Abstract
Heart tissues from hibernating mammals, such as ground squirrels, are able to endure hypothermia, hypoxia and other extreme insulting factors that are fatal for human and nonhibernating mammals. This study was designed to understand adaptive mechanisms involved in intracellular Ca2+ homeostasis in cardiomyocytes from the mammalian hibernator, ground squirrel, compared to rat. Electrophysiological and confocal imaging experiments showed that the voltage-dependence of L-type Ca2+ current (I Ca) was shifted to higher potentials in ventricular myocytes from ground squirrels vs. rats. The elevated threshold of I Ca did not compromise the Ca2+-induced Ca2+ release, because a higher depolarization rate and a longer duration of action potential compensated the voltage shift of I Ca. Both the caffeine-sensitive and caffeine-resistant components of cytosolic Ca2+ removal were more rapid in ground squirrels. Ca2+ sparks in ground squirrels exhibited larger amplitude/size and much lower frequency than in rats. Due to the high I Ca threshold, low SR Ca2+ leak and rapid cytosolic Ca2+ clearance, heart cells from ground squirrels exhibited better capability in maintaining intracellular Ca2+ homeostasis than those from rats and other nonhibernating mammals. These findings not only reveal adaptive mechanisms of hibernation, but also provide novel strategies against Ca2+ overload-related heart diseases.
Introduction
Intracellular Ca2+ regulates a wide variety of cellular processes, including excitation-contraction (E-C) coupling, gene expression, enzyme regulation, cell growth and cell death [1]–[3]. The intracellular Ca2+ concentration ([Ca2+]i) needs be regulated precisely to ensure homeostatic operation of physiological systems. Impaired regulation of intracellular Ca2+ under pathologic, ischemic and hypothermic conditions leads to cellular dysfunction and global disorders. For example, the inability to elevate [Ca2+]i to a required level following an excitation is a major pathogenic mechanism involved in heart failure [1], [4]. On the other hand, excessive elevation of intracellular Ca2+, a status known as Ca2+ overload, has been proven to be deleterious to almost all cell types, and can be associated with either necrotic or apoptotic cell death [2], [3], [5]. In the heart, abnormal handling of intracellular Ca2+ may induce severe arrhythmias and ventricular fibrillation [6]–[8].
Heart tissues from hibernating mammals demonstrate enhanced resistance against many insulting factors that cause heart dysfunction in humans and non-hibernators [9], [10]. Hibernating mammals, which usually maintain their body temperature around 37°C, can down-regulate their body temperature to only a few degrees in winter [11]. In contrast to the heart arrest and severe arrhythmia at low body temperatures in humans and non-hibernating mammals [9], [12], [13], circulation and respiration are well maintained, although at much lowered rates, in hibernating mammals during hibernation [11], [12]. Moreover, hibernators also exhibit functional stability against pathologic or stressful stimuli in general. For example, in hedgehog hearts, epicardial application of aconitine, administration of high concentration of CaCl2, combined procaine and adrenaline treatment or ligation of coronary artery branches cannot trigger the ventricular fibrillation that would commonly occur in non-hibernator hearts [9]. Ground squirrels can survive the 4.5% low oxygen that is fatal for rats [14]. It is therefore suggested that the cardiovascular system of hibernating mammals is a nature's model for arrhythmia resistance and hypoxia tolerance [9], [10]. Therefore, Understanding the adaptive mechanisms of hibernation is not only of important biological significance, but will also provide strategies for solving medical problems.
Given these unique adaptations, it is intriguing how the intracellular Ca2+ homeostasis is maintained against so many pathogenic/insulting factors. In heart cells, most of the Ca2+ cycling components are involved in E-C coupling, where the Ca2+ influx thorough the voltage gated L-type Ca2+ channels (I Ca) activates the Ca2+ release from the ryanodine receptors (RyRs) on the sarcoplasmic reticulum (SR) [1], [15]. Ca2+ entering the cytosol is either taken back to the SR by the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), or removed via Na+/Ca2+ exchange and other Ca2+ transporting systems [16], [17]. A few studies have shown that hibernators tend to down-regulate the L-type Ca2+ current and up-regulate the SR Ca2+ uptake and release capacity when they hibernate [18]–[21]. These findings demonstrat that a coordinated remodeling of cellular Ca2+ handling is a part of the adaptive preparation for maintaining forceful contraction under hibernating conditions. However, previous studies have not fully explained why hibernating mammals, even in their awake state, are still resistant against arrhythmogenic and hypoxic perturbations.
In order to test the hypothesis that hibernators are adapted for better maintaining intracellular Ca2+ homeostasis than other mammals, we systemically characterized the Ca2+ cycling and E-C coupling processes in heart cells from ground squirrels in comparison with commonly used animal models.
Results
Intracellular Ca2+ homeostasis under varying temperature
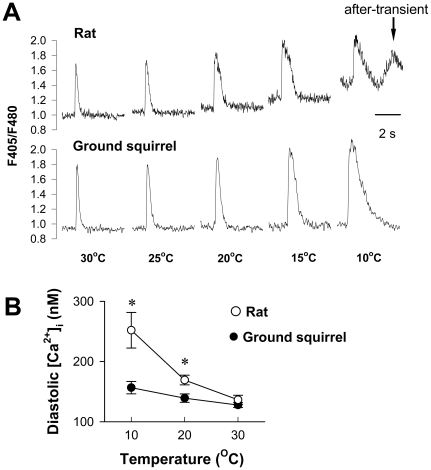
The intracellular Ca2+ dynamics during field stimulation in ventricular myocytes from rats and ground squirrels at varying temperature are illustrated in Figure 1A. The Ca2+ transients exhibited an increased background and decreased amplitude in rat cells but not in ground squirrel cells when the temperature was lowered from 30°C to 10°C. Below 15°C, most Ca2+ transients were accompanied by “after-transients” (Figure 1A arrow) in rats, which were never observed in ground squirrels. Calibration of indo-1 fluorescence showed that the diastolic Ca2+ level was increased from 136±7 nmol/L at 30°C to 252±29 nmol/L at 10°C in rats but was changed little in ground squirrels (Figure 1B). As a consequence, myocardium from ground squirrels was able to maintain vigorous contractile strength with complete relaxation at the low temperature, and avoided the low temperature-induced incomplete relaxation and “after-contractions” that occurred in rat myocardium (Figure S1). These results provided direct evidence that beating heart cells from hibernating mammals are able to keep homeostatic intracellular Ca2+ at varying temperatures.
Figure 1. Temperature-dependence of Ca2+ transient in ventricular myocytes from rats and ground squirrels.
(A) Typical calcium transients presented as the ratio of fluorescence at 405 nm vs. 480 nm (F405/F480). The arrow denotes an “after-transient”. (B) Diastolic [Ca2+]i of the Ca2+ transients in rat cells (n = 9 cells from 5 animals) were significantly higher than that in ground squirrel cells (n = 5 cells from 3 animals) at low temperatures. *P<0.05.
Ca2+ influx and membrane potential
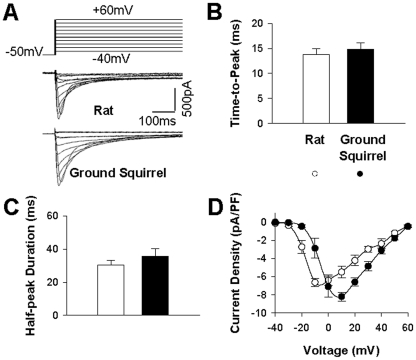
In cardiac cells, I Ca through L-type Ca2+ channels is the major pathway of Ca2+ entry, and is also the trigger for Ca2+-induced Ca2+ release from the sarcoplasmic reticulum. We used the whole-cell patch clamp technique to record I Ca in single ventricular myocytes when the membrane was depolarized to various voltages (V m) from the holding potential of −50 mV (Figure 2A). We included in the pipette 10 mmol/L EGTA to avoid Ca2+-induced feedback to I Ca. Measurement of the time course of I Ca showed that the time-to-peak (Figure 2B) and half-peak duration (Figure 2C) were similar in ground squirrels and rats, indicating similar activation and inactivation kinetics in these two species. The I Ca−V m curves were both bowl-shaped crossing the same reversal potential (Figure 2D). However, the voltage-dependence of I Ca differed. Both the activation threshold and the peak activation potential were shifted ∼10 mV more positive in ground squirrels than in rats. As the results, the I Ca amplitude at 20 mV was about one order of magnitude lower in ground squirrels than in rats.
Figure 2. Voltage-dependence of Ca2+ current.
(A) Ca2+ currents were activated by depolarizations from a holding potential of −50 mV to test potentials ranging from −40 mV to +60 mV with 10-mV steps. (B) The time-to-peak and (C) Half-peak duration was measured at 0 mV. (D) The current-voltage (I Ca−V m) relation in rats (n = 5 from 5 animals) and ground squirrels (n = 5 from 4 animals).
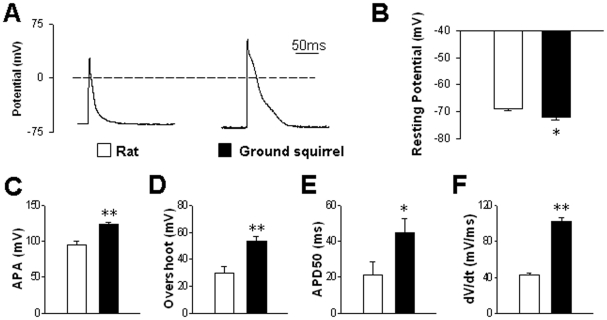
The right-shift of I Ca−V m curve in ground squirrel cells tends to result in later activation of I Ca during an action potential. To examine whether the ground squirrel action potential is indeed coordinated with the voltage dependence of I Ca, we performed whole-cell current clamp experiments (Figure 3A). When the pipette current was clamped at zero, we recorded resting potentials around −70 mV in both cell types (Figure 3B).
Figure 3. Action potentials evoked by current pulses.
(A) Typical examples of action potentials in ventricular myocytes from rats (left) and ground squirrels (right). (B) Resting membrane potentials were compared between rats (n = 18 cells from 7 animals) and ground squirrels (n = 21 cells from 7 animals). (C) Action potential amplitude (APA), (D) overshoot, (E) half-repolarization duration (APD50) and (F) maximum dV/dt of depolarization were compared between rats (n = 7 cells from 5 animals) and ground squirrels (n = 6 cells from 5 animals). *P<0.05 and **P<0.01.
Upon 2-ms current pulse stimulation, action potentials firing in ground squirrel cells shared similar triangle morphology as in rat cells (Figure 3A) and most other rodent cardiomyocytes [22]. Notably, the amplitude of action potential (APA) was significantly higher in ground squirrels (Figure 3C). The higher APA was basically attributable to their much larger overshoot (Figure 3D). Also, ground squirrel cells exhibited longer half-repolarization duration (Figure 3E) and faster depolarization rate (dV/dt) (Figure 3F). Larger depolarization rate and longer duration both facilitate more robust activation of the high-threshold I Ca in ground squirrels.
Evoked and spontaneous Ca2+ release
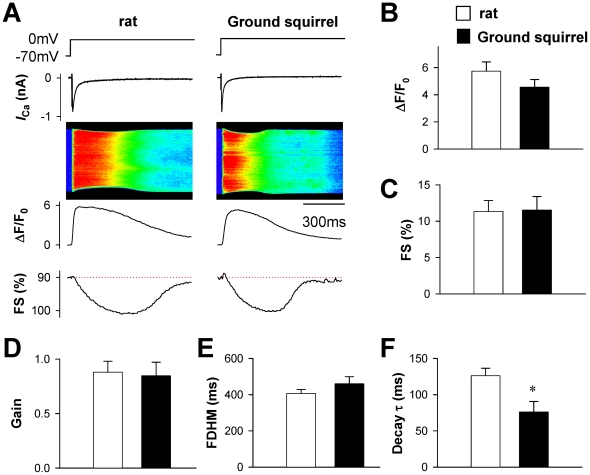
To compare the I Ca-evoked Ca2+ release from the sarcoplasmic reticulum, we recorded the Ca2+ transients by confocal line-scan imaging (Figure 4A) when the cells were depolarized to 0 mV. Given that the I Ca density was similar between two species (data not shown, but similar to Figure 2C), the amplitude of Ca2+ transients (Figure 4B) and fractional cell shortening (Figure 4C) were basically comparable between rats and ground squirrels. The gain of E-C coupling, defined as the Ca2+ transient amplitude per unit I Ca density, was also about the same (Figure 4D). Notably, although the full-duration at half-maximum (FDHM) was similar between two species (Figure 4E), the time constant of the second half of the Ca2+ transient decay was significantly shorter in ground squirrels than in rats (Figure 4F).
Figure 4. Ca2+ transients evoked by depolarization to 0 mV.
(A) Typical examples of simultaneous recording of I Ca and Ca2+ transient (image and ΔF/F0) in ventricular myocytes from rats (left) and ground squirrels (right). The fractional shortening (FS) was determined by edge-detection of Ca2+ transients. (B) The amplitude of Ca2+ transient (ΔF/F0) and (C) the fractional shortening (FS) reflecting the amplitude of contraction were compared between rats (n = 12 cells from 8 animals) and ground squirrels (n = 16 cells from 8 animals). (D) The gain of E-C coupling was calculated as the ratio between ΔF/F0 and I Ca density. (E) The full-duration at half-maximal (FDHM) and (F) the time constant (τ) of second half decay of Ca2+ transient were compared between rats (n = 12 cells from 8 animals) and ground squirrels (n = 16 cells from 8 animals).
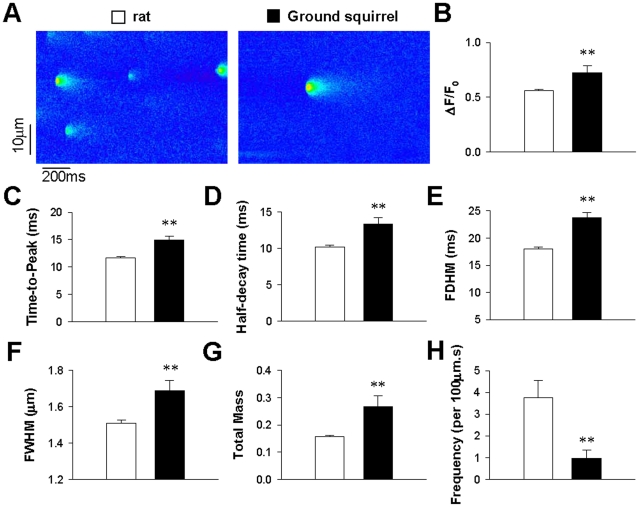
Although the global Ca2+ release following an I Ca trigger appeared similar in two species, the characteristics of local RyR Ca2+ release, as reflected in Ca2+ sparks [23], was diffeent. Confocal imaging of quiescent ventricular myocytes (Figure 5A) showed that spontaneous Ca2+ sparks in ground squirrels exhibited higher amplitude than in rats (Figure 5B). The higher amplitude was at least partially attributable to prolonged RyR Ca2+ release, as reflected by the longer time-to-peak (Figure 5C). As the half-decay time of Ca2+ spark was also prolonged (Figure 5D), the FDHM in ground squirrels was much longer than that in rats (Figure 5E). Prior to the present study, Ca2+ sparks in rats had been shown to be brighter than other animal species. Here, Ca2+ sparks in ground squirrels exhibited an even larger full-width at half-maximum (FWHM, Figure 5F) and signal mass (Figure 5G), and thus may represent the largest Ca2+ spark among all characterized mammal ventricular cells. The larger spark size in ground squirrels was partially due to higher SR Ca2+ load than in rats (Figure S2).
Figure 5. Spontaneous Ca2+ sparks.
(A) Line-scan images of spontaneous Ca2+ sparks in ventricular myocytes from rats (left) and ground squirrels (right). (B) The spark amplitude (ΔF/F0). (C) The time-to-peak. (D) The half-decay time. (E) The full duration at half-maximum (FDHM). (F) The full width at half-maximum (FWHM). (G) The total signal mass presented in arbitrary units (A.U.). (H) The frequency of Ca2+ sparks calculated by dividing the total number of sparks by the product of distance (µm) and time (s) of line scanning. **P<0.01 (n = 270 sparks in 6 rats and 53 sparks in 5 ground squirrels).
Although spontaneous Ca2+ sparks in ground squirrels are brighter, the Ca2+ spark frequency was less than 1/4 that in rats (Figure 5H). The lower frequency of Ca2+ sparks reflects less frequent leak from the SR Ca2+ store, which is essential for intracellular Ca2+ homeostasis.
Ca2+ removal
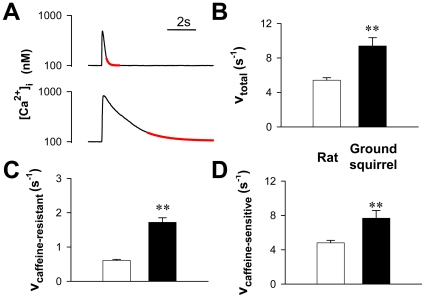
During the contraction-relaxation cycle, timely removal of Ca2+ from cytosol is important for intracellular Ca2+ homeostasis. During the whole-cell voltage clamp, the faster decay of Ca2+ transients in ground squirrel cells suggested a more rapid clearance of intracellular Ca2+ than in rat cells (Figure 4F). Under field stimulation conditions, the late decay of Ca2+ transients (Figure 6A upper) also exhibited a higher rate constant in ground squirrels than in rats (Figure 6B). To assess the mixed contributions of SERCA pumping, Na+/Ca2+ exchange, mitochondrial Ca2+ uptake and other possible mechanisms [16], we blocked SR Ca2+ uptake by perfusing the cells with 20 mmol/L caffeine (Figure 6A lower). The caffeine-resistant component of Ca2+ removal comprised a minor part in the total Ca2+ removal and was similar in both species (Figure 6C). Thus, the major species difference of Ca2+ removal was due to the higher rate of caffeine-sensitive SR Ca2+ uptake (Figure 6D), which reflected higher SERCA activity in ground squirrel cells.
Figure 6. Analysis of Ca2+ removal mechanisms.
(A) Representative Ca2+ transients induced by electrical stimulation (upper) and 20 mmol/L caffeine (lower) in a rat cardiac myocyte. The red lines illustrate the fitting of the lower 20% of Ca2+ transients. (B) Rate constants of total Ca2+ removal. (C) Rate constants of the caffeine-resistant component. (D) Rate constants of the caffeine-sensitive component were calculated as the difference between the total and the caffeine-resistant component. ** P<0.01 (n = 19 cells from 3 rats and 14 cells from 3 ground squirrels).
Relationship between intracellular Ca2+ and contraction
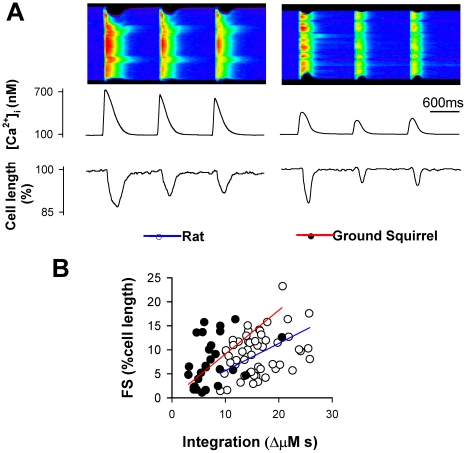
In cardiomyocytes from both species, repetitive field stimulation at 1-s intervals evoked a series of Ca2+ transients/contractions with varying amplitude (Figure 7A). To examine whether the myofiliments in ground squirrels are more sensitive to Ca2+ in generating a contraction, we investigated the relationship between cell shortening and Ca2+ transient integral (Figure 7B). Linear regression indeed revealed a higher contraction/transient ratio in ground squirrels, suggesting a higher Ca2+ signaling efficiency in generating contractions.
Figure 7. Relationship between Ca2+ transients and cell shortening.
(A) Ca2+ transients (upper image and plot) and cell length (lower plot) in response to 1 Hz electrical field stimulation in rat (left) and ground squirrel (right) cells. (B) The fractional shortening (FS) of each contraction was plotted against the integration of Ca2+ transients. Color lines indicate linear regression.
Discussion
Strategies of adaptation
Hibernation involves multiple insulting factors that are fatal for non-hibernating mammals, such as violent change of body temperature, sustained deep hyperthermia during hibernation, as well as intensified sympathetic innervation during arousal. It is therefore intriguing to understand why the cardiovascular system of hibernating mammals can maintain functional stability against these extreme factors. In the present study, we have for the first time systemically compared major Ca2+ cycling processes and intracellular Ca2+ homeostasis between hibernating and nonhibernating species. Compared to those in rats and most other mammals, heart cells from ground squirrels have evolved several adaptive strategies for more optimal and efficient maintenance of intracellular Ca2+ homeostasis:
1) Elevated threshold for Ca2+ entry
Excessive Ca2+ entry is a major reason of intracellular overload in many pathogenic processes [2], [3], [5]. In the deep hyperthermia condition during hibernation, the resting potential declines due to slowed turnover rate of sodium pumps and compromised transmembrane ionic gradients [24]. Therefore, our finding on the right-shift of I Ca−V m curve in ground squirrels has significant adaptive significance. The high threshold of I Ca would lower the risk of spontaneous I Ca activation, and is thus helpful in preventing excessive Ca2+ entry during the resting state. As E-C coupling highly depends on the profile of action potentials [25], the higher threshold of I Ca would be expected to compromise the activation of E-C coupling. Interestingly, there seems to have been a co-evolution between L-type Ca2+ channels and action potentials in ground squirrels. The more negative diastolic potential enhances L-type channel availability; the higher depolarization rate accelerates channel activation; and the longer duration of action potential increases Ca2+ influx. These factors well compensates the right-shift of I Ca−V m curve, and adaptively corrects the probability of Ca2+ channel activation.
2) Suppressed leak from Ca2+ store
Ca2+ leak from intracellular Ca2+ stores is another major factor interfering with intracellular Ca2+ homeostasis [8], [16]. A higher leakage imposes a heavier burden on energy-based Ca2+ transports [26]. In situations of compromised metabolism, such as hypothermia or recovery from hypoxia, excessive Ca2+ leak triggers a vicious cycle of Ca2+-induced Ca2+ release, and leads to irreversible process of Ca2+ mishandling and cell damage [26]. In heart cells from ground squirrels, spontaneous spark frequency is quite low. Even taking into consideration the larger Ca2+ leak signal mass in ground squirrels, the ensemble Ca2+ spark signal mass per unit time, i.e. the product of individual spark signal masse and spark frequency, is only about 1/3 of that in rat cells. The suppressed SR Ca2+ leak in ground squirrel cells is an important part of the Ca2+ homeostatic adaptation.
3) Accelerated rate of cytosolic Ca2+ removal
In essence, intracellular Ca2+ homeostasis is a balance between continuous Ca2+ removal from the cytosol and continuous Ca2+ entry/leak. In addition to the higher threshold of Ca2+ entry from L-type Ca2+ channels and less RyR Ca2+ leak from the SR, cardiomyocytes from ground squirrels also exhibit faster removal rate of cytosol Ca2+. During a Ca2+ transient, the caffeine-sensitive component of Ca2+ uptake by SERCA is no doubt the major driving force behind the faster Ca2+ removal in ground squirrels.
In addition to SR Ca2+ pumping, the caffeine-resistant component of Ca2+ removal, although secondary in the decay of a Ca2+ transient, plays an important role in maintaining intracellular Ca2+ homeostasis during the resting state [16]. This component usually involves Na+/Ca2+ exchanger and Ca2+-ATPase on the cell membrane, as well as Ca2+ uptake by mitochondria [16]. Although it is not known whether ground squirrels have additional Ca2+ transport mechanisms, their caffeine-insensitive component appears to be much more powerful than in rats. During hibernation, the heart rate drops to several beats per minute [11]. It is therefore expected that the stronger caffeine-insensitive Ca2+ transports are of great adaptive importance in maintaining Ca2+ homeostasis between the slow heart beats.
4) Enhanced Ca2+ signaling efficiency in generating contraction
For a functional reserve during hibernation, cells of hibernating mammals need to keep diastolic Ca2+ sufficiently low and systolic Ca2+ sufficiently high. In the context of temperature-dependent decrease of Ca2+ transport activities, these two demands become even more challenging. Ground squirrels gain a higher Ca2+ signaling efficiency in generating contractile strength by enhanced Ca2+ sensitivity of myofiliments. This adaptation not only lowers the Ca2+ requirement and its associated energy burden in generating sufficient pumping power, but also partially compensates the temperature-dependent decrease of myofiliment Ca2+ sensitivity [27] under hypothermic conditions, and is thus important for surviving hibernation.
Medical significance
Excessive elevation of resting Ca2+ has been proven to be deleterious to almost all cell types, and can be associated with either necrotic or apoptotic cell death [3], [5]. Abnormal handling of intracellular Ca2+ in the heart may induce severe arrhythmias and ventricular fibrillation [6]–[8]. The adaptive capability to maintain intracellular Ca2+ homeostasis in hibernator cells is beneficial in the following pathophysiological states:
Anti-arrhythmia
Many severe arrhythmias are caused by intercellular mishandling of intracellular Ca2+ homeostasis [6]–[8]. For example, leaky RyRs due to mutations underlies the catecholaminergic polymorphic ventricular tachycardia [8]. Intracellular Ca2+ overload usually leads to after-depolarizations, which is a major mechanism of arrhythmogenisis [16]. In contrast, hearts from the hedgehog, another hibernating mammal, are resistant to arrhythmogenic insults, including application of aconitine, high concentration extracellular calcium, procaine or adrenaline [9]. Our results show that cardiomyocytes from ground squirrels was able to avoid the low temperature-induced “after-transients” that occurs in rat hearts. The anti-arrhythmic property of the hibernator heart appears to be tightly linked to their Ca2+ homeostatic mechanisms, as increasing RyR leak and interfering Ca2+ uptake by low dose of caffeine can reproduce the arrhythmic after-contractions in ground squirrels [12].
Surgery and transplantation
Hypothermic anesthesia is often used in by-pass surgery to lower the demand of blood supply [28]. The American Heart Association suggests that the body temperature during surgery should be controlled above 30°C, because further decrease of body temperature will cause ventricular fibrillation and irreversible damage of organ function [29]. In contrast, hibernating mammals are able to regulate their body temperature between 37°C and freezing point without endangering their life. The ability for hibernator cells to keep intracellular Ca2+ homeostasis is one of the key mechanisms underlying their tolerance of hyperthermia. Also, organ preservation under low temperature is a major challenge in transplantation [28]. Intracellular Ca2+ homeostasis is again a key issue limiting the time window for organ transplantation [30]. Therefore, hyperthermia-resistant mechanisms in hibernating mammals, including those involved in intracellular Ca2+ homeostasis, are expected to provide strategies for improving the clinical practice in hypothermic anesthesia and organ preservation. One successful example is that a hibernation-inducing trigger obtained from hibernating woodchucks prolongs the survival time of auto-perfused canine multiorgan preparation, including working heart, lung, kidney, etc., from an average of 16 hours to 43 hours [31]. This study produced one of the longest average survival times for organ preservation.
In 2007, the entire genome of thirteen-lined ground squirrels (Spermophilus tridecemlineatus) was roughly sequenced, and deeper sequencing is ongoing. With the application of modern research tools on hibernation research, the mechanisms underlying the adaptation of hibernating mammals, including those involved in Ca2+ homeostasis, will be understood in more detail. These mechanisms will be expected to provide new ideas for developing therapeutic strategies against diseases/disorders involving intracellular Ca2+ mishandling.
Materials and Methods
Ethics Statement
The investigation conforms with the Guide for Care and Use of Laboratory animals published by the US National Institutes of Health. Animal trapping and experiments were approved by the Institutional Animal Care and Use Committee of Peking University (Permit Numbers: lsc-wangsq-1 and lac-tianyl-2). All surgery was performed under 20% ethylurethanm (i.p. injection 5 ml/kg) anaesthesia, and all efforts were made to minimize suffering.
Cell preparation
The ground squirrel used in the present study, Citellus dauricus, is a species dominant in Northeast Asia, which belongs to the same genus as those live in North America, e.g., Spermophilus tridecemlineatus. The animals were trapped in the field of Zhanbei County in Hebei Province, China. Ground squirrels were kept in active state in an environment of natural photoperiod at room temperature. The Sprague-Dawley rats were housed by and purchased from the Laboratory Animal Center of Peking University. The hearts were rapidly excised from adult ground squirrels (200–250 g) or rats (200–250 g) under anaesthesia and mounted on a Langendorff apparatus. Ventricular cardiac myocytes from animals were enzymatically isolated as previously reported [32]. Myocytes were stored in a solution containing (in mM) 137 NaCl, 4.0 KCl, 1.0 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 glucose and 10 HEPES, pH 7.35 adjusted with NaOH, and were used the same day they were isolated. The functional features of the prior non-hibernating state were assumed to be preserved after cell isolation, because isolated cardiomyocytes from hibernating and non-hibernating individuals exhibited distinct functional performances [21] that were in good agreement with those observed in cardiac tissues [18]–[20].
Measurement of intracellular Ca2+ concentration
When indo-1 fluorescence was used to determine intracellular Ca2+ concentration, cells were incubated in Tyrode's solution containing 2.5 µmol/L indo-1 AM for about 10 min in the dark at 37°C. The fluorescence was exited by a UV laser and measured by ASCS meridian 575UV confocal microscope using 485 nm and 405 nm band-pass filters as reported previously [33]. Intracellular Ca2+ concentration was measured at different temperatures, and calculated with formula [Ca2+]i = kA(R−Rmin)/(Rmax−R), where kA was an apparent constant determined by calibration [23], R is the ratio of fluorescence at 405 nm vs. 485 nm, Rmax and Rmin were the R in Ca2+ free and Ca2+-saturated buffers, respectively.
Whole-cell patch clamp
Whole-cell Ca2+ current were recorded at room temperature with 4 mmol/L 4-aminopyridine and 0.02 mmol/L tetrodotoxin in the bath solution, as reported reviously [34]. The pipettes were filled with (in mmol/L) CsCl 105, MgCl2 5, Na2ATP 5, TEA-Cl 15, EGTA 11, CaCl2 1, and HEPES 10 (pH was adjusted to 7.2 with CsOH). When Ca2+ transients were recorded simultaneously, the pipette electrode was filled with (in mmol/L) CsCl 127, NaCl 10, MgCl2 1, MgATP 5, TEA-Cl 15, HEPES 10 and fluo-4 pentapotassium 0.2 (pH 7.2 adjusted with CsOH). Leak current subtraction was performed Offline.
Membrane potential was recorded at room temperature under whole-cell current clamp mode. The pipettes were filled with intracellular solution which contained (in mmol/L) KCl 125, MgCl2 6, Na2ATP 5, EGTA 0.2 and HEPES 10 (pH was adjusted to 7.2 with KOH). Action potentials were evoked by 2-ms current pulses.
Confocal imaging of Intracellular Ca2+
Ca2+ indicators were loaded into myocytes either via the pipette electrode in patch-clamp experiments [34]or otherwise by incubation in a Tyrode's solution containing 20 µmol/L fluo-4 AM or fluo-5F AM (Molecular Probes) at 37°C for 5 min. The fluorescence was measured with Zeiss LSM-510 or LSM-5Live laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). Images were acquired at the room temperature (unless otherwise specified) in the line-scan mode. The Ca2+ level was either reported as the fluorescence normalized by its resting level (F/F0), or calculated according to the formula [Ca2+]i = kd·R/(kd/C0+1−R) [35], where R = F/F0, kd was set to 1.1 mol/L for fluo-4 and 2.3 mol/L for fluo-5N. The threshold for Ca2+ spark detection was set at 1.25 F/F0. Cell contraction was measured by edge detection and presented as the fractional shortening of cell length.
Data analysis
The data are presented as means ± s.e.m. Statistical significance was determined using Student-t test unless otherwise specified. A P value<0.05 was considered to be statistically significant.
Supporting Information
Contraction of papillary heart muscles in rats (left) and ground squirrels (right) in response to 0.2 Hz field stimulation at 8°C. Note the after-contractions and elevated resting tension in the rat.
(TIF)
SR Ca2+ load of ventricular myocytes at room temperature (RT) and 10°C were measured in rats and ground squirrels by perfusing the cells with 20 mmol/L caffeine after 15 min indo-1 AM loading. SR load was reported as the ratio of fluorescence at 405 nm vs. 485 nm.
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the 973 Major State Basic Research Development Program of China (2011CB809101); the 863 Program (2007AA02Z457); the National Natural Science Foundation of China (30730013); the National Institutes of Health, USA (NIH R01 TW007269); and the Intramural Research Program of the National Institute on Aging, NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Bio. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Orrenius S, Zhivotovsky B. Nicotera P: Regulation of cell death (2003) The calcium-apoptosis link. Nat Rev Mol Cell Biol. 4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 4.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 5.Kitakaze M, Weisman HF, Marban E. Contractile Dysfunction and ATP Depletion after Transient Calcium Overload in Perfused Ferret Hearts. Circulation. 1988;77:685–695. doi: 10.1161/01.cir.77.3.685. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations: are they linked to ventricular fibrillation? J Cardiovasc Electrophysiol. 1993;4:473–489. doi: 10.1111/j.1540-8167.1993.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 7.Kihara Y, Morgan JP. Intracellular Calcium and Ventricular-Fibrillation - Studies in the Aequorin-Loaded Isovolumic Ferret Heart. Circ Res. 1991;68:1378–1389. doi: 10.1161/01.res.68.5.1378. [DOI] [PubMed] [Google Scholar]
- 8.Wehrens XHT, Lehnart SE, Huang F, Vest JA, Reiken SR, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 9.Johansson BW. The hibernator heart–nature's model of resistance to ventricular fibrillation. Cardiovascular research. 1996;31:826–832. doi: 10.1016/0008-6363(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang SQ, Lakatta EG, Cheng H, Zhou ZQ. Adaptive mechanisms of intracellular calcium homeostasis in mammalian hibernators. J Exp Biol. 2002;205:2957–2962. doi: 10.1242/jeb.205.19.2957. [DOI] [PubMed] [Google Scholar]
- 11.Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and torporin mammals and birds. New York: Academic Press; 1982. [Google Scholar]
- 12.Wang SQ, Huang YH, Liu KS, Zhou ZQ. Dependence of myocardial hypothermia tolerance on sources of activator calcium. Cryobiology. 1997;35:193–200. doi: 10.1006/cryo.1997.2040. [DOI] [PubMed] [Google Scholar]
- 13.Nordrehaug JE. Sustained ventricular fibrillation in deep accidental hypothermia. British medical journal (Clinical research ed.) 1982;284:867–868. doi: 10.1136/bmj.284.6319.867-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alecy LG, Lundy EF, Kluger MJ, Harker CT, LeMay DR, et al. Beta-hydroxybutyrate and response to hypoxia in the ground squirrel. Comp Biochem Physiol B. 1990;96:189–193. doi: 10.1016/0305-0491(90)90361-v. [DOI] [PubMed] [Google Scholar]
- 15.López-López JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- 16.Bers DM. Excitation-contraction coupling and cardiac contractile force. 2nd Edition ed. Dordrecht (the Netherlands): Kluwer Academic Publishers; 2001. [Google Scholar]
- 17.Lalli MJ, Yong J, Prasad V, Hashimoto K, Plank D, et al. Sarcoplasmic reticulum Ca2+ ATPase (SERCA) 1a structurally substitutes for SERCA2a in the cardiac sarcoplasmic reticulum and increases cardiac Ca2+ handling capacity. Circ Res. 2001;89:160–167. doi: 10.1161/hh1401.093584. [DOI] [PubMed] [Google Scholar]
- 18.Kondo N, Shibata S. Calcium Source for Excitation-Contraction Coupling in Myocardium of Nonhibernating and Hibernating Chipmunks. Science. 1984;225:641–643. doi: 10.1126/science.6740332. [DOI] [PubMed] [Google Scholar]
- 19.Kondo N. Excitation-contraction coupling in myocardium of nonhibernating and hibernating chipmunks: effects of isoprenaline, a high calcium medium, and ryanodine. Circ Res. 1986;59:221–228. doi: 10.1161/01.res.59.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Alekseev AE, Markevich NI, Korystova AF, Terzic A, Kokoz YM. Comparative analysis of the kinetic characteristics of L-type calcium channels in cardiac cells of hibernators. Biophys J. 1996;70:786–797. doi: 10.1016/S0006-3495(96)79618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yatani A, Kim SJ, Kudej RK, Wang Q, Depre C, et al. Insights into cardioprotection obtained from study of cellular Ca2+ handling in myocardium of true hibernating mammals. Am J Physiol Heart Circ Physiol. 2004;286:H2219–H2228. doi: 10.1152/ajpheart.01096.2003. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman BF, Cranefield PF. Electrophysiology of the heart. New York: McGraw-Hill; 1960. [Google Scholar]
- 23.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 24.Kamm KE, Zatzman ML, Jones AW. Maintenance of ion concentration gradients in the cold in aorta from rat and ground squirrel. Am J Physiol. 1979;237:C17–C22. doi: 10.1152/ajpcell.1979.237.1.C17. [DOI] [PubMed] [Google Scholar]
- 25.Harris DM, Mills GD, Chen XW, Kubo H, Berretta RM, et al. lterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ Res. 2005;96:543–550. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- 26.Hochachka PW. Defense strategies against hypoxia and hypothermia. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Wang LCH, Belke DD. Effects of temperature and pH on cardiac myofilament Ca2+ sensitivity in rat and ground squirrel. Am J Physiol. 1993;264:R104–R108. doi: 10.1152/ajpregu.1993.264.1.R104. [DOI] [PubMed] [Google Scholar]
- 28.Lampe JW, Becker LB. State of the art in therapeutic hypothermia. Annual review of medicine. 2011;62:79–93. doi: 10.1146/annurev-med-052009-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Heart Association. American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112:IV-84–IV-138. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 30.Weir MR. Therapeutic Benefits of Calcium-Channel Blockers in Cyclosporine-Treated Organ Transplant Recipients - Blood-Pressure Control and Immunosuppression. Am J Med. 1991;90:S32–S36. doi: 10.1016/0002-9343(91)90483-e. [DOI] [PubMed] [Google Scholar]
- 31.Chien S, Oeltgen PR, Diana JN. Two-day preservation of major organs with autoperfusion multiorgan preparation and hibernation induction trigger. J Thorac Cardiovasc Surg. 1991;102:224–234. [PubMed] [Google Scholar]
- 32.Fu Y, Zhang GQ, Hao XM, Wu CH, Chai Z, et al. Temperature dependence and thermodynamic properties of Ca2+ sparks in rat cardiomyocytes. Biophys J. 2005;89:2533–2541. doi: 10.1529/biophysj.105.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SQ, Zhou ZQ. Alpha-stat calibration of indo-1 fluorescence and measurement of intracellular free calcium in rat heart cells at different temperatures. Life Sci. 1999;65:871–875. doi: 10.1016/s0024-3205(99)00317-3. [DOI] [PubMed] [Google Scholar]
- 34.Xu M, Zhou P, Xu SM, Liu Y, Feng X, et al. Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol. 2007;5(2):e21. doi: 10.1371/journal.pbio.0050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannell MB, Berlin JR, Lederer WJ. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987;238:1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contraction of papillary heart muscles in rats (left) and ground squirrels (right) in response to 0.2 Hz field stimulation at 8°C. Note the after-contractions and elevated resting tension in the rat.
(TIF)
SR Ca2+ load of ventricular myocytes at room temperature (RT) and 10°C were measured in rats and ground squirrels by perfusing the cells with 20 mmol/L caffeine after 15 min indo-1 AM loading. SR load was reported as the ratio of fluorescence at 405 nm vs. 485 nm.
(TIF)