Godfrey and Rossjohn discuss the varied ways to turn on NKT cells in the context of recent findings.
Abstract
Natural killer T (NKT) cells are CD1d-restricted, lipid antigen–reactive T cells with powerful immunoregulatory potential. The prototypic antigen for NKT cells is a marine sponge–derived glycolipid, α-galactosylceramide (α-GalCer), but this is not normally encountered in the mammalian environment. Thus, there is great interest in the identification of more physiological stimuli for NKT cells, and numerous studies have shown that NKT cells are capable of responding to a range of microbial lipid-based antigens. Two new studies expand our understanding of environmental NKT cell stimuli, with one showing that CD1d-restricted NKT cell antigens are present within common house dust extract (HDE), whereas the other shows that NKT cells can respond to innate stimuli irrespective of the presence of foreign microbial antigens. Collectively, these two investigations indicate that NKT cells are far more likely to encounter foreign antigens, or innate activating signals, than previously recognized, suggesting a more central role for these cells in the immune system.
NKT cells are lipid antigen (Ag)–reactive, CD1d-restricted T cells that express a limited array of αβ T cell receptors (TCRs). The most widely studied NKT cells are known as type 1 or classical NKT cells. They are present in mice and humans and are defined by their expression of an invariant TCR-α chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans) paired with particular TCR-Vβ chains (Vβ2, 7, or 8 in mice and Vβ11 in humans). Type 1 NKT cells are also characterized by their ability to recognize the prototypic CD1d-restricted glycosphingolipid Ag α-GalCer, which is a marine sponge–derived compound that has potent immunoregulatory potential. Type 2 NKT cells are distinct from type 1 NKT cells in that they express a more diverse αβTCR repertoire; these cells recognize CD1d-restricted lipid Ags such as sulfatide, but they do not recognize α-GalCer (Godfrey et al., 2010b). Although type 2 NKT cells are likely to have a unique and important role in the immune system, the remainder of this article will focus on type 1 NKT cells.
The growing repertoire of NKT cell antigens
For many years, immunologists struggled to explain the existence of a highly conserved T cell lineage that appeared to be specific for Ags derived from such a seemingly innocuous source as marine sponges. However, it is now clear that NKT cells are activated by naturally occurring microbial Ags such as α-glucuronosylceramide and α-galacturonosylceramide from Sphingomonas spp. (Kinjo et al., 2005; Mattner et al., 2005; Sriram et al., 2005), α-galactosyldiacylglycerol from Borrelia burgdorferi (Kinjo et al., 2006), phosphatidylinositol-mannosidase from Mycobacterium bovis BCG (Fischer et al., 2004), α-glucosyldiacylglycerol from Streptococcus pneumoniae (Kronenberg, M., personal communication), and a cholesteryl α-glucoside from Helicobacter pylori (Chang et al., 2011). Furthermore, NKT cells can also respond to self-lipid–based Ags, including β-linked glycosphingolipids β-galactosylceramide (β-GalCer), β-glucosylceramide (β-GlcCer), isoglobotrihexosylceramide (iGb3), and disialoganglioside, as well as self-phospholipid Ags such as phosphatidylethanolamine, phosphatidylinositol, and phosphatidylcholine (Godfrey et al., 2010a; Venkataswamy and Porcelli, 2010). Thus, NKT cells may be busier than initially realized in dealing with a broad range of Ags from a variety of different sources, both exogenous and endogenous. There is also great interest in the use α-GalCer derivatives that have the potential to skew NKT cell–mediated responses toward Th1 or Th2 directions, which may lead to tailored NKT cell–based immunotherapy (Venkataswamy and Porcelli, 2010). Remarkably, the NKT TCR seems to recognize this diverse range of Ags by acting as a pattern recognition receptor in which the docking of NKT TCR-CD1d-Ag is conserved, regardless of the Vβ usage (Borg et al., 2007; Scott-Browne et al., 2007; Pellicci et al., 2009; Mallevaey et al., 2011).
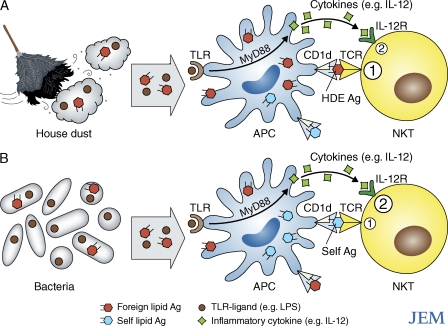
Two papers in the latest issue of JEM provide critical new insight into the physiological factors that activate NKT cells. In one paper, Wingender et al. demonstrates that NKT cells respond to Ags present in environmentally ubiquitous HDE. In the second paper, Brigl et al. provides evidence that the NKT cell response to a diverse range of bacterial infections appears to occur in a microbial Ag-independent and an IL-12– and Toll-like receptor (TLR)–dependent manner, even when the infectious organisms produce NKT cell Ags (Fig. 1).
Figure 1.
Two pathways of NKT cell activation in response to common environmental stimuli. (A) HDE is essentially ubiquitous, and most samples were found to contain CD1d-restricted Ags (orange hexagons) that are recognized by NKT TCRs (Wingender et al., 2011) and TLR ligands (brown circles; Batzer et al., 2007). Both components are important for the adjuvant effects of HDE (Batzer et al., 2007; Wingender et al., 2011). (B) Some, but not all, bacteria carry CD1d-restricted lipid Ags that are recognized by NKT TCRs, yet NKT cells respond to most bacterial infections. Irrespective of whether a particular strain carries these Ags, the NKT cell response is dominated by innate, TLR, and MyD88-dependent signaling that results in IL-12 (green squares)–mediated stimulation of NKT cells, rather than by bacterial glycolipid Ag recognition (Brigl et al., 2011). The response is also at least partly CD1d dependent, again, irrespective of the presence of bacterial CD1d-restricted lipid Ags, suggesting the recognition of self-lipid Ags in conjunction with the IL-12–mediated stimulation (Brigl et al., 2011). Thus, there are at least two pathways for NKT cell activation: (1) TCR mediated and (2) cytokine mediated. NKT TCR-mediated recognition of foreign lipid-based Ags presented by CD1d that appears to be important for the adjuvant effects of HDE (A), but less so for the response to bacterial infection, whereas innate, inflammatory cytokine–mediated stimulation of NKT cells appears to dominate the response to bacterial infection (B). In the context of bacterial infections, NKT cells may concurrently recognize self- (blue hexagons) or bacterial (orange hexagons) glycolipids, but regardless, the innate (IL-12– and TLR-mediated) stimuli appear to be critical for NKT cell activation.
CD1d-restricted NKT cell antigens in common HDE
HDE contains immunoregulatory adjuvant-like factors that can either enhance or suppress Th2 immune responses and airway hyperreactivity, depending on the dosing regimen (Ng et al., 2006; Lam et al., 2008). This suggests that components of HDE may be important in the development or pathogenesis of asthma, but conversely, may also provide immunotherapeutic opportunities for this disease (Ng et al., 2006). Wingender et al. (2011) now show that Ags present within HDE activate NKT cells in a CD1d-restricted, TCR-dependent manner. Moreover, NKT cell activation is critical for the adjuvant activity of HDE, the effects of which were markedly diminished in TCR-Jα18−/− NKT cell–deficient mice. The NKT cell response to HDE resulted in bystander NK cell activation, increased airway inflammation, and a stronger adaptive immune response to a model Ag (ovalbumin) that was co-administered with HDE. Furthermore, the adaptive immune response to ovalbumin resulted in a positive feedback loop that further enhanced the NKT cell response. Many studies have suggested a role for NKT cells in the development or pathogenesis of airway hyperreactivity and asthma (Umetsu and Dekruyff, 2010), and airway disease can be artificially induced by intranasal administration of α-GalCer (Meyer et al., 2006). However, the physiological mechanism of NKT cell activation and the identity and role of lipid-based Ags in association with such diseases is unclear. One study demonstrated the presence of CD1d-restricted phospholipid Ags (phosphatidylethanolamine and phosphatidylcholine) derived from allergy-inducing plant pollens, although these appeared to favor type 2 NKT cell stimulation (Agea et al., 2005), whereas the response to HDE as documented by Wingender et al. (2011) involved type 1 NKT cells. Interestingly, despite the fact that at least half of NKT cells in naive mice are CD4+, the majority of the HDE-responsive NKT cells appeared to be CD4−, many of which produced IL-17 (Wingender et al., 2011). This is further evidence for the existence of functionally distinct NKT cell subsets, and may indicate an important role for IL-17–producing NKT cells in this model of airway hyperreactivity, which is reminiscent of some earlier studies (Michel et al., 2007; Pichavant et al., 2008; Umetsu and Dekruyff, 2010). The critical questions that arise from these studies are whether HDE-mediated NKT cell stimulation promotes asthma in humans, and what is the nature of the HDE Ags involved? Although NKT cells are present in the lungs of asthmatic humans, the extent to which they are involved in disease is unclear and controversial (Thomas et al., 2010; Umetsu and Dekruyff, 2010). Both mouse and human NKT cells responded to HDE samples, and there was a rough correlation between the response of mouse and human NKT cells to each fraction, although some samples preferentially stimulated human or mouse NKT cells (Wingender et al., 2011). Furthermore, the hierarchy of HDE sample potency varied between different NKT cell hybridomas expressing different NKT TCR-Vβs, suggesting the presence of more than one NKT stimulatory Ag within HDE and that NKT TCR-Vβ chains determine the affinity for these Ags. This is consistent with previous work showing how the Vβ repertoire shapes the responsiveness to lipid Ags (Mallevaey et al., 2009). Preliminary studies suggested that the Ag activity was not derived from house dust mites, and that it was not a glycosphingolipid (Wingender et al., 2011). It is possible that the HDE Ags are of bacterial origin, and further investigations into different HDE components are clearly warranted. Regardless of the nature of these Ags, their presence in the majority of HDE samples tested suggests that we are literally surrounded by CD1d-restricted NKT cell Ags with the potential to exacerbate asthma and allergic airway disease. Whether NKT cells are also responsible for HDE-mediated suppression of AHR (Ng et al., 2006; Lam et al., 2008) was not investigated in the study by Wingender et al. (2011), but will be important to determine as it may represent a novel means of immunotherapy.
NKT cells respond to infection through innate stimuli, regardless of the presence of microbial CD1d-restricted Ag
NKT cells play an important role in many different types of infection, including bacteria, viruses, parasites, and fungal pathogens, regardless of whether the infectious organisms carry known NKT cell Ags (Brigl and Brenner, 2010). Two pathways are thought to exist for NKT cell activation in response to infection: one where NKT cells are activated through direct recognition of microbial glycolipid Ags via their TCR (Kinjo et al., 2005; Mattner et al., 2005; Sriram et al., 2005; Kinjo et al., 2006) and another that occurs in the absence of known microbial Ags because of the ability of NKT cells to respond to innate or inflammatory stimuli, possibly in conjunction with self-glycolipid Ag recognition (Brigl et al., 2003; Mattner et al., 2005; Paget et al., 2007; Salio et al., 2007; Brigl and Brenner, 2010; Darmoise et al., 2010). Brigl et al. (2011) provide intriguing new data suggesting that innate stimuli appear to be the major means of NKT cell activation, even with bacteria such as Sphingomonas spp. and S. pneumoniae that carry known NKT cell Ags. They demonstrated that the NKT cell response to bacterial glycolipid Ag α-glucuronosylceramide (GSL-1) was CD1d dependent, but IL-12 and TLR independent, and drove both IFN-γ and IL-4 production by NKT cells. In contrast, the response to whole bacteria (heat-killed or live) was IL-12 and TLR dependent and predominantly led to IFN-γ production with little or no IL-4. This includes the response to Sphingomonas, from which GSL-1 is derived. However, a role for CD1d is also important regardless of whether the bacteria expressed NKT cell Ags, probably reflecting a role for the recognition of self-glycolipid that synergizes with the innate IL-12– and/or other TLR-dependent signals.
These findings also touch on the HDE study (Wingender et al., 2011) discussed in the previous section, raising the question of whether HDE-derived Ags, or self-CD1d–restricted Ags combined with innate signals, are more important for the NKT cell-dependent response to HDE in vivo, which is also known to be TLR-dependent (Ng et al., 2006; Lam et al., 2008). However, production of both IFN-γ and IL-4 in the HDE model suggests a more prominent role for HDE-derived Ag recognition by NKT cells in this model. The precise identity of self-glycolipid ligands recognized by NKT cells in association with innate signaling remains unclear, but β-linked glycosphingolipids are good candidates (Stanic et al., 2003; Mattner et al., 2005; Paget et al., 2007; Salio et al., 2007; Darmoise et al., 2010). Collectively, these data suggest that NKT cell recognition of bacterial Ags, such as GSL-1, is at most redundant for their antimicrobial function.
This raises the important question of why NKT cells express TCRs that are highly conserved through evolution from mice to humans, with specificity for foreign glycolipid Ags—this must be of some benefit to the host. A simple explanation might be that the existence of multiple mechanisms for NKT cell stimulation in response to infection provides greater protection. For example, it is possible that Ag-specific NKT cell activation is important during stages of the antimicrobial immune response different than those tested in this study. Indeed, NKT cell activation by α-GalCer, the prototypic foreign NKT cell Ag, has a major impact on DC activation and the magnitude and quality of subsequent adaptive immune responses (Fujii et al., 2007; Hermans et al., 2007; Guillonneau et al., 2009) that were underway at the time of NKT cell activation (Cerundolo et al., 2009). Moreover, an enhanced adaptive immune response was observed in the HDE study by Wingender et al. (2011).Thus, Ag-specific NKT cell activation might be important for the development of adaptive immunity and/or immunological memory in infectious organisms, which was not assessed in the study by Brigl et al. (2011). It is clear that IL-12 does not promote the full spectrum of NKT cell functionality, such as production of IL-4 and possibly other important factors (Brigl et al., 2011). Determining the extent to which IL-12 and other Ag-independent inflammatory stimuli can mimic Ag-dependent NKT cell responses, and the downstream influence of these innate signals on adaptive immunity and memory, will be an important goal for future studies in the field.
Future directions
In addition to demonstrating that NKT cells are far more likely to encounter Ags or other stimulatory factors than previously appreciated, these new studies touch on some of the key issues in the NKT cell field. One study demonstrates how NKT cell activation can promote airway disease, thus having deleterious effects on the host (Wingender et al., 2011), whereas the other shows the beneficial effects of NKT cells via their importance in microbial immunity (Brigl et al., 2011). There are also published examples where NKT cells play an immunosuppressive rather than immunostimulatory role (Godfrey and Kronenberg, 2004). This raises the question of whether therapeutic NKT cell activation to boost antimicrobial or anticancer immunity might trigger undesirable side effects, and whether there are ways to safely and effectively harness the potent immunomodulatory potential of these cells? This is likely to depend on the type of Ags used, and the dose, timing, and context of NKT cell activation. What are the key self-Ags that underpin the development and self-reactivity of NKT cells, and what is the relationship between self-NKT cell Ags and foreign (microbial/environmental) NKT cell Ags? Do innate signals produced in response to infection result in a different repertoire of self-Ags to those involved in NKT cell development and maintenance, and is this important for self-Ag–mediated NKT cell stimulation? What Ags are responsible for maintaining the evolutionarily conserved NKT TCR specificity? Presumably, these Ags have a critical role to play in NKT cell biology. Whereas microbial glycolipid Ags appear to be the most potent agonists for NKT cells, perhaps the ability to recognize self-glycolipid Ags such as β-GalCer, β-GlcCer, or iGb3 presented by CD1d is more important for these cells. It is critical that we develop a more thorough understanding of the antigenic targets, and the role of the NKT TCR and/or other factors in the maintenance and activation of these cells. What are the biological consequences of TCR variability within the broader NKT cell population, and do microbial and/or self-glycolipid Ag-reactive subsets of NKT cells exist? There is a growing body of evidence to suggest that this is the case, even within the classical type 1 NKT cell pool where TCR-β variations can influence Ag reactivity and autoreactivity (Schümann et al., 2006; Mallevaey et al., 2009, 2011; Pellicci et al., 2009; Matulis et al., 2010; Wei et al., 2006; Wun et al., 2011). Also, despite clear differences in α-GalCer reactivity that distinguishes type 1 and 2 NKT cells, do the functions and natural antigenic targets of these cells otherwise overlap?
Our understanding of NKT cell biology, and our ability to manipulate these cells as a form of therapy, is critically dependent on our understanding of the factors that regulate and activate these cells. The two studies appearing in this issue have shown us that we are surrounded by Ags and other stimuli that turn on NKT cells. Now we just need to determine the consequences of these findings and whether they can be used to our advantage.
Acknowledgments
A National Health and Medical Research Council (NHMRC) Principal Research Fellowship supports D.I. Godfrey. J. Rossjohn is supported by an Australian Research Council Federation Fellowship. D.I. Godfrey and J. Rossjohn are also supported by NHMRC program and project grants, the Australian Research Council, and the Cancer Council of Victoria.
There authors have no conflicting financial interests.
References
- Agea E., Russano A., Bistoni O., Mannucci R., Nicoletti I., Corazzi L., Postle A.D., De Libero G., Porcelli S.A., Spinozzi F. 2005. Human CD1-restricted T cell recognition of lipids from pollens. J. Exp. Med. 202:295–308 10.1084/jem.20050773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer G., Lam D.P., Paulus P., Boasen J., Ng N., Horner A.A. 2007. Using house dust extracts to understand the immunostimulatory activities of living environments. Immunobiology. 212:491–498 10.1016/j.imbio.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg N.A., Wun K.S., Kjer-Nielsen L., Wilce M.C., Pellicci D.G., Koh R., Besra G.S., Bharadwaj M., Godfrey D.I., McCluskey J., Rossjohn J. 2007. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 448:44–49 10.1038/nature05907 [DOI] [PubMed] [Google Scholar]
- Brigl M., Brenner M.B. 2010. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin. Immunol. 22:79–86 10.1016/j.smim.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Brigl M., Bry L., Kent S.C., Gumperz J.E., Brenner M.B. 2003. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4:1230–1237 10.1038/ni1002 [DOI] [PubMed] [Google Scholar]
- Brigl M., Tatituri R.V., Watts G.F.M., Bhowruth V., Leadbetter E.A., Barton N., Cohen N.R., Hsu F.-F., Besra G.S., Brenner M.B. 2011. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208:1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V., Silk J.D., Masri S.H., Salio M. 2009. Harnessing invariant NKT cells in vaccination strategies. Nat. Rev. Immunol. 9:28–38 10.1038/nri2451 [DOI] [PubMed] [Google Scholar]
- Chang Y.J., Kim H.Y., Albacker L.A., Lee H.H., Baumgarth N., Akira S., Savage P.B., Endo S., Yamamura T., Maaskant J., et al. 2011. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J. Clin. Invest. 121:57–69 10.1172/JCI44845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmoise A., Teneberg S., Bouzonville L., Brady R.O., Beck M., Kaufmann S.H., Winau F. 2010. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 33:216–228 10.1016/j.immuni.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Scotet E., Niemeyer M., Koebernick H., Zerrahn J., Maillet S., Hurwitz R., Kursar M., Bonneville M., Kaufmann S.H., Schaible U.E. 2004. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA. 101:10685–10690 10.1073/pnas.0403787101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Shimizu K., Hemmi H., Steinman R.M. 2007. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 220:183–198 10.1111/j.1600-065X.2007.00561.x [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Kronenberg M. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114:1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.I., Pellicci D.G., Patel O., Kjer-Nielsen L., McCluskey J., Rossjohn J. 2010a. Antigen recognition by CD1d-restricted NKT T cell receptors. Semin. Immunol. 22:61–67 10.1016/j.smim.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Stankovic S., Baxter A.G. 2010b. Raising the NKT cell family. Nat. Immunol. 11:197–206 10.1038/ni.1841 [DOI] [PubMed] [Google Scholar]
- Guillonneau C., Mintern J.D., Hubert F.X., Hurt A.C., Besra G.S., Porcelli S., Barr I.G., Doherty P.C., Godfrey D.I., Turner S.J. 2009. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc. Natl. Acad. Sci. USA. 106:3330–3335 10.1073/pnas.0813309106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans I.F., Silk J.D., Gileadi U., Masri S.H., Shepherd D., Farrand K.J., Salio M., Cerundolo V. 2007. Dendritic cell function can be modulated through cooperative actions of TLR ligands and invariant NKT cells. J. Immunol. 178:2721–2729 [DOI] [PubMed] [Google Scholar]
- Kinjo Y., Wu D., Kim G., Xing G.W., Poles M.A., Ho D.D., Tsuji M., Kawahara K., Wong C.H., Kronenberg M. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 434:520–525 10.1038/nature03407 [DOI] [PubMed] [Google Scholar]
- Kinjo Y., Tupin E., Wu D., Fujio M., Garcia-Navarro R., Benhnia M.R., Zajonc D.M., Ben-Menachem G., Ainge G.D., Painter G.F., et al. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7:978–986 10.1038/ni1380 [DOI] [PubMed] [Google Scholar]
- Lam D., Ng N., Lee S., Batzer G., Horner A.A. 2008. Airway house dust extract exposures modify allergen-induced airway hypersensitivity responses by TLR4-dependent and independent pathways. J. Immunol. 181:2925–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallevaey T., Scott-Browne J.P., Matsuda J.L., Young M.H., Pellicci D.G., Patel O., Thakur M., Kjer-Nielsen L., Richardson S.K., Cerundolo V., et al. 2009. T cell receptor CDR2 beta and CDR3 beta loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 31:60–71 10.1016/j.immuni.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallevaey T., Clarke A.J., Scott-Browne J., Young M.H., Pellicci D.G., Patel O., Matsuda J.L., Mccluskey J., Godfrey D.I., Marrack P., et al. 2011. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 34:315–326 10.1016/j.immuni.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J., Debord K.L., Ismail N., Goff R.D., Cantu C., III, Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 434:525–529 10.1038/nature03408 [DOI] [PubMed] [Google Scholar]
- Matulis G., Sanderson J.P., Lissin N.M., Asparuhova M.B., Bommineni G.R., Schümperli D., Schmidt R.R., Villiger P.M., Jakobsen B.K., Gadola S.D. 2010. Innate-like control of human iNKT cell autoreactivity via the hypervariable CDR3beta loop. PLoS Biol. 8:e1000402 10.1371/journal.pbio.1000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E.H., Goya S., Akbari O., Berry G.J., Savage P.B., Kronenberg M., Nakayama T., Dekruyff R.H., Umetsu D.T. 2006. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl. Acad. Sci. USA. 103:2782–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M.L., Keller A.C., Paget C., Fujio M., Trottein F., Savage P.B., Wong C.H., Schneider E., Dy M., Leite-de-Moraes M.C. 2007. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204:995–1001 10.1084/jem.20061551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng N., Lam D., Paulus P., Batzer G., Horner A.A. 2006. House dust extracts have both TH2 adjuvant and tolerogenic activities. J. Allergy Clin. Immunol. 117:1074–1081 10.1016/j.jaci.2006.03.025 [DOI] [PubMed] [Google Scholar]
- Paget C., Mallevaey T., Speak A.O., Torres D., Fontaine J., Sheehan K.C., Capron M., Ryffel B., Faveeuw C., Leite de Moraes M., et al. 2007. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 27:597–609 10.1016/j.immuni.2007.08.017 [DOI] [PubMed] [Google Scholar]
- Pellicci D.G., Patel O., Kjer-Nielsen L., Pang S.S., Sullivan L.C., Kyparissoudis K., Brooks A.G., Reid H.H., Gras S., Lucet I.S., et al. 2009. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 31:47–59 10.1016/j.immuni.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichavant M., Goya S., Meyer E.H., Johnston R.A., Kim H.Y., Matangkasombut P., Zhu M., Iwakura Y., Savage P.B., DeKruyff R.H., et al. 2008. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 205:385–393 10.1084/jem.20071507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio M., Speak A.O., Shepherd D., Polzella P., Illarionov P.A., Veerapen N., Besra G.S., Platt F.M., Cerundolo V. 2007. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl. Acad. Sci. USA. 104:20490–20495 10.1073/pnas.0710145104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann J., Mycko M.P., Dellabona P., Casorati G., MacDonald H.R. 2006. Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J. Immunol. 176:2064–2068 [DOI] [PubMed] [Google Scholar]
- Scott-Browne J.P., Matsuda J.L., Mallevaey T., White J., Borg N.A., McCluskey J., Rossjohn J., Kappler J., Marrack P., Gapin L. 2007. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat. Immunol. 8:1105–1113 10.1038/ni1510 [DOI] [PubMed] [Google Scholar]
- Sriram V., Du W., Gervay-Hague J., Brutkiewicz R.R. 2005. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J. Immunol. 35:1692–1701 10.1002/eji.200526157 [DOI] [PubMed] [Google Scholar]
- Stanic A.K., De Silva A.D., Park J.J., Sriram V., Ichikawa S., Hirabyashi Y., Hayakawa K., Van Kaer L., Brutkiewicz R.R., Joyce S. 2003. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by beta-D-glucosylceramide synthase deficiency. Proc. Natl. Acad. Sci. USA. 100:1849–1854 10.1073/pnas.0430327100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.Y., Chyung Y.H., Luster A.D. 2010. Natural killer T cells are not the predominant T cell in asthma and likely modulate, not cause, asthma. J. Allergy Clin. Immunol. 125:980–984 10.1016/j.jaci.2010.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D.T., Dekruyff R.H. 2010. Natural killer T cells are important in the pathogenesis of asthma: the many pathways to asthma. J. Allergy Clin. Immunol. 125:975–979 10.1016/j.jaci.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataswamy M.M., Porcelli S.A. 2010. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin. Immunol. 22:68–78 10.1016/j.smim.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D.G., Curran S.A., Savage P.B., Teyton L., Bendelac A. 2006. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J. Exp. Med. 203:1197–1207 10.1084/jem.20060418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender G., Rogers P., Batzer G., Myung S.L., Bai D., Pei B., Khurana A., Kronenberg M., Horner A.A. 2011. Invariant NKT cells are crucial for airway inflammation induced by environmental antigens. J. Exp. Med. 208:1151–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun K.S., Cameron G., Patel O., Pang S.S., Pellicci D.G., Sullivan L.C., Keshipeddy S., Young M.H., Uldrich A.P., Thakur M., et al. 2011. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 34:327–339 10.1016/j.immuni.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]