Abstract
The parasitic protozoan Leishmania donovani is the causative organism for visceral leishmaniasis (VL) which persists in the host macrophages by deactivating its signaling machinery resulting in a critical shift from proinflammatory (Th1) to an anti-inflammatory (Th2) response. The severity of this disease is mainly determined by the production of IL-12 and IL-10 which could be reversed by use of effective immunoprophylactics. In this study we have evaluated the potential of Arabinosylated Lipoarabinomannan (Ara-LAM), a cell wall glycolipid isolated from non pathogenic Mycobacterium smegmatis, in regulating the host effector response via effective regulation of mitogen-activated protein kinases (MAPK) signaling cascades in Leishmania donovani infected macrophages isolated from BALB/C mice. Ara-LAM, a Toll-like receptor 2 (TLR2) specific ligand, was found to activate p38 MAPK signaling along with subsequent abrogation of extracellular signal–regulated kinase (ERKs) signaling. The use of pharmacological inhibitors of p38MAPK and ERK signaling showed the importance of these signaling pathways in the regulation of IL-10 and IL-12 in Ara-LAM pretreated parasitized macrophages. Molecular characterization of this regulation of IL-10 and IL-12 was revealed by chromatin immunoprecipitation assay (CHIP) which showed that in Ara-LAM pretreated parasitized murine macrophages there was a significant induction of IL-12 by selective phosphorylation and acetylation of histone H3 residues at its promoter region. While, IL-10 production was attenuated by Ara-LAM pretreatment via abrogation of histone H3 phosphorylation and acetylation at its promoter region. This Ara-LAM mediated antagonistic regulations in the induction of IL-10 and IL-12 genes were further correlated to changes in the transcriptional regulators Signal transducer and activator of transcription 3 (STAT3) and Suppressor of cytokine signaling 3 (SOCS3). These results demonstrate the crucial role played by Ara-LAM in regulating the MAPK signaling pathway along with subsequent changes in host effector response during VL which might provide crucial clues in understanding the Ara-LAM mediated protection during Leishmania induced pathogenesis.
Introduction
The parasitic protozoan Leishmania donovani, the causative agent of visceral leishmaniasis (VL), resides and multiplies in host macrophages [1]. Activated macrophages eliminate the intracellular parasite [2] by initiating host-protective anti-leishmanial responses. To counteract these anti-leishmanial responses parasites deploys different immune evasion strategies to survive within the macrophages [3]. Leishmania-induced macrophage dysfunctions have been correlated mainly with depletion of microbicidal molecules (nitric oxide (NO) and reactive oxygen species) [4], [5], and altered signaling events resulting in skewing the T helper cells (Th) to disease promoting Th2 subset that consequently suppress the host protective Th1 subset [6]. The outcome of infection in leishmaniasis is mainly determined by the Th1 versus Th2 effector response and the generation of IL-12 and IL-10 by the infected macrophages is important for this decision [7].
Several immunoprophylactics have been pursued to combat VL, with varying degrees of success. Recently Arabinosylated-lipoarabinomannan (Ara-LAM), an immunomodulator has emerged as an effective immunoprophylactic tool against VL. It confers protection during leishmanial pathogenesis via Toll-like receptor 2 (TLR2) signaling mediated nuclear factor-κB (NF-κB) translocation and concomitant induction of the proinflammatory mediators [8].
In addition to NF-κB activation, TLR signaling can also activate mitogen-activated protein kinases (MAPK) signaling cascades which include extracellular signal–regulated kinase (ERKs), p38 MAPKs, and c-Jun NH2-terminal kinases (JNK) [9]. Most of the effector functions in response to extracellular cues are regulated by (MAPK) [10], [11]. The parasite-triggered reciprocal MAPK signaling via p38MAPK and ERK1/2 govern the counteracting immune response of the host cell resulting in differential expression of IL-12 and IL-10 in macrophages during Leishmania infection [12]. p38 MAPK activation results in histone modifications at the IL-12p40 promoter loci, making it more accessible for the recruitment of NF-kB leading to transcriptional induction of IL-12 [13]. In contrast, enhanced IL-10 transcription is associated with ERK1/2 activation leading to phosphorylation and acetylation of histone H3 at the IL-10 promoter loci which facilitates the binding of Signal transducer and activator of transcription 3 (STAT3) to the IL-10 promoter resulting in enhanced IL-10 transcription [14]. Moreover activated STAT3 attenuates the transcription of proinfllammatory mediators with the help of Suppressor of cytokine signaling 3 (SOCS3) inductions [15], [16], [ and 17]. Previous work from our laboratory has shown that Ara-LAM is involved in IL-12 induction and IL-10 attenuation during infection demonstrating the suitability of it as a potential candidate for immunotherapy to cure VL. But, how Ara-LAM treatment of parasitized macrophages leads to epigenetic modification at the locus of these two counteractive cytokine genes leading to their transcriptional regulation and the involvement of MAPK signaling in this regard is yet to be explored.
In the present study, we have found that Ara-LAM, a TLR-2 ligand confers protection against leishmanial pathogenesis via reciprocal regulation of MAPK signaling. This Ara-LAM mediated regulation of MAPK signaling resulted in antagonistic regulation of IL-12 and IL-10 in host macrophages. Detailed investigation at the molecular level showed that Ara-LAM could induce IL-12 by selective phosphorylation and acetylation of histone H3 residues at the IL-12p40 promoter region while attenuated IL-10 production by abrogating such histone H3 modification at IL-10 promoter in parasitized macrophages. This antagonistic regulation of effector response by Ara-LAM in the form of IL-10 and IL-12 was further linked to STAT3 and SOCS3 which were found to be crucial in regulating the host protective immune response in leishmania infected macrophages.
Results
1. ERK and p38 MAP kinases differentially regulate Ara-LAM-mediated generation of macrophage effector molecules in Leishmania donovani infected macrophages
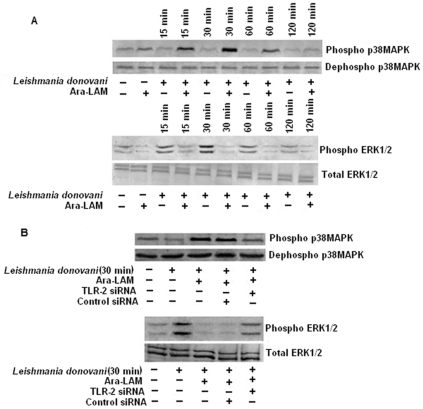
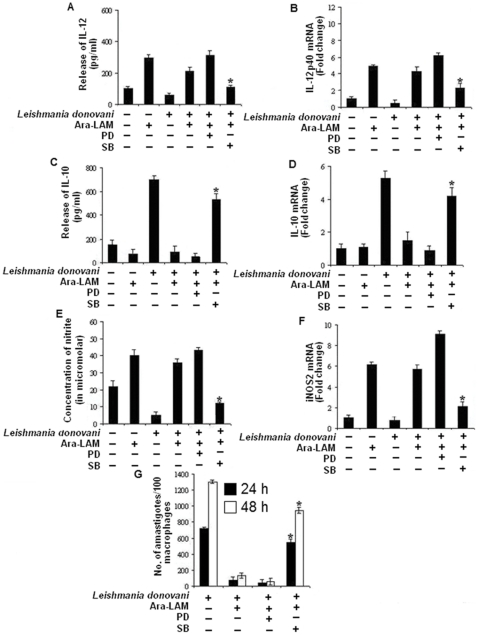
Ara-LAM has been reported to confer protection against leishmanial pathogenesis via TLR2 signaling–mediated induction of the proinflammatory response [8]. However, it is unclear whether Ara-LAM can modulate the p38 and ERK1/2 MAPK signaling molecules which play differential role in the leishmanial pathogenesis [12]. We found that at an early time point, Ara-LAM stimulated phosphorylation of p38MAPK was much higher than infected macrophages; in contrast, ERK1/2 phosphorylation was abrogated in Ara-LAM treated parasitized macrophages compared to that in infected macrophages (figure 1A ). Interestingly, gene silencing of TLR-2 in infected macrophages reverses the Ara-LAM mediated regulation of MAPK family (figure 1B ). The MAPKs are key regulators of IL-10, IL-12 generation and NO production [18], [19]; leishmanial parasite leads to impaired effector response by suppressing p38MAPK induced IL-12, NO secretion while augmenting ERK-1/2 induced IL-10 production [12]. As Ara-LAM leads to significant protection during leishmania infection via a Th1 polarized anti-parasitic response [8], we further probed Ara-LAM induced leishmanicidal activity in the presence of p38MAPK and ERK inhibitors. Interestingly, preincubation of cells with PD098059 (an ERK inhibitor) followed by Ara-LAM treatment in parasitized macrophages caused slight increase in host protective IL-12 (figure 2A, 2B ) and NO generation (figure 2E, 2F ) along with concomitant decrease in IL-10 production (figure 2C, 2D ) both at the protein and mRNA level resulting in reduction in intracellular parasite load compared to Ara-LAM treated parasitized macrophages (figure 2G ). In contrast SB203580 (p38 MAPK inhibitor) treatment of infected macrophages almost completely abrogated Ara-LAM induced generation of IL-12 (figure 2A,2B ) and NO (figure 2E,2F ) whilst enhancing IL-10 production (figure 2C,2D ) both at the protein and mRNA level resulting in drastic increase in intracellular parasite load (figure 2G ), compared to Ara-LAM treated parasitized macrophages. In contrast, treatment of cells with the drugs (PD098059 and SB203580) after adding Ara-LAM and parasite resulted in similar levels of IL-12 p40, NO and IL-10 (both at the protein as well as the mRNA level) as compared to only Ara-LAM treated parasitized macrophages (data not shown). Interestingly, PD098059 and SB203580 itself had no effect on the levels of NO, IL-12p40 and IL-10 in Leishmania infected macrophages both at the protein as well as the mRNA level (figure 2 ).
Figure 1. Ara-LAM mediated changes in MAPK signaling cascade in Leishmania donovani–infected macrophages.
Macrophages were pretreated with Ara-LAM for 3 hr or in some cases followed by Leishmania infection for 15, 30, 60, and 120 min. The cells were then lysed and subjected to Western blot with anti-pp38MAPK, p38MAPK and pERK1/2, ERK1/2, as described in Materials and Methods (A). In separate experimental sets, cells were transfected with control siRNA or TLR2-specific siRNA for 24 hr washed and then treated with Ara-LAM followed by L. donovani for 30 min. Western blot analysis was performed to analyze the expression of anti-pp38MAPK, p38MAPK and pERK1/2, ERK1/2 (B).
Figure 2. Ara-LAM mediated effector function depends on the reciprocal activation of p38MAPK and ERK–1/2.
Peritoneal macrophages (2×106cells/mL) were treated with SB203580 (SB) or PD098059 (PD) for 2 h, followed by Ara-LAM treatment for 3 hr. The cells were then infected with Leishmania parasite for 24 h and assayed for the levels of IL-12 (A) and IL-10 (C) in the culture supernatant by ELISA as described in Methods. ELISA data are expressed as means standard deviations of values from triplicate experiments that yielded similar observations. Macrophages cultured in a 24-well plate (1×106 cells/mL) were pretreated and infected as described above and assayed for the levels of extracellular NO as described in the Materials and Methods (E). Asterisks indicate statistically significant induction of nitrite generation, compared with Ara-LAM–pretreated infected macrophages. *P<.001. From a separate set of cells (2×106 cells/mL) RNA was isolated and levels of mRNA expression for IL-12p40 (B), IL-10 (D), inducible nitric oxide synthase 2 (iNOS2) (F) were determined by quantitative RT-PCR. Results are presented as changes (nfold) relative to uninfected control cells. The data represent the mean values± standard deviation of results from 3 independent experiments that all yielded similar results. In a separate experiment, the macrophages were cultured in coverglasses treated with SB or PD for 2 h, subsequently followed by 3 hr of Ara-LAM treatment and 4 h of Leishmania infection. After indicated time of incubation intracellular parasite number were assessed as described in methods. Pretreatment with SB significantly inhibited Ara-LAM –mediated parasite killing compared with levels in corresponding Ara-LAM pretreated infected controls (G). *P<.001 for SB.
2. Ara-LAM stimulation of infected macrophages resulted in histone modifications and altered transcription factor binding at the IL-10 and IL-12 promoter region via reciprocal regulation of MAPK pathway
To get the mechanistic insight by which Ara-LAM reduces IL-10 and augmentes IL-12 synthesis during leishmanial pathogenesis, we analyzed various histone modifications at the IL-10 and IL-12 locus by Chromatin immunoprecipitation assay (CHIP).
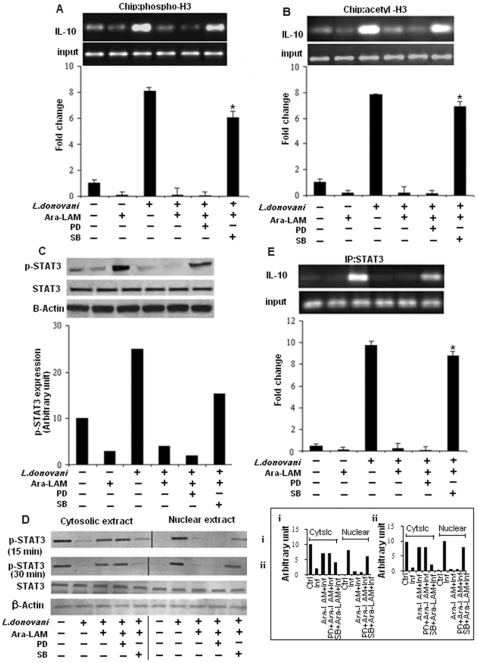
High level of IL-10 production in leishmania-infected macrophages was found to be associated with increased histone H3 phosphorylation (figure 3A ) and acetylation (figure 3B ) at the IL-10 promoter which was abrogated in Ara-LAM pretreated parasitized macrophages. Interestingly, ERK inhibition prior to Ara-LAM treatment in parasitized macrophages slightly reduced both histone H3 phosphorylation (figure 3A ) and acetylation (figure 3B ) at IL-10 locus compared to only Ara-LAM treated parasitized macrophages. In contrast there was significant increase in histone H3 phosphorylation (figure 3A ) and acetylation (figure 3B ) at IL-10 locus in parasitized macrophages treated with both p38 inhibitor SB203580 and Ara-LAM, compared to only Ara-LAM pretreated parasitized macrophages. Because STAT3 is an obligate factor required for IL-10 gene transcription [20], we explored whether Ara-LAM could modulate the STAT3 expression during leishmanial infection. Ara-LAM pretreatment of host macrophages, abrogated parasite induced phosphorylation (figure 3C ) and nuclear translocation (figure 3D ) of STAT3 at early time points. Strikingly, inhibiting ERK resulted in decreased phosphorylation (figure 3C ) and subsequent nuclear translocation (figure 3D ) of STAT3 in Ara-LAM treated parasitized macrophages while p-38 inhibition reverses the phenomena and significantly increases the nuclear translocation of phosphorylated STAT3 compared to only Ara-LAM treated parasitized macrophages (figure 3C,3D ). CHIP assays were performed to examine the binding of STAT3 to the IL-10 promoter. Unstimulated control cells exhibited virtually no binding of STAT3 to the IL-10 promoter. Ara-LAM pretreatment significantly reduced the parasite induced binding of STAT3 to the IL-10 promoter (figure 3E ). Furthermore, inhibiting ERK prior to Ara-LAM treatment in infected macrophages, caused further decrease in STAT3 binding to the IL-10 promoter while inhibition of p38 in Ara-LAM treated macrophages resulted in significant increase in the binding of STAT3 to the IL-10 promoter when compared to only Ara-LAM treated infected sets (figure 3E ).
Figure 3. Histone H3 modifications at the IL-10 promoter in Ara-LAM treated infected macrophages.
Murine macrophages (1×106 cells/mL) were treated with SB or PD for 2 h, subsequently followed by Ara-LAM treatment for 3 hr and Leishmania infection for 45 min. After 45 min of incubation, ChIP assays were conducted as described in Materials and Methods. Immunoprecipitations were performed using Abs specific to phosphorylated H3 (IP phospho-H3) (A) or IP acetyl-H3 (B), and conventional RTPCR or quantitative real-time PCR was performed using primers specific to the IL-10 promoter. *P<.001 compared with Ara-LAM–pretreated infected macrophages. In a separate experiment, the macrophages were treated as described above followed by infection with Leishmania parasite. After 24 hr of incubation, the cells were lysed and subjected to Western blot with anti-pSTAT3, and STAT3 as described in Materials and Methods (C). Peritoneal macrophages (3×106 (cells/mL) were treated and infected by Leishmania for 15, 30 min as described in the legends of figure 1 . Cytosolic and nuclear protein extracts were prepared for Western blot analysis as described in material method to analyze the nuclear translocation of pSTAT3 and STAT3 (D). The blot shown is a representative of experiments performed in triplicate. Band intensities were analyzed by densitometry (i,ii). (inset). In a separate experiment murine macrophages (1×106 cells/mL) were treated and infected with Leishmania parasite as described above. After 45 min of incubation, Immunoprecipitations were conducted using STAT3 (IP: STAT3) specific Abs. Conventional RT-PCR or quantitative real-time PCR was performed for amplifying the putative STAT3 binding sites of the IL-10 promoter. *P<.001 compared with Ara-LAM–pretreated infected macrophages.
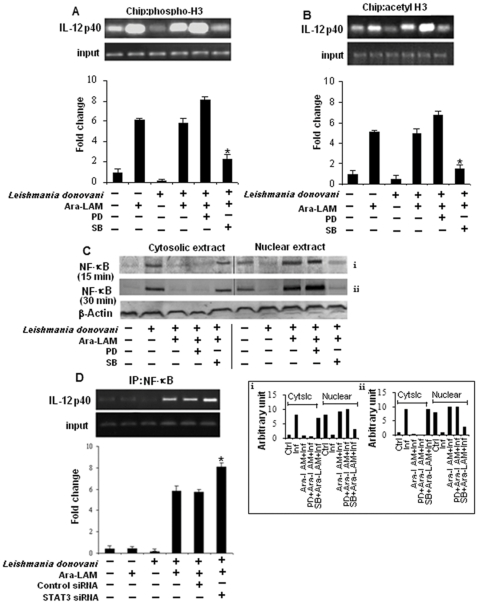
Transcriptional induction of IL-12 gene requires histone modification of the promoter region, a mast for NF-kB recruitment, which plays a pivotal role in IL-12 transcription and is regulated by STAT3 [13], [15]. CHIP assay showed infected macrophages pretreated with Ara-LAM had higher levels of phosphorylated (figure 4A ) and acetylated (figure 4B ) H3 associated with IL-12p40 promoter relative to infected macrophages. Moreover, ERK inhibition in presence of Ara-LAM caused further increase in histone H3 phosphorylation (figure 4A ) and acetylation (figure 4B ) at IL-12p40 locus while p-38 inhibition significantly attenuated such Ara-LAM mediated histone modification at this locus in infected macrophages compared to only Ara-LAM treated parasitized macrophages (figure 4A,4B ).
Figure 4. Hstone H3 modifications at the IL-12 promoter in Ara-LAM treated infected macrophages.
Murine macrophages (1×106cells/mL) were treated and infected as described in the Figure 3 legend. After 45 min of incubation, ChIP assays were conducted as described in Materials and Methods. Immunoprecipitations were performed using Abs specific to phosphorylated H3 (IP phospho-H3) (A) or IP acetyl-H3 (B), and conventional RT-PCR or quantitative real-time PCR was performed using primers specific to the IL-12p40 promoter. *P<.001 compared with Ara-LAM–pretreated infected macrophages. In a separate experiment peritoneal macrophages were treated and infected by Leishmania for 15, 30 min as described in Figure 3 legend. Cytosolic and nuclear protein extracts were analyzed for the nuclear translocation of NF-κB (C). The blot shown is a representative of experiments performed in triplicate. Band intensities were analyzed by densitometry (i,ii) (inset). Peritoneal macrophages (1×106 cells/mL) were transfected with control siRNA or STAT3-specific siRNA, subsequently followed by Ara-LAM treatment (for 3 hr) and Leishmania infection for 45 min. Immunoprecipitations were conducted using NF-κB specific Abs. Conventional RT-PCR or quantitative real-time PCR was performed for amplifying the putative NF-κB binding sites of the IL-12p40 promoter. *P<.001 compared with Ara-LAM–pretreated infected macrophages.
Ara-LAM induced nuclear translocation of NF-kB was p38 dependent as p38 inhibition showed marked reduction in the nuclear translocation of NF-kB in infected macrophages (figure 4C ). Because, STAT3 plays a decisive role in the recruitment of NF-kB to the IL-12p40 promoter region [15], we investigated Ara-LAM mediated recruitment of NF-kB to the IL-12p40 promoter region under STAT3 silenced condition. There was significant enhanced recruitment of NF-kB to the IL-12p40 promoter in STAT3 knocked down macrophages, as compared to only Ara-LAM treated parasitized macrophages (figure 4D ), thereby pointing towards the critical role of STAT3 in down-regulating IL-12 gene expression in parasitized macrophages under Ara-LAM-treatment.
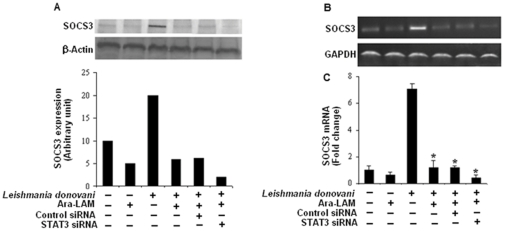
3. Ara-LAM induced attenuated SOCS3 expression is STAT3 dependent during VL
STAT3 activation plays a critical role in SOCS3 gene expression which in turn suppresses macrophage function [16]. As Ara-LAM treatment reduces the parasite induced STAT3 phosphorylation, we further studied its effect on SOCS3 expression, a downstream signaling intermediate of STAT3 pathway. Pretreatment of parasitized macrophages with Ara-LAM resulted in significant abrogation of the expression of SOCS3 both at the protein (figure 5A ) and m-RNA level (figure 5B, 5C ) compared to infected macrophages. To further examine whether Ara-LAM suppresses the expression of SOCS3 via the effective regulation of STAT3 signaling, we knock down STAT3 genes using STAT3 specific small interfering RNA (siRNA). STAT3 silencing of Ara-LAM treated parasitized macrophages resulted in higher reduction of SOCS3 expression as compared to only Ara-LAM treated parasitized macrophages which is not statistically significant. (figure 5A, 5B, and 5C ). These findings clearly suggest that Ara-LAM negatively regulates the STAT3 signaling leading to marked reduction in SOCS3 expression.
Figure 5. STAT3 silencing potentiate Ara-LAM mediated abrogation of SOCS3 expression in Leishmania-donovani infected macrophages.
Peritoneal macrophages (2×106 cells/mL) were transfected with control siRNA or STAT3-specific siRNA, subsequently followed by Ara-LAM treatment (for 3 hr) and Leishmania infection for 24 hr. The cells were then lysed and subjected to Western blot with anti-SOCS3 antibody as described in Materials and Methods (A). In a separate experiment macrophages were transfected with either control-siRNA or STAT3 specific siRNA, subsequently followed by Ara-LAM treatment for 3 hr and Leishmania infection for another 3 h. RNA was isolated and semi quantitative RT-PCR analyses for SOCS3 and GAPDH were done. Data represented here are from one of three independent experiments, all of which yielded similar results (B). Changes in expression of SOCS3 mRNA were also determined by quantitative real-time PCR. Results are presented as changes (n-fold) relative to uninfected control cells. The experiment was repeated 3 times, yielding similar results (C). *P<.001 compared with infected macrophages. The comparison of SOCS3 expression between Ara-LAM treated infected macrophages and STAT3 siRNA group did not show any statistical significance.
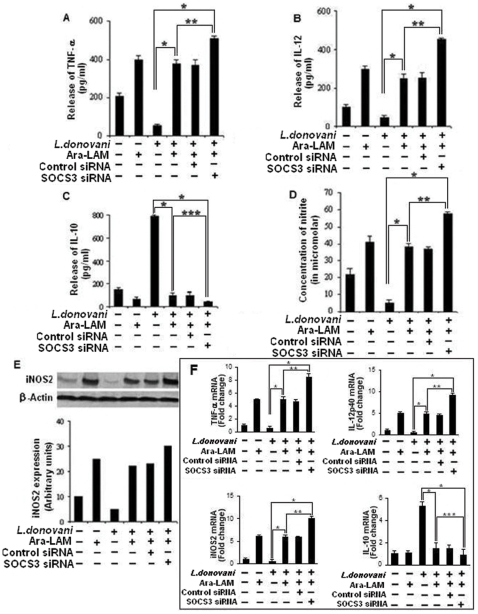
4. SOCS3 silencing significantly enhances host protective proinflammatory response and antigen presentation in Ara-LAM treated infected macrophages
Previous study with Ara-LAM showed that it could confer protection against leishmanial pathogenesis via induction of the proinflammatory response [8]. As SOCS3 has been speculated to participate in inhibition of macrophage activation [17], we tested whether SOCS3 interferes with Ara-LAM -stimulated signal transduction events in Leishmania donovani infected macrophages. We observed that SOCS3 silencing could markedly increase the Ara-LAM induced production of proinflammatory mediators such as IL-12 (figure 6B, 6F ), TNF-α, (figure 6A, 6F ) NO (figure 6D, 6E, 6F ) along with the downregulation of immunosupressive cytokine IL-10 (figure 6C, 6F ) in parasitized macrophages both at the protein and mRNA level. These results suggest that Ara-LAM leads to the production of host protective proinflammatory response by supressing SOCS3 functioning.
Figure 6. SOCS3 silencing significantly enhances host protective proinflammatory response generation by Ara-LAM in infected macrophages.
Peritoneal macrophages (2×106 cells/mL) were transfected with control siRNA or SOCS3-specific siRNA followed by Ara-LAM treatment (for 3 hr) and Leishmania infection for 24 h and assayed for the levels of TNF- α (A), IL-12 (B), and IL-10 (C) in the culture supernatant by ELISA, as described in Methods. ELISA data are expressed as means standard deviations of values from triplicate experiments that yielded similar observations. *P<.001 compared with infected macrophages, **P<.005 compared with Ara-LAM–pretreated infected macrophages. ***<.01 compared to Ara-LAM–pretreated infected macrophages. In a separate experiment macrophages were cultured and treated as described above and assayed for the levels of extracellular NO as described in the Materials and Methods (D). *P<.001 compared with infected macrophages, **P<.005 compared with Ara-LAM–pretreated infected macrophages. In a separate set macrophages were transfected and treated with Ara-LAM as described above followed by Leishmania infection for 24 hr. Western blot analysis was performed to analyze the expression of inducible nitric oxide synthase. The blot shown is a representative of experiments performed in triplicate. Band intensities were analyzed by densitometry (E). Peritoneal macrophages were cultured and treated with Ara-LAM as described above followed by Leishmania infection for 3 hr. Changes in mRNA expression of IL-12p40, TNF- α, IL-10, NO were determined by real-time PCR analysis Results are presented as changes (n-fold) relative to uninfected control cells. The experiment was repeated 3 times, yielding similar results; data are expressed as means ± standard deviations. (F). *P<.001 compared with infected macrophages, **P<.005 compared with Ara-LAM–pretreated infected macrophages.
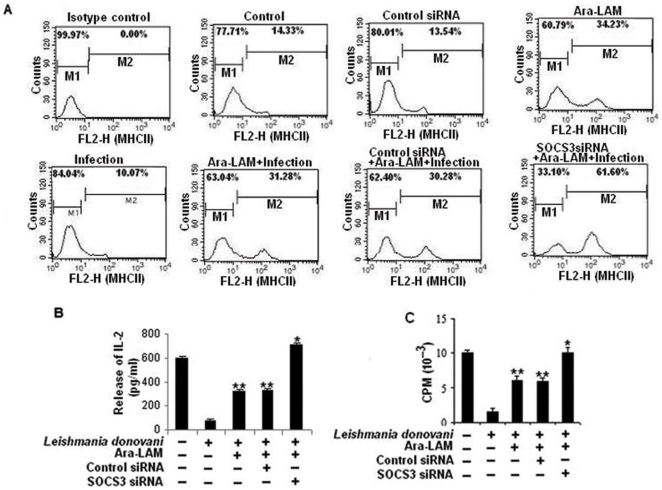
SOCS3 has also been reported to down regulate major histocompatibility -II (MHC-II) expression resulting in decreased antigen presentation by macrophages [21], a hallmark of leishmanial pathogenesis [22]. Fascinatingly, Ara-LAM treatment prominently restores MHC-II expression in L. donovani–infected macrophages (figure 7A ). We also investigated the role of Ara-LAM on the antigen presentation capability of infected macrophages by measuring their ability to activate λ peptide stimulated T cell hybridoma 9H3.5 cells in terms of IL-2 production which is important for T effector cell functioning. In case of infected macrophage, there was a significant impairment of antigen presentation compared with uninfected macrophages (figure 7C ) along with lower IL-2 secretion from T cell hybridoma 9H3.5 when co-cultured with infected macrophages (figure 7B ). Interestingly the release of IL-2 from 9H3.5 cells and antigen presentation ability could be effectively increased under Ara-LAM pretreatment in infected macrophages. (figure 7B,7C ). Moreover, parasitized macrophages in which SOCS3 was knocked down prior to Ara-LAM treatment resulted in enhanced MHC-II expression (figure 7A ) as reflected by greatly increased antigen presentation (figure 7C ) and higher IL-2 production from 9H3.5 cells (figure 7B ) demonstrating that SOCS3 interferes with antigen presentation and decreased SOCS3 was further potentiating Ara-LAM mediated antigen presentation ability of infected macrophages.
Figure 7. Ara-LAM induced MHC-II expression, antigen presentation ability of infected macrophages increased under SOCS3 silenced condition.
Peritoneal macrophages (2×106 cells/mL) were transfected with control siRNA or SOCS3-specific siRNA, subsequently followed by Ara-LAM treatment (for 3 hr) and Leishmania infection for 4 hr as mentioned above. After 24 hr of incubation treated macrophages were analyzed by flow cytometry for MHCII (FL2-H) expression as described in material method (A). Data are from 1 of 3 experiments conducted in the same way with similar results. In a separate set, normal and treated macrohages either uninfected or infected were pulsed with λR12–26, and then were incubated with T-cell hybridoma 9H 3.5. The culture supernatants were analysed for the presence of IL-2 by ELISA as described in the methods (B). Incorporation of 3H-Thymidine in the IL-2 dependent cell line HT-2 was assessed in presence of the cultured supernatant. Results are expressed as mean ± SD of 5 replicate experiments (C). *P<.001, **P<.005 compared with infected macrophages.
Discussion
Leishmania-induced deactivation of macrophage functions are due to defects in the signaling pathways, which facilitates the parasite to infect and propagate within the cell [23]. Recently the capacity of Leishmania to suppress macrophage function has been linked to alterations in MAPK signaling cascades [12], [24], [25]. To revert this parasite induced changes in MAPK signaling, novel immunomodulators are being evaluated for their functionality in VL. Ara-LAM, a potential immunomodulator strongly initiates the host protective immune response during VL via TLR-2 mediated proinflammatory responses [8]. But its role in regulating the MAPK signaling pathway has not been explored till date.
Recently it has been demonstrated that the differential activation of the MAPKs regulates cytokine production. CD40, a costimulatory molecule with host-protective anti-leishmanial function, could induce both IL-10 and IL-12 by differential activation of p38 MAPK and ERK-1/2, respectively. This reciprocal regulation of p38 MAPK and ERK1/2 has been shown to be responsible for the protection versus susceptibility to infection [12], [24].
Ara-LAM pretreatment restored the impaired p38MAPK phosphorylation in parasitized macrophages and abrogated the ERK1/2 phosphorylation via TLR-2 mediated downstream signaling (figure 1 ) thus shifting the macrophageal microenvironment from anti-inflammatory (IL-10) towards proinflammatory (IL-12) host protective immune response (figure 2 ). Our finding is consistent with other studies which have also highlighted the involvement of this novel molecule, Ara-LAM, in inducing proinflammatory responses (IL-8, TNF-α) in macrophages via interacting with TLR-2 [26], [27]. Additionally, Ara-LAM has been demonstrated as a major component responsible for the pro-inflammatory activity of the whole bacteria (M. smegmatis and M. fortuitum) [28].
Having identified the reciprocal alteration in Ara-LAM mediated production of IL-12 and IL-10 during VL, we studied whether Ara-LAM could modulate the chromatin (specifically histone H3) at the locus of these two counteractive cytokine genes since covalent modification to histones of chromatin can lead to differential gene expression. Our study revealed that Ara-LAM pretreatment leads to IL-12 induction via histone H3 phosphorylation (figure 4A ) and acetylation (figure 4B ) of chromatin at IL-12 locus along with IL-10 attenuation by abrogating the parasite induced histone H3 phosphorylation (figure 3A ) and acetylation (figure 3B ) at IL-10 locus during leishmanial pathogenesis. Thus Ara-LAM mediated gene-specific chromatin modifications are found to be associated with transient silencing of IL-10 genes and priming of the IL-12 gene.
To further ascertain the role of MAPKs in the regulation of counteracting cytokines IL-10 and IL-12, we investigated Ara-LAM mediated transcriptional alteration of these cytokine genes in the presence of their respective pharmacological inhibitors. Our results implicated that the inhibition of p38 MAPK pathway in Ara-LAM treated parasitized macrophages abrogates chromatin remodeling and nuclear translocation of NF-kB thereby attenuating IL-12 transcription (figure 4A, 4B, 4C) and simultaneously reverses Ara-LAM mediated attenuated IL-10 production in parasitized macrophages via increased histone H3 phosphorylation, acetylation and enhanced STAT3 recruitment to the IL-10 promoter leading to the induction of IL-10 transcription (figure 3A,3B,3D,3E). In contrast ERK inhibition of Ara-LAM treated parasitized macrophages leads to further increase in IL-12 transcription (figure 4A, 4B, 4C) along with significant reduction of the IL-10 (figure 3A,3B,3D,3E) compared to Ara-LAM treatment only. Therefore, this study suggested a crucial role of MAPK in Ara-LAM mediated modulation of IL-12 and IL-10 gene transcription during leishmanial pathogenesis.
The signaling pathways which direct IL-10-mediated inhibition of IL-12 production at the level of transcription is well documented [12]. Recent observations have suggested that absence of STAT3 resulted in enhanced lipopolysacharide induced IL-12p40 gene expression in IL-10_ / _ mice due to enhanced NF-κB recruitment to the IL-12p40 promoter [15]. Our observation also indicated that STAT3 silencing significantly enhances Ara-LAM mediated recruitment of NF-κB to the IL-12 p40 promoter (figure 4D ) indicating that STAT3 negatively regulates Ara-LAM mediated transcription of IL-12 gene during leishmanial pathogenesis.
Moreover, STAT3 activation leads to the induction of SOCS3, which plays a decisive role in inactivation of macrophage function [16]. SOCS3 is critical for the survival strategy of mycobacteria [29], leishmania [30], listeria [31] and RNA viruses [32] in the host macrophages. Moreover silencing of the SOCS3 gene resulted in a marked reversal of the immunosuppressive effect of IL-10 on many inflammatory mediators including cytokines, chemokines, and IFN-inducible, apoptotic and TNF ligand/receptor genes [33].
Ara-LAM treatment lead to significant reduction in SOCS3 expression by inactivation of STAT3 (figure 5A, 5B, 5C ). These changes resulted in significant induction of host protective proinflammatory responses (figure 6 ), MHC-II expression, and antigen presentation capability of infected macrophages (figure 7A, 7C ) along with enhanced T cell effector functioning as evident from higher IL-2 production from co-cultured T cells (figure 7B ). Further confirmation about the role of SOCS3 in the generation of host protective immune responses in Ara-LAM treated infected sets was done by SOCS3 silencing which was suggestive of SOCS3 hindrance in Ara-LAM mediated induction of proinflammatory response (figure 6 , 7A, 7B, 7C ).
In summary, our current findings provide a detailed molecular mechanistic insight of Ara-LAM mediated IL-12 and IL-10 production in Leishmania donovani infected macrophages where MAPK signaling via differential regulation of p38 and ERK plays an important role in generating host protective immune response during leishmanial pathogenesis.
Materials and Methods
Reagents and Chemicals
RPMI-1640 medium, M-199 medium (M199), penicillin and streptomycin, SB203580 (p38MAPK inhibitor), PD098059 (ERK inhibitor) and TRI Reagent were from Sigma (St Louis, MO, USA). Fetal calf serum (FCS) was obtained from Gibco BRL (Grand Island, NY, USA) and ELISA Assay Kit (Quantikine M) for tumour necrosis factor (TNF)-α, IL-12, IL-10 were from R&D Systems (Minneapolis, MN, USA). dNTPs, RevertAidTM M-MuLV Reverse Transcriptase, oligo dT, RNase inhibitor and other chemicals required for cDNA synthesis were from Fermentas (USA). Anti-phospho-H3 and acetyl-H3 Abs were purchased from Abcam and chromatin immunoprecipitation (ChIP) assay kits were purchased from Millipore. SOCS3, STAT3 siRNA were procured from Santa Cruze biotech.
T-cell hybridoma and lambda repressor peptide
9H3.5 (I-Ad restricted T-cell hybridoma) specific for lambda repressor N-terminal sequence 12–26 [LEDARRLKAIYEKKK, defined as λR 12–26]. T-cell hybridomas and peptides were kind gifts of Professor M. L. Gefter, Massachusetts Institute of Technology, Cambridge, MA, USA. The IL-2 dependent cell line HT-2 (mouse T helper cell line) was obtained from American Type Culture Collection. All cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 2-ME (5×10−5 M) at 5% CO2 in humidified atmosphere.
Animals and parasites
BALB/c mice were purchased from the National Centre for Laboratory Animal Sciences, India. For each experiment 8–10 mice (4–6 weeks old) were used, regardless of sex. L. donovani organisms (strain MHOM/IN/1983/AG-83) were maintained in Medium 199 (Sigma) plus 10% fetal calf serum (Gibco). Amastigotes were prepared as described elsewhere [34]. Stationary-phase promastigotes obtained by suitable transformation were used for experiments. All experimental protocols were given prior approval by the institutional animal ethics committee.
Isolation and purification of Ara-LAM
Ara-LAM was isolated as described elsewhere [35] Lipopolysaccharide contamination was checked by the Limulus test and was <25 ng/mg in Ara-LAM. The noncytotoxic dose of Ara-LAM was 3 µg/mL [36].
Peritoneal macrophage preparation
Macrophages were isolated by peritoneal lavage with ice-cold phosphate-buffered saline at 48 h after intraperitoneal injection of 1.0 mL of sterile 4% thioglycolate broth (Difco). Cells were cultured as described elsewhere [37].The adherent cell population was cultured for 48 h prior to any treatment, to achieve the resting state.
Uptake and intracellular multiplication
For assessing the activity of Ara-LAM against the amastigote stage of parasite, peritoneal macrophages cultured on glass cover slips were pretreated with Ara-LAM (3 µg/ml) for 3 h, followed by infection with L.donovani promastigotes at a ratio of 1∶10 for the indicated time periods; macrophages were then fixed and stained as described elsewhere [38] for calculation of the number of intracellular parasites. When indicated, the uptake and multiplication of L.donovani was studied in the presence and absence of the p38MAPK inhibitor SB203580 (10 µg/mL), or ERK inhibitor PD098059 (100 µmol/L; Sigma) [12].
Cytokine enzyme-linked immunosorbent assay (ELISA)
The conditioned medium of macrophage culture was assayed for mouse cytokines and chemokines with use of the sandwich ELISA kit (Quantikine M; R&D Systems). The assay was performed according to the manufacturer's instructions.
Preparation of cell lysate and immunoblot analysis
Cell lysates were prepared as described elsewhere [39]. Equal amounts of protein (50 µg) were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and were subsequently transferred to a nitrocellulose membrane. The membrane was blocked overnight with 3% bovine serum albumin in Tris-saline buffer (pH, 7.5), and immunoblotting was performed to detect Inducible Nitric Oxide Synthase 2 (iNOS2) and phosphorylated or dephosphorylated forms of p38MAPK, ERK1/2, STAT3 and β-Actin as described elsewhere [40].
Nitrite generation
Nitrite level in culture was measured using the Nitric Oxide Colorimetric Assay kit (Boehringer Mannheim Biochemicals) [41]. Cell-free supernatants were collected from different experimental sets at different time points of infection, and nitrite levels were estimated in accordance with the manufacturer's instructions. Data were expressed in micromoles of nitrite.
Antigen presentation assay
Antigen presenting ability of infected macrophages was studied by their ability to present λR12–26 to T-cell hybridoma (9H 3.5). The antigen presenting cells (APC) were incubated for 24 h with specific peptide and T-cell hybridoma in complete RPMI medium in a 37°C incubator. The culture supernatants were analysed for the presence of IL-2 by growing an IL-2 dependent cell line HT-2 in the supernatants. HT-2 (104 cells per well) was incubated with a 50% concentration of culture supernatant for 48 h. The cells were then pulsed with 1 µCi of 3H-Thymidine [6.7 Ci/mmole, New England Nuclear, Boston, NE, USA)] for the last 18 h [42]. The incorporation of radioactive thymidine was then assessed by a scintillation counter (Packard).
Flow cytometry
BALB/c-derived macrophages were stained with phycoerythrin (PE) labeled anti-MHCII antibodies. The cells were analyzed by a FACSVantage™ flow cytometer (Becton Dickinson).
Preparation of nuclear and cytoplasmic extracts
The nuclear extracts were prepared from normal and infected macrophages as described elsewhere [43]. Briefly, sedimented cells were resuspended in hypotonic buffer (10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.2 mM PMSF, and 0.5 mM DTT) and allowed to swell on ice for 10 min. Cells were homogenized in a homogenizer. The nuclei were separated by spinning at 3300×g for 5 min at 4°C. The supernatant was used as the cytoplasmic extract. The nuclear pellet was extracted in nuclear extraction buffer (20 mM HEPES (pH 7.9), 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM PMSF, and 0.5 mM DTT) for 30 min on ice and centrifuged at 12,000×g for 30 min. The supernatant was used as nuclear extract.
CHIP assay
CHIP assays were conducted using the CHIP Assay kit following the manufacturers
Protocol (Millipore). Briefly, 1×106 peritoneal macrophages were plated overnight in six-well plates. Cells were stimulated as described in figures, and then fixed for 10 min at 37°C in 1% paraformaldehyde. Cells were washed on ice with ice-cold HBSS containing 1 mM PMSF, harvested and then lysed in SDS lysis buffer. DNA was sheared by ultrasonication using a High Intensity Ultrasonic Processor (hielscher) for 3×10 s pulses at 20% amplitude. Lysates were cleared by centrifugation and diluted in ChIP dilution buffer. Lysates were precleared using salmon sperm DNA/protein A-agarose and a sample of “input DNA” was collected at this point. Protein-DNA complexes were immunoprecipitated with 5 µg of Ab overnight at 4°C. Ab-protein-DNA complexes were then captured using salmon sperm DNA/protein A-agarose for 1 h at 4°C. After washing beads with low and high salt, LiCl, and TE buffers, the protein/DNA complexes were eluted using 1% SDS, 0.1 M NaHCO3 buffer and disrupted by heating at 65°C for 4 h. DNA was then extracted using phenol/chloroform extraction and ethanol precipitation. PCR was conducted using promoter specific primers: IL-10 promoter (STAT3 binding region): sense 5′-TCATGCTGGGATCTGAGCTTCT-3′, antisense 5′-CGGAAGTCACCTTAGCACTCAGT-3′ (94°C, 15 s; 56°C, 30 s; 72°C, 1 min, 35cycles); IL-12p40 promoter (NF-κB binding site): sense 5′-AGTATCTC TGCCTCCTTCCTT-3′, antisense 5′-GCAACACTGAAAACTAGTGTC-3′ (initial denaturation at 95°C for 3 min; amplification cycles at 95°C for 30 s, 58°C for 1 min, and 72°C for 1 min; and a final extension at 72°C for 10 min, 40cycles). PCR amplified product was subsequently size fractioned on 2% agarose gel, stained with ethidium bromide and visualized under UV-light. For relative quantitation of promoter levels, real-time PCR was also performed. For real-time PCR samples were normalized to input DNA controls.
Isolation of RNA and real-time polymerase chain reaction
Total RNA extracted from macrophages (TRI reagent; Sigma) according to the standard protocol [44], [45] was reverse transcribed using Revert Aid M-MuLV reverse transcriptase (Fermentas). Real-time polymerase chain reaction (PCR) was performed using SYBR Green mix and the ABI 7500 real-time PCR system (Applied Biosystems). Glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) was used as a reference. Sequences of the PCR primers are listed in Table 1. The reaction conditions consisted of an initial activation step (5 min at 95°C) and cycling step (denaturation for 30 s at 94°C, annealing for 30 s at 58°C, and extension for 1 min at 72°C for 40 cycles), after which melt curve analysis was performed. Detection of the dequenched probe, calculation of threshold cycles, and further analysis of these data were done using Sequence Detector software (version 1.4; Applied Biosystems). Relative changes in iNOS2 and cytokine messenger RNA (mRNA) expression were compared with unstimulated control, normalized to GAPDH, and quantified by the 2ΔΔCt method.
Table 1. Sequences of the PCR primers.
| Gene | Sequence of Primers |
| iNOS2 | Forward 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′Reverse 5′-GGCTGTCAGAGCCTCG TGGCTTTGG-3′ |
| IL-10 | Forward 5′-CGGGAAGACAATAACTG-3′Reverse 5′-CATTTCCGATAAGGCTTGG-3′ |
| IL-12p40 | Forward 5′-CAACATCAAGAGCAGTAGCAG-3′Reverse 5′TACTCCCAGCTGACCTCCAC-3′ |
| TNF-α | Forward 5′-GGCAGGTCTACTTTGGAGTCATTGC-3′Reverse 5′-ACATTCGAGGCTCCAGTGAATTCGG-3′ |
| SOCS-3 | Forward 5′-GCGGGCACCTTTCTTATCC-3′Reverse 5′-TCCCCGACTGGGTCTTGAC-3′ |
| GAPDH | Forward 5′-CAAGGCTGTGGGCAAGGTCA-3′Reverse 5′-AGGTGGAAGAGTGGGAGTTGCTG-3′ |
Isolation of RNA and RT-PCR
RNA was isolated according to the standard protocol [44], [45]. Briefly, total RNA extracted from macrophages (TRI reagent; Sigma) according to the standard protocol [44], [45] was reverse transcribed using Revert Aid M-MuLV reverse transcriptase (Fermentas). The cDNA encoding the SOCS3, GAPDH gene was amplified using specific primers as listed in Table 1. PCR amplification was conducted in a reaction volume of 50 µl using a Perkin Elmer Gen Amp PCR system 2400 and 0.5 unit of Taq polymerase set for 35 cycles (denaturation: at 94°C for 30 s; annealing: at 58°C for 30 s; extension: at 72°C for 30 s) PCR amplified product was subsequently size fractioned on 2% agarose gel, stained with ethidium bromide and visualizedunder UV-light.
Densitometry analysis
Immunoblots were analyzed using a model GS-700 Imaging Densitometer and Molecular Analyst (version 1.5; Bio-Rad Laboratories).
Statistical analysis
The in vitro cultures were performed in triplicate. The data, represented as mean values ±standard deviations, are from 1 experiment that was performed at least 3 times. Student's t test was employed to assess the significance of the differences between the mean values of control and experimental groups. A P value of.05 was considered to be significant, and a P value <.001 was considered to be highly significant.
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All experimental animal protocols received prior approval from the Institutional Animal Ethical Committee (Bose Institute, Registration Number: 95/99/CPCSEA).
Acknowledgments
We are grateful to Director, Bose Institute (Kolkata) for his continuous encouragement. We cordially thank Mr. Debasish Majumdar and Mr. Prabal Gupta for their technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by Council of Scientifc Industrial Research, India (Grant No 37/1280/07/EMR-II) and Division of Molecular Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Descoteaux A, Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol. 1989;9:5223–7. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray HW, Spitalny GL, Nathan CF. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985;134:1619–1622. [PubMed] [Google Scholar]
- 3.Russell DG, Talamas-Rohana P. Leishmania and the macrophage: a marriage of Inconvenience. Immunol Today. 1989;10:328–33. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- 4.Murray HW. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982;129:351–7. [PubMed] [Google Scholar]
- 5.Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990;144:4794–7. [PubMed] [Google Scholar]
- 6.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 7.Bacellar O, D'oliveira A, Jr, Jerônimo S, Carvalho EM. IL-10 and IL-12 are the main regulatory cytokines in visceral leishmaniasis. Cytokine. 2000;12:1228–31. doi: 10.1006/cyto.2000.0694. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya P, Bhattacharjee S, Gupta G, Majumder S, Adhikari A, et al. Arabinosylated lipoarabinomannan-mediated protection in visceral leishmaniasis through up-regulation of toll-like receptor 2 signaling: an immunoprophylactic approach. J Infect Dis. 2010;202:145–55. doi: 10.1086/653210. [DOI] [PubMed] [Google Scholar]
- 9.Pan ZK. Toll-like receptors and TLR-mediated signaling: more questions than answers. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L918–20. doi: 10.1152/ajplung.00381.2003. [DOI] [PubMed] [Google Scholar]
- 10.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–22. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 11.Clayton AL, Mahadevan LC. MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett. 2003;546:51–8. doi: 10.1016/s0014-5793(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 12.Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat Med. 2004;10:540–4. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- 13.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;1:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 14.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–77. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 15.Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–96. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- 16.Qasimi P, Ming-Lum A, Ghanipour A, Ong CJ, Cox ME, et al. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumor necrosis factor alpha and nitric oxide production by macrophages. J Biol Chem. 2006;281:6316–24. doi: 10.1074/jbc.M508608200. [DOI] [PubMed] [Google Scholar]
- 17.Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, et al. Molecular mechanism of lipopolysaccharide-induced SOCS3 gene expression in macrophages and microglia. J Immunol. 2007;179:5966–76. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- 18.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, et al. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–12. [PubMed] [Google Scholar]
- 19.Suttles J, Milhorn DM, Miller RW, Poe JC, Wahl LM, et al. CD40 signaling of monocyte inflammatory cytokine synthesis through an ERK1/2-dependent pathway. A target of interleukin (il)-4 and il-10 anti-inflammatory action. J Biol Chem. 1999;274:5835–42. doi: 10.1074/jbc.274.9.5835. [DOI] [PubMed] [Google Scholar]
- 20.Murray PJ. STAT3-mediated anti-inflammatory signaling. Biochem Soc Trans. 2006;34:1028–31. doi: 10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiations in vitro and in vivo. J Immunol. 2006;177:1679–88. doi: 10.4049/jimmunol.177.3.1679. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty D, Banerjee S, Sen A, Banerjee KK, Das P, et al. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J Immunol. 2005;175:3214–24. doi: 10.4049/jimmunol.175.5.3214. [DOI] [PubMed] [Google Scholar]
- 23.Reiner NE. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today. 1994;15:374–381. doi: 10.1016/0167-5699(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 24.Awasthi A, Mathur R, Khan A, Joshi BN, Jain N, et al. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. J Exp Med. 2003;197:1037–43. doi: 10.1084/jem.20022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2007;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 27.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003;85:153–66. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 28.Bohsali A, Abdalla H, Velmurugan K, Briken V. The non-pathogenic mycobacteria M. smegmatis and M. fortuitum induce rapid host cell apoptosis via a caspase-3 and TNF dependent pathway. BMC Microbiol. 2010;10:237. doi: 10.1186/1471-2180-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai K, Kurita-Ochiai T, Ochiai K. Mycobacterium bovis bacillus Calmette-Guérin infection promotes SOCS induction and inhibits IFN-gamma-stimulated JAK/STAT signaling in J774 macrophages. FEMS Immunol Med Microbiol. 2003;39:173–80. doi: 10.1016/S0928-8244(03)00231-1. [DOI] [PubMed] [Google Scholar]
- 30.de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, et al. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol. 2003;33:2822–31. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 31.Stoiber D, Stockinger S, Steinlein P, Kovarik J, Decker T. Listeria monocytogenes modulates macrophage cytokine responses through STAT serine phosphorylation and the induction of suppressor of cytokine signaling 3. J Immunol. 2001;166:466–72. doi: 10.4049/jimmunol.166.1.466. [DOI] [PubMed] [Google Scholar]
- 32.Suhrbier A, La Linn M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003;24:165–8. doi: 10.1016/s1471-4906(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 33.Dennis V, Kaushal D, Dixit S, Mehra S, Singh S, et al. SOCS3 and IL-10 anti-inflammatory activity in Lyme disease. The FASEB Journal. 2008;22:860.17. [Google Scholar]
- 34.Hart DT, Vickerman K, Coombs GH, Quick A. Simple method for purifying L. mexicana amastigotes in large numbers. Parasitology. 1981;82:345–355. doi: 10.1017/s0031182000066889. [DOI] [PubMed] [Google Scholar]
- 35.Majumder N, Dey R, Mathur RK, Datta S, Maitra M, et al. An unusual pro-inflammatory role of interleukin-10 induced by arabinosylated lipoarabinomannan in murine peritoneal macrophages. Glycoconj J. 2006;23:675–686. doi: 10.1007/s10719-006-9017-9. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharjee S, Majumder N, Bhattacharyya P, Bhattacharyya S, Majumdar S. Immunomodulatory role of arabinosylated lipoarabinomannan on Leishmania donovani infected murine macrophages. Indian J Biochem Biophys. 2007;44:366–372. [PubMed] [Google Scholar]
- 37.Fahey TJ, Tracey KJ, Tekamp-Olson P, Cousens LS, Jones WG, et al. Macrophage inflammatory protein-1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 38.Bhattacharyya S, Ghosh S, Dasgupta B, Mazumder D, Roy S, Majumdar S. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J Infect Dis. 2002;185:1704–8. doi: 10.1086/340820. [DOI] [PubMed] [Google Scholar]
- 39.Majumdar S, Kane LH, Rossi MW, Volpp BD, Nauseef WM, et al. Protein kinase C isotypes and signal transduction in human neutrophils: selective substrate specificity of calcium dependent b-PKC and novel calcium independent n-PKC. Biochim Biophys Acta. 1993;1176:276–286. doi: 10.1016/0167-4889(93)90056-u. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh S, Bhattacharyya S, Sirkar M, Sa GS, Das T, et al. Leishmania donovani suppresses activator protein-1 and NF-kB in host macrophages via ceramide generation: involvement of extracellular signal regulated kinase. Infect Immun. 2002;70:6828–6838. doi: 10.1128/IAI.70.12.6828-6838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, et al. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 42.Roy S, Scherer MT, Briner TJ, Smith JA, Gefter LM. Murine MHC polymorphism and T cell specificities. Science. 1989;244:572–5. doi: 10.1126/science.2470147. [DOI] [PubMed] [Google Scholar]
- 43.Yaron A, Gonen H, Alkalay I, Hatzobai A, Jung S, et al. Inhibition of NF-κ-B cellular function via specific targeting of the I-κ-B-ubiquitin ligase. EMBO J. 1997;16:6486–6494. doi: 10.1093/emboj/16.21.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chomezynasky P, Sacchi N. A single step method of RNA isolation by guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, , NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]