lot) in HCASMCs. The degree of estrogen induced up-regulation of hSlo1 transcripts (∼30%) observed here in HAoSMCs is consistent with that reported in the guinea pig aorta (25). Interestingly, estrogen was unable to promote transcriptional activation of hSlo1 in one lot of HAoSMCs, which were isolated from the donor who died of intracerebral hemorrhage/stroke (data not shown). Thus, our present data indicate that estrogen induces higher hSlo1 transcript levels in healthy primary human smooth muscle cells and is consistent with estrogen promoting transcription of the hSlo1 gene.
FIGURE 1.
Estrogen increases hSlo1 transcript levels in primary HCASMCs and HAoSMCs. A, an example of real-time PCR reactions showing fluorescence intensity (in relative fluorescence units, RFU) versus PCR cycle number for hSlo1 and the housekeeping gene human GAPDH in HAoSMCs treated with 100 nm estrogen or vehicle (control). B, melting curves of hSlo1 and GAPDH PCR products from estrogen and vehicle (control) treated samples. C, agarose gel electrophoresis of the real-time PCR products (at the end of the 25th cycle) showing the expected size hSlo1 product (313 bp) with higher intensity in the estrogen treated sample than in control. A–C, correspond to the same experiment; error bars in A–B are from triplicates. D, mean % of hSlo1 transcript levels after estrogen stimulation relative to control (vehicle treated). Experiments were done in triplicate with HAoSMCs from two different donors and HCASMCs from a single donor as detailed under “Experimental Procedures.” *, significantly different (p < 0.05) from respective control.
hERα Activation Leads to Potent Transcription of hSlo1
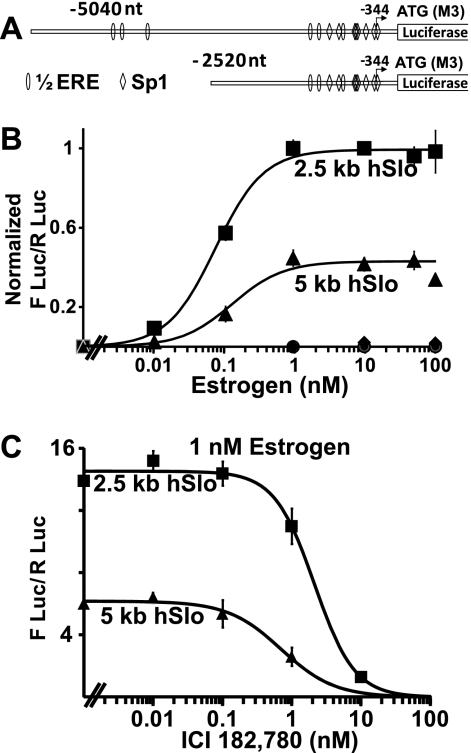
We have previously shown that activation of ERα promotes transcription of mSlo1 through a genomic mechanism by directly binding to the DNA (21). To determine whether hERα employs the same mechanism to promote transcription of hSlo1, we studied two 5′ constructs containing 2520 nt (2.5 kb) and 5040 nt (5 kb) upstream of the third possible ATG translation initiation codon (encoding Met-3) of hSlo1 (23). Fig. 2A shows a scheme of these two constructs, where the adenosine of Met-3 has been assigned position +1 and position −344 marks the transcription start site reported previously (22). Both constructs share 67.7 and 61.1% sequence identity with the corresponding mSlo1 promoter fragments, respectively. For simplicity, we designated the 2520 nt and 5040 nt sequences as 2.5 kb and 5 kb hSlo1 promoters (though they include 344 nt of 5′-untranslated region). Similar to the mSlo1 promoter, the hSlo1 promoter contains ½EREs and Sp1 sites; however, in contrast to mSlo1, hSlo1 lacks quasi-perfect EREs. Because the latter played a key role in promoting mSlo1 transcription by activated ERα, the lack of this type of site in hSlo1 hints to a differing mechanism of ERα action on hSlo1 transcription. As a prelude to testing this hypothesis, we investigated the ability of hERα to promote transcription of hSlo1. HeLa cells were transfected with promoter-luciferase constructs with or without cotransfection of hERα. Saturable dose-dependent curve in Fig. 2B shows that estrogen can promote transcription of hSlo1 (filled squares and triangles) and that this process requires hERα, as hERα untransfected cells (filled diamonds and circles) were unable to promote transcription of hSlo1 upon addition of estrogen. Doses as low as 0.01 nm estrogen were sufficient to promote transcription with both the 2.5- and 5-kb hSlo1 constructs. Although the strength of estrogen action differs in the two constructs (probably due to the presence of inhibitory sequences in the 5 kb construct), the estrogen concentration needed to observe 50% of the maximal effect (EC50) was similar for both promoters (0.07 ± 0.01 nm for the 2.5 kb, and 0.06 ± 0.007 nm for the 5 kb; n = 3 independent transfections; ± S.E. of the fit). These values are significantly lower than those observed for mSlo1 (EC50 of 0.4 nm for a 2.9 kb and 0.34 for a 4.9-kb construct) (21). Confirming the need for activated ERα, the anti-estrogen ICI 182,780 successfully antagonized estrogen-mediated up-regulation of hSlo1 transcription in a dose-dependent manner (Fig. 2C). The half-maximal inhibitory concentration (IC50) was 2.12 ± .29 nm for 2.5 kb hSlo1 and 0.7 ± 0.13 nm ICI 182,780 for 5 kb hSlo1 (n = 3 independent transfections; ± S.E. of the fit). Addition of 10 nm ICI 182,780 almost completely blocked the up-regulatory action of estrogen on hSlo1 transcription. In summary, transcriptional activation of hSlo1 is mediated by activated ERα at much lower concentrations than required for the mouse gene. This finding also points to a different mode of activation of hSlo1 from that of the mouse gene.
FIGURE 2.
Estrogen activates transcription of the hSlo1 promoter. A, schematics of hSlo1 promoter-luciferase constructs containing 5040 nucleotides (5 kb) and 2520 (2.5 kb) nucleotides upstream of the third possible translational start site encoding Met-3 (M3). Sites of ½ERE and Sp1- binding sites are depicted. Arrow marks the main transcription start site reported previously (22). B, hSlo1 promoter constructs cotransfected with hERα 2.5 kb (■) and 5 kb (▴) respond to estrogen in a dose-dependent manner (n = 6 independent experiments). Estrogen does not stimulate hSlo1 5-kb (♦) or 2.5-kb (●) promoter constructs in the absence of transfected hERα. Data were normalized to the maximum value of the 2.5-kb promoter. Normalized values were fitted to a Hill function of the form: Normalized FLuc/RLuc = Emax/(1 + (EC50/[estrogen])N), where Emax is maximum level of luciferase activity (1.0), EC50 = concentration of estrogen needed for 50% activation, and N = Hill coefficient. C, estrogen antagonist ICI 182,780 inhibits estrogen-induced up-regulation of hSlo1 transcription in a dose-dependent manner. The 2.5-kb (■) and 5-kb (▴) hSlo1 constructs were stimulated with 1 nm estrogen or 1 nm estrogen plus the indicated doses of ICI 182,780 (n = 3). Activity value with estrogen alone was taken as maximal activity for fitting purposes. IC50 values are given in the text. IC50 was calculated with a Hill function: FLuc/RLuc = Emax/(1 + ([ICI 182,780]/IC50)N), where Emax is maximum level of luciferase activity, IC50 = concentration of ICI 182,780 needed for 50% inhibition of estrogen response, and N = Hill coefficient.
hERα DNA Binding Domain and Sp1 Are Dispensable for Estrogen-activated hERα Transcriptional Activation of hSlo1
Both the 2.5- and 5-kb hSlo1 5′ sequences contain ½EREs, which could be recognized by hERα, allowing the receptor direct binding to hSlo1 DNA. In addition, the hSlo1 promoter contains the high affinity Sp1 (GGGGCGGGGC) but not AP-1 (TGA(C/G)TCA) sites (Fig. 2A), which are commonly used (combined or independently to ½EREs) for ERα-mediated transcription (6). Other regulatory sequences through which ERα indirectly associates to target genes are STAT 5 (signal transducer and activator of transcription 5), cyclic AMP response element, and NF-κB (14, 16, 26), but palindromic cyclic AMP response element (5′-TGACGTCA-3′) (27) and STAT 5 (TTCC(A>T)GGAA) (28) sites are absent in the 5-kb hSlo1 promoter; whereas NF-κB target genes are inhibited by ERα (26). These characteristics left two main possibilities that could explain hSlo1 transcriptional regulation by activated ERα via a genomic mechanism: (i) direct binding of hERα to ½EREs in hSlo1 promoter and/or (ii) an Sp1-mediated mechanism, which were assessed here.
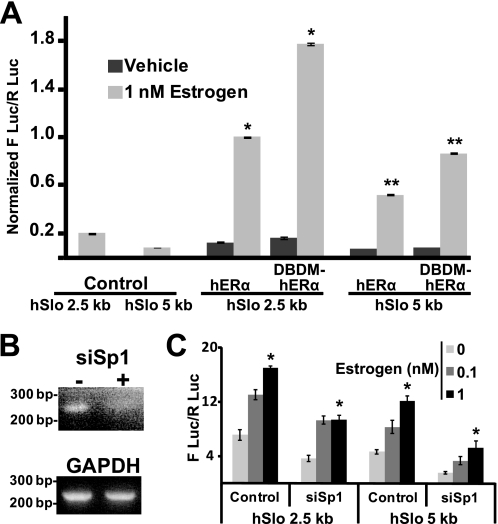
A direct binding of hERα to the hSlo1 promoter was tested by utilizing a hERα mutant that does not bind to DNA (E203A, G204A DNA binding domain mutant called here DBDM-hERα) (7, 24). HeLa cells were transfected with 2.5- and 5-kb hSlo1 constructs together with empty pcDNA3 vector (Control), hERα, or DBDM-hERα, and induced with 1 nm estrogen (light gray bars) or vehicle alone (dark gray bars) (Fig. 3A). Promoter activities with vehicle alone in hERα expressing cells were within background levels as they were close to those produced by empty pcDNA3 vector and 1 nm estrogen (control). As expected, 1 nm estrogen produced a significant increase in both hSlo1 promoter activities when hERα was transfected.
FIGURE 3.
Binding of hERα to DNA or Sp1 are dispensable for estrogen-mediated hSlo1 transcription. A, promoter activation assay of 2.5- or 5-kb constructs in HeLa cells cotransfected with empty vector (control), or with wild type hERα, or DBDM-hERα. Samples were stimulated with 1 nm estrogen (light gray bars) or with vehicle (dark gray bars) (n = 3). Values were normalized to estrogen-induced activity of hERα and 2.5 kb hSlo1 promoter expressing cells. * and **, significantly different from each other. B, RT-PCR analysis of HeLa cells cotransfected (+) or not (−) with siRNA directed against Sp1. GAPDH amplification is shown as control. C, cells expressing hERα and 2.5-kb or 5-kb constructs were cotransfected or not (control) with Sp1 siRNA and stimulated with vehicle (zero estrogen) or with estrogen (0.1 and 1 nm) (n = 3). *, significantly different from zero estrogen (vehicle).
Surprisingly, when DBDM-hERα was expressed, the stimulatory effect of 1 nm estrogen on hSlo1 promoter activities was not decreased (as observed for mSlo1, (21)) but on the contrary, estrogen was able to stimulate transcription even further than that achieved by wild type hERα. This was true for both, the 2.5- and 5-kb hSlo1 promoters. Estrogen increased hSlo1 2.5 kb promoter activity by 7.9 ± 0.11-fold (n = 3) when hERα was expressed and was augmented to 11.1 ± 0.34-fold (n = 3; p = 0.008) in the presence of DBDM-hERα; likewise, estrogen increased hSlo1 5-kb promoter activity by 7.4 ± 0.57-fold (n = 3) when hERα was expressed, and potentiated to 10.8 ± 0.80-fold (n = 3; p = 0.027) in the presence of DBDM-hERα. These results demonstrate that the DNA binding domain of hERα is dispensable for its estrogen-induced up-regulation of hSlo1 transcription. Thus, it is safe to postulate that, unlike with mSlo1, direct DNA binding of activated-ERα to hSlo1 promoters is not the mechanism by which ERα promotes transcription of hSlo1.
To determine whether Sp1 transcription factor is required for ERα-mediated transcription of hSlo1, we performed siRNA knockdown of Sp1. RT-PCR was performed to corroborate appropriate decrease in Sp1 transcript expression (Fig. 3B). Although Sp1 knockdown decreased basal activity of hSlo1 promoters; siSp1 failed to significantly abolish/decrease stimulation of hSlo1 gene transcription by estrogen-activated ERα for both the 2.5- and 5-kb hSlo1 promoters (Fig. 3C). At 1 nm estrogen, stimulation of the 2.5-kb hSlo1 promoter was 2.38 ± 0.26-fold (n = 3) in control versus 2.56 ± 0.36-fold (n = 3) (p = 0.67) in siSp1-treated cells. The same trend was observed for the 5-kb promoter where 1 nm estrogen caused a 2.60 ± 0.23-fold (n = 3) increase in activity in control and 3.39 ± 0.78-fold (n = 3) (p = 0.19) increase after Sp1 knockdown. In this respect, the hSlo1 gene also behaves distinctly from mSlo1. Together, these data rule out classical genomic mechanisms for estrogen activation of hSlo1 mediated by hERα direct binding to the hSlo1 promoter or by interacting with the Sp1 transcription factor, suggesting that unlike that for the mouse, hSlo1 regulation by hERα may occur through a nongenomic signaling mechanism. Thus, we next tested the possible role of c-Src tyrosine kinase, Rho, and PI3K, which are known signaling pathways triggered by activated ERα (10) and used DBDM-hERα to prevent hERα from binding to DNA.
c-Src Inhibits Basal hSlo1 Expression, whereas PI3K Plays a Role in Nongenomic-to-genomic Action of hERα
HeLa cells were cotransfected either with empty vector (control) or with DBDM-hERα and the 2.5-kb hSlo1 promoter reporter constructs. The 2.5-kb promoter and the DBDM-hERα mutant were chosen because the former had the largest activity (the 5 kb may contain a repressor element as shown in Figs. 2 and 3), and the latter prevents, as mentioned earlier, hERα binding to target DNA and thus, would be better suited for analysis of a signaling mediated mechanism.
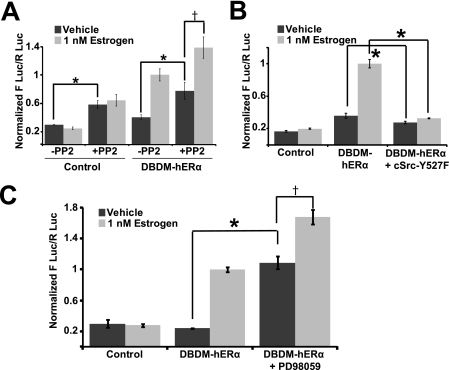
The possible role of Src tyrosine kinase was tested by inhibiting Src with PP2 (Fig. 4A). Cells were treated with vehicle (DMSO and ethanol) or with 1 nm estrogen plus/minus PP2.
FIGURE 4.
c-Src and ERK are estrogen-independent tonic repressors of hSlo1 transcription. A–C, activity of the 2.5-kb hSlo1 promoter in control (no hERα) and DBDM-hERα in the absence (vehicle, dark gray bars) or presence of 1 nm estrogen (light gray bars) (n = 3 each set). A, PP2 (10 μm) significantly increased hSlo1 basal activity in the absence (control) or presence of DBDM-hERα (dark gray bars) and did not prevent estrogen-induced activation by DBDM-hERα (†, significantly different) (n = 6). B, consistent with A, constitutively active c-Src (c-Src-Y527F mutant) decreased hSlo1 basal activation and even prevented estrogen-induced DBDM-hERα mediated hSlo1 transcription (n = 3). C, similar to c-Src inhibition by PP2 (A), inhibition of ERK activity by 25 μm PD98059 stimulated basal hSlo1 transcription and did not prevent DBDM-hERα mediated transcriptional activation (n = 3). In this and following figure, values were normalized to estrogen-induced activity of the DBDM-hERα+ hSlo1 promoter expressing cells in the absence of inhibitors or c-Src-Y527F. * and †, significantly different (p < 0.05). Note that control and DBDM-hERα values are the same for Figs. 3B, and 4, A and B, as experiments were run simultaneously.
Src inhibition with 10 μm PP2 did not prevent estrogen-activated DBDM-ERα induced stimulation of hSlo1 (fold increase was 2.56 ± 0.28, n = 6 in the absence and 1.8 ± 0.34, n = 6 in the presence of PP2; p = 0.25), which as expected for a receptor-dependent mechanism was absent in cells lacking DBDM-hERα (control). However, PP2 significantly increased basal activity (vehicle, dark gray bars) of the hSlo1 promoter regardless of the presence or absence (control) of DBDM-hERα. Moreover, this increase in basal activity was to the same extent in control (2.03 ± 0.18 fold increase, n = 6) and in DBDM-hERα (1.94 ± 0.34 fold increase, n = 6, respectively) transfected cells supporting a hERα-independent mechanism of hSlo1 repression by c-Src. Overall, the results suggest that Src activity does not participate in ligand-activated DBDM-hERα-induced transcription of hSlo1 but instead functions as a chronic repressor of hSlo1 transcription. Thus, we wondered whether constitutively active c-Src could shut down hSlo1 activity to a degree that ligand-activated hERα could no longer stimulate hSlo1.
Cotransfection of the constitutively active mutant clone of c-Src (Y527F) (29, 30) slightly but significantly decreased basal activity from 0.36 ± 0.03 to 0.27 ± 0.01 (n = 3; p = 0.01) and completely prevented hERα-mediated transcription of hSlo1 (Fig. 4B). These results are consistent with the idea that c-Src can mediate repression of hSlo1. Because gene activation by c-Src has been shown to require ERK as mediator (18), ERK activity was indirectly inhibited with 25 μm MEK1 inhibitor, PD98059. Accordingly, inhibition of MEK1 (Fig. 4C) resulted in increased basal transcription of hSlo1 (asterisk, dark gray bars) as observed with inhibition of Src with PP2 (Fig. 4A); and in retention of activated-DBDM-hERα ability to enhance hSlo1 activity (dagger). This correlation supports a role of MEK1-ERK signaling in Src-mediated repression of the hSlo1 gene.
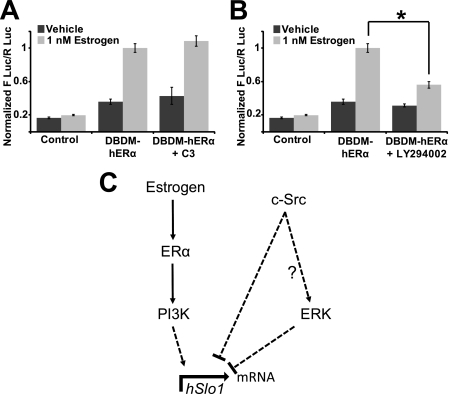
To determine whether Rho is required for ERα mediated regulation of hSlo1, Rho activity was inhibited by cotransfection of the C3 transferase, which functions to inactivate Rho proteins but not closely related monomeric G-proteins (e.g. Rac1 or Cdc42) via ADP-ribosylation. Inhibition of Rho signaling did not affect estrogen-activated hERα mediated transcription of hSlo1 (Fig. 5A).
FIGURE 5.
PI3K mediates estrogen activated hERα transcriptional activation of hSlo1. A and B, activity of the 2.5-kb hSlo1 promoter in control (no hERα) and DBDM-hERα in the absence (vehicle, dark gray bars) or presence of 1 nm estrogen (light gray bars) (n = 3 each set). A, blocking Rho activity by cotransfection with C3 transferase was unable to prevent estrogen-induced activation of hSlo1 by DBDM-hERα. B, blocking PI3K activity with 25 μm LY294002 reduced estrogen-induced activation of hSlo1 by DBDM-hERα. C, scheme of hSlo1 transcriptional regulation. Estrogen via ERα and PI3K stimulate hSlo1 transcription. c-Src and ERK inhibit hSlo1 basal transcription. Dashed lines depict possible intermediates in the signaling cascade. ?, whether ERK is downstream of c-Src in this system is unknown. *, significantly different (p < 0.05).
Finally, we inhibited PI3K activity with 10 μm LY294002. Cells were transfected with empty vector (control) or with DBDM-hERα and treated with or without estrogen and LY294002. Inhibition of PI3K resulted in reduced activated DBDM-hERα mediated hSlo1 transcription as compared with untreated cells (Fig. 5B). The fold increase by 1 nm estrogen in the absence of LY294002 was 2.857 ± 0.28, n = 3 and was reduced to 1.80 ± 0.11 by the inhibitor (n = 3, p = 0.039). In summary, our results revealed that c-Src and ERK function to repress hSlo1 transcription, whereas PI3K is a mediator of the nongenomic/signaling mechanism of action for gene transcription of hSlo1 by ERα (Fig. 5C).
DISCUSSION
Despite nearly two decades since human Slo1 cDNA cloning (23, 31), the mechanisms behind its gene transcriptional regulation have remained unknown with the exception of a single manuscript describing its basic promoter characteristics and mapping the main transcription start site (22). Here, we reveal that both estrogen-activated hERα (via PI3K) and c-Src regulate hSlo1 gene transcription in HeLa cells.
hSlo1 versus mSlo1 Regulation by Estrogen and Underlying Mechanisms
Although both hSlo1 (this work) and mSlo1 (21) genes are up-regulated by estrogen via activation of ERα (as assessed by lack of estrogenic effect in the absence of receptor and by the antagonistic action of the anti-estrogen ICI 182,780; Fig. 2), several important differences were made evident in the present studies: (i) estrogen regulation of hSlo1 is much more potent (∼6-fold) than mSlo1; (ii) opposite to mSlo1, the hSlo1 5-kb promoter lacks quasi-perfect EREs; (iii) the DNA-binding domain mutant of hERα (DBDM-hERα) or knocking down Sp1 did not decrease estrogen-action on hSlo1 as it does in mSlo1, ruling out direct or tethered (via Sp1) genomic mechanisms of action on hSlo1 by activated-hERα; and (iv) activated ERα utilizes a nongenomic/signaling mechanism to upregulate hSlo1 as opposed to the genomic mechanism underlying mSlo1 up-regulation (21). These species differences underscore the importance of studying the human Slo1 gene for future pharmacological interventions relevant to human health.
Interestingly, the hERα mutant lacking the DNA-binding domain functioned better than the wild type hERα to transcribe hSlo1, suggesting that in the case of the mutant all receptors were available for extranuclear activation of signaling cascades enhancing the up-regulation of hSlo1 transcription. The data imply that the usage of a signaling mechanism in humans results in a more efficient way for ERα to transduce estrogen action into hSlo1 transcriptional activation.
PI3K Role in hERα Nongenomic Transcriptional Activation of hSlo1
As stated previously, several signaling cascades like Src-MAPK, PI3K, and Rho pathways have been linked to nongenomic-to-genomic actions of ERα, where signaling cascades have as an end result transcriptional activation of target genes (11–14, 32). ERα has been shown to activate Src and in turn Ras and the MAPK pathway (18). c-Src and PI3K can also work in concert as mediators of ERα transcription (10). For example, estradiol induced expression of cyclin D1 can be blocked by the dominant-negative forms of p85 regulatory/adapter subunit of PI3K and c-Src (33). However, our results discarded a role for Src-MAPK (and of Rho) in ERα induced activation of the hSlo1 gene and uncovered PI3K as one underlying mechanism. As discussed below for c-Src, distinct roles of PI3K (activation of hSlo1 transcription by activated hERα) and c-Src (basal inhibition of hSlo1 transcription independent of hERα) were uncovered, suggesting that hERα interacts with PI3K but not with c-Src in our system.
Because blockade of PI3K results in an ∼40% decrease in ligand-activated ERα action; it is likely that ERα uses additional signaling mechanisms or perhaps yet to be discovered tethering transcription factors for its action on hSlo1 transcription. Yet, the results demonstrate that PI3K is one mechanism underlying hERα nongenomic-to-genomic transcriptional activation of hSlo1.
Noncanonical Regulation of hSlo1 Transcription by c-Src
Although c-Src plays a key role in ERα intracellular signaling (14), our results show that c-Src does not contribute to hERα positive regulation of hSlo1 transcription. Instead, our results demonstrate that c-Src inhibits basal transcription of hSlo1 independently of estrogen or hERα as Src inhibition with PP2 increased basal transcriptional activity in the same way in their absence or presence (Fig. 4A). The small but significant inhibitory effect of constitutively active c-Src mutant on hSlo1 basal activity (Fig. 4B, vehicle) may be explained by endogenous c-Src in HeLa cells already exerting a tonic inhibition of the hSlo1 promoter. However, it is remarkable that the repression of hSlo1 basal activity by constitutively active c-Src could not be surpassed by estrogen stimulation of DBDM-hERα (Fig. 4B). A diagram depicting our findings is shown in Fig. 5C.
Physiological Implications
c-Src action as a repressor of hSlo1 transcription shown in this work, together with the ability of c-Src to inhibit hSlo1 channel activity (34) points to c-Src as a multi-level negative regulator of hSlo1 expression and function.
The dependence of hSlo1 on estrogen for expression leads to interesting questions regarding the physiological links between levels of estrogen in the body, Slo1 protein levels and the resulting physiological impact. It is well known that estrogen levels are important for proper vascular health (35, 36). Slo1 channels are also integral in the physiology of the vasculature (1, 37), and here we have shown that estrogen increases hSlo1 transcript expression in primary human smooth muscle cells (Fig. 1). Declining serum estrogen levels of women after menopause may result in decreased levels of hSlo1 and other genes involved in regulation of vascular tone and may explain why hypertension increases as the individual ages. A drop in estrogen levels may lead to a drop in hSlo1 transcription/expression as observed in aging human coronaries (38) and consequently, propensity toward high blood pressure. This may open the possibility of treating hypertension by drugs targeted at increasing expression of hSlo1, one such way may include drugs that block c-Src.
Acknowledgments
We thank Dr. Bert W. O'Malley (Baylor College of Medicine) for providing the hERα clone, and Dr. Melanie H. Cobb (UT Southwestern) for C3 in pEF-Myc.
This work was supported, in whole or in part, by National Institutes of Health Grants HL54970 (to L. T.), HL088640 (to E. S.), and Postdoctoral Fellowship NS 007101 (to S. M. D.).
- ERα
- estrogen receptor α
- DBDM-hERα
- DNA binding domain mutant of human ERα
- ERE
- estrogen response element
- ½ERE
- site(s) containing only half (5 bp) of the complete palindromic ERE-binding sequence
- HAoSMCs
- human aortic smooth muscle cells
- HCASMCs
- human coronary artery smooth muscle cells.
REFERENCES
- 1. Eichhorn B., Dobrev D. (2007) Naunyn Schmiedebergs Arch. Pharmacol. 376, 145–155 [DOI] [PubMed] [Google Scholar]
- 2. Lu R., Alioua A., Kumar Y., Eghbali M., Stefani E., Toro L. (2006) J. Physiol 570, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanaka Y., Koike K., Toro L. (2004) J. Smooth Muscle Res. 40, 125–153 [DOI] [PubMed] [Google Scholar]
- 4. Du W., Bautista J. F., Yang H., Diez-Sampedro A., You S. A., Wang L., Kotagal P., Lüders H. O., Shi J., Cui J., Richerson G. B., Wang Q. K. (2005) Nat. Genet. 37, 733–738 [DOI] [PubMed] [Google Scholar]
- 5. Ledoux J., Werner M. E., Brayden J. E., Nelson M. T. (2006) Physiology 21, 69–78 [DOI] [PubMed] [Google Scholar]
- 6. O'Lone R., Frith M. C., Karlsson E. K., Hansen U. (2004) Mol. Endocrinol. 18, 1859–1875 [DOI] [PubMed] [Google Scholar]
- 7. Jakacka M., Ito M., Weiss J., Chien P. Y., Gehm B. D., Jameson J. L. (2001) J. Biol. Chem. 276, 13615–13621 [DOI] [PubMed] [Google Scholar]
- 8. Philips A., Chalbos D., Rochefort H. (1993) J. Biol. Chem. 268, 14103–14108 [PubMed] [Google Scholar]
- 9. Wu-Peng X. S., Pugliese T. E., Dickerman H. W., Pentecost B. T. (1992) Mol. Endocrinol. 6, 231–240 [DOI] [PubMed] [Google Scholar]
- 10. Fu X. D., Simoncini T. (2008) IUBMB Life 60, 502–510 [DOI] [PubMed] [Google Scholar]
- 11. Segars J. H., Driggers P. H. (2002) Trends Endocrinol. Metab. 13, 349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kousteni S., Bellido T., Plotkin L. I., O'Brien C. A., Bodenner D. L., Han L., Han K., DiGregorio G. B., Katzenellenbogen J. A., Katzenellenbogen B. S., Roberson P. K., Weinstein R. S., Jilka R. L., Manolagas S. C. (2001) Cell 104, 719–730 [PubMed] [Google Scholar]
- 13. Shupnik M. A. (2004) Oncogene 23, 7979–7989 [DOI] [PubMed] [Google Scholar]
- 14. Fox E. M., Andrade J., Shupnik M. A. (2009) Steroids 74, 622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stoica G. E., Franke T. F., Moroni M., Mueller S., Morgan E., Iann M. C., Winder A. D., Reiter R., Wellstein A., Martin M. B., Stoica A. (2003) Oncogene 22, 7998–8011 [DOI] [PubMed] [Google Scholar]
- 16. Björnström L., Sjöberg M. (2002) Mol. Endocrinol. 16, 2202–2214 [DOI] [PubMed] [Google Scholar]
- 17. Wade C. B., Dorsa D. M. (2003) Endocrinology 144, 832–838 [DOI] [PubMed] [Google Scholar]
- 18. Cheskis B. J. (2004) J. Cell. Biochem. 93, 20–27 [DOI] [PubMed] [Google Scholar]
- 19. Levin E. R. (2005) Mol. Endocrinol. 19, 1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su L. F., Knoblauch R., Garabedian M. J. (2001) J. Biol. Chem. 276, 3231–3237 [DOI] [PubMed] [Google Scholar]
- 21. Kundu P., Alioua A., Stefani E., Toro L. (2007) J. Biol. Chem. 282, 27478–27492 [DOI] [PubMed] [Google Scholar]
- 22. Dhulipala P. D., Kotlikoff M. I. (1999) Biochim. Biophys. Acta 1444, 254–262 [DOI] [PubMed] [Google Scholar]
- 23. Wallner M., Meera P., Ottolia M., Kaczorowski G. J., Latorre R., Garcia M. L., Stefani E., Toro L. (1995) Receptors Channels 3, 185–199 [PubMed] [Google Scholar]
- 24. DeNardo D. G., Cuba V. L., Kim H., Wu K., Lee A. V., Brown P. H. (2007) Mol. Cell. Endocrinol. 277, 13–25 [DOI] [PubMed] [Google Scholar]
- 25. Jamali K., Naylor B. R., Kelly M. J., Rønnekleiv O. K. (2003) Endocrine 20, 227–237 [DOI] [PubMed] [Google Scholar]
- 26. Kalaitzidis D., Gilmore T. D. (2005) Trends Endocrinol. Metab. 16, 46–52 [DOI] [PubMed] [Google Scholar]
- 27. Montminy M. R., Gonzalez G. A., Yamamoto K. K. (1990) Trends Neurosci. 13, 184–188 [DOI] [PubMed] [Google Scholar]
- 28. Grimley P. M., Dong F., Rui H. (1999) Cytokine Growth Factor Rev. 10, 131–157 [DOI] [PubMed] [Google Scholar]
- 29. Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. (1986) Science 231, 1431–1434 [DOI] [PubMed] [Google Scholar]
- 30. Courtneidge S. A. (1985) EMBO J. 4, 1471–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dworetzky S. I., Trojnacki J. T., Gribkoff V. K. (1994) Brain Res. Mol. Brain Res. 27, 189–193 [DOI] [PubMed] [Google Scholar]
- 32. Fu X., Gong M. C., Jia T., Somlyo A. V., Somlyo A. P. (1998) FEBS Lett. 440, 183–187 [DOI] [PubMed] [Google Scholar]
- 33. Castoria G., Migliaccio A., Bilancio A., Di Domenico M., de Falco A., Lombardi M., Fiorentino R., Varricchio L., Barone M. V., Auricchio F. (2001) EMBO J. 20, 6050–6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alioua A., Mahajan A., Nishimaru K., Zarei M. M., Stefani E., Toro L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14560–14565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xing D., Nozell S., Chen Y. F., Hage F., Oparil S. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qiao X., McConnell K. R., Khalil R. A. (2008) Gend. Med. 5, S46–64 [DOI] [PubMed] [Google Scholar]
- 37. Ghatta S., Nimmagadda D., Xu X., O'Rourke S. T. (2006) Pharmacol. Ther. 110, 103–116 [DOI] [PubMed] [Google Scholar]
- 38. Marijic J., Li Q., Song M., Nishimaru K., Stefani E., Toro L. (2001) Circ. Res. 88, 210–216 [DOI] [PubMed] [Google Scholar]