Abstract
Calpains make up a family of Ca2+-dependent intracellular cysteine proteases that include ubiquitously expressed μ- and m-calpains. Both are heterodimers consisting of a distinct large catalytic subunit (calpain 1 for μ-calpain and calpain 2 for m-calpain) and a common regulatory subunit (calpain 4). The physiological roles of calpain remain unclear in the organs, including the heart, but it has been suggested that calpain is activated by Ca2+ overload in diseased hearts, resulting in cardiac dysfunction. In this study, cardiac-specific calpain 4-deficient mice were generated to elucidate the role of calpain in the heart in response to hemodynamic stress. Cardiac-specific deletion of calpain 4 resulted in decreased protein levels of calpains 1 and 2 and showed no cardiac phenotypes under base-line conditions but caused left ventricle dilatation, contractile dysfunction, and heart failure with interstitial fibrosis 1 week after pressure overload. Pressure-overloaded calpain 4-deficient hearts took up a membrane-impermeant dye, Evans blue, indicating plasma membrane disruption. Membrane repair assays using a two-photon laser-scanning microscope revealed that calpain 4-deficient cardiomyocytes failed to reseal a plasma membrane that had been disrupted by laser irradiation. Thus, the data indicate that calpain protects the heart from hemodynamic stresses, such as pressure overload.
Keywords: Calcium, Calpain, Cardiac Muscle, Heart, Membrane Function, Protease, Protein Degradation, Heart Failure
Introduction
Calpains are a family of neutral Ca2+-activated cysteine proteases that are believed to participate in basic Ca2+-mediated intracellular processes (1, 2). There are 14 calpain gene family members in mammals. Two ubiquitous forms of calpains have been identified, μ- and m-calpains, which are activated by micromolar and millimolar Ca2+ concentrations, respectively. Both are heterodimers consisting of an 80-kDa catalytic subunit (calpain 1 for μ-calpain and calpain 2 for m-calpain, which are encoded by the Capn1 and Capn2 genes, respectively) and a common 28-kDa regulatory subunit (calpain 4/CAPNS1 (calpain small-1), which is encoded by the Capns1 gene).
Limited proteolysis of specific substrate proteins by calpain in distinct subcellular locations leads to their altered function in cells, and this makes calpain an important regulator of cellular signaling mechanisms (1, 2). The in vivo physiological function of calpain remains to be elucidated, although many potential substrate proteins have been identified. Loss-of-function studies in mice have been performed to determine the physiological functions of μ- and m-calpains (3–7). Homozygous deletion of Capns1 abolishes the expression and activity of both μ- and m-calpains, resulting in embryonic lethality with apparent defects in the cardiovascular system and erythropoiesis (3, 6, 8). Disruption of Capn2 also results in pre-implantation embryonic lethality (5). Thus, calpain is essential for embryonic development.
Mouse embryonic fibroblasts derived from calpain 4-deficient mice have been used to study functions of calpain in vitro. These studies indicate that calpains play important roles in cell migration, actin cytoskeleton organization, apoptosis, autophagy, plasma membrane repair, and membrane blebbing (9–14).
Calpain activity is increased in a wide variety of pathological conditions associated with Ca2+ overload (15). In the heart, increased calpain activation has been observed in pressure-overloaded myocardium, and attenuation of calpain activation by its inhibitor was found to improve contractile function (16, 17). Similarly, calpain activation and cleavage of its substrate have been reported during ischemic preconditioning and myocardial infarction (18–22), and some of these events are accompanied by programmed cell death and necrosis. These data imply a detrimental role of calpain in the diseased heart. However, loss of myocardial calpain activity by forced expression of calpastatin, an endogenous inhibitor of calpain, results in progressive dilated cardiomyopathy (23). Previously reported studies relied on small molecule inhibitors that lack complete calpain sensitivity, on overexpression of calpain or calpastatin, or on in vitro proteolysis of the putative calpain substrates. In this study, cardiac-specific calpain 4-deficient mice were generated to elucidate the role of calpain in the heart in response to pressure overload. This is the first report to identify an in vivo role of the ubiquitous calpains in the stress response.
EXPERIMENTAL PROCEDURES
Generation of Cardiac-specific Calpain 4-deficient Mice
This study was carried out under the supervision of the Animal Research Committee of Osaka University and in accordance with the Guidelines for Animal Experiments of Osaka University and the Japanese Act on Welfare and Management of Animals (No. 105). A 9.3-kb SalI-SacI fragment that included exons 1–9 from the mouse 129/SvJ genomic library was used for the targeting construct. The targeting construct was developed by inserting the loxP site into the SpeI site located 813 bp upstream from exon 7 and loxP sites along with the PGK-neo gene into the BamHI site located 176 bp downstream from exon 9. The targeting vector contained 7.5 kb of the homologous DNA upstream from the loxP-PGK-neo-loxP cassette site and 1.8 kb of the homologous DNA downstream from the third loxP site. PCR, Southern blotting, and karyotyping analyses were performed to obtain embryonic stem clones exhibiting the desired homologous recombination and normal karyotype. Highly chimeric mice, generated by aggregating these targeted embryonic stem cells into BDF1 blastocysts, were bred with C57B/6J mice. To remove the selection marker gene, PGK-neo, and to obtain a type II deletion, F1 mice with germ line transmission of the loxP-targeted Capns1 allele were cross-bred with EIIa-Cre mice, resulting in heterozygous Capns1-floxed mice without PGK-neo. α-MHCCre mice in the C57B/6J background (24) were mated with Capns1-floxed mice. Primers used for PCR screening were H1F (5′-AGAGAATACATTGCAGGATAGG-3′) and H2R (5′-TATAATGAGATCTGGTGGCCTC-3′) (700-bp PCR product) and primers 246F (5′-CGTCTAAGAAACCATTATTATCATGAC-3′) and 246R (5′-ATGGCCAGTACTAGTGAACCTCTTCGA-3′) (170-bp PCR product when PGK-neo was floxed out).
Transverse Aortic Constriction, Echocardiography, Hemodynamic Analysis, and Drug Administration
Transverse aortic constriction (TAC)3 operation and hemodynamic measurements were performed as described previously (25). To perform echocardiography on awakened mice, ultrasonography (SONOS-5500, equipped with a 15-MHz linear transducer, Philips Medical Systems) was used. Noninvasive measurements of blood pressure were carried out on mice anesthetized with 2.5% Avertin (26). Osmotic minipumps (ALZET Model 1002) were used to administer isoproterenol at a dose of 50 mg/kg of body weight for 2 weeks.
Histological Analysis
Hematoxylin/eosin or Azan-Mallory staining was performed on paraffin-embedded sections. The cross-sectional areas of cardiomyocytes were determined as described previously (25). The fibrotic area of the total area was assessed by computer-aided image analysis of Azan-Mallory-stained tissue sections. Three fields at 100-fold magnification for each mouse (n = 3) were captured and assessed. To detect apoptotic cells, triple staining with terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL), propidium iodide, and anti-α-sarcomeric actin antibody (Sigma) was performed on paraffin-embedded sections (25). Mice were injected intraperitoneally with Evans blue dye (1 mg of dye/0.1 ml of PBS/10 g of body weight) 3 h before TAC operation. Twenty-four h after TAC, serial frozen sections of cardiac muscle were studied for dye uptake (27).
Southern and Western Blot Analyses
Southern blot analysis of embryonic stem cells or mouse hearts was performed as reported previously (25). Genomic DNA was isolated from mouse hearts, digested with PstI, and subjected to Southern blot analysis (see Fig. 2B). The probe used was an 892-bp PCR fragment containing exons 4–6. Protein homogenates were subjected to Western blot analysis using antibodies against calpains 1, 2, and 4 (Abcam).
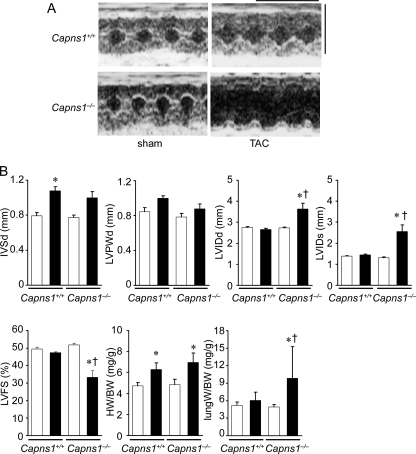
FIGURE 2.
Development of cardiomyopathy in Capns1−/− mice in response to pressure overload. Capns1−/− mice were analyzed 7 days after TAC. A, representative M-mode echocardiographic tracings. Scale bars = 0.2 s and 5 mm, respectively. B, echocardiographic and physiological parameters. Open and closed bars represent sham- and TAC-operated groups, respectively. Sham-operated Capns1+/+ mice (n = 7), TAC-operated Capns1+/+ mice (n = 12), sham-operated Capns1−/− mice (n = 8), and TAC-operated Capns1−/− mice (n = 13) are shown. Values are expressed as the mean ± S.E. *, p < 0.05 versus the corresponding sham-operated group; †, p < 0.05 versus TAC-operated Capns1+/+ mice. IVSd, diastolic interventricle septum wall thickness; LVPWd, diastolic LV posterior wall thickness; LVIDd, end-diastolic LV internal dimension; LVIDs, end-systolic LV internal dimension; LVFS, LV fractional shortening; HW, heart weight; BW, body weight.
Membrane Repair Assay
The in vitro membrane assay was performed on adult cardiomyocytes isolated from 10-week-old mice with some modifications (12). The isolated cardiomyocytes (28) were imaged using a two-photon confocal laser-scanning microscope (Olympus FV100MPE) and subjected to laser wounding at 37 °C in Tyrode's solution containing 140 mm NaCl, 5 mm KCl, 1 mm MgCl2, 10 mm Hepes (pH 7.0), and 10 mm glucose in the presence of 4 μm FM® 1-43FX dye (Invitrogen) and 100 mm 2,3-butanedione monoxime (Sigma) with 1 mm Ca2+. A 5-μm diameter half-circle area of the surface cardiomyocytes was irradiated with a 720-nm laser at 10% of maximum power for 300 ms. Intracellular Ca2+ was measured using a Fluo-4 direct calcium assay kit (Invitrogen). Calcium measurements were performed with excitation at 473 nm and fluorescence emission at 520 nm. The background fluorescence was subtracted off-line, and fluorescence (F) was normalized to the fluorescence before laser injury (F0) and reported as F/F0. The in situ membrane repair assay (27) was conducted on the whole heart with some modifications. The whole heart was placed in Tyrode's solution in the presence of 2 μm FM 1-43FX dye and 100 mm 2,3-butanedione monoxime with 1 mm Ca2+. To induce membrane damage, a 5 × 200-μm rectangular area of the heart was irradiated with a 720-nm laser at maximum power at 30 μm from the surface for 500 ms. Images at 30 μm from the surface of the heart were obtained with a two-photon confocal laser-scanning microscope.
Statistics
Results are expressed as the mean ± S.E. Statistical analyses were performed using Statcel2 software (OMS Publishing Inc., Tokorozawa, Japan). Comparisons between two groups were performed using Student's t test. One-way analysis of variance with Bonferroni post hoc test was used for multiple comparisons. p < 0.05 was considered statistically significant.
RESULTS
Generation of Mice Lacking Calpain 4 in the Heart
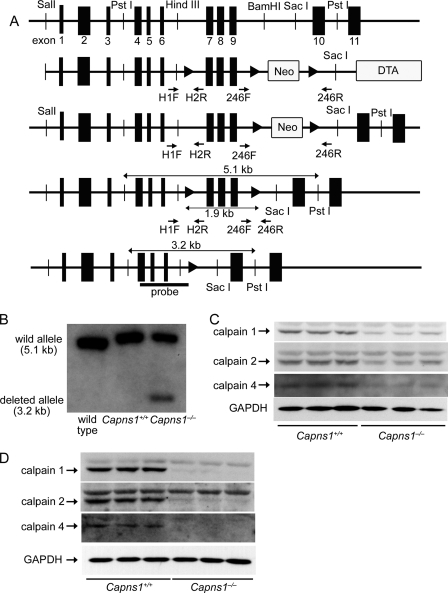
To obtain cardiac-specific calpain 4-deficient mice, the Capns1 gene was conditionally inactivated by inserting loxP sites in introns 6 and 9 (Fig. 1A). Selection cassettes comprising a neomycin resistance gene (neo) for positive selection and a diphtheria toxin gene for negative selection were positioned between two loxP sites downstream from the floxed exons and at the 3′-end of the targeting vector, respectively. Mice that harbored the Capns1-floxed allele and the phosphoglycerate kinase PGK-neo cassette selection marker gene were crossed with EIIa-Cre mice to obtain heterozygous Capns1-floxed mice without the PGK-neo cassette (29). The homozygous Capns1-floxed mice (Capns1flox/flox) appeared normal and were externally indistinguishable from littermates of other genotypes. To disrupt the Capns1 gene selectively in the myocardium, Capns1flox/flox mice were crossed with mice expressing the Cre recombinase under the control of the α-myosin heavy chain promoter (α-MHCCre mice) (24) to obtain Capns1flox/flox:α-MHCCre+ mice (Capns1−/−). Capns1flox/flox:α-MHCCre− littermates (Capns1+/+) were used as controls. Southern blot analysis of the mouse hearts indicated successful recombination in Capns1flox/flox:α-MHCCre+ hearts (Fig. 1B). Immunoblotting revealed an ∼77% reduction of calpain 4 protein in the Capns1−/− hearts (Fig. 1C). When adult cardiomyocytes were isolated as a partially purified single cell preparation, calpain 4 protein in Capns1−/− cardiomyocytes was not detected. In Capns1−/− mice, 50 and 31% reductions were observed in the levels of calpains 1 and 2, respectively, in whole heart homogenates and ∼96 and 80% reductions in the levels of calpains 1 and 2, respectively, in a partially purified adult cardiomyocyte preparation (Fig. 1D). These results agree with previous reports that demonstrated that ablation of Capns1 results in a loss of calpain 1 and 2 proteins (3, 6, 8).
FIGURE 1.
Targeted modification of the Capns1 gene. A, schematic structures of genomic Capns1 sequences, the targeting construct, the targeted allele, the type II deletion, and the Capns1−/− allele (from top to bottom). The arrowheads represent loxP sites. The targeting construct includes the PGK-neo cassette flanked by loxP sites and a diphtheria toxin gene (DTA). The arrows correspond to the primer sequences for PCR screening. The bar labeled probe corresponds to the sequence used for Southern blotting analysis in B. B, genomic analysis of in vivo hearts. Genomic DNA was isolated from mouse hearts, digested with PstI, and analyzed by Southern blotting with the probe. C and D, protein expression of the calpain family in whole heart homogenates (C) and in partially purified adult cardiomyocytes (D) from 10-week-old mice.
Normal Cardiac Structure and Function in Calpain 4-deficient Mice
Capns1−/− and Capns1+/+ mice were born at the expected Mendelian ratio of 48:52, respectively. Capns1−/− mice were normal at birth, and their external appearance was indistinguishable from that of their littermates. The mice grew to adulthood and were fertile. There was no difference in survival at 1 year between Capns1−/− and Capns1+/+ mice. Capns1−/− hearts showed no evidence of cardiac morphological defects. Histological examination of hearts from 10-week-old Capns1−/− mice revealed no myofibrillar disarray, necrosis, or fibrosis (data not shown). Physiological and echocardiographic parameters were not significantly different between Capns1−/− and Capns1+/+ mice (Table 1). Thus, Capns1−/− mice had normal global cardiac structure and function.
TABLE 1.
Physiological, echocardiographic, and hemodynamic parameters in Capns1+/+ and Capns1−/− mice at base line
Data are expressed as the mean ± S.E. There were no significant differences between Capns1+/+ and Capns1−/− mice in any parameters. IVSd, diastolic interventricle septum wall thickness; LVIDd, end-diastolic LV internal dimension; LVIDs, end-systolic LV internal dimension; LVPWd, diastolic LV posterior wall thickness; LVFS, LV fractional shortening.
| Capns1+/+ (n = 8) | Capns1−/− (n = 9) | |
|---|---|---|
| Body weight (g) | 24.7 ± 0.7 | 24.3 ± 0.5 |
| Tibia length (mm) | 18.0 ± 0.1 | 17.9 ± 0.1 |
| Heart weight (mg) | 111.5 ± 2.4 | 113.1 ± 3.0 |
| Lung weight (mg) | 127.0 ± 3.8 | 129.6 ± 3.0 |
| Heart weight/body weight (mg/g) | 4.52 ± 0.11 | 4.66 ± 0.09 |
| Lung weight/body weight (mg/g) | 5.14 ± 0.10 | 5.35 ± 0.14 |
| Capns1+/+ (n = 10) | Capns1−/− (n = 10) | |
|---|---|---|
| IVSd (mm) | 0.77 ± 0.08 | 0.79 ± 0.08 |
| LVIDd (mm) | 2.66 ± 0.03 | 2.73 ± 0.03 |
| LVIDs (mm) | 1.36 ± 0.11 | 1.35 ± 0.05 |
| LVPWd (mm) | 0.77 ± 0.02 | 0.77 ± 0.02 |
| LVFS (%) | 48.9 ± 1.0 | 50.5 ± 0.4 |
| Heart rate (/min) | 664.8 ± 17.0 | 655.5 ± 22.5 |
Development of Congestive Heart Failure in Response to Pressure Overload in Calpain 4-deficient Mice
Ten-week-old Capns1−/− and Capns1+/+ mice were subjected to TAC. In wild-type mice, pressure overload by TAC induced cardiac hypertrophy without any signs of contractile dysfunction 1 week after the operation and heart failure 4 weeks after the operation (25). One week after TAC, echocardiographic analysis was performed (Fig. 2, A and B). One week after sham operation, the end-diastolic and end-systolic left ventricle (LV) internal dimensions and LV fractional shortening, an index of contractility, were not significantly different between Capns1−/− and Capns1+/+ mice. However, end-diastolic and end-systolic LV internal dimensions were significantly elevated, and LV fractional shortening was significantly reduced in TAC-operated Capns1−/− mice compared with both sham-operated Capns1−/− and TAC-operated Capns1+/+ mice (Fig. 2B). Furthermore, the lung/body weight ratio, an index of lung congestion, was significantly elevated in TAC-operated Capns1−/− mice compared with the other groups. These findings indicate that Capns1−/−mice develop congestive heart failure in response to pressure overload.
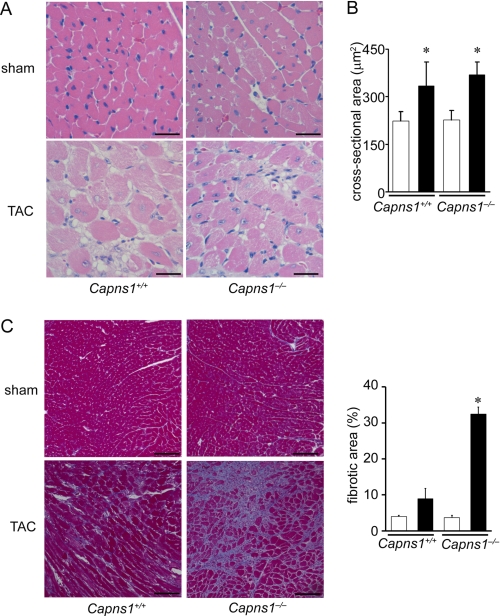
Although TAC increased the heart/body weight ratio and cardiomyocyte cross-sectional area in both Capns1−/− and Capns1+/+ mice, there was no significant difference between the two groups (Figs. 2B and 3, A and B). Thus, calpain is not related to pressure overload-induced cardiac hypertrophy. Azan-Mallory staining revealed interstitial and perivascular fibrosis in both TAC-operated Capns1−/− and Capns1+/+ hearts, but the extent of fibrosis in Capns1−/− mice was greater than in Capns1+/+ mice (Fig. 3C).
FIGURE 3.
Histological analysis of Capns1−/− hearts 7 days after TAC. A, hematoxylin/eosin-stained heart sections. Scale bars = 25 μm. B, cardiomyocyte cross-sectional area measured in the sections in A. Open and closed bars represent sham- and TAC-operated groups, respectively. Values are expressed as the mean ± S.E. *, p < 0.05 versus the corresponding sham-operated group. C, Azan-Mallory-stained heart sections. Quantification of fibrotic area is shown in the graph. Scale bars = 100 μm. Open and closed bars represent sham- and TAC-operated groups, respectively. Values are expressed as the mean ± S.E. *, p < 0.05 versus all other groups.
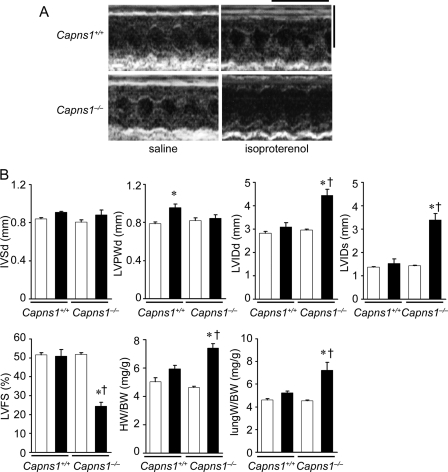
To confirm that calpain protects the heart against external stress, the role of calpain was examined in β-adrenergic stimulation-induced cardiac remodeling. Because β-adrenergic hyperactivation is observed in failing hearts, the isoproterenol infusion model has been employed to elucidate molecular mechanisms underlying cardiac remodeling (28). Isoproterenol infusion for 2 weeks induced LV chamber dilatation and cardiac dysfunction in Capns1−/− mice but not in Capns1+/+ mice (Fig. 4). Thus, calpain has a protective role in response to external stresses such as pressure overload and β-adrenergic stimulation.
FIGURE 4.
β-Adrenergic stress-induced cardiac dysfunction in Capns1−/− mice. Isoproterenol or saline was administered to Capns1−/− and Capns1+/+ mice for 2 weeks. A, representative images of transthoracic M-mode echocardiographic tracing 2 weeks after an infusion of isoproterenol. Scale bars = 0.2 s and 5 mm, respectively. B, echocardiographic and physiological parameters. Values are expressed as the mean ± S.E. (n = 4 in each group). Open and closed bars represent saline- and isoproterenol-treated groups, respectively. *, p < 0.05 versus the corresponding saline-treated group; †, p < 0.05 versus isoproterenol-treated Capns1+/+ mice. IVSd, diastolic interventricle septum wall thickness; LVPWd, diastolic LV posterior wall thickness; LVIDd, end-diastolic LV internal dimension; LVIDs, end-systolic LV internal dimension; LVFS, LV fractional shortening; HW, heart weight; BW, body weight.
Defective Membrane Repair in Calpain 4-deficient Hearts
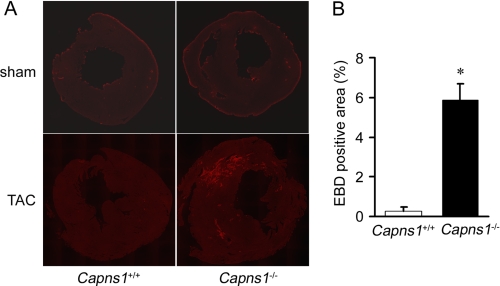
In this study, we attempted to elucidate the molecular mechanism underlying the beneficial role of calpain against pressure overload. Calpain exists in the cytosol as an inactive form, translocates to membranes, and is activated at the membrane in response to increases in cellular calcium (2, 30, 31). First, the structural stability of the sarcolemma was tested by examining Evans blue dye uptake into TAC-operated Capns1−/− hearts. In Capns1−/− mice, sporadic Evans blue dye-positive cardiomyocytes were observed 24 h after TAC. This was not the case with hearts from TAC-operated Capns1+/+ or sham-operated Capns1−/− mice (Fig. 5, A and B).
FIGURE 5.
Plasma membrane damage in Capns1−/− hearts. A, uptake of Evans blue dye in the hearts of Capns1−/− and Capns1+/+ mice following TAC or sham operation. B, Evans blue dye (EBD)-positive area per LV cross-sectional area in TAC-operated Capns1−/− (n = 6) and Capns1+/+ (n = 4) mice. Values are expressed as the mean ± S.E. *, p < 0.05.
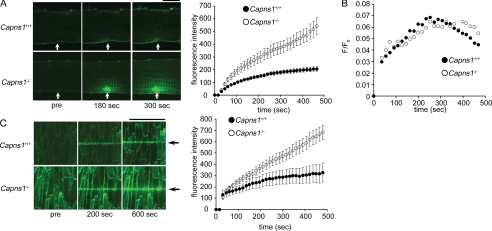
Hemodynamic stress can cause membrane damage by two different mechanisms: enhanced susceptibility to the stress and defective membrane repair. To visualize real-time sealing in injured cardiomyocytes, a laser-based assay was used to measure resealing kinetics in adult cardiomyocytes isolated from Capns1−/− mice (12). In this assay, staining of internal membranes by an otherwise membrane-impermeable dye, FM 1-43FX, was monitored microscopically over time after laser disruption. Resealing blocked further dye entry through the disruption and halted cell staining. Therefore, the measured whole cell fluorescence reached a plateau upon the completion of resealing. Although the Capns1+/+ cardiomyocytes resealed within 250 s, the Capns1−/− cardiomyocytes continued to take up FM 1-43FX dye for at least 480 s (Fig. 6A). These results indicate that membrane resealing was impaired in the Capns1−/− cardiomyocytes. When the intracellular Ca2+ concentration was measured using the Ca2+-sensitive fluorescent dye Fluo-4, an increase in the fluorescent signal was detected at the position of laser exposure. The time course of Fluo-4 fluorescence during the resealing process was similar for Capns1−/− and Capns1+/+ cardiomyocytes, but Capns1+/+ cardiomyocytes tended to show lower fluorescence intensity compared with Capns1−/− mice thereafter (Fig. 6B). The power of laser irradiation was varied to estimate its threshold for detection of the staining of internal membranes. There was no significant difference in the minimum level of laser power (6% of maximum laser power) to detect membrane damage between Capns1−/− and Capns1+/+ cardiomyocytes.
FIGURE 6.
Impaired plasma membrane repair in Capns1−/− cardiomyocytes. A, time course of membrane sealing after laser injury in isolated cardiomyocytes. Capns1−/− and Capns1+/+ cardiomyocytes were injured with a pulsed laser in the presence of FM 1-43FX with 1 mm Ca2+. Left panel, representative images; right panel, fluorescence versus time (n = 12–14). Scale bar = 10 μm. Arrows indicate laser damage. B, time course of Fluo-4 fluorescence during the resealing process. Capns1−/− and Capns1+/+ cardiomyocytes were loaded with Fluo-4 and injured with a pulsed laser with 1 mm Ca2+. The background fluorescence was subtracted off-line, and fluorescence (F) was normalized to the fluorescence before laser injury (F0) and reported as F/F0. F/F0 versus time is shown. C, hearts from Capns1−/− and Capns1+/+ mice were damaged with a linear laser in the presence of FM 1-43FX with 1 mm Ca2+. Images at 30 μm from the surface of the heart were obtained. Left panel, representative images 0 (pre), 200, and 600 s after laser irradiation; right panel, fluorescence versus time (n = 3). Scale bar = 100 μm. Arrows indicate laser damage.
To confirm impaired membrane repair in Capns1−/− cardiomyocytes, an in situ membrane repair assay was performed on the surface cardiomyocytes of the heart (27). The membranes of cardiomyocytes were wounded by maximum power irradiation with an infrared laser in the presence of FM 1-43FX dye. Images were obtained at 30 μm from the surface of the heart. After laser injury, the FM 1-43FX fluorescence intensity initially increased but halted within 100–150 s in Capns1+/+ hearts, whereas the fluorescence intensity continuously increased in Capns1−/− hearts (Fig. 6C). Although a subtle effect of calpain deletion on membrane fragility cannot be ruled out, the impaired membrane repair would lead to enhanced susceptibility to stress in Capns1−/− mice.
DISCUSSION
In this study, we generated cardiac-specific calpain 4-deficient mice to elucidate a role of calpain in the heart. Because ablation of Capns1 results in a loss of calpain 1 and 2 proteins (3, 6, 8), calpain 4-deficient mice or cells have been used to study functions of μ- and m-calpains. We have shown that Capns1−/− mice had normal global cardiac structure and function, indicating that the ubiquitous calpain in cardiomyocytes is not essential for cardiac development in mice. Homozygous deletion of Capns1 results in embryonic lethality with apparent defects in the cardiovascular system (3, 6, 8). Therefore, calpain in non-cardiomyocytes appears to play an important role in the development of the cardiovascular system, or the residual non-floxed-out cardiomyocytes contribute to normal cardiac development.
Furthermore, calpain was shown to play a beneficial role in response to hemodynamic stress. The cardioprotective role of calpain was confirmed by the experiment showing that a β-adrenergic stimulant induced LV chamber dilatation and cardiac dysfunction in Capns1−/− mice. This is the first report to identify the in vivo role of the ubiquitous calpains in the stress response using a loss-of-function technique. The data from this study are not in agreement with previous work from other laboratories that demonstrated that calpain causes the alteration of a number of specific proteins, leading to subcellular remodeling and cardiac dysfunction (17, 32–34). This discrepancy may be caused by the use of non-selective calpain inhibitors or a non-physiological substrate. However, calpain reportedly promotes both apoptosis and survival signals in response to different cell death stimuli (10). Further investigation will be necessary to elucidate the role of calpain regarding cell death in other experimental models such as ischemia-reperfusion.
In vitro studies using Capns1−/− cells indicate that the protective roles of calpain come from regulation of apoptosis, autophagy, proliferation, actin cytoskeleton organization, and focal adhesion or membrane repair (7, 9–12, 14). The anti-apoptotic role of calpain has been reported (10, 14). In fact, a 3-fold increase was observed in the number of TUNEL-positive cardiomyocytes in TAC-operated Capns1−/− hearts (data not shown). Although the anti-apoptotic effect of calpain may contribute to the observed phenotypes in TAC-operated Capns1−/− hearts, the level of apoptosis in Capns1−/− hearts appears to be too low to develop cardiomyopathy within 1 week of TAC (35). We have reported previously that autophagy protects the heart from hemodynamic stress (28). However, in this study, we did not see a significant suppression of autophagic activity in TAC-operated Capns1−/− hearts as estimated by Western blotting of LC3, which is a molecular marker for autophagy. Furthermore, sarcomere disorganization in Capns1−/− hearts was not observed. Because cardiomyocytes are terminally differentiated cells, proliferation or differentiation does not seem to be involved in the protective function of calpain. In this study, we showed that the structural stability of the plasma membrane was reduced in TAC-operated Capns1−/− hearts and that membrane resealing was impaired in the isolated Capns1−/− cardiomyocytes. The sustained membrane damage could cause cardiomyocyte loss and replacement fibrosis in TAC-operated Capns1−/− hearts. However, the possibility that a mechanism other than impaired membrane repair is responsible for the development of cardiomyopathy in Capns1−/− hearts cannot be excluded.
The mechanism of membrane resealing is now well characterized at the cellular level. A large plasma membrane disruption elicits Ca2+-activated homotypic vesicle fusion locally and rapidly. The “patch” vesicles thus formed then fuse exocytotically with the plasma membrane surrounding the defect site, restoring barrier continuity (36, 37). Removal of the actin cytoskeleton at the damaged area of the plasma membrane is a critical early event for resealing. Although identification of the protein components of this process is under way, several candidate repair proteins have been identified, including vesicle SNARE (soluble NSF attachment protein receptor) and target SNARE (38), dysferlin (27, 39), annexin A1 (40), and calpain (12). Dysferlin-deficient mice show significant Evans blue uptake in cardiomyocytes, impaired sarcolemma repair, and cardiomyopathy after stress exercise, similar to what was observed in Capns1−/− hearts in response to pressure overload. This supports the original hypothesis that calpain is involved in plasma membrane repair in pressure-overloaded hearts. Calpain activity is required for cytoskeletal remodeling after membrane disruption (12). Removal of the damaged actin network mediated through calpain activation may serve to prepare the wound area for subsequent events that initiate restoration of the cytoskeleton and membrane sealing. The exact role of calpain in the membrane repair process should be investigated.
In conclusion, this study provides clear evidence that calpain protects the heart from hemodynamic stress. The results suggest the need to reassess the rationale for the application of calpain inhibitors as therapeutic agents to prevent pathological activation of calpain in heart failure.
Acknowledgments
We thank Prof. Alan Wells (University of Pittsburgh) for calpain 2 cDNA and Kana Takada for technical assistance.
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology.
- TAC
- transverse aortic constriction
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- LV
- left ventricle.
REFERENCES
- 1. Goll D. E., Thompson V. F., Li H., Wei W., Cong J. (2003) Physiol. Rev. 83, 731–801 [DOI] [PubMed] [Google Scholar]
- 2. Suzuki K., Hata S., Kawabata Y., Sorimachi H. (2004) Diabetes 53, S12–S18 [DOI] [PubMed] [Google Scholar]
- 3. Arthur J. S., Elce J. S., Hegadorn C., Williams K., Greer P. A. (2000) Mol. Cell. Biol. 20, 4474–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azam M., Andrabi S. S., Sahr K. E., Kamath L., Kuliopulos A., Chishti A. H. (2001) Mol. Cell. Biol. 21, 2213–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dutt P., Croall D. E., Arthur J. S., Veyra T. D., Williams K., Elce J. S., Greer P. A. (2006) BMC Dev. Biol. 6, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmerman U. J., Boring L., Pak J. H., Mukerjee N., Wang K. K. (2000) IUBMB Life 50, 63–68 [DOI] [PubMed] [Google Scholar]
- 7. Shimada M., Greer P. A., McMahon A. P., Bouxsein M. L., Schipani E. (2008) J. Biol. Chem. 283, 21002–21010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan Y., Dourdin N., Wu C., De Veyra T., Elce J. S., Greer P. A. (2006) Genesis 44, 297–303 [DOI] [PubMed] [Google Scholar]
- 9. Dourdin N., Bhatt A. K., Dutt P., Greer P. A., Arthur J. S., Elce J. S., Huttenlocher A. (2001) J. Biol. Chem. 276, 48382–48388 [DOI] [PubMed] [Google Scholar]
- 10. Tan Y., Wu C., De Veyra T., Greer P. A. (2006) J. Biol. Chem. 281, 17689–17698 [DOI] [PubMed] [Google Scholar]
- 11. Demarchi F., Bertoli C., Copetti T., Tanida I., Brancolini C., Eskelinen E. L., Schneider C. (2006) J. Cell Biol. 175, 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mellgren R. L., Zhang W., Miyake K., McNeil P. L. (2007) J. Biol. Chem. 282, 2567–2575 [DOI] [PubMed] [Google Scholar]
- 13. Larsen A. K., Lametsch R., Elce J., Larsen J. K., Thomsen B., Larsen M. R., Lawson M. A., Greer P. A., Ertbjerg P. (2008) Biochem. J. 411, 657–666 [DOI] [PubMed] [Google Scholar]
- 14. Bertoli C., Copetti T., Lam E. W., Demarchi F., Schneider C. (2009) Oncogene 28, 721–733 [DOI] [PubMed] [Google Scholar]
- 15. Huang Y., Wang K. K. (2001) Trends Mol. Med. 7, 355–362 [DOI] [PubMed] [Google Scholar]
- 16. Greyson C. R., Schwartz G. G., Lu L., Ye S., Helmke S., Xu Y., Ahmad H. (2008) J. Mol. Cell. Cardiol. 44, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mani S. K., Shiraishi H., Balasubramanian S., Yamane K., Chellaiah M., Cooper G., Banik N., Zile M. R., Kuppuswamy D. (2008) Am. J. Physiol. Heart Circ. Physiol. 295, H314–H326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iwamoto H., Miura T., Okamura T., Shirakawa K., Iwatate M., Kawamura S., Tatsuno H., Ikeda Y., Matsuzaki M. (1999) J. Cardiovasc. Pharmacol. 33, 580–586 [DOI] [PubMed] [Google Scholar]
- 19. Papp Z., van der Velden J., Stienen G. J. (2000) Cardiovasc. Res. 45, 981–993 [DOI] [PubMed] [Google Scholar]
- 20. Chen M., Won D. J., Krajewski S., Gottlieb R. A. (2002) J. Biol. Chem. 277, 29181–29186 [DOI] [PubMed] [Google Scholar]
- 21. Singh R. B., Chohan P. K., Dhalla N. S., Netticadan T. (2004) J. Mol. Cell. Cardiol. 37, 101–110 [DOI] [PubMed] [Google Scholar]
- 22. Khalil P. N., Neuhof C., Huss R., Pollhammer M., Khalil M. N., Neuhof H., Fritz H., Siebeck M. (2005) Eur. J. Pharmacol. 528, 124–131 [DOI] [PubMed] [Google Scholar]
- 23. Galvez A. S., Diwan A., Odley A. M., Hahn H. S., Osinska H., Melendez J. G., Robbins J., Lynch R. A., Marreez Y., Dorn G. W., 2nd (2007) Circ. Res. 100, 1071–1078 [DOI] [PubMed] [Google Scholar]
- 24. Yamaguchi O., Watanabe T., Nishida K., Kashiwase K., Higuchi Y., Takeda T., Hikoso S., Hirotani S., Asahi M., Taniike M., Nakai A., Tsujimoto I., Matsumura Y., Miyazaki J., Chien K. R., Matsuzawa A., Sadamitsu C., Ichijo H., Baccarini M., Hori M., Otsu K. (2004) J. Clin. Invest. 114, 937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishida K., Yamaguchi O., Hirotani S., Hikoso S., Higuchi Y., Watanabe T., Takeda T., Osuka S., Morita T., Kondoh G., Uno Y., Kashiwase K., Taniike M., Nakai A., Matsumura Y., Miyazaki J., Sudo T., Hongo K., Kusakari Y., Kurihara S., Chien K. R., Takeda J., Hori M., Otsu K. (2004) Mol. Cell. Biol. 24, 10611–10620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hikoso S., Yamaguchi O., Nakano Y., Takeda T., Omiya S., Mizote I., Taneike M., Oka T., Tamai T., Oyabu J., Uno Y., Matsumura Y., Nishida K., Suzuki K., Kogo M., Hori M., Otsu K. (2009) Circ. Res. 105, 70–79 [DOI] [PubMed] [Google Scholar]
- 27. Han R., Bansal D., Miyake K., Muniz V. P., Weiss R. M., McNeil P. L., Campbell K. P. (2007) J. Clin. Invest. 117, 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M., Omiya S., Mizote I., Matsumura Y., Asahi M., Nishida K., Hori M., Mizushima N., Otsu K. (2007) Nat. Med. 13, 619–624 [DOI] [PubMed] [Google Scholar]
- 29. Lakso M., Pichel J. G., Gorman J. R., Sauer B., Okamoto Y., Lee E., Alt F. W., Westphal H. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molinari M., Carafoli E. (1997) J. Membr. Biol. 156, 1–8 [DOI] [PubMed] [Google Scholar]
- 31. Shao H., Chou J., Baty C. J., Burke N. A., Watkins S. C., Stolz D. B., Wells A. (2006) Mol. Cell. Biol. 26, 5481–5496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao W. D., Atar D., Liu Y., Perez N. G., Murphy A. M., Marban E. (1997) Circ. Res. 80, 393–399 [PubMed] [Google Scholar]
- 33. Tsuji T., Ohga Y., Yoshikawa Y., Sakata S., Abe T., Tabayashi N., Kobayashi S., Kohzuki H., Yoshida K. I., Suga H., Kitamura S., Taniguchi S., Takaki M. (2001) Am. J. Physiol. Heart Circ. Physiol. 281, H1286–H1294 [DOI] [PubMed] [Google Scholar]
- 34. Suryakumar G., Kasiganesan H., Balasubramanian S., Kuppuswamy D. (2010) J. Cardiovasc. Pharmacol. 55, 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wencker D., Chandra M., Nguyen K., Miao W., Garantziotis S., Factor S. M., Shirani J., Armstrong R. C., Kitsis R. N. (2003) J. Clin. Invest. 111, 1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McNeil P. L., Terasaki M. (2001) Nat. Cell Biol. 3, E124–E129 [DOI] [PubMed] [Google Scholar]
- 37. McNeil P. L., Steinhardt R. A. (2003) Annu. Rev. Cell Dev. Biol. 19, 697–731 [DOI] [PubMed] [Google Scholar]
- 38. Bi G. Q., Alderton J. M., Steinhardt R. A. (1995) J. Cell Biol. 131, 1747–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bansal D., Miyake K., Vogel S. S., Groh S., Chen C. C., Williamson R., McNeil P. L., Campbell K. P. (2003) Nature 423, 168–172 [DOI] [PubMed] [Google Scholar]
- 40. McNeil A. K., Rescher U., Gerke V., McNeil P. L. (2006) J. Biol. Chem. 281, 35202–35207 [DOI] [PubMed] [Google Scholar]