Abstract
We have demonstrated that SlyA activates fimB expression and hence type 1 fimbriation, a virulence factor in Escherichia coli. SlyA is shown to bind to two operator sites (OSA1 and OSA2), situated between 194 and 167 base pairs upstream of the fimB transcriptional start site. fimB expression is derepressed in an hns mutant and diminished by a slyA mutation in the presence of H-NS only. H-NS binds to multiple sites in the promoter region, including two sites (H-NS2 and H-NS3) that overlap OSA1 and OSA2, respectively. Mutations that disrupt either OSA1 or OSA2 eliminate or reduce the activating effect of SlyA but have different effects on the level of expression. We interpret these results as reflecting the relative competition between SlyA and H-NS binding. Moreover we show that SlyA is capable of displacing H-NS from its binding sites in vitro. We suggest SlyA binding prevents H-NS binding to H-NS2 and H-NS3 and the subsequent oligomerization of H-NS necessary for full inhibition of fimB expression. In addition, we show that SlyA activates fimB expression independently of two other known regulators of fimB expression, NanR and NagC. It is demonstrated that the rarely used UUG initiation codon limits slyA expression and that low SlyA levels limit fimB expression. Furthermore, Western blot analysis shows that cells grown in rich-defined medium contain ∼1000 SlyA dimers per cell whereas those grown in minimal medium contain >20% more SlyA. This study extends our understanding of the role that SlyA plays in the host-bacterial relationship.
Keywords: Bacterial Adhesion, Bacterial Genetics, Bacterial Pathogenesis, Bacterial Transcription, DNA Recombination, Escherichia coli, Gene Expression, Virulence Factors, Fimbriae, Phase Variation
Introduction
Bacterial-host attachment is a key step in colonization and pathogenesis. Although the type 1 fimbrial adhesin of Escherichia coli is produced by the majority of non-pathogenic as well as pathogenic strains of the bacterium, it has been implicated as a virulence factor in urinary tract and other infections (1–4). Type 1 fimbriate cells attach to uroplakin receptors in the bladder to facilitate invasion and the subsequent formation of intracellular communities thought to be required for chronic-recurrent UTI (5, 6). The adhesin is able to deliver LPS to TLR4 and even directly activate the TLR4-MyD88 pathway (5–7). This interaction produces an innate immune response in the host, including the release of pro-inflammatory cytokines IL-6, IL-8, and TNF-α (5, 6).
Like many cell surface virulence factors, type 1 fimbriation is controlled by phase variation that produces a mixture of expressing (fimbriate) and non-expressing (afimbriate) bacteria. Phase variation of type 1 fimbriation in E. coli requires the site-specific inversion of a short (∼300 bp) segment of DNA (fimS) that contains a promoter for the fimbrial structural operon (8–10). Inversion is catalyzed by tyrosine family recombinases FimB and FimE, which are encoded by genes situated adjacent to the fimbrial structural genes (11, 12). Whereas FimB promotes low frequency (<10−2 per cell per generation) phase switching in either direction, FimE can generate rates of fimbriate to afimbriate switching as high as 0.8 per cell per generation (13–15).
The expression of both fimB and fimE, as well as the inversion itself, are controlled by multiple signals including the availability of the branched chain amino acids and alanine, temperature, sialic acid (Neu5Ac), and N-acetylglucosamine (GlcNAc) (15–17) among others. In addition to the signals noted above, fimB expression, and hence type 1 fimbriation, is also enhanced by the alarmones guanosine tetra- and pentaphosphate ((p)ppGpp)2 (18). The response to many of the signals described above should decrease the fimbriate cell population during host inflammation, and we have proposed the raison d'être for the regulation observed in E. coli K-12 is to help balance the host-parasite interaction (16, 17, 19). Inversion of fimS can also be catalyzed by homologous recombinases encoded at a distant location in some clinical isolates (20, 21), raising the possibility that OFF-to-ON phase switching is less sensitive to such signals in strains showing greater pathogenicity.
The MarR-family member SlyA was originally identified as a regulator of virulence in Salmonella, where it is required for intracellular survival and systemic pathogenesis (22). SlyA is also found in E. coli where it was first shown to activate expression of the cryptic hemolysin gene hlyE (also known as clyA or sheA) (23). Proteomic analysis of the SlyA regulons of enteroinvasive E. coli (EIEC) and Salmonella revealed that SlyA positively or negatively controls the expression of over 30 proteins in each bacterium (24). This study, together with more recent work (25), shows that there is little overlap in the SlyA regulons of the two organisms. Perhaps surprisingly, all of the SlyA regulon members identified in EIEC, including those involved in heat and acid stress responses and a variety of metabolic functions, are also found in E. coli K-12. However, SlyA has also been shown to activate expression of the K5 capsule, which is a virulence factor in UPEC (26, 27).
Notwithstanding these differences between the SlyA regulons of E. coli and Salmonella noted above, in all cases where the mechanism of SlyA control has been characterized in detail, SlyA regulates gene expression by interacting with the abundant nucleoid-associated protein H-NS (27–30). However while SlyA antagonizes H-NS repression of the majority of genes which it activates in both E. coli and Salmonella, remodeling of the H-NS nucleoprotein complex facilitates activation of K5 capsule in E. coli (27). Here we show that SlyA antagonizes the inhibitory effect of H-NS on fimB expression. SlyA is thus a novel activator of type 1 fimbriation in E. coli.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, Media, and Growth Conditions
Descriptions and genotypes of bacterial strains are listed as supplemental material [strains-pdf]. All the bacterial strains used are derivatives of the E. coli K-12 strain MG1655 (31). All plasmids used for allelic exchange are derivatives of the chloramphenicol resistant, temperature-sensitive vector pMAK705 (32). All reagents were obtained from Sigma unless otherwise indicated. Media used included L broth (5 g of sodium chloride (Fisher Scientific), 5 g of yeast extract (Oxoid), and 10 g of tryptone (Becton-Dickinson & Co.) per liter) and L agar (L broth with 1.5% agar (Difco)). Sucrose agar used to select recombinant bacteria following allelic exchange was L agar supplemented with 6% sucrose in the absence of sodium chloride (33). The antibiotics chloramphenicol (25 μg/ml), tetracycline (10 μg/ml) and kanamycin (25 μg/ml) were included in selective media as required. Minimal MOPS medium was prepared as described by Neidhardt et al. (34), supplemented with 10 mm thiamine and 0.4% glycerol (Fisher Scientific). To prepare rich-defined medium, minimal MOPS medium was further supplemented with bases, vitamin B supplement and amino acids as originally reported (34). Liquid cultures were grown aerobically at 37 °C, and culture densities were monitored spectrophotometrically at 420 or 600 nm.
Analysis of fimB Expression and FimB Recombination
fimB expression was measured using a FimB-LacZ fusion situated in the chromosome at fim as described previously (16). Mutant fim regulatory alleles used in this study, including scanning-replacement mutations in which adjacent segments of the fimB promoter were replaced by 14–15 bp of heterologous DNA, were cloned into derivatives of the temperature-sensitive vector pMAK705 (32), and allelic exchange was then used to transfer the mutations into the chromosome at fim using sacB and sucrose counter-selection as described previously (33). P1 transduction was carried out using P1vir by standard procedures (35). β-Galactosidase assays were conducted as described by Miller (36), following growth in either rich defined or minimal medium at 37 °C with rapid aeration to an A600 of 0.2. Experiments were repeated at least twice, and the values shown represent the mean of at least four samples with 95% confidence intervals included for each value.
FimB recombination was measured as described previously (15). Bacteria were first plated onto lactose-MacConkey indicator medium to isolate phase OFF colonies. A single colony was subsequently inoculated into RD media, and grown at 37 °C to an A420 of ∼0.1. The cells were then diluted into 25 tubes to a density of ∼0.3 cells per tube, and grown for ∼22 generations at 37 °C with rapid aeration. The proportion of phase ON to phase OFF cells was determined by plating samples of the culture (5–7 duplicates) onto lactose-MacConkey indicator medium. The values shown represent the average obtained, together with the minimum and maximum.
Purification of SlyA
Cultures of E. coli strain BL21(DE3) containing slyA-pGS21a (GST-His-tagged SlyA overexpression plasmid constructed by GenScript) were grown in LB supplemented with 125 μg/ml ampicillin, shaking at 200 rpm at 37 °C. To overexpress the GST-His-tagged SlyA fusion protein, isopropyl β-d-thiogalactopyranoside was added to the cultures to a final concentration of 1 mm when the cultures reached an A600 of 0.6. Following a further 3 h of incubation, the cultures were cooled on ice for 10 min, and the cells were then pelleted by centrifugation at 8000 × g for 10 min at 4 °C. Cell pellets were frozen at −20 °C and then resuspended in Lysis Buffer (50 mm Tris-HCl, 2 mm EDTA, pH 8.0). Lysozyme (100 μg/ml) and Triton X-100 (0.1%) were added and the mixture was incubated for 20 min at room temperature. For DNase I treatment, MgCl2 (10 mm) and DNase I (20 μg/ml) were added, and samples were incubated at room temperature for a further 30 min, or until the viscosity was reduced. Supernatant was collected by centrifugation at 12,000 × g for 10 min at 4 °C prior to loading onto a glutathione-Sepharose 4B column (GE Healthcare) equilibrated with Binding Buffer (140 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 1.8 mm KH2PO4, pH 7.3). The column was then washed with 5 column volumes of Binding Buffer three times. The GST-His-tagged SlyA was eluted by adding 0.5 ml of Elution Buffer (50 mm Tris-HCl, 0.25 m NaCl, 10 mm reduced glutathione, pH 8.0) per ml bed volume of glutathione-Sepharose 4B. Fractions enriched for GST-His-tagged SlyA were pooled and dialyzed against 50 mm Tris-HCl, pH 8.0. The GST-His tag was removed by cleavage with His-tagged recombinant tobacco etch virus protease (Promega). The cleavage reaction was applied to a Nickel affinity column and tag-free SlyA was eluted with a 50 mm imidazole solution (50 mm imidazole, Tris-HCl 50 mm, 0.25 m NaCl, pH 8.0). Purified SlyA was finally dialyzed against 50 mm Tris-HCl, pH 8.0.
Western Blot Analysis of SlyA
1.5 mg of purified SlyA protein was prepared for antibody generation at Eurogentec. After immunization of 2 rabbits with 4 injections each during 28 days (Speedy polyclonal packages), preimmune, medium, and final bleeds were collected. The final bleed serum only was used for Western blot analysis. For Western blot analysis, E. coli strains were grown in rich defined or minimal media with rapid aeration to reach an A600 of 0.2. Cells were cooled on ice for 10 min, pelleted by centrifugation at 8000 × g for 10 min at 4 °C, and then lysed and treated with DNase I as described above. After centrifugation at 12,000 × g for 10 min at 4 °C, the supernatant of cell lysates was collected, and the concentration of total protein was determined by the Bradford method. 68.5 ng of purified SlyA and 62 μg of total protein from cell extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 5% stacking and 17.5% separating gels. The proteins were transferred from the gel onto polyvinylidene difluoride membrane (PVDF, Millipore) for 1 h by using a current of 0.15–0.20 A. The membrane was blocked by incubation with 5% Dried Skimmed Milk in PBS/T (phosphate-buffered saline + 0.2% Tween 20) for 40 min at room temperature, then incubated with primary antibody (1:1000 rabbit serum) for 1 h at room temperature. After three washes with PBS/T, the membrane was incubated with secondary antibody (1:5000 goat anti-rabbit IgG-peroxidase, Sigma-Aldrich) for 30 min at room temperature, and again washed three times with PBS/T. Proteins were visualized with enhanced chemiluminescence. Western blot films were scanned and processed using ImageJ to quantify SlyA. The plot area of SlyA bands of each strain were measured and compared with the purified SlyA plot area. The number of SlyA dimers were estimated by comparing the total weight of protein per E. coli cell (0.156 pg in rich-defined medium and 0.45 pg in minimal medium as reported by Bremer and Dennis (37)) to the weight of a SlyA dimer (0.543 × 10−7 pg).
DNA Manipulations
Plasmid DNA was isolated with a kit from Qiagen. Restriction and DNA modifying enzymes were purchased from either Promega or New England Biolabs. PWO DNA polymerase used in PCR reactions was obtained from Roche. PCR and restriction endonuclease digestions were carried out according to the manufacturers' recommendations. Deletion and replacement mutations were constructed using standard PCR techniques (38) and/or restriction endonuclease digestions, and used to replace wild type sequences cloned into derivatives of the temperature-sensitive vector, pMAK705 (32). Replacement mutations rm21–35, 14–17 bp in length, each contained a SacII restriction endonuclease site (5′-CCGCGG). Replacement mutations rm39–41, 12 bp in length, were identical and contained a BsiWI restriction endonuclease site (5′-CGTACG). DNA sequencing was performed by Lark Technologies/Cogenics (now Beckman Coulter Genomics). Kanamycin resistance cassettes of Keio collection origin (39) were cured as described (40). All other molecular genetic procedures were carried out according to standard protocols (38, 41).
EMSA and DNaseI Footprinting
Purified tag-free SlyA was produced by GenScript, and H-NS was obtained as a generous gift from Sylvie Rimsky. The fim DNA fragment used in EMSA was generated using primers EMSAfim03f (5′-CCCGGATCCGTAGTGACCAAAGC) and EMSAfim03r (5′-CCCGTCGACATAAAAAATTCAGC). A positive control for SlyA experiments containing slyA promoter DNA was generated using primers slyAf3 (5′-CCCCGGATCCTGACGGTAACCAAATGCAGCAATACATTTG) and EMSAsly01r (5′-CCCGTCGACGATGGTCTATCAGAGCACG), spanning the region reported to contain a SlyA binding site (27). A negative control was amplified from pBluescript as previously reported (21). Polyacrylamide gels were cast containing 5% acrylamide (Bio-Rad) and 2% glycerol (Fisher Scientific). Reaction buffer contained 10 mm Tris (Fisher Scientific) pH 9.0, 50 mm KCl (Fisher Scientific), and 0.1% Triton X-100, as used previously (42). Reactions were carried out in a final volume of 10 μl. Each DNA fragment was incubated at a final concentration of 11 nm with varying concentrations of SlyA or H-NS for 10 min, before addition of 2 μl of loading buffer (40% sucrose, 35 mm Tris, pH 8.0). Electrophoresis was immediately carried out in 1× TBE at 160 V before staining for 15 min in 0.5 μg/ml ethidium bromide.
The fim03 fragment (Fig. 1) was made by PCR with either the fim03f or fim03r oligonucleotide labeled by [γ-32P]ATP and polynucleotide kinase. DNase footprinting was carried out in two different buffers: the H-NS buffer described by Bouffartigues et al. (43) (2007) 40 mm Hepes, 60 mm potassium glutamate, pH 8.0, 5 mm MgCl2, 0.05% Nonidet P40, 5 mm DTT, 0.5 mg/ml BSA, and the “SlyA” buffer described by Zhao et al. (44) 10 mm Tris, pH 7.5, 10 mm NaCl, 1 mm MgCl2, 1 mm EDTA, 5 mm DTT, 5% glycerol, 0.5 mg/ml BSA. Complexes were formed at 25 °C in a final volume of 40 μl and DNase attack was carried out for generally 1 min with a final concentration of 50 ng/ml DNaseI. H-NS protects DNA from DNaseI and digestion times were increased in the presence of >100 nm H-NS. Reactions were stopped with 100 μl of aqua phenol pH 8.0 and 200 μl of 0.4 m sodium acetate (pH 5.0) 2.5 mm EDTA, 10 μg/ml sonicated herring sperm DNA was added. The digested DNA was phenol extracted, ethanol precipitated, and separated on 6% denaturing acrylamide gels. The radioactive gels were analyzed by phosphoimagery. The size marker is pBR322 DNA digested with MspI (New England Biolabs) and labeled with [γ-32P]ATP and polynucleotide kinase.
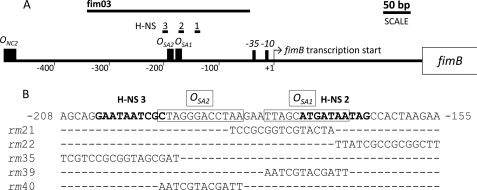
FIGURE 1.
Organization of the region upstream of the fimB open reading frame. A, location of operator sites for NagC (ONC2) and SlyA (OSA1 and OSA2), together with the fimB promoter, are indicated. The regions bound by H-NS for which a match to a consensus H-NS binding site (51) was identified are also shown (H-NS1 to H-NS3). The fim DNA included in the amplicon used for EMSA and DNase I footprinting experiments is likewise indicated (fim03). The NagC binding site ONC2 was reported previously (45). B, wild type nucleotide sequence encompassing OSA1 and OSA2 are boxed. The nucleotide sequences of matches to H-NS binding site consensus are labeled (H-NS2 and H-NS3) and are emphasized in bold text. The mutated sequences designated rm21, rm22, rm35, rm39, and rm40 are shown beneath.
RESULTS
SlyA Activates fimB Expression
A set of adjacent scanning-replacement mutations, extending from −38 to −184 bp upstream of the fimB transcription start site, were constructed and characterized in a chromosomal FimB-LacZ protein fusion background. This was initially carried out as part of an analysis of how the regulators NanR and NagC activate fimB expression (45). Whereas none of these mutations affected the responses to NanR or NagC (data not shown), the first two adjacent mutations (rm21 and rm22; Fig. 1) did diminish fimB expression (Fig. 2A). The nucleotide sequence mutated in rm21 and rm22 contains a close match (5′-TTAGCATGATAA; hereafter called OSA1; boxed in Fig. 1B) to a consensus recognition sequence for the transcriptional regulator SlyA (5′-TTAGCAAGCTAA (42)), suggesting that SlyA might be an activator of fimB expression.
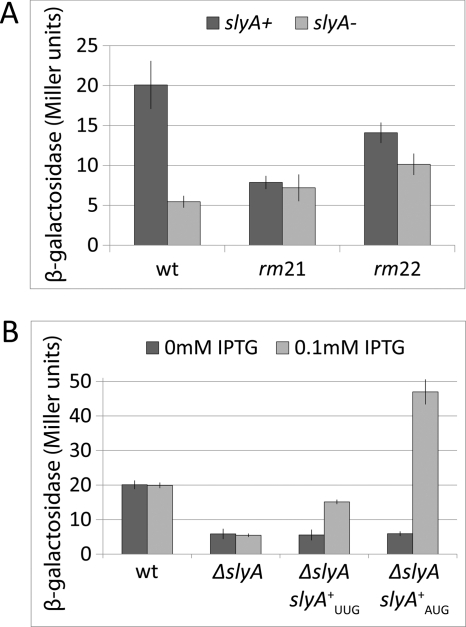
FIGURE 2.
The effects of replacement mutations and slyA on the β-galactosidase produced by a FimB-LacZ fusion. A, effects of mutations rm21 and rm22 in the presence of a wild type (dark bars) and deleted (light bars) slyA gene. Error bars indicate 95% confidence values calculated from four replicate experiments. Strains BGEC905, KCEC1243, KCEC1077, KCEC1271, KCEC1079, and KCEC1273, listed in supplemental material [strains-pdf], were grown in rich-defined glycerol medium and processed as described under “Experimental Procedures.” B, effect of 0.1 mm IPTG (light bars) versus 0 mm IPTG (dark bars) in wild type, ΔslyA and ΔslyA lacUV5-slyA backgrounds. Strains BGEC905, KCEC1334, KCEC1494, and KCEC1765 were used. Error bars and experimental conditions as A.
To test this hypothesis, the ΔslyA mutation of the Keio collection (39) was transduced into the FimB-LacZ fusion. It was found that expression of the fusion was diminished almost 4-fold in the mutant background following growth in rich-defined MOPS glycerol medium (Fig. 2). Moreover, in contrast to NanR and to NagC (data not shown), rm21 and rm22 had little effect on fimB expression in the absence of SlyA (Fig. 2A). In a control experiment it was also found that the effect of the ΔslyA mutation on fimB expression was complemented by an ectopic copy of slyA placed in the chromosome at lac. This construct (lacUV5-slyAUUG) included the slyA open reading frame, together with 65 bp of upstream DNA, placed downstream of the lacZYA promoter (Fig. 2B).
SlyA Binds to the fimB Regulatory Region
To determine whether SlyA binds to the putative operator OSA1, the interaction of SlyA with the fimB promoter region was investigated by EMSA and DNase I footprinting (Fig. 3). For this analysis, a 297-bp PCR product that includes 282 bp of fim sequences, extending from −45 to −327 bp upstream of the fimB transcriptional start site (Fig. 1) was used.
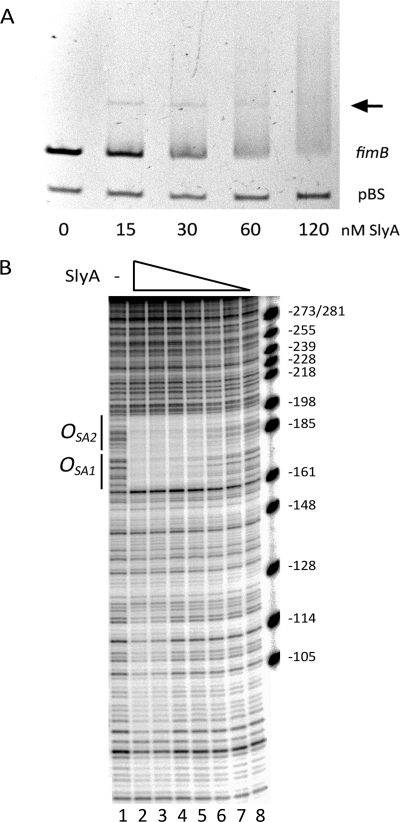
FIGURE 3.
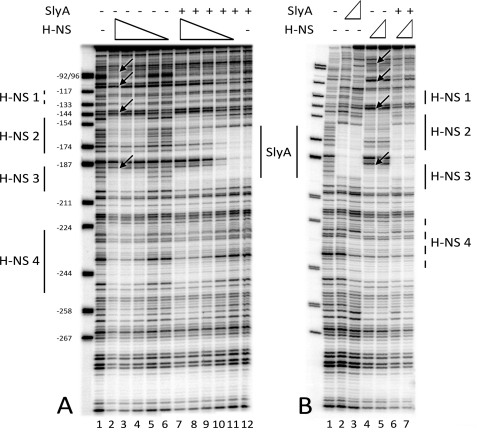
The interaction of SlyA with the fimB promoter region in vitro. A, effect of 0, 15, 30, 60, and 120 nm SlyA dimer on the electophoretic mobility of DNA amplicons fim03 and pBS (each 11 nm). Amplicon fim03 is 282 bp of the region upstream of fimB and includes OSA1 and OSA2 (Fig. 1). Amplicon pBS is a negative control as previously described (27). The SlyA-DNA complex is indicated with an arrow. Samples were separated on a 5% polyacrylamide gel. Electrophoresis was carried out at 160 V for 35 min as described under “Experimental Procedures.” B, DNase I footprinting. The fim03 fragment, labeled at the fim03r end, was mixed with decreasing concentrations of SlyA for 15 min at 25 °C before digestion with DNaseI. Lane 1, no SlyA; lane 2, 1 μm SlyA; lane 3, 500 nm; lane 4, 250 nm; lane 5, 125 nm; lane 6, 62.5 nm; lane 7, 31 nm; lane 8, 15.6 nm. The products were analyzed on a 6% denaturing polyacrylamide gel. Regions protected by SlyA are indicated. The marker is pBR322 digested with MspI, and the sizes of the fragments were used to calculate their positions relative to the transcriptional start site of fimB (+1).
In EMSA, SlyA produced a loss of the free fim DNA, whereas it had no effect on the pBluescript DNA negative control as expected (27) (Fig. 3A). SlyA also bound to its own promoter as expected from previous work (27, 42) (data not shown). However, SlyA produced only a faint discrete mobility-shifted band with either the fim (arrow in Fig. 3A) or slyA substrates. In repeat experiments, between 16 and 27 nm SlyA dimer was required to diminish the amount of the free fim DNA by 50% (not shown).
DNase I footprinting demonstrated that SlyA bound to a region of DNA ∼40 bp long, spanning OSA1 and extending around 20 bp further upstream relative to the fimB transcriptional start site (Fig. 3B). A DNaseI hypersensitive site near position −161 appears to delimit the downstream protected region. Thus SlyA binds to OSA1 as predicted. The nucleotide sequence adjacent to OSA1contains a second potential SlyA operator site (5′-CTAGGGACCTAA; hereafter called OSA2), situated 3 bp upstream of OSA1. Both OSA1 and OSA2 are altered by rm21 (Fig. 1). The whole 40 bp region is simultaneously protected by different concentrations of SlyA suggesting it corresponds to a single site. Despite the fact that the SlyA consensus is only 12 bp long, most SlyA-protected regions described in the literature are at least 40 bp long and are often bounded by hypersensitive DNaseI cleavages (27, 28). A weaker level of protection was also observed in a region closer to the fimB promoter. However, mutation of this region did not alter the response of fimB expression to SlyA and the potential importance of this region was not investigated further (data not shown).
Mutagenesis of Potential SlyA Operators
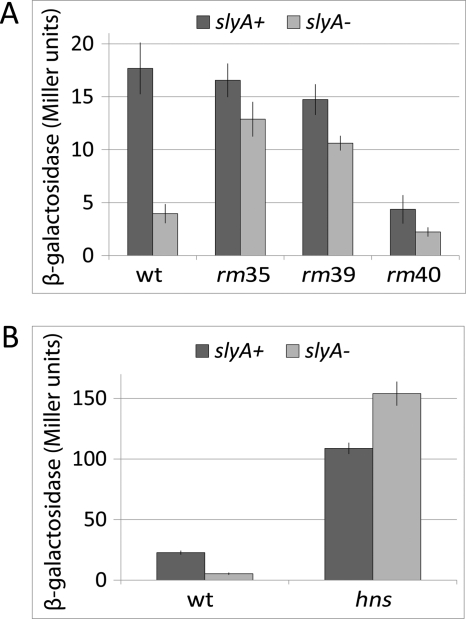
To examine the possible roles of OSA1and OSA2 further, two additional mutations which targeted OSA1 (rm39) and OSA2(rm40) specifically were constructed and their effects on fimB expression were characterized (Fig. 1 and 4A). Surprisingly, although the OSA2 mutation decreased fimB expression substantially in the slyA+ background, the OSA1 mutation affected fimB expression to only a small extent. However, in both of the rm slyA double mutants, the rm mutations each largely suppressed the activating effect of SlyA on fimB expression, supporting the hypothesis that both OSA1 and OSA2 are SlyA operators as proposed.
It is notable that although all the four mutations rm21, 22, 39, and 40 eliminated the majority of the slyA mediated stimulation of fimB expression they had different effects on the basal fimB expression in the ΔslyA mutant. Expression in the rm22 and rm39 mutants, and to a lesser extent the rm21 mutant, actually increased. One hypothesis to explain these results is that the mutations rm21, rm22, and rm39 also affect the binding of a repressor of fimB expression.
SlyA Activates fimB Expression by Antagonizing H-NS Repression
It has been shown that SlyA antagonizes H-NS repression of hlyE expression, apparently by steric hindrance when the two proteins compete for overlapping operators (28, 48). Moreover it is known that H-NS inhibits fimB expression (49). As shown in Fig. 4B, the activating effect of SlyA on fimB expression is suppressed in an hns mutant. Indeed in the absence of H-NS, SlyA actually seems to inhibit fimB expression. While these results demonstrate that SlyA can exert opposing effects on fimB expression, it is apparent that SlyA has a net activating effect on fimB expression in the wild type background by somehow antagonizing H-NS repression.
FIGURE 4.
The effects of replacement mutations and hns deletion on the β-galactosidase produced by a FimB-LacZ fusion in the presence and absence of SlyA. A, effects of mutations rm35, rm39, and rm40 in the wild type (dark bars) and ΔslyA mutant (light bars). Strains BGEC905, KCEC1243, KCEC1831, KCEC1854, KCEC2014, KCEC2016, KCEC2020, and KCEC2022 were used. B, effect of an hns::mTn10 mutation in the presence of the wild type (dark bars) or deleted (light bars) slyA gene. Strains BGEC905, KCEC1243, KCEC755, and KCEC1300 were used. Error bars and experimental conditions are described in Fig. 2.
H-NS Binding to the fimB Upstream Region with and without SlyA
Prior work showed that H-NS binds in the vicinity of the fimB promoter, but the location of the binding sites was not determined (49). In DNase I footprinting experiments, we have found that H-NS bound to an extensive region of the fimB promoter, producing specific protection at short (10–20 bp) sequences in a region that extended from about 500 bp upstream to about 150 bp downstream of the transcriptional start site (data not shown) similar to that observed at other H-NS sites (for examples, see (29, 43)).
As predicted from the results described above, H-NS bound to regions overlapping the DNA sequence protected by SlyA. In particular, H-NS protected two regions (called H-NS2 and H-NS3) which overlapped the SlyA binding site, and more weakly a third region (H-NS4) further upstream (Figs. 1 and 5A, lanes 2–4). The H-NS2 and H-NS3 (5′-ATGATAATAG and GCGATTATTC, appearing on the top and bottom stands in Fig. 1, respectively) each contain 5/10 bp matches to the H-NS site (5′-TCGATAAATT) proposed by Lang et al. (51). H-NS4 does not contain a discernable match.
FIGURE 5.
Competition between H-NS and SlyA binding upstream of the fimB promoter. A, binding of H-NS to the fim03 fragment labeled at the fim03f end. Decreasing concentrations of H-NS were mixed with the fim03 DNA with and without SlyA in the “HNS” buffer. Lane 1, no proteins; lanes 2 and 7, 400 nm H-NS; lanes 3 and 8, 200 nm H-NS; lanes 4 and 9, 100 nm H-NS; lanes 5 and 10, 50 nm H-NS; lanes 6 and 12, 25 nm H-NS; lanes 7–12 also contained 2 μm SlyA. B, H-NS binding with and without SlyA in the “SlyA” buffer. Lane 1, no proteins; lane 2, 50 nm SlyA; lane 3, 1 μm SlyA; lane 4, 50 nm H-NS; lane 5, 100 nm H-NS; lane 6, 1 μm SlyA, and 50 nm H-NS; lane 7, 1 μm SlyA and 100 nm H-NS. Proteins were incubated for 15 min at 25 °C before treatment with DNase I. Products were analyzed on 6% denaturing polyacrylamide gels. Regions protected by H-NS and SlyA are indicated. The arrows indicate hypersensitive DNaseI cleavages on the DNA in the presence of H-NS, which are observed in the “SlyA” buffer (B) but not in the “H-NS” buffer (H-NS). The marker is pBR322 digested with MspI, and the sizes of the fragments were used to calculate their positions relative to the transcriptional start site of fimB (+1).
Adding SlyA (2 μm) had no effect on H-NS binding and only when H-NS binding was lost (at 25 and 50 nm H-NS) was SlyA binding to its site detected (Fig. 5A, lanes 5, 6, 11, and 12). These experiments were performed in a Hepes-glutamate buffer previously described for use with H-NS (43). We noted however that many workers have preferred to study SlyA binding using lower salt buffers (42, 44). Using the buffer used by Zhao et al. (44), SlyA bound to the same sites although with higher affinity (Fig. 5B, lanes 2 and 3). In this buffer H-NS also bound to the H-NS2 and H-NS3 sites, but protection was extended in the direction of fimB (site H-NS1) and reduced at H-NS4 (Fig. 5B, lanes 4 and 5). H-NS1 actually contains a 7/10 match to the H-NS consensus. In addition, there were several hypersensitive DNaseI cleavages (indicated by arrows), which were not observed in the “H-NS” buffer, suggesting that the structure of the H-NS-DNA complex is dependent upon the ionic conditions. This observation is in agreement with a recent publication which demonstrated that H-NS binds to DNA in two different modes, a “stiffening” mode and a “bridging” mode, depending upon salt and divalent cation composition (50). The low salt, low Mg2+ “SlyA” buffer is compatible with the “stiffening” mode of H-NS binding, which was proposed to be implicated in the gene silencing function of H-NS. Thus, it is interesting to note that in the “SlyA” buffer, SlyA was capable of displacing H-NS from its binding sites, although it did require a 10-fold molar excess of SlyA (lanes 6 and 7). It should also be noted that these experiments were performed on linear DNA and the native supercoiled chromosome could influence the binding characteristics.
Mutation rm21, which might be expected to disrupt both OSA1 and H-NS2, seems mainly to eliminate SlyA activation. This suggests that H-NS binding to H-NS3 alone might be sufficient for repression of fimB expression. However the more substantial increase in fimB expression seen in the ΔslyA derivatives of the other OSA1 mutants rm22 and rm39 (Figs. 2A and 4) relative to the single ΔslyA mutant suggests that this is not the case. We have not been able to detect a decrease in H-NS binding to the fimB promoter PCR fragment in these mutants by EMSA (data not shown), though, suggesting that small local changes in H-NS binding can affect the overall repression of fimB expression by H-NS. The consensus match within H-NS3 overlaps with the SlyA operator OSA2 by 1 bp. We note that the rm40 mutation of the SlyA operator OSA2 coincidently improves the homology between the H-NS consensus and the match contained within H-NS3 by altering the first base of this sequence from G to T (the C to A transversion on the top strand shown in Fig. 1). Thus the unexpectedly large decrease in fimB expression seen in the rm40 mutant might reflect both diminished SlyA binding to OSA2 coupled to increased binding of H-NS to H-NS3.
We have constructed an additional scanning-replacement mutation that extends from OSA2 further upstream of fimB that partially replaces OSA2 while replacing the H-NS3 sequence entirely (rm35, Fig. 1). Analysis of the effects of this mutation on fimB expression shows that while it has very little effect in the wild type (slyA+) background, it produces a 3-fold increase in the slyA mutant background, implying that H-NS binding has been significantly diminished (Fig. 4A). This result supports the hypothesis that H-NS binding to H-NS3 inhibits fimB expression and that SlyA binding to the overlapping operator site OSA2 counteracts this.
SlyA Limits fimB Expression and Produces Differential Control of fimB Expression in Rich and Minimal Medium
The slyA open reading frame starts with an unusual UUG codon (46), suggesting that a poor level of translation initiation of slyA limits the protein expression. In agreement with this hypothesis, it was found that replacing the native UUG initiation codon with an AUG codon enhanced the level of SlyA that was detected by Western blot analysis over 5-fold (Fig. 6). The lacUV5-slyAAUG construct also increased SlyA levels to well above that produced by the wild type and enhanced fimB expression to above wild type levels too (Figs. 6 and 2B, respectively). Thus poor translation initiation of slyA limits slyA expression and hence fimB expression.
FIGURE 6.
Western blot analysis of slyA expression. 68.5 ng of purified SlyA (P; lane 1), or 62 μg of total protein isolated from the strains indicated (lanes 2–8), were subject to SDS-PAGE and Western blot analysis as described under “Experimental Procedures.” Extracts were made from the wild type strain (BGEC905) grown in rich-defined glycerol medium (wt R; lane 2) or minimal glycerol medium (wt M; lane 3). All the additional strains analyzed (lanes 4–8) were grown in rich-defined glycerol medium. All extracts were prepared from cultures grown to an A600 = 0.2. Lane 4 (slyA) ΔslyA mutant (KCEC1334); lanes 5 and 6, the lacUV5-slyAUUG construct (KCEC1494); lanes 7 and 8, the lacUV5-slyAAUG construct (KCEC1765). The lac fusion constructs were grown in the absence (−I) or presence (+I) of 1 mm IPTG as indicated.
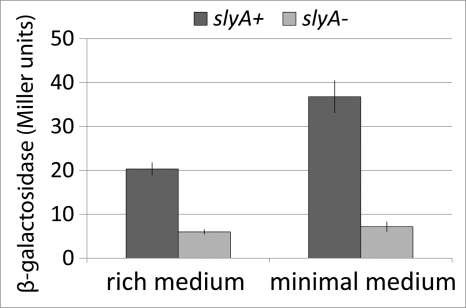
The level of expression of poorly-translated proteins generally rise as growth rate declines because of diminished competition with highly translated ribosomal and other proteins for limiting translation factors (47). This suggested that SlyA should have a larger effect on fimB expression in minimal medium than it would in rich medium. In agreement with this prediction, SlyA levels were higher in minimal medium than in rich medium (Fig. 6) and the effect of SlyA on fimB expression was enhanced too (Fig. 7). We estimated that there are ∼1036 dimers (the mean of duplicate estimates of 1012 and 1060) of SlyA in exponential-phase cells grown in rich defined medium and ∼21% more SlyA in cells grown in minimal medium (the mean of 1280 and 1230). It should be noted that in estimating the levels of SlyA in the different media, we have taken into account the ∼3-fold higher total protein content of cells grown in rich medium than in minimal medium (37).
FIGURE 7.
The effects of media on the β-galactosidase produced by a FimB-LacZ fusion in the presence and absence of SlyA. Strains BGEC905 (wild type; dark bars) and KCEC1334 (ΔslyA mutant, light bars) were used. Error bars and experimental conditions are described in Fig. 2.
The Effect of SlyA on the Response of fimB Expression to NanR and NagC
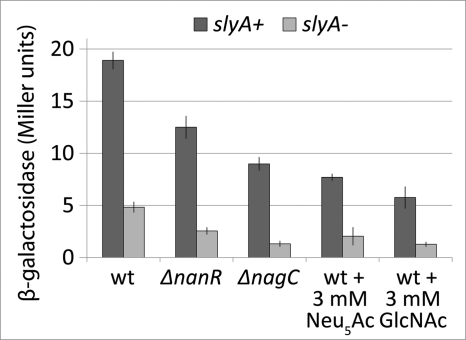
The mutations rm21 and rm22 have no effect on the ability of either NanR or NagC to activate fimB expression (data not shown), suggesting that SlyA controls fimB expression independently of either NanR or NagC. As anticipated, combination of the slyA and nanR or nagC mutations decreased fimB expression levels further than did the single mutations alone, as did inclusion of either Neu5Ac (the inducer of NanR) or GlcNAc (the inducer of NagC) in the growth medium of the slyA mutant (Fig. 8). Thus fimB expression is activated by multiple factors, with SlyA having a greater effect than either NanR or NagC have.
FIGURE 8.
The effects of NanR and NagC and their respective inducers Neu5Ac and GlcNAc on the β-galactosidase produced by a FimB-LacZ fusion in the presence and absence of SlyA. Strains BGEC905 (wild type), KCEC357 (ΔnanR), and KCEC505 (ΔnagC) (dark bars) and ΔslyA mutant derivatives (light bars) of these strains KCEC1334, KCEC1275, and KCEC1278, respectively, were used. Error bars and experimental conditions are described in Fig. 2. 3 mm Neu5Ac or GlcNAc were included in the medium as indicated.
The Effect of SlyA on Type 1 Fimbriation
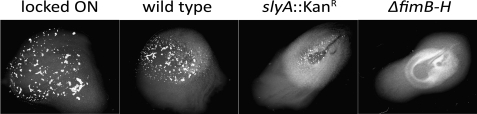
Low levels of fimB expression limit FimB-mediated recombination, and hence OFF-to-ON phase switching, under the conditions used in this study (16). As anticipated, the rate of FimB recombination was over 12-fold lower in the slyA mutant (Table 1). Furthermore, as judged by a decreased level of yeast cell agglutination, deletion of slyA in the wild type Fim+ background (strain MG1655) also diminished type 1 fimbriation as expected (Fig. 9).
TABLE 1.
The effect of a slyA mutation on FimB inversion in rich-defined glycerol medium at 37° C from OFF-to-ON per cell per generation
Cells were grown for approximately 22 generations as described by Gally et al. (15). The mean, together with minimum and maximum values obtained for replicate cultures, are shown. The strains used are listed in supplemental materials.
| Genotype | Inversion frequency (× 10−4) |
|---|---|
| Wild type | 7.23 (1.1–29.2; AAEC370A) |
| slyA | 0.58 (0.0–1.1; KCEC1360) |
FIGURE 9.
Yeast agglutination experiment showing clumping indicative of type 1 fimbriation. Strains were grown to A600 2.0 in rich-defined glycerol medium and mixed with a suspension of S. cerevisiae in a 1:1 ratio. Samples were incubated at room temperature for 30 s and then photographed. Strains shown are AAEC554 (constitutively expressing type 1 fimbriae; locked ON), MG1655 (wild type), KCEC1235 (ΔslyA::KanR), and AAEC072A (afimbriate; ΔfimA-H).
In contrast, SlyA had almost no effect on the expression of a FimE-LacZ fusion (data not shown). Moreover, the level of yeast agglutination of a strain containing the fim switch locked in the ON or fimbriate orientation was unaffected by SlyA (data not shown). Thus the effect of SlyA on fimB expression described in detail above seems to be sufficient to explain how SlyA affects both FimB recombination and type 1 fimbriation.
DISCUSSION
The phase-variable expression of type 1 fimbriation in E. coli is regulated by a range of environmental signals. Some of these, such as the availability of the branched-chain amino acids and alanine, directly affect the DNA inversion controlling phase variation mediated by both FimB and FimE to alter phase switching from both the afimbriate to fimbriate phase and vice versa. Others, however, including exogenous sialic acid and N-acetylglucosamine, exert a specific effect on the direction of phase variation by controlling fimB expression selectively. Here we identify SlyA as a novel and specific activator of fimB expression and hence type 1 fimbriation.
In initial experiments it was found that mutagenesis of the DNA sequence extending from −184 to −155 bp upstream of the fimB transcriptional start site diminished fimB expression. Inspection of the wild type sequence in this region showed that it contains a close match (5′-TTAGCATGATAA; extending from −179 to −168 bp) to the consensus binding site for SlyA (5′-TTAGCAAGCTAA) proposed by Stapleton et al. (42). The fim sequence, which we have called OSA1, differs from the consensus at only two positions (1A changed to T and 3C changed to A, according to the numbering adopted by Haider et al. (5′ −6T−5T−4A−3G−2C−1A1A2G3C4T5A6A)) (52). Furthermore the sequences 5′ −6T−5T−4A and 5′ 4T5A6A, shown by Haider et al. (52) to be most important for SlyA binding, are conserved in OSA1. In DNase I footprinting experiments it was shown that SlyA binds to OSA1 thus protecting the DNA from digestion. Deletion of slyA diminished fimB expression and this defect was complemented by an ectopic copy of slyA placed under control of the lacUV5 promoter at lac. Moreover mutations that disrupt OSA1 (rm21, 22, and 39) greatly diminish the activating effect of SlyA on fimB expression. These results show that SlyA is an activator of fimB expression and that OSA1 is required for this effect. However the SlyA binding site characterized by DNase I footprinting extends further upstream than OSA1 and includes a second potential SlyA operator (5′-CTAGGGACCTAA; OSA2, extending from −194 to −183) three bases pairs upstream of OSA1. OSA2 differs from the SlyA consensus at four positions (−6T changed to C, −2C changed to G, −1A changed to G and 2G changed to C). Mutations that disrupt OSA2 (rm35 and rm40) also significantly reduce the activating effect of SlyA on fimB expression. Thus SlyA requires the region covering both OSA1 and OSA2 to be able to activate fimB expression fully.
H-NS is an inhibitor of fimB expression and was shown previously by EMSA to bind to the fimB promoter region (49). Here we have shown that the activating effect of SlyA on fimB expression is suppressed in the absence of H-NS. In common with a number of other systems which SlyA controls (29, 48, 53), it thus seems that SlyA activates fimB expression by somehow preventing H-NS repression. We have found that H-NS protects a series of short (10–20 bp) sequences within a region extending from at least −500 bp to around +150 bp downstream of the fimB promoter from attack by DNase I, suggesting that SlyA could activate fimB expression by preventing H-NS binding to the fimB promoter. At least two models have been proposed for how SlyA antagonizes H-NS binding in other systems: “displacement,” implying direct competition between the two proteins for overlapping binding sites, and “remodeling,” where the binding of SlyA alters, but does not prevent, H-NS binding (28, 29, 30). Two of the H-NS binding sites that we have identified (H-NS2 and H-NS3) overlap the SlyA operator sites OSA1 and OSA2, respectively, and at higher concentrations, SlyA does prevent H-NS binding to both H-NS2 and H-NS3 under one of the conditions tested. On the other hand, we see no evidence in footprinting experiments that the two proteins can bind simultaneously to the overlapping operator sites. However we do note that SlyA might affect H-NS binding outside the immediate SlyA protected regions; for example the hypersensitive cleavages in the downstream part of the H-NS footprint of Fig. 5B (near positions −110 and −80 lanes 4 and 5) are also attenuated in the presence of SlyA (Fig. 5B, lanes 6 and 7).
Mutations of OSA1 (rm21, 22, and 39) that would be expected to diminish both SlyA and H-NS binding to their overlapping sites have only a modest effect on fimB expression in the otherwise wild type background. We note that a similar effect was reported for mutations simultaneously affecting overlapping SlyA and H-NS binding sites in the phoPQ promoter of Salmonella typhimurium (54). Conversely, a mutation of OSA2 (rm40) that would, if anything, be expected to enhance H-NS binding to H-NS3 by increasing the match to consensus, decreased fimB expression to a very low level. We propose that H-NS binding to H-NS2 and H-NS3 serve as nucleation sites for oligomerization of H-NS along the DNA that is necessary for full inhibition of fimB expression (51, 55, 56). We suggest that the simplest interpretation of our data is that SlyA activates fimB expression by binding to OSA1 and OSA2 to prevent, presumably by steric hindrance, H-NS binding to the overlapping operator sites H-NS2 and H-NS3, respectively.
We have shown previously that fimB expression is activated by the regulators NanR (sialic acid-responsive) and NagC (N-acetylglucosamine 6P-responsive) that bind to operators (ONR and ONC1 + ONC2 respectively) located between around 760 and 470 bp upstream of the fimB promoter (16, 17, 45). One possibility that we have considered is that SlyA affects the ability of these proteins to activate fimB expression. However, mutants lacking SlyA remain fully responsive to NanR and to NagC and their respective inducers Neu5Ac and GlcNAc. Thus fimB expression is under complex and independent regulation by multiple factors.
We have been unable to show an effect of ppGpp on the binding activity of SlyA for fimB, as has been reported for SlyA binding to the divergent pagD-pagC promoters of Salmonella (44). These results emphasize the complexity of the regulation of fimB expression and of FimB recombination and the need for further analysis of both.
We estimate there are ∼1000 SlyA dimers present in exponential-phase cells grown in rich defined glycerol medium. SlyA, along with Lrp, somehow enhances the inhibitory effect that type 1 fimbriation exerts over motility (57). While the basis for this effect requires further investigation, these and our results show that SlyA not only activates type 1 fimbriation, but that it also helps to coordinate adherence with other cellular functions. Overall SlyA controls a regulon of at least 40 proteins in EIEC (24) and our observation that SlyA is moderately abundant is consistent with this observation. Among these, SlyA represses the expression of ivy (ykfE), which encodes an inhibitor of C-lysozyme. The intact outer membrane is an effective barrier against lysozyme, and we suppose that slyA expression might be suppressed, and hence ivy induced, if the bacterial outer membrane is damaged or even if its integrity is threatened by host defenses or other stresses. Both type 1 fimbriae and the K5 capsule are attached to the outer membrane, and we suggest that SlyA may contribute to a novel signaling pathway linking cell structural robustness to the expression of these cell surface virulence factors.
Our results also show that slyA expression is limited by its poorly translated UUG initiation codon. These observations are consistent with the conclusion that SlyA both limits fimB expression and that it has a greater effect on fimB expression in minimal medium than it does in rich-defined medium. However, these results also suggest that oxidative and nitrosative stress, which limit methionine biosynthesis (58, 59), could lead to even lower levels of slyA translation and hence fimB expression. Because SlyA controls fimB expression independently of either NanR or NagC, such an effect could restrict fimB expression very substantially when combined with enhanced levels of sialic acid in the course of host inflammation (16, 19, 45). We thus propose that the regulation of fimB expression by SlyA provides an additional mechanism that helps to limit type 1 fimbriation, and hence host adherence and invasion, when host defensive mechanisms are activated.
Supplementary Material
This work was supported by Grants GR076360/Z/05/Z and 085931/Z/08/Z from the Wellcome Trust. The work in Paris was supported by funds from the CNRS (UPR9073).

The on-line version of this article (available at http://www.jbc.org) contains supplemental materials.
- ppGpp
- guanine tetraphosphate
- EIEC
- enteroinvasive E. coli
- NagC
- N-acetylglucosamine 6P-responsive.
REFERENCES
- 1. Connell H., Agace W., Klemm P., Schembri M., Mårild S., Svanborg C. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 9827–9832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahrani-Mougeot F. K., Buckles E. L., Lockatell C. V., Hebel J. R., Johnson D. E., Tang C. M., Donnenberg M. S. (2002) Mol. Microbiol. 45, 1079–1093 [DOI] [PubMed] [Google Scholar]
- 3. Teng C. H., Xie Y., Shin S., Di Cello F., Paul-Satyaseela M., Cai M., Kim K. S. (2006) Infect Immun. 74, 5609–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnich N., Darfeuille-Michaud A. (2007) World J. Gastroenterol. 13, 5571–5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhakal B. K., Kulesus R. R., Mulvey M. A. (2008) Eur J. Clin. Invest. (suppl.) 2, 2–11 [DOI] [PubMed] [Google Scholar]
- 6. Wiles T. J., Kulesus R. R., Mulvy M. A. (2008) Exp. Mol. Pathol. 85, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mossman K. L., Mian M. F., Lauzon N. M., Gyles C. L., Lichty B., Mackenzie R., Gill N., Ashkar A. A. (2008) J. Immunol. 181, 6702–6706 [DOI] [PubMed] [Google Scholar]
- 8. Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 5724–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blomfield I. C. (2001) Adv. Microb. Physiol. 45, 1–49 [DOI] [PubMed] [Google Scholar]
- 10. van der Woude M. W., Bäumler A. J. (2004) Clin. Microbiol. Rev. 17, 581–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klemm P. (1986) EMBO J. 5, 1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gally D. L., Leathart J., Blomfield I. C. (1996) Mol. Microbiol. 21, 725–738 [DOI] [PubMed] [Google Scholar]
- 13. Blomfield I. C., McClain M. S., Princ J. A., Calie P. J., Eisenstein B. I. (1991) J. Bacteriol. 173, 5298–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McClain M. S., Blomfield I. C., Eisenstein B. I. (1991) J. Bacteriol. 173, 5308–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gally D. L., Bogan J. A., Eisenstein B. I., Blomfield I. C. (1993) J. Bacteriol. 175, 6186–6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Labany S., Sohanpal B. K., Lahooti M., Akerman R., Blomfield I. C. (2003) Mol. Microbiol. 49, 1109–1118 [DOI] [PubMed] [Google Scholar]
- 17. Sohanpal B. K., El-Labany S., Lahooti M., Plumbridge J. A., Blomfield I. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 16322–16327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aberg A., Shingler V., Balsalobre C. (2006) Mol Microbiol 60, 1520–1533 [DOI] [PubMed] [Google Scholar]
- 19. Lahooti M., Roesch P. L., Blomfield I. C. (2005) J Bacteriol 187, 6273–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bryan A., Roesch P., Davis L., Moritz R., Pellett S., Welch R. A. (2006) Infect. Immun. 74, 1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie Y., Yao Y., Kolisnychenko V., Teng C. H., Kim K. S. (2006) Infect Immun. 74, 4039–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fass E., Groisman E. A. (2009) Curr. Opin. Microbiol. 12, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oscarsson J., Mizunoe Y., Uhlin B. E., Haydon D. J. (1996) Mol Microbiol 20, 191–199 [DOI] [PubMed] [Google Scholar]
- 24. Spory A., Bosserhoff A., Rhein C., von Goebel W., Ludwig A. (2002) J. Bacteriol. 184, 3549–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Navarre W. W., Halsey T. A., Walthers D., Frye J., McClelland M., Potter J. L., Kenney L. J., Gunn J. S., Fang F. C., Libby S. J. (2005) Mol. Microbiol. 56, 492–508 [DOI] [PubMed] [Google Scholar]
- 26. Whitfield C. (2006) Annu. Rev. Biochem. 75, 39–68 [DOI] [PubMed] [Google Scholar]
- 27. Corbett D., Bennett H. J., Askar H., Green J., Roberts I. S. (2007) J. Biol. Chem. 282, 33326–33335 [DOI] [PubMed] [Google Scholar]
- 28. Lithgow J. K., Haider F., Roberts I. S., Green J. (2007) Mol Microbiol 66, 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perez J. C., Latifi T., Groisman E. A. (2008) J. Biol. Chem. 283, 10773–10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stoebel D. M., Free A., Dorman C. J. (2008) Microbiology 154, 2533–2545 [DOI] [PubMed] [Google Scholar]
- 31. Guyer M. S., Reed R. R., Steitz J. A., Low K. B. (1981) Cold Spring Harb. Symp. Quant. Biol. 45, 135–140 [DOI] [PubMed] [Google Scholar]
- 32. Hamilton C. M., Aldea M., Washburn B. K., Babitzke P., Kushner S. R. (1989) J. Bacteriol. 171, 4617–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blomfield I. C., Vaughn V., Rest R. F., Eisenstein B. I. (1991) Mol. Microbiol. 5, 1447–1457 [DOI] [PubMed] [Google Scholar]
- 34. Neidhardt F. C., Bloch P. L., Smith D. F. (1974) J. Bacteriol. 119, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silhavy T. J., Berman M. L., Enquist L. W. (1984) Experiments with Gene Fusions, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 36. Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, New York [Google Scholar]
- 37. Bremer H., Dennis P. P. (1996) in Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology (Neidhardt F. C., Curtiss I. R., Ingraham J. L., Lin E. C. C., Low K. B., Magasani B., et al., eds) Vol 2, pp. 1553–1566, American Society for Microbiology, Washington D. C [Google Scholar]
- 38. Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Struhl K. (1987) Current Protocols in Molecular Biology, John Wiley and Sons, New York [Google Scholar]
- 39. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Syst. Biol. 2, 2006–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 42. Stapleton M. R., Norte V. A., Reads R. C., Green J. (2002) J. Biol. Chem. 277, 17630–17637 [DOI] [PubMed] [Google Scholar]
- 43. Bouffartigues E., Buckle M., Badaut C., Travers A., Rimsky S. (2007) Nat. Struct. Mol. Biol. 14, 441–448 [DOI] [PubMed] [Google Scholar]
- 44. Zhao G., Weatherspoon N., Kong W., Curtiss R., 3rd, Shi Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20924–20929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sohanpal B. K., Friar S., Roobol J., Plumbridge J. A., Blomfield I. C. (2007) Mol. Microbiol. 101, 16322–16327 [DOI] [PubMed] [Google Scholar]
- 46. Kawakami T., Kaneko A., Okada N., Imajoh-Ohmi S., Nonaka T., Matsui H., Kawahara K., Danbara H. (1999) Microbiol. Immunol. 43, 351–357 [DOI] [PubMed] [Google Scholar]
- 47. Liang S. T., Xu Y. C., Dennis P., Bremer H. (2000) J. Bacteriol. 182, 3037–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wyborn N. R., Stapleton M. R., Norte V. A., Roberts R. E., Grafton J., Green J. (2004) J. Bacteriol. 186, 1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Donato G. M., Lelivelt M. J., Kawula T. H. (1997) J. Bacteriol. 179, 6618–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y., Chen H., Kenney L. J., Yan J. (2010) Genes Dev. 24, 339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lang B., Blot N., Bouffartigues E., Buckle M., Geertz M., Gualerzi C. O., Mavathur R., Muskhelishvili G., Pon C. L., Rimsky S., Stella S., Babu M. M., Travers A. (2007) Nucleic Acids Res. 35, 6330–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haider F., Lithgow J. K., Stapleton M. R., Nirte V. A., Roberts R. E., Green J. (2008) Int. Microbiol. 11, 245–250 [DOI] [PubMed] [Google Scholar]
- 53. Westermark M., Oscarsson J., Mizunoe Y., Urbonaviciene J., Uhlin B. E. (2000) J. Bacteriol. 182, 6347–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Song H., Kong W., Weatherspoon N., Qin G., Tyler W., Turk J., Curtis R., 3rd, Shi Y. (2008) J. Biol. Chem. 283, 28158–28168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fang F. C., Rimsky S. (2008) Curr. Opin. Microbiol. 11, 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dorman C. J., Kane K. A. (2009) FEMS Microbiol. Rev. 33, 587–592 [DOI] [PubMed] [Google Scholar]
- 57. Simms A. N., Mobley H. L. (2008) J. Bacteriol. 190, 3747–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hondorp E. R., Matthews R. G. (2004) PloS Biol 2, e336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flatley J., Barrett J., Pullan S. T., Hughes M. N., Green J., Poole R. K. (2005) J. Biol. Chem. 280, 10065–10072 [DOI] [PubMed] [Google Scholar]
- 60. Blomfield I. C., Calie P. J., Eberhardt K. J., McClain M. S., Eisenstein B. I. (1993) J. Bacteriol. 175, 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McClain M. S., Blomfield I. C., Eberhardt K. J., Eisenstein B. I. (1993) J. Bacteriol. 175, 4335–4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.