Abstract
The physiological roles of taurine, a product of cysteine degradation and one of the most abundant amino acids in the body, remain elusive. Taurine deficiency leads to heart dysfunction, brain development abnormalities, retinal degradation, and other pathologies. The taurine synthetic pathway is proposed to be incomplete in astrocytes and neurons, and metabolic cooperation between these cell types is reportedly needed to complete the pathway. In this study, we analyzed taurine synthesis capability as reported by incorporation of radioactivity from [35S]cysteine into taurine, in primary murine astrocytes and neurons, and in several transformed cell lines (human (SH-SY5Y) and murine (N1E-115) neuroblastoma, human astrocytoma (U-87MG and 1321 N1), and rat glioma (C6)). Extensive incorporation of radioactivity from [35S]cysteine into taurine was observed in rat glioma cells as well as in primary mouse astrocytes and neurons, establishing the presence of an intact taurine synthesis pathway in these cells. Interestingly, exposure of cells to cysteine or cysteamine resulted in elevated intracellular hypotaurine without a corresponding increase in taurine levels, suggesting that oxidation of hypotaurine limits taurine synthesis in cells. Consistent with its role as an organic osmolyte, taurine synthesis was stimulated under hypertonic conditions in neurons.
Keywords: Amino Acid, Brain Metabolism, Metabolism, Oxidation-reduction, Sulfur, cysteine, Taurine
Introduction
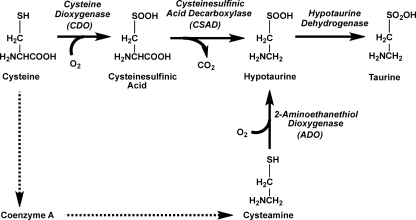
Taurine, or ethanesulfonic acid, is one of the most abundant amino acids in the body whose physiological role remains elusive. Its biosynthesis involves the sequential oxidation of cysteine to cysteinesulfinic acid, catalyzed by cysteine dioxygenase, decarboxylation by cysteinesulfinate decarboxylase, and oxidation of the resulting hypotaurine to taurine by a putative hypotaurine dehydrogenase (Fig. 1). The latter enzyme has, however, not been purified to homogeneity and remains uncharacterized (1–3). In fact, it has been suggested that oxidation of hypotaurine to taurine might occur via a nonenzymatic reaction in cells (4–6). Hypotaurine can also be produced via oxidation of cysteamine, the end product of coenzyme A degradation (Fig. 1). Cysteamine oxidation is catalyzed by 2-aminoethanethiol dioxygenase, which is widely expressed in mammalian tissues (7).
FIGURE 1.
Schematic showing pathways for hypotaurine and taurine synthesis in mammals.
Although it is generally believed that taurine cannot be further metabolized and is excreted by the kidney or as a bile component, there are several older reports in the literature on the conversion of taurine to isethionic acid in mammalian tissues (8–11). Various roles have been ascribed to taurine, including regulation of osmotic pressure (12–14), neuromodulatory (3, 15) and immunomodulatory (16) factors, and as an antioxidant for detoxifying hypochlorous acid, forming taurine chloramine (12). Taurine deficiency is associated with heart dysfunction, brain development abnormalities, retinal degradation, and other pathologies (3, 17). Taurine is actively transported into cells by a specific Na+-dependent transporter (TAUT) and accumulates to high concentrations particularly in the liver, muscle, and retina. Genetic disruption of the taut gene encoding the taurine transporter results in decreased tissue taurine levels and is associated with a number of abnormalities, including blindness, a low capacity for physical exercise, and decreased fertility (18).
Regulatory mechanisms exist in cells to stabilize intracellular volume and rely on a variety of inorganic ions and organic osmolytes, including taurine, which is abundant. In the brain, small molecule osmolytes like taurine might be better suited than ions for volume regulation because significant ion fluxes would perturb the transmembrane potential and, therefore, electrical activity (19). In fact, taurine efflux and intracellular accumulation under hypotonic and hypertonic conditions, respectively, have been observed in astrocytes, neurons, and brain slices (13, 20, 21). Taurine levels are highest in the developing brain at a time when the concentration of other amino acids is relatively low.
In the brain, biosynthesis of taurine is postulated to involve metabolic cooperation between astrocytes and neurons. Neurons reportedly lack cysteinesulfinic acid decarboxylase (22) and are thought to either rely on astrocytes for the provision of hypotaurine (23) or to acquire taurine via transport. Interestingly, coculture of neurons and astrocytes leads to a 10-fold decrease in the hypotaurine:taurine ratio in astrocytes (23). These results were interpreted as evidence for intercellular metabolic coupling, i.e. the presence of neurons modulates the hypotaurine:taurine ratio in astrocytes, whereas the presence of astrocytes increases neuronal taurine content. However, these labeling studies were performed with supraphysiological concentrations of cysteine rather than the relevant extracellular form of this amino acid, cystine, raising questions about their biological relevance (23).
Cultured astrocytes, unlike neurons, exhibit fairly high concentrations of hypotaurine. However, the significance of hypotaurine accumulation in astrocytes is unclear (24). In the whole brain, hypotaurine is estimated to be present at approximately 1% of taurine levels (25). Hypotaurine is postulated to function as an antioxidant, and astrocytic hypotaurine is believed to be exported to neurons, where it is converted to taurine (3, 23, 24). Labeling rat astrocytes with [13C]cysteine results in labeling of intra- and extracellular hypotaurine and taurine, which were detected by NMR spectroscopy (24). It was estimated that after 72 h of labeling, 35% of the hypotaurine and 23% of the taurine pools were labeled.
In this study, we analyzed taurine synthesis capability in several transformed cell lines and in primary murine astrocytes and neurons. We report robust incorporation of radioactivity from [35S]cysteine into taurine in rat glioma cells (C6) as well as in primary mouse astrocytes and neurons. The rate of [35S]cysteine accumulation into the taurine pool in primary and transformed cell lines is comparable with its rate of accumulation in the glutathione pool, indicating the quantitative significance of the endogenous taurine synthesis pathway. Taurine synthesis was stimulated under hypertonic conditions in neurons but not in astrocytes. Interestingly, conditions that lead to elevated intracellular hypotaurine do not result in a corresponding increase in taurine levels, suggesting that oxidation of hypotaurine limits taurine synthesis, at least in brain cells.
EXPERIMENTAL PROCEDURES
Cell Culture Conditions
Human neuroblastoma SH-SY5Y and human astrocytoma U-87 MG cell lines were obtained from ATCC. Human astrocytoma 1321 N1, mouse neuroblastoma N1E-115, and rat glioma C6 cell lines were obtained from Dr. Steven Fisher (University of Michigan). Primary astrocytes and neurons were prepared from BalbC mice as described previously (26, 27). The protocols for animal handling were approved by the University Committee on Use and Care of Animals, University of Michigan.
All transformed cell lines (i.e. astrocytomas, neuroblastomas, and glioma) were cultured in 6-well plates with 100% medium change. The following media were used for cultures of transformed cell lines: DMEM/F12 (Invitrogen) for SH-SY5Y, DMEM (Invitrogen) for 1321 N1 and N1E-115, and Eagle's Minimal Essential Medium (ATCC) for U-87 MG and C6. All media contained 10% FBS (Hyclone, South Logan, UT), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen).
Astrocytes were cultured for three passages in DMEM/F12 medium containing 10% heat- inactivated FBS (Hyclone), 100 units/ml penicillin, and 100 μg/ml streptomycin. At the end of the third passage, primary astrocytes were seeded in 6-well (2 × 106 cells/well), 12-well (1 × 106 cells/well), or 24-well (5 × 105 cells/well) plates and incubated for a week (with 50% of the medium being changed every third day). The purity of astrocytes was determined as described previously (27) and was ≥ 93%.
Neurons were cultured in 6-well (6 × 105 cells/well) or 24-well (1 × 105 cells/well) plates in neurobasal medium (Invitrogen) containing B27 supplement (1×) (Invitrogen), 2 mm l-glutamine and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively) on laminin- (Invitrogen) and poly-D-lysine- (Sigma) coated glass coverslips (26, 27). The purity of neurons was assessed by staining with antibody to anti-β III tubulin (TUJ1) (Millipore, Billerica, MA) and found to be > 95% as visualized microscopically.
In all cases, cells were cultured with 0.5, 1, and 2 ml of medium/well in 24-, 12-, and 6-well plates, respectively. At the beginning of each experiment, 100% of the medium was changed for all cell types except neurons, for which 50% of the medium was changed. Simultaneously with the medium change, other reagents were added as desired.
To prepare the hypertonic medium, 10 μl of 5 m NaCl solution was added per ml of incubation medium. To detect incorporation of cysteine into different metabolites, 0.2 μl of [35S]cysteine (PerkinElmer Life Sciences), 10 mCi/ml, > 1000 Ci/mmol) was added per 1 ml of incubation medium. All stock solutions were sterilized by filtration through a 0.2-μm filter.
Incubation of human astrocytoma U-87 MG cells and primary mouse astrocytes in media supplemented with dialyzed FBS was performed as follows. Astrocytoma cells were split into new plates in medium supplemented either with 10% standard or dialyzed FBS (Hyclone). After 3 days of incubation, the medium was changed (standard or dialyzed, respectively), and 48 h later, cells were harvested. For primary astrocytes, 100% of the medium was replaced with standard medium or with medium supplemented with 10% dialyzed FBS. Three to four days later, a second 100% medium change was done (standard or dialyzed, respectively), and 48 h later, cell samples were collected. Cells incubated in the media supplemented with standard FBS were used as controls. The use of dialyzed FBS did not appear to affect the appearance of primary astrocytes or rat glioma cells nor influence their growth rate under our experimental conditions.
Sample Preparation and Metabolite Analysis
To measure taurine levels in the culture medium, 100 μl of the medium was mixed with 100 μl of 10% TCA. For analysis of intracellular metabolite levels, the culture medium was aspirated, cells were washed twice with cold PBS, scraped with a plastic spatula, and resuspended in a small volume of PBS. For analysis of thiol metabolites, 100 μl of cell suspension was mixed with an equal volume of metaphosphoric acid solution (16.8 mg/ml HPO3, 2 mg/ml EDTA, 9 mg/ml NaCl). For taurine and hypotaurine analysis, 100 μl of cell suspension was mixed with 100 μl of 10% TCA. For protein analysis, 30 μl of cell suspension was mixed with 30 μl of lysis buffer (20 mm HEPES (pH 7.5), 25 mm KCl, 0.5% Nonidet P-40, 10 μl/ml protease inhibitor mixture for mammalian cells and tissue extracts (Sigma), 25 μg/ml tosyllysine chloromethylketone, 17 μg/ml phenylmethylsulfonyl fluoride). When cultures from 12- or 24-well plates were used, cells from 2 or 4 wells, respectively, were pooled prior to processing. Samples were stored at −80 °C until use. For metabolite and protein analysis, samples were thawed, vortexed, and centrifuged, and supernatants were used.
Thiols (GSH, GSSG, and cysteine) were analyzed following derivatization with iodoacetic acid and dinitrofluorobenzene followed by separation on a 300 × 3.9 mm μ Bondapak-NH2 HPLC column (Waters, Milford, MA) as described previously (28). To measure incorporation of radioactivity into thiol metabolites, HPLC fractions corresponding to the metabolite peaks were collected, and radioactivity in the fractions was measured using a liquid scintillation counter.
Taurine and hypotaurine concentrations were analyzed as described previously (29). Briefly, samples were neutralized to pH 7–8 with saturated K2CO3 solution and diluted 5- (medium samples) or 10-fold (cell samples) with sodium borate buffer (0.2 m (pH 9.6)). A 50-μl aliquot of diluted sample was derivatized with 25 μl of o-phthaldialdehyde solution (15 mm o-phthaldialdehyde, 0.2% β-mercaptoethanol (v/v), 10% methanol (v/v) in 0.2 m sodium borate buffer (pH 9.6)) for 1 min at 10 °C. A 10-μl aliquot of the derivatized sample was then injected into a 4.6 × 150 mm ZORBAX Eclipse XDB-C18 (5 μm) analytical HPLC column (Agilent, Santa Clara, CA) and eluted at a flow rate of 1 ml/min with a gradient of methanol (38% to 56% from 0 to 10 min, 56% to 80% from 10 to 15 min, 80% from 15 to 20 min) in 0.1 m sodium acetate buffer (pH 4.75). Eluted peaks were detected by fluorescence at 340 nm excitation and 450 nm emission wavelengths. To measure incorporation of radioactivity into taurine and hypotaurine, the neutralized samples were diluted 1:1 with the borate buffer, and after derivatization with o-phthaldialdehyde solution, 50 μl of each sample was injected into the column. Eluted peaks were detected by absorption at 340 nm. HPLC fractions corresponding to taurine and hypotaurine peaks were collected, and radioactivity in these fractions was measured using a liquid scintillation counter.
Incorporation of radioactivity in the protein pool was measured in precipitates obtained after addition of 10% TCA to cell samples. The protein precipitate was washed twice with 6% TCA and then dissolved in 100 μl of 0.5 m NaOH and counted in a liquid scintillation counter. Approximately 2–4.5% of the total extracellular radioactivity was incorporated into intracellular protein and metabolite pools in 10 h. We note that the extracellular volume is approximately 100-fold greater than the intracellular volume under our cell culture conditions.
All intracellular parameters were normalized to protein concentration in cell suspension. The protein concentration in samples was measured using the Bradford reagent (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard.
RESULTS
Cysteamine and Cystamine Increase Hypotaurine but Not Taurine Levels in Transformed Human Brain-derived Cell Lines
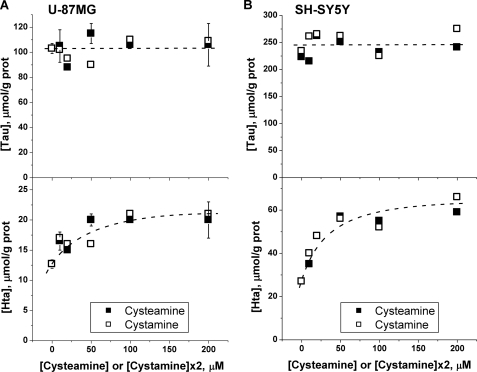
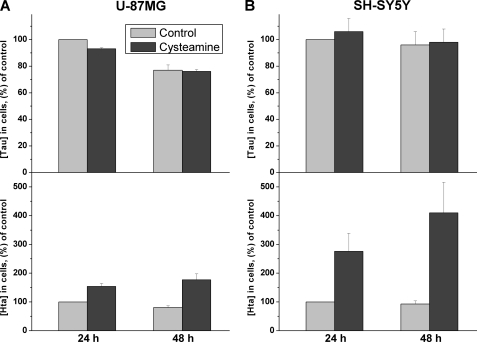
In preliminary experiments, we have found that incubation of human astrocytoma (U-87 MG) and neuroblastoma (SH-SY5Y) cells with varying concentrations of cysteamine or its oxidized disulfide form, cystamine, results in an approximately 2- to 4-fold increase in intracellular hypotaurine concentrations (Fig. 2). The dependence of hypotaurine accumulation on cysteamine or cystamine concentration indicates that 1) cystamine is rapidly converted to cysteamine because the dependence of hypotaurine concentration on cysteamine versus 2× cystamine is identical and 2) that hypotaurine formation is saturated at approximately 50 μm cysteamine in both cell lines. In contrast to hypotaurine concentrations, which remain significantly elevated over a 48-h period following exposure to cysteamine (Fig. 3) or cystamine, taurine concentrations were not similarly increased during prolonged incubation of cells with these precursors (Figs. 2 and 3). This result does not support the hypothesis that conversion of hypotaurine to taurine occurs nonenzymatically. Instead, it demonstrates that the capacity for enzymatic conversion of hypotaurine to taurine is either absent or that the conversion is tightly regulated in these cell lines.
FIGURE 2.
Elevation of intracellular hypotaurine does not affect taurine levels in human astrocytoma (U-87MG) (A) or human neuroblastoma (SH-SY5Y) (B) cells. Cells were incubated for 24 h with the indicated concentrations of cysteamine or cystamine in the medium, and intracellular taurine and hypotaurine concentrations were determined as described under “Experimental Procedures.” Symbols in A represent the mean ± S.D. of two independent samples.
FIGURE 3.
Time-dependent changes in intracellular taurine and hypotaurine concentrations in human astrocytoma (U-87MG) (A) and human neuroblastoma (SH-SY5Y) (B) cells, incubated with 200 μm extracellular cysteamine. The data represent the mean ± S.D. n = 4 and 6 for U-87MG and SH-SY5Y cells, respectively.
The Pathway for Oxidation of Cysteine to Taurine Is Intact in Primary Astrocytes and Neurons but Is Not Uniformly Preserved in Transformed Cell Lines
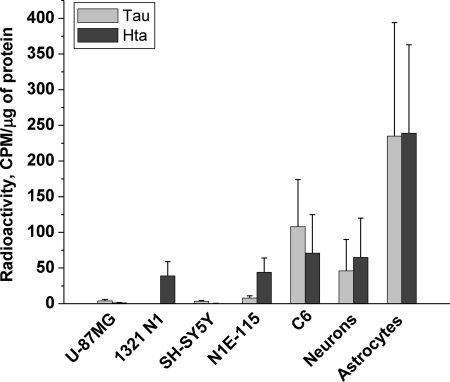
Next, we examined whether the pathway for taurine biosynthesis from cysteine is intact in several brain-derived transformed cell lines. For this, we monitored incorporation of [35S]cysteine into hypotaurine and taurine (Fig. 4). Of the five lines that were tested, only the rat glioma line, C6, showed significant incorporation of radioactivity into taurine (Fig. 4).
FIGURE 4.
Incorporation of [35S] from cysteine to taurine and hypotaurine in different cell types. Cells were incubated with 2 μCi/ml of [35S]cysteine in the medium for 10 h, and incorporation of radioactivity into the taurine and hypotaurine pools was determined as described under “Experimental Procedures.” Data are the mean ± S.D. n = 2 for U-87MG, 1321 N1, SH-SY5Y, and N1E-115 cell lines and n = 11, 4, and 6 for the C6 cell line, neurons, and astrocytes, respectively. CPM, counts per minute.
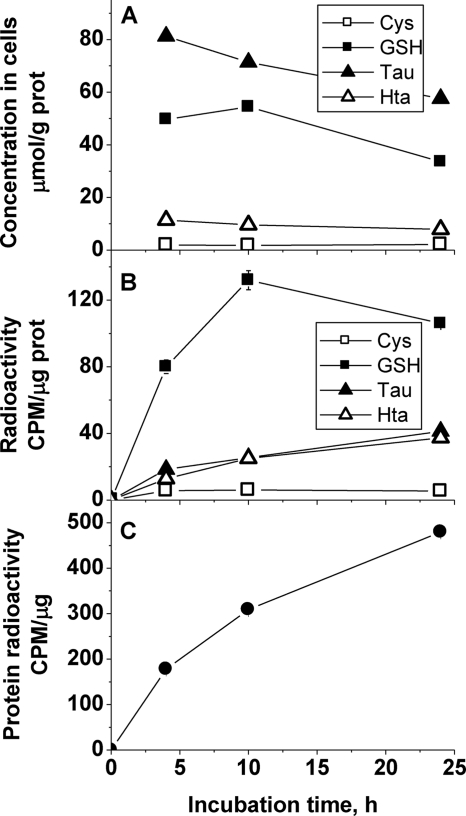
To estimate how much cysteine is partitioned into taurine versus protein and glutathione, incorporation of [35S]cysteine into these pools was tracked in the C6 cell line (Fig. 5). Although the concentrations of cysteine and hypotaurine were stable over 24 h, glutathione and taurine levels declined. However, the specific radioactivity increased in all metabolite pools within the first 10 h of incubation with the exception of cysteine. Radioactive cysteine added to the incubation medium equilibrates with the intracellular cysteine pool within 1–2 h, after which the specific radioactivity of intracellular cysteine is more or less constant. These results suggest a fairly high turnover of the glutathione, taurine, and hypotaurine pools. Although the taurine concentration is approximately 1.5-fold higher than glutathione in the C6 cell line, the initial rate of radiolabel incorporation into glutathione, determined within the first 10 h of incubation, is approximately 2-fold greater than in the hypotaurine plus taurine pools (Fig. 5), suggesting a higher rate of turnover in the glutathione pool.
FIGURE 5.
Time-dependent changes in concentrations of sulfur metabolites (A), incorporation of radioactivity into sulfur metabolites (B), and incorporation of radioactivity into proteins (C) in C6 rat glioma cells following incubation with [35S]-cysteine. Symbols represent the mean ± S.D. of two separate samples. Representative data from four independent experiments are shown. CPM, counts per minute.
Significant incorporation of radioactive cysteine into the protein pool, which was substantially greater than into other metabolite pools was observed in C6 cells, consistent with their rapidly proliferating phenotype.
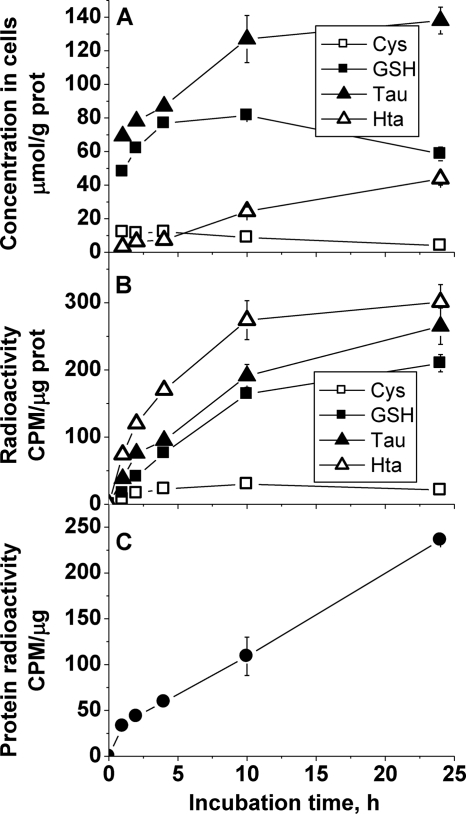
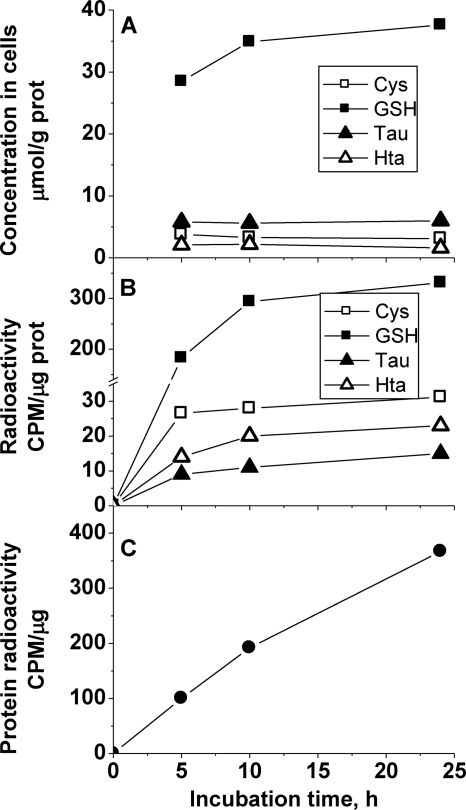
Partitioning of cysteine into the various pools was also monitored in primary neurons and astrocytes (Figs. 6 and 7). Both the absolute and relative concentrations of sulfur tied up in the glutathione and taurine pools showed cell line-specific variations. Thus, the relative concentration of taurine was approximately 1.5-fold higher than of glutathione in the rat glioma cell line (Fig. 5) and up to 2-fold higher in murine astrocytes (Fig. 6). However, the absolute concentration of these metabolites was approximately 2-fold higher in murine astrocytes compared with the rat glioma cell line. In contrast, the glutathione concentration was approximately 5-fold higher than taurine in murine neurons (Fig. 7). It should be noted, however, that the low taurine concentration in cultured neurons might be partially due to the absence of taurine from the neuron culture medium, which contained taurine-free B27 supplement instead of FBS. All other culture media used in this study were supplemented with 10% FBS, which contains taurine. Thus, cells could accumulate taurine from the medium via transmembrane transport (as also discussed below for experiments comparing cells cultured with normal versus dialyzed FBS).
FIGURE 6.
Time-dependent changes in sulfur metabolite concentrations (A), incorporation of radioactivity into sulfur metabolites (B), and incorporation of radioactivity into proteins (C) in primary mouse astrocytes following incubation with [35S]cysteine. Symbols represent the mean ± S.D. of two separate samples. Representative data from four independent experiments are shown. CPM, counts per minute.
FIGURE 7.
Time-dependent changes in sulfur metabolite concentrations (A), incorporation of radioactivity into sulfur metabolites (B), and incorporation of radioactivity into proteins (C) in primary mouse neurons following incubation with [35S]cysteine. Representative data from three independent experiments are shown. CPM, counts per minute.
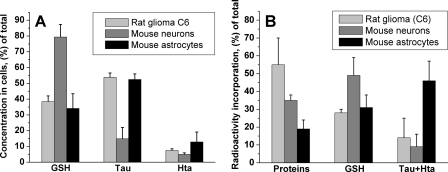
Cell-specific patterns of sulfur metabolite concentrations and distribution of [35S]cysteine between the metabolite and protein pools were seen (Fig. 8). In the fast-growing glioma cell line, approximately 55% of the radioactivity was associated with the protein pool at 10 h in comparison with approximately 35% in neurons and approximately 20% in astrocytes. The proportion of radiolabel incorporation into the glutathione pool was similar in all three cell types (approximately 30–50%). Astrocytes incorporated substantially more radioactivity in the combined hypotaurine/taurine pool (approximately 50%) than the other two cell types.
FIGURE 8.
Relative concentrations of sulfur metabolites (A) and incorporation of radioactivity into protein and metabolite pools (B) after 10 h of incubation with [35S]cysteine. Metabolite concentrations and radioactivity were normalized per gm of cell protein. The data summarize results from multiple repeats of experiments shown in Figs. 5–7 and represent the mean ± S.D. n = 8, 8, and 3 for rat glioma cells, astrocytes, and neurons, respectively.
Conversion of Hypotaurine to Taurine Is Limiting in Cells That Have an Intact Taurine Synthetic Pathway
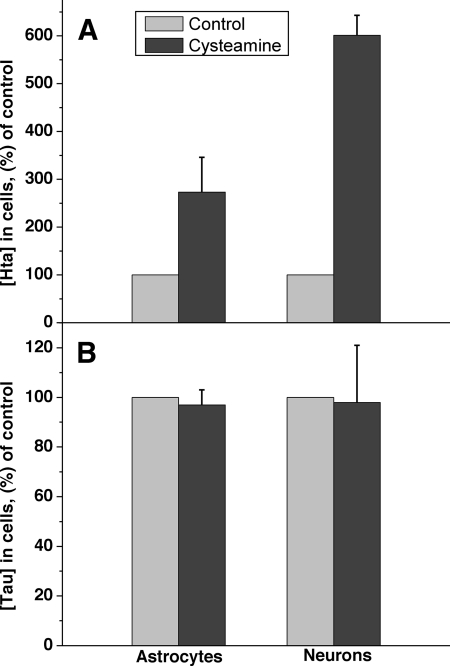
Because we observed that several transformed cell lines convert cysteamine to hypotaurine but not to taurine (Figs. 2 and 3), we investigated this issue with primary astrocytes and neurons, which convert cysteine to taurine. As with the U-87 MG and SH-SY5Y cell lines, addition of cysteamine to murine astrocytes or neurons led to a significant elevation of hypotaurine levels (Fig. 9A). Furthermore, hypotaurine levels also increased in response to supplementation with cysteine or cysteine sulfinic acid (not shown). However, a corresponding increase in the concentration of taurine was not observed (Fig. 9B).
FIGURE 9.
Incubation with exogenous cysteamine elicits an increase in intracellular hypotaurine but not taurine in cells capable of taurine synthesis. Cells were incubated for 48 h in the presence of 200 μm of cysteamine, and the intracellular hypotaurine (A) and taurine (B) concentrations were measured as described under “Experimental Procedures.” The concentrations are represented as percentage of untreated controls. Data are the mean ± S.D. n = 6 and 3 for astrocytes and neurons, respectively.
Taurine Import Is a Significant Contributor to the Intracellular Taurine Pool in the Astrocytomas but Not Primary Astrocytes
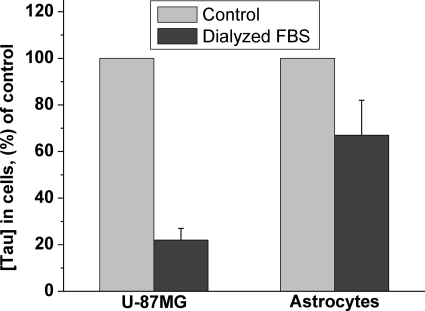
To assess the contribution of taurine import to the intracellular pool size, primary astrocytes and the U-87MG astrocytoma cell line were cultured in media containing dialyzed FBS and hence lacking detectable levels of taurine. The concentration of taurine in the culture medium supplemented with 10% FBS is approximately 15 μm, which, over time, can be concentrated to the millimolar levels seen inside cells. When cells were cultured in the media containing dialyzed FBS, the concentration of taurine decreased by 80% in the U-87 MG cell line, which is unable to synthesize taurine (Fig. 10). In contrast, the taurine concentration in primary astrocytes, which can synthesize taurine, diminished by approximately 35% (Fig. 10). Because neurons are cultured in a serum-free medium lacking taurine, the intracellular taurine pool in these cells must be derived via synthesis.
FIGURE 10.
Intracellular concentrations of taurine in the human astrocytoma cell line (U-87MG) and in primary murine astrocytes cultured in media containing untreated or dialyzed FBS as described under “Experimental Procedures.” Data are the mean ± S.D. n = 6 and 18 for U-87MG and astrocytes, respectively.
The U-87MG Astrocytoma Cells Activate Taurine Transport under Hypertonic Culture Conditions
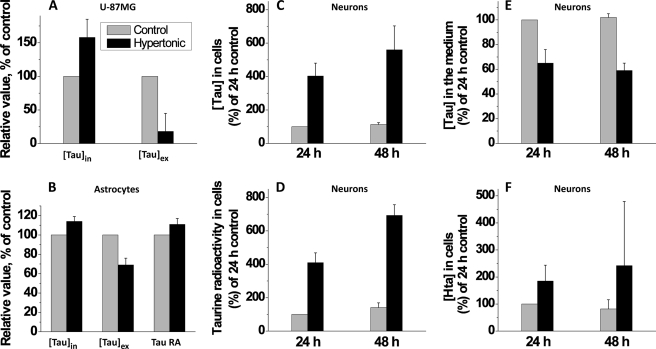
A major role ascribed to taurine in the central nervous system is osmoregulation. To test this hypothesis, we first examined the response of the U-87MG cell line, which is unable to synthesize taurine, to an increase in extracellular osmolarity (from 300 to 400 mosmole). Over a 48-h period, intracellular taurine levels in U-87MG cells increased by approximately 50% in response to hypertonic culture conditions, and this increase was accompanied by a significant decrease in extracellular taurine concentration (Fig. 11A).
FIGURE 11.
Hypertonic conditions increase intracellular taurine concentrations. A, human astrocytoma cells (U-87MG) were incubated for 48 h in normal (300 mosmol) or hypertonic (400 mosmol) medium. Data are the mean ± S.D. n = 8. B, primary mouse astrocytes were incubated for 48 h in normal (300 mosmol) or hypertonic (400 mosmol) medium, supplemented with [35S]cysteine. Data are the mean ± S.D. n = 4. C, primary mouse neurons were incubated for 48 h in normal (300 mosmol) or hypertonic (400 mosmol) medium supplemented with [35S]cysteine. [Tau]in, [Tau]ex, and Tau RA indicate taurine concentration in cells, in the medium, and radioactivity associated with the intracellular taurine pool, respectively. Data represent the mean ± S.D. n = of five to seven independent experiments.
Astrocytes Activate Taurine Synthesis and Transport under Hypertonic Culture Conditions
In response to hypertonic culture conditions, taurine concentration increased 14% in 48 h in primary astrocytes (Fig. 11B). The intracellular increase in taurine was accompanied by a more modest decrease in extracellular taurine levels versus the change observed with U-87 MG cells. To assess whether taurine synthesis was activated under hypertonic conditions, incorporation of the radiolabel from [35S]cysteine into taurine was monitored. An approximately 11% increase in radiolabel incorporation into the taurine pool was observed under hyperosmotic versus isoosmotic conditions (Fig 11B).
Neurons Activate Taurine Synthesis and Transport under Hypertonic Culture Conditions
Exposure of primary neurons to hypertonic culture conditions resulted in an approximately 6-fold increase in intracellular taurine concentration (Fig. 11C). This was accompanied by a corresponding increase in radiolabel incorporation from [35S]cysteine into taurine and a modest decrease in extracellular taurine concentrations (Fig. 11, D and E).
DISCUSSION
Despite the quantitatively significant investment of sulfur in the taurine pool, fundamental questions about the allocation of the biosynthetic responsibility between glial and neuronal cells for taurine synthesis are mired in controversy and remain unanswered. Thus, conflicting models have been proposed for the synthesis of taurine, primarily on the basis of localization studies on cysteine dioxygenase and cysteine sulfinic acid decarboxylase in neurons versus astrocytes (30) and propose 1) that the pathway is initiated in neurons and that cysteine sulfinic acid is exported to astrocytes or 2) that the pathway is initiated in astrocytes and that hypotaurine is exported to neurons. In addition, the contribution of the cysteamine pathway to taurine synthesis in these cells is not known. In this study, we have assessed the ability of primary and transformed brain-derived cells to synthesize taurine from cysteine and characterized the changes in taurine synthesis and transport in response to hypertonic culture conditions. We demonstrate that although incubation of brain-derived cell lines, primary astrocytes and neurons with cysteamine or cysteine, leads to a significant accumulation of intracellular hypotaurine, a corresponding increase in taurine concentration is not observed. These results suggest strongly that conversion of hypotaurine to taurine is catalyzed and that it is regulated. Although a putative hypotaurine dehydrogenase has been described (1–3), it has not been purified to homogeneity and characterized. Intracellular taurine pools are substantial; In the 1–50 mm range, depending on the tissue, the organism, sex, and age (3, 31, 32). Hence, unregulated oxidation of hypotaurine to taurine, which is coupled to reduction of an as yet unidentified cofactor, would result in large perturbations in its intracellular redox potential and, therefore, must be regulated. This suggests that unlike most metabolic pathways where the rate-limiting step is early in the sequence, in the taurine synthetic pathway, the ultimate step is at least partially rate-determining.
Incorporation of the radiolabel from [35S]cysteine into taurine in primary murine astrocytes and neurons and in the C6 rat glioma cell line provide unequivocal evidence for the capacity of these cell types to oxidize cysteine to taurine. These results lay to rest the controversy over whether or not the taurine biosynthetic pathway is intact in individual brain cell types (30). Metabolic cooperation between astrocytes and neurons has been proposed as a strategy for taurine biosynthesis. The controversy likely stems from the inability of many (but not all) transformed cell lines to convert cysteine to taurine as demonstrated in this study, which might be due to the repression of cysteine dioxygenase in transformed lines (34). In fact, our data confirm an earlier report demonstrating the ability of cultured rat astrocytes to synthesize taurine from cysteine (35).
Glutathione and taurine represent quantitatively significant pools of highly reduced and oxidized organic sulfur species, respectively. By tracing the fate of [35S]cysteine, we have estimated its partitioning into the glutathione and taurine pools in primary neurons, astrocytes, and in a glioma cell line (Fig. 8). We find cell line-specific patterns in incorporation of a radiolabel into these metabolite pools. Thus, in the rat glioma line and in murine neurons, 2- and 5-fold more radioactivity is incorporated into glutathione compared with the hypotaurine/taurine pool. In murine astrocytes the situation is reversed, and approximately 1.5-fold more radioactivity is incorporated into the hypotaurine/taurine pool compared with glutathione (Fig. 8). In all three cell types, turnover of the hypotaurine/taurine pool is comparable with that of glutathione and, thus, relatively rapid.
Proteins represent a third large intracellular sink for cysteine. As anticipated for the rapidly growing rat glioma cell line, the largest fraction of radiolabel incorporation from cysteine was into the protein pool. Astrocytes showed the lowest accumulation in the protein pool, whereas in neurons, the radioactivity in the protein pool was intermediate between the glutathione and taurine/hypotaurine pools. Interestingly, in all three cell types, 30–50% of the radiolabel was partitioned toward glutathione synthesis.
In the human astrocytoma U-87MG cell line, a significant (approximately 80%) depletion of the intracellular taurine pool was observed when cells were grown in taurine-free medium (Fig. 10). This result is consistent with U-87MG cells being dependent solely on taurine import for maintaining the intracellular pool of this metabolite. In contrast, the intracellular taurine pool in astrocytes is less susceptible to exogenous depletion of this metabolite because these cells are capable of taurine synthesis.
Cells possess volume regulatory mechanisms to deal with fluctuations in osmolarity. Cell swelling leads to extrusion of ions/osmolytes and, subsequently, to regulatory volume decrease (36). Cell shrinkage has the converse effect and leads to ion/osmolyte accumulation that achieves regulatory volume increase. Some metabolic pathways are known to be sensitive to cell volume changes. For instance, cell swelling increases glycogen synthesis and inhibits glycolysis, which serves to decrease the cellular concentration of carbohydrates (36). The effect of osmolarity changes on the sulfur metabolic pathway is not known.
The sensitivity of taurine transport to changes in the osmolarity of the culture medium has been noted previously (29, 37, 38). Hypoosmotic conditions stimulate taurine efflux from cells, whereas hyperosmotic conditions lead to taurine accumulation. Osmosensitive taurine release appears to occur via a volume-sensitive organic anion channel and is blocked by anion channel inhibitors (39). Interestingly, one such inhibitor significantly improves recovery in an experimental model for brain trauma/hypoxia (40). In cultured astrocytes, a 50% decrease in osmolarity leads to a 40-fold increase in taurine efflux rate (35). We find that the taurine concentration increases in the U-87MG astrocytoma line in response to hypertonic shock and is accompanied by a dramatic decrease in extracellular taurine levels. This result is consistent with activation of taurine influx into cells under hyperosmotic conditions. A similar response was observed in primary astrocytes, although the increase in intracellular taurine was due to both import and increased synthesis.
A dramatic increase in intracellular taurine levels was observed in murine neurons in response to hyperosmotic shock. The increase was due in part to increased import and in part to activation of taurine synthesis. The rise in intracellular neuronal taurine concentration in the face of constant extracellular taurine concentration (Fig. 11, C and E) can only be explained by activation of taurine synthesis under these conditions.
Pathological conditions that elicit neural cell swelling include ischemia, traumatic head injury and brain edema, hypoglycemia, prolonged hypoxia, hepatic encephalopathy, and hyponatremia. The latter is a common clinical condition resulting from volume dysregulation and affects between 2.5–5.0% of hospitalized patients and disproportionately affects the young and the elderly (41, 42). Depending on the magnitude, hyponatremia can elicit symptoms ranging from emesis and headaches to respiratory arrest, coma, and brain damage (33). Given the clinical relevance, the responsiveness of neurons and astrocytes to changes in osmotic conditions warrants investigation.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grant DK64959.
REFERENCES
- 1. Sumizu K. (1962) Biochim. Biophys. Acta 63, 210–212 [DOI] [PubMed] [Google Scholar]
- 2. Oja S. S., Kontro P. (1981) Biochim. Biophys. Acta 677, 350–357 [DOI] [PubMed] [Google Scholar]
- 3. Huxtable R. J. (1989) Prog. Neurobiol. 32, 471–533 [DOI] [PubMed] [Google Scholar]
- 4. Fiori A., Costa M. (1969) Acta Vitaminol. Enzymol. 23, 204–207 [PubMed] [Google Scholar]
- 5. Ricci G., Dupré S., Federici G., Spoto G., Matarese R. M., Cavallini D. (1978) Physiol. Chem. Phys. 10, 435–441 [PubMed] [Google Scholar]
- 6. Fellman J. H., Green T. R., Eicher A. L. (1987) Adv. Exp. Med. Biol. 217, 39–48 [DOI] [PubMed] [Google Scholar]
- 7. Dominy J. E., Jr., Simmons C. R., Hirschberger L. L., Hwang J., Coloso R. M., Stipanuk M. H. (2007) J. Biol. Chem. 282, 25189–25198 [DOI] [PubMed] [Google Scholar]
- 8. Read W. O., Welty J. D. (1962) J. Biol. Chem. 237, 1521–1522 [PubMed] [Google Scholar]
- 9. Peck E. J., Jr., Awapara J. (1967) Biochim. Biophys. Acta 141, 499–506 [DOI] [PubMed] [Google Scholar]
- 10. Huxtable R., Bressler R. (1972) J. Nutr. 102, 805–814 [DOI] [PubMed] [Google Scholar]
- 11. Sturman J. A., Hepner G. W., Hofmann A. F., Thomas P. J. (1975) J. Nutr. 105, 1206–1214 [DOI] [PubMed] [Google Scholar]
- 12. Huxtable R. J. (1992) Physiol. Rev. 72, 101–163 [DOI] [PubMed] [Google Scholar]
- 13. Tuz K., Ordaz B., Vaca L., Quesada O., Pasantes-Morales H. (2001) J. Neurochem. 79, 143–151 [DOI] [PubMed] [Google Scholar]
- 14. Morales I., Dopico J. G., Sabate M., Gonzalez-Hernandez T., Rodriguez M. (2007) Am. J. Physiol. Cell Physiol. 292, C1934–1941 [DOI] [PubMed] [Google Scholar]
- 15. Albrecht J., Schousboe A. (2005) Neurochem. Res. 30, 1615–1621 [DOI] [PubMed] [Google Scholar]
- 16. Schuller-Levis G. B., Park E. (2003) FEMS Microbiol. Lett. 226, 195–202 [DOI] [PubMed] [Google Scholar]
- 17. Sturman J. A. (1993) Physiol. Rev. 73, 119–147 [DOI] [PubMed] [Google Scholar]
- 18. Warskulat U., Heller-Stilb B., Oermann E., Zilles K., Haas H., Lang F., Häussinger D. (2007) Methods Enzymol. 428, 439–458 [DOI] [PubMed] [Google Scholar]
- 19. Banerjee R., Vitvitsky V., Garg S. K. (2008) Trends Biochem. Sci. 33, 413–419 [DOI] [PubMed] [Google Scholar]
- 20. Hussy N., Deleuze C., Brès V., Moos F. C. (2000) Adv. Exp. Med. Biol. 483, 227–237 [DOI] [PubMed] [Google Scholar]
- 21. Bothwell J. H., Rae C., Dixon R. M., Styles P., Bhakoo K. K. (2001) J. Neurochem. 77, 1632–1640 [DOI] [PubMed] [Google Scholar]
- 22. Tappaz M., Almarghini K., Do K. (1994) Adv. Exp. Med. Biol. 359, 257–268 [DOI] [PubMed] [Google Scholar]
- 23. Brand A., Richter-Landsberg C., Leibfritz D. (1997) Cell Mol. Biol. 43, 645–657 [PubMed] [Google Scholar]
- 24. Brand A., Leibfritz D., Hamprecht B., Dringen R. (1998) J. Neurochem. 71, 827–832 [DOI] [PubMed] [Google Scholar]
- 25. Perry T. L., Hansen S. (1973) J. Neurochem. 21, 1009–1011 [DOI] [PubMed] [Google Scholar]
- 26. Garg S. K., Banerjee R., Kipnis J. (2008) J. Immunol. 180, 3866–3873 [DOI] [PubMed] [Google Scholar]
- 27. Garg S. K., Kipnis J., Banerjee R. (2009) J. Neurochem. 108, 1155–1166 [DOI] [PubMed] [Google Scholar]
- 28. Garg S. K., Yan Z., Vitvitsky V., Banerjee R. (2009) in Methods in Redox Signaling, pp. 7–11, Mary Ann Liebert, New York [Google Scholar]
- 29. Foster D. J., Vitvitsky V. M., Banerjee R., Heacock A. M., Fisher S. K. (2009) J. Neurochem. 108, 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dominy J., Eller S., Dawson R., Jr. (2004) Neurochem. Res. 29, 97–103 [DOI] [PubMed] [Google Scholar]
- 31. Nakamura H., Yatsuki J., Ubuka T. (2006) Amino Acids 31, 27–33 [DOI] [PubMed] [Google Scholar]
- 32. Worden J. A., Stipanuk M. H. (1985) Comp. Biochem. Physiol. B 82, 233–239 [DOI] [PubMed] [Google Scholar]
- 33. Fisher S. K., Cheema T. A., Foster D. J., Heacock A. M. (2008) J. Neurochem. 106, 1998–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dominy J. E., Jr., Hwang J., Stipanuk M. H. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E62–69 [DOI] [PubMed] [Google Scholar]
- 35. Beetsch J. W., Olson J. E. (1998) Am. J. Physiol. 274, C866–874 [DOI] [PubMed] [Google Scholar]
- 36. Lang F., Busch G. L., Ritter M., Völkl H., Waldegger S., Gulbins E., Häussinger D. (1998) Physiol. Rev. 78, 247–306 [DOI] [PubMed] [Google Scholar]
- 37. Hoffmann E. K., Lambert I. H., Pedersen S. F. (2009) Physiol. Rev. 89, 193–277 [DOI] [PubMed] [Google Scholar]
- 38. Lambert I. H. (2004) Neurochem. Res. 29, 27–63 [DOI] [PubMed] [Google Scholar]
- 39. Jackson P. S., Strange K. (1993) Am. J. Physiol. 265, C1489–1500 [DOI] [PubMed] [Google Scholar]
- 40. Kimelberg H. K., Cragoe E. J., Jr., Nelson L. R., Popp A. J., Szarowski D., Rose J. W., Woltersdorf O. W., Jr., Pietruszkiewicz A. M. (1987) Cent. Nerv. Syst. Trauma. 4, 3–14 [DOI] [PubMed] [Google Scholar]
- 41. Bhardwaj A. (2006) Ann. Neurol. 59, 229–236 [DOI] [PubMed] [Google Scholar]
- 42. Lien Y. H., Shapiro J. I., Chan L. (1991) J. Clin. Invest. 88, 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]