Abstract
Autophagy has been recently demonstrated to control cell and tissue homeostasis, including the functions of various metabolic tissues. However, it remains unclear whether autophagy is critical for the central nervous system and particularly the hypothalamus for exerting metabolic regulation. Using autophagy-related protein 7 (Atg7) as an autophagic marker, this work showed that autophagy was highly active in the mediobasal hypothalamus of normal mice. In contrast, chronic development of dietary obesity was associated with autophagic decline in the mediobasal hypothalamus. To investigate the potential role of autophagy in the hypothalamic control of metabolic physiology, a mouse model was developed with autophagic inhibition in the mediobasal hypothalamus using site-specific delivery of lentiviral shRNA against Atg7. This model revealed that hypothalamic inhibition of autophagy increased energy intake and reduced energy expenditure. These metabolic changes were sufficient to increase body weight gain under normal chow feeding and exacerbate the progression of obesity and whole-body insulin resistance under high-fat diet feeding. To explore the underlying mechanism, this study found that defective hypothalamic autophagy led to hypothalamic inflammation, including the activation of proinflammatory IκB kinase β pathway. Using brain-specific IκB kinase β knockout mice, it was found that the effects of defective hypothalamic autophagy in promoting obesity were reversed by IκB kinase β inhibition in the brain. In conclusion, hypothalamic autophagy is crucial for the central control of feeding, energy, and body weight balance. Conversely, decline of hypothalamic autophagy under conditions of chronic caloric excess promotes hypothalamic inflammation and thus impairs hypothalamic control of energy balance, leading to accelerated development of obesity and comorbidities.
Keywords: Autophagy, Brain, Inflammation, Jun N-terminal Kinase (JNK), NF-κB Transcription Factor, Obesity
Introduction
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved process that is essential for the maintenance of cellular homeostasis (1, 2). In response to intracellular nutrient depletion or stress, such as protein misfolding or organelle dysfunctions, cells employ an autophagic reaction to digest intracellular contents and supply energy (1, 2). Various experimental models have been developed to consistently demonstrate that impairment of autophagy can underlie the development of diverse diseases such as infections (3–5), cancers (6, 7), muscle diseases (2, 8), aging (2, 9), heart diseases (2, 10), and neurodegenerative disorders (11–13). During the past years, molecular studies have elucidated that the process of autophagy is critically mediated by a group of autophagy-related proteins (Atg) that function to formulate autophagosome (1, 2). Among Atg proteins, Atg7 plays a key role in the autophagic response, and Atg7 loss of function is sufficient to impair the autophagic function of cells (11–17). Indeed, mice with whole-body Atg7 knockout died within 24 h after birth, presumably because of nutrient and energy depletion (14). On the other hand, animal models with tissue-specific Atg7 ablation or inhibition have revealed that the baseline autophagy is normally required for the physiological actions of individual tissues and organs (11–17). More recently, the relevance of autophagy to metabolic diseases such as type 2 diabetes (T2D)2 and lipid disorders has increasingly begun to receive research attention. To date, research has explored a number of peripheral metabolic tissues, including the liver (18–21), skeletal muscle (22), pancreatic β cells (23–25), and fat cells (26, 27). In general, the findings in these studies have led to an overall view that autophagy defects can impair metabolic functions to promote T2D and related diseases (18–25), although impairment of fat adipogenesis via autophagic dysfunction can be exceptionally beneficial in terms of the counteraction against fat expansion and obesity development (26, 27).
From another perspective, recent research has increasingly appreciated the important functions of the hypothalamus in regulation of feeding, body weight, and energy balance (28, 29). More recently, metabolic inflammation in the hypothalamus was identified to mediate the neural dysregulations of energy and metabolic balance, which led to the development of obesity-T2D syndrome (30–40). The accountable pathways include IκB kinase β (IKKβ) and NF-κB pathway (30–34) and upstream inputs such as MyD88 (35), endoplasmic reticulum stress (30, 31, 36), and JNK signaling (38–40). However, it remains unexplored whether autophagy in the hypothalamus is crucial for the central control of metabolic physiology and related metabolic diseases, despite the fact that impairment of autophagy in the CNS can promote neurodegenerative disorders (11–13). Interestingly, a few recent studies have reported that autophagic defects can lead to the induction of inflammation and, in particular, the activation of NF-κB in tumor cells or immune cells (41–45). In this background, this work aimed to investigate the potential roles of hypothalamic autophagy in the central control of energy and body weight balance and related disease and also to test the possible connection of hypothalamic autophagic alteration with inflammatory changes of the hypothalamus in causing disease development.
EXPERIMENTAL PROCEDURES
Animals
IKKβlox/lox mice have been described previously (30). Nestin-Cre mice and C57BL/6 mice were from The Jackson Laboratory. All these mouse lines were maintained in a C57BL/6 strain background. Nestin-Cre mice were backcrossed with IKKβlox/lox mice for > 25 generations over 6 years. Mice were housed in standard conditions and maintained on a normal chow or a high-fat diet (HFD, Research Diets, Inc.). The Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine approved all animal procedures.
Metabolic Phenotyping
Mouse body weight was regularly measured, and food intake was determined on a daily basis by individual housing. Overnight-fasted mice were intraperitoneally injected with glucose (2 g/kg of body weight) for the glucose tolerance test. Blood glucose levels were measured at the indicated time points post-injection with a LifeScan blood glucose monitoring system. The mouse body composition was measured using an EchoMRITM whole body composition analyzer (Echo Medical Systems). The physiological markers of energy expenditure, including O2 consumption and CO2 production, were measured using the metabolic chambers (Columbus Instruments, Inc.) at the Diabetes Research and Training Center core facility of the Albert Einstein College of Medicine. O2 consumption and CO2 production were normalized by lean body mass of mice. Energy expenditure was calculated using the formula energy expenditure = (3.815 + 1.232 × VCO2/VO2) × VO2, where VCO2 and VO2 were expressed as ml/kg/h corrected by body weight according to the literature (46). Blood insulin and leptin levels were determined using ELISA kits (Chrystal Chem).
Tissue Dissection and Western Blot Analyses
As described previously (30), the hypothalamus of mice was cut along the anterior border of the optic chiasm, the posterior border of the mammillary body, the upper border of the anterior commissure, and the lateral border halfway from the lateral sulcus in the ventral side. Tissue lysis, protein extraction, and Western blot analyses were performed as described previously (30). Proteins were dissolved in a lysis buffer and separated by SDS/PAGE for Western blot analyses. Primary antibodies included rabbit anti-Atg7 (Abcam, Inc.), anti-Atg5 (Abgent), anti-LC3 (Novus), anti-phosphorylated NF-κB, anti-NF-κB (RelA), anti-phosphorylated IKKα/β, anti-IKKβ, anti-β-actin (Cell Signaling Technology, Inc.), and anti-IκBα (Santa Cruz Biotechnology, Inc.). Secondary antibody was HRP-conjugated anti-rabbit IgGs (Pierce). The densitometric analyses of Western blotting images were performed using Image-Pro Plus software (Media Cybernetics).
Quantitative RT-PCR
Total RNA from the tissues was extracted using TRIzol (Invitrogen). The Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) system (Promega) was used to synthesize cDNA, and PCR was performed and quantified using SYBR Green real-time PCR Master Mix (Applied Biosystems). Data were normalized by mRNA levels of β-actin.
Cardiac Perfusion and Immunostaining
Mice under anesthesia were transcardially perfused with 4% PFA, and brains were post-fixed in 4% PFA for 4 h and infiltrated with 20–30% sucrose. Brain sections of 20-μm thickness were made using a cryostat at −20 °C. Fixed brain sections were blocked with serum of the appropriate species, penetrated with 0.2% Triton X-100, and treated with primary antibodies, including rabbit anti-Atg7 (Abcam, Inc.) and mouse anti-NeuN (Chemicon), and subsequently reacted with fluorescent Alexa Fluor 488 or 555 secondary antibody (Invitrogen). Images were captured under a confocal microscope.
Lentiviruses and Hypothalamic Injection
The lentiviral vector of shRNA against mouse Atg7 and matched control lentiviral vector were purchased from Sigma. Lentiviruses were produced from HEK293T cells through cotransfection of target plasmids with their packaging plasmids. Lentiviruses were purified by ultracentrifugation. As described previously (30), an ultraprecise stereotax was employed to bilaterally inject lentiviruses into the mediobasal hypothalamus (predominantly the arcuate nucleus) at the coordinates of 1.5 mm posterior to the bregma, 0.3 mm lateral to the midline, and 5.8 mm below the surface of the skull.
Statistical Analyses
Two-tailed Student's t tests were used for two-group comparisons. Analysis of variance and appropriate post hoc analyses were used for comparisons of more than two groups. Data were presented as mean ± S.E. p < 0.05 was considered statistically significant.
RESULTS
Autophagic Marker/Mediator Atg7 Is Abundantly Expressed in the Mediobasal Hypothalamus
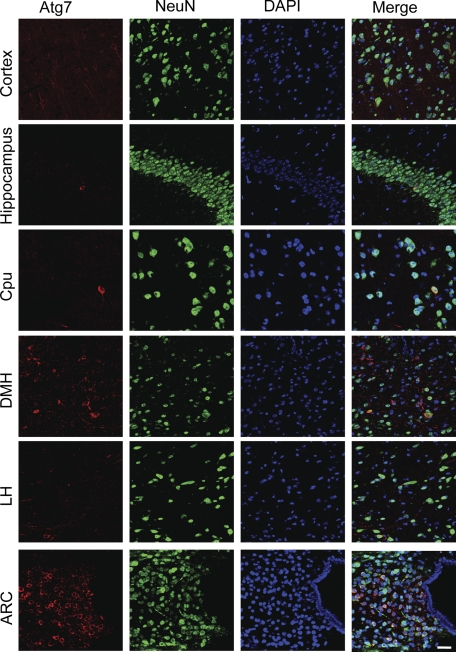
Atg proteins are a group of key components that function to form autophagosome and mediate autophagic activity (1, 2). Among Atg proteins, Atg7 is essential for the autophagic response, and, indeed, Atg7 ablation or inhibition is sufficient to impair the autophagic function of cells (11–17). To investigate the potential role of autophagy in the hypothalamus, we first profiled the distribution of Atg7 protein in the hypothalamus of normal mice (standard C57BL/6 adult males). Using the immunostaining of Atg7, we found that Atg7 was broadly expressed in various brain regions, including the hypothalamus (Fig. 1). Interestingly, we observed that compared with all other brain and hypothalamic regions, Atg7 protein levels in the hypothalamic arcuate nucleus was most abundant (Fig. 1). To understand types of neural cells in which Atg7 was expressed most, we performed coimmunostaining of Atg7 with a neuronal marker, NeuN. As shown in Fig. 1, Atg7 was expressed predominantly in the cytoplasm of most neurons, which were visualized through the nuclear staining of NeuN. Because the literature has well established that Atg7 represents a molecular marker that indicates the activity of autophagy (11, 14, 19), our data indicated that autophagy was highly active in the hypothalamic arcuate nucleus of normal mice.
FIGURE 1.
Distribution of Atg7 in the brain and hypothalamus. Coimmunostaining of Atg7 (red) with neuronal marker NeuN (green) was performed for the sections across various brain areas and hypothalamic regions of normal C57BL/6 mice (chow-fed adult males). DAPI staining (blue) reveals the nucleus of all cells in the sections. Images were merged to show Atg-immunoreactive cells among the total cell population in the sections. Data show representative immunostaining in the cortex, hippocampus, caudate putamen (Cpu), dorsal medial hypothalamus (DMH), lateral hypothalamus (LH), and arcuate nucleus (ARC). At least three mice per brain regions were analyzed. Scale bar = 100 μm.
Chronic Obesogenic Conditions via HFD Feeding Impair Autophagy in the Hypothalamus
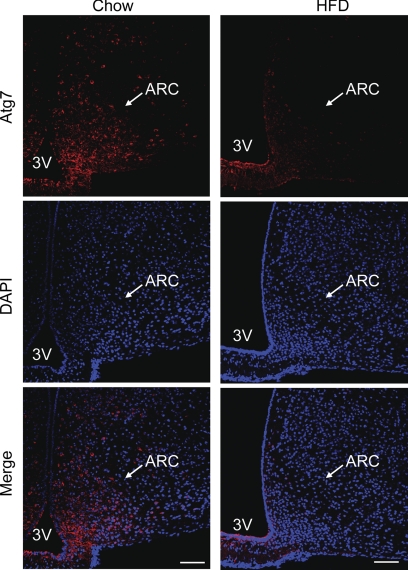
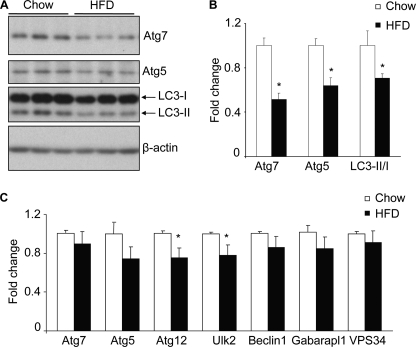
The arcuate nucleus of the hypothalamus has been known as the central regulator of feeding and body weight balance (28, 29). Defects of neuronal activities or molecular signaling in the arcuate nucleus can cause feeding and energy imbalance, leading to the development of obesity and related diseases (28, 29). Hence, the data in Fig. 1 inspired us to examine whether autophagic changes in the hypothalamic arcuate nucleus could be significant for the hypothalamic pathogenesis of obesity. We first explored whether hypothalamic autophagy might be affected in C57BL/6 mice, with obesity induced by chronic HFD feeding. Using Atg7 immunostaining, we found that Atg7 protein levels were generally reduced in many brain regions but most strikingly in the arcuate nucleus by chronic (approximately 4–5-month) HFD feeding (Fig. 2). Subsequently, Western blot analysis was employed to quantitatively compare the hypothalamic profiles of autophagic markers in normal chow-fed versus HFD-fed mice. Results revealed that chronic HFD feeding significantly decreased the expression of autophagic markers in the hypothalamus, as evidenced by a reduction of 49% in Atg7 protein and 36% in Atg5 protein (Fig. 3, A and B). Furthermore, we determined the conversion of microtubule-associated protein light chain 3 (LC3) from LC3-I to LC3-II, a molecular process that is key for autophagic response (1, 2). Indeed, the protein ratio of LC3-II to LC3-I in the hypothalamus of HFD-fed mice was significantly lower than that in chow-fed mice (Fig. 3, A and B). In addition, we employed real-time RT-PCR to measure the mRNA levels of genes that encode various autophagic components, including Atg7, Atg5, Atg12, Ulk2, Beclin-1, VPS34, and Gabarapl-1. Data demonstrated that hypothalamic mRNA levels of these genes were basically similar between HFD-fed mice and chow-fed controls (Fig. 3C). Taken together, these data suggest that impairment of hypothalamic autophagy under chronic HFD feeding was induced mainly through posttranslational processes rather than gene transcriptional processes.
FIGURE 2.
Effect of HFD feeding on Atg7 in hypothalamic arcuate nucleus. Male C57BL/6 mice were maintained on a normal chow diet versus an HFD, and brain sections were then prepared for Atg7 immunostaining (red). Data show Atg7 immunostaining across the hypothalamic arcuate nucleus (ARC) of chow-fed versus HFD-fed C57BL/6 mice. DAPI staining (blue) reveals the nucleus of all cells in the sections. At least five mice per group were analyzed. Data represent approximately 4–5 months of HFD versus chow feeding. 3V, third ventricle. Scale bar = 100 μm.
FIGURE 3.
Effects of HFD feeding on autophagy markers in the hypothalamus. Male C57BL/6 mice were maintained on a normal chow diet versus an HFD. The hypothalamus was harvested for the measurements of the indicated autophagic markers by Western blot analyses (A and B) and real-time RT-PCR analyses (C). Quantification of Western blot analyses are presented in B. LC3-II/I, ratio of LC3-II to LC3-I. *, p < 0.05; n = 3 (B) and 10 (C) per group. Data are presented as mean ± S.E.
Defect of Hypothalamic Autophagy Causes Positive Energy Balance and Systemic Insulin Resistance
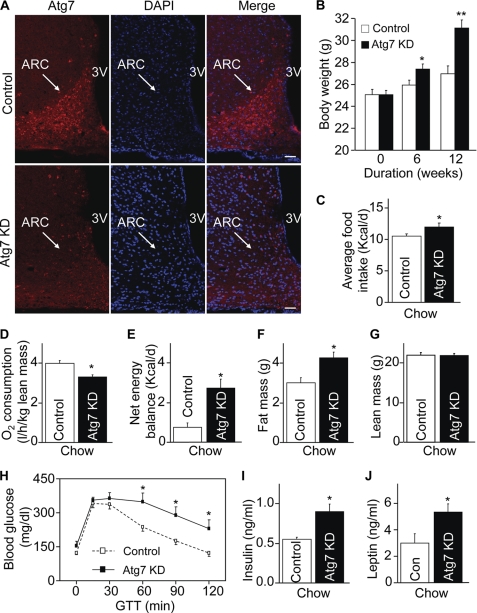
We next questioned if autophagic activity in the hypothalamic arcuate nucleus might be critical for the hypothalamic regulation of feeding, energy balance and body weight. Our experimental approach aimed to induce autophagic dysfunction broadly in the hypothalamic arcuate nucleus, which can mimic the changes of mice induced by chronic HFD feeding, as shown in Fig. 2. To do this, we employed site-specific RNA interference to knock down Atg7 via hypothalamic delivery of lentiviral shRNA against mouse Atg7. Normal C57BL/6 mice (chow-fed adult males) were injected bilaterally with lentiviral Atg7 shRNA versus matched control shRNA into the mediobasal hypothalamic region (MBH), which predominantly targeted the arcuate nucleus. The mice with MBH-directed Atg7 knockdown were termed MBH/Atg7 KD mice. The technique of MBH-directed injection has been used extensively in our research (31, 47), and post-hoc analysis verified that injection did not cause hypothalamic lesions in both MBH/Atg7 KD mice and control mice. Using Atg7 immunostaining, we confirmed that site-specific Atg7 knockdown was successful in MBH/Atg7 KD mice (Fig. 4A). Following injection, mice continued to be maintained on the standard normal chow. Despite normal caloric contents of the chow diet, MBH/Atg7 KD mice showed a significant increase in weight gain, which was detectable on week 6 and became more evident on week 12 post-injection (Fig. 4B). The phenotype of increased body weight gain was attributed to elevated energy intake (Fig. 4C) and impaired energy expenditure (D), which caused a positive increase in estimated net energy balance (E). Locomotive activities were nonetheless comparable between MBH/Atg7 KD mice and controls. MRI scanning confirmed that body weight gain of MBH/Atg7 KD mice was attributed to fat mass expansion but not lean body mass increase (Fig. 4, F and G). Consistent with the obesity-prone change, MBH/Atg7 KD mice showed a cluster of pre-T2D disorders such as glucose intolerance (Fig. 4H), hyperinsulinemia (I), and hyperleptinemia (J). In sum, all these data indicate that the central control of feeding, body weight, and metabolic physiology requires the functional integrality of autophagy in the mediobasal hypothalamus.
FIGURE 4.
Metabolic phenotype of mice with MBH-specific Atg7 knockdown on chow feeding. C57BL/6 mice (chow-fed adult males) with Atg7 knockdown (Atg7 KD) versus the control were generated using bilateral injections of the MBH with lentiviruses containing shRNA against mouse Atg7 or the matched control shRNA. A, Atg7 immunostaining (red) of brain sections was performed to verify Atg7 knockdown in the MBH. DAPI staining (blue) reveals the nuclei of all cells in the sections. Scale bar = 50 μm. KD, knockdown. B and C, longitudinal follow-up of body weight (B) and average daily food intake (C) of chow-fed mice over 12 weeks post-MBH injection. *, p < 0.05; **, p < 0.01 (comparisons between two mouse groups at the matched time points that are underlined); n = 12–13 per group. Data are presented as mean ± S.E. D, metabolic chamber assessment of O2 consumption was performed for a subgroup of chow-fed mice at week 5 post injection. O2 consumption was corrected by lean body mass of mice. *, p < 0.05; n = 4 per group. Data are presented as mean ± S.E. E, net energy balance was calculated by subtracting energy expenditure ((3.815 + 1.232 × VCO2/VO2) × VO2, Columbus Instruments) from energy intake for mice at week 5 post-injection. *, p < 0.05; n = 4 per group. Data are presented as mean ± S.E. F and G, MRI assessment of fat mass (D) and lean mass (E) was performed for a subgroup of chow-fed mice at 5 weeks post-injection. *, p < 0.05; n = 6–8 per group. Data are shown as mean ± S.E. H–J, a subgroup of chow-fed mice was analyzed for glucose tolerance (H), fasting blood insulin concentration (I), and fasting blood leptin concentration (J) at week 10 post-injection. *, p < 0.05; n = 6 per group. Data are shown as mean ± S.E. GTT, glucose tolerance test; Con, control.
Defect of Hypothalamic Autophagy Markedly Exacerbates Development of Obesity and Systemic Insulin Resistance
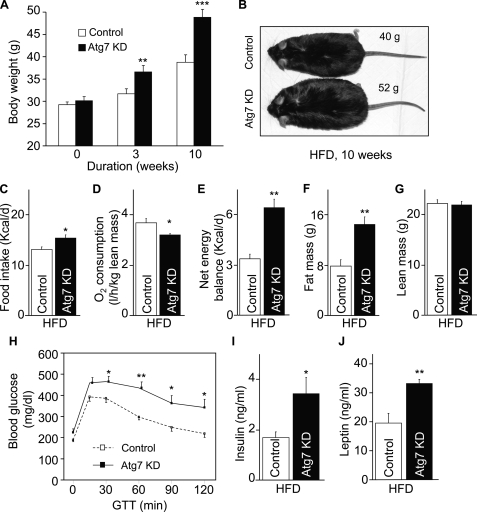
Data in Fig. 4 have shown that inhibition of hypothalamic autophagy can promote weight gain even under normal chow feeding. We further examined whether this effect could lead to exacerbated development of obesity-T2D disorders under HFD feeding condition. To test this question, we placed MBH/Atg7 KD mice and matched controls under HFD feeding after lentiviral shRNA injection. As shown in Fig. 5A, Atg7 ablation markedly potentiated HFD-induced body weight gain, even after a short period (3 weeks) of HFD feeding. Over a 10-week follow-up, MBH/Atg7 KD mice developed obesity much more severely compared with HFD-fed controls (Fig. 5, A and B). The development of obesity in MBH/Atg7 KD mice was mediated by increased positive energy balance that resulted from exaggerated energy intake and impaired energy expenditure (Fig. 5, C–E). Locomotive activities slightly decreased in HFD-fed MBH/Atg7 KD mice compared with HFD-fed controls. The obesity phenotype of MBH/Atg7 mice was further confirmed by the MRI scanning data showing that fat mass but not lean mass increased remarkably (Fig. 5, F and G). Along with the obesity phenotype, HFD-fed MBH/Atg7 KD mice displayed a morbid magnitude of glucose intolerance, hyperinsulinemia, and hyperleptinemia (Fig. 5, H–J). Altogether, these data indicate that hypothalamic autophagy decline can disrupt the central regulation of energy balance as well as systemic glucose/insulin homeostasis, leading to strong pathogenic effects toward the development of obesity-T2D under obesogenic conditions.
FIGURE 5.
Metabolic phenotype of mice with MBH-specific Atg7 knockdown under HFD feeding. Body weight-matched C57BL/6 mice (chow-fed adult males) with Atg7 knockdown (Atg7 KD) versus the control were generated using bilateral injections of the MBH with lentiviruses containing shRNA against mouse Atg7 or the matched control shRNA. Mice were switched to HFD feeding post-lentiviral injection. A–C, longitudinal follow-up of body weight (A), obesity appearance (B), and average daily food intake (C) of HFD-fed mice at the indicated time points post-MBH injection. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 10 - 12 per group. Data are presented as mean ± S.E. KD, knockdown. D, metabolic chamber assessment of energy expenditure was performed for a subgroup of HFD-fed mice at week 3 post-injection. *, p < 0.05; n = 4 per group. Data are presented as mean ± S.E. E, net energy balance was calculated by subtracting energy expenditure ((3.815 + 1.232 × VCO2/VO2) × VO2, Columbus Instruments) from energy intake for mice at week 3 post-injection. **, p < 0.01; n = 4 per group. Data are presented as mean ± S.E. F and G, MRI assessment of fat mass (F) and lean mass (G) was performed at 3 weeks post-injection. **, p < 0.01; n = 12 per group. Data are shown as mean ± S.E. H–J, mice were analyzed with a glucose tolerance at week 4 post-injection (H) and for fasting blood insulin (I) and leptin (J) concentrations at week 10 post-injection. *, p < 0.05; **, p < 0.01; n = 6–12 per group. Data are shown as mean ± S.E. GTT, glucose tolerance test.
Defective Hypothalamic Autophagy Induces IKKβ/NF-κB Activation and Inflammatory Changes in the Hypothalamus
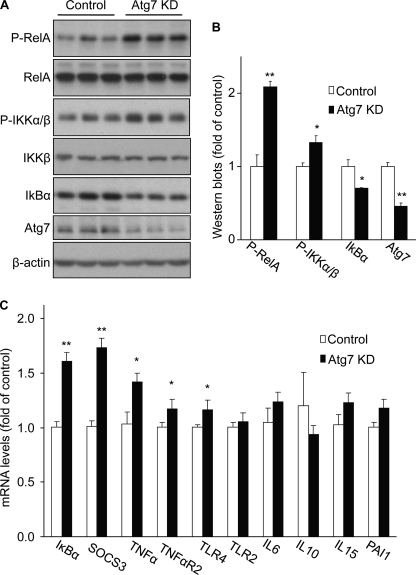
Next, we investigated the molecular basis that can link defective hypothalamic autophagy to the outcome of energy imbalance and obesity. We were intrigued by recent studies showing that autophagic defects cause inflammatory response through IKKβ/NF-κB activation in tumor cells or immune cells (41–44). Notably, recent literature has revealed that the central pathogenesis of obesity involves the effects of hypothalamic IKKβ/NF-κB (30–34) and related signaling (38–40). Against this background, we examined if dysfunction of hypothalamic autophagy could affect the IKKβ/NF-κB signaling pathway. Normal C57BL/6 mice received lentiviral injections of Atg7 shRNA versus control shRNA into the MBH. Hypothalamus was harvested from the mice at 1 week post-viral injection, a time point necessary for the occurrence of Atg7 gene knockdown prior to evident changes in body weight. Using Western blot analysis, we confirmed that Atg7 shRNA reduced the hypothalamic Atg7 protein levels by 55% (Fig. 6, A and B). Western blot analysis was further used to determine the IKKβ/NF-κB signaling pathway. As shown in Fig. 6, the phosphorylation levels of NF-κB subunit RelA increased by > 2-fold in response to hypothalamic Atg7 knockdown. The phosphorylation levels of IKKα/β were also examined because IKKα/β phosphorylation is classically required for NF-κB activation (48). Data in Fig. 6 showed that Atg7 knockdown significantly increased IKKα/β phosphorylation in the hypothalamus. Our Western blot analyses also examined IκBα, the prototypical inhibitory protein of NF-κB that is degraded through IKKβ-directed phosphorylation (48). Indeed, hypothalamic IκBα protein levels significantly decreased following hypothalamic Atg7 knockdown (Fig. 6, A and B). Consistent with this finding, Atg7 knockdown increased the expression of several NF-κB-dependent inflammatory genes in the hypothalamus (Fig. 6C). Collectively, these data indicate that defective hypothalamic autophagy can induce hypothalamic inflammation via IKKβ/NF-κB activation in the hypothalamus.
FIGURE 6.
Effects of Atg7 knockdown on IKKβ/NF-κB and related inflammation in the hypothalamus. C57BL/6 mice (chow-fed adult males) with Atg7 knockdown (Atg7 KD) versus the control were generated using bilateral injections of the MBH with lentiviruses containing Atg7 shRNA or matched control shRNA. Hypothalami were harvested at 1 week post-injection and analyzed for IKKβ/NF-κB signaling by Western blotting (A and B) and for gene expression of related inflammatory molecules by real-time RT-PCR (C). P-RelA, phosphorylated RelA; P-IKKα/β, phosphorylated IKKα/β. The bar graphs in B show the quantification analysis of Western blot analyses. *, p < 0.05; **, p < 0.01; n = 3 per group (B) and 6 per group (C). Data are shown as mean ± S.E.
IKKβ Ablation Counteracts the Obese Effect of Defective Hypothalamic Autophagy
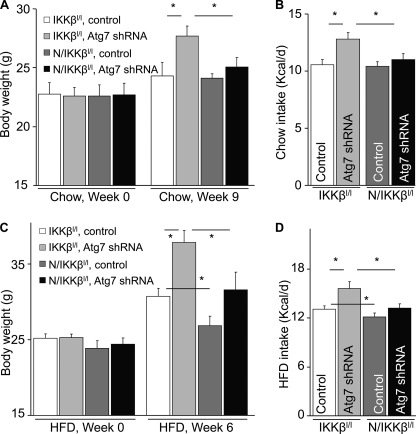
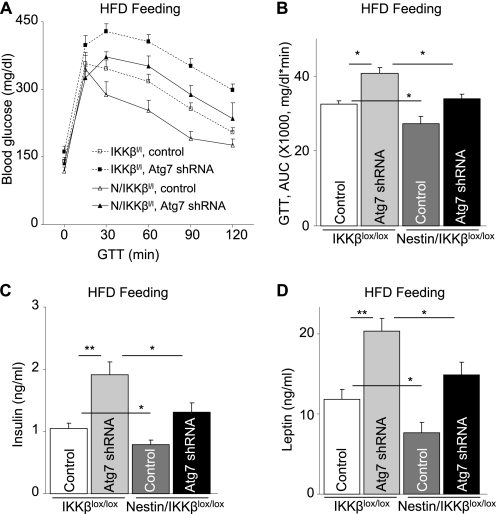
The subsequent question was, could IKKβ/NF-κB be important for the disease effect of defective hypothalamic autophagy? To answer this question, we tested whether inhibition of IKKβ/NF-κB could abolish the metabolic changes displayed in MBH/Atg7 KD mice. In these experiments, brain-specific IKKβ knockout mice, termed Nestin/IKKβlox/lox mice, were employed that were generated by crossing Nestin-Cre mice with IKKβlox/lox mice as described previously (30). Nestin/IKKβlox/lox mice and littermate controls IKKβlox/lox mice received intra-MBH injections of lentiviral Atg7 shRNA or control shRNA. Mice were maintained on either a chow diet or a HFD. Data in Fig. 7, A and B, demonstrate that IKKβ knockout significantly abrogated the overeating and body weight-promoting effects of MBH-directed Atg7 knockdown under chow feeding conditions. Also, importantly, although Atg7 knockdown in the MBH promoted the control mice to develop obesity in association with hyperphagia under HFD feeding condition, the effects of Atg7 knockdown were prevented significantly by IKKβ knockout in the brain (Fig. 7, C and D). Consistent with the obesity-resistant phenotype, Nestin/IKKβlox/lox mice prevented Atg7 knockdown from causing glucose intolerance (Fig. 8, A and B), hyperinsulinemia (C), and hyperleptinemia (D). Thus, in conjunction with Fig. 4 showing that Atg7 knockdown activated IKKβ/NF-κB, these data can suggest that IKKβ/NF-κB represents a downstream player for the deleterious metabolic effects of defective hypothalamic autophagy in the pathogenesis of obesity and related T2D.
FIGURE 7.
IKKβ ablation in the brain prevents the metabolic effects of MBH-Atg7 knockdown. Nestin/IKKβlox/lox mice (N/IKKβl/l) and littermate controls (IKKβlox/lox mice, IKKβl/l) were maintained under chow feeding since weaning. At an adult age, mice were bilaterally injected with lentivirus to deliver Atg7 shRNA or control shRNA to the MBH. Following injection, mice were maintained on either chow feeding (A and B) or HFD feeding (C and D). The longitudinal body weight (A and C) and daily food intake (B and D) of these mice were followed up. *, p < 0.05; n = 6–8 per group. Data are presented as mean ± S.E.
FIGURE 8.
IKKβ ablation in the brain prevents the metabolic effects of MBH-Atg7 knockdown. Nestin/IKKβlox/lox mice (N/IKKβl/l) and littermate controls (IKKβlox/lox mice, IKKβl/l) were maintained under chow feeding since weaning. At an adult age, mice with matched body weight were bilaterally injected with lentivirus to deliver either Atg7 shRNA or control shRNA to the MBH. Following injection, mice were maintained on HFD feeding. Mice were analyzed for glucose tolerance (A and B), fasting blood insulin (C), and leptin (D) concentrations at week 6 post-injection. The bar graphs in B show the area under curve (AUC) values of the glucose tolerance test (GTT) presented in A. *, p < 0.05; **, p < 0.01; n = 6–8 per group. Data are presented as mean ± S.E.
DISCUSSION
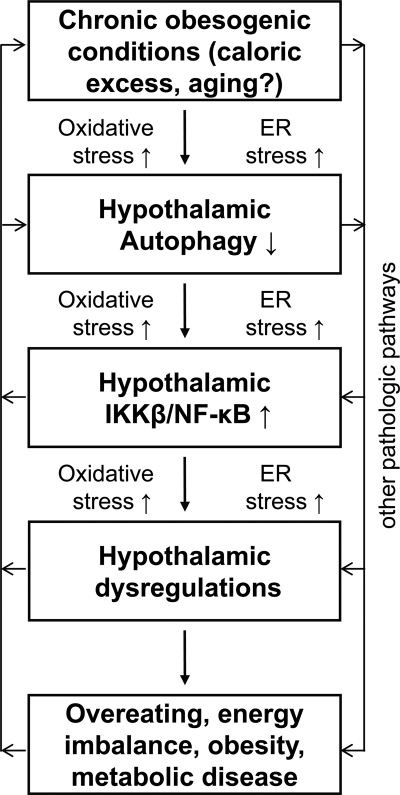
This study investigated whether and how hypothalamic autophagy is involved in the CNS control of metabolic physiology and disease. Our results show that autophagy is abundantly active in the mediobasal hypothalamus of normal mice but impaired during the chronic development of dietary obesity. Compromised function of hypothalamic autophagy causes overeating and impairs energy expenditure, leading to increases in weight gain under chow feeding and exacerbated progression of obesity-T2D syndrome under HFD feeding. Mechanistic studies further reveal that defect of hypothalamic autophagy can activate the hypothalamic IKKβ/NF-κB pathway to induce the disease outcomes. Overall, this study can conclude that hypothalamic autophagy is essential for the physiological functions of the hypothalamus in maintaining metabolic homeostasis and preventing metabolic disease, and, on the other hand, defective hypothalamic autophagy can employ inflammatory changes to potentiate the development of obesity and related metabolic problems (Fig. 9).
FIGURE 9.
Model for hypothalamic defect of autophagy in the pathogenesis of obesity and related disease. Chronic obesogenic conditions (including caloric excess and possibly aging) can reduce the function of hypothalamic autophagy, leading to IKKβ/NF-κB activation and inflammation in the hypothalamus. As a result, the hypothalamic function of regulating body weight and metabolic homeostasis is impaired, which potentiates the development of obesity and related metabolic diseases. In conjunction with the literature, oxidative stress and ER stress are likely involved in causing hypothalamic defect of autophagy, and, on the other hand, the onset of defective hypothalamic autophagy is predicted to also promote these intracellular stresses. In sum, all these changes can sustain IKKβ/NF-κB activation and related inflammation in the hypothalamus and thereby exacerbate the progression of obesity and related metabolic disease.
Autophagy is a fundamental function of cells for controlling intracellular homeostasis, and impairment of autophagy has been shown to cause or aggravate diverse types of diseases (1–13). Recently, the metabolic relevance of autophagy to the pathogenesis of T2D and related metabolic syndromes has been appreciated increasingly (18–27). However, almost all the studies were based on peripheral metabolic tissues such as liver, skeletal muscle, pancreatic β cells, and fat cells (18–27), and it remains to be explored whether autophagy in the CNS (particularly in the hypothalamus, which is known as the headquarters for metabolic regulation) could be important for metabolic physiology or disease. In this work, we found that autophagic activity is normally robust in the mediobasal region of the hypothalamus, a critical region that has been established to direct the central regulation of feeding, energy balance, body weight, and systemic glucose/insulin homeostasis (28, 29). Notably, chronic development of obesity under HFD feeding is evidently accompanied by reduction of autophagic activities in the mediobasal hypothalamus. Inspired by this observation, we then employed site-specific shRNA delivery approach to broadly inhibit autophagy in the mediobasal hypothalamus. An advantage of this approach is that it can delineate the overall effect of autophagy inhibition across the mediobasal hypothalamus. Also, because we applied shRNA-directed gene knockdown to adult animals, it eliminated the potential confounding effects of defective autophagy on hypothalamic development. The results reveal that defective autophagy in the mediobasal hypothalamus can lead to overeating, impaired energy expenditure, increased weight gain, as well as systemic glucose intolerance and insulin resistance. In general, the findings are in line with the overall observations in neuroscience research that autophagy dysfunctions of the brain are associated with neurodegenerative disorders (11–13). It also aligns with research that has shown that autophagy in the peripheral metabolic tissues is important for metabolic homeostasis of the body (18–25). On the other hand, we recognize that the hypothalamus is highly heterogeneous and that the mediobasal hypothalamus contains diverse neuronal subpopulations, including proopiomelanocortin (POMC) neurons and agouti-related protein (AGRP) neurons, which have opposite actions in regulation of feeding and body weight. The possibility exists that autophagic defects in POMC neurons versus AGRP neurons may have different effects on obesity and related diseases. This possibility can be more predictable if autophagic changes might have effects on neurogenesis in the hypothalamus of adult animals. Despite these remaining issues, the findings in this work have clearly shown that the net outcome of adulthood-onset, non-selective autophagic inhibition in the mediobasal region of the hypothalamus is obesogenic and pro-diabetic.
Research during the past several years has revealed that hypothalamic inflammation can act as a causal factor for the development of energy imbalance, obesity, and T2D (30–40). In this study, we found that defect of hypothalamic autophagy leads to hypothalamic inflammation via activation of the IKKβ/NF-κB pathway. Further, we showed that inhibition of IKKβ/NF-κB in the brain is able to significantly abrogate the obesogenic effect of defective hypothalamic autophagy. Such an induction of hypothalamic inflammation by autophagic dysfunction is accountable, at least partly, for the effect of defective hypothalamic autophagy in promoting obesity. Indeed, the inhibitory effect of autophagy on inflammation in the hypothalamus is in line with recent observations in tumor cells or immune cells (41–45). For example, increased NF-κB activation was found in autophagy-deficient tumor cells (41, 42), and loss of autophagy in macrophages was shown to induce inflammasome activation and inflammatory cytokines (43). Conversely, induction of autophagy by energy restriction was demonstrated to reduce NF-κB activity or cytokine production in intestinal cells (44) or macrophages (45). Thus, in the context of recent literature showing the roles of endoplasmic reticulum (ER) stress and oxidative stress in causing hypothalamic inflammation (30–40), the findings here provide another intracellular event that also underlies obesogenic activation of IKKβ/NF-κB and obesity development. On the other hand, we should point out that induction of defective autophagy in the hypothalamus requires long-term (4–5 months) HFD feeding, and this time course seems to be also necessary for the induction of hepatic autophagic defect by HFD feeding (19). Thus, the mechanism of hypothalamic autophagic defect is mostly applicable for middle-/late-stage rather than early-stage obesity development.
Question still remains regarding how a defect of hypothalamic autophagy is induced under chronic HFD feeding. We have noted that in the literature oxidative stress and ER stress were shown to cause autophagic dysfunction in certain tissues and cells (2). In conjunction with our recent work showing the connection between ER stress and IKKβ/NF-κB in the hypothalamus (30), we predict that intracellular stresses, such as ER stress and also oxidative stress, can contribute to the functional decline of hypothalamic autophagy at multiple steps (Fig. 9). In addition, it is worthwhile to mention that abnormal mammalian target of rapamycin (mTOR) activation and hyperinsulinemia have been reported to mediate autophagic inhibition in peripheral tissues, such as the liver, under the conditions of obesity (2, 20, 49). Thus, it is also possible that the induction of defective hypothalamic autophagy under chronic HFD feeding is attributed to hyperinsulinemia and perhaps even hyperleptinemia because leptin can also activate hypothalamic mTOR (47). Overall, although future research is anticipated to investigate the detailed mechanisms of defective hypothalamic autophagy in an obesogenic environment, this study can provide an initial set of evidence to indicate that defective hypothalamic autophagy significantly promotes the central pathogenesis of obesity and related problems and that the underlying molecular basis at least involves the induction of proinflammatory IKKβ/NF-κB pathway.
Acknowledgments
We thank the members of the Cai laboratory for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK078750 and R01 AG031774 (to D. C.). This work was also supported by the internal support of the Albert Einstein College of Medicine (to D. C.). Part of this work was presented in 71st Annual Meeting of the American Diabetes Association.
- T2D
- type 2 diabetes
- IKKβ
- IκB kinase β
- HFD
- high-fat diet
- LC3
- light chain 3
- MBH
- mediobasal hypothalamus
- ER
- endoplasmic reticulum
- POMC
- proopiomelanocortin
- AGRP
- agouti-related protein
- mTOR
- mammalian target of rapamycin.
REFERENCES
- 1. Yorimitsu T., Klionsky D. J. (2005) Cell Death. Differ. 12, 1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine B., Kroemer G. (2008) Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shintani T., Klionsky D. J. (2004) Science 306, 990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirkegaard K., Taylor M. P., Jackson W. T. (2004) Nat. Rev. Microbiol. 2, 301–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levine B. (2005) Cell 120, 159–162 [DOI] [PubMed] [Google Scholar]
- 6. Levine B., Deretic V. (2007) Nat. Rev. Immunol. 7, 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathew R., Karantza-Wadsworth V., White E. (2007) Nat. Rev. Cancer 7, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka Y., Guhde G., Suter A., Eskelinen E. L., Hartmann D., Lüllmann-Rauch R., Janssen P. M., Blanz J., von Figura K., Saftig P. (2000) Nature 406, 902–906 [DOI] [PubMed] [Google Scholar]
- 9. Jia K., Levine B. (2007) Autophagy 3, 597–599 [DOI] [PubMed] [Google Scholar]
- 10. Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M., Omiya S., Mizote I., Matsumura Y., Asahi M., Nishida K., Hori M., Mizushima N., Otsu K. (2007) Nat. Med. 13, 619–624 [DOI] [PubMed] [Google Scholar]
- 11. Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. (2006) Nature 441, 880–884 [DOI] [PubMed] [Google Scholar]
- 12. Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. (2006) Nature 441, 885–889 [DOI] [PubMed] [Google Scholar]
- 13. Lee J. A., Gao F. B. (2009) J. Neurosci. 29, 8506–8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., Kominami E., Tanaka K., Chiba T. (2005) J. Cell Biol. 169, 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mortensen M., Ferguson D. J., Edelmann M., Kessler B., Morten K. J., Komatsu M., Simon A. K. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 832–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pua H. H., Dzhagalov I., Chuck M., Mizushima N., He Y. W. (2007) J. Exp. Med. 204, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takamura A., Komatsu M., Hara T., Sakamoto A., Kishi C., Waguri S., Eishi Y., Hino O., Tanaka K., Mizushima N. (2011) Genes Dev. 25, 795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim J. S., Nitta T., Mohuczy D., O'Malley K. A., Moldawer L. L., Dunn W. A., Jr., Behrns K. E. (2008) Hepatology 47, 1725–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang L., Li P., Fu S., Calay E. S., Hotamisligil G. S. (2010) Cell Metab. 11, 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu H. Y., Han J., Cao S. Y., Hong T., Zhuo D., Shi J., Liu Z., Cao W. (2009) J. Biol. Chem. 284, 31484–31492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A. M., Czaja M. J. (2009) Nature 458, 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. (2009) Cell Metab. 10, 507–515 [DOI] [PubMed] [Google Scholar]
- 23. Fujitani Y., Kawamori R., Watada H. (2009) Autophagy 5, 280–282 [DOI] [PubMed] [Google Scholar]
- 24. Ebato C., Uchida T., Arakawa M., Komatsu M., Ueno T., Komiya K., Azuma K., Hirose T., Tanaka K., Kominami E., Kawamori R., Fujitani Y., Watada H. (2008) Cell Metab. 8, 325–332 [DOI] [PubMed] [Google Scholar]
- 25. Jung H. S., Chung K. W., Won Kim J., Kim J., Komatsu M., Tanaka K., Nguyen Y. H., Kang T. M., Yoon K. H., Kim J. W., Jeong Y. T., Han M. S., Lee M. K., Kim K. W., Shin J., Lee M. S. (2008) Cell Metab. 8, 318–324 [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y., Goldman S., Baerga R., Zhao Y., Komatsu M., Jin S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 19860–19865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh R., Xiang Y., Wang Y., Baikati K., Cuervo A. M., Luu Y. K., Tang Y., Pessin J. E., Schwartz G. J., Czaja M. J. (2009) J. Clin. Invest. 119, 3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elmquist J. K., Flier J. S. (2004) Science 304, 63–64 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz M. W., Porte D., Jr. (2005) Science 307, 375–379 [DOI] [PubMed] [Google Scholar]
- 30. Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. (2008) Cell 135, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Purkayastha S., Zhang H., Zhang G., Ahmed Z., Wang Y., Cai D. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 2939–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi S. J., Kim F., Schwartz M. W., Wisse B. E. (2010) Am. J. Physiol. Endocrinol. Metab. 298, E1122-E1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oh-I S., Thaler J. P., Ogimoto K., Wisse B. E., Morton G. J., Schwartz M. W. (2010) Am. J. Physiol. Endocrinol. Metab. 299, E47-E53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Posey K. A., Clegg D. J., Printz R. L., Byun J., Morton G. J., Vivekanandan-Giri A., Pennathur S., Baskin D. G., Heinecke J. W., Woods S. C., Schwartz M. W., Niswender K. D. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E1003-E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kleinridders A., Schenten D., Könner A. C., Belgardt B. F., Mauer J., Okamura T., Wunderlich F. T., Medzhitov R., Brüning J. C. (2009) Cell Metab. 10, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ozcan L., Ergin A. S., Lu A., Chung J., Sarkar S., Nie D., Myers M. G., Jr., Ozcan U. (2009) Cell Metab. 9, 35–51 [DOI] [PubMed] [Google Scholar]
- 37. De Souza C. T., Araujo E. P., Bordin S., Ashimine R., Zollner R. L., Boschero A. C., Saad M. J., Velloso L. A. (2005) Endocrinology 146, 4192–4199 [DOI] [PubMed] [Google Scholar]
- 38. Belgardt B. F., Mauer J., Wunderlich F. T., Ernst M. B., Pal M., Spohn G., Brönneke H. S., Brodesser S., Hampel B., Schauss A. C., Brüning J. C. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 6028–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sabio G., Cavanagh-Kyros J., Barrett T., Jung D. Y., Ko H. J., Ong H., Morel C., Mora A., Reilly J., Kim J. K., Davis R. J. (2010) Genes Dev. 24, 256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Unger E. K., Piper M. L., Olofsson L. E., Xu A. W. (2010) Endocrinology 151, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mathew R., Karp C. M., Beaudoin B., Vuong N., Chen G., Chen H. Y., Bray K., Reddy A., Bhanot G., Gelinas C., Dipaola R. S., Karantza-Wadsworth V., White E. (2009) Cell 137, 1062–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moscat J., Diaz-Meco M. T. (2009) Cell 137, 1001–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saitoh T., Fujita N., Jang M. H., Uematsu S., Yang B. G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., Tanaka K., Kawai T., Tsujimura T., Takeuchi O., Yoshimori T., Akira S. (2008) Nature 456, 264–268 [DOI] [PubMed] [Google Scholar]
- 44. Fujishima Y., Nishiumi S., Masuda A., Inoue J., Nguyen N. M., Irino Y., Komatsu M., Tanaka K., Kutsumi H., Azuma T., Yoshida M. (2011) Arch. Biochem. Biophys. 506, 223–235 [DOI] [PubMed] [Google Scholar]
- 45. Crişan T. O., Plantinga T. S., van de Veerdonk F. L., Farcaş M. F., Stoffels M., Kullberg B. J., van der Meer J. W., Joosten L. A., Netea M. G. (2011) PLoS ONE 6, e18666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Gouill E., Jimenez M., Binnert C., Jayet P. Y., Thalmann S., Nicod P., Scherrer U., Vollenweider P. (2007) Diabetes 56, 2690–2696 [DOI] [PubMed] [Google Scholar]
- 47. Morris D. L., Rui L. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E1247-E1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delhase M., Hayakawa M., Chen Y., Karin M. (1999) Science 284, 309–313 [DOI] [PubMed] [Google Scholar]
- 49. Yin X. M., Ding W. X., Gao W. (2008) Hepatology 47, 1773–1785 [DOI] [PubMed] [Google Scholar]