Abstract
Local translation of dendritic mRNAs plays an important role in neuronal development and synaptic plasticity. Although several hundred putative dendritic transcripts have been identified in the hippocampus, relatively few have been verified by in situ hybridization and thus remain uncharacterized. One such transcript encodes the protein neuronatin. Neuronatin has been shown to regulate calcium levels in non-neuronal cells such as pancreatic or embryonic stem cells, but its function in mature neurons remains unclear. Here we report that neuronatin is translated in hippocampal dendrites in response to blockade of action potentials and NMDA-receptor dependent synaptic transmission by TTX and APV. Our study also reveals that neuronatin can adjust dendritic calcium levels by regulating intracellular calcium storage. We propose that neuronatin may impact synaptic plasticity by modulating dendritic calcium levels during homeostatic plasticity, thereby potentially regulating neuronal excitability, receptor trafficking, and calcium dependent signaling.
Introduction
Local translation of mRNAs in neuronal dendrites provides a means for rapidly eliciting site-specific changes in protein levels during neuronal development and synaptic plasticity. Dendritic localization of poly-A containing RNAs and translation machinery, such as ribosomes and translation factors, enables protein synthesis hundreds of microns from the soma [1], [2], [3], [4]. Gene expression profiling of isolated dendrites identified as many as 450 putative dendritic mRNAs in the hippocampus [5], [6], [7], [8]. However, only a handful of mRNAs, including Arc (activity regulated cytoskeletal protein), Eef1α (eukaryotic elongation factor 1 α), and CaMKIIα (Ca2+/CaM dependent kinase IIα) have been verified as being dendritically localized and/or translated in response to stimuli, such as those that induce long-term potentiation (LTP), long-term depression (LTD), or homeostatic plasticity [9], [10], [11], [12], [13].
One uncharacterized dendritic mRNA encodes the protein neuronatin (NNAT) which was first identified in embryonic rat brain and subsequently shown to be enriched in isolated dendrites [5], [14]. NNAT is expressed in rat as two alternatively spliced isoforms, encoding an 81 or 54 amino acid protein (NNATα or β) [14]. Its levels are highest early in brain development, with the NNATα isoform being expressed at E7–10 and the β isoform appearing at E11–14, during the onset of neurogenesis. NNATα and β levels continue to increase during neurogenesis (between E16–19) and decrease postnatally [15]. The Nnat gene is also maternally imprinted and contains a neuron-restrictive silencer element [16], [17]. Non-neuronal data from pancreatic beta and 3T3-L1 cells shows that NNAT resides in the endoplasmic reticulum (ER) and modulates intracellular Ca2+stores [18], [19]. NNAT is strikingly similar to phospholamban (PLN), an ER-resident Ca2+ regulator found in cardiac muscle. Both proteins bear α-helical membrane domains and highly basic cytoplasmic tails [14]. However, even though the mechanism by which PLN regulates Ca2+ by SERCA pump inhibition has been studied extensively, much less is known about the cellular and molecular function of NNAT in mature neurons, particularly within the dendrite [20].
Local intracellular Ca2+ concentrations are tightly controlled and compartmentalized in neurons [21]. Due to its potent effects, cytoplasmic Ca2+ is rapidly cleared by mechanisms such as extrusion into the ER or mitochondrial uptake [22], [23], [24]. Aberrant Ca2+ levels may result in abnormal synaptic development and learning-related plasticity, and may contribute to cognitive disorders such as Fragile X Syndrome [25], [26], [27], [28], [29]. Calcium signaling, particularly in dendrites and spines, has been tied to LTP and LTD induction, as well as spine morphology [22], [26], [30], [31], [32]. Local translation of dendritically localized transcripts such as calmodulins, CaMKIIá, visinin-like protein-1, NMDA (N-methyl-D-aspartic acid), and AMPA (2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid) receptors may be a means for regulating dendritic Ca2+ signaling [5],[33],[34],[35],[36],[37]. Although uncharacterized in neurons, NNAT may also belong to this group due to the dendritic localization of its mRNA and its ability to regulate intracellular Ca2+ [18],[19].
Since local translation and Ca2+ regulation are critical during synaptic plasticity, and given its ability to modulate intracellular Ca2+ in non-neuronal cells, we asked how NNAT translation was locally regulated and if it might be involved in dendritic Ca2+ signaling in mature hippocampal neurons. We also examined a potential interaction between Nnat mRNA and FMRP (Fragile X Mental Retardation Protein), an RNA binding protein that regulates dendritic mRNA localization and translation, and whose absence or loss-of-function is thought to underlie Fragile X syndrome [38],[39],[40]. Here, we report that NNAT is indeed dendritically translated in mature neurons during homeostatic plasticity and that it likely regulates dendritic calcium by modulation of intracellular Ca2+ stores.
Results
Localization of neuronatin mRNA and protein in mature hippocampal dendrites
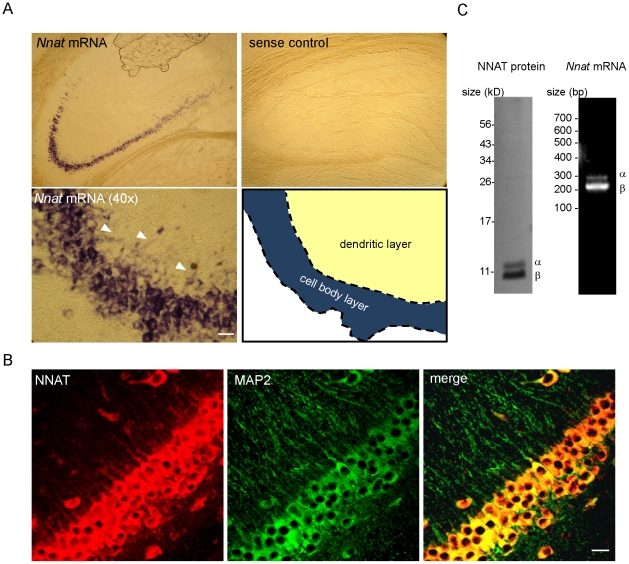
To understand the function of NNAT in mature neurons, we first examined NNAT expression in rat hippocampal tissue (P21). At this age, neurons in the hippocampus have formed synaptic connections and are capable of displaying forms of learning-related and homeostatic plasticity [41],[42]. In vivo, Nnat mRNA was somatodendritically expressed, but its expression was restricted primarily to hippocampal CA2 and CA3 regions (Fig. 1A). Our data agrees with the Allen Mouse Brain Atlas (http://www.brainatlas.org), which further shows expression in the caudate, hypothalamus, and amygdala. Immunohistochemistry in rat hippocampal slice also showed somatodendritic NNAT protein expression in CA2 and CA3 as demonstrated by co-localization with the somatodendritic marker MAP2 (microtubule-associated protein 2) (Fig. 1B, Fig. S1A). NNAT intensity was strongest in proximal dendrites. NNAT antibody specificity was verified by immunostaining HeLa cells overexpressing NNAT (Fig. S1B). Both NNAT isoforms were present in the P21 and adult hippocampus as confirmed by Western blot and RT-PCR (Fig. 1C and Fig. S1C).
Figure 1. Neuronatin is expressed in the P21 rat hippocampus.
(A) Nnat in situ hybridization (ISH) in hippocampus visualized using alkaline phosphatase. Nnat mRNA is present in hippocampal regions CA2 and 3. Arrowheads denote dendritic localization. Scale bar: 20 µm. Schematic of cell body and dendritic layer boundary in 40× image is shown (bottom right panel). (B) NNAT immunofluorescent staining in the CA2 region of hippocampus showing dendritic localization, NNAT (red), MAP2 (green). Scale bar: 20 µm. (C) left, Western blot for NNAT and right, RT-PCR for Nnat mRNA using rat hippocampal tissue showing both α and β isoforms.
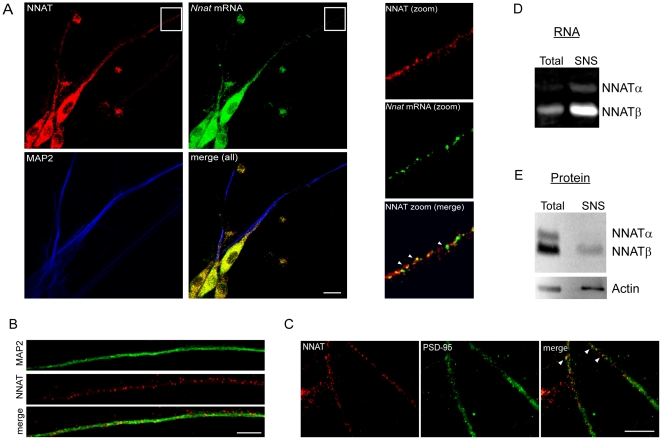
We next used fluorescent in situ hybridization (FISH) combined with immunocytochemistry (ICC) to examine the intracellular localization of Nnat mRNA and protein in mature hippocampal cultures. Both were observed in distal dendrites (>150 µm) as demonstrated by co-localization of Nnat mRNA with MAP2 (Fig. 2A, Fig. S2A). NNAT puncta were also observed along the length of the dendrite, but only partially co-localized with the synaptic marker PSD-95, suggesting that NNAT expression is not restricted to synapses (Fig. 2B and C). NNAT did not co-localize with the axonal marker β III tubulin by immunofluorescence (data not shown). NNAT mRNA and protein were observed in both excitatory and inhibitory neurons as determined by cell morphology [43].
Figure 2. NNAT mRNA and protein are present in dendrites and synapses.
(A) NNAT protein (red) in cultured hippocampal neurons; Nnat mRNA (green); somatodendritic marker, MAP2 (blue). Scale bar: 10 µm. Zoom of region boxed in white. Arrowheads denote sites of co-localization. (B) NNAT puncta along the dendrite as shown by immunofluorescence, MAP2 (green), NNAT (red), and merge. Scale bar: 10 µm. (C) Right, immunocytochemistry shows partial NNAT co-localization with synapses using antibodies against NNAT (red) and the synaptic marker PSD-95 (green), arrowheads in merge denote sites of co-localization. Scale bar: 10 µm. (D) NNATα and â mRNAs are enriched in synaptoneurosomes (SNS) as analyzed by RT-PCR (25 cycles) using equal amounts of total or SNS RNA. (E) NNAT protein levels in total hippocampal (total) versus SNS as assessed by immunoblot. Actin levels were used as a loading control.
To examine synaptic enrichment, we assessed levels of Nnatα and β mRNA and protein in synaptoneurosomes (SNS), a biochemically enriched synaptic preparation [11],[44],[45]. Interestingly, enrichment of both Nnatα and β transcripts was not reflected at the protein level, implying that translational control may play a significant role in regulating synaptic NNAT expression (Fig. 2D). PSD-95 (a synaptic marker) and histone 3 (a somatic marker) protein levels were used to verify synaptoneurosome enrichment relative to whole tissue (Fig. S2B).
Dendritic NNAT translation in response to TTX/APV treatment
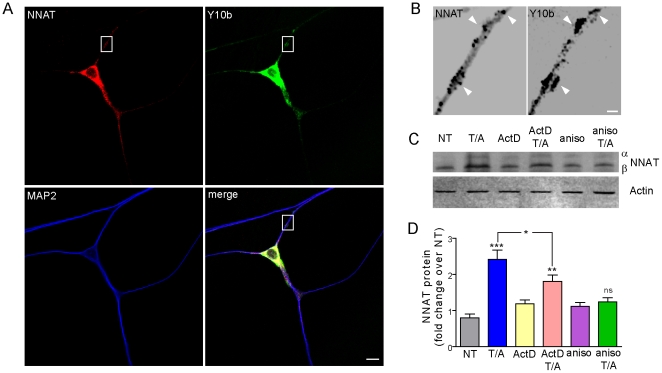
To determine whether Nnat might be locally translated, we first examined if NNAT protein co-localized with dendritic translation hot spots, characterized as discrete ribosome-containing sites along the dendrite [46]. Co-immunofluorescent staining of NNAT and ribosomes (using the Y10b antibody, which recognizes the 5.8S ribosomal subunit) showed co-localization between dendritic NNAT puncta and ribosomes in cultured hippocampal dendrites (Fig. 3A and B) [47].
Figure 3. Cell-wide NNAT protein levels are induced following TTX/APV treatment.
(A) Immunofluorescent staining in cultured hippocampal neurons using an antibody for NNAT (red), the 5.8S RNA antibody Y10b (green), and MAP2 (blue). Scale bar: 10 µm. (B) Magnified view of boxed region in (A) showing localization of NNAT and Y10b signal. Grayscale used to enhance contrast. Arrowheads denote sites of NNAT and ribosome co-localization. Scale bar: 1 µm. (C) NNAT is translationally regulated by TTX/APV as examined by immunoblot using lysates from hippocampal cultures treated with TTX/APV for 8 hrs in the presence or absence of transcriptional or translational inhibitors. Actin was used as loading control. (D) Quantification of total NNAT protein levels following treatment (expressed as fold change over no treatment (NT). Values were all n = 5 (3 independent experiments), where *** p<0.001, ** p<0.01, * p<0.05).
NMDA receptor blockade with APV ((2R)-amino-5-phosphonovaleric acid) in the presence of TTX (tetrodotoxin), induces a rapid, local protein synthesis dependent form of homeostatic plasticity [41],[48]. Since TTX/APV treatment increases synaptic Ca2+ permeability, we wanted to examine whether NNAT, with its putative role in Ca2+ regulation, might also be translated in response to TTX/APV treatment [11],[41],[49],[50]. We observed a 2.4-fold increase in total NNAT levels after 8 hrs of TTX/APV in cultured hippocampal neurons (Fig. 3C and D). To determine if this induction was transcription-dependent, neurons were pretreated with a transcriptional inhibitor, actinomycin D (ActD). ActD pretreatment reduced TTX/APV-induced NNAT levels by 25%, implying a transcriptional component to the cell-wide increase in NNAT (Fig. 3C and D). We also pretreated neurons with a protein synthesis inhibitor, anisomycin, to ensure that NNAT induction was translation-dependent. As expected, anisomycin pretreatment completely abolished any increase in NNAT protein levels (Fig. 3C and D).
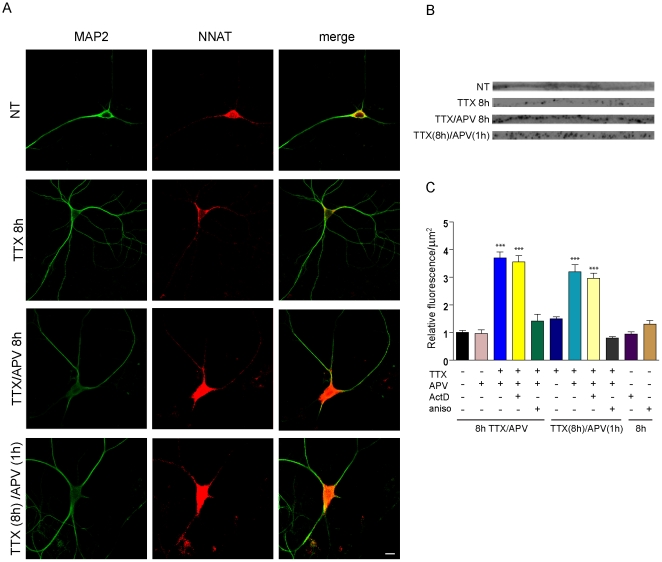
To specifically determine whether the increase in NNAT represents dendritic protein synthesis, we performed immunofluorescent staining in cultured neurons using antibodies against NNAT and MAP2 (Fig. 4A and B). Upon TTX/APV treatment, we observed a 3.3-fold increase in dendritic signal. Importantly, even in the presence of ActD, we continued to observe a 3-fold increase in dendritic NNAT levels following TTX/APV. By contrast, pretreatment with the translation inhibitor anisomycin completely blocked this increase. Neither ActD nor anisomycin alone had any effect on NNAT baseline levels (Fig. 4C). These data indicate that an increase in translation, not transcription, is responsible for the elevated dendritic NNAT levels in response to TTX/APV treatment.
Figure 4. Neuronatin is dendritically translated in response to TTX/APV.
(A) Cultured hippocampal neurons were immunostained for MAP2 and NNAT after no treatment, or treatment with 8 h TTX, 8 h TTX/APV, or TTX (8 h)+APV(1 h). Scale bar: 10 µm. (B) Magnified 30 µm straightened dendritic segments taken from neurons treated as in (A) showing NNAT distribution. Images were grayscaled to enhance contrast. (C) Quantification showing NNAT fluorescence in response to TTX/APV as a function of dendritic area (in µm2). All values were compared to no treatment (NT). Sample size: NT, n = 39; 8 h APV, n = 14; 8 h TTX/APV, n = 30; ActD+8 h TTX/APV, n = 30; aniso+TTX/APV, n = 12; 8 h TTX alone, n = 38; TTX (8 h)+APV (1 h), n = 18, TTX (8 h)+APV(1 h) ActD, n = 22; TTX (8 h)+APV(1 h) aniso, n = 27, actinomycin D (ActD), n = 20, anisomycin (aniso), n = 12. *** p<0.001). Each condition was performed using at least 3 independent batches of cultures.
Since 8 hrs of TTX/APV treatment allows ample time for proteins to be somatically synthesized then transported to distal dendritic sites, we sought to further delineate the dendritic contribution to the NNAT increase by using short inhibition of NMDAR activity (with APV) following action potential blockade as shown by Sutton et al. [41]. We treated neurons with TTX for 8 hrs, applied APV only during the last hour, and observed an anisomycin-sensitive 2.2-fold increase in dendritic NNAT levels; TTX alone had no effect (Fig. 4A–C). This effect persisted even during transcriptional inhibition with ActD, supporting the notion that NNAT is dendritically translated (Fig. 4A and C). Similar effects were also observed using another translation inhibitor, cycloheximide (Fig. S3).
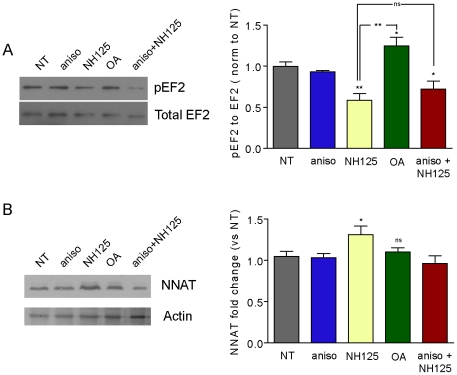
During TTX/APV treatment, NMDAR-mediated Ca2+ influx is inhibited, suppressing EF2K (elongation factor 2 kinase) activity, resulting in enhanced translation [51]. To see if TTX/APV-induced NNAT translation could occur as a result of modulating EF2 phosphorylation, we treated SNS with either NH125 (an EF2K inhibitor) or 2 nM okadaic acid (OA), which inhibits EF2 dephosphorylation via protein phosphatase 2a [51],[52],[53]. SNS provide the advantage of being rapidly obtainable from tissue and are amenable to pharmacological manipulation and biochemical analysis [54]. We first confirmed pharmacological control of EF2 phosphorylation and observed that levels of phospho-EF2 decreased in response to NH125 and increased as a result of OA treatment (Fig. 5A). In response to NH125 treatment, we observed a 1.3-fold increase in NNAT levels; no significant increase was observed in response to okadaic acid (Fig. 5B). Inhibition of translation with anisomycin attenuated the NH125-induced increase in NNAT protein. Although stimuli other than TTX/APV blockade may also influence EF2 phosphorylation, our data show that NNAT can be translated at the synapse in an EF2-dependent manner.
Figure 5. NNAT translation in synaptoneurosomes is regulated by EF2 phosphorylation.
(A) SNS were treated with anisomycin (aniso), NH125 (an EF2 kinase inhibitor), 2 nM okadaic acid (OA) (an inhibitor of PP2A), or anisomycin+NH125, followed by immunoblot of phosphorylated (pEF2) and total EF2. Values were graphed as a ratio of phospho- to total EF2 and normalized to no treatment (n = 6 per group). (B) Total NNAT levels in synaptoneurosomes treated as in (A). Values were normalized to actin and compared to the no treatment group. Sample size was n = 7 (4 independent experiments) per group. ** p<0.01, * p<0.05).
Neuronatin overexpression increases baseline calcium levels
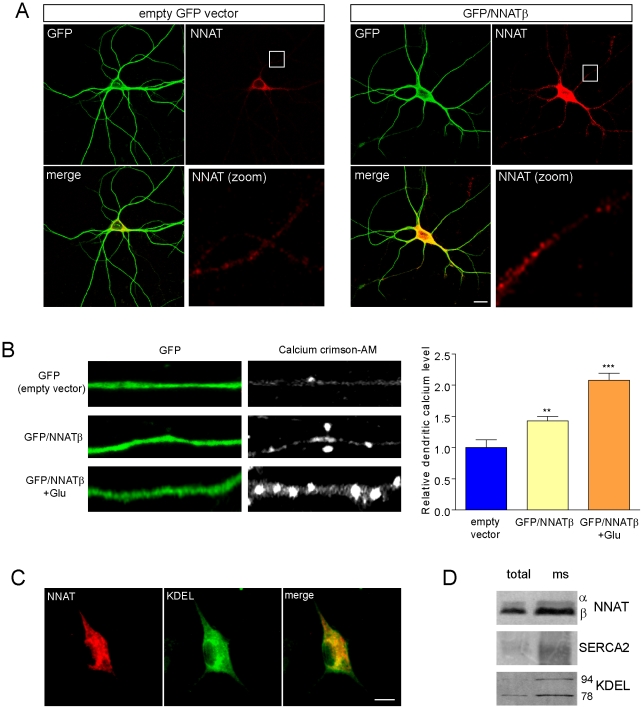
NNAT overexpression in non-neuronal cell types modulates intracellular Ca2+ storage, resulting in elevated cytoplasmic Ca2+ levels [18],[19].To see if NNAT could regulate dendritic Ca2+ levels, we overexpressed NNATβ, the predominant isoform, in mature hippocampal cultures followed by loading with Calcium Crimson-AM, a BAPTA-based Ca2+ indicator dye [55],[56]. NNATβ was overexpressed using a dual expression construct containing GFP and the full length rat Nnatβ mRNA sequence (including 5′ and 3′ UTRs to preserve translational control and localization) driven by separate promoters (GFP/NNATβ) (Fig. 6A). Neurons overexpressing NNATβ (as identified by GFP fluorescence) exhibited a 1.3-fold increase in dendritic Ca2+ levels compared to those transfected with the empty vector (Fig. 6B). A brief five minute glutamate application was used to verify that Ca2+ had not reached ceiling levels.
Figure 6. NNATβ overexpression results in elevated dendritic Ca2+.
(A) Neurons were transfected with the empty GFP vector (left) or the dual expression GFP/NNATβ (right), allowed to express for 8 hrs, and immunostained for NNAT (red) and GFP (green). Scale bar: 10 µm. (B) Left, neurons transfected with empty vector or GFP/NNATβ were loaded with Calcium Crimson-AM, and dendritic GFP and Calcium Crimson signals were acquired. GFP/NNATβ transfected cells were also treated with 40 µM glutamate (5 min) following Calcium Crimson loading to further induce Ca2+ levels. Calcium Crimson signal was grayscaled to enhance contrast. Values expressed as ratios of Calcium Crimson normalized to GFP fluorescence (empty vector, n = 12 dendrites, 4 neurons; GFP/NNATâ, n = 12 dendrites, 6 neurons; GFP/NNATβ+Glu, n = 10 dendrites, 5 neurons, **p<0.01, ***p<0.001). Experiments were performed using 2 independent batches of cultures. (C) NNAT is localized to the endoplasmic reticulum (ER). Cultured neurons co-immunostained with NNAT (red) and an ER marker against KDEL (green). Scale bar: 5 µm. (D) NNAT is 1.8-fold enriched in microsomes (ms) generated from hippocampi compared to total protein. Microsome enrichment was assessed using antibodies against ER markers Grp94 (2.1-fold enrichment), Grp78 (2.7-fold enrichment), and SERCA2 (3.0-fold enrichment).
NNAT regulates calcium by antagonizing SERCA pump activity
Given the structural similarities between NNAT and phospholamban, and the presence of SERCA in neuronal dendrites and synapses, we hypothesized that NNAT might function as a SERCA pump regulator (Fig. S2B) [57]. ER localization of NNAT has been previously observed in other cell types, but has not been examined in mature neurons [18],[58]. We verified NNAT localization to neuronal ER by co-immunostaining NNAT with a KDEL ER-marker antibody and observed perinuclear co-localization, consistent with ER distribution (Fig. 6C). Microsomes (an ER-enriched preparation) generated from rat hippocampi, also showed a 1.8-fold enrichment of NNAT (Fig. 6D). Microsome purity was verified by immunoblotting with the KDEL (which recognizes Grp78 and Grp94) or SERCA2 antibody (Fig. 6D).
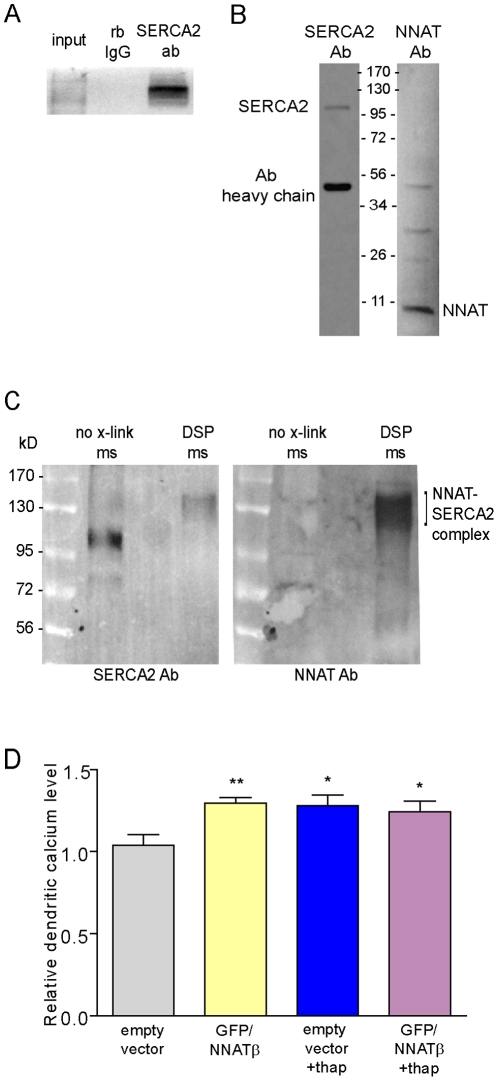
We next examined whether NNAT interacts with the SERCA pump. Using an antibody against SERCA2, the predominant neuronal isoform, we were able to co-immunoprecipitate (co-IP) NNAT from hippocampal microsomes suggesting a possible association (Fig. 7A and B). However, to show a more direct association, we first crosslinked microsomes using DSP (dithiobis [succinimidyl propionate]), followed by co-IP using the SERCA2 antibody. In uncrosslinked microsomes, we detected SERCA2 at 114 kD by Western blot (Fig. 7C, left). Upon crosslinking and co-IP, however, we observed a size-shifted SERCA2-containing complex at ∼120–150 kD (Fig. 7C, left). Re-probing of the same blot with the NNAT antibody revealed that this complex also contained NNAT (Fig. 7C, right).
Figure 7. Neuronatin is associated with SERCA2 in the hippocampus.
(A) SERCA2 antibody immunoprecipitation efficiency compared to rabbit IgG. 2.5% of input or immunoprecipitated samples were loaded. (B) NNAT was co-immunoprecipitated from microsomes using SERCA2 antibody. Pulldown was confirmed by immunoblotting with SERCA2 or NNAT antibody. (C) Microsomes were crosslinked with dithiobis [succinimidyl propionate] (DSP) followed by SERCA2 immunoprecipitation and immunoblot analysis. SERCA2 antibody (left) recognizes a 114 kD band in uncrosslinked microsomes and a size-shifted complex at 120–150 kD after crosslinking and IP. Right, membrane was stripped and reprobed with NNAT antibody, revealing the presence of NNAT within the 120–150 kD complex. (D) Overexpression of GFP/NNATβ occludes Ca2+ elevation by thapsigargin (GFP/NNATβ+thap), as measured using Calcium Crimson-AM, suggesting that NNAT antagonizes SERCA pump activity (empty vector: n = 24 dendrites, 14 neurons; GFP/NNATâ: n = 33 dendrites, 14 neurons; empty vector+thap: 18 dendrites, 8 neurons; GFP/NNATβ+thap: 19 dendrites, 8 neurons; GFP/NNATβ+thap+Glu: 19 dendrites, 9 neurons. **p<0.01, *p<0.05). Experiments were performed using at least 2 independent batches of cultures.
Given this association, we hypothesized that the elevation in dendritic Ca2+ following NNAT overexpression was due to increased NNAT inhibition of SERCA pump activity. If this were true, we would expect NNAT overexpression to occlude Ca2+ induction by a SERCA inhibitor, such as thapsigargin. Indeed, no additive Ca2+ increase was observed following thapsigargin treatment in neurons transfected with GFP/NNATâ, while in empty GFP vector transfected neurons, thapsigargin increased dendritic Ca2+ by 1.23-fold (Fig. 7D). Similar to before, overexpression of GFP/NNATâ resulted in a 1.25-fold increase in dendritic Ca2+. To exclude the possibility that NNATβ overexpression induced a Ca2+ ceiling effect, following thapsigargin, we treated GFP/NNATâ transfected neurons with an additional 5 min pulse of glutamate (to activate extracellular Ca2+ influx or intracellular Ca2+ release) and observed a 1.54-fold increase in Ca2+ over the GFP/NNATβ transfected baseline levels (data not shown).
Fragile X Mental Retardation Protein binds Nnat mRNA
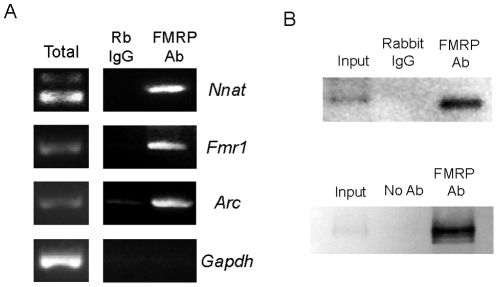
A screen for mRNAs associated with FMRP (Fragile X Mental Retardation protein) by Miyashiro et al., suggested a putative association between FMRP and Nnat mRNA [38]. Recent work has also shown that the loss of FMRP impairs TTX/APV-induced homeostatic plasticity [59]. Using hippocampal tissue, we confirmed that Nnat mRNA associates with FMRP by RNA co-IP using an FMRP antibody (Fig. 8A). Binding specificity was verified by enrichment of two known FMRP binding targets, Arc and Fmr1 [10],[60] and the lack of Gapdh enrichment in the immunoprecipitated sample (Fig. 8A) [39],[61]. Immunoprecipitation of FMRP protein was specific as assessed by rabbit IgG and no antibody negative controls (Fig. 8B). However, when attempting to co-immunoprecipitate Nnat mRNA and FMRP from hippocampal tissue at high stringency using ultraviolet crosslinking (which is specific for protein-nucleic acid interactions) and an SDS-containing wash buffer, we were unable to detect a direct association between FMRP and Nnat mRNA [38],[62]. This lack of association by UV crosslinking suggests the intriguing possibility that an unknown intermediate, such as a protein or RNA species, may facilitate the interaction between FMRP and Nnat mRNA.
Figure 8. Nnat mRNA associates with FMRP.
(A) RNA co-immunoprecipitation (non-UV crosslinked) followed by RT-PCR using FMRP antibody or rabbit IgG for Nnat mRNA. Known FMRP binding targets, Arc and Fmr1, are enriched in the FMRP immunoprecipitated sample, whereas Gapdh, a non-target, is not. Based on product size, Nnatβ was the predominant FMRP-binding isoform. RT-PCR using total hippocampal RNA is shown in the left column. (B) FMRP immunoprecipitation efficiency was tested using 2.5% input or immunoprecipitated hippocampal protein, probed with FMRP antibody and compared to rabbit IgG or no antibody.
Discussion
Although high embryonic and early postnatal expression has suggested significant roles for NNAT during neuronal development, its function in mature neurons has not been examined. Here, we show that Nnat mRNA is expressed and dendritically translated during homeostatic plasticity in mature hippocampal neurons. Moreover, we have demonstrated that it regulates dendritic Ca2+ levels by antagonizing SERCA pump activity. Given the importance of Ca2+ signaling during neuronal events, together with data that Nnat mRNA associates with FMRP, we propose that NNAT may play a crucial role in synaptic and possibly cognitive function.
The presence of ER throughout dendrites and synapses provides a readily accessible Ca2+ source, potentially allowing NNAT to modulate local intracellular Ca2+, and thus influence site-specific events, such as LTP and LTD [21],[63],[64]. Since the ER is continuous between the dendritic shaft and synapse, transport along the ER membrane could facilitate the localization of dendritically translated NNAT to the synapse. Thus, induction of synaptic NNAT levels would not be limited exclusively to translation at the synapse. Rather, such a mechanism would allow dendritic protein synthesis to contribute to synaptic NNAT levels as well [65],[66].
TTX/APV-induced homeostatic plasticity regulates dendritic translation by modulating the state of EF2 phosphorylation [51]. Our data here shows that synaptic Nnat mRNA translation is indeed sensitive to EF2 phosphorylation; however, NNAT induction was relatively modest, suggesting that other factors may be involved. Retinoic acid has recently been implicated in TTX/APV mediated homeostatic plasticity [11]. Molecularly, RARα (retinoic acid receptor α) binds to consensus motifs in the 5′UTR of certain mRNAs thus repressing their translation [67]. Unpublished data from our lab suggests that while RA does affect NNAT protein levels, Nnat mRNA does not bind RARá, and is probably not subject to direct translational regulation by RARα. We are currently investigating additional molecular mechanisms at play, including possible cis-acting motifs in the 5′ and 3′ untranslated regions that might underlie the translational control or localization of Nnat mRNA. Our data also shows that TTX/APV results in a transcription-dependent increase in NNAT. Although we have yet to investigate the underlying mechanism, previous reports have shown that TTX-induced homeostatic plasticity can induce transcription via CaMKIV [68].
Many FMRP-regulated mRNAs are present in dendrites and it has been proposed that their aberrant translation or mislocalization is an underlying cause of Fragile X syndrome [69],[70],[71]. We observed here that Nnat mRNA associates with FMRP, but likely through an indirect interaction. Recently, Edbauer and colleagues reported that a subset of microRNAs associate with FMRP to regulate synapse structure and function [61]. Such an intermediate might also participate in the FMRP-Nnat interaction. Interestingly, certain hallmarks of the Fmr1−/− mouse (a model of Fragile X syndrome), including enhanced mGluR-LTD, prolonged epileptiform bursts in hippocampal CA3, and elongated dendritic spines are consistent with the notion of perturbed Ca2+ signaling [26],[69],[72]. We are currently conducting studies in the Fmr1 −/− mouse to investigate whether these might also be linked to perturbed NNAT levels.
Using dendritic protein synthesis to regulate Ca2+ signaling presents an attractive mechanism as precise, spatial control of Ca2+ is essential for synapse formation, elimination and various forms of learning-related plasticity [21]. NNAT is well-suited as one such regulator as its local synthesis would allow for sustained changes in cytoplasmic Ca2+ levels. However, the precise molecular events governing the interaction between NNAT and the SERCA pump remains an important question to be answered. That NNAT contains several putative sites of posttranslational modification, including phosphorylation, implicates the involvement of additional signaling pathways. Modification at such sites could facilitate the integration of multiple signaling events at or near the synapse, resulting in the tuning of intracellular Ca2+ signals and any associated downstream pathways [73].
In summary, our data support that NNAT functions as a dendritic Ca2+ regulator whose levels are locally controlled in an activity-dependent manner. Its potential involvement in cognitive disorders, such as Fragile X syndrome, makes NNAT an appealing candidate for future study. Interestingly, phenylketoneuria patients, some of whom display autism-like symptoms, also exhibit abnormally high levels of NNAT [74],[75]. We posit therefore, that understanding the action and regulation of NNAT may shed light on the molecular basis of certain forms of cognitive impairment, particularly those associated with aberrant Ca2+ signaling. In a broader sense, our study also underscores a relationship between local translation and Ca2+ signaling, demonstrating the functional richness of dendritically localized mRNAs and the pressing need for their characterization.
Materials and Methods
Antibodies
Information on antibodies can be found in the Supplementary Information section.
Primers and constructs
Primer sequences and cloning strategies can be found in the Supplementary Information section.
Animals
Sprague-Dawley rats used in these experiments were housed at the Joint Science Department and handled according to guidelines outlined and approved by the Institutional Animal Care and Use Committee at the Joint Science Department of the Claremont Colleges. Animals were euthanized using CO2 followed by decapitation and tissue collection.
Calcium imaging
Calcium imaging was performed as described in Korkotian and Segal [76],[77]. Further details can be found in the Supplementary Information section.
SERCA2 crosslinking and co-immunoprecipitation
Microsomes were prepared from P21 hippocampal tissue as described previously [78]. For immunoprecipitation, SERCA2 antibody or rabbit IgG was prepared by prebinding with Protein A/G beads (Santa Cruz Biotechnology, Santa Cruz, CA) equilibrated in NP-40 lysis buffer (1% Nonidet P-40 in PBS, pH 7.4) containing protease inhibitor. DSP (dithiobis [succinimidylpropionate])(Pierce, Rockford, IL) was dissolved in dry DMSO at 25 mM and used on microsomes at 2 mM and incubated for 2 h on ice and quenched at 4°C overnight in 20 mM Tris, pH 7.5. Crosslinked microsomes were pelleted at 140,000× g at 4°C for 1 h. The supernatant was removed and the microsomes resuspended in NP-40 lysis buffer with protease inhibitor and lysed at 4°C overnight with agitation. Lysates were precleared with Protein A/G beads, then incubated with antibody-bound beads at 4°C overnight and samples prepared in 2× SDS sample loading sample (crosslinked samples were prepared in the absence of 5% β-mercaptoethanol).
FMRP RNA co-immunoprecipitation
RNA co-immunoprecipitation was performed as described previously [79] with the following exceptions: hippocampal tissue was Dounce homogenized in RNAse-free lysis buffer containing 0.5% Nonidet P-40 in PBS, 300 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 20 mM Tris, pH 7.4, 5 mM DTT, 1 mg/mL yeast tRNA, protease inhibitors and 40 U/mL RNAseOut (Invitrogen, Carlsbad, CA) and cleared by centrifugation. Antibody was prebound to protein A/G beads in lysis buffer. Lysates were also precleared using Protein A/G beads equilibrated in lysis buffer. The FMRP antibody used for IP (Cell Signaling, Cat#4317) was against a peptide spanning amino acids 536–593 of human origin, which is similar to that used by others [80]. After overnight incubation with lysates, antibody-bound beads were washed three times with lysis buffer, then twice with lysis buffer containing 650 mM NaCl as previously described [79]. RNA was extracted directly from beads using Trizol (Invitrogen, Carlsbad, CA). Immunoprecipitation by UV crosslinking was performed as described previously [67].
Microscopy
All images were taken using either a Zeiss Pascal LSM 510 confocal microscope and LSM software or a Nikon Eclipse 90i epifluorescent microscope using Metamorph software (Universal Imaging, Downington, PA).
Statistical Analysis
All statistics were performed using one-way analysis of variance followed by post hoc analysis with a Newman-Keuls multiple comparison test.
Additional experimental procedures can be found in the Methods S1.
Supporting Information
(A) NNAT expression extends into distal dendrites in hippocampal slice. Top left, 40× merged image from Figure 2B, MAP2 (green), NNAT (red). Top right, magnified image (in order to highlight NNAT expression >100 µm from the soma (arrowhead). Asterisk denotes a possible interneuron in the dendritic layer. Scale bar: 10 µm. (B) NNAT antibody is specific for NNAT by immunocytochemistry. HeLa cells were transfected with NNATβ or empty vector (pCI-Neo), then immunostained using the NNAT antibody (green). Cells were counterstained with propidium iodide (red). Scale bar: 50 µm. (C) left, Western blot for NNAT and right, RT-PCR for Nnat mRNA using P21 and adult (7 month old) rat hippocampal tissue showing both α and β isoforms.
(TIF)
(A) Sense control for Nnat fluorescent in situ hybridization. Top left panel, Nnat sense control (green), top right panel, NNAT immunofluorescence (red), bottom left, MAP2 (blue), bottom right, merge. Scale bar: 10 µm. (B) Synaptoneurosome enrichment was assessed by Western blot using antibodies against Histone 3 (cell body marker) or PSD-95 (synaptic marker) on equal amounts of total or synaptoneurosome (SNS) protein samples. SERCA2 is also present in SNS.
(TIF)
TTX/APV-induced local NNAT synthesis is inhibited by the translation inhibitor, cycloheximide. Quantification summarizing NNAT fluorescence as a function of dendritic area (in µm2) in response to TTX/APV treatment in the presence or absence of cycloheximide. All values were compared to no treatment (NT) using one-way ANOVA followed by Newman-Keuls multiple comparison test (sample size: NT, n = 34 dendrites; 8 h TTX/APV, n = 39 dendrites; actinomycin D (ActD), n = 35 dendrites; ActD+8 h TTX/APV, n = 33 dendrites; cycloheximide (CHX), n = 11 dendrites; CHX+TTX/APV, n = 13 dendrites, *** p<0.001). Experiments were performed using at least 3 batches of independent cultures.
(TIF)
(DOCX)
Acknowledgments
The authors thank Kelsey Martin (UCLA) for reagents and technical assistance, and Florence Lee (UCSF), Dillon Chen (Mount Sinai School of Medicine), Marta Soden (University of Washington) and Kwok-On Lai (HKUST) for helpful reading of the manuscript and comments. The authors also thank Lu Chen (Stanford) and Marta Soden for providing the pFAN construct, Judy Samistribootr for assistance with in situ hybridization in brain slice, members of the Poon lab for helpful discussion, the Harvey Mudd College Department of Biology (particularly Elaine Guerra and Suheilah Abdallah), and the Joint Science Department at the Claremont Colleges for animal housing.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by a Howard Hughes Medical Institute Teaching and Research Postdoctoral Fellowship (Award# 52006301) to M.M.P. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 2.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleiman R, Banker G, Steward O. Differential subcellular localization of particular mRNAs in hippocampal neurons in culture. Neuron. 1990;5:821–830. doi: 10.1016/0896-6273(90)90341-c. [DOI] [PubMed] [Google Scholar]
- 5.Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26:13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberwine J, Belt B, Kacharmina JE, Miyashiro K. Analysis of subcellularly localized mRNAs using in situ hybridization, mRNA amplification, and expression profiling. Neurochem Res. 2002;27:1065–1077. doi: 10.1023/a:1020956805307. [DOI] [PubMed] [Google Scholar]
- 7.Zhong J, Zhang T, Bloch LM. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian QB, Nakayama K, Okano A, Suzuki T. Identification of mRNAs localizing in the postsynaptic region. Brain Res Mol Brain Res. 1999;72:147–157. doi: 10.1016/s0169-328x(99)00214-4. [DOI] [PubMed] [Google Scholar]
- 9.Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, et al. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, Park JM, Kim S, Kim JA, Shepherd JD, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci. 2005;25:7199–7209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB. Tetanic stimulation leads to increased accumulation of Ca(2+)/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J Neurosci. 1999;19:7823–7833. doi: 10.1523/JNEUROSCI.19-18-07823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph R, Dou D, Tsang W. Molecular cloning of a novel mRNA (neuronatin) that is highly expressed in neonatal mammalian brain. Biochem Biophys Res Commun. 1994;201:1227–1234. doi: 10.1006/bbrc.1994.1836. [DOI] [PubMed] [Google Scholar]
- 15.Joseph R, Dou D, Tsang W. Neuronatin mRNA: alternatively spliced forms of a novel brain-specific mammalian developmental gene. Brain Res. 1995;690:92–98. doi: 10.1016/0006-8993(95)00621-v. [DOI] [PubMed] [Google Scholar]
- 16.Dou D, Joseph R. Cloning of human neuronatin gene and its localization to chromosome-20q 11.2–12: the deduced protein is a novel “proteolipid’. Brain Res. 1996;723:8–22. doi: 10.1016/0006-8993(96)00167-9. [DOI] [PubMed] [Google Scholar]
- 17.Kagitani F, Kuroiwa Y, Wakana S, Shiroishi T, Miyoshi N, et al. Peg5/Neuronatin is an imprinted gene located on sub-distal chromosome 2 in the mouse. Nucleic Acids Res. 1997;25:3428–3432. doi: 10.1093/nar/25.17.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joe MK, Lee HJ, Suh YH, Han KL, Lim JH, et al. Crucial roles of neuronatin in insulin secretion and high glucose-induced apoptosis in pancreatic beta-cells. Cell Signal. 2008;20:907–915. doi: 10.1016/j.cellsig.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Suh YH, Kim WH, Moon C, Hong YH, Eun SY, et al. Ectopic expression of Neuronatin potentiates adipogenesis through enhanced phosphorylation of cAMP-response element-binding protein in 3T3-L1 cells. Biochem Biophys Res Commun. 2005;337:481–489. doi: 10.1016/j.bbrc.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 20.Colyer J. Phosphorylation States of Phospholamban. Annals of the New York Academy of Sciences. 1998;853:79–91. doi: 10.1111/j.1749-6632.1998.tb08258.x. [DOI] [PubMed] [Google Scholar]
- 21.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 22.Yuste R, Majewska A, Cash SS, Denk W. Mechanisms of calcium influx into hippocampal spines: heterogeneity among spines, coincidence detection by NMDA receptors, and optical quantal analysis. J Neurosci. 1999;19:1976–1987. doi: 10.1523/JNEUROSCI.19-06-01976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majewska A, Brown E, Ross J, Yuste R. Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J Neurosci. 2000;20:1722–1734. doi: 10.1523/JNEUROSCI.20-05-01722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Korkotian E, Segal M. Release of calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1999;96:12068–12072. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris KM. Calcium from internal stores modifies dendritic spine shape. Proc Natl Acad Sci U S A. 1999;96:12213–12215. doi: 10.1073/pnas.96.22.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohmann C. Calcium signaling and the development of specific neuronal connections. Prog Brain Res. 2009;175:443–452. doi: 10.1016/S0079-6123(09)17529-5. [DOI] [PubMed] [Google Scholar]
- 29.Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman DA, Sprengel R, Sakmann B. Molecular dissection of hippocampal theta-burst pairing potentiation. Proc Natl Acad Sci U S A. 2002;99:7740–7745. doi: 10.1073/pnas.092157999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- 32.Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- 33.Berry FB, Brown IR. CaM I mRNA is localized to apical dendrites during postnatal development of neurons in the rat brain. J Neurosci Res. 1996;43:565–575. doi: 10.1002/(SICI)1097-4547(19960301)43:5<565::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 34.Mayford M, Baranes D, Podsypanina K, Kandel ER. The 3′-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci U S A. 1996;93:13250–13255. doi: 10.1073/pnas.93.23.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, et al. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci. 2006;26:8339–8351. doi: 10.1523/JNEUROSCI.0472-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benson DL. Dendritic compartmentation of NMDA receptor mRNA in cultured hippocampal neurons. Neuroreport. 1997;8:823–828. doi: 10.1097/00001756-199703030-00004. [DOI] [PubMed] [Google Scholar]
- 37.Lai KO, Zhao Y, Ch'ng TH, Martin KC. Importin-mediated retrograde transport of CREB2 from distal processes to the nucleus in neurons. Proc Natl Acad Sci U S A. 2008;105:17175–17180. doi: 10.1073/pnas.0803906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 39.Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 40.Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, et al. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, et al. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 42.Gray EE, Fink AE, Sarinana J, Vissel B, O'Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- 43.Benson DL, Watkins FH, Steward O, Banker G. Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol. 1994;23:279–295. doi: 10.1007/BF01188497. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MW, Chotiner JK, Watson JB. Isolation and characterization of synaptoneurosomes from single rat hippocampal slices. J Neurosci Methods. 1997;77:151–156. doi: 10.1016/s0165-0270(97)00120-9. [DOI] [PubMed] [Google Scholar]
- 45.Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, et al. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J Neurosci. 1985;5:2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 47.Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- 49.Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y, et al. Phosphorylation of AMPA Receptors Is Required for Sensory Deprivation-Induced Homeostatic Synaptic Plasticity. PLoS One. 2011;6:e18264. doi: 10.1371/journal.pone.0018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 52.Redpath NT, Proud CG. The tumour promoter okadaic acid inhibits reticulocyte-lysate protein synthesis by increasing the net phosphorylation of elongation factor 2. Biochem J. 1989;262:69–75. doi: 10.1042/bj2620069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins E, Sim AT. Regulation of neuronal PP1 and PP2A during development. Methods Mol Biol. 1998;93:79–102. doi: 10.1385/0-89603-468-2:79. [DOI] [PubMed] [Google Scholar]
- 54.Weiler IJ, Greenough WT. Potassium ion stimulation triggers protein translation in synaptoneurosomal polyribosomes. Mol Cell Neurosci. 1991;2:305–314. doi: 10.1016/1044-7431(91)90060-2. [DOI] [PubMed] [Google Scholar]
- 55.Bolsover S, Ibrahim O, O'Luanaigh N, Williams H, Cockcroft S. Use of fluorescent Ca2+ dyes with green fluorescent protein and its variants: problems and solutions. Biochem J. 2001;356:345–352. doi: 10.1042/0264-6021:3560345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nath S, Goodwin J, Engelborghs Y, Pountney DL. Raised calcium promotes alpha-synuclein aggregate formation. Mol Cell Neurosci. 2011;46:516–526. doi: 10.1016/j.mcn.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Segal M, Vlachos A, Korkotian E. The spine apparatus, synaptopodin, and dendritic spine plasticity. Neuroscientist. 2010;16:125–131. doi: 10.1177/1073858409355829. [DOI] [PubMed] [Google Scholar]
- 58.Lin HH, Bell E, Uwanogho D, Perfect LW, Noristani H, et al. Neuronatin (Nnat) Promotes Neural Lineage in Embryonic Stem Cells via Ca(2+) Signaling. Stem Cells. 2010 doi: 10.1002/stem.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soden ME, Chen L. Fragile X Protein FMRP Is Required for Homeostatic Plasticity and Regulation of Synaptic Strength by Retinoic Acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, et al. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 2001;20:4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, et al. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 63.Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc Natl Acad Sci U S A. 2009;106:15055–15060. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 65.Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 66.Schuman EM, Dynes JL, Steward O. Synaptic regulation of translation of dendritic mRNAs. J Neurosci. 2006;26:7143–7146. doi: 10.1523/JNEUROSCI.1796-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RARalpha. Proc Natl Acad Sci U S A. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 69.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 74.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 75.Surendran S, Tyring SK, Matalon R. Expression of calpastatin, minopontin, NIPSNAP1, rabaptin-5 and neuronatin in the phenylketonuria (PKU) mouse brain: possible role on cognitive defect seen in PKU. Neurochem Int. 2005;46:595–599. doi: 10.1016/j.neuint.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Korkotian E, Segal M. Regulation of dendritic spine motility in cultured hippocampal neurons. J Neurosci. 2001;21:6115–6124. doi: 10.1523/JNEUROSCI.21-16-06115.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korkotian E, Segal M. Fast confocal imaging of calcium released from stores in dendritic spines. Eur J Neurosci. 1998;10:2076–2084. doi: 10.1046/j.1460-9568.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 78.Gurd JW, Jones LR, Mahler HR, Moore WJ. Isolation and partial characterization of rat brain synaptic plasma membranes. J Neurochem. 1974;22:281–290. doi: 10.1111/j.1471-4159.1974.tb11591.x. [DOI] [PubMed] [Google Scholar]
- 79.Karginov FV, Conaco C, Xuan Z, Schmidt BH, Parker JS, et al. A biochemical approach to identifying microRNA targets. Proc Natl Acad Sci U S A. 2007;104:19291–19296. doi: 10.1073/pnas.0709971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, et al. On BC1 RNA and the fragile X mental retardation protein. Proc Natl Acad Sci U S A. 2008;105:734–739. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) NNAT expression extends into distal dendrites in hippocampal slice. Top left, 40× merged image from Figure 2B, MAP2 (green), NNAT (red). Top right, magnified image (in order to highlight NNAT expression >100 µm from the soma (arrowhead). Asterisk denotes a possible interneuron in the dendritic layer. Scale bar: 10 µm. (B) NNAT antibody is specific for NNAT by immunocytochemistry. HeLa cells were transfected with NNATβ or empty vector (pCI-Neo), then immunostained using the NNAT antibody (green). Cells were counterstained with propidium iodide (red). Scale bar: 50 µm. (C) left, Western blot for NNAT and right, RT-PCR for Nnat mRNA using P21 and adult (7 month old) rat hippocampal tissue showing both α and β isoforms.
(TIF)
(A) Sense control for Nnat fluorescent in situ hybridization. Top left panel, Nnat sense control (green), top right panel, NNAT immunofluorescence (red), bottom left, MAP2 (blue), bottom right, merge. Scale bar: 10 µm. (B) Synaptoneurosome enrichment was assessed by Western blot using antibodies against Histone 3 (cell body marker) or PSD-95 (synaptic marker) on equal amounts of total or synaptoneurosome (SNS) protein samples. SERCA2 is also present in SNS.
(TIF)
TTX/APV-induced local NNAT synthesis is inhibited by the translation inhibitor, cycloheximide. Quantification summarizing NNAT fluorescence as a function of dendritic area (in µm2) in response to TTX/APV treatment in the presence or absence of cycloheximide. All values were compared to no treatment (NT) using one-way ANOVA followed by Newman-Keuls multiple comparison test (sample size: NT, n = 34 dendrites; 8 h TTX/APV, n = 39 dendrites; actinomycin D (ActD), n = 35 dendrites; ActD+8 h TTX/APV, n = 33 dendrites; cycloheximide (CHX), n = 11 dendrites; CHX+TTX/APV, n = 13 dendrites, *** p<0.001). Experiments were performed using at least 3 batches of independent cultures.
(TIF)
(DOCX)