Abstract
In this work, we set out to identify and characterize the calcium occluded intermediate(s) of the plasma membrane Ca2+-ATPase (PMCA) to study the mechanism of calcium transport. To this end, we developed a procedure for measuring the occlusion of Ca2+ in microsomes containing PMCA. This involves a system for overexpression of the PMCA and the use of a rapid mixing device combined with a filtration chamber, allowing the isolation of the enzyme and quantification of retained calcium. Measurements of retained calcium as a function of the Ca2+ concentration in steady state showed a hyperbolic dependence with an apparent dissociation constant of 12 ± 2.2 μm, which agrees with the value found through measurements of PMCA activity in the absence of calmodulin. When enzyme phosphorylation and the retained calcium were studied as a function of time in the presence of LaIII (inducing accumulation of phosphoenzyme in the E1P state), we obtained apparent rate constants not significantly different from each other. Quantification of EP and retained calcium in steady state yield a stoichiometry of one mole of occluded calcium per mole of phosphoenzyme. These results demonstrate for the first time that one calcium ion becomes occluded in the E1P-phosphorylated intermediate of the PMCA.
Keywords: ATPases, Calcium ATPase, Calcium Transport, Membrane Proteins, Phosphorylation, Calcium Occlusion, Plasma Membrane Calcium ATPase
Introduction
The plasma membrane calcium ATPase (PMCA)3 is a calmodulin-modulated P-type ATPase responsible for the maintenance of low intracellular concentrations of Ca2+ in most eukaryotic cells. It couples the transport of Ca2+ out of cells with the hydrolysis of ATP into ADP and inorganic phosphate. PMCAs consist of a single polypeptide chain of 127,000 to 137,000 Da. Mammalian PMCAs are encoded by four separate genes (PMCA1–4), and additional isoforms are generated via alternative RNA splicing, which augments the number of variants to >20 (1).
The current kinetic model for PMCA function proposes that the enzyme exists in two main conformations, E1 and E2. E1 has a high affinity for Ca2+ and is readily phosphorylated by ATP, whereas E2 has a low affinity for Ca2+ and can be phosphorylated by Pi. After binding of intracellular Ca2+ to high affinity sites, E1 can be phosphorylated by ATP with formation of the intermediate E1P. After a conformational transition to E2P, Ca2+ would be released to the extracellular medium from low affinity sites, followed by the hydrolysis of the phosphoenzyme to E2 and a new conformational transition to E1 (Fig. 1) (2). During some stages of the reaction cycle, Ca2+ becomes occluded, i.e. trapped in the enzyme machinery while it is transported from one side to the other side of the membrane. The principal aim of this study is to identify and kinetically characterize the intermediate(s) of the PMCA containing occluded calcium. Evidence for occlusion in Na,K-ATPase and sarcoplasmic reticulum Ca2+-ATPase (SERCA) has been well established (3), and a good deal of information exists about the occlusion and deocclusion steps of the transported cations in these pumps. Na+ and K+ are occluded in the E1P and E2 intermediates of the Na+/K+-ATPase (4), respectively, and Ca2+ becomes occluded in the E1P intermediate of the SERCA (5).
FIGURE 1.
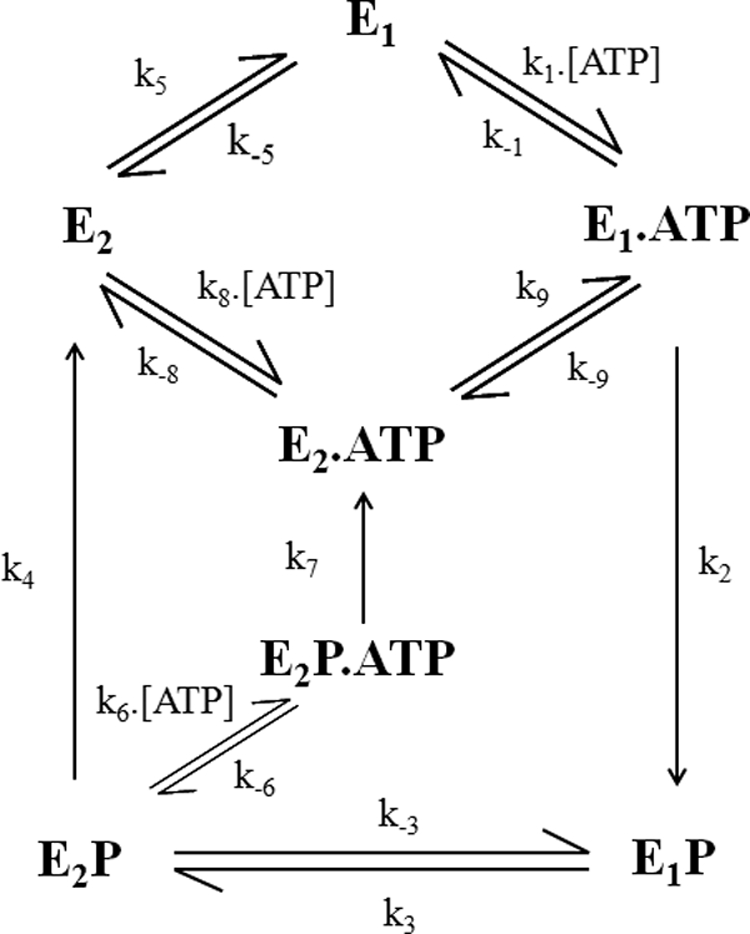
Kinetic model of the PMCA proposed by Rega and Garrahan (2).
By analogy with SERCA, one would expect (5) that Ca2+ occlusion occurs in the E1P state of the PMCA. However, it has not been possible until now to obtain definitive experimental evidence of such a phenomenon, mainly because PMCA in microsomal preparations obtained from natural sources is not sufficiently abundant and pure. To overcome this difficulty, we have used a procedure to overexpress PMCA in Sf9 insect cells using a baculovirus system and also to isolate the microsomal membranes. Binding of Ca2+ to these membranes was measured as a function of time using a method (6) that combines a quench-flow apparatus with a rapid filtration device, with a time resolution of 3.5 ms. The procedure included inhibition of endogenous SERCA and the permeabilization of microsomal vesicles using the pore-forming peptide alamethicin, which prevents 45Ca2+ accumulation (7). Our results suggest that a single calcium ion is occluded in E1P of the PMCA and that the formation of this phosphoenzyme and the occlusion of calcium are simultaneous events.
EXPERIMENTAL PROCEDURES
Expression of PMCA in Sf9 Cells
The Sf9 cells (derived from pupal ovarian tissue of the fall armyworm Spodoptera frugiperda) were grown in suspension at 27 °C in Grace Medium supplemented with 10% fetal bovine serum, 1% Pluronic, and 1× antibiotic-antimycotic (Invitrogen, catalog no. 15240-062). The expression for protein production was carried out by infecting Sf9 cells in suspension in complete Grace Medium with the recombinant virus at a multiplicity of infection of 1–2. After 48 h of incubation at 27 °C in the dark, the cells were harvested. A 250-ml culture gave 250·106 cells. The cells were washed with phosphate-buffered saline buffer containing 1 mm EDTA and protease inhibitors, quickly frozen, and then kept at −80 °C until microsome processing.
Recombinant Baculovirus
The viral stocks for expression of human PMCA4b have been described (8) and were kindly provided by Ariel J. Caride and Adelaida G. Filoteo (Mayo Clinic, Rochester, MN).
Amplification of Recombinant Baculovirus
The viral stocks were amplified using a multiplicity of infection of 0.1–0.2 following standard procedures, and the titer of the amplified stock was determined. The viral stock was kept at 4 °C in the dark.
Microsomal Preparation
Crude microsomal membranes from Sf9 cells were prepared essentially as described for COS cells with some minor modifications (9). After washing the cells with phosphate-buffered saline containing increased amounts of protease inhibitors, 10 μg/ml aprotinin, and 4 μg/ml leupeptin, the cell pellets were immediately frozen in aliquots of 250·106 cells until processing. The volumes of buffers used for homogenizing the relatively large cell pellets were also increased two or three times to maintain the concentrations required to promote formation of inside-out vesicles. Per 250·106 cells, 7 ml of hypotonic and 7 ml of homogenization buffers were used. Aliquots of microsomes were stored in liquid nitrogen.
Reagents and Reaction Conditions
(45Ca)CaCl2, [γ-32P]ATP, and 125I were obtained from Perkin-Elmer Life Sciences. All other reagents were of analytical grade.
All experiments were performed at 25 °C in medium containing 30 mm MOPS (pH 7.4 at 25 °C), 120 mm KCl, 3 mm MgCl2, 200 nm thapsigargin, and enough CaCl2 to obtain the concentration of free Ca2+ indicated in the figures. The concentrations of other components varied according to the experiments and are indicated in the figure legends. For measuring time courses of phosphoenzyme and Ca2+ retained in the millisecond-second timescale, we used a rapid mixing apparatus SFM4 from Bio-Logic.
Measurement of Free Ca2+ Concentrations
The Ca2+ concentration in the incubation medium was measured using a selective Ca2+ electrode (93–20, Orion Research, Inc.), as described by Kratje et al. (10).
Thapsigargin Treatment
Thapsigargin (octanoic acid derivative of azulene[4,5-b]furan) was obtained from Sigma (catalog no. T9033). Thapsigargin was dissolved in dimethyl sulfoxide to a concentration of 153.6 μm. Dilutions of this solution were added to a suspension containing the protein. The final concentration of dimethyl sulfoxide never exceeded 0.1% in volume. Controls of Ca2+-ATPase activity with and without dimethyl sulfoxide showed no significant differences. In agreement with Sagara et al. (11), we found that inhibition of SERCA activity was achieved after a 15-min incubation of the microsomal preparation with 200 nm thapsigargin at 25 °C. This inhibition lasted for at least 3 min after addition of Ca2+. Therefore, all experiments were performed within this time frame.
Measurements of ATPase Activity
This was measured as the amount of [32P]Pi released from [γ-32P]ATP, according to a slight modification of the method described by Schwarzbaum et al. (12). Incubation time was short enough to prevent the hydrolysis of >10% of the ATP present and to ensure initial rate conditions. Enzyme concentration was 60 μg of total protein/ml, and blanks were included in an assay in the same medium without Ca2+ in the presence of 1 mm EGTA. The medium contained 5 mm NaN3 and 0.5 mm ouabain.
Measurements of Calcium Retained
Ca2+ retained was measured using the method of Rossi et al. (6) where the rapid mixing apparatus is connected to a quenching-and-washing chamber (supplemental Fig. S1), through a suitable polyethylene tubing. In a typical experiment, one volume of a microsomal preparation suspended in a solution with 30 mm MOPS (pH 7.4 at 25 °C), 120 mm KCl, and 400 nm thapsigargin was mixed with the same volume of a solution containing the same concentrations of MOPS and KCl, plus 6 mm MgCl2 and enough ATP and (45Ca2+)CaCl2 to obtain the concentrations of the nucleotide and of free Ca2+ indicated in the figures. For some experiments, 100 μm LaIII was also included in the latter solution. Measurements were carried out at 25 °C. Reactions were quenched after the appropriate time by injecting the reaction mixture into the quenching-and-washing chamber at a flow rate of 1–5 ml. s−1. During the injection process, the fluid was mixed with an ice-cold washing solution flowing at a rate of 30–40 ml/s and then filtered through a Millipore filter (AA, 0.8-μm pore size) placed in the quenching-and-washing chamber to retain the microsomal suspension that includes the enzyme. From control experiments using a microsomal preparation covalently labeled with [125I]TID-PC ([125I]TID (3-(trifluromethyl)-3-(m-[125I]iodophenyl)diazirin)) (13), and measuring the radioactivity on the Millipore filters of 0.22–0.80-μm pore size after a quenching and washing run, at least 99% of the labeled enzyme was recovered irrespective of the filter pore size.
To ensure that the initial temperature in the quenching-and-washing chamber was 1–2 °C and that the flow was constant, ∼50 ml of washing solution was allowed to run through the filter prior to the injection of the reaction mixture, and 240 ml of washing solution was applied to the filter from that moment. The composition of the washing solution was 10 mm Tris, 10 mm EDTA, pH 7.4, at 2 °C. Control experiments show that the washing procedure effectively removes the unbound 45Ca2+. After the washing solution was drained, the filter was removed, dried under a lamp, and counted for 45Ca2+ radioactivity in a scintillation counter. This was converted into nanomoles of Ca2+ using the specific activity value of the 45Ca2+ in the reaction mixture. Retained Ca2+ was considered equal to the 45Ca2+ radioactivity retained by the enzyme after subtracting the blank values. These were estimated from the amount of 45Ca2+ retained by the filters in the presence of enzyme that was heat-inactivated for 2 h at 50 °C (see supplemental Figs. S2 and S3).
Determination of Phosphorylated Intermediates
The phosphorylated intermediates (EP), were measured as the amount of acid-stable 32P incorporated in the enzyme from [γ-32P]ATP after stopping the reaction with an ice-cold solution containing 10% trichloroacetic acid. The isolation of the intermediate was performed according to two methodologies.
In the first methodology, the suspension was transferred to Millipore filters (Type GS, 0.22 μm pore size) where the phosphoenzyme was washed with 20 ml of a solution of 10% trichloroacetic acid and 50 mm H3PO4. For the blanks, similar experiments were done with heat-inactivated enzyme.
In the second methodology, when the microsomes were phosphorylated with high concentration of ATP, we used the method described by Echarte et al. (14). The phosphorylation reaction was stopped, and the tubes were spun down at 7000 rpm for 3.5 min at 4 °C. The samples were then washed once with 7% TCA, 150 mm H3PO4, and once with double-distilled water and processed for SDS-PAGE. For this purpose, the pellets were dissolved in a medium containing 150 mm Tris-HCl (pH 6.5 at 14 °C), 5% SDS, 5% DTT, 10% glycerol, and bromphenol blue (sample buffer). Electrophoresis was performed at pH 6.3 (14 °C) in a 7.5% polyacrylamide gel. The reservoir buffer was 175 mm MOPS, pH 6.5, with 0.1% SDS. Migration of the sample components took place at 14 °C, with a current of 60 mA until the tracking dye reached a distance of ∼10 cm from the top of the gel. Gels were stained, dried, and exposed to a Storage Phosphoscreen of Molecular Dynamics (Amersham Biosciences). Unsaturated autoradiograms and stained gels were scanned with an HP Scanjet G2410 scanner. Analysis of the images was performed with GelPro Analyzer. EP quantification was achieved as described in Echarte et al. (14).
Alamethicin Treatment
Alamethicin was obtained from Sigma (catalog no. A4665). This peptide was dissolved in 60% (v/v) ethanol to a concentration of 20 mg/ml (7). This solution was added to a suspension containing the protein. Final concentrations of alamethicin added to the microsomes are expressed on a weight basis relative to microsomal protein. The final ethanol concentration of the microsomes after adding alamethicin never exceeded 0.1%, and for all of the experiments, an equivalent amount of ethanol was added to control membranes not treated with alamethicin.
Data Analysis
Theoretical equations were fitted to the results by nonlinear regression based on the Gauss-Newton algorithm using commercial programs (Excel and Sigma-Plot for Windows, the latter being able to provide not only the best fitting values of the parameters but also their S.E.). The goodness of fit of a given equation to the experimental results was evaluated by the corrected AIC criterion defined in Equation 1,
where N is the number of data, P is the number of parameters plus one, and SS is the sum of weighted square residual errors (15). Unitary weights were considered in all cases, and the best equation was chosen as that giving the lower value of AICC. The AIC criterion is based on information theory and selects an equation among several possible equations on the basis of its capacity to explain the results using a minimal number of parameters.
RESULTS
Calcium Retained by Microsomal Vesicles Expressing PMCA
According to earlier findings on SERCA (16), one would expect to observe occlusion of Ca2+ in PMCA as a fast phase early in the time course of 45Ca2+ uptake by microsomal vesicles.
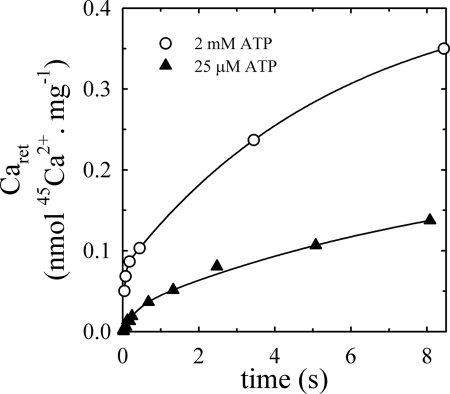
Fig. 2 shows the amount of 45Ca2+ retained by microsomal vesicles (Caret) measured as a function of time in media with 25 μm or 2000 μm ATP, 3 mm MgCl2, and 60 μm [45Ca] Ca2+ at 25 °C. For both concentrations of ATP, the time course can be described by the sum of two increasing exponential functions of time,
where A1 and A2 are maximal amounts of Caret and k1 and k2 are rate coefficients. The best fitting values of the parameters are shown in Table 1, where it appears that A1 and k1, as well as k2 increase with the concentration of ATP. If one assumes that the fast component is due to the occlusion of Ca2+ whereas the slow one reflects the accumulation of Ca2+ into vesicles of the microsomal preparation, A1 should be on the order of the amount of Ca2+-ATPase, while A2 (but not necessarily A1) should decrease upon addition of a permeabilizing agent. Results of experiments to test these predictions are described in the following paragraphs.
FIGURE 2.
Time course of retained calcium by microsomal preparations of PMCA at 25 μm or 2 mm ATP. Measurements of retained calcium by hPMCA4b were carried out at 25 °C in a reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 0.2 μm thapsigargin (△), 25 μm or 2000 μm ATP (○), and enough (45Ca)CaCl2 to give concentrations of 100 μm free Ca2+.
TABLE 1.
Best fitting values of the parameters of Equation 2 adjusted to the data in Fig. 2
| Parameters | 25 μm ATP | 2000 μm ATP |
|---|---|---|
| A1 (nmol Ca2+·mg−1) | 0.0269 ± 0.0041 | 0.07686 ± 0.00001 |
| k1 (s−1) | 2.963 ± 0.562 | 23.48 ± 0.01 |
| A2 (nmol Ca2+·mg−1) | 0.1939 ± 0.031 | 0.3541 ± 0.0001 |
| k2 (s−1) | 0.1045 ± 0.0291 | 0.1746 ± 0.0001 |
Enzyme Concentration
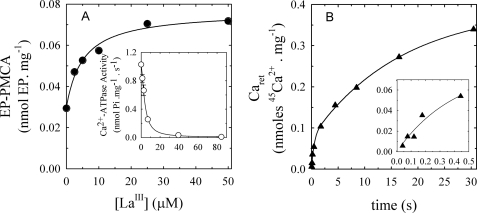
LaIII is known to prevent the Mg2+-dependent transition E1P→E2P, acting noncompetitively with respect to Ca2+ and ATP (17, 18). Thus, the amount of PMCA can be evaluated by measuring the concentration of enzyme phosphorylated from [γ-32P]ATP in the presence of this inhibitor, producing accumulation of E1P with the inhibition of the ATPase activity (17, 18).
Fig. 3A shows the amount of phosphorylated intermediates, EP (= [E1P] + [E2P]), as a function of [LaIII]. EP increased with the concentration of LaIII along the following hyperbolic function,
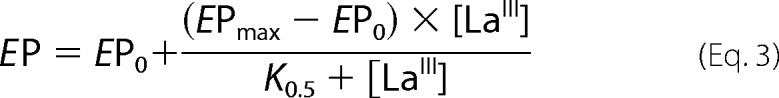 |
where EP0 and EPmax are the amounts of PMCA phosphorylated in the absence of lanthanum and in the presence of nonlimiting concentrations of the inhibitor, respectively, and K0.5 is the concentration of LaIII at which Ep = (EPmax + EP0)/2. The best fitting values for EP0, EPmax, and K0.5 were 0.030 ± 0.002 nmol EP·mg−1, 0.0765 ± 0.0024 nmol EP·mg−1, and 5.25 ± 1.33 μm, respectively. In the inset of Fig. 3A, we plotted Ca2+-ATPase activity as a function of the concentration of LaIII. The best fit to the experimental data were a hyperbolic decreasing function (see legend to Fig. 3), where the Ki for LaIII was 3.5 ± 0.8 μm (cf. with the value of K0.5(La) for EP formation). The time course of Caret in the presence of 50 μm LaIII was measured under similar conditions, although using a different preparation (Fig. 3B). Best fitting values using equation 2 were A1 = 0.0774 ± 0.0072 nmol·mg−1, A2 = 0.314 ± 0.014 nmol·mg−1, k1 = 2.17 ± 0.38 s−1, and k2 = 0.059 ± 0.007 s−1, respectively. Note that the value of A1 is of the same order of magnitude as that of EPmax above, which can be taken as evidence that the first fast phase of the time course of calcium retained is due to the occlusion of Ca2+ in the PMCA. Moreover, although LaIII almost completely inhibits the ATPase activity, it does not significantly decrease the fraction of the slow phase in the time course of Ca2+ retained.
FIGURE 3.
Determination of amount of active PMCA and calcium retained in microsomes. A, dependence of PMCA phosphorylation on lanthanum concentration. The PMCA phosphorylation was measured at 25 °C in reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 25 μm ATP, and enough (45Ca)CaCl2 to give 100 μm of free Ca2+. Inset, Ca2+-ATPase activity as a function of [LaIII] was measured in the same conditions as phosphorylation. The continuous line corresponds to a plot of A = (A0 × Ki)/(Ki + [LaIII]), where A is the Ca2+-ATPase activity, A0 is the Ca2+-ATPase activity in the absence of lanthanum, [LaIII] is lanthanum concentration, and Ki is the inhibitory constant for lanthanum. B, time course of calcium retained by microsomal preparations of PMCA. Measurements of calcium retained by hPMCA4b were carried out at 25 °C in reaction medium containing 60 μg/ml total protein, 0.2 μm thapsigargin, 3 mm MgCl2, 25 μm ATP; 50 μm LaCl3, and enough (45Ca)CaCl2 to give concentrations of 100 μm free Ca2+. The inset shows the data for the initial 0.5 s at an expanded scale.
Effect of Alamethicin on Ca2+ Accumulation by Microsomes
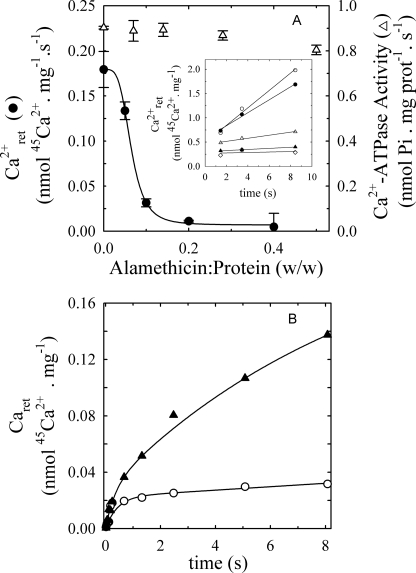
If the slow phase of the time course in Figs. 2 and 3B is due to the uptake of Ca2+ into vesicles, addition of permeabilizing agents should facilitate the washing out of Ca2+ in the quenching and washing chamber and accordingly remove a possible confounding factor for the detection of Ca2+-occluded states in the PMCA. We tested this by treating the microsomal membranes with alamethicin, a peptide that forms large pores allowing the passage of organic molecules of considerable size such as ATP. Alamethicin has been used previously in transport studies of the SERCA (7) and determinations of occlusion of Rb+ in the H+/K+-ATPase (19).
Following incubation of microsomal membranes with different concentrations of alamethicin for 30 min at 25 °C, retained calcium was measured as a function of time from 1.5 to 8.5 s (Fig. 4A, inset). In Fig. 4A, we plotted both the Ca2+-ATPase activity and the slopes of the curves of retained calcium versus time as a function of the alamethicin concentration. The data show that, as [alamethicin] increases, the slope decreases, whereas the PMCA activity remains nearly constant, at least up to 0.3 mg of alamethicin per mg of total protein. In a similar experiment (data not shown), we found that it was safe to use this peptide in a concentration of 0.4 mg per mg of total protein without affecting the enzyme activity. Based on these results, we performed subsequent experiments adding alamethicin at a concentration of 0.2–0.4 mg per mg of total protein. This substantially reduced (but did not totally eliminate) the interference due to the microsomal uptake and accumulation of Ca2+ in our measurements of Caret. As a further refinement of these measurements we also took into account a slow residual linear component of bound Ca2+ that was observed as well for heat inactivated enzyme and was therefore subtracted as background (supplemental Fig. S2).
FIGURE 4.
Effects of alamethicin on calcium retained and ATPase activity in PMCA4b-expressing microsomes. A, effect of alamethicin on the velocity of calcium retention and Ca2+-ATPase activity. The retained calcium was measured at 25 °C in reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 2000 μm ATP, and enough (45Ca)CaCl2 to give concentrations of 60 μm free Ca2+. The inset shows the calcium retained over time as a function of different ratios of alamethicin:total protein. Each slope line represents the velocity of calcium retention at a given alamethicin:protein ratio. Measurements of Ca2+-ATPase activity were carried out at 25 °C in medium containing 60 μg/ml total protein, 3 mm MgCl2, 2000 μm ATP, and enough CaCl2 to give concentrations of 60 μm free Ca2+. B, time course of calcium retained by microsomal preparations of hPMCA4b treated with or without alamethicin. The amount of bound calcium was measured at 25 °C in medium containing 60 μg/ml total protein, 3 mm MgCl2, 25 μm ATP without (▴) or with (○) 12 μg/ml alamethicin and enough (45Ca)CaCl2 to give concentrations of 60 μm free Ca2+.
Fig. 4B compares the time course of calcium retained measured either in the absence of alamethicin or in the presence of 0.2 mg alamethicin per mg of total protein. Notice that LaIII was not present in this experiment. Although the time course with no added alamethicin shows a behavior similar to that in Fig. 2 and can be described by the same function of time, results obtained in the presence of alamethicin are well fitted by a single increasing exponential function of time plus a slow linear component. The faster exponential component of both curves has similar values (0.0269 ± 0.0041 nmol Ca2+ ·mg−1 in the absence and 0.0224 ± 0.0012 nmol Ca2+·mg−1 in the presence of alamethicin) and rate coefficient (2.963 ± 0.764 s−1 in the absence and 2.336 ± 0.439 s−1 in the presence of alamethicin). This indicates that alamethicin permits efficient washing of the permeabilized vesicles without affecting the capacity of PMCA to bind calcium.
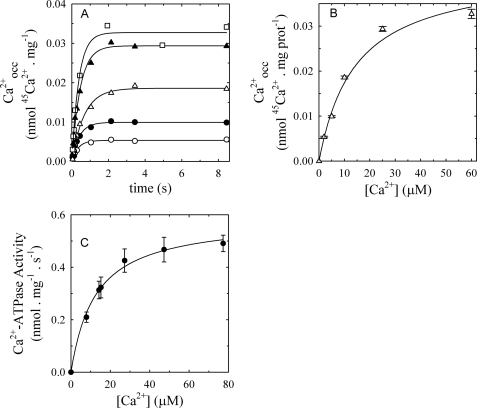
Steady-state Level of Calcium Occluded and Ca2+-ATPase Activity as a Function of [Ca2+]
If the retained calcium that accumulates in the presence of alamethicin corresponded to a reaction intermediate of the PMCA, its steady-state amount should vary with the concentration of a substrate with the same affinity as that of the ATPase activity. To test this, we measured the amount of retained calcium in the steady state (Fig. 5, A and B) as well as the Ca2+-ATPase activity (Fig. 5C) as a function of [Ca2+]. Fig. 5A shows the time courses of retained calcium, after subtracting the small linear component due to nonspecific binding (see supplemental Fig. S3), measured at different [Ca2+]. All the curves can be described by a single increasing exponential function of time,
where A is the steady-state amount of the “specific” retained Ca2+, and k is an apparent rate coefficient. The best fitting values of A from Fig. 5A were plotted as a function of [Ca2+] as shown in Fig. 5B.
FIGURE 5.
Steady-state level of retained calcium and Ca2+-ATPase activity as a function of [Ca2+]. A, the retained calcium was measured at 25 °C in reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 25 μm ATP, 12 μg/ml alamethicin, and enough (45Ca)CaCl2 to give 2 μm free Ca2+ (○); 5 μm free Ca2+ (●); 10 μm free Ca2+ (△); 25 μm free Ca2+ (▴); and 60 μm free Ca2+ (□). B, plot of the values of maximal retained Ca2+ as a function of the free Ca2+ concentration as obtained from an analysis of the data in A. C, Ca2+-ATPase activity measurements were carried out at 25 °C in reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 25 μm ATP, and 12 μg/ml alamethicin enough CaCl2 to give different concentrations of free Ca2+.
Both the value of A from Equation 4 and the Ca2+-ATPase activity can be described by a rectangular hyperbola as a function of [Ca2+],
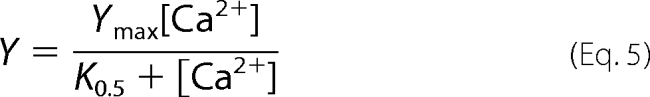 |
where Ymax is the Ca2+-ATPase activity or the value of A when the calcium concentration tends to infinity, and K0.5 represents the [Ca2+] at which the half-maximum effect is achieved. The best fitting values of K0.5 were 12.5 ± 1.3 μm and 12.7 ± 2.2 μm for activity and occluded calcium, respectively. The fact that these values are not significantly different from each other indicates that the retained calcium measured in media with alamethicin is due to an intermediate of the reaction cycle, probably that containing occluded calcium.
Stoichiometry of Occluded Calcium
As shown in Fig. 3, the amount of PMCA, evaluated as the maximal concentration of EP that accumulates as E1P in the presence of LaIII, is of the same order of magnitude as the size of the fast component of the time course of Caret. Because the yield of PMCA expressed in Sf9 cells varies between different preparations, a strictly quantitative comparison between Caret and EP requires performing experiments using the same preparation under the same conditions. Results of parallel experiments in media with LaIII measuring steady-state levels of both EP and Ca2+ occluded, and the ratio Caocc/EP are shown in Table 2 for two different microsomal preparations. Although the values of both experimentally determined levels vary between the preparations, the ratio Caocc/EP is nearly equal to 1, which is consistent with the stoichiometry of one Ca2+ transported per molecule of ATP hydrolyzed (20–22) and with the hypothesis that, in the presence of ATP, calcium is occluded in the E1P conformation of the PMCA.
TABLE 2.
Comparison of E1P and occluded calcium
Phosphorylated PMCA was measured in steady state at 25 °C in reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 25 μm [γ-32P]ATP, 12 μg/ml alamethicin, 50 μm LaIII, and enough CaCl2 to give 60 μm of free Ca2+. The occluded calcium was measured in steady state at 25 °C in reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 25 μm ATP, 12 μg/ml alamethicin, 50 μm LaIII, and enough [45Ca]CaCl2 to give 60 μm of free Ca2+. Note that the EP for microsomes 1 was measured by the method described by Echarte et al. (14) and the EP for microsomes 2 was measured by filtration (see “Experimental Procedures”).
| Ratio CaOcc/E1P |
||
|---|---|---|
| Microsomes 1 | Microsomes 2 | |
| CaOcc (nmol·mg−1) | 0.100 ± 0.002 | 0.048 ± 0.001 |
| E1P (nmol·mg−1) | 0.095 ± 0.007 | 0.045 ± 0.003 |
| Ratio | 1.045 ± 0.103 | 1.064 ± 0.086 |
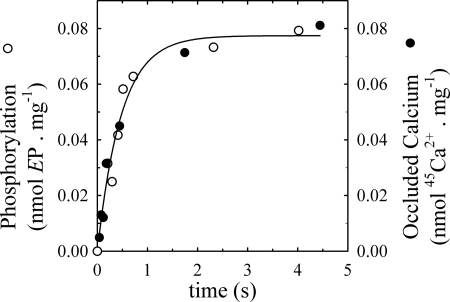
Time Course of E1P and Occluded Calcium
To determine whether calcium occlusion in the PMCA is concomitant with the formation of the E1P phosphorylated enzyme intermediate, we measured the time course of EP and calcium occlusion under the same experimental conditions. Fig. 6 shows that both phosphorylation and calcium occlusion increase simultaneously and can be described by a single exponential as a function of time adjusted to Equation 4, with best fitting values of k = 2.10 ± 0.30 s−1 and 2.09 ± 0.31 s−1, for E1P and occluded calcium, respectively. Furthermore, the data reveal a stoichiometry of 1:1 for calcium occlusion and PMCA phosphorylation over time, showing that both reactions occur concomitantly.
FIGURE 6.
Time course of phosphorylated intermediate formation and occluded calcium in the presence of lanthanum. The phosphorylation of hPMCA4b and the amount of occluded calcium were measured at 25 °C in reaction medium containing 60 μg/ml total protein, 3 mm MgCl2, 25 μm ATP, 50 μm LaIII, and enough CaCl2 to give concentrations of 60 μm free Ca2+.
DISCUSSION
This work provides the first conclusive evidence that Ca2+ becomes occluded in the plasma membrane Ca2+-ATPase, with a stoichiometry of one Ca2+ transported per ATP hydrolyzed. The evidence is based on the following findings. First, during ATPase activity, the uptake of Ca2+ by microsomes enriched in PMCA shows a time course with a fast component whose amplitude is compatible with the amount of enzyme, and a slow component, which is related to accumulation of Ca2+ into vesicles. In support of this interpretation, addition of the pore-forming peptide alamethicin tends to eliminate the slow component without affecting the fast one. Second, measurements in the presence of alamethicin and LaIII show that the time course of the fast component of Ca2+ uptake is concomitant with that of enzyme phosphorylation, with a stoichiometry of one Ca2+ retained per phosphorylation site. Third, steady-state measurements of calcium retained and ATPase activity in the presence of alamethicin exhibit the same K0.5 for Ca2+. These results indicate that Ca2+ becomes occluded in the E1P intermediate of the PMCA, with a stoichiometry of one Ca2+ per phosphorylation site, but they do not rule out the possibility that other intermediates of the reaction cycle can occlude Ca2+ as well.
Steps toward Detailed Kinetic Model for PMCA
Although there is a large body of information on the kinetics of the PMCA, most of this information has not been incorporated into a detailed unified model because of the heterogeneity of the data. The main reason for this has been the lack of a reliable standard preparation of the pump. Unlike for other P-type ATPases, there are no known tissue sources rich enough in a single isoform of PMCA. Even the purified erythrocyte preparation is a mixture of PMCA4b and PMCA1b (23). Additionally, methodological challenges have made it very difficult to obtain reliable measurements of phosphorylated species at physiological (millimolar) concentrations of ATP. One of the main differences between purified enzyme preparations from erythrocytes and the insect cell-derived recombinant enzyme preparation used in this work is that in the former case the enzyme is solubilized in detergents and included in phospholipid micelles, whereas in the latter it remains embedded in the lipid bilayer of the plasma membrane. Therefore, besides the fact that the PMCA is kept in its original environment, an important advantage of the Sf9 insect cell-expressed PMCA preparation is the possibility of isolating the enzyme containing occluded ions by filtration on Millipore-type membranes. This, plus the use of alamethicin as a permeabilizing agent for efficient washing out of unbound Ca2+, provides an excellent system to measure occlusion of this cation in the PMCA.
By analogy with Ca2+ occlusion in SERCA and Na+ occlusion in Na,K-ATPase (4, 5), Ca2+ occlusion is thought to occur in the E1P state of the PMCA. To detect Ca2+-occluded states the strategy therefore must be to work under conditions where the expected amount of E1P is maximal, and its rate of breakdown is minimal. These conditions can be deduced from kinetic studies determining the ADP-sensitive phosphoenzyme (24), but an alternative approach is to perform the reactions in the presence of LaIII (17, 18), which blocks the Mg2+-dependent E1P to E2P transition. In PMCA purified from erythrocytes, the steady-state amount of EP increases with the concentration of ATP along a nonhyperbolic curve, indicating a complex mechanism for nucleotide hydrolysis during calcium transport (14). ATP not only accelerates the dephosphorylation reaction, as reported by Garrahan and Rega (25), but it also modifies the rate of conformational transition E1P→E2P, accelerating this reaction at millimolar concentrations of Mg2+. However, in this study, we have shown that occluded Ca2+ can be detected even in the presence of 3 mm MgCl2. This raises the question whether Ca2+ occlusion could also take place in intermediates other than E1P.
Occluded Cations as Intermediates of Ca2+ Transport Cycle of PMCA
In SERCA and Na,K-ATPase pumps, studies of cation occlusion gave crucial information on how the pumps couple the exergonic hydrolysis of ATP to uphill movement of the transported cations (3). According to Glynn and Karlish (3), occluded ions are defined as those that can “escape from the pump only after a change in the pump machinery that represents a step (or steps) in the working cycle.” For instance, when SERCA is phosphorylated by ATP to give the ADP-sensitive form of phosphoenzyme (E1P), two Ca2+ ions bound to high affinity, Ca2+-selective sites at the cytoplasmic surface become occluded. A change in conformation of the phosphoenzyme to E2P makes these sites accessible from the inner (lumenal) surface of the sarcoplasmic reticulum, and the phosphoryl group is transferred to water. Release of the occluded Ca2+ ions and hydrolysis of the phosphoenzyme are followed by a spontaneous change in conformation of the dephosphoenzyme back to a form that can bind Ca2+ ions at the cytoplasmic surface with high affinity. Using erythrocyte ghosts permeabilized with the ionophore A23187, Moreira et al. (26) reported evidence for occluded Ca2+ in the E1P intermediate of PMCA. However, according to their results, ∼1.5 mol of Ca2+ are occluded per mole of enzyme. This value is in contrast with the proposed stoichiometry of 1 Ca2+ transported per ATP hydrolyzed by the PMCA (20, 21, 22). In our hands4 (not shown, but see Ref. 26), the preparation of erythrocyte ghosts, which is not highly enriched in PMCA, presents serious problems for filtration through Millipore-type filters, and washing out of Ca2+ from the ghosts using the ionophore A23187 is slow and inefficient. Ca2+ occlusion is a very rapid transient process (on the second-subsecond scale), and passage of Ca2+ ions through the ionophore can take a few minutes, producing an accumulation of the cation, which impedes reliable measurements of Ca2+ occlusion. In addition, Moreira et al. (26) used reaction blanks for Ca2+ occlusion with ATP but not LaIII, a condition that can still lead to the formation of occluded Ca2+, as we have shown in this work.
Perspectives
Like the phosphorylated states of the enzyme, which are intermediates during the hydrolysis of ATP, the occluded states of the enzyme are thought to be true intermediates during the transport of cations (27). The kinetic study of these two types of intermediates in parallel experiments will yield very important information for the construction of a detailed reaction model of the plasma membrane Ca2+-ATPase. Future experiments using the methodology employed in this paper will also include a comparative kinetic study of Ca2+ occlusion in different PMCA isoforms. Of particular interest will be studies on PMCA2, which differs significantly from PMCA4 in activation kinetics and basal activity (28). These studies may then also be extended to PMCA mutants with changes in amino acids thought to be involved in the state transitions linked to Ca2+ occlusion.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health, Fogarty International Center Grant R03TW006837. This work was also supported by Agencia Nacional de Promoción Científica y Tecnológica, Consejo Nacional de Investigaciones Científicas y Técnicas, and Universidad de Buenos Aires, Ciencia y Técnica from Argentina.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
M. S. Ferreira-Gomes, R. M. González-Lebrero, M. C. de La Fuente, E. E. Strehler, R. C. Rossi, and J. P. F. C. Rossi, unpublished observations.
- PMCA
- plasma membrane calcium pump
- [125I]TID-PC/16
- 1-O-hexadecanoyl-2-O-[9-[[[2-[125I]iodo-4-(trifluoromethyl-3H- diazirin-3-yl)benzyl]oxy]carbonyl] nonanoyl]-sn-glycero-3-phosphocholine.
REFERENCES
- 1. Strehler E. E., Zacharias D. A. (2001) Physiol. Rev. 81, 21–50 [DOI] [PubMed] [Google Scholar]
- 2. Rega A. F., Garrahan P. J. (1986) The Ca2+ Pump of Plasma Membranes, CRC Press Inc., Boca Raton, FL [Google Scholar]
- 3. Glynn I. M., Karlish S. J. (1990) Annu. Rev. Biochem. 59, 171–205 [DOI] [PubMed] [Google Scholar]
- 4. Glynn I. M., Richards D. E. (1982) J. Physiol. 330, 17–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takisawa H., Makinose M. (1983) J. Biol. Chem. 258, 2986–2992 [PubMed] [Google Scholar]
- 6. Rossi R. C., Kaufman S. B., González Lebrero R. M., Nørby J. G., Garrahan P. J. (1999) Anal. Biochem. 270, 276–285 [DOI] [PubMed] [Google Scholar]
- 7. Ritov V. B., Murzakhmetova M. K., Tverdislova I. L., Menshikova E. V., Butylin A. A., Avakian, Yu T., Yakovenko L. V. (1993) Biochim. Biophys. Acta 1148, 257–262 [DOI] [PubMed] [Google Scholar]
- 8. Caride A. J., Penheiter A. R., Filoteo A. G., Bajzer Z., Enyedi A., Penniston J. T. (2001) J. Biol. Chem. 276, 39797–39804 [DOI] [PubMed] [Google Scholar]
- 9. Verma A. K., Enyedi A., Filoteo A. G., Strehler E. E., Penniston J. T. (1996) J. Biol. Chem. 271, 3714–3718 [DOI] [PubMed] [Google Scholar]
- 10. Kratje R. B., Garrahan P. J., Rega A. F. (1983) Biochim. Biophys. Acta 731, 40–46 [DOI] [PubMed] [Google Scholar]
- 11. Sagara Y., Fernandez-Belda F., de Meis L., Inesi G. (1992) J. Biol. Chem. 267, 12606–12613 [PubMed] [Google Scholar]
- 12. Schwarzbaum P. J., Kaufman S. B., Rossi R. C., Garrahan P. J. (1995) Biochim. Biophys. Acta 1233, 33–40 [DOI] [PubMed] [Google Scholar]
- 13. Weber T., Brunner J. (1995) J. Am. Chem. Soc. 117, 3084–3095 [Google Scholar]
- 14. Echarte M. M., Levi V., Villamil A. M., Rossi R. C., Rossi J. P. (2001) Anal. Biochem. 289, 267–273 [DOI] [PubMed] [Google Scholar]
- 15. Akaike H. (1974) IEEE Transactions of Automatic Control 19, 716–723 [Google Scholar]
- 16. Sumida M., Tonomura Y. (1974) J. Biochem. 75, 283–297 [DOI] [PubMed] [Google Scholar]
- 17. Luterbacher S., Schatzmann H. J. (1983) Experientia 39, 311–312 [DOI] [PubMed] [Google Scholar]
- 18. Herscher C. J., Rega A. F. (1996) Biochemistry 35, 14917–14922 [DOI] [PubMed] [Google Scholar]
- 19. Montes M. R., Spiaggi A. J., Monti J. L., Cornelius F., Olesen C., Garrahan P. J., Rossi R. C. (2011) Biochim. Biophys. Acta 1808, 316–322 [DOI] [PubMed] [Google Scholar]
- 20. Schatzmann H. J. (1973) J. Physiol. 235, 551–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen F. L., Hinds T. R., Vincenzi F. F. (1978) J. Membr. Biol. 41, 361–376 [DOI] [PubMed] [Google Scholar]
- 22. Clark A., Carafoli E. (1983) Cell Calcium. 4, 83–88 [DOI] [PubMed] [Google Scholar]
- 23. Strehler E. E., James P., Fischer R., Heim R., Vorherr T., Filoteo A. G., Penniston J. T., Carafoli E. (1990) J. Biol. Chem. 265, 2835–2842 [PubMed] [Google Scholar]
- 24. Andersen J. P., Jørgensen P. L., Møller J. V. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 4573–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garrahan P. J., Rega A. F. (1978) Biochim. Biophys. Acta 513, 59–65 [DOI] [PubMed] [Google Scholar]
- 26. Moreira O. C., Rios P. F., Barrabin H. (2005) Biochim. Biophys. Acta 1708, 411–419 [DOI] [PubMed] [Google Scholar]
- 27. Kaufman S. B., González-Lebrero R. M., Schwarzbaum P. J., Nørby J. G., Garrahan P. J., Rossi R. C. (1999) J. Biol. Chem. 274, 20779–20790 [DOI] [PubMed] [Google Scholar]
- 28. Caride A. J., Filoteo A. G., Penheiter A. R., Pászty K., Enyedi A., Penniston J. T. (2001) Cell Calcium 30, 49–57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.