Abstract
Recent studies indicate that oxidative stress mediates salt-sensitive hypertension. To test the hypothesis that the renal epithelial sodium channel (ENaC) is a target of oxidative stress, patch clamp techniques were used to determine whether ENaC in A6 distal nephron cells is regulated by hydrogen peroxide (H2O2). In the cell-attached configuration, H2O2 significantly increased ENaC open probability (Po) and single-channel current amplitude but not the unit conductance. High concentrations of exogenous H2O2 are required to elevate intracellular H2O2, probably because catalase, the enzyme that promotes the decomposition of H2O2 to H2O and O2, is highly expressed in A6 cells. The effect of H2O2 on ENaC Po was enhanced by 3-aminotriazole, a catalase inhibitor, and abolished by overexpression of catalase, indicating that intracellular H2O2 levels are critical to produce the effect. However, H2O2 did not directly activate ENaC in inside-out patches. The effects of H2O2 on ENaC Po and amiloride-sensitive Na+ current were abolished by inhibition of phosphatidylinositide 3-kinase (PI3K). Confocal microscopy data showed that H2O2 elevated phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) in the apical membrane by stimulating PI3K. Because ENaC is stimulated by PI(3,4,5)P3, these data suggest that H2O2 stimulates ENaC via PI3K-mediated increases in apical PI(3,4,5)P3.
Keywords: Epithelial Cell; Inositol Phospholipid; Intracellular Processing; Reactive Oxygen Species (ROS); Sodium Channels; ENaC; Hydrogen Peroxide; Confocal Microscopy; Patch Clamp Technique; Phosphatidylinositol 3,4,5-Trisphosphate
Introduction
ENaC2 in the distal nephron plays an important role in maintaining Na+ homeostasis and consequently controls systemic blood pressure. Excess sodium reabsorption due to enhanced ENaC activity can lead to hypertension, as is seen in Liddle syndrome (1, 2) and other genetic diseases (3). Surprisingly, whether high salt intake induces hypertension by targeting ENaC has not been examined. High salt intake can elevate both superoxide (O2⨪) (4, 5) and H2O2 (6) in the kidney by stimulating NAD(P)H oxidase (7–9), and O2⨪ can increase aldosterone-mediated stimulation of ENaC by scavenging nitric oxide (NO) (10). However, it is still unknown whether H2O2, a product of O2⨪, also stimulates ENaC.
H2O2 may be more important than other reactive oxygen species (ROS) in the pathogenesis of salt-sensitive hypertension. There are two lines of evidence that support a role for H2O2 in salt-sensitive hypertension. First, transgenic mice overexpressing the human catalase gene are found to be less sensitive to hypertension-inducing agents, such as norepinephrine and angiotension II (11). Second, among ROS, H2O2 is a relatively stable and mild oxidant, suggesting that it may have a longer effect than O2⨪. In this context, H2O2 acts as an intracellular messenger that modulates the function of several types of transmembrane proteins (12), including ATP-sensitive potassium (KATP) channels (13). Intracellular H2O2 concentrations are balanced between the rate of production determined by NAD(P)H oxidase-mediated production of O2⨪ and the rate of degradation determined by catalase and glutathione peroxidase (14).
ROS, including H2O2, stimulate the non-receptor tyrosine kinase, c-Src (15). c-Src is known to be directly associated with p85, the regulatory subunit of PI3K (16). Tyrosine phosphorylation of p85 reduces its inhibitory effect on PI3K (17). These studies indicate that ROS may stimulate PI3K and consequently promote the synthesis of PI(3,4,5)P3. We (18–20) and others (21, 22) have previously shown that PI(3,4,5)P3 stimulates ENaC. Taken together, these studies suggest that H2O2 may stimulate ENaC by elevating PI(3,4,5)P3 through activation of PI3K. In the present study, using patch clamp techniques combined with confocal microscopy analysis of apical PI(3,4,5)P3 levels, we show that H2O2 stimulates ENaC in A6 distal nephron cells via PI3K-dependent elevation of PI(3,4,5)P3 in the apical membrane.
MATERIALS AND METHODS
Cell Culture
Xenopus A6 distal nephron cells were purchased from the American Type Culture Collection (Manassas, VA). Under previously used culture conditions, basal activity of ENaC in A6 cells is relatively high (23). It is difficult to see and analyze any stimulatory effect when basal ENaC activity is high. Therefore, in the present study, we modified the culture medium to reduce basal ENaC activity. The medium contained 2 parts of DMEM/F-12 (1:1) medium (Invitrogen), 1 part of H2O, 15 mm NaHCO3 (total Na+ = 101 mm, which is ideal for amphibian A6 cells), 2 mm l-glutamine, 10% fetal bovine serum (Invitrogen), 25 units/ml penicillin, 25 units/ml streptomycin. A6 cells were cultured in plastic flasks in the presence of 1 μm aldosterone at 26 °C and 4% CO2. After the cells became 70% confluent in plastic flasks, A6 cells were plated on the polyester membrane of either Snapwell inserts (Corning Costar Co.) for patch clamp experiments) or Transwell inserts (Corning Costar Co.) for confocal microscopy experiments and measurement of amiloride-sensitive Na+ current). The cells were cultured for 3–4 weeks to allow them to be fully polarized before each experiment.
Patch Clamp Recordings
Both cell-attached and inside-out recordings of ENaC single-channel currents from A6 distal nephron cells were carried out using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). As described previously (24), prior to the experiments, A6 cells cultured on the polyester membrane of Snapwell inserts were thoroughly washed with NaCl solution containing 100 mm NaCl, 3.4 mm KCl, 1 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES; pH was adjusted to 7.4 with NaOH. The glass micropipette was filled with NaCl solution (the pipette resistance is 7–10 megaohms). In cell-attached experiments, NaCl solution was used for both the luminal and basolateral bath. For experiments using the inside-out patch clamp configuration, KCl solution was used for the cytoplasmic bath, which contained 100 mm KCl, 5 mm NaCl, 1 mm MgCl2, and 10 mm HEPES (50 nm free Ca2+ after titration with 1 mm EGTA); pH was adjusted to 7.2 with KOH. Single-channel currents were obtained with applied pipette potentials of either 0 mV for cell-attached recordings or +60 mV for inside-out recordings, filtered at 1 kHz, and sampled every 50 μs with Clampex 8.0 software. All experiments were conducted at room temperature. The total numbers of functional channels in the patch were estimated by observing the number of peaks detected on the current amplitude histograms during an at least 20-min recording period including the period after application of H2O2. Each experiment was started after the first 2-min recordings when the ENaC activity had stabilized. The open probability (Po) of ENaC before (−3–0 min) and after each experimental manipulation (every 3-min period) was calculated using Clampfit 9.0.
Measurement of Amiloride-sensitive Na+ Current
After they were cultured on the polyester membrane of Transwell inserts for 3–4 weeks, A6 cells were fully polarized and formed a cell monolayer. The voltage (V) and resistance (R) across the cell monolayer were measured with electrodes connected to a volt ohm meter, the EVOM (World Precision Instruments, Sarasota, FL). The transepithelial current (I) was calculated according to Ohm's law (I = V/R) and corrected for the surface area of the polyester membrane. Because H2O2 could alter the membrane potential by stimulating iberiotoxin-sensitive K+ channels (data not shown), we used the amiloride-sensitive Na+ current (INa) rather than the transepithelial current to analyze ENaC activity. To calculate the amiloride-sensitive INa, the total transepithelial current was subtracted with the current after blockade of ENaC with 1 μm amiloride.
Transfection of A6 Cells with Enhanced Green Fluorescence Protein-tagged Pleckstrin Homology Domain of Akt (EGFP-PH-Akt)
In this set of experiments, A6 cells were plated on the polyester membrane of Transwell inserts at a high density to allow the cells to be confluent within 3 days. To facilitate transfection of confluent A6 cells, we treated confluent A6 cells with Ca2+-free and Mg2+-free PBS (Invitrogen). The PBS was modified with H2O (3 parts of PBS with 1 part of H2O) to match the osmolarity of amphibian cells. After treatment with the modified PBS for 20 min (to break the tight junctions), cells were incubated with transfection reagent containing EGFP-PH-Akt DNA construct and Lipofectamin 2000 for 6 h and then incubated with regular culture medium for 2 days before performing the confocal microscopy experiments.
Confocal Microscopy Imaging
To detect PI(3,4,5)P3 levels in A6 cells, A6 cells were transiently transfected with EGFP-PH-Akt. To detect the levels of intracellular ROS, A6 cells were stained with a membrane-permeable, ROS-sensitive, fluorescent probe, 2′,7′-dichlorodihydrofluorescein diacetate. Prior to the confocal miscroscopy experiments, cells were washed twice with NaCl solution. Immediately following each experimental manipulation, the polyester membrane that supports the A6 cell monolayer was quickly excised and mounted on a glass slide with a drop of NaCl solution to keep the cells alive. Confocal microscopy xy or xz scanning of A6 cells was accomplished within 5 min. xy optical sections were performed to provide a flat view of the cells near the apical membrane, across the lateral membrane, or near the basal membrane. xz optical sections were also performed to provide a lateral view of the cells. In each set of experiments, images were taken using the same parameter settings.
Measurement of Intracellular H2O2
It has been suggested that aquaporins are required for H2O2 to move across the cell membrane (25). After the cells were challenged with exogenous H2O2, the levels of intracellular H2O2 remained unknown. Therefore, H2O2 concentrations in A6 cells after treatment with exogenous H2O2 were measured. After treatment with H2O2 at different concentrations for 10 min, cells were washed five times in ice-cold NaCl solution. Cells were then bathed in calcium- and magnesium-free PBS for 15 min and treated with trypsin for 1–2 min. Cells were collected by centrifuging at 600 × g for 5 min, washed twice with NaCl solution, and then suspended in 350 μl of NaCl solution. H2O2 concentrations in live A6 cells were measured using the Amplite fluorimetric hydrogen peroxide assay kit (ABD Bioquest, Sunnyvale, CA) according to the manufacturer's directions. Briefly, a 50-μl cell suspension was incubated with an equal volume mixture of Amplite Red peroxidase substrate and horseradish peroxidase for 30 min at room temperature. The signal at 570 nm in a 96-well microtiter plate was detected and recorded by a microplate reader. Cell number was counted and used to calculate intracellular H2O2 according to the standard curve.
Western Blotting
A6 cells were cultured as described above. Cell lysates (100 μg) were loaded and electrophoresed on 10% SDS-polyacrylamide gels for 60–90 min. Gels were blotted onto polyvinylidene fluoride (PVDF) membranes for 1.5 h at 50 V. After 1 h of blocking with 5% BSA-PBST buffer, PVDF membranes were incubated with primary antibody (1:10,000 dilution) of rabbit monoclonal antibody to catalase (Abcam; ab76024) overnight at 4 °C and then incubated with horseradish peroxidase (HRP)-conjugated sheep anti-rabbit IgG secondary antibody (1:10,000 dilution; GE Healthcare) for 1 h after four vigorous washes. Finally, blots were visualized with chemiluminescence using the ECL Plus Western blotting detection system (GE Healthcare).
Chemicals
Most chemicals were purchased from Sigma-Aldrich. H2O2 was purchased from Fisher. H2O2 was diluted with NaCl solution before it was added to either luminal or basolateral bath. All of the solutions were either premade and stored in a −20 °C freezer or freshly made before the experiments. All of the concentrations listed in this study represent the final concentrations.
Statistical Analysis
Data are reported as mean values ± S.E. Statistical analysis was performed with SigmaPlot and SigmaStat software (Jandel Scientific). Paired t test was used for comparisons between pre- and post-treatment activities. Analysis of variance was used for multiple comparisons among various treatment groups or at different time points of one treatment group. Results were considered significant if p was <0.01.
RESULTS
Basolateral H2O2 Stimulates ENaC in Cell-attached Patches
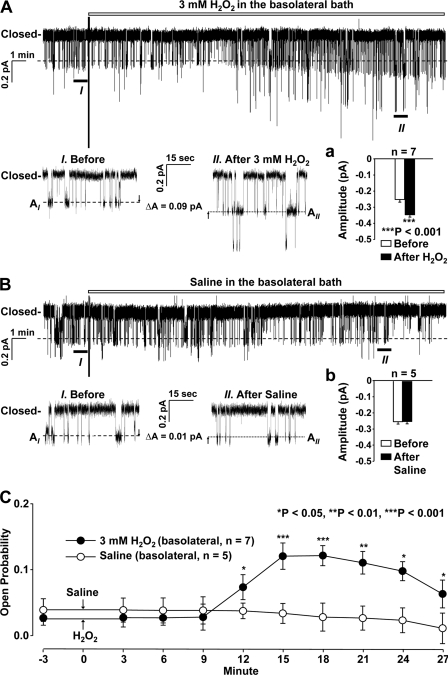
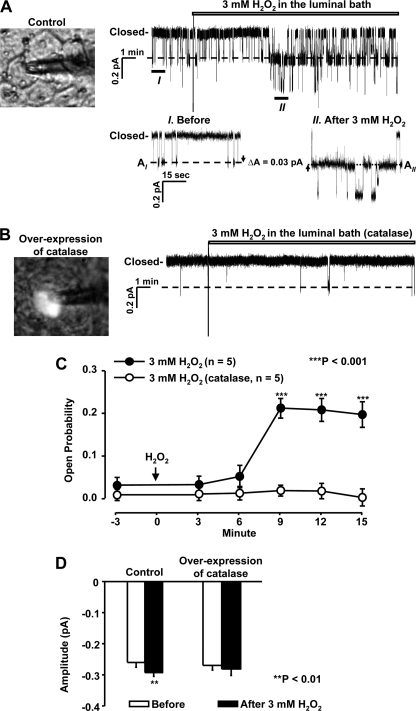
Recent studies suggest that elevation of H2O2 in the kidney participates in salt-sensitive hypertension (6, 26), implying that the activity of sodium transporters might be increased by H2O2. In the present study, cell-attached patch clamp experiments were performed to determine whether H2O2 would stimulate ENaC. Because ENaC in A6 distal nephron cells is well characterized, we used the A6 cell line as a model for sodium-transporting epithelial cells. We intended to determine the role of intracellular rather than extracellular H2O2 in regulating ENaC. Therefore, H2O2 was applied outside the patch pipette to the cells rather than to inside the pipette with the expectation that high concentrations of extracellular H2O2 would drive H2O2 to move across the cell membrane and increase intracellular H2O2. Our results show that the addition of 3 mm H2O2 to the basolateral bath significantly increased both ENaC open probability (Po) and single-channel current amplitude with a latency of about 12 min (Fig. 1). In contrast, the addition of saline (NaCl solution, used for making up H2O2 stock solution) to the basolateral bath did not affect ENaC activity. These data show that H2O2 stimulates ENaC from the basolateral side in distal nephron cells but with a long latency.
FIGURE 1.
Basolateral H2O2 elevates ENaC open probability (Po) and single-channel current amplitude in A6 distal nephron cells. A, a representative single-channel record from a cell-attached patch before and after the addition of 3 mm H2O2 to the basolateral bath (inset panel a shows increases in single-channel current amplitude after H2O2). B, a representative single-channel record from a cell-attached patch before and after the addition of saline (used both for the bath and for making up H2O2 stock solution) to the basolateral bath (inset panel b shows no change in single-channel current amplitude after saline). I and II, zoomed-in regions. Downward events represent channel openings. C, summary plots of ENaC (Po) before and after the addition of either H2O2 (filled circles) or saline (open circles) to the basolateral bath. Each circle represents mean Po calculated from 3-min single-channel records in A6 cells. Error bars, S.E.
Luminal H2O2 Also Stimulates ENaC in Cell-attached Patches
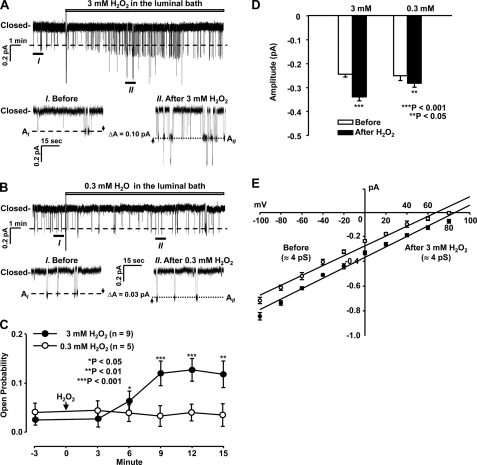
In the above experiments, H2O2 was applied to the basolateral bath. If H2O2 stimulates ENaC by causing intracellular oxidative stress, it must pass through the basolateral membrane in order to reach the apical membrane where ENaC is located. Therefore, we examined the effects of H2O2 applied in the luminal bath on ENaC. Compared with basolateral H2O2, luminal H2O2 at the same concentration of 3 mm also significantly increased both ENaC Po and single-channel current amplitude but with a shorter latency of about 6 min (Fig. 2, A, C, and D). The current-voltage relationship showed that H2O2 did not alter ENaC unit conductance (Fig. 2E). In contrast, the addition of 0.3 mm H2O2 to the luminal bath had no effect on ENaC Po and only slightly increased ENaC single-channel current amplitude (Fig. 2, B, C, and D).
FIGURE 2.
Luminal H2O2 also elevates ENaC Po and single-channel current amplitude in A6 distal nephron cells. A and B, representative single-channel records from cell-attached patches in A6 cells before and after the addition of either 3 mm or 0.3 mm H2O2 to the luminal bath. C, summary plots of ENaC Po before and after the addition of either 3 mm (filled circles) or 0.3 mm H2O2 (open circles) to the luminal bath. D, summary plots of single-channel current amplitude before and after H2O2. E, the current-voltage (I-V) relationship before and after 3 mm H2O2. Error bars, S.E.
A High Concentration of Exogenous H2O2 Is Required to Cause Intracellular Oxidative Stress
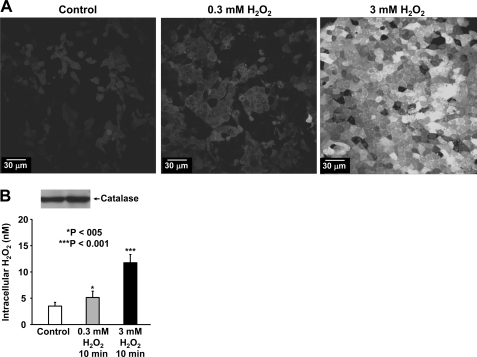
It has been reported that high concentrations of H2O2 are required to produce significant acute effects in some types of cells (27, 28). However, it remains unknown whether high concentrations of extracellular H2O2 are necessary to elevate intracellular H2O2 and produce significant oxidative stress. Therefore, the relationship between exogenous H2O2 load and intracellular oxidative stress was determined. The data show that exogenous H2O2 does not significantly elevate intracellular ROS until the concentration of exogenous H2O2 was in the millimolar range (Fig. 3A). To determine whether the oxidative stress is due to elevation of intracellular H2O2, intracellular H2O2 was measured using the AmpliteTM hydrogen peroxide assay kit. Luminal exposure of A6 cells to relatively high concentrations of H2O2 does increase intracellular H2O2 from 3.5 ± 0.2 (control; n = 9) to 5.1 ± 0.4 nm (0.3 mm H2O2 for 10 min; n = 9; p < 0.05) or 12.2 ± 0.7 nm (3 mm H2O2 for 10 min; n = 9; p < 0.001) (Fig. 3B). This small but significant change suggests that either the permeability of the cell membranes to H2O2 is low or the rate of H2O2 degradation is high. Because the level of intracellular H2O2 is tightly controlled by catalase, we performed Western blot experiments and found that catalase is highly expressed in A6 cells (Fig. 3B, inset). These data suggest that a high concentration of exogenous H2O2 is required to elevate intracellular H2O2 because catalase rapidly breaks down H2O2 that entered the cells.
FIGURE 3.
Exogenous H2O2 causes intracellular oxidative stress. A shows that luminal H2O2 at 3 mm, but not at 0.3 mm, significantly elevated intracellular ROS. Confluent A6 cells were stained with 2′,7′-dichlorodihydrofluorescein diacetate, a membrane-permeable fluorescent probe detecting intracellular ROS. These confocal microscopy data represent consistent results from three experiments. B shows that the elevation was due to increases in intracellular H2O2 in A6 cells. Intracellular H2O2 was determined using the AmpliteTM hydrogen peroxide assay kit (ABD Bioquest, Sunnyvale, CA). The inset shows Western blot of catalase in A6 cells. The cells were under control conditions (left) or treated with either 0.3 mm (middle) or 3 mm H2O2 (right). Error bars, S.E.
Inhibition of Catalase Facilitates H2O2 Stimulation of ENaC
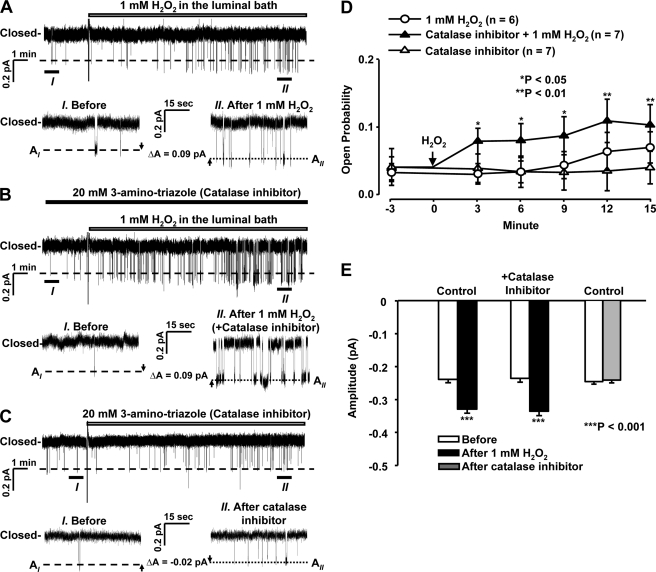
If exogenously added H2O2 stimulates ENaC by elevating intracellular H2O2, but the intracellular concentration of H2O2 is kept low by the action of catalase, then inhibition of catalase should facilitate the effect of exogenous H2O2 on ENaC activity. To test this hypothesis, A6 cells were exposed to 1 mm H2O2 in the absence (control) and presence of 20 mm 3-aminotriazole, a catalase inhibitor. Under control conditions, 1 mm H2O2 tended to elevate ENaC Po but not in a statistically significant manner. In contrast, after inhibition of catalase with 3-aminotriazole, the effect of 1 mm H2O2 on ENaC Po became statistically significant (Fig. 4). Inhibition of catalase did not alter the effect of 1 mm H2O2 on ENaC single-channel current amplitude, indicating that H2O2 may elevate ENaC current amplitude through another pathway that is very sensitive and already saturated by 1 mm H2O2 alone. 3-Aminotriazole alone did not acutely affect the basal activity of ENaC in A6 cells. This lack of effect is probably due to low basal H2O2 production when A6 cells were in the patch chamber and bathed with glucose-free saline at room temperature. These data suggest that H2O2 regulates ENaC via an intracellular mechanism.
FIGURE 4.
Inhibition of catalase enhances the effect of H2O2 on ENaC Po. A, representative single-channel record from a cell-attached patch before and after the addition of 1 mm H2O2 to the luminal bath. B, representative single-channel record from a cell-attached patch before and after the addition of 1 mm H2O2 to the luminal bath in the presence of 20 mm 3-aminotriazole, a catalase inhibitor. C, representative single-channel record from a cell-attached patch before and after the addition of 20 mm 3-aminotriazole. D, summary plots of ENaC Po before and after the addition of 1 mm H2O2 alone (open circles), 1 mm H2O2 in the presence of a catalase inhibitor (20 mm 3-aminotriazole; filled triangles) or the catalase inhibitor alone (20 mm 3-aminotriazole; open triangles) to the luminal bath. E, summary plots of single-channel current amplitude before and after H2O2 in the absence (left bars) or presence (middle bars) of the catalase inhibitor. The catalase inhibitor alone served as another control (right bars). Error bars, S.E.
Overexpression of Catalase Abolishes H2O2 Stimulation of ENaC
The data described above support the hypothesis that highly expressed catalase may account for the resistance of A6 cells to H2O2. To determine whether exogenous H2O2 stimulates ENaC by elevating intracellular H2O2, we overexpressed catalase in A6 cells. To monitor the expression of this enzyme, we subcloned mitochondrial catalase into pEYFP-C1 vector to form the pEYFP-C1-mito-Cat construct. A6 cells were then transiently transfected with this construct. The cells expressing this exogenous catalase were identified by yellow fluorescence. In an A6 cell that was under transfecting conditions but did not express this catalase, the addition of 3 mm H2O2 to the luminal bath elevated both ENaC Po and single-channel current amplitude (Fig. 5A) but failed to produce an effect on ENaC in an A6 cell that expressed the exogenous catalase (the cell with the fluorescence; Fig. 5B). These results suggest that the effect of exogenous H2O2 on ENaC activity is highly dependent on the levels of intracellular H2O2.
FIGURE 5.
Overexpression of a catalase abolishes H2O2 stimulation of ENaC. A and B, representative single-channel records from cell-attached patches in A6 cells either under control conditions (A) or when overexpressing a catalase (B) before and after the addition of 3 mm H2O2 to the luminal bath. C, summary plots of ENaC Po before and after 3 mm H2O2 in A6 cells either under control conditions (filled circles) or when overexpressing a catalase (open circles). D, moderate increases in single-channel current amplitude after the addition of 3 mm H2O2 in control A6 cells but not in the cells overexpressing a catalase. Error bars, S.E.
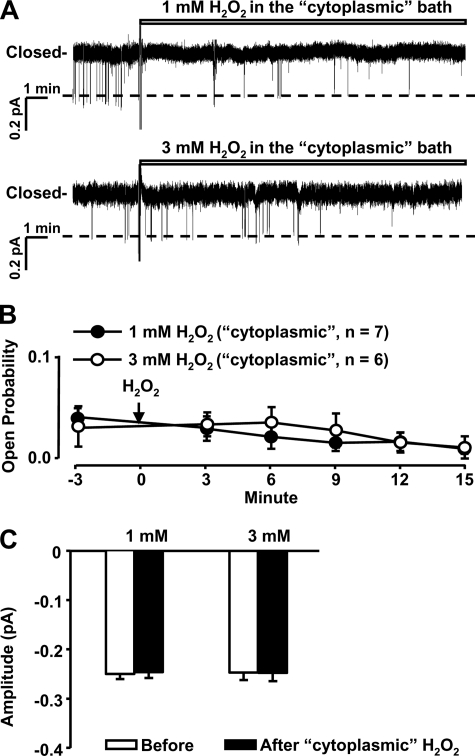
H2O2 Does Not Affect ENaC Activity in Inside-out Patches
Previously reported studies suggested that H2O2 stimulates voltage-gated K+ channels of the Kv7 family by directly oxidizing cysteine residues of this channel protein (29). Therefore, we originally hypothesized that H2O2 might stimulate ENaC by oxidizing intracellular cysteine residues of ENaC. However, using the inside-out configuration, the cytosolic surface of ENaC was exposed to either 1 or 3 mm H2O2. We found that H2O2 did not alter either ENaC PO (Fig. 6, A and C) or ENaC single-channel current amplitude (Fig. 6C). These data suggest that H2O2 stimulates ENaC probably not by directly oxidizing ENaC but rather via activation of signal transduction pathways.
FIGURE 6.
Exposure of the cytoplasmic side of ENaC to H2O2 does not affect its activity. A, a representative inside-out patch showing that ENaC activity was unchanged after the addition of 1 mm or 3 mm H2O2 to the “cytoplasmic” bath. B, summary plots of ENaC Po before and after the addition of 1 mm (filled circles) or 3 mm H2O2 (open circles) to the “cytoplasmic” bath. C, no change in single-channel current amplitude after the addition of 1 or 3 mm H2O2. +60 mV was applied to the patch pipette. Error bars, S.E.
Inhibition of PI3K Abolishes the Effect of H2O2 on ENaC PO
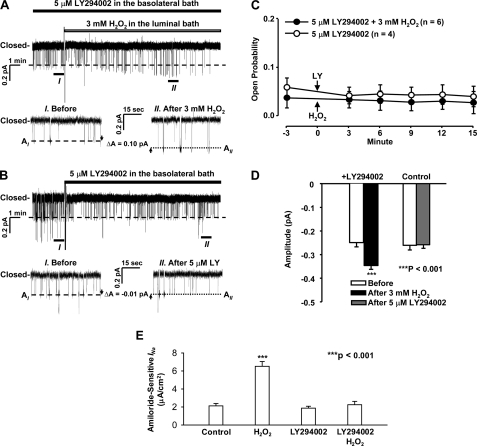
To determine whether PI3K mediates the effects of H2O2 on ENaC activity, prior to the addition of 3 mm H2O2 to the luminal bath, A6 cells were pretreated with 5 μm LY294002, a PI3K inhibitor, for 5 min. Previous studies suggested that PI(3,4,5)P3 is produced in the inner leaflet of the basolateral membrane and can freely diffuse in the inner leaflet to reach the apical membrane (30). Therefore, the PI3K inhibitor was added to the basolateral bath. We found that after the pretreatment, H2O2 no longer elevated ENaC Po but still increased ENaC single-channel current amplitude (Fig. 7, A–D). In contrast, LY294002 alone only slightly reduced ENaC Po when basal ENaC Po was low and had no effect on ENaC single-channel current amplitude. These data suggest that the effect of H2O2 on ENaC Po, but not on ENaC single-channel current amplitude, is mediated by a PI3K-dependent pathway.
FIGURE 7.
Inhibition of PI3K abolishes the effect of H2O2 on ENaC Po. A, a representative single-channel record from a cell-attached patch before and after the addition of 3 mm H2O2 to the luminal bath in the presence of 5 μm LY294002 (added to the basolateral bath), a PI3K inhibitor. B, a representative single-channel record from a cell-attached patch before and after the addition of 5 μm LY294002 to the basolateral bath. C, summary plots of ENaC Po before and after the addition of either 3 mm H2O2 to the luminal bath in the presence of 5 μm LY294002 (filled circles) or 5 μm LY294002 alone to the basolateral bath (open circles). D, summary plots of single-channel current amplitude before and after either 3 mm H2O2 in the presence of 5 μm LY294002 (left bars) or 5 μm LY294002 alone (right bars). E, summary plots of amiloride-sensitive INa. A6 cells were in control conditions, apically treated with 3 mm H2O2 for 15 min, basolaterally treated with 5 μm LY294002 for 15 min, or basolaterally pretreated with 5 μm LY294002 for 5 min followed by apical treatment with 3 mm H2O2 for 15 min. Error bars, S.E.
Inhibition of PI3K Abolishes the Effect of H2O2 on Amiloride-sensitive Na+ Current
To confirm the results from single-channel recordings, amiloride-sensitive Na+ current (INa) was measured. As shown in Fig. 7E, under control conditions when A6 cells were cultured with our modified culture medium, base-line INa was 2.1 ± 0.1 μA/cm2 (n = 10). After the A6 cell monolayer was apically treated with 3 mm H2O2 for 15 min, INa was increased to 6.5 ± 0.2 μA/cm2 (n = 10, p < 0.001). However, H2O2 failed to increase INa (2.3 ± 0.1 μA/cm2, n = 10) in A6 cells pretreated with 5 μm LY294002 for 5 min. Treatment of the cells with 5 μm LY294002 alone for 15 min did not alter INa, probably because PI3K is inactive in A6 cells under basal conditions. These results are consistent with the data from patch clamp single-channel recordings.
H2O2 Induces PI3K-dependent Elevation of PI(3,4,5)P3 in the Apical Membrane of A6 Cells
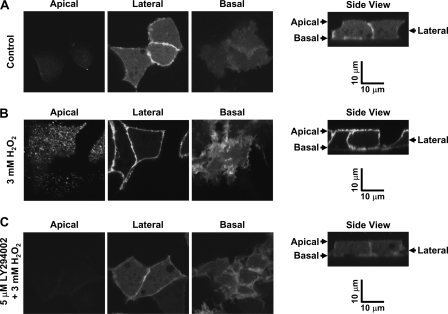
Because our previous studies have shown that PI(3,4,5)P3, one of the PI3K products, stimulates ENaC (18–20), H2O2 may stimulate ENaC via a PI3K-dependent elevation of PI(3,4,5)P3 in the apical membrane. To test this hypothesis, a previously established method (31) was used to track PI(3,4,5)P3 in living A6 cells. The cells were transiently transfected with the EGFP-PH-Akt construct in which the PH domain could selectively bind to PI(3,4,5)P3. Consistent with previous studies (31), the data showed that under basal conditions, PI(3,4,5)P3 was undetectable in the apical membrane of A6 cells but was mainly located in the lateral and basal membranes. Treatment of the cells with 3 mm H2O2 for 15 min induced the appearance of PI(3,4,5)P3 in the apical membrane. In contrast, when the cells were pretreated with 5 μm LY294002 for 5 min to inhibit PI3K, H2O2 failed to induce apical PI(3,4,5)P3 production (Fig. 8). Because our patch clamp data showed that inhibition of PI3K abolished the effect of H2O2 on ENaC Po, these confocal microscopy data suggest that apical PI(3,4,5)P3 production mediates H2O2 stimulation of ENaC.
FIGURE 8.
H2O2 induces PI3K-dependent elevation of PI(3,4,5)P3 in the apical membrane of A6 cells. Left images show confocal microscopy XY sections of apical, lateral, and basal membranes of A6 cells. Right images show XZ sections A6 cells. The cells were transiently transfected with GFP-Akt-PHD to monitor PI(3,4,5)P3 localization. The experiments were performed either under control conditions (A) or when the cells were treated with 3 mm H2O2 for 15 min in the absence (B) or the presence of 5 μm LY294002 (added to the basolateral bath) (C). The data represent three sets of experiments showing similar results. Error bars, S.E.
DISCUSSION
The present study shows that H2O2 increases both ENaC activity and apical PI(3,4,5)P3 via a PI3K-dependent pathway. Because we previously showed that PI(3,4,5)P3 stimulates ENaC in A6 cells (18–20), H2O2 probably stimulates ENaC by causing a PI3K-dependent elevation of PI(3,4,5)P3 in the apical membrane. However, PI(3,4,5)P3 should first be produced in the inner leaflet of the basolateral membrane and then diffused in the inner leaflet to the apical membrane because the PI3K inhibitor LY294002 was added to the basolateral bath. This hypothesis is supported by previous studies, which showed that PI(3,4,5)P3 can laterally diffuse in the inner leaflet of epithelial cell membranes from the basolateral membrane domain, where it is generated, to the apical membrane domain (30). Theoretically, the apical PI(3,4,5)P3 would be rapidly degraded because the lipid phosphatase PTEN (phosphatase and tensin homolog) is located in the apical membrane (31). However, a sustained elevation of apical PI(3,4,5)P3 was observed after treatment of the cells with H2O2. This is not surprising because H2O2 not only activates PI3K but also inactivates PTEN by oxidizing its cysteine residues (32). Because the cytosolic domains of ENaC also contain cysteine residues, we reasoned that H2O2 may stimulate ENaC by oxidizing these residues. However, results from experiments using the inside-out or excised patch configuration show that H2O2 is unable to affect ENaC activity. The results suggest that H2O2 does not directly regulate ENaC but rather alters ENaC activity via a PI3K-dependent pathway.
In the present study, exogenous H2O2 was used as a tool to manipulate the levels of intracellular H2O2. In A6 cells, a high exogenous concentration of H2O2 is required to counteract the efficient catalase present in these cells, elevate intracellular H2O2, and regulate ENaC. However, H2O2, even at 10 mm, did not result in either cell lysis or apoptosis of A6 cells (supplemental Fig. 1). Based on these data, the concentrations of H2O2 used for the current study (up to 3 mm) should not result in any nonspecific effects due to cellular damage. Therefore, such high concentrations of H2O2 can be used as a tool to manipulate the levels of intracellular H2O2 in A6 cells. This argument is probably also true for neurons because in the hippocampus, the IC50 for extracellular H2O2 to affect postsynaptic potentials is nearly 6 mm (33). Because, in the present study, H2O2 was applied outside the patch pipette (not to ENaC in the patch), it remains to be determined whether extracellular H2O2 could also directly regulate ENaC by oxidizing cysteine and/or histidine residues in the extracellular loops of ENaC subunits.
Compared with previously published studies from us and others (23, 34), ENaC mean Po presented in this study is relatively low. In previous reports, ENaC mean Po was calculated according to the number of active channels in the patch estimated under resting conditions. This estimation excluded any silent channels present in the patch. The mean Po reflects only the value of active channels. In the present study, we found that the number of active channels was elevated after treatment with H2O2. This elevation is due to activation of silent channels rather than insertion of new channels in the patch because previous work also with A6 cells suggests that insertion of new channels never occurred in a patch that had already been formed (35). Therefore, the elevated number of channels after H2O2 was used to calculate base-line ENaC Po. This Po reflects the mean Po value of both active and silent channels. Therefore, the Po value reported here is much lower than those published previously. However, the open time of each active channel is still several s (see the zoom-in single-channel traces in each figure).
In order to clearly see the stimulatory effect of H2O2 on ENaC activity, we modified culture medium to reduce basal ENaC activity (see “Methods and Materials”). Compared with the culture medium we used previously (23), the modified culture medium contained less NaHCO3 (15 mm versus 25 mm). Because recent studies have shown that HCO3− stimulates ENaC (36), less HCO3− in culture medium may account, at least in part, for low basal ENaC activity observed in the present study. We noticed that inhibition of PI3K did not reduce basal ENaC Po and amiloride-sensitive Na+ current. Because our confocal microscopy data showed that PI(3,4,5)P3 was undetectable in the apical membrane of A6 cells under basal conditions, we argue that lack of PI(3,4,5)P3 might account for both the low basal ENaC activity and the insensitivity to the PI3K inhibitor, LY294002. However, it remains unknown whether our modified culture conditions could affect the levels of apical PI(3,4,5)P3.
Our data showed that H2O2 increased ENaC single-channel current amplitude. Because H2O2 did not alter ENaC single-channel conductance, the increased current amplitude is probably caused by elevation of the driving force for Na+ influx. First, the electrical driving force (the membrane potential) may be elevated because H2O2 stimulates both KATP channels (13) and large conductance, calcium- and voltage-activated potassium (BK) channels (37). Second, the chemical driving force (Na+ concentration gradient) may also be elevated because H2O2 also stimulates Na+,K+-ATPase (38), which could decrease intracellular Na+. Investigation of the underlying mechanism will be the direction of our future studies. We noticed that the effect of H2O2 on single-channel amplitude in the control experiment shown in Fig. 5 was less significant than those observed in any other experiments. Because fully polarized epithelial cells are difficult to transfect, poorly polarized cells were used in the experiments shown in Fig. 5. In addition, prior to the transfection, the cells were serum-deprived for 24 h. Therefore, we argue that poor polarization and serum deprivation may minimize the effect of H2O2 on ENaC single-channel current amplitude, presumably by reducing functional KATP, BK, and/or Na+,K+-ATPase in the plasma membrane.
In summary, the present study provides the first evidence that H2O2 not only enhances ENaC Po by elevating PI(3,4,5)P3 in the apical membrane via activation of PI3K but also increases ENaC single-channel current amplitude via a PI3K-independent pathway. Because H2O2 in the kidney is elevated in salt-sensitive rats on a high salt diet (6), our in vitro data presented here imply a possible role of ENaC in salt-sensitive hypertension.
Supplementary Material
Acknowledgments
I thank You-You Liang, a research technician, who performed the experiments; Dr. A. Agarwal (Division of Nephrology, University of Alabama at Birmingham), who provided the catalase DNA construct; and Dr. T. Balla (Sections on Molecular Signal Transduction, Program for Developmental Neuroscience, NICHD, National Institutes of Health, Bethesda, MD), who provided the EGFP-PH-Akt DNA construct.
This work was supported, in whole or in part, by National Institutes of Health Grant 5R01-DK067110 (to H.-P. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- ENaC
- epithelial sodium channel
- Po
- open probability
- PI(3,4,5)P3
- phosphatidylinositol 3,4,5-trisphosphate
- ROS
- reactive oxygen species
- EGFP
- enhanced green fluorescent protein
- PH
- pleckstrin homology.
REFERENCES
- 1. Warnock D. G. (1999) Curr. Hypertens. Rep. 1, 158–163 [DOI] [PubMed] [Google Scholar]
- 2. Warnock D. G. (2001) Am. J. Med. Sci. 322, 302–307 [DOI] [PubMed] [Google Scholar]
- 3. Williams S. S. (2007) Curr. Opin. Pediatr. 19, 192–198 [DOI] [PubMed] [Google Scholar]
- 4. Mori T., O'Connor P. M., Abe M., Cowley A. W., Jr. (2007) Hypertension 49, 1336–1341 [DOI] [PubMed] [Google Scholar]
- 5. Abe M., O'Connor P., Kaldunski M., Liang M., Roman R. J., Cowley A. W., Jr. (2006) Am. J. Physiol. Renal Physiol. 291, F350–F357 [DOI] [PubMed] [Google Scholar]
- 6. Taylor N. E., Cowley A. W., Jr. (2005) Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1573–R1579 [DOI] [PubMed] [Google Scholar]
- 7. Taylor N. E., Glocka P., Liang M., Cowley A. W., Jr. (2006) Hypertension 47, 692–698 [DOI] [PubMed] [Google Scholar]
- 8. Gill P. S., Wilcox C. S. (2006) Antioxid. Redox Signal. 8, 1597–1607 [DOI] [PubMed] [Google Scholar]
- 9. Kitiyakara C., Chabrashvili T., Chen Y., Blau J., Karber A., Aslam S., Welch W. J., Wilcox C. S. (2003) J. Am. Soc. Nephrol. 14, 2775–2782 [DOI] [PubMed] [Google Scholar]
- 10. Yu L., Bao H. F., Self J. L., Eaton D. C., Helms M. N. (2007) Am. J. Physiol. Renal Physiol. 293, F1666–F1677 [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y., Griendling K. K., Dikalova A., Owens G. K., Taylor W. R. (2005) Hypertension 46, 732–737 [DOI] [PubMed] [Google Scholar]
- 12. Veal E. A., Day A. M., Morgan B. A. (2007) Mol. Cell 26, 1–14 [DOI] [PubMed] [Google Scholar]
- 13. Avshalumov M. V., Rice M. E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11729–11734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhee S. G., Yang K. S., Kang S. W., Woo H. A., Chang T. S. (2005) Antioxid. Redox Signal. 7, 619–626 [DOI] [PubMed] [Google Scholar]
- 15. Giannoni E., Buricchi F., Raugei G., Ramponi G., Chiarugi P. (2005) Mol. Cell. Biol. 25, 6391–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. (1991) Cell 65, 91–104 [DOI] [PubMed] [Google Scholar]
- 17. Cuevas B. D., Lu Y., Mao M., Zhang J., LaPushin R., Siminovitch K., Mills G. B. (2001) J. Biol. Chem. 276, 27455–27461 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Z. R., Chou C. F., Wang J., Liang Y. Y., Ma H. P. (2010) Pflugers Arch. 459, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma H. P., Chou C. F., Wei S. P., Eaton D. C. (2007) Pflugers Arch. 455, 169–180 [DOI] [PubMed] [Google Scholar]
- 20. Ma H. P., Saxena S., Warnock D. G. (2002) J. Biol. Chem. 277, 7641–7644 [DOI] [PubMed] [Google Scholar]
- 21. Pochynyuk O., Tong Q., Staruschenko A., Ma H. P., Stockand J. D. (2006) Am. J. Physiol. Renal Physiol. 290, F949–F957 [DOI] [PubMed] [Google Scholar]
- 22. Pochynyuk O., Tong Q., Staruschenko A., Stockand J. D. (2007) J. Physiol. 580, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao H. F., Zhang Z. R., Liang Y. Y., Ma J. J., Eaton D. C., Ma H. P. (2007) Am. J. Physiol. Renal Physiol. 293, F1178–F1186 [DOI] [PubMed] [Google Scholar]
- 24. Wang J., Zhang Z. R., Chou C. F., Liang Y. Y., Gu Y., Ma H. P. (2009) Am. J. Physiol. Renal Physiol. 296, F284–F290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bienert G. P., Møller A. L., Kristiansen K. A., Schulz A., Møller I. M., Schjoerring J. K., Jahn T. P. (2007) J. Biol. Chem. 282, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 26. Chen Y. F., Cowley A. W., Jr., Zou A. P. (2003) Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R827–R833 [DOI] [PubMed] [Google Scholar]
- 27. Lin M. J., Yang X. R., Cao Y. N., Sham J. S. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1598–L1608 [DOI] [PubMed] [Google Scholar]
- 28. Zhang W., Tong Q., Conrad K., Wozney J., Cheung J. Y., Miller B. A. (2007) Am. J. Physiol. Cell Physiol. 292, C1746–C1758 [DOI] [PubMed] [Google Scholar]
- 29. Gamper N., Zaika O., Li Y., Martin P., Hernandez C. C., Perez M. R., Wang A. Y., Jaffe D. B., Shapiro M. S. (2006) EMBO J. 25, 4996–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blazer-Yost B. L., Vahle J. C., Byars J. M., Bacallao R. L. (2004) Am. J. Physiol. Cell Physiol. 287, C1569–C1576 [DOI] [PubMed] [Google Scholar]
- 31. Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. (2007) Cell 128, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rhee S. G., Kang S. W., Jeong W., Chang T. S., Yang K. S., Woo H. A. (2005) Curr. Opin. Cell Biol. 17, 183–189 [DOI] [PubMed] [Google Scholar]
- 33. Nisticò R., Piccirilli S., Cucchiaroni M. L., Armogida M., Guatteo E., Giampà C., Fusco F. R., Bernardi G., Nisticò G., Mercuri N. B. (2008) Br. J. Pharmacol. 153, 1022–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu L., Helms M. N., Yue Q., Eaton D. C. (2008) Am. J. Physiol. Renal Physiol. 295, F1519–F1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marunaka Y., Eaton D. C. (1991) Am. J. Physiol. 260, C1071–C1084 [DOI] [PubMed] [Google Scholar]
- 36. Pech V., Pham T. D., Hong S., Weinstein A. M., Spencer K. B., Duke B. J., Walp E., Kim Y. H., Sutliff R. L., Bao H. F., Eaton D. C., Wall S. M. (2010) J. Am. Soc. Nephrol. 21, 1928–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barlow R. S., El-Mowafy A. M., White R. E. (2000) Am. J. Physiol. Heart Circ. Physiol. 279, H475–H483 [DOI] [PubMed] [Google Scholar]
- 38. Bełtowski J., Wójcicka G., Trzeciak J., Marciniak A. (2006) Peptides 27, 3234–3244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.