PLZF-expressing NKT cells establish residence at intravascular locations, failing to enter the circulation because of constitutive interactions with LFA-1 and ICAM-1.
Abstract
Innate-like NKT cells conspicuously accumulate within the liver microvasculature of healthy mice, crawling on the luminal side of endothelial cells, but their general recirculation pattern and the mechanism of their intravascular behavior have not been elucidated. Using parabiotic mice, we demonstrated that, despite their intravascular location, most liver NKT cells failed to recirculate. Antibody blocking experiments established that they were retained locally through constitutive LFA-1–intercellular adhesion molecule (ICAM) 1 interactions. This unprecedented lifelong intravascular residence could be induced in conventional CD4 T cells by the sole expression of promyelocytic leukemia zinc finger (PLZF), a transcription factor specifically expressed in the NKT lineage. These findings reveal the unique genetic and biochemical pathway that underlies the innate intravascular surveillance program of NKT cells.
NKT cells are CD1d-restricted T cells that express a conserved semi-invariant Vα14(huVα24)-Jα18/Vβ8,7,2(huVβ11) TCR. They constitute a separate lineage of innate-like T cells emerging from thymic development as effector cells able to explosively release IL-4, IL-13, and also IFN-γ upon primary stimulation (Matsuda and Gapin, 2005; Bendelac et al., 2007; Godfrey et al., 2010). NKT cells characteristically express high levels of the BTB-POZ transcription factor promyelocytic leukemia zinc finger (PLZF) encoded by the gene Zbtb16, which is not only necessary for their effector differentiation but can also transfer this program upon ectopic (transgenic) expression in conventional MHC-restricted T cells (Kovalovsky et al., 2008, 2010; Raberger et al., 2008; Savage et al., 2008).
A majority of NKT cells recognize the same microbial lipids characterized by a galactose in α linkage to a ceramide or a diacylglycerol backbone (Kinjo et al., 2005, 2006; Mattner et al., 2005; Sriram et al., 2005). They also recognize the self glycosphingolipid isoglobotrihexosylceramide (iGb3; Zhou et al., 2004). Other self- and foreign lipids have been identified as ligands for a smaller fraction of NKT cells (Fox et al., 2009). NKT cells are not only involved in microbial defense, as expected, but they also contribute to immune responses during viral infections, cancer, and various inflammatory and allergic states (Brigl and Brenner, 2004; Godfrey and Kronenberg, 2004). In some cases where pathogens do not appear to express ligands for direct NKT cell recognition, it has been demonstrated that the endogenous ligand iGb3 is up-regulated through toll-like receptor–mediated down-regulation of its lysosomal degrading enzyme α-galactosidase A (Darmoise et al., 2010).
The homing and recirculation patterns of NKT cells are unique among T or B cell lineages. It has long been known that NKT cells are particularly abundant among liver lymphocytes (Ohteki and MacDonald, 1994), but only recently was their peculiar location and motility revealed by intravital imaging. In CXCR6-GFP knockin mice, GFPbright cells, which are mostly NKT cells, were found to crawl on the luminal surface on sinusoidal endothelial cells without directional bias at a speed of 10 µm/min (Geissmann et al., 2005). Thus, NKT cells are particularly well suited to intercept bloodborne pathogens such as Borrelia that are inoculated through tick bite and whose lipid antigens can be displayed by CD1d-expressing Kupffer cells in the liver sinusoids (Lee et al., 2010). Ehrlichia is another bacterial genus inoculated by tick bites and specifically recognized by NKT cells in a CD1d-dependent manner (Mattner et al., 2005). This early innate interaction between NKT cells and Kupffer cells contributes to the formation of infectious granulomas. The location of NKT cells in other tissues, however, has remained obscure as a result of their relative rarity and the lack of a reliable staining method. In vitro CFSE-labeled and reinjected NKT cells were seen in the interfollicular region of the lymph node (Barral et al., 2010), but the isolation procedure using TCR reagents likely induced some activation and may have altered homing. The location of NKT cells within the spleen and lung has not been elucidated.
The mechanisms underlying the adhesion and crawling behavior of NKT cells in the liver sinusoids are not well understood. In particular, CXCR6-deficient NKT cells normally homed to the liver, although they exhibited decreased survival (Geissmann et al., 2005) or function (Shimaoka et al., 2007; Germanov et al., 2008). LFA-1–deficient mice exhibited a selective and profound decrease in liver NKT cells, but whether LFA-1 was required for liver retention of normal NKT cells or for the development of liver-tropic NKT cells was debated (Emoto et al., 1999; Ohteki et al., 1999). Mice deficient in the LFA-1 ligand intercellular adhesion molecule (ICAM) 1 did not show a corresponding decrease (Emoto et al., 1999; Ohteki et al., 1999) and blocking experiments using anti–LFA-1 and anti–ICAM-1 antibodies have not been reported. Although NKT cells express an unusual pattern of chemokine receptors and integrins, no other chemokine receptor or integrin deficiencies have been associated with a specific NKT cell defect and surprisingly little is known about their homing pattern and recirculation dynamics (Johnston et al., 2003; Thomas et al., 2003, 2007; Geissmann et al., 2005; Shimaoka et al., 2007; Semmling et al., 2010).
In this study, we generated parabiotic mouse pairs to investigate the dynamics of NKT cell exchange and recirculation and we adapted a tetramer staining method to stain NKT cells in different tissues. Our findings demonstrate that most NKT cells were located in the T cell zones of lymphoid tissues but, unlike other T cells, they did not recirculate and instead established very long-term residence in these locations. In the liver, despite their location inside the sinusoid capillaries, NKT cells also failed to exchange over a period of 2 mo, implying that they largely restricted their movements within the confines of the liver capillary bed. A similar intravascular residence pattern was demonstrated in the lung. Injection of blocking antibodies against LFA-1 and ICAM-1 induced the rapid release of liver NKT cells into the peripheral blood, demonstrating the critical role of these integrins in the long-term intravascular residence of NKT cells. These unusual properties could be transferred to conventional T cells by ectopic expression of PLZF, the signature transcription factor of the NKT cell lineage. Thus, PLZF directs a broad innate effector lymphocyte program where homing and recirculation properties are optimized to rapidly confront bloodborne, lymph-borne, or airborne pathogens in different tissues.
RESULTS
Intravascular compartments of NKT cells
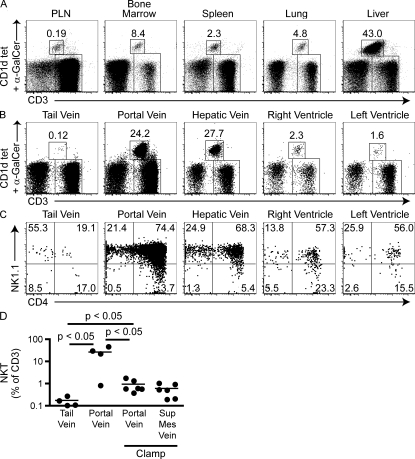
Fig. 1 A illustrates the well known heterogeneous tissue distribution of CD1d-α-galactosylceramide+ (α-GalCer) NKT cells in C57BL/6 mice, shown as percentage of CD3+ T cells, with particular accumulation in the liver but also spleen, bone marrow, and lung compared with peripheral lymph nodes and peripheral blood. The frequency of NKT cells in the latter compartments is low at 0.2% of T cells on average, reflecting the down-regulation of CD62L expression in these effector-type cells. However, when blood was collected from portal and hepatic veins, using a needle directed toward the liver and mounted on a syringe for aspiration, ∼25% of T cells on average were NKT cells. Blood similarly collected from right and left heart ventricles, which connect to the pulmonary artery and vein, respectively, was 10–20× enriched in NKT cells compared with peripheral blood. These high frequencies suggest that the accumulation of NKT cells in the microvascular compartment, previously established for the liver (Geissmann et al., 2005), may be observed in other organs such as the lungs. These accumulated blood NKT cells were mainly CD4+, whereas those in the peripheral blood were mostly CD4− (Fig. 1 C). Further proof that the high concentration of NKT cells in blood drawn from portal vein was of hepatic rather than portal origin was obtained by placing a clamp near the hilum of the liver and drawing blood from the portal system with the needle pointing away from the liver. The frequency of NKT cells in portal blood was <1% on average (Fig. 1 D). A similar frequency was observed in the superior mesenteric vein, which drains into the portal vein.
Figure 1.
Tissue and blood distribution of NKT cells. (A and B) Representative dot plots pregated on B220-negative cells and showing tissue (A) and blood (B) Vα14 NKT cells identified as CD1d-α-GalCer tetramer+ CD3ε+ cells. Numbers show percentages of Vα14 NKT cells among CD3ε+ cells. Note that the blood samples of portal and hepatic vein, and right and left heart ventricles, were drawn by aspiration through a needle directed toward the liver and the lung, respectively. (C) Representative dot plots showing CD4 and NK1.1 expression by gated CD1d-α-GalCer tetramer+ CD3ε+ cells. Data are representative of three experiments with four to six mice per group. (D) Compilation of experiments showing the percentage of NKT cells among CD3ε+ cells recovered from tail vein and portal vein blood drawn as in B or from portal vein and superior mesenteric vein after clamping the portal vein at the liver hilum (clamp). Horizontal bars represent means.
Anatomical location of NKT cells in secondary lymphoid tissues
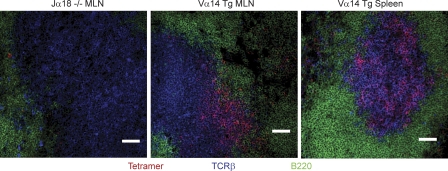
The anatomical location of NKT cells in spleen and lymph node was investigated using a CD1d-α-GalCer tetramer-based staining method to visualize these cells in Vα14-Jα18 TCR-α transgenic mice. Fig. 2 shows that NKT cells were present in the T cell zone of the lymph node and in the periarteriolar sheath of the spleen, along with other TCR-β+ cells, but rarely found in the B cell follicles, in the microvasculature, or in the red pulp. This picture was consistent with results observed in tissues of nontransgenic mice, although the low natural frequency of NKT cells in these mice made it difficult to ascertain every individual cell stained by the tetramer method (unpublished data). Thus, NKT cells in secondary lymphoid tissues are mostly extravascular and located in the T cell zone among conventional T cells.
Figure 2.
Anatomical location of NKT cells in mesenteric lymph node and spleen. Mesenteric lymph nodes (MLN) and spleens were harvested from Jα18 KO or Vα14-Jα18 transgenic mice and frozen sections were stained with CD1d-α-GalCer tetramers in red, TCR-β in blue, and B220 in green. Data are representative of three experiments with one mouse per group. Bars, 50 µm.
Recirculation studies in parabiotic mice
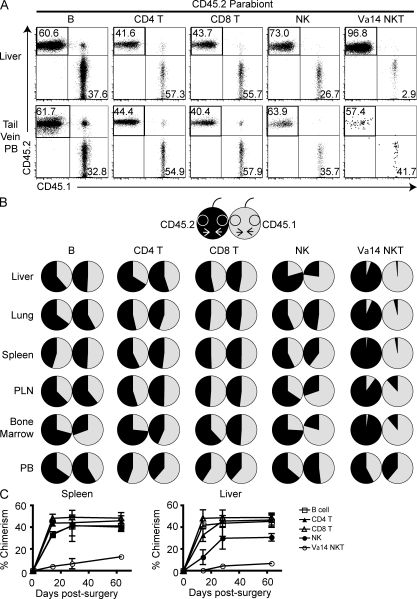
To study the NKT cell recirculation pattern, we generated parabiotic mice with pairs of CD45.1 and CD45.2 congenic animals. During the healing process after surgery, capillary anastomoses form between the two vascular systems, allowing for exchange of cells circulating in peripheral blood. Parabiotic mice were studied between 2 and 8 wk after surgery, a period during which bone marrow and thymic cell progenitors failed to exchange (Wright et al., 2001; Liu et al., 2007; for example, only ∼5% of double-positive thymocytes were of parabiotic partner origin at week 8 [not depicted]). As previously established, conventional CD4 and CD8 T cells and B cells rapidly recirculated and equilibrated within 2 wk (Fig. 3). NK cells also largely recirculated in most tissues, although full exchange was not observed in liver and bone marrow. In marked contrast, NKT cells in liver and lung, but also in lymphoid tissues such as spleen, lymph node, and bone marrow, failed to recirculate even after 8 wk. Thus, parabiotic mice harbored <5–10% congenic NKT cells 2 mo after surgery. In contrast, the peculiar population of CD4− NKT cells found in the peripheral blood rapidly equilibrated. Thus, NKT cells in organs and in lymphoid tissues appeared to be resident populations that did not exchange, even if they resided inside the vascular compartment.
Figure 3.
Parabiotic mice do not exchange NKT cells. CD45.1 and CD45.2 congenic C57BL/6 mice joined in parabiotic pairs at 6 wk of age were examined 4 wk later (A and B) or at various time points as indicated (C). (A) Representative FACS dot plots from liver and from tail vein peripheral blood (PB) of the CD45.2 member of a parabiotic pair after 28 d of parabiosis show the relative frequencies of CD45.2 (host) and CD45.1 (parabiotic partner) cells among B (B220+ CD3ε−), CD4 T (B220− CD3ε+ CD4+ CD1d-α-GalCer−), CD8 T (B220− CD3ε+ CD8α+), NK (NK1.1+ CD3ε−), and Vα14 NKT (B220− CD3ε+ CD1d-α-GalCer+) cells. (B) Pie charts indicate the relative frequencies of CD45.2 (black) and CD45.1 (gray) cells in each member of a representative parabiotic pair after 28 d. Data are shown for various lymphocyte subsets and tissues as indicated. (C) Summary of chimerism for B (open square), CD4 T (filled triangle), CD8 T (open triangle), NK (filled circle), and Vα14 NKT (open circle) cells in the liver and spleen at different times after surgery for all parabionts. Each data point represents mean ± SEM. Days 14 and 28 are from two experiments with two parabiotic pairs per group and day 63 is from one experiment with two parabiotic pairs per group.
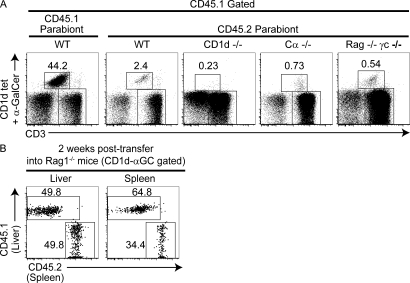
The failure to recirculate could be related to the saturation of a putative NKT cell niche in the parabiotic partner. However, in parabiosis experiments joining WT with CD1d-deficient mice, the CD1d-deficient partner also failed to attract WT NKT cells (Fig. 4 A). Likewise, NKT cells failed to populate TCR-Cα–deficient (lacking T cells) and RAG-γc double-deficient (lacking T, B, NK, and other innate immune cells) parabionts, whereas conventional T cells and B cells quickly repopulated these “empty” mice.
Figure 4.
NKT cell homing in empty mice and after intravenous transfer. (A) FACS dot plots show the Vα14 NKT cell percentage among CD45.1+ CD3ε+ liver cells 30 d after parabiosis. The leftmost dot plot is from the WT CD45.1 parabiont and the four other dot plots are from CD45.2 parabionts of WT, CD1d KO, TCR-α KO, and Rag-γc double KO origin. Data are representative of three each of WT CD1d KO, WT TCR-α KO, or WT Rag-γc double KO parabiotic pairs. (B) A Rag1−/− recipient of a mixture of liver (CD45.2) and splenic (CD45.1) cells from Vα14-Jα18 transgenic (Tg) mice was analyzed after 2 wk. Lymphocytes harvested from spleen and liver were gated on CD3ε+ CD1d-α-GalCer+ NKT cells and displayed in the CD45.1/CD45.2 dot plots, with rectangular gates showing the NKT cells of liver (CD45.2) and spleen (CD45.1) origin. Data are representative of one experiment where two individual Rag1−/− recipient mice received mixtures containing 3.2–4.0 × 106 NKT cells:6.8–10.8 × 104 NKT cells were recovered in the liver and 1.0–1.3 × 104 NKT cells in the spleen.
NKT cell transfers
Because NKT cells do not spontaneously leave their tissue of residence, we extracted them from the spleen or liver of Vα14-Jα18 transgenic mice and intravenously injected them into CD45 congenic RAG KO hosts. To avoid any potential interference with the natural homing properties of these cells, they were not stained with TCR antibody or tetramer reagents which may induce significant activation. In addition, to directly test for potential biases in tissue homing, we injected mixtures of CD45.1 splenic and CD45.2 liver NKT cells, asking whether splenic (extravascular) and liver (intravascular) NKT cells would exhibit differential homing properties. Strikingly, splenic and liver NKT cells homed to both spleen and liver, irrespective of their origin (Fig. 4 B). These experiments suggest therefore that, whether extracted from the intravascular liver pool or the extravascular splenic pool, tissue-resident NKT cells home equally well to either location upon intravenous transfer.
Biochemical mechanism of intravascular residency
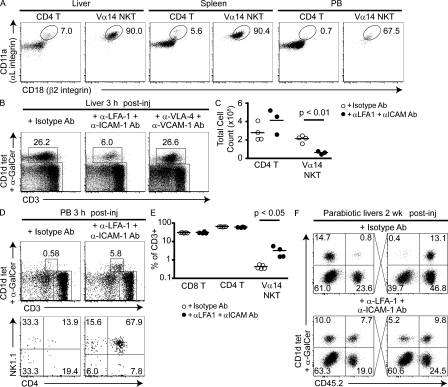
LFA-1–deficient mice exhibited a selective decrease in frequency of NKT cells in the liver and liver NKT cells expressed a very high level of LFA-1 compared with other CD4 T cells (Emoto et al., 1999). We confirmed the very high expression of LFA-1 in liver and found a similar pattern in the spleen (Fig. 5 A). However, we found that NKT cells recirculating in the peripheral blood had significantly lower LFA-1 levels, suggesting a role for LFA-1 in the resident phenotype. To directly test the role of LFA-1 in vascular adhesion, we injected mice with various anti-integrin antibodies, including anti–LFA-1, ICAM-1, very late antigen (VLA) 4, and vascular cell adhesion molecule (VCAM) 1 alone or in combinations. Modest but inconsistent drops of NKT cells in both relative frequency and absolute number were observed in the liver with anti–LFA-1 but not the other antibodies (unpublished data). Next, we injected a combination of anti–LFA-1 and anti–ICAM-1. Within 3 h, we observed a fivefold drop on average in the NKT cell numbers recovered from the liver (Fig. 5, B and C). This massive drop was accompanied by a 10-fold increase of the frequency of NKT cell in the peripheral recirculating blood, which now exceeded that of lymphoid tissues (Fig. 5, D and E). Because of this massive release of liver NKT cells in the general circulation, it was not possible to test whether LFA-1 also controlled the resident phenotype in lymphoid tissues. Characteristically, the cells released in the circulation were predominantly of the CD4+ phenotype found in tissue rather than in peripheral blood. No changes were observed after injection of anti–VLA-4 + anti–VCAM-1 (Fig. 5 B).
Figure 5.
LFA-1 controls the intravascular residence of liver NKT cells. (A) Representative dot plots gated on liver, spleen, and peripheral blood CD1d-α-GalCer+ Vα14 NKT cells and CD1d-α-GalCer− CD4 T cells show LFA-1 (αLβ2 integrin) staining. Data are representative of three experiments with two to four mice per group. In one such experiment, mean fluorescence intensity (MFI) ± SEM of CD11a (αL) was 27,069 ± 553 for liver NKT, 25,725 ± 1741 for spleen NKT, and 16,574 ± 971 for peripheral blood NKT. Mean fluorescence intensity of CD18 (β2) was 1,274 ± 194 for liver NKT, 680 ± 30 for spleen NKT, and 419 ± 23 for peripheral blood NKT. The mean fluorescence intensities of CD11a and CD18 NKT cells in spleen and liver were significantly higher than in peripheral blood (P < 0.05). (B) Percentage of liver NKT lymphocytes 3 h after injection of either 200 µg isotype control antibody or 200 µg anti–LFA-1 + 100 µg anti–ICAM-1. Dot plots were gated on B220-negative cells. (C) Summary plot showing absolute numbers of liver CD4 T (excluding CD1d-α-GalCer+ cells) or Vα14 NKT cells in individual mice after treatment with antibodies as indicated. Horizontal bars represent means. Data shown are from one experiment and are representative of three experiments with two to four mice per group. (D) NKT lymphocytes in tail vein peripheral blood 3 h after injection of antibodies as in B. Dot plots in the top row are gated on B220− lymphocytes and show percentages of Vα14 NKT cells among CD3ε+ cells. Dot plots in the bottom row show the CD4 versus NK1.1 phenotype of gated NKT cells. Data are representative of two experiments with two to three mice per group. (E) Summary plot showing the percentage of CD4 T, CD8 T, and CD1d-α-GalCer+ NKT cells among CD3ε+ PBL in individual mice 3 h after injection of control or anti-integrin antibodies. Horizontal bars represent means. (F) Recirculation of liver NKT cells in parabiotic mice treated with a single injection of anti–LFA-1 + anti–ICAM-1, or isotype control and examined 2 wk later. Data are representative of three anti–LFA-1/ICAM-1–treated parabiotic pairs and two isotype-treated pairs.
As blockade of LFA-1–ICAM-1 interactions led to the release of liver-resident NKT cells into the blood circulation, it was possible that the recirculating NKT cells might now reach the liver of a parabiotic partner. Indeed, parabiotic mice treated with the same anti–LFA-1 + anti–ICAM-1 combination, but not isotype control, developed extensive recirculation between their liver NKT cell pools (Fig. 5 F). Thus, 2 wk after antibody treatment, although control isotype-treated parabiotic mice had only 4.6% of their liver NKT cells originating from the parabiotic partner, the pairs treated with anti–LFA-1 + anti–ICAM-1 had 47% on average. These results establish that LFA-1 and ICAM-1 are key integrins mediating the adhesion to sinusoidal cells and strongly suggest, therefore, that the very high levels of LFA-1 constitutively expressed by NKT cells cause their long-term residence in the liver.
The transcription factor PLZF is sufficient to induce LFA-1 up-regulation and liver accumulation of NKT cells
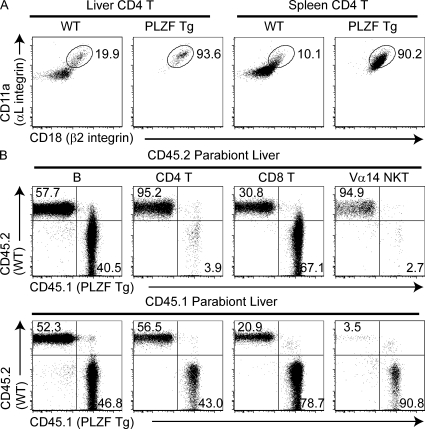
PLZF transgenic mice expressing the signature transcription factor PLZF under the cd4 promoter exhibit a massive conversion of naive MHC class II–restricted CD4 T cells into effector cells capable of producing both IL-4 and IFN-γ (Savage et al., 2008). This effector conversion, which occurred during thymic development, was not accompanied by cell division and was associated with a redirection of CD4 cells from lymph node and blood to tissues. We examined adhesion receptors and found that PLZF transgenic CD4 T cells also uniformly overexpressed LFA-1 (Fig. 6 A). To test whether, like natural NKT cells, PLZF transgenic CD4 cells were resident rather than recirculating cells, we generated parabiotic pairs between WT and PLZF transgenic mice. Fig. 6 B shows that the transgenic CD4 T cells did not recirculate to the WT organs, whereas, in marked contrast, the WT CD4 T cells promptly recirculated to the transgenic organs. Thus, PLZF expression not only induced high LFA-1 expression but also conferred the long-term tissue-resident phenotype upon expression in conventional CD4 T cells.
Figure 6.
PLZF induces high LFA-1 expression and long-term tissue residence. (A) Dot plots gated on liver and spleen CD4 T (excluding CD1d-α-GalCer+ cells) show LFA-1 (αLβ2 integrin) staining in WT or PLZF transgenic mice. Data are representative of one experiment with two mice per group. (B) Parabiotic pairs joining CD45.1 PLZF transgenic (Tg) and CD45.2 WT mice. Dot plots show the CD45.2 and CD45.1 staining of gated B, CD4 T, CD8 T, and Vα14 NKT cells in the liver of the WT parabiont (top row) or the PLZF transgenic parabiont (bottom row) after 25 d of parabiosis. Data are representative of one experiment with two pairs of parabiotic mice.
DISCUSSION
This paper reveals several unexpected characteristics of the recirculation and homing pattern of NKT cells and elucidates key biochemical and molecular mechanisms underlying these unusual properties. Although sinusoidal retention and crawling are well documented behaviors for many cell types, this mode of locomotion is transient, limited to the acute phase of immune response for effector T cells (Bartholomäus et al., 2009), monocytes (Auffray et al., 2007), and PMNs (Phillipson et al., 2006; McDonald et al., 2010) invading an organ or to a maturation step for immature B cells in the bone marrow (Pereira et al., 2009). In the case of NKT cells (Geissmann et al., 2005), the results of the long-term parabiosis experiments presented in this study imply that this behavior can be prolonged for weeks and, therefore, that these innate-like lymphocytes constitutively wander within the confines of the sinusoid capillary bed of the liver for periods of time that can exceed their lifespan. This is consistent with their suggested role of vascular surveillance for blood-borne pathogens that are captured by Kupffer cells (Lee et al., 2010). Relevant examples of such pathogens include Borrelia and Ehrlichia (Mattner et al., 2005; Tupin et al., 2008), both of which are injected intravenously through tick bite. In addition, NKT cells can recognize lipids presented by CD1d-expressing hepatic stellate cells (Ito cells) and hepatocytes, which are easily accessible through the fenestrated capillaries of the liver (Winau et al., 2007).
Although direct visualization of NKT cells has not been reported in other organs, our findings support the notion that NKT cells also accumulate in the vascular compartment of the lung. Indeed, contrasting with a 0.2% frequency of NKT cells in peripheral tail vein, 2% of cells obtained through needle aspiration of the lung vascular system were NKT cells. Although this frequency is well below the extraordinary frequencies found in samples aspirated through the hepatic vein or the portal vein, it is consistent with a significant accumulation of NKT cells in the vascular compartment of the lung, with important implications for innate allergic responses in this organ. For example, we have recently found that airborne exposure to microbial NKT ligands resulted in rapid translocation of NKT cells from the vascular compartment to the lung interstitium and bronchoalveolar space with subsequent recruitment of eosinophils (unpublished data).
Cell adhesion and locomotion involves reversible binding between various integrins, such as LFA-1, Mac-1, VLA-4, LPAM-1 (lymphocyte Peyer’s Patch adhesion molecule 1), and their endothelial receptor ICAMs, VCAM-1, and MAdCAM-1 (mucosal vascular addressin cell adhesion molecule 1). Our results clearly identify LFA-1 and ICAM-1 as major mediators of the endothelial adhesion of NKT cells. Although blocking of either LFA-1 or ICAM-1 alone had little effect on liver NKT cells, the combination resulted in rapid release of liver NKT cells into the peripheral circulating blood. In contrast, blocking with anti–VLA-4 and anti–VCAM-1, which play a substantial role in the intraluminal crawling and transmigration of conventional effector T cells (Bartholomäus et al., 2009), had no effect. The release of liver NKT cells was seen as early as 30 min after injection and was complete by 3 h, as indicated by a loss of liver NKT cells and a >10-fold corresponding increase of their frequency in the peripheral blood. Furthermore, a single injection of anti–LFA-1 and anti–ICAM-1 was sufficient to induce extensive recirculation of NKT cells between parabiotic mice, as judged by the near equilibration achieved in the liver 2 wk after antibody blockade.
Integrin-mediated adhesion typically relies on inside-out activation, for example by signaling through chemokine molecules arrayed on the surface of endothelial cells or by TCR signaling (Gomez-Rodriguez et al., 2007; Abram and Lowell, 2009). In the case of NKT cells, the precise mechanisms enabling these interactions remain to be elucidated. Notably, previous studies based on transgenic expression of CD1d in the thymus only, or on NKT cell transfer into CD1d-deficient hosts, showed normal accumulation in the liver (McNab et al., 2005; Wei et al., 2005), implying that CD1d recognition, and therefore TCR stimulation, is not a prerequisite for sinusoidal retention. Crawling was also unimpeded in mice treated with anti-CD1d antibody (Lee et al., 2010). An alternative possibility is that chemokines constitutively arrayed on the luminal side of liver endothelial cells in the healthy state might maintain LFA-1 activation. However, neither genetic ablation of CXCR6, the major chemokine receptor expressed by NKT cells, nor treatment with pertussis toxin, an inhibitor of most G protein–coupled chemoreceptors, could induce the detachment of NKT cells from the liver microvasculature (Geissmann et al., 2005; Lee et al., 2010). Notably, NKT cells also normally accumulated in the liver of germ-free mice, ruling out a requirement for microbial exposure (Park et al., 2000).
Although the NKT cells present in tissues did not recirculate, those in the peripheral blood readily equilibrated in parabiotic mice. This apparent paradox may be explained by two observations. First, the circulating NKT cells had mostly a CD4-negative phenotype, whereas the tissue-resident cells were mostly CD4+, suggesting that they belonged to different subsets. Second, of relevance to the mechanism of vascular residence, circulating NKT cells expressed significantly fewer LFA-1 receptors than their tissue-resident counterparts. In any case, this observation supports the emerging heterogeneity of NKT cells, as pointed out recently (Godfrey et al., 2010), and raises further caution about the representative value of human studies that are restricted to the circulating pool of NKT cells.
Our study also clarifies the anatomical location of NKT cells in secondary lymphoid tissues. A previous study suggested a clustering of eGFP+ TCR-β+ cells, presumably including NKT cells, in the periarteriolar lymphoid sheath of 4get mice after injection of anti-CD3 antibody (Stetson et al., 2003), but a direct identification of NKT cells before stimulation was not provided. A previous study showed that purified and reinjected CFSE-labeled NKT cells migrated to the paracortical region of the lymph node (Barral et al., 2010), a result which seemed to be in conflict with the remarkable endothelial adhesion and intravascular location of NKT cells in the liver. In the paracortical location, NKT cells adopted a random walk (Barral et al., 2010) different from their crawling behavior on the surface of endothelial cells in the liver microvasculature (Geissmann et al., 2005). One potential caveat in these experiments was the need to stain NKT cells with CD1d tetramer or anti–TCR-β antibody for purification before in vivo injection. The resulting TCR cross-linking might have induced some activation and changed the properties of NKT cells. Our specific staining in tissue section with CD1d tetramers not only confirmed that NKT cells did preferentially reside in the T cell zone of the lymph node and were conspicuously absent from the B cell follicles but also extended this observation to the spleen. This type of anatomical location is well suited for immediate responses to microbial ligands brought to the draining lymph node (Barral et al., 2010) and is consistent with their innate mission of rapid response to microbial invasion.
The presence of noncommunicating pools of NKT cells with such drastic differences in location and locomotion, as found in the liver microvasculature and in the lymphoid organs, raises the question of whether they belong to different specialized subsets or whether they represent essentially the same population with a flexible behavior in distinct environments. Our transfer experiments using mixtures of splenic and hepatic NKT cells clearly demonstrated that NKT cells in these locations had a similar ability to colonize either tissue. However, other studies have highlighted the existence of tissue-specific NKT cell subsets. For example, NKT cells in peripheral lymph nodes stably expressed retinoic acid receptor–related orphan receptor γt and produced IL-17 (Doisne et al., 2009), and a subset of splenic and lung NKT cells expressed the IL-25 receptor (Terashima et al., 2008). Thus, as different genetic subprograms may be associated with NKT cells in different tissues, it is possible that NKT cells in some locations would exhibit special homing and adhesion properties.
Finally, our studies identified PLZF, the signature transcription factor of NKT cells (Kovalovsky et al., 2008; Savage et al., 2008), as an essential inducer of their homing and adhesion behavior. Thus, whereas PLZF-deficient mice lacked liver NKT cells (Kovalovsky et al., 2008; Savage et al., 2008), the current study shows that PLZF transgenic conventional CD4 T cells not only acquired the very high LFA-1 expression pattern but also demonstrated the same liver-resident phenotype as NKT cells. These findings indicate that PLZF genetically controls the vascular surveillance program of NKT cells, at least in part through the induction of high level of LFA-1.
In summary, this study demonstrates that NKT cells exhibit constitutive LFA-1–ICAM-1–mediated interactions that underlie a unique lifelong patrolling behavior within the confines of the microcirculation of the liver and likely also the lung. Together with their rapid cytokine and chemokine responses, this property makes them uniquely positioned for rapid response within the infected organ itself, as recently visualized in the context of infection by Borrelia (Lee et al., 2010). Because this behavior can be induced through the expression of PLZF alone, our study emphasizes the central role of this signature transcription factor in orchestrating the complex functional and circulatory properties that characterize innate-like effector T cells.
MATERIALS AND METHODS
Mice.
C57BL/6 (Ly5.2), B6.SJL-PtprcaPep3b/BoyJ (CD45.1), CD1d-deficient (Cd1d1/Cd1d2−/−), TCR-α–deficient (tcra−/−), and Rag-1−/− mice in the C57BL/6 background were purchased from The Jackson Laboratory. C57BL/6 Rag-γc double-deficient (Rag2−/− il2rg−/−) mice were obtained from Taconic. C57BL/6 Jα18−/− mice were obtained from M. Taniguchi (Riken Research Center for Allergy and Immunology, Yokohama, Japan; Cui et al., 1997). Vα14-Jα18 TCR-α transgenic mice (Griewank et al., 2007) and PLZF transgenic mice (driven by the cd4 promoter) were described previously (Savage et al., 2008). Unless otherwise stated, 6–10-wk-old mice were used in experiments. All mice were raised in a specific pathogen-free environment at the University of Chicago, and experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Antibodies and flow cytometry.
CD1d-α-GalCer tetramers were prepared as previously described (Benlagha et al., 2000). Primary or fluorochrome-labeled monoclonal antibodies for flow cytometry against mouse B220 (RA3-6B2), CD1d (1B1), CD3ε (17A2), CD4 (RM4-5), CD8α (53–6.7), CD11a (2D7), CD18 (M18/2), CD24 (M1/69), CD29 (HMβ1-1), CD44 (IM7), CD41 (MWReg30), CD45.1 (A20), CD45.2 (104), CD49a (Ha31/8), CD49b (DX5), CD49d (9C10 [MFR4.B] or R1-2), CD49e (5H10-27 [MFR5]), CD49f (GoH3), CD51 (RMV-7), CD61 (2C9.G2 [HMβ3-1]), CD62L (MEL-14), CD104 (346-11A), LPAM-1 (DATK32), NK1.1 (PK136), and TCR-β (H57-597) were purchased from eBioscience, BD, or BioLegend. Samples were collected on a LSRII (BD) using FACSDiva software (BD), and data were analyzed using FlowJo (Tree Star).
Single cell preparation.
Blood was either isolated from tail vein or was aspirated into a syringe through a needle inserted into the portal, hepatic, left, or right ventricle, with the needle directed toward the liver or the lung, respectively, while mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. Lung and liver were perfused with PBS, harvested, minced, and digested for 30 min in RPMI with Blendzyme 3 (Roche) and DNase (Roche) at 37°C. The remaining cell slurry was strained through 70-µm mesh and pelleted by centrifugation. The cell pellet was resuspended in 44% Percoll, layered over 66% Percoll gradient, and spun at 600 g. Bone marrow was isolated by flushing bones with RPMI. Spleen, thymus, and lymph node cells were isolated by pressing through cell strainers.
Immunofluorescence and microscopy.
Mesenteric lymph nodes and spleen were placed in Tissue-Tek OCT and were flash frozen on dry ice and stored at −80°C. Cryostat sections 7 mm in thickness were transferred to slides. Slides were blocked with 1% BSA and then incubated with CD1d-α-GalCer allophycocyanin (APC) tetramer (1:10), washed, and then stained with PE anti–TCR-β (Armenian hamster; 1:400), FITC anti-B220 (rat; 1:200), and anti-APC (rabbit; 1:1,000; Dako), washed, and stained with cyanine (Cy) 3 anti–Armenian hamster (1:1,000; Jackson ImmunoResearch Laboratories), Cy5 anti–rabbit (1:1,000; Jackson ImmunoResearch Laboratories), and FITC anti–rat (1:500; Jackson ImmunoResearch Laboratories) and then washed, and Prolong Gold (Invitrogen) was added to slides plus coverslip. Note that a critical aspect of this procedure was the immediate processing of the staining reaction in the absence of fixation. Tissue was analyzed on a microscope (DM IRE2; Leica) with a digital camera (ORCA-ER; Hamamatsu).
Parabiosis surgery.
Mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. A linear incision was made from the scapulae to the lower abdomen on opposing sides of each member of the pair. Animals were placed side by side and skin edges sewn together.
Adoptive transfer of NKT cells.
Single cell suspensions from liver and spleen of CD45.2 and CD45.1 Vα14-Jα18 transgenic mice, respectively, were isolated by centrifugation over Lympholyte and mixed at ∼1:2 ratio of Vα14 NKT cells. 20 × 106 cells of the mixture (containing ∼4 × 106 NKT cells) were injected i.v. into C57BL/6.Rag1−/− hosts.
Blocking antibody experiments.
Blocking antibodies against mouse CD11a (LFA-1; M17/4), CD54 (ICAM-1; YNI.7.4), CD49d (VLA-4; PS/2), and CD106 (VCAM-1; M/K-2.7) and rat IgG2a Control (2A3) were purchased from BioXCell and injected iv 3 h before tissue harvest. Injections consisted of 200 µg isotype control antibody, 100 µg anti–LFA-1, 200 µg anti–ICAM-1, 100 µg VLA-4, or 200 µg VCAM-1 blocking antibodies. These quantities were based on previously determined saturating antibody concentrations (Pereira et al., 2009).
Statistical analysis.
Statistical analysis was performed in Prism (GraphPad Software) with the unpaired Student’s t test.
Acknowledgments
We thank members of the Bendelac laboratory for discussions, advice, and help at various stages of the study, the National Institute of Allergy and Infectious Diseases tetramer facility for CD1d tetramers, and the University of Chicago Animal Resource Center.
This work was supported by National Institutes of Health grants AI038339 and AI053725 (A. Bendelac), CA69212 (A.D. Luster), and the Sandler Program for Asthma Research (A. Bendelac). A. Bendelac is a Howard Hughes Medical Institute Investigator.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- α-GalCer
- α-galactosylceramide
- ICAM
- intercellular adhesion molecule
- PLZF
- promyelocytic leukemia zinc finger
- VCAM
- vascular cell adhesion molecule
- VLA
- very late antigen
References
- Abram C.L., Lowell C.A. 2009. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27:339–362 10.1146/annurev.immunol.021908.132554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 317:666–670 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- Barral P., Polzella P., Bruckbauer A., van Rooijen N., Besra G.S., Cerundolo V., Batista F.D. 2010. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat. Immunol. 11:303–312 10.1038/ni.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomäus I., Kawakami N., Odoardi F., Schläger C., Miljkovic D., Ellwart J.W., Klinkert W.E., Flügel-Koch C., Issekutz T.B., Wekerle H., Flügel A. 2009. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 462:94–98 10.1038/nature08478 [DOI] [PubMed] [Google Scholar]
- Bendelac A., Savage P.B., Teyton L. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25:297–336 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- Benlagha K., Weiss A., Beavis A., Teyton L., Bendelac A. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895–1903 10.1084/jem.191.11.1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M., Brenner M.B. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817–890 10.1146/annurev.immunol.22.012703.104608 [DOI] [PubMed] [Google Scholar]
- Cui J., Shin T., Kawano T., Sato H., Kondo E., Toura I., Kaneko Y., Koseki H., Kanno M., Taniguchi M. 1997. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 278:1623–1626 10.1126/science.278.5343.1623 [DOI] [PubMed] [Google Scholar]
- Darmoise A., Teneberg S., Bouzonville L., Brady R.O., Beck M., Kaufmann S.H., Winau F. 2010. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 33:216–228 10.1016/j.immuni.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne J.M., Becourt C., Amniai L., Duarte N., Le Luduec J.B., Eberl G., Benlagha K. 2009. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J. Immunol. 183:2142–2149 10.4049/jimmunol.0901059 [DOI] [PubMed] [Google Scholar]
- Emoto M., Mittrücker H.W., Schmits R., Mak T.W., Kaufmann S.H. 1999. Critical role of leukocyte function-associated antigen-1 in liver accumulation of CD4+NKT cells. J. Immunol. 162:5094–5098 [PubMed] [Google Scholar]
- Fox L.M., Cox D.G., Lockridge J.L., Wang X., Chen X., Scharf L., Trott D.L., Ndonye R.M., Veerapen N., Besra G.S., et al. 2009. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 7:e1000228 10.1371/journal.pbio.1000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F., Cameron T.O., Sidobre S., Manlongat N., Kronenberg M., Briskin M.J., Dustin M.L., Littman D.R. 2005. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3:e113 10.1371/journal.pbio.0030113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanov E., Veinotte L., Cullen R., Chamberlain E., Butcher E.C., Johnston B. 2008. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J. Immunol. 181:81–91 [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Kronenberg M. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114:1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.I., Stankovic S., Baxter A.G. 2010. Raising the NKT cell family. Nat. Immunol. 11:197–206 10.1038/ni.1841 [DOI] [PubMed] [Google Scholar]
- Gomez-Rodriguez J., Readinger J.A., Viorritto I.C., Mueller K.L., Houghtling R.A., Schwartzberg P.L. 2007. Tec kinases, actin, and cell adhesion. Immunol. Rev. 218:45–64 10.1111/j.1600-065X.2007.00534.x [DOI] [PubMed] [Google Scholar]
- Griewank K., Borowski C., Rietdijk S., Wang N., Julien A., Wei D.G., Mamchak A.A., Terhorst C., Bendelac A. 2007. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 27:751–762 10.1016/j.immuni.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B., Kim C.H., Soler D., Emoto M., Butcher E.C. 2003. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J. Immunol. 171:2960–2969 [DOI] [PubMed] [Google Scholar]
- Kinjo Y., Wu D., Kim G., Xing G.W., Poles M.A., Ho D.D., Tsuji M., Kawahara K., Wong C.H., Kronenberg M. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 434:520–525 10.1038/nature03407 [DOI] [PubMed] [Google Scholar]
- Kinjo Y., Tupin E., Wu D., Fujio M., Garcia-Navarro R., Benhnia M.R., Zajonc D.M., Ben-Menachem G., Ainge G.D., Painter G.F., et al. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7:978–986 10.1038/ni1380 [DOI] [PubMed] [Google Scholar]
- Kovalovsky D., Uche O.U., Eladad S., Hobbs R.M., Yi W., Alonzo E., Chua K., Eidson M., Kim H.J., Im J.S., et al. 2008. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 9:1055–1064 10.1038/ni.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D., Alonzo E.S., Uche O.U., Eidson M., Nichols K.E., Sant’Angelo D.B. 2010. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J. Immunol. 184:6746–6755 10.4049/jimmunol.1000776 [DOI] [PubMed] [Google Scholar]
- Lee W.Y., Moriarty T.J., Wong C.H., Zhou H., Strieter R.M., van Rooijen N., Chaconas G., Kubes P. 2010. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 11:295–302 10.1038/ni.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Waskow C., Liu X., Yao K., Hoh J., Nussenzweig M. 2007. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 8:578–583 10.1038/ni1462 [DOI] [PubMed] [Google Scholar]
- Matsuda J.L., Gapin L. 2005. Developmental program of mouse Valpha14i NKT cells. Curr. Opin. Immunol. 17:122–130 10.1016/j.coi.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Mattner J., Debord K.L., Ismail N., Goff R.D., Cantu C., III, Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 434:525–529 10.1038/nature03408 [DOI] [PubMed] [Google Scholar]
- McDonald B., Pittman K., Menezes G.B., Hirota S.A., Slaba I., Waterhouse C.C., Beck P.L., Muruve D.A., Kubes P. 2010. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 330:362–366 10.1126/science.1195491 [DOI] [PubMed] [Google Scholar]
- McNab F.W., Berzins S.P., Pellicci D.G., Kyparissoudis K., Field K., Smyth M.J., Godfrey D.I. 2005. The influence of CD1d in postselection NKT cell maturation and homeostasis. J. Immunol. 175:3762–3768 [DOI] [PubMed] [Google Scholar]
- Ohteki T., MacDonald H.R. 1994. Major histocompatibility complex class I related molecules control the development of CD4+8− and CD4−8− subsets of natural killer 1.1+ T cell receptor-α/β+ cells in the liver of mice. J. Exp. Med. 180:699–704 10.1084/jem.180.2.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T., Maki C., Koyasu S., Mak T.W., Ohashi P.S. 1999. Cutting edge: LFA-1 is required for liver NK1.1+TCR alpha beta+ cell development: evidence that liver NK1.1+TCR alpha beta+ cells originate from multiple pathways. J. Immunol. 162:3753–3756 [PubMed] [Google Scholar]
- Park S.H., Benlagha K., Lee D., Balish E., Bendelac A. 2000. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur. J. Immunol. 30:620–625 [DOI] [PubMed] [Google Scholar]
- Pereira J.P., An J., Xu Y., Huang Y., Cyster J.G. 2009. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat. Immunol. 10:403–411 10.1038/ni.1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson M., Heit B., Colarusso P., Liu L., Ballantyne C.M., Kubes P. 2006. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 203:2569–2575 10.1084/jem.20060925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberger J., Schebesta A., Sakaguchi S., Boucheron N., Blomberg K.E., Berglöf A., Kolbe T., Smith C.I., Rülicke T., Ellmeier W. 2008. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc. Natl. Acad. Sci. USA. 105:17919–17924 10.1073/pnas.0805733105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage A.K., Constantinides M.G., Han J., Picard D., Martin E., Li B., Lantz O., Bendelac A. 2008. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 29:391–403 10.1016/j.immuni.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmling V., Lukacs-Kornek V., Thaiss C.A., Quast T., Hochheiser K., Panzer U., Rossjohn J., Perlmutter P., Cao J., Godfrey D.I., et al. 2010. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat. Immunol. 11:313–320 10.1038/ni.1848 [DOI] [PubMed] [Google Scholar]
- Shimaoka T., Seino K., Kume N., Minami M., Nishime C., Suematsu M., Kita T., Taniguchi M., Matsushima K., Yonehara S. 2007. Critical role for CXC chemokine ligand 16 (SR-PSOX) in Th1 response mediated by NKT cells. J. Immunol. 179:8172–8179 [DOI] [PubMed] [Google Scholar]
- Sriram V., Du W., Gervay-Hague J., Brutkiewicz R.R. 2005. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J. Immunol. 35:1692–1701 10.1002/eji.200526157 [DOI] [PubMed] [Google Scholar]
- Stetson D.B., Mohrs M., Reinhardt R.L., Baron J.L., Wang Z.E., Gapin L., Kronenberg M., Locksley R.M. 2003. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198:1069–1076 10.1084/jem.20030630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A., Watarai H., Inoue S., Sekine E., Nakagawa R., Hase K., Iwamura C., Nakajima H., Nakayama T., Taniguchi M. 2008. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 205:2727–2733 10.1084/jem.20080698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.Y., Hou R., Boyson J.E., Means T.K., Hess C., Olson D.P., Strominger J.L., Brenner M.B., Gumperz J.E., Wilson S.B., Luster A.D. 2003. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J. Immunol. 171:2571–2580 [DOI] [PubMed] [Google Scholar]
- Thomas S.Y., Banerji A., Medoff B.D., Lilly C.M., Luster A.D. 2007. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J. Immunol. 179:1901–1912 [DOI] [PubMed] [Google Scholar]
- Tupin E., Benhnia M.R., Kinjo Y., Patsey R., Lena C.J., Haller M.C., Caimano M.J., Imamura M., Wong C.H., Crotty S., et al. 2008. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA. 105:19863–19868 10.1073/pnas.0810519105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D.G., Lee H., Park S.H., Beaudoin L., Teyton L., Lehuen A., Bendelac A. 2005. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J. Exp. Med. 202:239–248 10.1084/jem.20050413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winau F., Hegasy G., Weiskirchen R., Weber S., Cassan C., Sieling P.A., Modlin R.L., Liblau R.S., Gressner A.M., Kaufmann S.H. 2007. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 26:117–129 10.1016/j.immuni.2006.11.011 [DOI] [PubMed] [Google Scholar]
- Wright D.E., Wagers A.J., Gulati A.P., Johnson F.L., Weissman I.L. 2001. Physiological migration of hematopoietic stem and progenitor cells. Science. 294:1933–1936 10.1126/science.1064081 [DOI] [PubMed] [Google Scholar]
- Zhou D., Mattner J., Cantu C., III, Schrantz N., Yin N., Gao Y., Sagiv Y., Hudspeth K., Wu Y.P., Yamashita T., et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science. 306:1786–1789 10.1126/science.1103440 [DOI] [PubMed] [Google Scholar]