Abstract
Emphysema is one of the characteristic features of chronic obstructive pulmonary disease, which is caused mainly by cigarette smoking. Recent data have suggested that apoptosis and cell cycle arrest may contribute to the development of emphysema. In this study, we addressed the question of whether and how cigarette smoke affected Akt, which plays a critical role in cell survival and proliferation. In normal human lung fibroblasts, cigarette smoke extract (CSE) caused cell death, accompanying degradation of total and phosphorylated Akt (p-Akt), which was inhibited by MG132. CSE exposure resulted in preferential ubiquitination of the active Akt (myristoylated), rather than the inactive (T308A/S473A double mutant) Akt. Consistent with cytotoxicity, CSE induced a progressive decrease of phosphorylated human homolog of mouse double minute homolog 2 (p-HDM2) and phosphorylated apoptosis signal regulating kinase 1 (p-ASK1) with concomitant elevation of p53, p21, and phosphorylated p38 MAPK. Forced expression of the active Akt reduced both CSE-induced cytotoxicity and alteration in HDM2/p53/p21 and ASK1/p38 MAPK, compared with the inactive Akt. Of note, CSE induced expression of the tetratrico-peptide repeat domain 3 (TTC3), known as a ubiquitin ligase for active Akt. TTC3 siRNAs suppressed not only CSE-induced Akt degradation but also CSE-induced cytotoxicity. Accordingly, rat lungs exposed to cigarette smoke for 3 months showed elevated TTC3 expression and reduced Akt and p-Akt. Taken together, these data suggest that cigarette smoke induces cytotoxicity, partly through Akt degradation via the ubiquitin-proteasome system, in which TTC3 acts as a ubiquitin ligase for active Akt.
Keywords: Akt, Cell Death, Chronic Obstructive Pulmonary Disease (COPD), Cigarette Smoke, Ubiquitination
Introduction
Chronic obstructive pulmonary disease (COPD),2 caused mainly by cigarette smoking, is characterized by emphysema, small airway remodeling, chronic bronchitis, and vascular remodeling (1). Although overwhelming protease activity due to persistent inflammation has long been suggested to play a pivotal role in the development of emphysema (1), recent data suggest that apoptosis, cell cycle arrest, and cellular senescence may contribute to the development of emphysema. Cigarette smoke causes apoptotic death in human lung fibroblasts and mouse lungs (2), inhibits cell proliferation, and induces cellular senescence in human lung fibroblasts, with elevation of p53 and p21 (3). Patients with emphysema have a higher percentage of type II alveolar epithelial cells and endothelial cells positive for p16 and p21, along with shortened telomeres, compared with asymptomatic smokers and nonsmokers (4). In a recent report, reduction in Werner syndrome protein by cigarette smoke extract (CSE) was suggested to be responsible for cigarette smoke-induced senescence and functional impairment in human lung fibroblasts (5). Of note, CSE-induced Werner syndrome protein decrease was prevented by lactacystin, a proteasome inhibitor.
The ubiquitin-proteasome system (UPS) is regarded as an important regulatory mechanism in cell cycle and growth (6). Sequential action of ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3) leads to Lys-48-linked conjugation of ubiquitin to the lysine residue of proteins destined to degradation by the 26 S proteasome (7). Even with a long history of studies of the UPS, only a few recent studies have addressed the pathogenic importance of the UPS in COPD. In addition to Werner syndrome protein (5), cigarette smoke causes degradation of histone deacetylase 2 (HDAC2) through the UPS after nitration at tyrosine residues (8) or phosphorylation at serine/threonine residues (9), probably resulting in glucocorticoid resistance in patients with COPD (10).
Akt, a serine/threonine kinase, suppresses apoptosis and regulates the cell cycle (reviewed in Refs. 11, 12). By growth factors or stresses, Akt is recruited to the plasma membrane and activated by phosphorylation in a PI3K-dependent manner. Activated Akt suppresses apoptosis through inactivation of pro-apoptotic factors, stabilization of anti-apoptotic factors, and maintenance of mitochondrial integrity. In addition, Akt phosphorylates and inhibits interaction of p21 with proliferating cell nuclear antigen and promotes assembly of cyclin D1·cyclin-dependent kinase 4 complex (13). Moreover, Akt phosphorylates the ubiquitin ligase mouse double minute homolog 2 (Mdm2), leading to ubiquitination and subsequent degradation of p53 (14). Furthermore, Akt phosphorylates apoptosis signal-regulating kinase (ASK1) (15), thus suppressing JNK and p38 MAPK activation and preventing apoptosis (16). As Akt enhances cell survival, decreased levels of p-Akt in cigarette smoke-exposed rat lungs (17) raise the question of the pathogenic role of Akt in development of emphysema.
By virtue of its critical role as a signaling hub, Akt is tightly regulated in various ways. One such mechanism is Akt degradation (18). VEGF deprivation (19) or TNF-α treatment (20) caused Akt degradation through the UPS and caspases, leading to endothelial cell death or insulin signaling impairment, respectively. In addition, Akt degradation through the UPS plays a physiological role in formation of neuronal polarity (21). Therefore, Akt degradation is recognized as one of the Akt regulatory mechanisms; however, only limited information on E3 ligases for Akt is available. The breast cancer susceptibility gene 1 (BRCA1) (22) and tetratrico-peptide repeat domain 3 (TTC3) (23) are known to cause p-Akt ubiquitination and proteasomal degradation. Opposite to the negative regulatory role of BRCA1 and TTC3, TNF receptor-associated factor 6 (TRAF6) causes Akt ubiquitination in a different manner, enhancing membrane localization and subsequent activation of Akt (24).
In this study, we demonstrate for the first time that cigarette smoke induces Akt degradation through the UPS, resulting in cell death associated with activation of death-signaling pathways in normal human lung fibroblasts (NHLFs). In addition, we show that TTC3 is a ubiquitin E3 ligase responsible for CSE-induced Akt ubiquitination and cytotoxicity and that cigarette smoke induces TTC3 expression in NHLFs and rat lungs.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Chemicals were purchased from Sigma and Calbiochem. Anti-total Akt, Akt1, Akt2, Akt3, anti-p-Akt (Ser-473), anti-p-Mdm2 (Ser-166), anti-Myc, HRP-conjugated anti-mouse IgG, and HRP-conjugated anti-rabbit IgG antibodies were purchased from Cell Signaling (Beverly, MA); anti-hemagglutinin (HA), anti-p53, anti-p21, and anti-ASK1, anti-p-ASK1 (Ser-83) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); anti-p38 MAPK and anti-p-p38 MAPK antibodies were purchased from BD Biosciences; and anti-GAPDH antibody was purchased from AbFrontier (Korea).
Cell Culture
NHLFs (Lonza, Rockland, ME) were grown in DMEM containing 10% heat-inactivated FBS (Invitrogen) and penicillin/streptomycin (Invitrogen). Passage 6–8 NHLFs were used in this study.
CSE Preparation
CSE was prepared as described previously (3), with slight modifications. Briefly, through one opening of a three-way stopcock, 10 ml of serum-free DMEM was drawn into a 50-ml plastic syringe. Subsequently, 40 ml of one puff-cigarette smoke (filtered cigarettes; Eighty Eight Light containing 8.5 mg of tar and 0.9 mg of nicotine per cigarette, KT & G, Korea) was drawn into the syringe and mixed with the medium by vigorous shaking, until cigarette smoke grossly disappeared in the syringe. One cigarette was used for each 10 ml of medium, and 13–15 puffs were taken from one cigarette. CSE was prepared no more than 30 min before use by one person (S. Y. Kim) in whole experiments. CSE solution filtered through an aseptic 0.22-μm filter was considered as 100%. Typically, optical absorbance of 100% CSE solution at 320 nm was about 5.0.
Plasmids
Akt plasmids were made as described previously (25), with modifications. In brief, the entire coding region of Akt1, generated by PCR from cDNA synthesized from total RNAs of HeLa cells, was cloned into a pcDNA3.1/Myc-His vector (Invitrogen) (wild-type Akt/Myc-His). Constitutively active Akt (Myr Akt/Myc-His) was made by addition of the myristoylation site to the N terminus of Akt1 through PCR using a forward (5′-GGAATTCCACCATGGGGAGCAGCAAGAGCAAGCCCAAGATGAGCGACGTGGCTATTG-3′) and a reverse (5′-CCGCTCGAGCGGGCCGTGCCGCTGGCCGAG-3′) primer. Inactive (T308A/S473A double mutant) Akt plasmid was created from WT Akt/Myc-His plasmid using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA), according to the manufacturer's instructions. Hemagglutinin-tagged ubiquitin (Ubi-HA) plasmid was kindly provided by Dr. Dirk Bohmann (University of Rochester, Rochester, NY). To observe Lys-48-linked Akt ubiquitination, we used wild-type and lysine mutant Ubi-HA plasmids of the same backbone that were kindly provided by Dr. Zhijian Chen (University of Texas Southwestern Medical Center, Dallas); WT Ubi-HA, K48R Ubi-HA, K48 Ubi-HA (all lysine residues except Lys-48 were replaced with arginine), and KO Ubi-HA (all lysine residues were replaced with arginine).
Transfection
Transient transfection was performed using HilyMax reagents (Dojindo, Japan), according to the manufacturer's instructions. Briefly, at 70% confluence in a 10-cm diameter culture dish, culture medium was replaced with fresh serum-free DMEM, and Akt/Myc-His plasmid (1 μg) was co-transfected with or without Ubi-HA plasmid (1 μg). Six hour later, the culture medium was replaced with DMEM containing 10% FBS and incubated for 24 h. Thereafter, transfected NHLFs were exposed to CSE for evaluation of Akt ubiquitination or seeded into 96-well plates for determination of cell viability. Seeded NHLFs were incubated overnight in DMEM containing 10% FBS prior to CSE exposure.
Cell Viability Assay
NHLFs were seeded at a density of 2,000 cells per well in a 96-well plate. The next day, NHLFs were washed with PBS and exposed to CSE in serum-free DMEM. Cell viability was checked with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Roche Applied Science), according to the manufacturer's instructions.
Western Blot Analysis
NHLFs were lysed with a lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 0.1% SDS, 1% Nonidet P-40, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 2 mm p-nitrophenyl phosphate, and a protease inhibitor mixture) on ice for 30 min. Following centrifugation at 14,000 × g for 20 min at 4 °C, proteins in supernatants were separated in a 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked with 5% skim milk for 1 h at room temperature and then incubated overnight with primary antibody (1:1,000) at 4 °C. After washing with 0.5% Tween 20 in Tris-buffered saline (TBS-T), membranes were incubated with HRP-conjugated secondary antibody (1:5,000). Proteins were visualized using ECL reagents (Amersham Biosciences) and detected with LAS-3000 (Fuji, Japan). Image densities were quantified with software (TINA, Germany).
Detection of Ubiquitinated Akt
Akt/Myc-His plasmid was co-transfected with Ubi-HA plasmid into NHLFs, as described above, and ubiquitinated Akt/Myc-His was detected by His tag pulldown assay. In brief, NHLFs were washed with PBS, lysed in 200 μl of denaturing lysis buffer (50 mm Tris-HCl, pH 7.4, 0.5% SDS, and 70 mm β-mercaptoethanol) by vortexing, and boiled for 15 min at 95 °C. The lysates were then diluted with 800 μl of buffer A (50 mm NaH2PO4, 300 mm NaCl, 10 mm imidazole, pH 8.0, protease inhibitor mixture, and 10 μm MG132) and incubated overnight with 50 μl of nickel-nitrilotriacetic acid beads (Qiagen) at 4 °C. Beads were washed five times with buffer B (50 mm NaH2PO4, 300 mm NaCl, and 20 mm imidazole, pH 8.0), and bound proteins were eluted by boiling in a mixture of 5× SDS-polyacrylamide gel loading buffer and buffer C (50 mm NaH2PO4, 300 mm NaCl, 250 mm imidazole, pH 8.0) (1:4). Exogenously introduced and ubiquitinated Akt were identified with anti-Myc and anti-HA antibodies, respectively, in Western blot.
Exposure of Rats to Cigarette Smoke
Animal experimental protocol in this study was reviewed and approved by the Institutional Animal Care and Use Committee of Asan Medical Center. Eight-week-old inbred male Lewis rats (Orient, South Korea) were exposed to the mainstream smoke of 20 filtered commercial cigarettes per day (Eighty Eight Lights, South Korea), 5 days per week for 3 months, as in our previous report (26). Control rats inhaled clean room air. Each group consisted of five rats.
RT-PCR
Expression of TTC3, TRAF6, β-actin, and GAPDH was estimated by RT-PCR. Total RNAs in NHLFs and rat lungs were isolated by TRIzol reagent (Invitrogen), and cDNA was synthesized with 1 μg of total RNAs and murine leukemia virus reverse transcriptase (Intron, Korea), according to the manufacturer's instructions. PCR primer sequences were as follows: human TRAF6, 5′-TTTATGGAGGAGATCCAGGG-3′ and 5′-ATTTTTGGAAGGGACGCTGG-3′; human TTC3, 5′-CTTGGCAATGGAAGAAGCTC-3′ and 5′-AATGACCCTTTGGCCAAGTG-3′; human β-actin, 5′-CTCATGACCACAGTCCATGC-3′ and 5′-TTCAGCTCTGGGATGACCTT-3′; rat ttc3, 5′-TTCAAAGGTGGCAGATGAGG-3′ and 5′-GTAACTCTGTAAAGGCCTGG-3′; rat gapdh, 5′-CTCATGACCACAGTCGATGC-3′ and 5′-TTCAGCTCTGGGATGACCTT-3′. PCR products were separated by 1% agarose gel and observed using a Gel-Doc system (Bio-Rad).
RNA Interference
Four types of siRNAs of TTC3 were synthesized as described previously (23), and scrambled RNAs were used as a negative control. A total of 100 pmol of scrambled RNAs or four types of TTC3 siRNAs (25 pmol each) was transfected into NHLFs at 70% confluence in a 10-cm diameter culture dish using HilyMax reagents in the same manner used for transfection of Akt plasmids. To assess the effect of TTC3 siRNA on Akt ubiquitination, Myr Akt/Myc-His and Ubi-HA plasmids were introduced to NHLFs 24 h after siRNA transfection.
Detection of Ubiquitinated Akt in Rat Lungs
Akt ubiquitination in rat lungs was confirmed by two methods, ubiquitinated protein pulldown and immunoprecipitation of Akt, followed by Western blot with anti-Akt and anti-Ubi antibodies, respectively. Proteins were extracted from rat lungs by homogenization in lysis buffer (50 mm HEPES, pH 7.5, 5 mm EDTA, 150 mm NaCl, 1% Triton X-100, protease inhibitor mixture, and phosphatase inhibitor mixture). For ubiquitinated protein pulldown, ubiquitinated proteins were enriched using a kit (ubiquitinated protein enrichment kit, Calbiochem), according to the manufacturer's instructions. Briefly, 500 μg of extracted proteins were incubated overnight with 50 μl of polyubiquitin affinity beads at 4 °C. The next day, the beads were washed three times with lysis buffer and suspended with 40 μl of 5× SDS-polyacrylamide gel loading buffer. After boiling and centrifugation, the supernatants were applied to an 8% SDS-polyacrylamide gel, and ubiquitinated Akt was detected by Western blot using anti-Akt antibody. For Akt immunoprecipitation, 200 μg of extracted proteins in 500 μl of lysis buffer were incubated overnight with 10 μl of anti-total Akt antibody at 4 °C. The next day, 50 μl of protein A/G beads (Santa Cruz Biotechnology) were incubated with the mixture at 4 °C. One hour later, the beads were washed three times with lysis buffer, followed by addition of 40 μl of 5× SDS-PAGE sample buffer. After boiling and centrifugation, ubiquitinated Akt was separated in an 8% SDS-polyacrylamide gel and identified with anti-Ubi antibody. Image densities were quantified with software (TINA, Germany).
Statistical Analysis
Data are expressed as the means ± S.D. and analyzed using analysis of variance, followed by the Tukey-Kramer method for multiple comparisons. p < 0.05 was considered statistically significant.
RESULTS
CSE Causes Cell Death and Akt Degradation through UPS
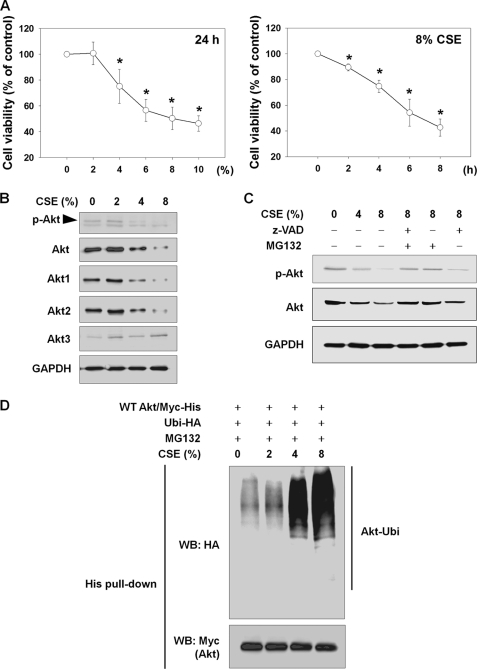
First, we determined CSE-induced cytotoxicity in NHLFs. NHLFs were exposed to CSE in the absence of serum, and cell viability was determined by an MTT assay. CSE from 4 to 10% significantly reduced cell viability in a concentration-dependent manner after 24 h (Fig. 1A, left panel), and 8% CSE caused rapid and progressive reduction in cell viability in a time-dependent manner (Fig. 1A, right panel). Next, we determined whether CSE might alter Akt and p-Akt levels. In parallel with reduction in cell viability, levels of total Akt, Akt1, Akt2, and p-Akt were also decreased by a 24-h CSE exposure in a concentration-dependent manner, whereas Akt3 levels were not changed (Fig. 1B). To evaluate involvement of caspases or the UPS in Akt and p-Akt reduction, NHLFs were treated with 10 μm MG132 (a proteasome inhibitor) or 10 μm Z-VAD (a broad spectrum caspase inhibitor) for the last 6 h during a 24-h period of exposure to CSE (Fig. 1C). After a 24-h period of exposure to 8% CSE, levels of Akt and p-Akt showed a dramatic drop, whereas Akt and p-Akt reduction was almost completely blocked by MG132. In addition, Z-VAD inhibited the decrease in Akt and p-Akt, but to a much lesser degree, compared with MG132. As we were encouraged by the finding that MG132 almost completely blocked CSE-induced Akt degradation, we attempted to determine whether CSE induced Akt degradation via Akt ubiquitination. To observe Akt ubiquitination, NHLFs were transfected with Myc-His-tagged wild-type Akt (WT Akt/Myc-His) and ubiquitin-hemagglutinin (Ubi-HA) plasmids, followed by exposure to CSE for 24 h with 1 μm MG132 to prevent Akt degradation by the 26 S proteasome (Fig. 1D). In a concentration-dependent manner, exposure to CSE resulted in dramatically induced Akt ubiquitination. Taken together, under the tested conditions, CSE caused Akt ubiquitination and degradation through the UPS in NHLFs.
FIGURE 1.
CSE causes cell death and Akt degradation through the UPS in NHLFs. A, CSE-induced cytotoxicity. To determine concentration- or time-dependent cytotoxicity, NHLFs were exposed to the indicated concentrations of CSE for 24 h or to 8% CSE for the indicated times in the absence of serum. Cell viability was evaluated by an MTT assay. Data represent the means ± S.D. Asterisks denote a statistically significant difference (p < 0.05) compared with the CSE-unexposed control. Number of each group is six. B, reduction in Akt and p-Akt by CSE exposure. After 24 h of CSE exposure, the reduction in total Akt, Akt1, Akt2, Akt3, and p-Akt (Ser-473) was assessed by Western blot. C, involvement of caspases and the proteasome in CSE-induced Akt reduction. NHLFs were exposed to CSE for 24 h. For the last 6 h, 10 μm Z-VAD or MG132 was added. D, Akt ubiquitination by CSE. NHLFs were transfected with the Myc-His-tagged wild-type Akt (WT Akt/Myc-His) and hemagglutinin-tagged ubiquitin (Ubi-HA) plasmids. After 24 h, NHLFs were exposed to CSE for another 24 h in serum-free DMEM with addition of 1 μm MG132 to prevent proteasomal degradation of ubiquitinated proteins. Ubiquitinated Akt was identified by His tag pulldown and Western blot (WB).
CSE Causes Early Akt Phosphorylation and Later Akt Reduction
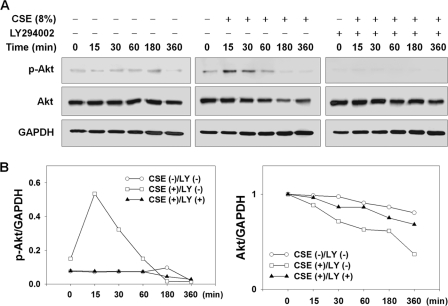
Phosphorylated (activated) Akt is known as a substrate for Akt E3 ligases (22, 23), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and nicotine, cigarette smoke components, rapidly activate Akt through PI3K (27); thus, we investigated the question of whether PI3K-mediated Akt phosphorylation might cause CSE-induced Akt degradation. In serum-deprived NHLFs pretreated with 10 μm LY294002 (a PI3K inhibitor), levels of Akt and p-Akt (Ser-473) were checked with lapse of CSE exposure time (Fig. 2). p-Akt levels showed an increase as early as 15 min and returned to the level before CSE exposure at 60 min. Thereafter, p-Akt was barely detected at 180 and 360 min after CSE exposure. Concomitantly, total Akt showed a continuous decrease, with noticeable reduction at 60, 180, and 360 min after CSE exposure. In contrast to an early rise in p-Akt, increase in total Akt was not observed at early time points. In comparison, in NHLFs pretreated with LY294002, CSE-induced total Akt reduction was significantly prevented. As expected, LY294002 induced complete blockade of both basal and CSE-induced Akt phosphorylation. In addition, LY294002 suppressed CSE-induced reduction in total Akt.
FIGURE 2.
CSE causes early Akt phosphorylation and later Akt reduction. NHLFs were deprived of serum for 6 h and pretreated with 10 μm LY294002 1 h prior to 8% CSE exposure. A, effect of LY294002 on CSE-induced reduction in Akt and p-Akt levels in NHLFs. B, quantitative results. Intensities of Akt and p-Akt are normalized to intensities of GAPDH.
CSE Induces Ubiquitination Preferentially in Active Akt
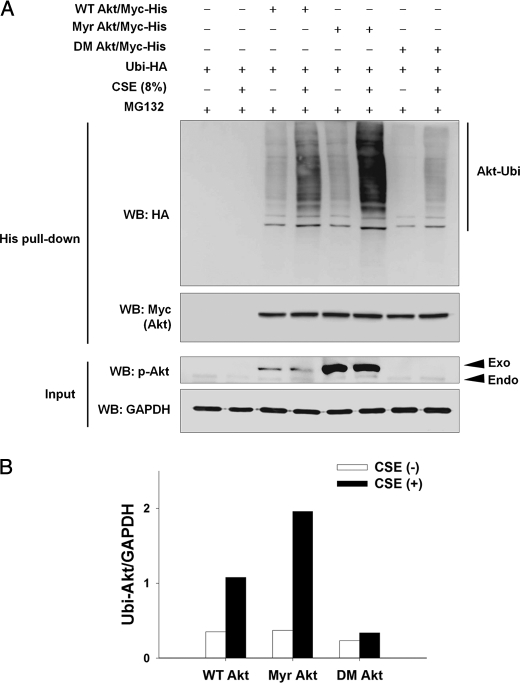
Based on the finding that PI3K-mediated Akt phosphorylation enhanced Akt degradation (Fig. 2), we hypothesized that phosphorylated and activated Akt might be preferential to inactive Akt as a substrate for ubiquitination. To prove the preferential ubiquitination of p-Akt, NHLFs were transfected with Myc-His-tagged wild-type Akt (WT Akt/Myc-His), constitutively active Akt (Myr Akt/Myc-His), or inactive Akt (double mutant T308A/S473A, DM Akt/Myc-His) plasmid with the Ubi-HA plasmid. Twenty four hours after transfection, NHLFs were exposed to 8% CSE for 8 h in the presence of 10 μm MG132, and ubiquitinated Akt was detected by His tag pulldown assay (Fig. 3). In NHLFs expressing WT Akt/Myc-His, CSE exposure resulted in a considerable increase of WT Akt ubiquitination. CSE-induced ubiquitination became much more prominent in Myr Akt than in WT Akt, whereas basal ubiquitination was similar between WT and Myr Akt without CSE exposure. In contrast to the prominent increase of ubiquitination in Myr Akt, CSE-induced ubiquitination was remarkably attenuated in DM Akt. Similarly, basal ubiquitination of DM Akt was lower than that of WT or Myr Akt.
FIGURE 3.
CSE induces preferential ubiquitination of active Akt. A, to determine preferential ubiquitination of active Akt, NHLFs were transfected with the Myc-His-tagged wild-type Akt (WT Akt/Myc-His), constitutively active myristoylated Akt (Myr Akt/Myc-His), or inactive Akt (T308A/S473A double mutant, DM Akt/Myc-His) plasmid together with the hemagglutinin-tagged ubiquitin (Ubi-HA) plasmid. Twenty four hours after transfection, NHLFs were exposed to 8% CSE for 8 h in the presence of 10 μm MG132. Ubiquitinated Akt was detected by His tag pulldown and Western blot (WB). Endo and Exo denote phosphorylated forms of endogenous and exogenously introduced Akt, respectively. GAPDH was used as an input control. B, quantitative results (ubiquitinated Akt/GAPDH).
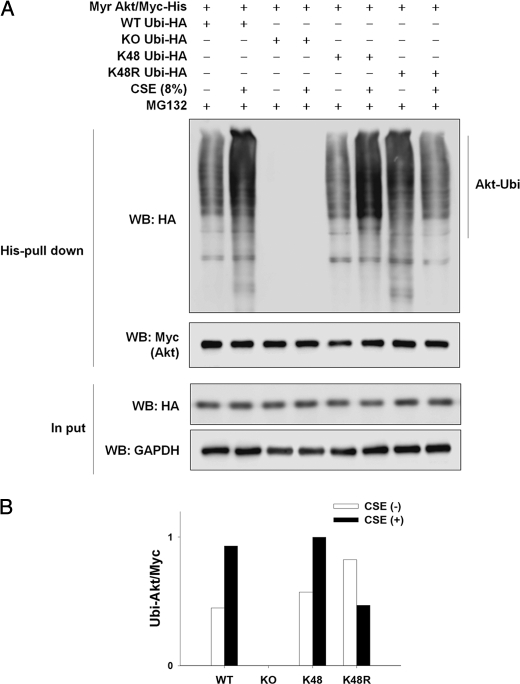
To determine whether CSE causes Lys-48-linked ubiquitination of Akt, Myr Akt/Myc-His plasmid was co-transfected into NHLFs with wild-type (WT) or lysine mutant (KO, all lysine residues were replaced with arginine; K48, all lysine residues except Lys-48 were replaced with arginine; K48R, only Lys-48 was replaced with arginine) Ubi-HA plasmid. In Fig. 4, CSE caused Akt ubiquitination in NHLFs expressing WT Ubi or K48 Ubi to a similar degree. In contrast, CSE exposure reduced Akt ubiquitination in NHLFs expressing K48R Ubi. As expected, in NHLFs expressing KO Ubi, no Akt ubiquitination was observed both in the absence and presence of CSE exposure.
FIGURE 4.
CSE induces Lys-48-linked ubiquitination of Akt. A, NHLFs were transfected with the Myr Akt/Myc-His plasmid together with the wild-type or lysine mutant HA-tagged Ubi plasmid (WT Ubi-HA, wild-type ubiquitin; KO Ubi-HA, all lysine residues replaced with arginine; K48 Ubi-HA, all lysine residues except Lys-48 replaced with arginine; K48R Ubi-HA, Lys-48R replaced with arginine). Twenty four hours after transfection, NHLFs were exposed to 8% CSE for 8 h in the presence of 10 μm MG132. Ubiquitinated Akt was detected by His tag pulldown and Western blot. Myc denotes exogenously introduced Myc-His-tagged Akt. GAPDH was used as an input control. B, quantitative results (ubiquitinated Akt/Myc).
CSE Alters HDM2/p53/p21 and ASK1/p38 Signaling Pathways and Leads to Cell Death via Decreased Akt Activity
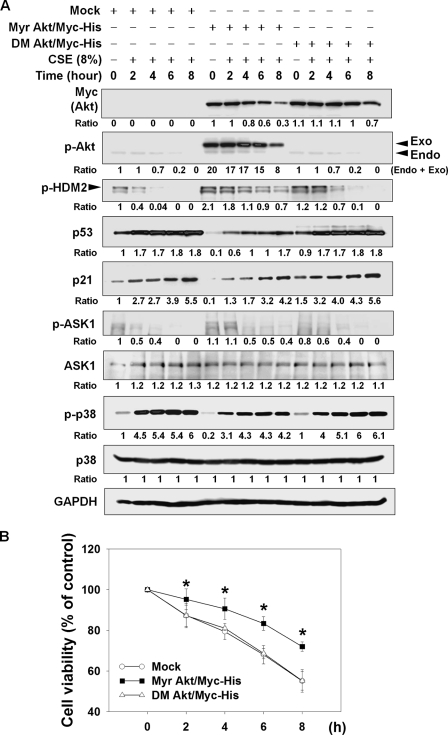
In the above experiments, we showed that CSE caused cell death and preferential ubiquitination and subsequent degradation of active Akt by the UPS. Akt is known to suppress cell death by regulating HDM2/p53/p21 and ASK1/p38 pathways, through stimulatory phosphorylation of Mdm2 at Ser-166 and Ser-186 (14) and inhibitory phosphorylation of ASK1 at Ser-83 (15), respectively. Therefore, we evaluated the question of whether CSE might affect these signaling pathways through Akt reduction. In NHLFs exposed to 8% CSE for 8 h, CSE exposure led to a progressive decrease of Akt, p-Akt, p-HDM2 (human homolog of Mdm2), and p-ASK1 in a time-dependent manner (Fig. 5A, Mock). Consistent with these changes, CSE exposure resulted in a prominent increase of p53, p21, and p-p38. Next, to assess the cause-result relationship between Akt reduction and alteration of these signaling pathways, we compared the alteration in these signaling molecules between NHLFs expressing active and inactive Akt. Whereas CSE caused a prominent reduction in p-HDM2 and p-ASK1 in NHLFs expressing inactive Akt (DM Akt/Myc-His), reduction in p-HDM2 and p-ASK1 was considerably lessened in NHLFs expressing active Akt (Myr Akt/Myc-His). Furthermore, induction of p53, p21, and p-p38 was much more prominent in inactive Akt-expressing NHLFs than in active Akt-expressing NHLFs throughout the entire observation period. Consistent with suppression of death-promoting signaling pathways by active Akt, CSE-induced cytotoxicity was significantly inhibited in active Akt-expressing NHLFs, compared with inactive Akt-expressing NHLFs (Fig. 5B). No difference in cytotoxicity was observed between NHLFs transfected with mock and inactive Akt (DM Akt/Myc-His) plasmids. These data imply that CSE induces cell death, possibly through reduction in Akt activity and subsequent alteration of HDM2/p53/p21 and ASK1/p38 pathways.
FIGURE 5.
CSE alters HDM2/p53/p21 and ASK1/p38 signaling pathways and leads to cell death via decreased Akt activity. A, suppression of CSE-induced alteration in the HDM2/p53/p21 and ASK1/p38 pathways by expression of active Akt. Twenty four hours after transfection of the mock, active Akt (Myr Akt/Myc-His), or inactive Akt (DM Akt/Myc-His) plasmid, NHLFs were exposed to 8% CSE in serum-free DMEM for the indicated times. Each protein was quantified after normalization to GAPDH. Endo and Exo denote phosphorylated forms of endogenous and exogenously introduced Akt, respectively. B, suppression of CSE-induced cytotoxicity by expression of active Akt in NHLFs. After 24 h of transfection of the plasmids, NHLFs were seeded on 96-well plates. The next day, NHLFs were exposed to 8% CSE for the indicated times. Cell viability was evaluated by the MTT assay. Data represent the means ± S.D. Asterisks denote statistically significant difference (p < 0.05, Meck versus Myr Akt/Myc-His). Number of each group is six.
TTC3 Mediates CSE-induced Akt Ubiquitination and Cytotoxicity in NHLFs
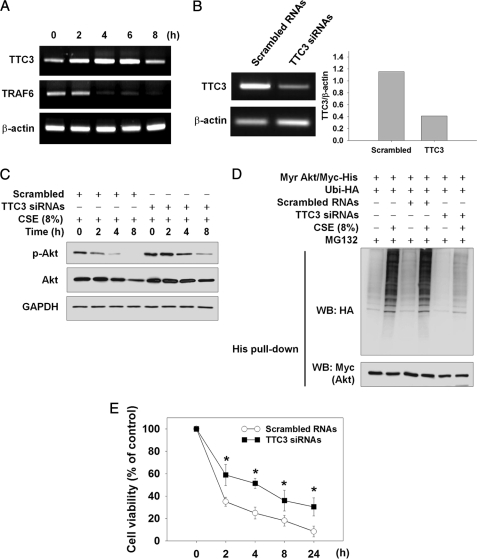
As TTC3 and TRAF6 are known as Akt ubiquitin E3 ligases for Akt degradation (23) and Akt activation (24), respectively, we attempted to determine whether CSE might regulate TTC3 and TRAF6 expression. CSE at 8% augmented TTC3 mRNA expression from 2 to 6 h after CSE exposure, and its expression subsided at 8 h (Fig. 6A). In contrast, TRAF6 mRNA was decreased from 4 to 8 h. To confirm involvement of TTC3 in CSE-induced Akt ubiquitination, TTC3 expression was suppressed by four types of TTC3 siRNAs. Considerably inhibited TTC3 mRNA expression by TTC3 siRNAs, compared with scrambled RNAs, was observed at 48 h after transfection (Fig. 6B). Knockdown of TTC3 expression by TTC3 siRNAs resulted in noticeable prevention of CSE-induced Akt and p-Akt reduction (Fig. 6C). In an additional experiment, we evaluated TTC3-mediated degradation of active Akt, because TTC3 is known to preferentially ubiquitinate active Akt (23). In NHLFs expressing active Akt (Myr Akt/Myc-His), TTC3 siRNAs prominently inhibited CSE-induced Akt ubiquitination, whereas scrambled RNAs did not affect Akt ubiquitination (Fig. 6D). Even without CSE exposure, basal ubiquitination of active Akt was lower in TTC3 siRNA-transfected NHLFs than in nontransfected or scrambled RNA-transfected NHLFs. Next, we attempted to determine whether or not knockdown of TTC3 expression might affect CSE-induced cell death. Compared with scrambled RNAs, TTC3 siRNAs significantly attenuated CSE-induced cytotoxicity at all time points from 2 to 24 h after CSE exposure (Fig. 6E).
FIGURE 6.
TTC3 mediates CSE-induced Akt ubiquitination and cytotoxicity in NHLFs. A, CSE-induced TTC3 expression. NHLFs were exposed to 8% of CSE for the indicated times. Expression of TTC3, TRAF6, and β-actin mRNA was assessed by RT-PCR. B, knockdown of TTC3 expression by TTC3 siRNAs. Forty eight hours after transfection with scrambled RNAs or four types of TTC3 siRNAs, TTC3 expression was evaluated by RT-PCR. C, prevention of CSE-induced Akt and p-Akt reduction by TTC3 knockdown. Forty eight hours after RNA transfection, NHLFs were exposed to 8% CSE. D, inhibition of ubiquitination of the active Akt (Myr Akt/Myc-His) by TTC3 knockdown. NHLFs were transfected with scrambled RNAs or TTC3 siRNAs and subsequently the Myr Akt/Myc-His and Ubi-HA plasmids. Twenty four hours later, NHLFs were exposed to 8% CSE for 8 h in the presence of 10 μm MG132. Ubiquitinated Akt was identified by His tag pulldown and Western blot (WB). E, suppression of CSE-induced cytotoxicity by TTC3 knockdown. Forty eight hours after siRNA transfection, NHLFs were seeded at a density of 2,000 cells per well on a 96-well plate. The next day, NHLFs were exposed to 8% CSE for the indicated times. Cell viability was determined by an MTT assay. Data represent the means ± S.D. Asterisks denote a statistically significant difference (p < 0.05, scrambled versus TTC3 siRNAs). Number of each group is six.
Cigarette Smoke Induces TTC3 Expression and Akt Degradation in Rat Lungs
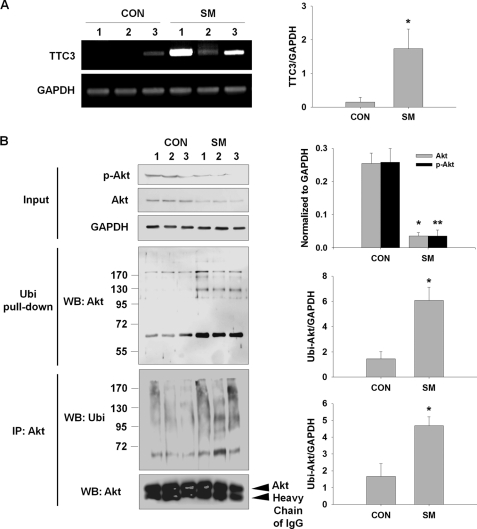
In the above experiments, we observed that CSE induced TTC3 expression and reduced Akt and p-Akt through TTC3-mediated ubiquitination of p-Akt and proteasomal degradation in NHLFs. To confirm these changes in vivo, we assessed Akt and TTC3 levels in rat lungs after exposure to cigarette smoke for 3 months. TTC3 mRNA levels were significantly elevated in cigarette smoke-exposed rats, compared with room air-exposed rats (Fig. 7A). In cigarette smoke-exposed rats (SM), Western blot showed that p-Akt and Akt levels were significantly lower than in room air-exposed rats (CON) (Fig. 7B, Input). According to the previous in vitro data, ubiquitinated Akt prominently increased in cigarette smoke-exposed rat lungs in both the ubiquitinated protein pulldown assay (Fig. 7B, Ubi Pull-down) and the Akt immunoprecipitation assay (Fig. 7B, IP: Akt).
FIGURE 7.
Cigarette smoke reduces Akt and p-Akt and induces ttc3 expression and Akt ubiquitination in rat lungs. Lewis male rats were exposed to cigarette smoke (SM) or clean room air (CON) for 3 months. A, induction of ttc3 expression. Expression of ttc3 and gapdh was assessed by RT-PCR. B, reduction in Akt and p-Akt and induction of Akt ubiquitination in rat lungs. Akt ubiquitination in rat lungs was confirmed by two methods, ubiquitinated protein pulldown (Ubi pull-down) and immunoprecipitation of Akt (IP: Akt), followed by Western blot (WB) with anti-Akt and anti-Ubi antibodies, respectively. Akt and p-Akt levels were determined before pulldown or immunoprecipitation (IP) (Input). Asterisks denote a statistically significant difference (p < 0.05) between the control and smoke groups. Number of each group is five.
DISCUSSION
In this study, we demonstrated the novel findings that cigarette smoke induced Akt degradation, possibly by TTC3-mediated Akt ubiquitination and proteasomal degradation, leading to cell death in NHLFs. Although Akt is regulated primarily by phosphorylation of Thr-308 in the activation loop and Ser-473 in the hydrophobic motif, mainly by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and mammalian target of rapamycin complex 2 (mTORC2) (11, 18), Akt dephosphorylation and degradation are suggested to contribute to Akt inactivation (18). Akt dephosphorylation by protein phosphatases may not necessarily lead to Akt degradation, and Akt can be degraded by the UPS-dependent pathway, caspase-dependent cleavage, and caspase-dependent ubiquitination (18). 4-Hydroxynonenal led to Akt degradation by caspase-3 in Jurkat cells (28), and TNF-α caused caspase-dependent Akt cleavage and Akt ubiquitination, both of which were blocked by a caspase-6 inhibitor in 3T3-L1 cells (20). In contrast, in this study, the broad spectrum caspase inhibitor, Z-VAD, partially prevented Akt decrease, whereas MG132 almost completely prevented Akt degradation. Therefore, degradation of Akt by the UPS appears to be a major pathway for Akt degradation in CSE-challenged NHLFs, and this discrepancy may originate from multiple Akt degradation pathways under different conditions.
Several E3 ligases for Akt ubiquitination, including TTC3 (23) and TRAF6, have been reported (24). TTC3 induces preferential ubiquitination of phosphorylated Akt and facilitates subsequent degradation (23). The TTC3 gene is located within the trisomic locus responsible for Down syndrome manifesting various developmental anomalies (29). Overexpressed TTC3 inhibits cell proliferation with suppression of the G2M-to-G1 transition, while TTC3 siRNAs oppositely induce cell proliferation (23). Furthermore, transfection of Myr Akt or TTC3 siRNAs results in recovery of p-Akt levels and cell cycle progression in Down syndrome cells exhibiting elevated TTC3 expression, reduced p-Akt, and accumulation in G2M phase. Similar to this finding, in this study, transfection of TTC3 siRNAs to NHLFs resulted in inhibition of CSE-induced Akt and p-Akt reduction and Akt ubiquitination, and prevented cell death (Fig. 6). Furthermore, TTC3 mRNA and ubiquitinated Akt were elevated, and Akt and p-Akt were decreased in rat lungs exposed to cigarette smoke (Fig. 7). Thus, TTC3 appears to play an important role in cigarette smoke-mediated lung injury, possibly via Akt degradation, which may induce cell death and prevent lung repair through inhibition of cell proliferation. It is of note that patients with Down syndrome have alveolar hypoplasia (30) and that impaired alveolar development is associated with decreased Akt activation in a hyperoxia-induced bronchopulmonary dysplasia model (31).
TRAF6 is also reported as a ubiquitin E3 ligase for Akt (24). In contrast to TTC3 that causes Lys-48-linked ubiquitination and degradation of Akt (23), TRAF6-mediated Lys-63-linked ubiquitination of Akt does not induce proteasomal degradation of Akt but activates Akt through translocation of ubiquitinated Akt to plasma membrane (24). Therefore, TRAF6 reduction that was caused by CSE exposure may enhance CSE-induced cytotoxicity. Therefore, deficiency in TRAF6 sensitizes mouse embryonic fibroblasts to serum deprivation and DNA-damaging agents (24). In addition, the DNA-damaging agents reduce Akt to a greater extent in Traf6−/− mouse embryonic fibroblasts than in Traf6+/+ mouse embryonic fibroblasts (24). In accordance, TRAF6-mediated Lys-63-linked Akt ubiquitination may inhibit Akt degradation. However, it still remains unknown whether TRAF6-mediated Akt ubiquitination can protect Akt from CSE-induced Lys-48-linked ubiquitination and degradation. In Fig. 4, Akt ubiquitination was reduced by CSE exposure in NHLFs expressing K48R Ubi, in contrast to increased Akt ubiquitination in NHLFs expressing WT Ubi or K48 Ubi. These data indicate that the nature of CSE-induced Akt ubiquitination (Lys-48-linked ubiquitination) may be different from that of Akt ubiquitination in the absence of CSE exposure (ubiquitination via lysine residues other than Lys-48). Fate of Akt ubiquitinated at lysine residues other than Lys-48 after CSE exposure and its pathological meaning need to be clarified in future investigations.
TTC3 has a nuclear localization signal and ubiquitinates nuclear Akt (23). However, in our study, Akt degradation by CSE does not appear to be confined to nuclei. In Myr Akt expressing NHLFs, CSE-induced Akt ubiquitination was observed in both the nuclear and cytosolic fractions (data not shown). Therefore, although TTC3 is known to act predominantly in nuclei, Akt ubiquitination in the cytosolic fraction may suggest the existence of other cytosolic E3 ligases for ubiquitination of active Akt in CSE-exposed NHLFs. In addition, we observed that cigarette smoke induced early Akt phosphorylation. Of particular interest, TTC3 is phosphorylated and activated by Akt in vitro (23). Thus, Akt degradation by TTC3 is understood as a negative feedback mechanism for termination of Akt activation; however, possible involvement of other kinases for TTC3 phosphorylation and activation in vivo is not excluded. Recently, the mammalian target of rapamycin complex 2 (mTORC2) was shown not only to phosphorylate Akt at Ser-473 but also to induce Akt degradation by the UPS, thus acting as a negative feedback regulator for termination of Akt activation (32). Hence, Akt degradation by the UPS appears to be a negative feedback mechanism for precise regulation of Akt, which is an important signaling hub for a variety of physiological responses. However, it remains unknown whether early Akt activation by exposure to cigarette smoke may enhance TTC3 activity and accelerate Akt degradation, leading to acceleration of cell death, rather than induction of cell survival and/or proliferation.
Because apoptosis and senescence are induced by cigarette smoke in vitro and in vivo (2–5), and Akt is a well known signaling hub for cell survival (11–16), suppression of Akt has been proposed to be related to cell death and dysfunction. However, only a few reports provide indirect evidence showing that Akt may be related to development of emphysema. In the context of emphysema development, the role of Akt is relatively well characterized in pulmonary vascular endothelial cells. VEGF receptor blockade caused Akt reduction in VEGFR-2 complex and development of emphysema (33). Cigarette smoke induced a reduction in protein levels and phosphorylation of VEGFR2 and endothelial nitric-oxide synthase (34), and cigarette smoke induced a decrease of PI3K, p-Akt, and p-eNOS in rat lungs (17). In emphysema development by VEGF blockade, a superoxide dismutase mimetic suppressed p-Akt reduction and emphysema development, suggesting that oxidative stress appears to mediate p-Akt reduction and cell death (35). Collectively, suppression of the VEGFR2-PI3K-Akt pathway by cigarette smoke, which directly and indirectly evokes oxidative stress, appears not only to affect cell survival but also to induce endothelial dysfunction.
Similarly, CSE causes suppression of proliferation (36), apoptosis (37), senescence (3), and inhibition of collagen gel contraction (38) in lung fibroblasts in vitro, and it reduces proliferation (39), chemotaxis, and collagen gel contraction (40) in lung fibroblasts from patients with COPD. Considering the structural support by lung fibroblasts, Akt may play an important role in maintenance of lung integrity by lung fibroblasts, because PI3K/Akt signaling is involved in the production of extracellular matrix proteins. In human lung fibroblasts, collagen 1A1 mRNA is stabilized in a PI3K-dependent manner (41), and TGF-β induces elastin expression, which is abolished by LY294002 or Akt2 siRNA in human lung fibroblasts (42). Similarly, fibronectin assembly is impaired in Akt1-null mouse embryonic fibroblasts (43). Therefore, considering the physiological role of lung fibroblasts and Akt involvement in cell survival/proliferation and synthesis of extracellular matrix proteins, cigarette smoke-induced Akt degradation may contribute to impairment of lung fibroblast-mediated repair and eventually culminate in emphysema development.
In this study, we observed that cigarette smoke reduced Akt and p-HDM2, together with induction of p53 and p21. In addition, forced expression of active Akt (Myr Akt/Myc-His) resulted in increased p-HDM2, reduced p53 and p21, and increased cell survival, compared with inactive Akt (DM Akt/Myc-His, Fig. 5). Akt is known to suppress cell death by activating phosphorylation of HDM2 and subsequent p-HDM2-mediated ubiquitination and degradation of p53 (14), which is upstream to p21 (44). Therefore, cigarette smoke is supposed to contribute to cell death, partly via Akt degradation and reduced phosphorylation of HDM2, resulting in an increase of p53 and p21. In human pulmonary artery endothelial cells, CSE induced p53, and inhibition of p53 by a pharmacological inhibitor or p53 siRNA resulted in significant blockade of cigarette smoke-induced apoptosis (45). In addition to p53 induction by decreased p-HDM2, cigarette smoke may exert its cytotoxic action by activation of p53 through the ASK1/p38 MAPK pathway. In cerebral endothelial cells, amyloid-β induced dephosphorylation of ASK1. Dephosphorylated ASK1 is released from 14-3-3, and activates p38 MAPK, leading to p53 phosphorylation and activation (46). Therefore, Akt acts as a survival signaling hub through negative control of multiple cytotoxic pathways.
In our recent study, we observed that bone marrow cell transplantation resulted in repair of emphysematous lungs damaged by chronic exposure to cigarette smoke (26). Of note, transplanted bone morrow cells suppressed apoptosis of alveolar septal cells and induced cell proliferation, along with Akt phosphorylation, implicating the importance of Akt activation in repair of emphysematous lungs. However, because of multiple facets of Akt, a cautious approach is needed to understand the role of Akt in COPD pathogenesis. Recently, Yoshida et al. (47) reported that rapamycin, a selective inhibitor of mTORC1, induced apoptosis of alveolar septal cells in room air-kept mice, although rapamycin induced an increase in the number of inflammatory cells in bronchoalveolar lavage fluid. However, paradoxically, rapamycin suppressed cigarette smoke-induced NF-κB activation and an increase in alveolar macrophages. As Akt activates mTORC1 (48) and is also involved in inflammation (49), suppression of Akt activity may be a therapeutic strategy in treatment of COPD, especially in an aggravating phase. However, Akt suppression may not guarantee prevention or recovery from emphysema and may even worsen it by acceleration of alveolar cell death. Therefore, the complicating roles of Akt in COPD must be further clarified in the context of both inflammation and emphysema for development of an appropriate therapeutic regimen.
In conclusion, we demonstrate for the first time that CSE induces Akt ubiquitination and proteasomal degradation, which led to cell death in NHLFs. In CSE-induced Akt degradation, preferential ubiquitination of active Akt by TTC3 was demonstrated. Finally, we confirmed increased TTC3 expression and Akt and p-Akt reduction in lungs of rats exposed to cigarette smoke for 3 months. These data may provide a clue to future study for investigation of a pathogenic role of Akt degradation and TTC3 induction in cigarette smoking-induced development of emphysema.
This research was supported by Grant 2010-0007209 from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology and by Grant A084753 from the Korea Healthcare Technology R&D Welfare & Family Affairs Project, Republic of Korea.
- COPD
- chronic obstructive pulmonary disease
- CSE
- cigarette smoke extract
- NHLF
- normal human lung fibroblasts
- UPS
- ubiquitin-proteasome system
- Ubi
- ubiquitin
- MTT
- 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- Z
- benzyloxycarbonyl
- DM
- double mutant.
REFERENCES
- 1. Churg A., Cosio M., Wright J. L. (2008) Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L612–L631 [DOI] [PubMed] [Google Scholar]
- 2. Park J. W., Kim H. P., Lee S. J., Wang X., Wang Y., Ifedigbo E., Watkins S. C., Ohba M., Ryter S. W., Vyas Y. M., Choi A. M. (2008) J. Immunol. 180, 4668–4678 [DOI] [PubMed] [Google Scholar]
- 3. Nyunoya T., Monick M. M., Klingelhutz A., Yarovinsky T. O., Cagley J. R., Hunninghake G. W. (2006) Am. J. Respir. Cell Mol. Biol. 35, 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsuji T., Aoshiba K., Nagai A. (2006) Am. J. Respir. Crit. Care Med. 174, 886–893 [DOI] [PubMed] [Google Scholar]
- 5. Nyunoya T., Monick M. M., Klingelhutz A. L., Glaser H., Cagley J. R., Brown C. O., Matsumoto E., Aykin-Burns N., Spitz D. R., Oshima J., Hunninghake G. W. (2009) Am. J. Respir. Crit. Care Med. 179, 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pagano M. (1997) FASEB J. 11, 1067–1075 [DOI] [PubMed] [Google Scholar]
- 7. Pickart C. M. (2001) Annu. Rev. Biochem. 70, 503–533 [DOI] [PubMed] [Google Scholar]
- 8. Osoata G. O., Yamamura S., Ito M., Vuppusetty C., Adcock I. M., Barnes P. J., Ito K. (2009) Biochem. Biophys. Res. Commun. 384, 366–371 [DOI] [PubMed] [Google Scholar]
- 9. Adenuga D., Yao H., March T. H., Seagrave J., Rahman I. (2009) Am. J. Respir. Cell Mol. Biol. 40, 464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes P. J. (2009) Annu. Rev. Physiol. 71, 451–464 [DOI] [PubMed] [Google Scholar]
- 11. Franke T. F. (2008) Oncogene 27, 6473–6488 [DOI] [PubMed] [Google Scholar]
- 12. Manning B. D., Cantley L. C. (2007) Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou B. P., Liao Y., Xia W, Spohn B., Lee M. H., Hung M. C. (2001) Nat. Cell Biol. 3, 245–252 [DOI] [PubMed] [Google Scholar]
- 14. Zhou B. P., Liao Y., Xia W., Zou Y., Spohn B., Hung M. C. (2001) Nat. Cell Biol. 3, 973–982 [DOI] [PubMed] [Google Scholar]
- 15. Kim A. H., Khursigara G., Sun X., Franke T. F., Chao M. V. (2001) Mol. Cell. Biol. 21, 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. (2001) EMBO Rep. 2, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marwick J. A., Edirisinghe I., Arunachalam G., Stevenson C. S., Macnee W., Kirkham P. A., Rahman I. (2010) J. Inflamm. 7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liao Y., Hung M. C. (2010) Am. J. Transl. Res. 2, 19–42 [PMC free article] [PubMed] [Google Scholar]
- 19. Riesterer O., Zingg D., Hummerjohann J., Bodis S., Pruschy M. (2004) Oncogene 23, 4624–4635 [DOI] [PubMed] [Google Scholar]
- 20. Medina E. A., Afsari R. R., Ravid T., Castillo S. S., Erickson K. L., Goldkorn T. (2005) Endocrinology 146, 2726–2735 [DOI] [PubMed] [Google Scholar]
- 21. Yan D., Guo L., Wang Y. (2006) J. Cell Biol. 174, 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiang T., Ohashi A., Huang Y., Pandita T. K., Ludwig T., Powell S. N., Yang Q. (2008) Cancer Res. 68, 10040–10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suizu F., Hiramuki Y., Okumura F., Matsuda M., Okumura A. J., Hirata N., Narita M., Kohno T., Yokota J., Bohgaki M., Obuse C., Hatakeyama S., Obata T., Noguchi M. (2009) Dev. Cell 17, 800–810 [DOI] [PubMed] [Google Scholar]
- 24. Yang W. L., Wang J., Chan C. H., Lee S. W., Campos A. D., Lamothe B., Hur L., Grabiner B. C., Lin X., Darnay B. G., Lin H. K. (2009) Science 325, 1134–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohn A. D., Takeuchi F., Roth R. A. (1996) J. Biol. Chem. 271, 21920–21926 [DOI] [PubMed] [Google Scholar]
- 26. Huh J. W., Kim S. Y., Lee J. H., Lee J. S., Ta Q. V., Kim M. J., Oh Y. M., Lee Y. S., Lee S. D. (2011) Am. J. Physiol. Lung Cell. Mol. Physiol., in press [Google Scholar]
- 27. West K. A., Brognard J., Clark A. S., Linnoila I. R., Yang X., Swain S. M., Harris C., Belinsky S., Dennis P. A. (2003) J. Clin. Invest. 111, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W., Akhand A. A., Takeda K., Kawamoto Y., Itoigawa M., Kato M., Suzuki H., Ishikawa N., Nakashima I. (2003) Cell Death Differ. 10, 772–781 [DOI] [PubMed] [Google Scholar]
- 29. Tsukahara F., Hattori M., Muraki T., Sakaki Y. (1996) J. Biochem. 120, 820–827 [DOI] [PubMed] [Google Scholar]
- 30. Cooney T. P., Thurlbeck W. M. (1982) N. Engl. J. Med. 307, 1170–1173 [DOI] [PubMed] [Google Scholar]
- 31. Alphonse R. S., Vadivel A., Coltan L., Eaton F., Barr A. J., Dyck J. R., Thébaud B. (2011) Am. J. Respir. Cell Mol. Biol. 44, 146–154 [DOI] [PubMed] [Google Scholar]
- 32. Wu Y. T., Ouyang W., Lazorchak A. S., Liu D., Shen H. M., Su B. (2011) J. Biol. Chem. 286, 14190–14198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kasahara Y., Tuder R. M., Taraseviciene-Stewart L., Le Cras T. D., Abman S., Hirth P. K., Waltenberger J., Voelkel N. F. (2000) J. Clin. Invest. 106, 1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edirisinghe I., Yang S. R., Yao H., Rajendrasozhan S., Caito S., Adenuga D., Wong C., Rahman A., Phipps R. P., Jin Z. G., Rahman I. (2008) FASEB J. 22, 2297–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tuder R. M., Zhen L., Cho C. Y., Taraseviciene-Stewart L., Kasahara Y., Salvemini D., Voelkel N. F., Flores S. C. (2003) Am. J. Respir. Cell Mol. Biol. 29, 88–97 [DOI] [PubMed] [Google Scholar]
- 36. Nakamura Y., Romberger D. J., Tate L., Ertl R. F., Kawamoto M., Adachi Y., Mio T., Sisson J. H., Spurzem J. R., Rennard S. I. (1995) Am. J. Respir. Crit. Care Med. 151, 1497–1503 [DOI] [PubMed] [Google Scholar]
- 37. Carnevali S., Petruzzelli S., Longoni B., Vanacore R., Barale R., Cipollini M., Scatena F., Paggiaro P., Celi A., Giuntini C. (2003) Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L955–L963 [DOI] [PubMed] [Google Scholar]
- 38. Carnevali S., Nakamura Y., Mio T., Liu X., Takigawa K., Romberger D. J., Spurzem J. R., Rennard S. I. (1998) Am. J. Physiol. 274, L591–L598 [DOI] [PubMed] [Google Scholar]
- 39. Holz O., Zühlke I., Jaksztat E., Müller K. C., Welker L., Nakashima M., Diemel K. D., Branscheid D., Magnussen H., Jörres R. A. (2004) Eur. Respir. J. 24, 575–579 [DOI] [PubMed] [Google Scholar]
- 40. Togo S., Holz O., Liu X., Sugiura H., Kamio K., Wang X., Kawasaki S., Ahn Y., Fredriksson K., Skold C. M., Mueller K. C., Branscheid D., Welker L., Watz H., Magnussen H., Rennard S. I. (2008) Am. J. Respir. Crit. Care Med. 178, 248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ricupero D. A., Poliks C. F., Rishikof D. C., Cuttle K. A., Kuang P. P., Goldstein R. H. (2001) Am. J. Physiol. Cell Physiol. 281, C99–C105 [DOI] [PubMed] [Google Scholar]
- 42. Kuang P. P., Zhang X. H., Rich C. B., Foster J. A., Subramanian M., Goldstein R. H. (2007) Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L944–L952 [DOI] [PubMed] [Google Scholar]
- 43. Somanath P. R., Kandel E. S., Hay N., Byzova T. V. (2007) J. Biol. Chem. 282, 22964–22976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu J., Zhang L. (2005) Biochem. Biophys. Res. Commun. 331, 851–858 [DOI] [PubMed] [Google Scholar]
- 45. Damico R., Simms T., Kim B. S., Tekeste Z., Amankwan H., Damarla M., Hassoun P. M. (2011) Am. J. Respir. Cell Mol. Biol. 44, 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu M. J., Hsu C. Y., Chen B. C., Chen M. C., Ou G., Lin C. H. (2007) J. Neurosci. 27, 5719–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoshida T., Mett I., Bhunia A. K., Bowman J., Perez M., Zhang L., Gandjeva A., Zhen L., Chukwueke U., Mao T., Richter A., Brown E., Ashush H., Notkin N., Gelfand A., Thimmulappa R. K., Rangasamy T., Sussan T., Cosgrove G., Mouded M., Shapiro S. D., Petrache I., Biswal S., Feinstein E., Tuder R. M. (2010) Nat. Med. 16, 767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zoncu R., Efeyan A., Sabatini D. M. (2011) Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Di Lorenzo A., Fernández-Hernando C., Cirino G., Sessa W. C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14552–14557 [DOI] [PMC free article] [PubMed] [Google Scholar]