Abstract
The limited success of dendritic cell (DC)-based immunotherapy in multiple myeloma is partly due to hepatocyte growth factor (HGF)-induced DC dysfunction. From a therapeutic standpoint, it is important to understand the molecular events involved in inhibition of DC activation/maturation by HGF. Because Bruton's tyrosine kinase (Btk) negatively regulates maturation and immunostimulatory function of DCs, a role for Btk in HGF-induced inhibition of both murine and human DCs was investigated. We demonstrate that Btk is a novel proximal component of HGF-induced c-MET (HGF receptor) signaling. Following HGF treatment, Btk binds to c-MET and becomes activated. Btk activation in turn blocks the NF-κB pathway and subsequent DC activation via the c-Src-PI3K-AKT-mammalian target of rapamycin (mTOR) pathway. Notably, Btk activation is necessary for HGF-induced association of c-Src and PI3K with c-MET. Furthermore, we provide the first evidence that HGF inhibits DC activation by inducing autocrine interleukin (IL)-10 secretion, which requires activation of Btk. Blocking activation of Btk and its downstream the c-Src-PI3K-AKT-mTOR pathway prevents HGF-induced IL-10 secretion by DCs. In addition, neutralization of IL-10 secretion from DCs impaired the inhibitory effect of HGF on DCs. Thus, our study identifies a novel role for Btk in HGF-induced DC inhibition.
Keywords: Dendritic Cells, Hepatocyte Growth Factor, Immunosuppression, NF-κB (NF-κB), Signal Transduction
Introduction
Hepatocyte growth factor (HGF)2 has been shown to influence various cellular functions in addition to its role in growth of hepatocytes (1–6). Some studies have further demonstrated an immunoregulatory role for HGF (4, 7). Indeed, HGF regulates activation and function of many immune cells including dendritic cells (DCs) (8–10). DCs are the most potent antigen-presenting cells of the immune system (APCs) exhibiting the unique ability to activate naïve T cells (11). Upon HGF treatment, DCs exhibit a tolerogenic phenotype characterized by a reduced capacity to secrete proinflammatory cytokines and mediate APC function (10). Consequently, HGF-tolerized DCs induce expansion of regulatory T (Treg) cells (10). A similar impairment of activation and APC function of HGF-treated DCs has been demonstrated by the Dohi and co-workers (12). Notably, this HGF-induced inhibition of DCs is thought to be an important reason for the reduced anti-tumor efficacy of DC-based immunotherapy in multiple myeloma (MM) (13). However, the molecular basis for HGF-induced inhibition of DCs remains ill-defined.
The pleiotropic protein HGF acts by binding to the tyrosine kinase receptor c-MET (14). In addition to DCs; B cells, monocytes, T cells, and neutrophils either express c-MET and/or exhibit cellular responses to HGF (15–19). c-MET is a 190 kDa heterodimer composed of a 45 kDa extracellular α-chain and a disulfide-linked 145 kDa β-chain (20). Both α and β chains are produced from a single-chain precursor upon proteolysis. The β subunit contains an extracellular region involved in ligand binding, a transmembrane region and an intracellular region containing a multifunctional docking site YVHVNATYVNV that binds SH2-containing proteins c-Src, Grb2-SOS complex, phospholipase C (PLC)-γ, and PI3K (21, 22). Among these signaling molecules PI3K plays a critical role in HGF-induced monocyte invasion, B cell adhesion and neutrophil migration (16, 17, 19).
Recently our group demonstrated a pivotal role for PI3K in HGF-induced inhibition of DCs. If activation of the PI3K/AKT pathway is blocked, HGF fails to suppress DC activation (23). In DCs, the inhibitory effect of HGF-induced PI3K/AKT signaling is primarily mediated via mammalian target of rapamycin (mTOR)-induced blockade of the nuclear factor-κB (NF-κB) pathway (23). The transcription factor NF-κB is a primary regulator of gene expression associated with activation, maturation and APC function of DCs (24–26). In addition, activation of c-Src via c-MET is essential for HGF-induced activation of the PI3K/AKT pathway and consequent inhibition of NF-κB signaling in DCs (23). Nevertheless, the proximal signaling events triggered in DCs due to c-MET binding of HGF are poorly defined.
A recent report indicated that Bruton's tyrosine kinase (Btk), a member of Tec nonreceptor tyrosine kinase family, negatively regulates maturation and APC function of DCs (27). However, the role of Btk in HGF-induced signaling is currently unknown. Accordingly, whether Btk plays any role in HGF-induced DC inhibition was investigated. Here we demonstrate that Btk participates in proximal signaling events transduced by c-MET in DCs after HGF binding and that activation of Btk is required for HGF-induced inhibition of DCs. Our findings reveal a novel role for Btk in DC immunoregulation by HGF.
EXPERIMENTAL PROCEDURES
Mice
BALB/c and C57/BL6 (B6) mice were maintained and bred under specific pathogen-free conditions. The use of mice was approved by the Institutional Animal Ethics Committee of the Institute of Microbial Technology, India. All animal experimentations were performed according to the National Regulatory Guidelines issued by CPSEA (Committee for the Purpose of Supervision of Experiments on Animals), Ministry of Environment and Forest, Govt. of India.
Preparation of Primary DCs
Bone marrow-derived DCs (BMDCs) and splenic DCs (sDCs) were prepared from male or female BALB/c and B6 mice between 8–12 weeks of age as described previously (24, 28). Flow cytometric analyses indicated ∼90% purity of both BMDCs and sDCs, based on CD11c expression.
To prepare human monocyte-derived DCs (huMoDCs), human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy donors provided from the Department of Transfusion Medicine, Fortis Hospital, Mohali, India, using Ficoll gradient. CD14+ monocytes were purified from PBMCs with αCD14 antibody (Ab)-magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). DCs were prepared from monocytes as described (29).
Pretreatment of DCs with HGF, αc-MET Ab, or Signaling Molecule Inhibitors
DCs (5 × 106/well) were pretreated with 60 ng/ml of recombinant human HGF (Sigma-Aldrich) for indicated times in RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, 1 mm sodium pyruvate, 1× non-essential amino acids, and 50 μm 2-mercaptoethanol (complete RPMI medium). Subsequently, DCs were washed twice, resuspended in complete medium, and stimulated with LPS (500 ng/ml) (Sigma-Aldrich) for specified times. In some experiments, DCs were treated with Btk inhibitor LFM-A13 (100 μm; Calbiochem, Gibbstown, NJ), c-Src inhibitor E804 (10 nm; Calbiochem), PI3K inhibitors wortmannin (Wort) (200 nm; Cell Signaling Technology, Danvers, MA) or Ly294002 (Ly) (50 μm; Cell Signaling Technology), mTOR inhibitor rapamycin (100 nm; Calbiochem), or 10 μg/ml blocking αc-MET Ab (23) or isotype control Ab (R&D Systems, Minneapolis, MN) for 1 h before HGF treatment. Alternatively, DCs were treated with HGF for 6 h. At the 2nd h of HGF treatment, 10 μg/ml neutralizing αIL-10 monoclonal Ab (30) or isotype control rat IgG2b Ab (BD Biosciences, Franklin Lakes, NJ) were added to the DC culture. After HGF treatment, DCs were washed and stimulated with LPS for specified times.
EMSA and Western Blotting
Nuclear and cytoplasmic extracts were prepared from DCs as described previously (31). EMSA was performed using 32P-labeled DNA probe containing NF-κB binding sites derived from MHC-I H2K promoter: 5′-CAGGGCTGGGGATTCCCCATCTCCACAGTTTCACTTC-3′ (30). A double-stranded OCT-1 DNA probe, 5′-TGTCGAATGCAAATCACTAGAA-3′ was used as control (30). Bands were visualized using a phosphorimager (Bio-Rad Molecular Imager FX).
Western blotting was performed as described (28). Membranes were probed with Abs specific for: IκBα, IκBβ, IκBϵ, IKK1, Btk, c-Src, and PI3K p110δ (Santa Cruz Biotechnology, Santa Cruz, CA); phospho-IκBα, phospho-IKK1/phospho-IKK2 (Ser-180/Ser-181), IKK2, phospho-AKT (Ser-473), AKT, phospho-Btk (Tyr-223), phospho-c-Src (Tyr-416), phospho-p70S6 kinase (phospho-p70S6K) (Thr-389), p70S6K, phospho-eukaryotic initiation factor 4E binding protein 1 (phospho-4E-BP1) (Thr-37/46), 4E-BP1, PI3K p110α, and PI3K p85α (Cell Signaling Technology); c-Met (Millipore, Billerica, MA); phosphotyrosine (phospho-Tyr) (BD Biosciences); and β-actin (Sigma-Aldrich). Binding of secondary HRP-labeled goat-αrabbit (Santa Cruz Biotechnology) or goat-αmouse (Sigma-Aldrich) Abs was determined by using SuperSignal West Pico or West Dura Chemiluminescent Substrate (Pierce). Densitometric analysis was performed using ImageJ software (National Institutes of Health).
DC Transfection with siRNAs
Transfection of DCs with 60 nm scrambled control siRNA (Santa Cruz Biotechnology), or siRNAs specific for mouse Btk (Dharmacon, Chicago, IL), human Btk (Sigma-Aldrich) or mouse c-Src (Santa Cruz Biotechnology) was carried out using Lipofectamine LTX (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. The efficiency of DC transfection with siRNA was determined by transfecting DCs with fluorescein isothiocyanate (FITC)-conjugated control siRNA, and determining the frequency of FITC-positive DCs by flow cytometry. Approximately, >80% of DCs were transfected under the conditions used. DCs were then subjected to different treatments as specified.
Immunoprecipitation of c-MET
DCs (5 × 106) were incubated with HGF for indicated times, and whole cell lysates prepared. Lysates were precleared with protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology) and c-MET was immunoprecipitated using protein A/G PLUS-agarose beads precoated with αc-MET Ab. Phosphorylation of c-MET was determined via Western blot using αphospho-Tyr Ab. For “pull-down” experiments, DCs were treated with HGF for various times, chilled on ice, resuspended in 1 ml of cell-permeable protein crosslinker dimethyl 3,3-dithiopropionimidate dihydrochloride (Sigma-Aldrich) in PBS (2 mg/ml) and incubated at room temperature for 20 min. Immunoprecipitation of c-MET from whole BMDC lysates was performed as above. In some experiments, DCs were treated with LFM-A13, or transfected with scrambled control siRNA or Btk-specific siRNA before HGF treatment. Western blots were probed with Abs specific for Btk, c-Src, and the PI3K subunits p85α, p110α, and p110δ.
IKK Assay
Immunoprecipitation of IKK complex from 700 μg of whole DC lysates was carried out using protein A/G PLUS-Agarose beads and rabbit polyclonal αIKK1Ab. In vitro kinase reaction was performed by incubating IKK signalosome-bead complex with the IκBα-GST substrate for 1 h at 30 °C in kinase buffer containing 20 μl of magnesium/ATP mixture (Millipore). Supernatants were collected and IKK activity was analyzed by measuring phosphorylation of the IκBα-GST substrate via immunoblot probed with αphospho-IκBα Ab.
Flow Cytometry
The following monoclonal Abs used for staining were purchased from BD Biosciences: PE-αmouse CD11c, FITC-αmouse CD40, FITC-αmouse CD80, and FITC-αmouse CD86. Stained cells were analyzed on FACSCalibur (BD Biosciences) using Cell Quest Pro software.
Measurement of Cytokine Production from DCs
BMDCs or huMoDCs (5 × 105/ml) were pretreated (or not) with HGF for 6 h, washed, and stimulated with LPS for additional 24 h. Supernatants were analyzed for interleukin (IL)-12p70 and TNFα secretion in triplicate using enzyme-linked immunosorbent assay (ELISA) kits specific for mouse IL-12p70 and mouse TNFα (BD Biosciences); and human IL-12p70 and human TNFα (eBioScience, San Diego, CA) following the manufacturer's instructions. In some experiments, BMDCs or huMoDCs (5 × 105/ml) were treated with HGF for specified times or left untreated. Supernatants were assayed for IL-10 and transforming growth factor β1 (TGFβ1) secretion in triplicate using mouse IL-10 (BD Biosciences), human IL-10 (eBioscience), and mouse TGFβ1 (eBioscience) ELISA kits.
Statistical Analysis
All statistical analyses were conducted by one-way ANOVA using the SigmaPlot 11.0 program. A probability level of ≤ 0.05 was considered significant. Data are represented as means ± S.E.
RESULTS
HGF Treatment Induces Binding of Btk to c-MET and Concomitant Btk Activation in DCs
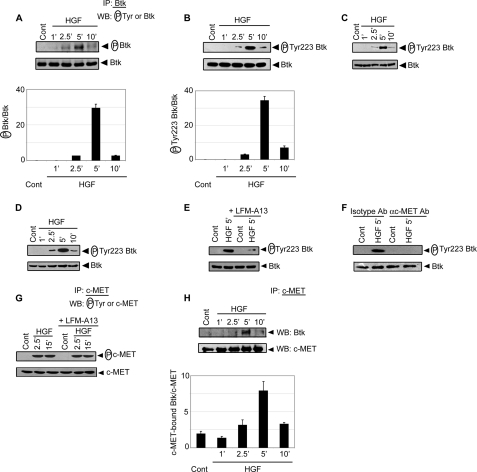
Studies demonstrated that HGF inhibits activation and maturation of DCs (10, 12). Furthermore, HGF-induced c-MET is known to form complexes with many SH2-containing signaling molecules (21). Because Btk contains SH2 domain and inhibits LPS-induced DC maturation (27, 32), a role for Btk in HGF-induced inhibition of DCs was investigated. Initially, whether HGF treatment induces Btk activation in DCs was examined. Immature BMDCs (CD11c+CD8α−) prepared from BALB/c mice were treated with HGF for varying times and Btk activation was determined by measuring Tyr phosphorylation of Btk as described (33). Treatment of BMDCs with HGF induced a transient increase in Tyr phosphorylation of Btk, which peaked within 5 min and returned to basal levels by 10 min after HGF treatment (Fig. 1A). The level of Btk protein, however, remained unchanged (Fig. 1A). A similar result was obtained when Btk activation in HGF-stimulated BMDCs, sDCs, and huMoDCs was reconfirmed by measuring phosphorylation of Btk Tyr-223 (Fig. 1, B–D). The latter is an indicator of in vivo Btk kinase activity (34), and therefore was analyzed to determine Btk activation in all subsequent experiments. Pretreatment of BMDCs with LFM-A13, a Btk-specific inhibitor (35), however, blocked HGF-stimulated Tyr-223 phosphorylation of Btk (Fig. 1E). Similarly, HGF-induced Btk Tyr-223 phosphorylation was significantly reduced upon BMDC pretreatment with blocking αc-MET Ab but not isotype control Ab (Fig. 1F). This data indicates that HGF-induced Btk activation is dependent on activation of c-MET. In contrast, Btk activation was not required for c-MET phosphorylation induced by HGF. As demonstrated in Fig. 1, E and G; HGF continued to promote c-MET phosphorylation regardless of whether Btk activation was inhibited by LFM-A13 treatment. Notably, immunoprecipitation of c-MET from whole BMDC lysates demonstrated formation of a Btk/c-MET complex in BMDCs treated with HGF for 5 min but not in untreated BMDCs (Fig. 1H). However, no significant difference in the level of c-MET protein was observed in untreated versus HGF-treated BMDC lysates (Fig. 1H). Together, these results demonstrate that HGF treatment induces recruitment of Btk to c-MET and concomitant Btk activation in DCs.
FIGURE 1.
Btk binds to c-MET and is activated upon HGF treatment of DCs. BMDCs (A, B, and E–H), sDCs (C) or huMoDCs (D) were treated with HGF for specified times. In some experiments (E–G), DCs were treated with Btk inhibitor LFM-A13 (E and G), or isotype control Ab or αc-MET Ab (F) prior to HGF treatment. A, Btk was immunoprecipitated from whole cell lysates and Western blots were probed for phosphotyrosine [(P)-Tyr] and Btk. The lower panel shows densitometric analysis representing the ratio of intensity of Tyr-phosphorylated Btk to Btk expression per unit area and is presented as an arbitrary unit. B–F, phosphorylation of Btk Tyr-223 in cytoplasmic extracts was determined via Western blot using αphospho-Tyr-223 Btk Ab. The same blot was reprobed for Btk protein. Densitometric readings (lower panel of B) indicate the ratio of intensity of Tyr-223-phosphorylated Btk to Btk expression per unit area and are represented as an arbitrary unit. G and H, c-MET was immunoprecipitated from whole cell lysates and Western blots were probed for phospho-Tyr (G), Btk (H) and c-MET (G and H) using the same membranes. The lower panel of H represents densitometric readings, which indicate the ratio of intensity of c-MET-bound Btk to c-MET expression per unit area, are presented as an arbitrary unit. For this and all other figures, control (Cont) lane represents DCs without any treatment. For all experiments, DCs derived from BALB/c mice were used unless DCs of other mouse strain are specified. Data are representative of three independent experiments.
Btk Functions Upstream of the c-Src-PI3K-AKT-mTOR Pathway
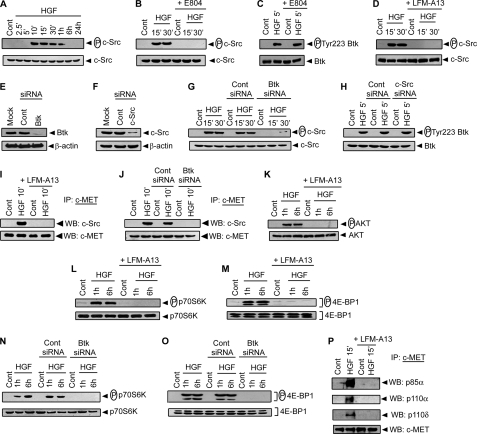
We previously demonstrated that HGF induces activation of c-Src in DCs (23). In addition, a number of studies indicate that Src family kinases including c-Src regulate Btk activation (36–38). Therefore, activation of Btk was expected to be a consequence of c-Src activation in HGF-treated DCs. To test this possibility, a temporal analysis of c-Src activation was performed in BMDCs treated with HGF. Activation of c-Src was assessed by c-Src Tyr-416 phosphorylation as described (23). An increased Tyr-416 phosphorylation was initially detected by 10 min after HGF treatment and persisted up to 1 h (Fig. 2A). On the other hand, Btk activation, as demonstrated in Fig. 1, A–D; was observed within 5 min after HGF treatment. This early activation of Btk compared with c-Src suggested that Btk might function upstream of c-Src in the HGF-induced signaling pathway in DCs. To confirm this possibility, the effect of BMDC pretreatment with Btk inhibitor LFM-A13 and c-Src inhibitor E804 on HGF-stimulated activation of Btk and c-Src was investigated. Interestingly, HGF-treated BMDCs continued to promote Tyr-223 phosphorylation of Btk despite inhibition of c-Src by E804 incubation (Fig. 2, B and C). In contrast, pretreatment of BMDCs with LFM-A13 blocked HGF-induced c-Src phosphorylation (Fig. 2D). To rule out the nonspecific effects of pharmacological inhibitors of Btk and c-Src, the effect of siRNA-mediated blockade of Btk and c-Src expression on HGF-induced activation of c-Src and Btk, respectively, was analyzed. Transfection of BMDCs with Btk- or c-Src-specific siRNA reduced Btk and c-Src expression by ∼80% (Fig. 2, E and F). Interestingly, BMDC transfection with Btk-specific but not scrambled control siRNA blocked HGF-induced phosphorylation of c-Src (Fig. 2G). In contrast, HGF-induced Tyr-223 phosphorylation of Btk was not affected by BMDC transfection with c-Src-specific siRNA (Fig. 2H). These data therefore suggest that c-Src acts downstream of Btk in the signaling cascade induced in DCs by HGF. Furthermore, immunoprecipitation experiments demonstrated that pretreatment of BMDCs with LFM-A13, or BMDC transfection with Btk-specific but not control siRNA prevented HGF-induced c-Src/c-MET complex formation without affecting the level of c-MET (Fig. 2, I and J). Finally, the effect of blocking Btk activation with LFM-A13 on activation of PI3K and mTOR, which function downstream of c-Src in HGF-induced signaling in DCs (23), was tested. Activation of PI3K and mTOR was determined by measuring phosphorylation of PI3K effector AKT, and mTOR effectors p70S6K and 4E-BP1, respectively. Pretreatment with LFM-A13 prevented HGF-induced phosphorylation of AKT, p70S6K, and 4E-BP1 (Fig. 2, K–M). Phosphorylation of p70S6K and 4E-BP1 induced by HGF was similarly inhibited in BMDCs transfected with Btk-specific but not control siRNA (Fig. 2, N and O). In addition, immunoprecipitation of c-MET from HGF-treated BMDC lysates demonstrated that association of PI3K subunits p85α, p110α, and p110δ with c-MET was markedly reduced when Btk activation was inhibited by LFM-A13 incubation (Fig. 2P). These data indicate that Btk activation induced by HGF is responsible for subsequent activation of the c-Src-PI3K-AKT-mTOR pathway, and binding of c-Src and PI3K to c-MET.
FIGURE 2.
Btk mediates HGF-induced activation of the c-Src-PI3K-AKT-mTOR pathway in DCs. BMDCs were either left untreated or treated with HGF for specified times. For experiments (B–D, and G–P), BMDCs were either pretreated with c-Src inhibitor E-804 (B and C) or Btk inhibitor LFM-A13 (D, I, K–M, and P), or transfected with scrambled control siRNA (Cont siRNA) (G, H, J, N, and O), Btk-specific siRNA (G, J, N, and O) or c-Src-specific siRNA (H) before HGF treatment. A, B, D, G, and K–O, Expression of phospho-c-Src versus c-Src (A, B, D, and G), phospho-AKT versus AKT (K), phospho-p70S6K versus p70S6K (L and N), and phospho-4E-BP1 versus 4E-BP1 (M and O) in whole cell lysates was detected via Western blot with the same blots. C and H, phosphorylation of Btk Tyr-223 in cytoplasmic extracts was determined via Western blot using αphospho-Tyr-223 Btk Ab. The same blot was reprobed for Btk protein. E and F, BMDCs were transfected with scrambled control siRNA (Cont siRNA) (E and F), or siRNA specific for Btk (E) or c-Src (F). Expression of Btk (E), c-Src (F), and β-actin (E and F) in whole cell lysates was detected via Western blot with the same blot. I, J, and P, c-MET was immunoprecipitated from whole cell lysates. Western blots were probed for c-Src (I and J), PI3K subunits p85α, p110α, and p110δ (P), and c-MET (I, J, and P) using the same membranes. Data are representative of three independent experiments.
HGF-induced DC Inhibition Requires Btk Activation
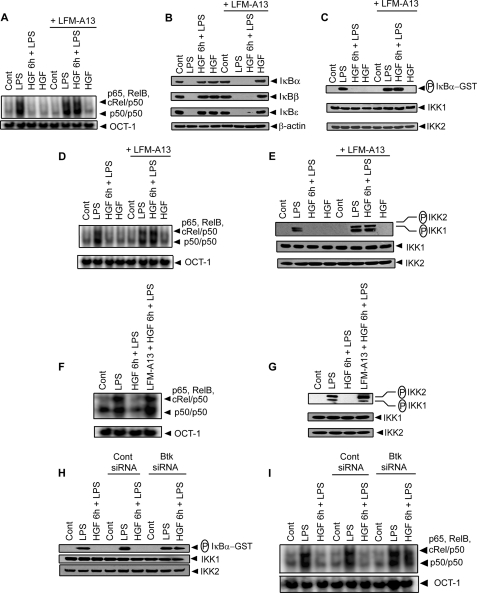
Previously our work demonstrated that HGF-induced activation of the c-Src-PI3K-AKT-mTOR pathway inhibits DC activation by blocking NF-κB signaling (23). Because, Btk has been demonstrated above as an upstream regulator of the c-Src-PI3K-AKT-mTOR pathway, a role for Btk in HGF-induced inhibition of NF-κB signaling was determined. BMDCs were pretreated with Btk inhibitor LFM-A13, incubated with HGF and then stimulated with LPS. As expected, LPS-induced NF-κB DNA binding as determined by EMSA was inhibited upon HGF treatment of BMDCs (Fig. 3A). This inhibitory effect of HGF, however, was blocked by pretreating BMDCs with LFM-A13 (Fig. 3A). In agreement with EMSA data, the upstream signaling events leading to NF-κB activation such as degradation of inhibitory IκB proteins and IκB kinase (IKK) activity were induced by LPS stimulation in BMDCs preincubated with LFM-A13, despite HGF treatment (Fig. 3, B and C). Similar to BMDCs, LPS-induced NF-κB DNA binding, and IKK activation as measured by phosphorylation of IKK1 and IKK2 were not inhibited by HGF in both sDCs and huMoDCs pretreated with LFM-A13 (Fig. 3, D–G). Furthermore, HGF no longer inhibited LPS-induced IKK activity and NF-κB DNA binding in BMDCs transfected with Btk-specific but not control siRNA (Fig. 3, H and I). The role of Btk in HGF-induced inhibition of NF-κB signaling in DCs is not intrinsic to BALB/c genotype. For instance, inhibition of Btk with LFM-A13 effectively blocked the inhibitory effects of HGF on LPS-induced NF-κB DNA binding and IKK activity in BMDCs derived from B6 mice also (supplemental Fig. S1). These findings suggest that Btk plays a pivotal role in mediating HGF-induced inhibition of NF-κB activation independently of the genotype of DCs.
FIGURE 3.
Activation of Btk is necessary for HGF-induced inhibition of NF-κB signaling in DCs. BMDCs (A–C), sDCs (D and E), or huMoDCs (F and G) were treated or not with Btk inhibitor LFM-A13. Alternatively, in some experiments (H and I), BMDCs were transfected with scrambled control siRNA (Cont siRNA) or Btk-specific siRNA. DCs were then incubated with HGF for 6 h or left untreated, and stimulated with LPS for 30 min. A, D, F, and I, nuclear NF-κB or OCT-1 DNA binding activity was determined via EMSA. B, cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin were detected via Western blot using the same blot. C and H, in vitro IKK activity was determined by measuring phosphorylation of an IκBα-GST substrate. IKK1 and IKK2 protein expression in immunoprecipitated samples were analyzed via Western blot. E and G, IKK phosphorylation was detected via Western blot and the same blot reprobed for IKK1 and IKK2 protein. Data are representative of three independent experiments.
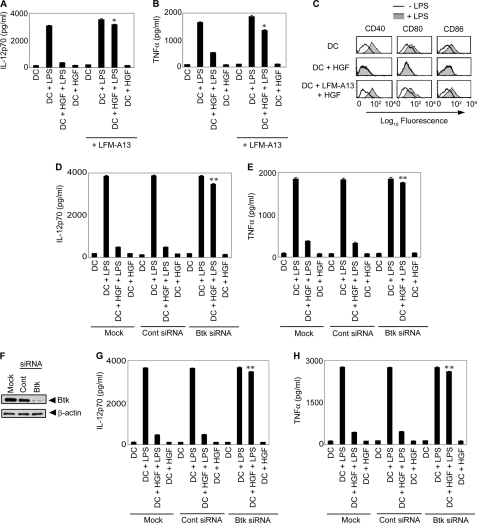
Next, whether Btk contributes to HGF-induced inhibition of DC activation at the cellular level was investigated. For this purpose, the effect of BMDC pretreatment with Btk inhibitor LFM-A13 on the capacity of HGF to inhibit LPS-stimulated up-regulation of costimulatory molecule (CD40, CD80, and CD86) expression and secretion of proinflammatory cytokines IL-12p70 and TNFα was determined. Pretreatment of BMDCs with HGF for 6 h completely inhibited LPS-stimulated secretion of IL-12p70 and TNFα (Fig. 4, A and B). LPS-induced up-regulation of CD40, CD80, and CD86 expression was also inhibited by HGF pretreatment of BMDCs (Fig. 4C). However, the inhibitory effect of HGF on the induction of costimulatory molecule expression and secretion of proinflammatory cytokines by LPS was blocked in BMDCs pretreated with LFM-A13 (Fig. 4, A–C). HGF also failed to inhibit LPS-stimulated secretion of IL-12p70 and TNFα by BMDCs transfected with Btk-specific but not control siRNA (Fig. 4, D and E). Similar to results obtained with murine BMDCs, huMoDC transfection with Btk-specific siRNA successfully silenced Btk expression (Fig. 4F), and markedly reduced the inhibitory effect of HGF on LPS-stimulated secretion of IL-12p70 and TNFα (Fig. 4, G and H). Collectively, these data demonstrate that HGF-induced inhibition of DCs is dependent on Btk activation.
FIGURE 4.
HGF-induced inhibition of DCs requires Btk activation. A–C, BMDCs were incubated with or without Btk inhibitor LFM-A13, treated with HGF for 6 h, and stimulated with LPS for 24 (A and B) or 48 h (C). Secretion of IL-12p70 (A) and TNFα (B) was measured via ELISA, and costimulatory molecule expression assessed via FACS (C). D, E, G, and H, BMDCs (D and E) or huMoDCs (G and H) were transfected with scrambled control (Cont siRNA) or Btk-specific siRNA, treated with HGF for 6 h and then stimulated with LPS for 24 h. IL-12p70 (D and G) and TNFα (E and H) secretion was measured by ELISA. F, huMoDCs were transfected with scrambled control (Cont siRNA) or Btk-specific siRNA. Expression of Btk and β-actin in whole cell lysates was detected via Western blot using the same blot. Data are the representative of three independent experiments and were analyzed using one-way ANOVA. *, p < 0.001 DC+LFM-A13+HGF+LPS versus DC+HGF+LPS; **, p < 0.001 DCs transfected with Btk-specific siRNA+HGF+LPS versus mock-transfected DCs+HGF+LPS or DCs transfected with control siRNA (Cont siRNA)+HGF+LPS.
Btk Activation Mediates HGF-induced Inhibition of DCs in an IL-10-dependent Manner
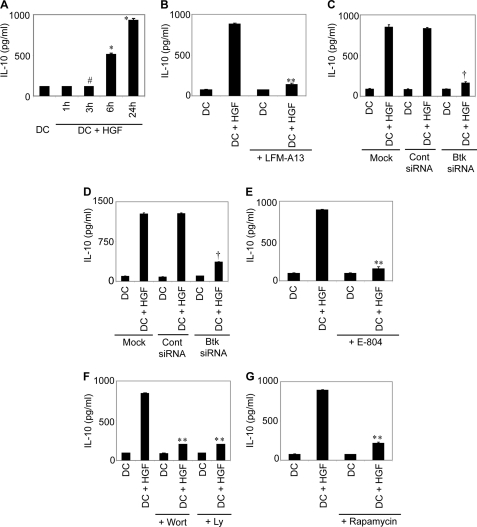
The anti-inflammatory cytokine IL-10 is known to exhibit inhibitory effect on DCs in an autocrine manner (39). Accordingly, whether DC inhibition by HGF is IL-10-dependent was investigated. At first, the ability of HGF, if any, to stimulate IL-10 secretion by DC was tested. Temporal analysis demonstrated that IL-10 secretion by BMDCs was initially detected at 6 h after HGF treatment and increased with time (Fig. 5A). In contrast to IL-10, secretion of another anti-inflammatory cytokine TGFβ1 by BMDCs was not induced despite HGF treatment (supplemental Fig. S2). Given that Btk is activated following HGF binding to c-MET (Fig. 1), and that LPS stimulation of DCs requires Btk activity for maximum production of IL-10, which inhibits DC APC function in an autocrine manner (27); the role for Btk in HGF-induced IL-10 production by DCs was examined. Pretreatment of BMDCs with Btk inhibitor LFM-A13 or BMDC transfection with Btk-specific siRNA effectively blocked HGF-induced IL-10 secretion by BMDCs (Fig. 5, B and C). Similarly, transfection of huMoDCs with Btk-specific siRNA but not control siRNA prevented HGF-induced IL-10 secretion (Fig. 5D). HGF also failed to induce secretion of IL-10 when activation of signaling molecules downstream of Btk such as c-Src, PI3K, and mTOR was inhibited by incubation with E-804, Wort or Ly, and rapamycin, respectively (Fig. 5, E–G). These data suggest that HGF stimulates IL-10 secretion from DCs by inducing activation of Btk and downstream the c-Src-PI3K-AKT-mTOR pathway.
FIGURE 5.
Btk activation is required for HGF-induced IL-10 secretion by DCs. A–C, and E–G, BMDCs were treated with HGF for indicated times (A) or 24 h (B, C, and E–G). For experiments (B, C, and E–G), BMDCs were either pretreated with Btk inhibitor LFM-A13 (B), c-Src inhibitor E-804 (E), PI3K inhibitors Wort or Ly (F), or mTOR inhibitor rapamycin (G); or transfected with scrambled control siRNA or Btk-specific siRNA (C) prior to HGF treatment. Secretion of IL-10 in culture supernatants was measured via ELISA. D, huMoDCs were transfected with scrambled control siRNA or Btk-specific siRNA, and treated with HGF for 24 h. IL-10 secretion was determined by ELISA. Data are the representative of three independent experiments and were analyzed using one-way ANOVA. #, p = 0.121 DC treated with HGF for 3 h versus DC; *, p < 0.001 DC treated with HGF for 6 or 24 h versus DC; **, p < 0.001 DC+LFM-A13+HGF, DC+E804+HGF, DC+Wort+HGF, DC+Ly+HGF or DC+rapamycin+HGF versus DC+HGF; †, p < 0.001 DCs transfected with Btk-specific siRNA+HGF versus mock-transfected DCs+HGF or DCs transfected with control siRNA (Cont siRNA)+HGF. Findings were considered significant with p values ≤0.05.
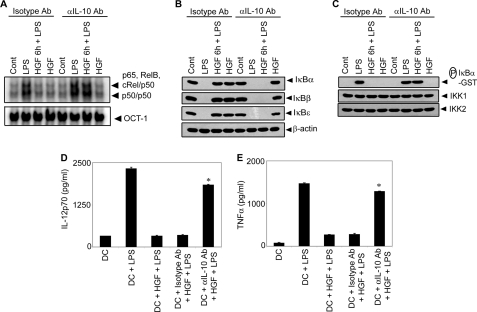
To test a functional role for IL-10 in HGF-induced inhibition of DCs, the effect of neutralization of IL-10 with αIL-10 Ab was investigated. Whereas HGF treatment inhibited LPS-induced NF-κB DNA binding, IκB degradation, and IKK activity in BMDCs treated with isotype control Ab, IL-10 neutralization with αIL-10 Ab blocked this effect (Fig. 6, A–C). Additionally, LPS-stimulated BMDCs treated with αIL-10 Ab but not isotype control Ab continued to secrete IL-12p70 and TNFα despite HGF treatment (Fig. 6, D and E). These findings demonstrate that IL-10 plays a key role in DC inhibition by HGF. Furthermore, activation of Btk and its downstream the c-Src-PI3K-AKT-mTOR pathway is required for HGF-induced IL-10 secretion by DCs.
FIGURE 6.
αIL-10 Ab blocks HGF-induced inhibition of NF-κB signaling and DC activation. A–C, BMDCs were pretreated with αIL-10 or isotype control Ab as described under “Experimental Procedures,” and incubated with HGF for 6 h. BMDCs were then stimulated with LPS for 30 min. Nuclear NF-κB or OCT-1 DNA binding activity was determined via EMSA (A), cytoplasmic IκBα, IκBβ, IκBϵ, and β-actin protein expression was detected by Western blot using the same blot (B), and in vitro IKK activity and IKK1 and IKK2 protein expression was determined as described in the Fig. 3 legend (C). D and E, BMDCs were either left untreated or pretreated with αIL-10 or isotype control Ab, and incubated with HGF as described above. Subsequently, BMDCs were stimulated with LPS for 24 h. IL-12p70 (D) and TNFα (E) secretion was measured via ELISA. Data are the representative of three independent experiments and were analyzed using one-way ANOVA. *, p < 0.001 DC+αIL-10 Ab+HGF+LPS versus DC+isotype Ab+HGF+LPS or DC+HGF+LPS.
DISCUSSION
Impairment of DC function by HGF is believed to be an important reason for the limited anti-tumor efficacy of DC-based immunotherapy in MM (13). To achieve the desired effectiveness for this therapeutic strategy in MM, a thorough understanding of the molecular basis for HGF-induced DC suppression is necessary. In this study, we provide a new insight into the early HGF-stimulated c-MET signaling events by establishing Btk as a novel proximal signaling effector. Furthermore, we show that Btk plays a pivotal role in immunoregulation of DCs by HGF.
Our finding that HGF treatment results in Btk activation in DCs (Fig. 1) reveals new information about the c-MET signaling events induced by HGF. Notably, Btk was found physically associated with c-MET following HGF treatment (Fig. 1H). However, it is currently unknown whether Btk directly binds to c-MET or associates with any adaptor protein. Furthermore, the need of Btk activation for HGF-induced inhibition of DCs (Fig. 4) establishes a novel role for Btk in DC immunoregulation by HGF. Btk is widely known for its crucial role in B cell development and function (40). A lack of Btk function results in immunodeficiency in both mice (X-linked immunodeficiency (Xid)) and humans (X-linked agammaglobulinemia (XLA)) due to defective B cell signaling (40, 41). Additionally, Btk activity is required for effector function of mast cells (42). On the contrary, evidence depicting the role of Btk in DC immunobiology is very limiting and contradictory. Whereas Btk defect was initially reported to have no effect on DC maturation (43), a recent study using btk−/− DCs demonstrates an inhibitory role for Btk in LPS-induced DC maturation (27). In agreement with the later report, our study indicates that HGF-induced Btk activation prevents DC activation/maturation. Indeed, the inhibitory effect of HGF on LPS-stimulated up-regulation of costimulatory molecule expression and secretion of proinflammatory cytokines was blocked by treating DCs with pharmacological inhibitors or siRNA specific for Btk (Fig. 4). Importantly, Btk caused HGF-induced inhibition of DCs by blocking activation of IKK and downstream NF-κB (Figs. 3 and 4). Our data differ from earlier reports demonstrating that Btk positively regulates NF-κB activation in B cells and a monocytic cell line upon stimulation with B cell-activating factor (BAFF) and LPS, respectively (44, 45). The inhibitory effect of Btk activation on NF-κB signaling therefore seems to be DC-specific and is established in the context of HGF-induced c-MET signaling.
We further analyzed how HGF-induced Btk blocks the NF-κB pathway and subsequent activation/maturation of DCs. Surprisingly, c-Src activation was observed as a downstream event of Btk activation in HGF-stimulated DCs (Fig. 2). Until now, Src family kinases including c-Src are considered as upstream regulators of Btk activity (36–38). However, our observations that c-Src exhibits delayed activation compared with that of Btk in response to HGF treatment, and the inhibitor of Btk and silencing of Btk expression block HGF-induced c-Src activation (Figs. 1 and 2) suggest that Btk is an upstream regulator of c-Src activity in HGF-induced signaling pathway. In addition, Btk activation was found necessary for establishing the c-MET/c-Src complex upon HGF treatment (Fig. 2, I and J). Recently, we have reported that c-Src mediates HGF-induced inhibition of DCs by activating the PI3K-AKT-mTOR pathway (23). In the current study, we demonstrate that activation of the PI3K/AKT pathway and mTOR by HGF requires activation of c-Src by Btk (Fig. 2). Furthermore, HGF-induced binding of PI3K complexes p85α/p110α and p85α/p110δ to c-MET is dependent on Btk activation (Fig. 2P). Our findings therefore establish an essential role for Btk in HGF-induced DC suppression and demonstrate that this effect of Btk is mediated through the c-Src-PI3K-AKT-mTOR pathway.
A recent study has reported that HGF promotes monocyte differentiation to IL-10-producing cells exhibiting DC like features (13). In addition, HGF-treated DCs induce differentiation of IL-10-producing Treg cells (10). However, the ability of HGF to induce IL-10 secretion by DCs has yet to be determined. The current study provides first evidence that HGF treatment induces secretion of anti-inflammatory cytokine IL-10 by DCs (Fig. 5). In addition, Btk activation is necessary for this effect of HGF. For instance, the capacity of DCs to secrete IL-10 following HGF treatment was significantly impaired by blocking Btk activation via siRNA and pharmacological inhibitor (Fig. 5). This finding is consistent with a recent report demonstrating an essential role for Btk in LPS-induced IL-10 secretion by DCs (27). Our results further suggest that Btk mediates HGF-induced IL-10 secretion by DCs via the c-Src-PI3K-AKT-mTOR pathway. This conclusion is supported by results demonstrating that HGF-induced IL-10 secretion is impaired in DCs pretreated with c-Src, PI3K and mTOR inhibitors (Fig. 5). The mechanism by which the c-Src-PI3K-AKT-mTOR pathway induces IL-10 secretion by DCs is not yet clear and currently under investigation. Nevertheless, our findings demonstrate a direct role for Btk and its downstream the c-Src-PI3K-AKT-mTOR pathway in HGF-stimulated secretion of IL-10 by DCs.
Because of the capacity of IL-10 to suppress DC activation (28, 39), the increased IL-10 production by DCs following HGF treatment would be expected to contribute to HGF-induced inhibition of DCs. This possibility was further spurred by the observation that IL-10 secretion by DCs was initially detected at 6 h of HGF treatment (Fig. 5A), which coincided with our previous finding that NF-κB activation in DCs is also inhibited after 6 h of HGF pretreatment (23). Indeed, the inhibitory effect of HGF on proinflammatory cytokine production by DCs is blocked by in vitro neutralization of IL-10 secreted by the same DCs (Fig. 6). Furthermore, HGF failed to inhibit activation of the NF-κB pathway in DCs if IL-10 secreted by DCs is neutralized (Fig. 6). These findings therefore suggest that autocrine IL-10 is necessary to mediate HGF-induced inhibition of NF-κB signaling and subsequent DC activation.
Overall, the current study for the first time demonstrates an involvement of Btk in proximal signaling events induced by HGF and that Btk via the c-Src-PI3K-AKT-mTOR pathway serves a novel and major role in HGF-induced inhibition of DC activation by blocking NF-κB signaling in an IL-10-dependent manner. Furthermore, Btk has been demonstrated here to play an essential role in induction of p85α/p110α and p85α/p110δ PI3K complexes by HGF. We have previously reported that these two PI3K complexes are required for HGF-induced inhibition of DCs (23). Our findings are noteworthy in view of the fact that suppression of DCs by HGF weakens the anti-tumor efficacy of DC-based immunotherapy in MM, and a very recent report suggesting PI3K/p110δ as a novel therapeutic target for MM (13, 46). Notably, an increased Btk expression in a human MM cell line resistant to dexamethasone, a drug commonly used for MM treatment, has also been demonstrated (47). Based on the evidences provided in the current study that Btk is an upstream regulator of PI3K activity and required for DC inhibition by HGF, Btk can be considered as a potential novel target for preventing HGF-induced DC dysfunction. In this way, breakdown of HGF-mediated DC suppression may provide a strategy to restore the efficacy of DC-based anti-tumor immunotherapy in MM.
Supplementary Material
Acknowledgments
We thank Dr. Kuldeep Singh (Fortis Hospital, Mohali, India) for providing human buffy coat and IMTECH animal house facility for providing animals required for experimentation.
This study was supported by a grant from the Department of Biotechnology, Government of India and an intramural grant from the Council of Scientific and Industrial Research, Government of India (to P. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- HGF
- hepatocyte growth factor
- APC
- antigen-presenting cell
- BAFF
- B cell-activating factor
- BMDC
- bone marrow-derived dendritic cell
- Btk
- Bruton's tyrosine kinase
- DC
- dendritic cell
- 4E-BP1
- eukaryotic initiation factor 4E-binding protein 1
- huMoDC
- human monocyte-derived dendritic cell
- IKK
- IκB kinase
- MM
- multiple myeloma
- mTOR
- mammalian target of rapamycin
- NF-κB
- nuclear factor-κB
- p70S6K
- p70S6 kinase
- Treg cells
- regulatory T cells
- sDC
- splenic dendritic cell.
REFERENCES
- 1. Bhargava M., Joseph A., Knesel J., Halaban R., Li Y., Pang S., Goldberg I., Setter E., Donovan M. A., Zarnegar R., Michalopoulos G. A., Nakamura T., Faletto D., Rosen E. M. (1992) Cell Growth Differ. 3, 11–20 [PubMed] [Google Scholar]
- 2. Nakamura T., Nawa K., Ichihara A. (1984) Biochem. Biophys. Res. Commun. 122, 1450–1459 [DOI] [PubMed] [Google Scholar]
- 3. Zarnegar R., Michalopoulos G. K. (1995) J. Cell Biol. 129, 1177–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ueki T., Kaneda Y., Tsutsui H., Nakanishi K., Sawa Y., Morishita R., Matsumoto K., Nakamura T., Takahashi H., Okamoto E., Fujimoto J. (1999) Nat. Med. 5, 226–230 [DOI] [PubMed] [Google Scholar]
- 5. Niemann C., Brinkmann V., Spitzer E., Hartmann G., Sachs M., Naundorf H., Birchmeier W. (1998) J. Cell Biol. 143, 533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson J., Dolcet X., Hilton M., Tolcos M., Davies A. M. (2004) Mol. Cell Neurosci. 27, 441–452 [DOI] [PubMed] [Google Scholar]
- 7. Yamaura K., Ito K., Tsukioka K., Wada Y., Makiuchi A., Sakaguchi M., Akashima T., Fujimori M., Sawa Y., Morishita R., Matsumoto K., Nakamura T., Suzuki J., Amano J., Isobe M. (2004) Circulation 110, 1650–1657 [DOI] [PubMed] [Google Scholar]
- 8. Futamatsu H., Suzuki J., Mizuno S., Koga N., Adachi S., Kosuge H., Maejima Y., Hirao K., Nakamura T., Isobe M. (2005) Circ. Res. 96, 823–830 [DOI] [PubMed] [Google Scholar]
- 9. Galimi F., Cottone E., Vigna E., Arena N., Boccaccio C., Giordano S., Naldini L., Comoglio P. M. (2001) J. Immunol. 166, 1241–1247 [DOI] [PubMed] [Google Scholar]
- 10. Benkhoucha M., Santiago-Raber M. L., Schneiter G., Chofflon M., Funakoshi H., Nakamura T., Lalive P. H. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 6424–6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hilkens C. M., Kalinski P., de Boer M., Kapsenberg M. L. (1997) Blood 90, 1920–1926 [PubMed] [Google Scholar]
- 12. Okunishi K., Dohi M., Nakagome K., Tanaka R., Mizuno S., Matsumoto K., Miyazaki J., Nakamura T., Yamamoto K. (2005) J. Immunol. 175, 4745–4753 [DOI] [PubMed] [Google Scholar]
- 13. Rutella S., Bonanno G., Procoli A., Mariotti A., de Ritis D. G., Curti A., Danese S., Pessina G., Pandolfi S., Natoni F., Di Febo A., Scambia G., Manfredini R., Salati S., Ferrari S., Pierelli L., Leone G., Lemoli R. M. (2006) Blood 108, 218–227 [DOI] [PubMed] [Google Scholar]
- 14. Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. (1991) Science 251, 802–804 [DOI] [PubMed] [Google Scholar]
- 15. Kurz S. M., Diebold S. S., Hieronymus T., Gust T. C., Bartunek P., Sachs M., Birchmeier W., Zenke M. (2002) Eur. J. Immunol. 32, 1832–1838 [DOI] [PubMed] [Google Scholar]
- 16. van der Voort R., Keehnen R. M., Beuling E. A., Spaargaren M., Pals S. T. (2000) J. Exp. Med. 192, 1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beilmann M., Vande Woude G. F., Dienes H. P., Schirmacher P. (2000) Blood 95, 3964–3969 [PubMed] [Google Scholar]
- 18. Adams D. H., Harvath L., Bottaro D. P., Interrante R., Catalano G., Tanaka Y., Strain A., Hubscher S. G., Shaw S. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 7144–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mine S., Tanaka Y., Suematu M., Aso M., Fujisaki T., Yamada S., Eto S. (1998) Lab. Invest. 78, 1395–1404 [PubMed] [Google Scholar]
- 20. Tulasne D., Paumelle R., Weidner K. M., Vandenbunder B., Fafeur V. (1999) Mol. Biol. Cell 10, 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., Graziani A., Panayotou G., Comoglio P. M. (1994) Cell 77, 261–271 [DOI] [PubMed] [Google Scholar]
- 22. Ponzetto C., Bardelli A., Maina F., Longati P., Panayotou G., Dhand R., Waterfield M. D., Comoglio P. M. (1993) Mol. Cell. Biol. 13, 4600–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singhal E., Sen P. (2011) Int. J. Biochem. Cell Biol. 43, 1134–1146 [DOI] [PubMed] [Google Scholar]
- 24. Weaver D. J., Jr., Poligone B., Bui T., Abdel-Motal U. M., Baldwin A. S., Jr., Tisch R. (2001) J. Immunol. 167, 1461–1468 [DOI] [PubMed] [Google Scholar]
- 25. Rescigno M., Martino M., Sutherland C. L., Gold M. R., Ricciardi-Castagnoli P. (1998) J. Exp. Med. 188, 2175–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poligone B., Weaver D. J., Jr., Sen P., Baldwin A. S., Jr., Tisch R. (2002) J. Immunol. 168, 188–196 [DOI] [PubMed] [Google Scholar]
- 27. Kawakami Y., Inagaki N., Salek-Ardakani S., Kitaura J., Tanaka H., Nagao K., Xiao W., Nagai H., Croft M., Kawakami T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhattacharyya S., Sen P., Wallet M., Long B., Baldwin A. S., Jr., Tisch R. (2004) Blood 104, 1100–1109 [DOI] [PubMed] [Google Scholar]
- 29. Iwama A., Osawa M., Hirasawa R., Uchiyama N., Kaneko S., Onodera M., Shibuya K., Shibuya A., Vinson C., Tenen D. G., Nakauchi H. (2002) J. Exp. Med. 195, 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haldar A. K., Yadav V., Singhal E., Bisht K. K., Singh A., Bhaumik S., Basu R., Sen P., Roy S. (2010) PLoS Pathog. 6, e1000907 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr. (1993) Mol. Cell. Biol. 13, 3301–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tzeng S. R., Pai M. T., Lung F. D., Wu C. W., Roller P. P., Lei B., Wei C. J., Tu S. C., Chen S. H., Soong W. J., Cheng J. W. (2000) Protein Sci. 9, 2377–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomlinson M. G., Kurosaki T., Berson A. E., Fujii G. H., Johnston J. A., Bolen J. B. (1999) J. Biol. Chem. 274, 13577–13585 [DOI] [PubMed] [Google Scholar]
- 34. Hantschel O., Rix U., Schmidt U., Bürckstümmer T., Kneidinger M., Schütze G., Colinge J., Bennett K. L., Ellmeier W., Valent P., Superti-Furga G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13283–13288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glassford J., Soeiro I., Skarell S. M., Banerji L., Holman M., Klaus G. G., Kadowaki T., Koyasu S., Lam E. W. (2003) Oncogene 22, 2248–2259 [DOI] [PubMed] [Google Scholar]
- 36. Rawlings D. J., Scharenberg A. M., Park H., Wahl M. I., Lin S., Kato R. M., Fluckiger A. C., Witte O. N., Kinet J. P. (1996) Science 271, 822–825 [DOI] [PubMed] [Google Scholar]
- 37. Afar D. E., Park H., Howell B. W., Rawlings D. J., Cooper J., Witte O. N. (1996) Mol. Cell. Biol. 16, 3465–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurosaki T., Kurosaki M. (1997) J. Biol. Chem. 272, 15595–15598 [DOI] [PubMed] [Google Scholar]
- 39. Corinti S., Albanesi C., la Sala A., Pastore S., Girolomoni G. (2001) J. Immunol. 166, 4312–4318 [DOI] [PubMed] [Google Scholar]
- 40. Khan W. N., Alt F. W., Gerstein R. M., Malynn B. A., Larsson I., Rathbun G., Davidson L., Müller S., Kantor A. B., Herzenberg L. A. (1995) Immunity 3, 283–299 [DOI] [PubMed] [Google Scholar]
- 41. Hashimoto S., Tsukada S., Matsushita M., Miyawaki T., Niida Y., Yachie A., Kobayashi S., Iwata T., Hayakawa H., Matsuoka H., Tsuge I., Yamadori T., Kunikata T., Arai S., Yoshizaki K., Taniguchi N., Kishimoto T. (1996) Blood 88, 561–573 [PubMed] [Google Scholar]
- 42. Hata D., Kawakami Y., Inagaki N., Lantz C. S., Kitamura T., Khan W. N., Maeda-Yamamoto M., Miura T., Han W., Hartman S. E., Yao L., Nagai H., Goldfeld A. E., Alt F. W., Galli S. J., Witte O. N., Kawakami T. (1998) J. Exp. Med. 187, 1235–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gagliardi M. C., Finocchi A., Orlandi P., Cursi L., Cancrini C., Moschese V., Miyawaki T., Rossi P. (2003) Clin. Exp. Immunol. 133, 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shinners N. P., Carlesso G., Castro I., Hoek K. L., Corn R. A., Woodland R. T., Woodland R. L., Scott M. L., Wang D., Khan W. N. (2007) J. Immunol. 179, 3872–3880 [DOI] [PubMed] [Google Scholar]
- 45. Jefferies C. A., Doyle S., Brunner C., Dunne A., Brint E., Wietek C., Walch E., Wirth T., O'Neill L. A. (2003) J. Biol. Chem. 278, 26258–26264 [DOI] [PubMed] [Google Scholar]
- 46. Ikeda H., Hideshima T., Fulciniti M., Perrone G., Miura N., Yasui H., Okawa Y., Kiziltepe T., Santo L., Vallet S., Cristea D., Calabrese E., Gorgun G., Raje N. S., Richardson P., Munshi N. C., Lannutti B. J., Puri K. D., Giese N. A., Anderson K. C. (2010) Blood 116, 1460–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chauhan D., Auclair D., Robinson E. K., Hideshima T., Li G., Podar K., Gupta D., Richardson P., Schlossman R. L., Krett N., Chen L. B., Munshi N. C., Anderson K. C. (2002) Oncogene 21, 1346–1358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.