IFN-γ stimulates blood-eating macrophages (hemophagocytes) by acting directly on macrophages to promote phagocytosis and uptake of blood cells.
Abstract
Cytopenias of uncertain etiology are commonly observed in patients during severe inflammation. Hemophagocytosis, the histological appearance of blood-eating macrophages, is seen in the disorder hemophagocytic lymphohistiocytosis and other inflammatory contexts. Although it is hypothesized that these phenomena are linked, the mechanisms facilitating acute inflammation-associated cytopenias are unknown. We report that interferon γ (IFN-γ) is a critical driver of the acute anemia observed during diverse microbial infections in mice. Furthermore, systemic exposure to physiologically relevant levels of IFN-γ is sufficient to cause acute cytopenias and hemophagocytosis. Demonstrating the significance of hemophagocytosis, we found that IFN-γ acts directly on macrophages in vivo to alter endocytosis and provoke blood cell uptake, leading to severe anemia. These findings define a unique pathological process of broad clinical and immunological significance, which we term the consumptive anemia of inflammation.
Although anemia is commonly observed in patients with sepsis or other severe infections (Vincent et al., 2002; Corwin et al., 2004; Walsh and Saleh, 2006; Napolitano et al., 2009), the connection between acute inflammation and cytopenias is not well understood. Although anemia in some acutely ill patients may be a result of obvious causes such as blood loss, hemodilution, or microangiopathic hemolysis, the etiology is often unknown (Abshire, 1996; Ballin et al., 2009; Rivera and Ganz, 2009). There is a critical need to better understand acute inflammation-associated cytopenias; however, because unexplained early cytopenias are associated with a poor prognosis in patients presenting with sepsis and other infections (Imran et al., 2005; Bateman et al., 2008; Reade et al., 2010). Unlike acutely developing cytopenias, anemias associated with chronic inflammation (anemia of chronic disease) have been extensively studied and are known to be caused by decreased production of erythrocytes (Agarwal and Prchal, 2009).
Unexplained acute cytopenias are also seen in the disorder hemophagocytic lymphohistiocytosis (HLH), which is a disease of excessive and abnormal immune activation associated with deficiencies of lymphocyte cytotoxic function (Filipovich, 2008). Development of cytopenias in these patients may be quite rapid, suggesting a consumptive etiology (although anti-RBC antibodies or hemolysis are not typically noted; Henter et al., 1998). The pathology seen in HLH is thought to be the result of a storm of inflammatory cytokines, including IFN-γ (Henter et al., 1991; Janka and zur Stadt, 2005; Janka, 2007), which has been correlated with poor prognosis (Henter et al., 1991; Ohga et al., 1993; Imashuku et al., 1994) and is necessary for the development of HLH in murine models (Jordan et al., 2004; Pachlopnik Schmid et al., 2009).
Hemophagocytosis (blood eating) is a term used to describe the histological appearance of macrophages engulfing blood cells. Although hemophagocytosis is characteristic of HLH, it is also seen in many instances of severe inflammation such as bacterial sepsis (Ito et al., 2006), influenza (Ando et al., 2006; Hsieh and Chang, 2006), malaria (Ohno et al., 1996; Zvulunov et al., 2002), leishmaniasis (Agarwal et al., 2006), and active rheumatologic disorders (Behrens et al., 2007; Parodi et al., 2009; Hinze et al., 2010). Although hemophagocytic macrophages are suspected to contribute to the development of cytopenias seen in HLH, this link has not been demonstrated. Hemophagocytosis remains largely uncharacterized; the triggers, the mechanisms by which hemophagocytic macrophages consume blood cells, and the consequences are all unknown.
Although IFN-γ has been linked to the pathogenesis of HLH in animal models, how it may be related to the phenomenon of hemophagocytosis is unknown. IFN-γ is known as a classical activator of macrophages, up-regulating antigen presentation and antimicrobial responses, including production of reactive oxygen species and induction of inducible nitric oxide synthase (Rosa et al., 1986; Cassatella et al., 1989; Kato et al., 1989; Deguchi et al., 1995; Boehm et al., 1997). Although cytopenias are not reported with typical intermittent dosing of IFN-γ (as used for certain immunodeficient patients; Marciano et al., 2004), early clinical trials using sustained infusions of the cytokine noted rapid induction of cytopenias in some recipients (Kurzrock et al., 1986; Quesada et al., 1987; Kuebler et al., 1990; Brown et al., 1991). Although IFN-γ is thought to suppress hematopoiesis (Zoumbos et al., 1984), the rapid onset of these cytopenias suggests a consumptive rather than hypoproductive etiology.
To better understand the mechanisms behind otherwise unexplained acute inflammation-associated cytopenias, we tested the hypothesis that hemophagocytosis is a significant cause of rapidly developing anemia and other cytopenias in severe inflammatory contexts. Furthermore, because of IFN-γ’s known role in HLH development, we hypothesized that it is the proximal cause of hemophagocytosis. To test these hypotheses, we examined various hematologic and histological parameters in mice during infection or during sterile IFN-γ infusion. We found that sustained systemic exposure of mice to IFN-γ, at physiological levels seen during diverse infections, is sufficient to cause a rapid-onset severe anemia. This anemia develops within days, defining it as a consumptive (as opposed to hypoproductive) process, and it occurs in the absence of apparent hemorrhage, autoantibodies, or hemolysis. However, it is associated with diffuse hemophagocytosis, and both the anemia and hemophagocytosis are dependent on the direct action of IFN-γ on macrophages in a STAT1- and IFN regulatory factor 1 (IRF-1)–dependent manner. Thus, these studies mechanistically define a novel pathological process, which we term the consumptive anemia of inflammation (CAI; to distinguish it from the hypoproductive anemia of chronic diseases). Furthermore, they uncover a new mechanism for unexplained acute inflammation–associated cytopenias by identifying hemophagocytosis as the underlying immunopathologic process.
RESULTS
Infection-associated inflammation leads to a severe consumptive anemia that is dependent on IFN-γ
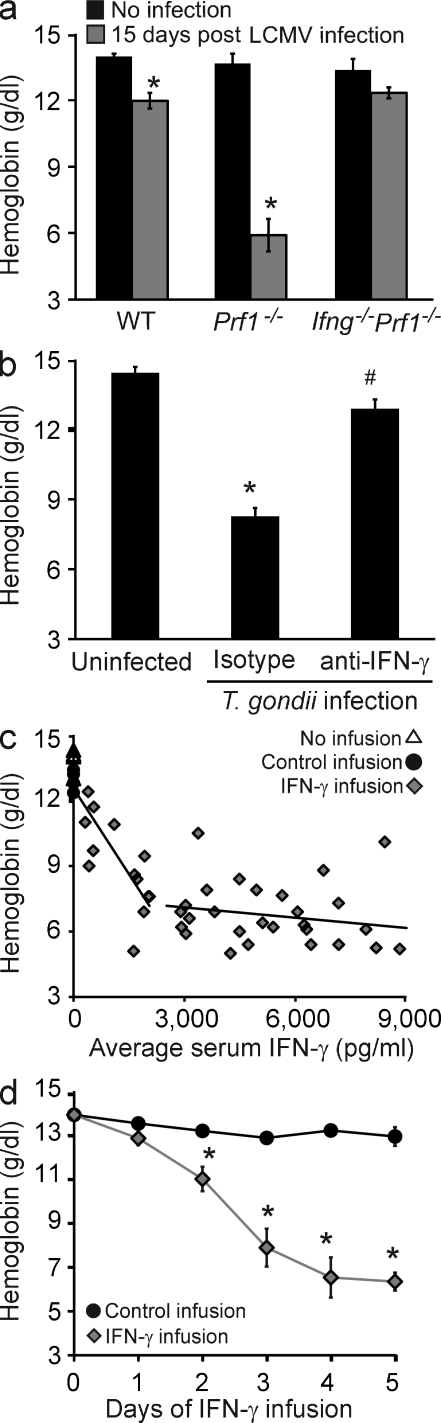
We have previously demonstrated that lymphocytic choriomeningitis virus (LCMV)–infected perforin-deficient (Prf1−/−) mice exhibit the clinical characteristics of human HLH, including high serum levels of IFN-γ and the development of pancytopenia (Jordan et al., 2004). We hypothesized that IFN-γ was vital to the development of disease-associated pathologies. To test this, Prf1−/− mice that were also IFN-γ deficient (Prf1−/−/Ifng−/−) were infected with LCMV (Fig. 1 a). On day 15 of infection, hemoglobin levels were assessed and we found that Prf1−/−/Ifng−/− mice were strikingly protected from the severe anemia seen in LCMV-infected Prf1−/− mice. In addition, WT mice (which have peak IFN-γ levels of 1,000–2,000 pg/ml after infection, compared with Prf1−/− mice with peak levels >10,000 pg/ml; Jordan et al., 2004) also developed anemia, albeit much milder.
Figure 1.
Sustained elevation of IFN-γ induces a severe consumptive anemia in physiological contexts and doses. (a) Blood hemoglobin concentrations were determined in WT, Prf1−/−, and Prf1−/−Ifng−/− mice before or 15 d after infection with LCMV. Data are mean values ± SEM. n = 5–9 mice per group combined from three experiments. *, P < 0.0001, compared with uninfected controls. (b) WT mice were left uninfected or were infected with T. gondii and, 4–5 d later, given either IFN-γ–neutralizing or isotype control antibody. 9 or 10 d after infection, hemoglobin concentrations were determined. Data are mean values ± SEM. n = 8–9 mice per group combined from two experiments. *, P < 0.0001, compared with all other points; #, P < 0.0001, compared with uninfected controls. (c) Mice were infused with various amounts of IFN-γ to achieve a range of serum levels of IFN-γ (x axis) for 5 d before blood hemoglobin concentrations were determined. The leftmost trend line includes control infusion mice (as 0 values). Each point represents an individual mouse. n = 53 mice. (d) WT mice were infused with saline or IFN-γ via osmotic pumps (to maintain levels >2,500 pg/ml in the IFN-γ group) and hemoglobin levels were assessed at the indicated times. Data are mean values ± SEM. n = 107 mice combined from five experiments. Zero time point represents mice that received no infusion. *, P ≤ 0.001, compared with control infusion.
The anemia observed in WT mice suggested that this process was not specific to Prf1−/− mice but was simply heightened by their excessive IFN-γ production. To test this hypothesis, WT mice were infected with Toxoplasma gondii (which is known to drive high serum levels of IFN-γ and has been reported to cause anemia; Mullarky et al., 2007) and were given either neutralizing anti–IFN-γ or isotype control antibody 4–5 d after infection. The T. gondii infection led to peak IFN-γ levels of 5,000–7,000 pg/ml in the isotype-treated animals (not depicted), and by day 9 or 10 these animals became severely anemic, a process which was prevented in the mice which received IFN-γ–neutralizing antibody (Fig. 1 b).
Observing IFN-γ–dependent acute anemia during two diverse infections, we hypothesized that IFN-γ would be sufficient, in the absence of infection, to induce rapid-onset anemia. To test this idea, IFN-γ was infused into uninfected animals via subcutaneously placed osmotic pumps to achieve various average serum levels over 5 d of infusion. We found that anemia developed in a dose-dependent fashion, reaching a plateau at a mean continuous exposure of ∼2,500 pg/ml (Fig. 1 c). Notably, this level is well within the physiological range of IFN-γ levels seen in LCMV-infected Prf1−/− mice, T. gondii–infected WT mice, or human patients with active HLH (Ohga et al., 1993; Imashuku et al., 1994, 1998; Jordan et al., 2004; Mullarky et al., 2007). Next, we assessed the kinetics of this response by infusing mice with IFN-γ to achieve levels of >2,500 pg/ml for various times. Over 5 d of IFN-γ infusion, WT mice developed severe anemia, which became apparent after 48 h of exposure (Fig. 1 d). In contrast, mice injected with a single large dose of IFN-γ (up to 100 µg i.p.) did not develop anemia on subsequent days (unpublished data), which may be related to the short half-life of IFN-γ (∼30 min in mice; Rutenfranz and Kirchner, 1988). The kinetics and severity of anemia observed with IFN-γ infusion mimicked those seen after infection. WT mice infected with T. gondii or Prf1−/− mice infected with LCMV did not display serum IFN-γ levels >2,500 pg/ml until day 4 or 6 of infection and did not develop anemia until after day 6 or 8 of infection, respectively (Jordan et al., 2004; not depicted). Furthermore, the mild (but consistent) anemia observed in LCMV-infected WT mice correlated well with these kinetic data. Although these animals develop potentially pathological peak IFN-γ levels (1,000–2,000 pg/ml), this level was maintained for <48 h.
We measured red cell indices of IFN-γ–infused mice and found that they were relatively unchanged (Fig. S1). The anemia that developed after IFN-γ infusion was a normocytic one, with no notable changes in peripheral morphology other than a compensatory reticulocytosis, which was evident by day 5. In addition to anemia, IFN-γ–infused mice developed leukopenia, neutropenia, and thrombocytopenia within 2 d of initiating the infusion (Fig. S1). The severity and the rapid development of the anemia defined it as a consumptive process rather than a result of decreased RBC production because murine RBCs have a lifespan of ∼40 d (Ishikawa-Sekigami et al., 2006; Kempe et al., 2006). Furthermore, the brisk reticulocytosis demonstrated that erythropoiesis was not significantly suppressed. The rapid onset of neutropenia and thrombocytopenia also suggested a consumptive process, but because of the shorter life spans of these blood elements it is difficult to rule out decreased production or trafficking (in the case of leukocytes) as a cause of these cytopenias. Because of this, we focused subsequent studies on understanding the nature of the consumptive anemia which develops in IFN-γ–exposed mice.
In each context (LCMV infection, T. gondii infection, and IFN-γ infusion), blood loss by internal hemorrhage in stool or urine was not detected (unpublished data). To rule out hemolysis as a likely cause of anemia, blood smear morphology, serum lactate dehydrogenase (LDH), and bilirubin levels, as well as RBC autoantibodies (direct and indirect Coombs assay) were all assessed (Fig. S1 and Fig. S2). With the exception of a mild increase in LDH (which was much less than that seen in control mice with intravascular hemolysis induced by human serum; Ino et al., 1987), no evidence of a hemolytic process was detected after IFN-γ infusion. Of note, moderate elevations of LDH are commonly observed in patients with active HLH. Thus, a unique and severe consumptive anemia develops in response to sustained exposure to elevated levels of IFN-γ, a process which we have termed the CAI to distinguish it from the hypoproductive anemia associated with chronic inflammatory processes.
Systemic IFN-γ exposure induces hemophagocytosis in vivo
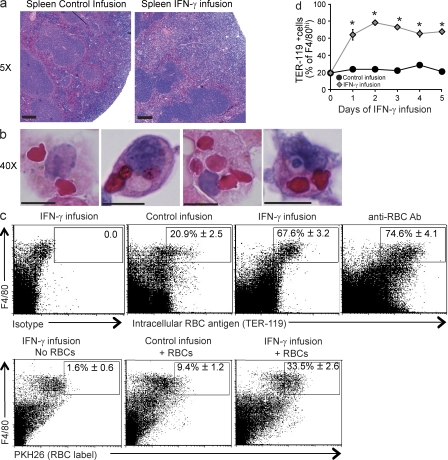
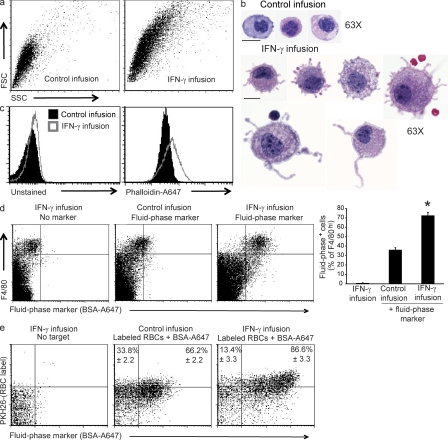
Because we observed a loss of almost 60% of circulating RBCs within 5 d, we reasoned that there should be evidence of RBC uptake in various tissues. First, we noted that spleens were enlarged, with an expansion of the red pulp/subcapsular space and a congested appearance of the red pulp of IFN-γ–exposed animals (Fig. 2 a and Fig. S3). We also noted an increase of macrophages in the liver and bone marrow, with a mild decrease of marrow cellularity (Fig. S3). Examination of spleen, liver, and bone marrow tissue sections, as well as cytospins from each of these tissues, revealed many examples of hemophagocytosis (Fig. 2 b and Fig. S4). We quantitated hemophagocytosis in the spleen by flow cytometric staining for intracellular RBC antigen in macrophages (Fig. 2 c). Intracellular localization of RBC antigen was assessed by first blocking surface-exposed antigen with saturating amounts of unlabeled antibody and then permeabilizing and staining (see Materials and methods). Consistent with the normal function of the spleen as a grooming organ for circulating RBCs, a fraction of macrophages demonstrated readily detectable RBC antigen within them before IFN-γ infusion. However, after IFN-γ infusion or administration of anti-RBC antibody (Jordan et al., 2003), most splenic macrophages were found to contain intracellular RBC antigen (Fig. 2 c). For additional corroboration, nonopsonized fluorescently labeled RBCs were injected intravenously into mice and found to be taken up by macrophages in mice receiving IFN-γ infusions (Fig. 2 c). We examined several macrophage-specific markers to identify which cells were most readily taking up RBCs. Bright expression of the F4/80 marker correlated best with RBC uptake, suggesting that splenic hemophagocytic macrophages are red pulp macrophages (not depicted; Schaller et al., 2002). Increased intracellular RBC antigen was detectable in splenic macrophages (F4/80hi) as early as 1 d after initiating IFN-γ infusion, i.e., preceding the onset of measurable anemia (Fig. 1 c and Fig. 2 d). Additionally, we found significant uptake of a neutrophil-specific antigen by F4/80hi macrophages after IFN-γ infusion (Fig. S4). This finding, and the appearance of nucleated debris within hemophagocytic macrophages, suggested they were taking up other cell types in addition to RBCs. Notably, our flow cytometry–based assay appears to be much more sensitive than light microscopy for detecting hemophagocytosis because cytospins of sorted F4/80+/TER-119+ cells revealed that only a fraction of these cells contained recognizable RBCs or nucleated cells, whereas nearly all appeared to be debris laden (unpublished data). Hemophagocytosis did not appear to be related to global increase in apoptosis because we did not observe a significant increase in apoptotic or necrotic splenocytes after IFN-γ infusion (unpublished data).
Figure 2.
Systemic IFN-γ exposure induces hemophagocytosis in vivo. (a and b) Brightfield micrographs of H&E-stained sections from mice infused with or without IFN-γ for 5 d of spleen sections (a; bars, 200 µm) or cytospun F4/80hi spleen cells (b; bars, 10 µm). (c, Top) mice were given 5-d IFN-γ infusions, 5-d control infusions, or injected with anti-RBC antibody 1 d before analyzing spleen cells. Spleen cells were permeabilized and stained intracellularly for RBC antigen (after blocking surface-exposed antigen with unlabeled antibody). (c, Bottom) Alternatively, RBCs were labeled with PKH26 and injected or not intravenously 24 h before spleen cell analysis. Dot plots are representative samples with mean percentages of n = 5–6 mice per group from two combined experiments (TER-119 staining) or n = 6–8 mice per group from three combined experiments (PKH26 labeling). Numbers represent the mean percentage TER+ or PKH+ of F4/80hi spleen cells ± SEM. (d) Mice were infused with IFN-γ for the indicated times and spleen cells were stained intracellularly (as in c) for RBC antigen. Data are mean values ± SEM. n = 6–11 mice per group combined from two experiments. *, P < 0.001 compared with control infusion.
IFN-γ acts directly on macrophages to induce hemophagocytosis, leading to CAI
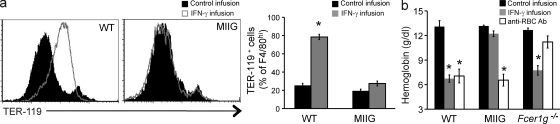
The kinetics of CAI and hemophagocytosis after IFN-γ infusion suggested that hemophagocytosis was causing the anemia we observed. Furthermore, the lack of widespread cell death or other changes in splenocytes or RBCs suggested that IFN-γ was acting primarily on macrophages to induce hemophagocytosis, which led to CAI. To test these hypotheses, we developed a transgenic mouse, which we have previously described, called the macrophages insensitive to IFN-γ (MIIG) mouse (Lin et al., 2009; Lykens et al., 2010). MIIG mice selectively express a dominant-negative mutant IFN-γ receptor in macrophage lineage cells and have a near complete blockade of IFN-γ signaling in these cells, whereas other cell types are able to produce and respond normally to this cytokine. When we challenged MIIG mice with IFN-γ infusion, we found that splenic macrophages from MIIG mice displayed no evidence of hemophagocytosis (Fig. 3 a). Thus, IFN-γ must act directly on macrophages in order for hemophagocytosis to be induced in vivo.
Figure 3.
IFN-γ acts directly on macrophages to induce hemophagocytosis and CAI. (a) WT and MIIG mice, which selectively lack IFN-γ signaling in macrophage-lineage cells, were given IFN-γ or control infusions for 5 d and intracellular RBC antigen was assessed in splenic F4/80hi macrophages by flow cytometry. Histograms are representative of three experiments and are gated onF4/80hi cells. Bar graph data are mean values ± SEM. n = 5–14 mice per group combined from three experiments. (b) WT, Fcer1g−/−, and MIIG mice were challenged with IFN-γ infusion (for 5 d) or anti-RBC antibody, and blood hemoglobin concentration was determined. Data are mean values ± SEM. n = 7–18 mice per group combined data from two or more experiments. *, P < 0.0001 compared with control infusion.
Next, we examined the induction of anemia in MIIG mice after IFN-γ infusion. When challenged with IFN-γ infusion, we found that MIIG mice were completely protected from CAI (Fig. 3 b). As an additional control condition, we challenged MIIG mice with anti-RBC antibody to test whether these mice were capable of developing a macrophage-dependent anemia (Jordan et al., 2003). MIIG mice had an identical response to that of WT animals to anti-RBC antibody injection, demonstrating that macrophages in these animals were capable of engulfing RBCs. This divergence between IFN-γ–induced anemia and antibody-induced anemia led us to also examine Fcer1g−/− mice, which have defective Fc receptor signaling. When we challenged them with antibody injection, Fcer1g−/− mice were largely protected from antibody-driven anemia. However, Fcer1g−/− mice developed CAI-like WT animals after IFN-γ infusion, demonstrating that hemophagocytosis is a distinct process from Fc receptor–mediated phagocytosis.
These findings demonstrate that hemophagocytic macrophages are more than just innocent bystanders during CAI, which has not been demonstrated before. Because IFN-γ must act directly on macrophages to induce them to become hemophagocytic, and because CAI does not develop in the absence of this process, IFN-γ–activated hemophagocytic macrophages appear to play a causal role in the induction of CAI. These findings are also consistent with our observation that hemophagocytosis precedes anemia in WT mice during IFN-γ infusion (Fig. 1 d and Fig. 2 d). Indeed, the fact that measurable hemophagocytosis precedes measurable anemia suggests that hemophagocytosis may be a rate-limiting step in the development of anemia.
Necessity of pathways downstream of the IFN-γ receptor
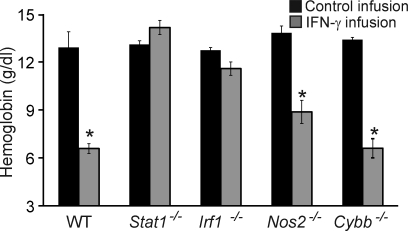
In all cell types, IFN-γ is known to signal through STAT1 and to induce transcription of numerous genes, including that of the transcription factor IRF-1. In turn, IRF-1 directly induces a large proportion of IFN-γ–induced genes (Dror et al., 2007). In macrophages, IFN-γ also prominently induces production of nitric oxide via the inducible nitric oxide synthase (NOS2) and reactive oxygen species, in part via Cybb (also called Gp91phox or NOX2). We tested the hypothesis that these classical IFN-γ signaling/response mediators are essential for CAI by challenging mice deficient in each of them. As expected, Stat1−/− mice infused for 5 d with IFN-γ were completely protected from CAI (Fig. 4). Notably, Irf1−/− mice were also protected from CAI after IFN-γ infusion, whereas Nos2−/− and Cybb−/− mice displayed only minimal or modest protection (Fig. 4). Together, these data indicate that proximal IFN-γ signaling mechanisms involving STAT1 and IRF-1 are necessary for the development of CAI in mice, whereas classical IFN-γ–induced macrophage effector molecules do not appear to be essential.
Figure 4.
IFN-γ–driven CAI is STAT1 and IRF-1 dependent but largely NOS2 and Cybb independent. WT, Stat1−/−, and Irf1−/−, Nos2−/−, and Cybb−/− mice were infused with IFN-γ over 5 d and blood hemoglobin concentrations were determined. Data are mean values ± SEM. n = 3–13 per group combined from two or more experiments. *, P ≤ 0.001 compared with control infusion
Cell surface and endocytic changes in hemophagocytic macrophages
The appearance of widespread hemophagocytosis suggested that tissue macrophage phenotypes were significantly altered by IFN-γ infusion. However, when we examined F4/80hi macrophages, we found no significant alterations in cell surface molecule expression beyond expected changes, such MHC up-regulation (unpublished data). However, after IFN-γ infusion we observed that macrophages in the spleen and other tissues increased markedly in size and complexity/side scatter, as assessed by flow cytometry (Fig. 5 a). Microscopic examination of cytospun peritoneal macrophages from control and IFN-γ–infused mice confirmed an increase in size and also revealed a dramatic increase in membrane protrusions and ruffles (Fig. 5 b). Splenic macrophages (obtained after collagenase digestion of disrupted spleens) displayed a similar phenotype but with a more prominent foamy or vacuolated morphology and less obvious membrane protrusions (unpublished data). We quantitated this increase in membrane ruffling by measuring F-actin content of macrophages, which would be expected to increase with more active membrane dynamics. Because the formation and degradation of F-actin is a very dynamic process, we stained peritoneal macrophages which were fixed immediately after lavage. We found a clear increase in F-actin after IFN-γ infusion in these cells (Fig. 5 c). Based on their foamy appearance and increased membrane ruffling, we hypothesized that macrophages in IFN-γ–infused mice had increased fluid-phase endocytosis, or pinocytosis. To examine this possibility, we directly examined pinocytic uptake of (inert) fluid-phase markers in vivo. We found that IFN-γ infusion markedly increased uptake of fluid-phase constituents by splenic, hepatic, and peritoneal macrophages, as indicated by uptake of intravenously administered fluorescently labeled bovine albumin (Fig. 5 d and not depicted). This finding is a novel one; IFN-γ has not previously been shown to influence macrophage pinocytosis. Similar increased fluid-phase uptake was also seen in peritoneal macrophages of IFN-γ–exposed mice when a soluble fluorescent dye (hydrazide salt of Alexa Fluor 647) was injected intraperitoneally (Fig. 6 and not depicted). In contrast, when we injected mice with fluorescent dextran, which is largely taken up via the macrophage mannose receptor, we saw no increase in uptake (Fig. S5), suggesting that IFN-γ exposure was not affecting all forms of macrophage endocytic uptake. Finally, injection of both labeled RBCs and fluid-phase markers showed that hemophagocytic macrophages were also the ones that most avidly took up fluid-phase markers in response to IFN-γ infusion (Fig. 5 e). Collectively, these data indicate that IFN-γ induces an increase in pinocytosis, which is associated with hemophagocytosis in splenic macrophages.
Figure 5.
IFN-γ infusion leads to increased size, membrane protrusions, F-actin assembly, and pinocytosis by macrophages. (a) 5 d after control or IFN-γ infusion, F4/80hi gated spleen cells were assessed by flow cytometry for forward/side scatter. Dot plots are representative of three experiments. (b) 3 d after control or IFN-γ infusion, mouse peritoneal cells were cytospun and stained for H&E. Bars, 10 µm. (c) 3 d after control or IFN-γ infusion, F-actin was measured in F4/80+ peritoneal cells by flow cytometric phalloidin-A647 staining. Histograms are representative of three experiments. (d) WT mice infused with IFN-γ for 1 d were injected with a fluid-phase marker (BSA-A647) and spleen cells were assessed 1 d later (while infusion continued) for uptake of this marker. Bar graph represents mean values ± SEM. n = 8–11 mice per group combined from three experiments. *, P < 0.001 compared with all conditions. (e) Mice infused with IFN-γ for 1 d were injected intravenously with PKH26-labeled RBCs along with BSA-A647 (as in d). 1 d later (while infusion continued), F4/80hi gated spleen cells were assessed for combined RBC and fluid-phase uptake by flow cytometry. Dot plots are representative two experiments. Numbers represent mean percentage of BSA+ of hemophagocytic (PKH+) F4/80hi spleen cells. n = 3–6 mice per group combined from two experiments.
Figure 6.
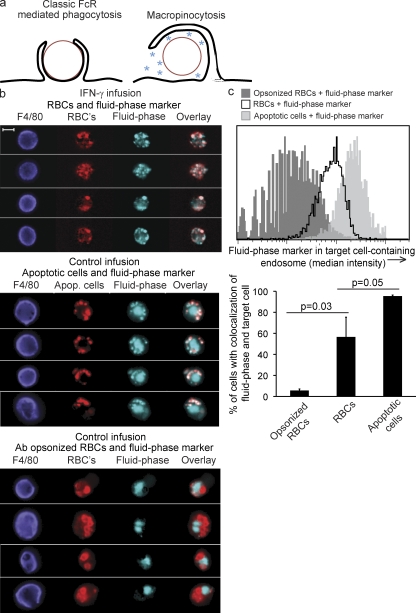
IFN-γ–induced hemophagocytosis is a macropinocytic process. (a) Depictions of fluid-phase exclusion (classical ligand-driven phagocytosis) versus fluid-phase inclusion (macropinocytosis). PKH26-labeled apoptotic cells or RBCs (with or without opsonizing antibody), along with a fluid-phase marker (soluble Alexa Fluor 647), were injected i.p. into mice which had been infused for 48 h with saline or IFN-γ. 45 min after injection, peritoneal cavities were lavaged and F4/80hi cells were assessed for colocalization of fluid-phase marker and target cells. (b) Example images of peritoneal macrophages. Bar, 10 µm. (c) Histograms (gated on F4/80hi PKH+ A647+ cells) quantitating fluid-phase inclusion (median intensity) in target cell–containing endosomes (PKH minimum intensity mask, expanded 1 pixel), representative of three experiments. Bar graphs represent percentage of colocalization ± SD of the mean of fluid-phase and target cells, combining two experiments. *, P = 0.03, one-tailed Student’s t test.
Hemophagocytosis is a macropinocytic process
Endocytosis may be divided broadly into classical ligand-driven phagocytosis and pinocytosis (Amyere et al., 2002; Conner and Schmid, 2003). Phagocytosis, a receptor-mediated process (e.g., Fc receptor or complement receptor), involves tight hugging of the cargo by the phagocyte’s membrane (a zipper-like mechanism) allowing little fluid-phase uptake. During pinocytosis, which is either spontaneous or induced by ligand/receptor stimulation, membrane extensions engulf cargo (which can include whole cells), incorporating some of the surrounding fluid phase into a loose pinosome (Fig. 6 a). Pinocytosis can be further divided into macro- and micropinocytosis, depending on whether the cargo/pinosome is greater or smaller than 1 micron. One well studied macropinocytic process is the uptake of apoptotic cells by phagocytes, which is sometimes referred to as efferocytosis (Hoffmann et al., 2001; Ogden et al., 2001; Gardai et al., 2005, 2006; Vandivier et al., 2006). Increased pinocytic uptake by hemophagocytic macrophages led us to hypothesize that hemophagocytosis is also a macropinocytic process in which fluid-phase constituents are taken up along with the blood cells.
We examined fluid-phase incorporation into hemophagocytic endosomes during very short-term in vivo experiments to further define this process. For these studies, we injected fluid-phase markers and dye-labeled RBCs intraperitoneally into mice that had received either IFN-γ or control infusions. Peritoneal macrophages were retrieved within 45 min and assessed using a flow microscopy technology to quantify colocalization of RBCs and fluid-phase dye. Control animals were injected with a fluid-phase marker along with dye-labeled apoptotic cells, as an example of macropinocytic uptake, or IgG-opsonized RBCs, for classical FcR-mediated phagocytosis. As expected, the fluid-phase marker was substantially excluded from phagosomes containing opsonized RBCs but was incorporated into endosomes containing apoptotic cells (Fig. 6, b and c). The endosomes containing hemophagocytosed RBCs (nonopsonized RBCs, injected into IFN-γ–infused mice) incorporated the fluid-phase markers, similar to those containing apoptotic cells (Fig. 6, b and c). Collectively, these data demonstrate that IFN-γ–induced hemophagocytosis is a macropinocytic process, with similarities to apoptotic cell uptake.
DISCUSSION
In the current study, we have demonstrated that an IFN-γ–dependent anemia develops acutely in the context of diverse infections or sterile cytokine infusion. This anemia is associated with the widespread appearance of hemophagocytosis in vivo, and both processes are dependent on IFN-γ signaling in macrophage lineage cells. Furthermore, IFN-γ–triggered hemophagocytosis is characterized as a macropinocytic process. These findings are summarized diagrammatically in Fig. S6. In addition to defining a new mechanism for the development of acute inflammation-associated cytopenias, these studies have provided the first evidence for the causal role of macrophages and the significance of hemophagocytosis in this pathological process. Critical aspects of the hemophagocytic response remain undefined, however. Future studies will focus on better understanding the receptors/ligands that are likely to govern uptake, as well as the signaling mechanisms downstream of STAT1 and IRF1 which drive hemophagocytosis. Although the current study has provided new mechanistic insights into poorly understood pathological processes, several caveats are applicable to our findings. First, although IFN-γ–driven CAI is a profound cause of infection-associated anemia in our studies, it is not likely to be the only cause of cytopenias in complex acutely ill patients. Other processes, such as blood loss, hemolysis, and/or decreased marrow output may be occurring, depending on the clinical context. Future studies will be needed to determine the relative contribution of hemophagocytosis/CAI to otherwise unexplained acute inflammation-associated cytopenias in human patients. Second, although we have demonstrated an essential role for IFN-γ in our studies, this does not rule out a potential role for other inflammatory cytokines or mediators in acute inflammation-associated cytopenias. Indeed, Milner et al. (2010) have demonstrated that IL-4 exposure can drive hemophagocytosis and mild anemia in experimental animals. Although we have assessed IFN-γ’s role in diverse infections (viral and parasitic), future studies examining other infections or inflammatory contexts (e.g., bacterial pathogens) will be needed to define the uniqueness of IFN-γ for promoting CAI. Third, although we conclude that hemophagocytosis is an essential part of IFN-γ–driven CAI, it remains possible that other IFN-γ–driven processes are contributing to the observed anemia. We conclude that hemophagocytosis is causal for CAI based on three lines of data: (1) these processes have the common mechanism that both are abolished in the absence of macrophage IFN-γ signaling; (2) hemophagocytosis correlates kinetically with anemia, preceding it by 1 d and continuing during its development; and (3) a massive number of RBCs are eliminated (∼1010) within 5 d of IFN-γ infusion and yet the only trace of this consumptive process is found intracellularly, within macrophages. Although hemophagocytosis is clearly important for the development of CAI, these data do not formally exclude the possibility that other IFN-γ–driven macrophage-mediated processes may also contribute to CAI.
A final caveat for interpreting our data are that even though our flow cytometric assays reveal a global up-regulation of RBC uptake, the histological appearance of hemophagocytosis is notably variable in human patients with HLH (or acute inflammation-associated cytopenias). In the case of HLH, this may be a result of specific aspects of disease development. It is commonly reported that hemophagocytosis is variably found at the onset of clinical disease but is readily demonstrated at later time points during active disease (Henter et al., 1998). Furthermore, the variable prevalence of hemophagocytosis may be related to sampling error or limitations of clinical sampling. Spleen tissue is rarely available for clinical assessment but appears to be the best tissue to examine in experimental animals. Our data also point to a third potential explanation for the variable clinical and experimental prevalence of hemophagocytosis; histologically demonstrable hemophagocytosis may only be the tip of an iceberg of cellular engulfment. In our controlled experimental studies, we find that most splenic macrophages acquire RBC antigens after IFN-γ infusion. When examined microscopically, most of these same cells appear to be laden with unidentifiable debris, whereas a subset contain probable RBCs and only a fraction contain clearly recognizable RBCs or nucleated cells (Fig. 2 and not depicted). This finding suggests that variations in breakdown of engulfed cells may play a major role in the histological phenomenon of hemophagocytosis.
This study has significant implications for the development of new translational therapies and for the deeper understanding of macrophage biology, immune-mediated pathologies, and hematologic processes. First, these studies provide a clear rationale for developing therapies that directly target IFN-γ–activated macrophages in patients with HLH and those with acute inflammation-associated cytopenias. Although inflammatory macrophages have been hypothesized to be important for the development of HLH and similar acute inflammatory pathologies (Abshire, 1996), their role has not been previously demonstrated. This study demonstrates, for the first time, the causal role of IFN-γ–activated hemophagocytic macrophages in driving cytopenias. Because this IFN-γ–driven pathological process occurs in both mutant and normal mice, it appears to be a pathophysiologic process that can be triggered in any individual with sufficient immune activation. The broader relevance of these findings is underscored by the fact that in some case series, nearly one third of patients admitted to hospital intensive care units with sepsis have unexplained cytopenias at presentation (Bateman et al., 2008), and such critically ill patients with early onset cytopenias or marrow hemophagocytosis have been found to have inferior survival rates (Strauss et al., 2004). Thus, this study provides a rationale for assessing hemophagocytosis in these various disease states and targeting this process for potential therapeutic benefit.
Second, these studies have significant implications for a better understanding of how IFN-γ influences macrophage biology. Although IFN-γ has for many years been described as a classical activator of macrophages, our data suggest that prolonged in vivo exposure triggers an additional unique and unexpected phenotype. Although hemophagocytosis was not triggered by a single dose of IFN-γ, continuous exposure over 24 h led to widespread uptake of RBCs. Although these kinetics will need to be studied in further detail, they suggest that new gene expression or other complex feedback mechanisms (Hu and Ivashkiv, 2009) may be driving the hemophagocytic response. Future studies will be needed to better define such processes. The current studies also have significant implications for better understanding of how inflammation influences apoptotic cell uptake by macrophages. The process of hemophagocytosis bears similarities to efferocytosis (uptake of apoptotic cells) in that they are both macropinocytic processes. Also, because IFN-γ has recently been demonstrated to increase apoptotic cell uptake by macrophages (Fernandez-Boyanapalli et al., 2010), it is possible that IFN-γ plays a significant role in altering apoptotic cell uptake in inflammatory environments. Although the proximal signals triggering cell uptake during hemophagocytosis are not known, engulfment is generally limited by “don’t eat me” signals that need to be actively circumvented or outweighed by “eat me” signals such as opsonization or phosphatidylserine exposure on the target cell (Oldenborg et al., 2000; Gardai et al., 2005). Induction of hemophagocytosis may involve alterations of macrophage receptiveness to don’t eat me signals, which may also alter apoptotic cell uptake. Future studies examining these signals, as well as macrophage membrane/cytoskeletal dynamics (such as microtubule formation; Binker et al., 2007) may deepen our understanding of the hemophagocytic response and other fundamental biological processes.
Third, the current study provides significant new insight into understanding how inflammation drives destructive pathologies. The current study has defined a new form of immune-mediated pathology, CAI, and defined its critical underlying mediators, signaling mechanisms, and cell types. Although our studies illustrate the pathological potential of IFN-γ–driven up-regulation of macrophage pinocytosis and hemophagocytosis, future studies may define the nonpathological role of this process in the immune response. IFN-γ generally acts locally (not as a hormone) and is secreted directionally from T cells toward interacting cells (Schroder et al., 2004; Huse et al., 2006). This cytokine may commonly be sustained at high levels in inflammatory microenvironments (undoubtedly above the threshold level for CAI we defined with systemic infusion), triggering localized changes in macrophage endocytosis. Perhaps only when such levels are sustained systemically, as occurs with certain infections or in individuals with HLH, the process becomes pathological. Although IFN-γ appears to be uniquely capable of driving CAI (Jordan et al., 2004), further study will be required to determine whether other inflammatory cytokines may have similar effects or whether they may modify the effects of IFN-γ. However, because IFN-γ is both necessary for induction of anemia in the context of acute infection and sufficient to induce anemia (and other cytopenias) in uninfected animals, this cytokine may be contributing to pathological cytopenias in a variety of clinical conditions.
Finally, these studies have increased our understanding of hematologic dynamics. CAI is a unique process that is distinct from the anemia of chronic inflammation because the former is an acute and consumptive process, whereas the latter involves alterations of iron metabolism and chronic suppression of hematopoiesis (Agarwal and Prchal, 2009). Even though IFN-γ may suppress hematopoiesis in certain contexts, our studies do not suggest that marrow suppression plays a significant role in the cytopenias we observed after short-term IFN-γ exposure. The brisk reticulocytosis we observed after 5 d of IFN-γ infusion also suggests that significant hemophagocytosis may be occurring more commonly than is appreciated in various clinical contexts. Gene expression studies examining peripheral blood from patients with HLH or those with systemic onset juvenile idiopathic arthritis have revealed a unique primitive erythropoietic signature (Hinze et al., 2010; Sumegi et al., 2011).This finding suggests that significant compensation is occurring for (sometimes occult) hemophagocytosis. Over 60 yr ago, Alexander et al. (1956) found that red blood cell life spans were significantly shortened in patients with rheumatoid arthritis. Remarkably, this shortened half-life correlated with a marker of systemic inflammation (erythrocyte sedimentation rate), both among patients and across time in individual patients. Although the anemia of chronic disease may also play a role in this patient population, such findings and our current data suggest that old assumptions about the physiology and pathology of the hematopoietic system should be reexamined.
MATERIALS AND METHODS
Mice
All mouse experiments were approved by the Cincinnati Children’s Hospital Medical Center International Animal Care and Use Committee. All mice were on a C57BL/6 (WT) background. WT, Irf1−/−, Prf1−/−, Ifng−/−, Nos2−/−, and Cybb−/− mice were obtained from The Jackson Laboratory. MIIG mice were described previously (Lykens et al., 2010). Fcer1g−/− mice were obtained from Taconic. Stat1−/− mice are “Poison” mice (ENU mutagenesis-derived mutant with complete loss of STAT1 function) and were a gift from K. Hoebe (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH).
Antibodies
In vivo antibodies.
TER-119 and 34-3C (anti-RBC antibodies; Fossati-Jimack et al., 1999), 20LC (isotype), and XMG1.2 (anti–IFN-γ–neutralizing antibody) were purified from hybridoma supernatants.
Flow cytometry.
F4/80-bio (clone bm8) and streptavidin-PerCP (BioLegend), streptavidin–Pacific blue (Invitrogen), TER-119-A647, and 20LC-A647 were labeled with Alexa Fluor 647 per the manufacturer’s protocol (Invitrogen). Hybridomas were gifts from E. Janssen (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) and S. Izui (University of Geneva, Geneva, Switzerland).
In vivo infection models
LCMV propagated in BHK21 cells was titered using a standard plaque assay (Hildeman et al., 1997). For infection, mice were injected i.p. with 200 PFU LCMV-WE. T. gondii (ME49 strain) infections were produced by injecting 50 cysts i.p. (purified from the brains of chronically infected mice). 0.5 mg of either 20LC (isotype control) or XMG1.2 (IFN-γ–neutralizing antibody) were injected i.p. T. gondii was a gift from A. Dias and J. Aliberti (both at Cincinnati Children’s Hospital Medical Center, Cincinnati, OH).
Infusion of IFN-γ and induction of anemia
Infusion of IFN-γ (PeproTech) was achieved by filling 3- or 7-d ALZET osmotic pumps (DURECT) with IFN-γ per the manufacturer’s instructions and placing them subcutaneously on the back of mice. Mice were bled <50 µl by tail every 2–3 d of infusion to measure serum levels of IFN-γ by standard ELISA techniques using anti–IFN-γ antibodies purified from hybridoma supernatants. As an alternative method for inducing anemia, 40–100 µg of TER-119/mouse (Fig. 3) or 150 µg of 34-3C/mouse (Fig. 2) were injected i.p. Mice were retroorbitally bled for complete blood count (CBC) analysis using a dedicated veterinary CBC machine (Hemavet950FS; Drew Scientific). In all experiments, RBC numbers correlated with hemoglobin levels. No mouse was bled more than once for CBC tests. Serum LDH and total and direct bilirubin were measured by the Cincinnati Children’s Hospital Clinical Research Laboratory using a commercial blood chemistry analyzer. Complement-mediated hemolysis was induced by injecting 0.3 ml i.v. of freshly collected human serum in to mice and waiting 30 min before collecting blood.
Tissue cell counts and spleen weights
Mice were weighed before pump placement, and then spleens were weighed after 5 d of control or IFN-γ infusion and calculated as a percentage of initial body weight. Bone marrow was flushed out of one tibia and femur, combined, and counted on a Coulter counter to determine cellularity.
Coombs: assessment of autoantibodies in mice
RBCs from mice given control or IFN-γ infusion for 5 d, RBCs from naive mice incubated in 50% serum from the infused mice, or naive RBCs incubated with different amounts of mouse anti–mouse RBC autoantibody to create a positive control curve were each incubated for 30 min at 4°C and then stained with pan anti–mouse IgG-FITC (Invitrogen) and analyzed on a flow cytometer.
Flow cytometry
Quantifying endocytosis.
After IFN-γ infusion, infection, or opsonizing antibody injection, spleen cells were first disassociated in collagenase (Liberase CI; Roche), incubated at 37°C for 20 min with agitation, and then incubated with 20 mM EDTA at 4°C and forced through a 100-mm filter, followed by fixation with 2% paraformaldehyde. Cells were stained with F4/80 and surface RBC antigen was blocked with unlabeled TER-119 (at 10× staining concentration). Cells were then permeabilized with 0.03% saponin and stained intracellularly for TER-119.
Blood from untreated mice was labeled with PKH26 (Sigma-Aldrich) as per the manufacturer’s protocol. 1010 labeled cells were injected i.v. into an untreated mouse for 1–3 d so damaged cells could be remodeled in vivo. These mice were then bled, and 109 RBCs were injected into control or IFN-γ–infused mice i.v. along with a fluid-phase marker (150 µg bovine serum albumin; Thermo Fisher Scientific) that had been conjugated to Alexa Fluor 647 (Invitrogen). 24 h later, spleen cells were processed by collagenasing as described in the previous paragraph and stained with F4/80 for flow cytometric analysis.
Up to 106 cells were acquired on the FACSCalibur using Cell Quest Pro software (BD) and analyzed using FCS Express (De Novo Software,). Isotype staining values (or percentages) were subtracted from TER-119 or PKH26 values (or percentages) for each condition assayed. Cells from collagenased spleens of control or IFN-γ–infused mice were stained intracellularly with clone NIMPR14 (a gift from S. Divanovic, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) to detect neutrophil antigen.
F-actin, dextran, or CD68 flow cytometric assays.
F-actin assembly was assessed by flow cytometric intracellular staining with phalloidin–Alexa Fluor 647 (Invitrogen). 100 µg Dextran (500 kD molecular mass; Invitrogen) conjugated to Alexa Fluor 647 was injected into mice 24 h after the start of IFN-γ or control infusion. Spleens were assessed 24 h later for uptake of dextran within the F4/80 population. Anti-CD68 antibody (AbD Serotec) was used to assess the percentage of macrophages in the bone marrow of mice given control or IFN-γ infusion.
Brightfield microscopy and histology
Cytospins.
RBCs or peritoneal cells or F4/80hi cells sorted by FACS (from bone marrow, liver, or spleen) from mice that received 3 d of control or IFN-γ infusion were cytospun onto slides and stained with H&E.
Tissue sections.
5-µm paraffin-embedded sections from 3 or 5 d after control or IFN-γ infusion from liver, bone marrow, and spleen were either stained with H&E or immunohistochemistry was performed as follows. Sections on slides were dehydrated and underwent antigen retrieval with 10 min of boiling in sodium citrate buffer, pH 6.0. Sections were blocked with goat serum and incubated with biotinylated anti-F4/80 (clone BM8; BioLegend), followed by incubation with goat anti-biotin secondary. Sections were then developed with DAB (brown color). Sections were incubated in an avidin/biotin block, followed by blocking with goat serum, and then incubated with rat anti-RBC antibody (cone TER-119; purified from hybridoma supernatants). This was followed by incubation with secondary antibody, as before, developed with VIP (purple color), and then stained with hematoxylin. All reagents for immunohistochemistry except for the primary antibodies were obtained from Vector Laboratories. Images were taken with either an AxioCam MRC-5 camera and AxioPlan2 microscope or an AxioCamICc3 camera on the Imager Z.1 microscope and analyzed with AxioVision 4.7 software (Carl Zeiss).
Quantitative fluorescent microscopy: ImageStream analysis
RBCs were obtained from untreated mice. Opsonized RBCs were incubated before injection for 45 min with 500 ng TER-119/107 RBCs. Apoptotic cells were the B0-97.10 T cell hybridoma cell line irradiated with UV light and incubated for 1 h at 37°C to achieve at least 40–50% annexin V (BD)–positive 7AAD (Sigma-Aldrich)-negative cells. 107 pregroomed PKH26-labeled RBCs (with or without opsonization) or 3 × 106 PKH26-labeled apoptotic cells were injected i.p into mice, along with 15 µg of the hydrazide salt of Alexa Fluor 647 (fluid-phase marker; Invitrogen). 45 min later, peritoneal cavities were lavaged. RBCs were lysed and remaining cells were stained with F4/80. 2 × 105 cells were acquired on the ImageStream machine using the IDEAS software (Amnis Corporation) and analyzed using the ImageStream INSPIRE software. Single focused F4/80+, PKH+, and A647+ cells with endosomes of a minimum PKH intensity were assessed. Colocalization of fluid-phase and ingested cells was determined by analyzing the image of the RBC-containing phagosome (PKH minimum intensity mask, expanded 1 pixel) and measuring the median intensity of A647 signal.
Statistical analysis
All studies were repeated at least twice with consistent results and with a minimum of three mice per group, although typically more (as indicated in figure legends). All p-values were calculated using a two-tailed Student’s t test unless otherwise noted.
Online supplemental material
Fig. S1 shows CBC indices and RBC morphology after IFN-γ infusion. Fig. S2 shows that IFN-γ–infused mice do not show evidence of a hemolytic process. Fig. S3 shows changes in morphology and cellularity of tissues after IFN-γ infusion. Fig. S4 shows hemophagocytic macrophages from bone marrow, spleen, and liver, as well as macrophage intracellular staining for neutrophil antigen after IFN-γ infusion. Fig. S5 shows that IFN-γ infusion does not increase the uptake of dextran by splenic macrophages. Fig. S6 is a summary diagram of the mechanisms of IFN-γ–induced hemophagocytosis and CAI. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20102538/DC1.
Acknowledgments
We would like to thank A. Dias and J. Aliberti for providing T. gondii, K. Hoebe for the STAT-1−/− mice, E. Janssen and S. Izui for supplying hybridomas, T. Willson for help with the immunohistochemistry, K. Risma for thoughtful discussion, and S. Divanovic for the NIMPR14 antibody.
This work is supported by NIH R01 HL091769 (M.B. Jordan), NIH R01 GM61031 (P.M. Henson), and a grant from the Histiocytosis Association of America.
The authors declare they have no competing financial interests.
E.E. Zoller designed and performed experiments and wrote the manuscript. C.E. Terrell and J.E. Lykens performed experiments. J. Aliberti contributed reagents and assisted with experimental design. A.H. Filipovich and P.M. Henson assisted with experimental design and manuscript editing. M.B. Jordan designed and performed experiments and wrote the manuscript.
Footnotes
Abbreviations used:
- CAI
- consumptive anemia of inflammation
- CBC
- complete blood count
- HLH
- hemophagocytic lymphohistiocytosis
- IRF-1
- IFN regulatory factor 1
- LCMV
- lymphocytic choriomeningitis virus
- LDH
- lactate dehydrogenase
- MIIG
- macrophages insensitive to IFN-γ
References
- Abshire T.C. 1996. The anemia of inflammation. A common cause of childhood anemia. Pediatr. Clin. North Am. 43:623–637 10.1016/S0031-3955(05)70425-9 [DOI] [PubMed] [Google Scholar]
- Agarwal N., Prchal J.T. 2009. Anemia of chronic disease (anemia of inflammation). Acta Haematol. 122:103–108 10.1159/000243794 [DOI] [PubMed] [Google Scholar]
- Agarwal S., Narayan S., Sharma S., Kahkashan E., Patwari A.K. 2006. Hemophagocytic syndrome associated with visceral leishmaniasis. Indian J. Pediatr. 73:445–446 10.1007/BF02758574 [DOI] [PubMed] [Google Scholar]
- Alexander W.R., Richmond J., Roy L.M., Duthie J.J. 1956. Nature of anaemia in rheumatoid arthritis. II. Survival of transfused erythrocytes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 15:12–20 10.1136/ard.15.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyere M., Mettlen M., Van Der Smissen P., Platek A., Payrastre B., Veithen A., Courtoy P.J. 2002. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int. J. Med. Microbiol. 291:487–494 10.1078/1438-4221-00157 [DOI] [PubMed] [Google Scholar]
- Ando M., Miyazaki E., Hiroshige S., Ashihara Y., Okubo T., Ueo M., Fukami T., Sugisaki K., Tsuda T., Ohishi K., et al. 2006. Virus associated hemophagocytic syndrome accompanied by acute respiratory failure caused by influenza A (H3N2). Intern. Med. 45:1183–1186 10.2169/internalmedicine.45.1736 [DOI] [PubMed] [Google Scholar]
- Ballin A., Lotan A., Serour F., Ovental A., Boaz M., Senecky Y., Rief S. 2009. Anemia of acute infection in hospitalized children-no evidence of hemolysis. J. Pediatr. Hematol. Oncol. 31:750–752 10.1097/MPH.0b013e3181b79696 [DOI] [PubMed] [Google Scholar]
- Bateman S.T., Lacroix J., Boven K., Forbes P., Barton R., Thomas N.J., Jacobs B., Markovitz B., Goldstein B., Hanson J.H., et al. ; Pediatric Acute Lung Injury and Sepsis Investigators Network 2008. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am. J. Respir. Crit. Care Med. 178:26–33 10.1164/rccm.200711-1637OC [DOI] [PubMed] [Google Scholar]
- Behrens E.M., Beukelman T., Paessler M., Cron R.Q. 2007. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J. Rheumatol. 34:1133–1138 [PubMed] [Google Scholar]
- Binker M.G., Zhao D.Y., Pang S.J., Harrison R.E. 2007. Cytoplasmic linker protein-170 enhances spreading and phagocytosis in activated macrophages by stabilizing microtubules. J. Immunol. 179:3780–3791 [DOI] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J.C. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749–795 10.1146/annurev.immunol.15.1.749 [DOI] [PubMed] [Google Scholar]
- Brown T.D., Goodman P.J., Fleming T., Macdonald J.S., O’Rourke T., Taylor S.A., Neefe J.R., Gaynor E. 1991. Phase II trial of recombinant DNA gamma-interferon in advanced colorectal cancer: a Southwest Oncology Group study. J. Immunother. 10:379–382 10.1097/00002371-199110000-00011 [DOI] [PubMed] [Google Scholar]
- Cassatella M.A., Hartman L., Perussia B., Trinchieri G. 1989. Tumor necrosis factor and immune interferon synergistically induce cytochrome b-245 heavy-chain gene expression and nicotinamide-adenine dinucleotide phosphate hydrogenase oxidase in human leukemic myeloid cells. J. Clin. Invest. 83:1570–1579 10.1172/JCI114054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S.D., Schmid S.L. 2003. Regulated portals of entry into the cell. Nature. 422:37–44 10.1038/nature01451 [DOI] [PubMed] [Google Scholar]
- Corwin H.L., Gettinger A., Pearl R.G., Fink M.P., Levy M.M., Abraham E., MacIntyre N.R., Shabot M.M., Duh M.S., Shapiro M.J. 2004. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit. Care Med. 32:39–52 10.1097/01.CCM.0000104112.34142.79 [DOI] [PubMed] [Google Scholar]
- Deguchi M., Sakuta H., Uno K., Inaba K., Muramatsu S. 1995. Exogenous and endogenous type I interferons inhibit interferon-gamma-induced nitric oxide production and nitric oxide synthase expression in murine peritoneal macrophages. J. Interferon Cytokine Res. 15:977–984 10.1089/jir.1995.15.977 [DOI] [PubMed] [Google Scholar]
- Dror N., Alter-Koltunoff M., Azriel A., Amariglio N., Jacob-Hirsch J., Zeligson S., Morgenstern A., Tamura T., Hauser H., Rechavi G., et al. 2007. Identification of IRF-8 and IRF-1 target genes in activated macrophages. Mol. Immunol. 44:338–346 10.1016/j.molimm.2006.02.026 [DOI] [PubMed] [Google Scholar]
- Fernandez-Boyanapalli R., McPhillips K.A., Frasch S.C., Janssen W.J., Dinauer M.C., Riches D.W., Henson P.M., Byrne A., Bratton D.L. 2010. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-γ in a nitric oxide-dependent manner. J. Immunol. 185:4030–4041 10.4049/jimmunol.1001778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovich A.H. 2008. Hemophagocytic lymphohistiocytosis and other hemophagocytic disorders. Immunol. Allergy Clin. North Am. 28:293–313 10.1016/j.iac.2008.01.010 [DOI] [PubMed] [Google Scholar]
- Fossati-Jimack L., Reininger L., Chicheportiche Y., Clynes R., Ravetch J.V., Honjo T., Izui S. 1999. High pathogenic potential of low-affinity autoantibodies in experimental autoimmune hemolytic anemia. J. Exp. Med. 190:1689–1696 10.1084/jem.190.11.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai S.J., McPhillips K.A., Frasch S.C., Janssen W.J., Starefeldt A., Murphy-Ullrich J.E., Bratton D.L., Oldenborg P.A., Michalak M., Henson P.M. 2005. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 123:321–334 10.1016/j.cell.2005.08.032 [DOI] [PubMed] [Google Scholar]
- Gardai S.J., Bratton D.L., Ogden C.A., Henson P.M. 2006. Recognition ligands on apoptotic cells: a perspective. J. Leukoc. Biol. 79:896–903 10.1189/jlb.1005550 [DOI] [PubMed] [Google Scholar]
- Henter J.I., Elinder G., Söder O., Hansson M., Andersson B., Andersson U. 1991. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 78:2918–2922 [PubMed] [Google Scholar]
- Henter J.I., Aricò M., Elinder G., Imashuku S., Janka G. 1998. Familial hemophagocytic lymphohistiocytosis. Primary hemophagocytic lymphohistiocytosis. Hematol. Oncol. Clin. North Am. 12:417–433 10.1016/S0889-8588(05)70520-7 [DOI] [PubMed] [Google Scholar]
- Hildeman D., Yañez D., Pederson K., Havighurst T., Muller D. 1997. Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. J. Virol. 71:9672–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze C.H., Fall N., Thornton S., Mo J.Q., Aronow B.J., Layh-Schmitt G., Griffin T.A., Thompson S.D., Colbert R.A., Glass D.N., et al. 2010. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res. Ther. 12:R123 10.1186/ar3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P.R., deCathelineau A.M., Ogden C.A., Leverrier Y., Bratton D.L., Daleke D.L., Ridley A.J., Fadok V.A., Henson P.M. 2001. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155:649–659 10.1083/jcb.200108080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S.M., Chang S.C. 2006. Insufficient perforin expression in CD8+ T cells in response to hemagglutinin from avian influenza (H5N1) virus. J. Immunol. 176:4530–4533 [DOI] [PubMed] [Google Scholar]
- Hu X., Ivashkiv L.B. 2009. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 31:539–550 10.1016/j.immuni.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M., Lillemeier B.F., Kuhns M.S., Chen D.S., Davis M.M. 2006. T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol. 7:247–255 10.1038/ni1304 [DOI] [PubMed] [Google Scholar]
- Imashuku S., Hibi S., Fujiwara F., Ikushima S., Todo S. 1994. Haemophagocytic lymphohistiocytosis, interferon-gamma-naemia and Epstein-Barr virus involvement. Br. J. Haematol. 88:656–658 10.1111/j.1365-2141.1994.tb05095.x [DOI] [PubMed] [Google Scholar]
- Imashuku S., Hibi S., Tabata Y., Sako M., Sekine Y., Hirayama K., Sakazaki H., Maeda N., Kito H., Shichino H., Mugishima H. 1998. Biomarker and morphological characteristics of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis. Med. Pediatr. Oncol. 31:131–137 [DOI] [PubMed] [Google Scholar]
- Imran M.N., Leng P.H., Yang S., Kurup A., Eng P. 2005. Early predictors of mortality in pneumococcal bacteraemia. Ann. Acad. Med. Singapore. 34:426–431 [PubMed] [Google Scholar]
- Ino Y., Sato T., Suzuki S., Iwaki M., Yoshikawa T. 1987. Inhibitory effects of FUT-175, a new synthetic protease inhibitor, on intravascular hemolysis by human serum in mice. Int. J. Immunopharmacol. 9:533–537 10.1016/0192-0561(87)90120-2 [DOI] [PubMed] [Google Scholar]
- Ishikawa-Sekigami T., Kaneko Y., Okazawa H., Tomizawa T., Okajo J., Saito Y., Okuzawa C., Sugawara-Yokoo M., Nishiyama U., Ohnishi H., et al. 2006. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 107:341–348 10.1182/blood-2005-05-1896 [DOI] [PubMed] [Google Scholar]
- Ito S., Takada N., Ozasa A., Hanada M., Sugiyama M., Suzuki K., Nagae Y., Inagaki T., Suzuki Y., Komatsu H. 2006. Secondary hemophagocytic syndrome in a patient with methicillin-sensitive Staphylococcus aureus bacteremia due to severe decubitus ulcer. Intern. Med. 45:303–307 10.2169/internalmedicine.45.1535 [DOI] [PubMed] [Google Scholar]
- Janka G.E. 2007. Familial and acquired hemophagocytic lymphohistiocytosis. Eur. J. Pediatr. 166:95–109 10.1007/s00431-006-0258-1 [DOI] [PubMed] [Google Scholar]
- Janka G., zur Stadt U. 2005. Familial and acquired hemophagocytic lymphohistiocytosis. Hematology (Am Soc Hematol Educ Program). 2005:82–88 [DOI] [PubMed] [Google Scholar]
- Jordan M.B., van Rooijen N., Izui S., Kappler J., Marrack P. 2003. Liposomal clodronate as a novel agent for treating autoimmune hemolytic anemia in a mouse model. Blood. 101:594–601 10.1182/blood-2001-11-0061 [DOI] [PubMed] [Google Scholar]
- Jordan M.B., Hildeman D., Kappler J., Marrack P. 2004. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 104:735–743 10.1182/blood-2003-10-3413 [DOI] [PubMed] [Google Scholar]
- Kato T., Kitaura M., Inaba K., Watanabe Y., Kawade Y., Muramatsu S. 1989. Suppression of macrophage Ia antigen expression by endogenous interferon-alpha/beta. J. Interferon Res. 9:393–405 10.1089/jir.1989.9.393 [DOI] [PubMed] [Google Scholar]
- Kempe D.S., Lang P.A., Duranton C., Akel A., Lang K.S., Huber S.M., Wieder T., Lang F. 2006. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 20:368–370 [DOI] [PubMed] [Google Scholar]
- Kuebler J.P., Goodman P.J., Brown T.D., Crawford E.D., Reitz C.L., Knight W.A., III, Kish J.A. 1990. Phase II study of continuous infusion recombinant gamma interferon in renal carcinoma. A Southwest Oncology Group study. Invest. New Drugs. 8:307–309 10.1007/BF00171843 [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Quesada J.R., Rosenblum M.G., Sherwin S.A., Gutterman J.U. 1986. Phase I study of i.v. administered recombinant gamma interferon in cancer patients. Cancer Treat. Rep. 70:1357–1364 [PubMed] [Google Scholar]
- Lin A.A., Tripathi P.K., Sholl A., Jordan M.B., Hildeman D.A. 2009. Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J. Virol. 83:8604–8615 10.1128/JVI.02477-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykens J.E., Terrell C.E., Zoller E.E., Divanovic S., Trompette A., Karp C.L., Aliberti J., Flick M.J., Jordan M.B. 2010. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J. Immunol. 184:877–885 10.4049/jimmunol.0902346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano B.E., Wesley R., De Carlo E.S., Anderson V.L., Barnhart L.A., Darnell D., Malech H.L., Gallin J.I., Holland S.M. 2004. Long-term interferon-gamma therapy for patients with chronic granulomatous disease. Clin. Infect. Dis. 39:692–699 10.1086/422993 [DOI] [PubMed] [Google Scholar]
- Milner J.D., Orekov T., Ward J.M., Cheng L., Torres-Velez F., Junttila I., Sun G., Buller M., Morris S.C., Finkelman F.D., Paul W.E. 2010. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 116:2476–2483 10.1182/blood-2009-11-255174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarky I.K., Szaba F.M., Kummer L.W., Wilhelm L.B., Parent M.A., Johnson L.L., Smiley S.T. 2007. Gamma interferon suppresses erythropoiesis via interleukin-15. Infect. Immun. 75:2630–2633 10.1128/IAI.01836-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano L.M., Kurek S., Luchette F.A., Corwin H.L., Barie P.S., Tisherman S.A., Hebert P.C., Anderson G.L., Bard M.R., Bromberg W., et al. ; American College of Critical Care Medicine of the Society of Critical Care Medicine; Eastern Association for the Surgery of Trauma Practice Management Workgroup 2009. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit. Care Med. 37:3124–3157 10.1097/CCM.0b013e3181b39f1b [DOI] [PubMed] [Google Scholar]
- Ogden C.A., deCathelineau A., Hoffmann P.R., Bratton D., Ghebrehiwet B., Fadok V.A., Henson P.M. 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781–795 10.1084/jem.194.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S., Matsuzaki A., Nishizaki M., Nagashima T., Kai T., Suda M., Ueda K. 1993. Inflammatory cytokines in virus-associated hemophagocytic syndrome. Interferon-gamma as a sensitive indicator of disease activity. Am. J. Pediatr. Hematol. Oncol. 15:291–298 [PubMed] [Google Scholar]
- Ohno T., Shirasaka A., Sugiyama T., Furukawa H. 1996. Hemophagocytic syndrome induced by Plasmodium falciparum malaria infection. Int. J. Hematol. 64:263–266 10.1016/0925-5710(96)00495-1 [DOI] [PubMed] [Google Scholar]
- Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. 2000. Role of CD47 as a marker of self on red blood cells. Science. 288:2051–2054 10.1126/science.288.5473.2051 [DOI] [PubMed] [Google Scholar]
- Pachlopnik Schmid J., Ho C.H., Chrétien F., Lefebvre J.M., Pivert G., Kosco-Vilbois M., Ferlin W., Geissmann F., Fischer A., de Saint Basile G. 2009. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med. 1:112–124 10.1002/emmm.200900009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A., Davì S., Pringe A.B., Pistorio A., Ruperto N., Magni-Manzoni S., Miettunen P., Bader-Meunier B., Espada G., Sterba G., et al. ; Lupus Working Group of the Paediatric Rheumatology European Society 2009. Macrophage activation syndrome in juvenile systemic lupus erythematosus: a multinational multicenter study of thirty-eight patients. Arthritis Rheum. 60:3388–3399 10.1002/art.24883 [DOI] [PubMed] [Google Scholar]
- Quesada J.R., Kurzrock R., Sherwin S.A., Gutterman J.U. 1987. Phase II studies of recombinant human interferon gamma in metastatic renal cell carcinoma. J. Biol. Response Mod. 6:20–27 [PubMed] [Google Scholar]
- Reade M.C., Weissfeld L., Angus D.C., Kellum J.A., Milbrandt E.B. 2010. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm. Med. 10:15 10.1186/1471-2466-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S., Ganz T. 2009. Animal models of anemia of inflammation. Semin. Hematol. 46:351–357 10.1053/j.seminhematol.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F.M., Cochet M.M., Fellous M. 1986. Interferon and major histocompatibility complex genes: a model to analyse eukaryotic gene regulation? Interferon. 7:47–87 [PubMed] [Google Scholar]
- Rutenfranz I., Kirchner H. 1988. Pharmacokinetics of recombinant murine interferon-gamma in mice. J. Interferon Res. 8:573–580 10.1089/jir.1988.8.573 [DOI] [PubMed] [Google Scholar]
- Schaller E., Macfarlane A.J., Rupec R.A., Gordon S., McKnight A.J., Pfeffer K. 2002. Inactivation of the F4/80 glycoprotein in the mouse germ line. Mol. Cell. Biol. 22:8035–8043 10.1128/MCB.22.22.8035-8043.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., Hume D.A. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163–189 10.1189/jlb.0603252 [DOI] [PubMed] [Google Scholar]
- Strauss R., Neureiter D., Westenburger B., Wehler M., Kirchner T., Hahn E.G. 2004. Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients—a postmortem clinicopathologic analysis. Crit. Care Med. 32:1316–1321 10.1097/01.CCM.0000127779.24232.15 [DOI] [PubMed] [Google Scholar]
- Sumegi J., Barnes M.G., Nestheide S.V., Molleran-Lee S., Villanueva J., Zhang K., Risma K.A., Grom A.A., Filipovich A.H. 2011. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. 117:e151–e160 10.1182/blood-2010-08-300046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandivier R.W., Henson P.M., Douglas I.S. 2006. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 129:1673–1682 10.1378/chest.129.6.1673 [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Baron J.F., Reinhart K., Gattinoni L., Thijs L., Webb A., Meier-Hellmann A., Nollet G., Peres-Bota D.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators 2002. Anemia and blood transfusion in critically ill patients. JAMA. 288:1499–1507 10.1001/jama.288.12.1499 [DOI] [PubMed] [Google Scholar]
- Walsh T.S., Saleh E.E. 2006. Anaemia during critical illness. Br. J. Anaesth. 97:278–291 10.1093/bja/ael189 [DOI] [PubMed] [Google Scholar]
- Zoumbos N.C., Djeu J.Y., Young N.S. 1984. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes in vitro. J. Immunol. 133:769–774 [PubMed] [Google Scholar]
- Zvulunov A., Tamary H., Gal N. 2002. Pancytopenia resulting from hemophagocytosis in malaria. Pediatr. Infect. Dis. J. 21:1086–1087 10.1097/00006454-200211000-00028 [DOI] [PubMed] [Google Scholar]