Abstract
Objective
To identify factors associated with increased 30-day mortality after advanced ovarian cancer debulking among elderly women.
Methods
A database linking Medicare records with the Surveillance, Epidemiology and End-Results (SEER) data was used to identify a cohort of 5,475 women aged 65 and older who had primary debulking surgery for stage III or IV epithelial ovarian cancer (diagnosed1995-2005). Women were stratified by acuity of hospital admission. Multivariable analysis was performed to identify patient and treatment related variables associated with 30-day mortality.
Results
Five-thousand four-hundred seventy-five women had surgery for advanced ovarian cancer, and the overall 30-day mortality was 8.2%. Women admitted electively had a 30-day mortality of 5.6 % (251/4517) and those admitted emergently had a 30-day mortality of 20.1% (168/835). Advancing age, increasing stage, and increasing comorbidity score were all associated with an increase in 30-day mortality (all p<0.05) among elective admissions. A high risk group of women admitted electively included those aged 75 or older with stage IV disease and women aged 75 or older with stage III disease and a comorbidity score of 1 or more. This group had an observed 30-day mortality of 12.7% (95%CI 10.7%-14.9%).
Conclusions
Age, cancer stage, and comorbidity scores may be helpful to stratify electively admitted patients based on predicted postoperative mortality. If validated in a prospective cohort, these factors may help identify women who may benefit from alternative treatment strategies.
Introduction
In 2010, an estimated 21,880 women were diagnosed and 13,850 women died of ovarian cancer in the US making it the most lethal gynecologic malignancy and standard treatment consists of cytoreductive surgery and platinum based chemotherapy (1, 2). The amount of residual tumor following primary cytoreduction is inversely related to outcomes, being one of the strongest prognostic factors (3, 4). Recently, improved success of complete cytoreduction by the incorporation of more extensive upper abdominal procedures has been reported at specialized cancer centers and these improvements have been associated with improved median survival (5, 6).
Extensive surgical procedures are associated with substantial post-operative morbidity and mortality. Published reports of 30-day mortality following primary debulking surgery for advanced ovarian cancer have recently been reviewed by Gerestein et. al.(7). The majority of published data consists of single institution reports, with an average 30-day mortality of 2.5%. Population-based reports from Denmark and the Netherlands have reported slightly higher 30-day mortality ranging from 2.5-4.4% (8, 9).
Almost half of American women are 65 or older when diagnosed with ovarian cancer (10). Increasing age has been strongly associated with increased post operative mortality following abdominal surgery(11). 30-day mortality among women over 80 with all stages of ovarian cancer has been reported to range from 5.4%-9.8% (7, 12). In addition to age, post-operative mortality may be related to patient characteristics such as medical co-morbidities and stage of disease, but most reports lack sufficient power to evaluate these associations. Others have suggested that hospital and surgeon characteristics or procedure volume may be related to short term outcomes following surgery for advanced ovarian cancer (13). The ability to identify subpopulations of patients at very high risk for poor post operative outcomes may allow providers to select patients that may benefit from an alternative treatment approach such as the use of neoadjuvant chemotherapy. Recent data have suggested that the use of neoadjuvant chemotherapy prior to cytoreductive surgery results in similar long term survival rates with lower post-operative morbidity and mortality in advanced ovarian cancer(14).
The objective of this study was to estimate 30-day mortality in a large population based cohort of elderly women with advanced ovarian cancer and identify patient and treatment characteristics associated with 30-day mortality. From this analysis we aimed to identify criteria that could be identified pre-operatively and used to predict post-operative mortality in elderly women admitted routinely for ovarian cancer surgery.
Materials and Methods
Internal Review Board approval was obtained from the Human Subjects Division of the University of Washington (IRB 37473)(15). Data for this analysis came from a linkage between the Surveillance Epidemiology, End Results (SEER) database provided by the National Cancer Institute (NCI) and Medicare healthcare claims records provided by the Center for Medical Services (CMS)(16). SEER registries identify 97% of all incident cancer cases among persons residing in SEER regions (14% of the US population in 1995 and 26% in 2005) (17), and 93% of persons in these registries over 65 had Medicare data successfully matched to SEER records in the linkage process(16).
This study identified all women over the age of 65 in the SEER-Medicare database diagnosed with ovarian cancer from January 1, 1995 to December 31, 2005. Women were included if they had American Joint Cancer Committee (AJCC) stage III or IV ovarian cancer (n=13,998). Women were excluded if they had a diagnosis based on autopsy or death certificate only, non-invasive pathology, disease that was not pathologically confirmed, non-epithelial malignancies, or they had a second primary malignancy diagnosed any time in the six months before or after the date of the ovarian cancer diagnosis (1488 excluded). Women had to be continuously covered by Medicare parts A+B and not be enrolled in an HMO from the 12 months prior to diagnosis and at least 9 months after diagnosis (4264 excluded). This study was further limited to the 5475 women from the above cohort who had evidence in Medicare records of a debulking surgery for their ovarian cancer. The first episode of cytoreductive surgery for ovarian cancer in the year after the diagnosis date was identified as defined below from the Medicare records which were available for claims through December 31, 2007.
SEER data was used to identify and categorize age (5 year groups), race (white, black or other) and marital status (married or unmarried). SEER registries were grouped according to geographic region (Northeast, Midwest, South or West). Population density of area of residence was categorized as defined in the SEER files. Median household income from zip code of residence was used as a proxy for socioeconomic status and was derived from 2000 census data included in the SEER files (categorized into quartiles low (1) to high(4)). Tumor stage, grade and histology were determined from SEER. Tumor grade was missing for over 20% of the subjects and so was not utilized as a variable in the analysis. Comorbidity score was determined using claims for the 12 months prior to ovarian cancer diagnosis to calculate the Deyo adaptation (18) of the Charleson comorbidity index (19, 20). One point is assigned for evidence of each of the following: dementia, congestive heart failure, coronary artery disease (heart attack, angina or revascularization), diabetes, hypertension, peripheral vascular disease, pulmonary disease, renal disease or stroke. Two points are assigned for previous malignancy and three points assigned for hepatic disease.
Hospital volume was determined as the number of cases from January 1, 1995-December 31, 2006 for hospitals that were located within SEER registry areas as indicated in the hospital files in the SEER-Medicare database. Hospital volume was categorized by the number of ovarian cancer cases over the study period as described by Schrag et al. as low (1-12), intermediate (13-28) and high (>28)(21). Only women whose surgeries occurred at hospitals located in SEER areas were included in the multivariable analysis that accounted for treatment variables to ensure that all Medicare cases for that hospital were identified. While this volume does not include non-Medicare cases, it has been shown to correlate with overall hospital volume and allow ranking of hospitals into volume categories(22). Ovarian cancer surgeon was determined by the use of a unique provider identification number when available on provider claims (when available) associated with ovarian cancer surgeries and surgeon volume was categorized into low (1-4), intermediate (5-25) and high (>25). Provider specialty was determined from both Medicare files and AMA files(23). Surgeon specialty was categorized as gynecological oncologist, gynecologist, surgeon or other/unknown.
Admission type was identified in 97.5% of the patients from the inpatient hospital billing records for the surgical episode. Patients were categorized as having an emergent admission if they were admitted through the emergency department or if the admitting physician indicated on the billing claim that the admission was an emergency.
Surgical treatment for ovarian cancer was identified in the MEDPAR files using ICD-9 procedure codes and in the physician claims using CPT codes indicating surgical resection of the primary tumor as previously described(24). Complexity of the primary surgery may influence 30-day mortality. Identification of upper abdominal procedures at the time of the primary surgery was performed by searching for International Classification of Disease-9 codes (ICD-9) in the inpatient billing records. Patients were classified as having an upper abdominal procedure if they were noted to have a liver (50.22, 50.3), diaphragm (34.81), spleen (41.2, 41.3, 41.5) or pancreatic (52.5, 52.6) resection. Large bowel resections were identified from ICD-9 codes (45.52, 45.7 45.8, 45.92, 45.95, 45.93, 45.94, 48.4, 48.5, 48.6) of the inpatient records. Chemotherapy was identified as previously defined if either the inpatient record, outpatient file or physician claims indicated that chemotherapy was given(15). Chemotherapy was classified as neoadjuvant if administered prior to the date of the primary surgical episode. Previous studies have determined a high level of agreement between Medicare data and chart review in the identification of surgery and chemotherapy among cancer patients (25, 26).
The primary outcome in this study was 30 day mortality after surgery, defined as death from any cause in the 30 days after the primary surgical episode. Death was identified from the Medicare records which is verified with the social security administration and captures all patient deaths regardless of location (in hospital, home, hospice etc).
The chi-squared test was used to compare the frequency distributions of categorical variables. All analyses were stratified based on the admission type and all models empirically included year of diagnosis as a confounding variable to account for possible temporal changes. Because the outcome of interest was not rare, a Poisson regression was used to model incident rate ratios which were interpreted as a relative risk for the outcome of interest. Outcomes at a particular institution may be related to unmeasured factors from the individual institution and thus as described previously a generalized estimating equation was used to account for clustering by hospital(27). Models were fit using generalized estimating equations with a Poisson family, a log link and the hospital identifier as a clustering variable. Variables of interest were classified as either patient (age, race, median household income, marital status, geographic region, size of area of residence, stage, histology and co-morbidity score) or treatment (hospital and surgeon volume, surgeon specialty, upper abdominal procedures, large bowel resection, neoadjuvant chemotherapy) related. The first model fit included all patient related variables significantly associated with 30-day mortality on univariable analysis. The second model included all variables in the first model and the treatment related variables found to be significant on univariable analysis. Model fit was assessed by the use of generalized Pearson residuals. All p values are 2 sided and a p<0.05 was considered significant. No statistical corrections were made for multiple comparisons. STATA SE version 11.0 (College Station, TX) was used for all calculations.
Lastly a decision tree was then constructed for women with routine admissions. Because we aimed to identify women who may benefit from alternative treatment strategies such as neoadjuvant chemotherapy, women who already received neoadjuvant chemotherapy were excluded from this tree (n=605). Similarly only women admitted routinely were included in this tree, as it was hypothesized that women admitted emergently were much more likely to have bowel obstructions and other acute symptoms that would dictate the need for immediate surgery, regardless of the surgical mortality. Only variables that were significant in the multivariable model were included and we limited variables in the analysis to the patient related variables as these were easily ascertained pre-operatively and objective. Age was dichotomized at age 75 (65-75 vs. 75+), stage was classified as III or IV and co-morbidity score was categorized as 0-1 vs. 2+. We were then able to categorize women into risk groups based on the observed 30 day mortality in these subgroups.
Results
Of the 5475 women having surgery for advanced ovarian cancer, 4517 (84.4%) had an elective admission, 835 (15.6%) were admitted emergently and 123 (2.2%) had an unknown admission status. Demographic, clinical and pathological characteristics are listed for the entire cohort and stratified by admission type in Table 1. Women admitted emergently tended to be slightly older (median age 76.9 vs. 75.1 years), with higher comorbidity scores, were more likely to have stage IV disease (41.9% vs. 32.9%), be of non-white race and be unmarried when compared to women with elective admissions (all p<0.001). Geographic variability was noted, with higher proportions of emergent admissions observed in women living in the Northeast and the Midwest than in the South and the West (p<0.001). Neoadjuvant chemotherapy was used in 649 (11.85%) patients, the majority of whom were admitted electively.
Table 1.
Demographic, Clinical and Pathologic Characteristics Stratified by Admission Type¥
| Entire Population n (column %) |
Elective Admission n (column %) |
Emergency Admission n (column %) |
|
|---|---|---|---|
| All patients | 5475 | 4517 (84.40) | 835 (15.60) |
|
| |||
| Age (years) | * | ||
|
| |||
| 65-69 | 1223 (22.34) | 1065 (23.58) | 132 (15.81) |
| 70-74 | 1584 (28.93) | 1346 (29.80) | 209 (25.03) |
| 75-79 | 1464 (26.74) | 1185 (26.23) | 249 (29.82) |
| 80-84 | 822 (15.01) | 647 (14.32) | 150 (17.96) |
| 85+ | 382 (6.98) | 247 (6.07) | 95 (11.38) |
|
| |||
| Race | * | ||
|
| |||
| White | 4927 (89.99) | 4077 (91.82) | 741 (89.17) |
| Black | 265 (4.48) | 193 (4.35) | 64 (7.70) |
| Other | 200 (3.65) | 170 (3.83) | 26 (3.13) |
|
| |||
| Median Household Income | * | ||
|
| |||
| First Quartile | 1263 (23.07) | 1011 (23.36) | 218 (27.39) |
| Second Quartile | 1302 (23.78) | 1064 (24.59) | 205 (25.75) |
| Third Quartile | 1312 (23.96) | 1089 (25.17) | 193 (24.25) |
| Fourth Quartile | 1367 (24.97) | 1163 (26.88) | 180 (22.61) |
|
| |||
| Marital Status | * | ||
|
| |||
| Married | 2511 (45.86) | 2223 (50.40) | 281 (34.99) |
| Not Married | 2819 (51.49) | 2188 (49.60) | 522 (65.01) |
|
| |||
| Region | * | ||
|
| |||
| Northeast | 1074 (19.62) | 803 (17.78) | 246 (29.46) |
| Midwest | 1097 (20.04) | 851 (18.84) | 211 (25.27) |
| South | 782 (14.28) | 661 (14.63) | 108 (12.93) |
| West | 2522 (46.06) | 2202 (48.75) | 270 (32.34) |
|
| |||
| Area of Residence | p=0.022 | ||
|
| |||
| Large Metropolitan | 3133 (57.22) | 2541 (56.25) | 516 (61.80) |
| Metropolitan | 1484 (27.11) | 1246 (27.58) | 213 (25.51) |
| Urban | 334 (6.10) | 279 (6.18) | 45 (5.39) |
| Less Urban | 414 (7.56) | 360 (7.97) | 46 (5.51) |
| Rural | 110 (2.01) | 91 (2.01) | 15 (1.80) |
|
| |||
| Stage | * | ||
|
| |||
| III | 3489 (63.73) | 2953 (65.38) | 466 (55.81) |
| IV | 1885 (34.43) | 1484 (32.85) | 350 (41.92) |
| “Distant” NOS | 101 (1.84) | 90 (1.77) | 19 (2.28) |
|
| |||
| Histology | * | ||
|
| |||
| Serous/Adenocarcinoma | 4206 (76.82) | 3494 (77.35) | 618 (74.01) |
| Mucinous | 204 (3.73) | 153 (3.39) | 47 (5.63) |
| Endometroid | 321 (5.86) | 264 (5.84) | 46 (5.51) |
| Clear Cell | 91 (1.66) | 80 (1.77) | 10 (1.20) |
| Other Epithelial | 653 (11.93) | 526 (11.64) | 114 (13.65) |
|
| |||
| Comorbidity Score | * | ||
|
| |||
| 0 | 3717 (67.89) | 3143 (69.58) | 487 (58.32) |
| 1 | 1163 (21.24) | 942 (20.85) | 199 (23.83) |
| 2 | 365 (6.67) | 274 (6.07) | 83 (9.94) |
| 3+ | 230 (4.20) | 158 (3.50) | 66 (7.90) |
Admission type was available for 5352/5475 (97.75%), not all totals add up to 100% because of rounding and missing data and women for whom admission type was unknown are not included in the elective or emergency admission columns.
indicates p<0.01 comparing elective admissions to emergent admissions
Hospital, surgeon and treatment characteristics are summarized in Table 2. Women admitted emergently were more likely to be operated on in low volume hospitals, by low volume physicians and by non-gynecologic oncologists (all p<0.001). There was no difference in the performance of upper abdominal procedures, but women admitted emergently were more likely to have large bowel resections during surgery (23.95% vs. 18.88%, p=0.001). Women admitted on an emergent basis were much less likely than those admitted routinely to have been previously treated with neoadjuvant chemotherapy (2.99% vs. 13.39%, p<0.001).
Table 2.
Surgery, Surgeon, and lospital Charact eristics Stratified by Admission Type¥
| Entire Population n (column %) |
Elective Admission n (column %) |
Emergency Admission n (column %) |
|
|---|---|---|---|
| No. of patients | 5475 | 4517 (84.40) | 835 (15.60) |
|
| |||
| Hospital Volume | * | ||
|
| |||
| Low (1-12 cases; n=499) | 1679 (30.67) | 1265 (28.01) | 364 (43.59) |
| Intermediate (13-28 cases; n=69) | 1254 (22.90) | 1065 (23.58) | 171 (20.48) |
| High (>28 cases; n=40) | 1867 (34.10) | 1626 (36.00) | 233 (26.71) |
| Non SEER area (n=137) | 675 (12.33) | 561 (12.42) | 77 (9.22) |
|
| |||
| Surgeon Specialty | * | ||
|
| |||
| Gynecological Oncologist (n=309) | 2824 (51.58) | 2496 (55.26) | 287 (34.37) |
| Gynecologist (n= 628) | 1223 (22.34) | 1010 (22.36) | 183 (21.92) |
| Surgeon (n=432) | 681 (12.44) | 465 (10.29) | 171 (20.48) |
| Other/Unknown | 747 (13.64) | 546 (12.09) | 194 (23.23) |
|
| |||
| Provider Volume | * | ||
|
| |||
| Low (1-4 cases; n=1233) | 1640 (29.95) | 1263 (27.96) | 308 (36.89) |
| Intermediate (5-25 cases; n=167) | 1797 (32.82) | 1570 (34.76) | 198 (23.17) |
| High (>25 cases; n=39) | 1408 (25.72) | 1232 (27.27) | 156 (18.68) |
| Missing | 630 (11.51) | 452 (10.01) | 173 (20.72) |
|
| |||
| Upper Abdominal Procedure | p=0.096 | ||
|
| |||
| Yes | 217 (3.96) | 191 (4.23) | 25 (2.99) |
| No | 5258 (96.04) | 4326 (95.77) | 810 (97.01) |
|
| |||
| Large Bowel Resection | * | ||
|
| |||
| Yes | 1072 (19.58) | 853 (18.88) | 200 (23.95) |
| No | 4403 (80.42) | 3664 (81.12) | 635 (76.05) |
|
| |||
| Neoadjuvant Chemotherapy | * | ||
|
| |||
| No | 4826 (88.15) | 3912 (86.61) | 810 (97.01) |
| Yes | 649 (11.85) | 605 (13.39) | 25 (2.99) |
Admission type was available for 5352/5475 (97.75%), not all totals add up to 100% because of rounding and missing data. Women for whom admission type was unknown are not included in the elective or emergency admission columns.
indicates p<0.01 comparing elective admissions to emergent admissions
The 30-day mortality among the entire cohort was 8.22%. Women admitted electively had a much lower 30-day mortality of 5.56% compared to 20.12% for those admitted emergently (p<0.001). In a univariable analysis (Table 3) age was strongly associated with 30 day mortality in both elective and emergent admissions (p<0.001 elective; p=0.03 emergent). Among those with elective admissions, 30- day mortality was over 5 times higher for women over age 85 compared to those age 65-69 (17.52% vs. 3.19%, p<0.001). When age was entered into the model as a continuous variable in the group, each additional year over 65 was associated with a 7.5% increase in the risk of 30-day morality (95%CI 1.06-1.10). The relationship between advancing age and 30-day mortality among women admitted emergently was not as strong, with less than a two-fold difference observed between the same groups (26.32% vs. 14.29%, p=0.03). As a continuous variable in this group, each increase in age of one year over 65 was associated with a 2.8% increase in the risk of 30-day mortality (95%CI 1.01-1.05). Marital status was associated with 30-day mortality, with unmarried women in all groups having over a two-fold increase in observed 30 day mortality (elective: 7.06% vs. 3.39%, p<0.001; emergent 23.75% vs13.88%, p=0.001). Stage IV disease and mucinous histology were associated with higher 30-day mortality for both emergent and elective admissions (all p<0.05). Increasing comorbidity score was strongly associated with higher 30-day mortality in all groups (all p<=0.001). No differences in 30-day mortality were observed in either group by race, median household income, geographical region or size of area of residence.
Table 3.
Unadjusted 30-Day Mortality Stratified by Admission Type*
| 30 Day Mortality Entire Population n=5475 (%) |
30 Day Mortality Elective Admit n=4517 (%) |
30 Day Mortality Emergency Admit n=835 (%) |
|
|---|---|---|---|
| All patients | 450/5475 (8.22) | 251/4517 (5.56) | 168/835 (20.12) |
|
| |||
| Age (years) | p<0.001 | p<0.001 | p=0.028 |
|
| |||
| 65-69 | 56/1223 (4.58) | 34/1065 (3.19) | 19/132 (14.39) |
| 70-74 | 86/1584 (5.43) | 49/1346 (3.64) | 32/209(15.31) |
| 75-79 | 132/1464 (9.02) | 71/1185 (5.99) | 54/249 (21.69) |
| 80-84 | 96/822 (11.68) | 49/647 (7.57) | 38/150 (25.33) |
| 85+ | 80/382 (20.94) | 48/274 (17.52) | 25/95 (26.32) |
|
| |||
| Race | p=0.428 | p=0.810 | p=0.784 |
|
| |||
| White | 393/4927 (7.98) | 218/4077 (5.35) | 147/741 (19.84) |
| Black | 27/265 (10.19) | 10/193 (5.18) | 15/64 (23.44) |
| Other | 17/200 (8.50) | 11/170 (6.47) | 5/26 (19.23) |
|
| |||
| Median Household Income | p=0.254 | p=0.295 | p=0.752 |
|
| |||
| First Quartile | 114/1263 (9.03) | 61/1011 (6.03) | 41/218 (18.81) |
| Second Quartile | 115/1302 (8.83) | 61/1064 (5.73) | 47/205 (22.93) |
| Third Quartile | 113/1312 (8.61) | 70/1089 (6.43) | 38/193 (19.69) |
| Fourth Quartile | 97/1367 (7.10) | 54/1163 (4.64) | 37/180 (20.56) |
|
| |||
| Marital Status | p<0.001 | p<0.001 | p=0.001 |
|
| |||
| Married | 133/2511 (5.30) | 86/2188 (3.39) | 39/281 (13.88) |
| Not Married | 302/2819 (10.71) | 157/2223 (7.06) | 124/522 (23.75) |
|
| |||
| Region | p=0.290 | p=0.964 | p=0.113 |
|
| |||
| Northeast | 91/1074 (8.47) | 42/803 (5.23) | 46/246 (18.70) |
| Midwest | 103/1097 (9.39) | 49/851 (5.76) | 44/211 (20.85) |
| South | 55/782 (7.03) | 38/661 (5.75) | 14/108 (12.96) |
| West | 201 (7.97) | 122/2202 (5.54) | 64/270 (23.70) |
|
| |||
| Area of Residence | p=0.725 | p=0.127 | p=0.868 |
|
| |||
| Large Metropolitan | 259/3133 (8.27) | 139/2541 (5.47) | 103/516 (19.96) |
| Metropolitan | 112/1484 (7.55) | 62/1246(4.98) | 44/213 (20.66) |
| Urban | 30/334 (8.98) | 17/279 (6.09) | 10/45 (22.22) |
| Less Urban | 39/414 (9.42) | 30/360 (8.33) | 7/46 (15.22) |
| Rural | 10/110 (9.09) | 3/91 (3.30) | 4/15 (26.67) |
|
| |||
| Stage | p<0.001 | p=0.001 | p=0.022 |
|
| |||
| III | 234/3489 (6.71) | 139/2953 (4.71) | 78/466 (16.74) |
| IV | 203/1885 (10.77) | 104/1484 (7.01) | 86/350 (24.57) |
|
| |||
| Histology | p<0.001 | p=0.041 | p=0.003 |
|
| |||
| Serous/Adenocarcinoma | 335/4206 (7.96) | 196/3494 (5.61) | 119/618 (19.26) |
| Mucinous/Endometroid/Clear Cell** | 66/616 (10.71) | 34/497 (6.84) | 26/103 (25.24) |
| Other Epithelial | 49/653 (7.50) | 21/526 (3.99) | 23/114 (20.18) |
|
| |||
| Comorbidity Score | p<0.001 | p<0.001 | p=0.001 |
|
| |||
| 0 | 249/3717 (6.70) | 146/3143 (4.65) | 84/487 (17.25) |
| 1 | 109/1163 (9.37) | 64/942 (6.79) | 38/199 (19.10) |
| 2 | 48/365 (13.15) | 24/274 (8.76) | 21/83 (25.30) |
| 3+ | 44/230 (19.13) | 17/158 (10.76) | 25/66 (37.88) |
|
| |||
| Hospital Volume | p<0.001 | p=0.089 | p=0.151 |
|
| |||
| Low (1-12 cases) | 186/1679 (11.08) | 85/1265 (6.72) | 84/364 (23.08) |
| Intermediate (13-28 cases) | 104/1254 (8.29) | 63/1065 (5.92) | 35/171 (20.47) |
| High (>28 cases) | 113/1867 (6.05) | 76/1626 (4.67) | 34/233 (25.25) |
| Non SEER area | 47/675 (6.96) | 27/561 (4.81) | 15/77 (19.48) |
|
| |||
| Surgeon Specialty | p<0.001 | p<0.001 | p<0.001 |
|
| |||
| Gynecological Oncologist | 156/2824 (5.52) | 108/2496 (4.33) | 40/287 (13.94) |
| Gynecologist | 84/1223 (6.87) | 43/1010 (4.26) | 34/183 (18.58) |
| Surgeon | 76/681 (11.16) | 29/465 (6.24) | 33/171 (19.88) |
| Other/Unknown | 134/747 (17.94) | 71/546 (13.00) | 60/194 (30.93) |
|
| |||
| Provider Volume | p<0.001 | p<0.001 | p<0.001 |
|
| |||
| Low (1-4 cases) | 143/1640 (8.72) | 65/1263 (5.15) | 58/308 (18.83) |
| Intermediate (5-25 cases) | 100/1797 (5.56) | 68/1570 (4.33) | 26/198 (13.13) |
| High (>25 cases) | 85/1408 (6.04) | 55/1232 (4.46) | 27/156 (17.31) |
| Missing | 122/630 (19.37) | 63/452 (13.94) | 57/173 (32.95) |
Admission type was available for 5352/5475 (97.75%), not all totals add up to 100% because of rounding and missing data and women for whom admission type was unknown are not included in the elective or emergency admission columns.
some categories have been combined and cell contents suppressed for confidentiality due to n<11, p values refer to the uncombined analysis and use all values.
When stratified by admission type, hospital volume was not associated with 30-day mortality (Table 3). Surgeon specialty and provider volume were associated with 30-day mortality, however this association appears to be mostly driven by the higher mortality observed when provider status was other/unknown (30-day mortality 17.94%) and procedure volume was missing (30 day mortality 19.37%). When cases with missing provider status and provider procedure volumes are excluded from the analysis all p values are not significant for an association with 30-day mortality. Women whose surgeries included upper abdominal procedures or large bowel resections had no significant difference in 30-day mortality. Among women admitted electively, those having neoadjuvant chemotherapy had more than 70% lower 30-day mortality than those without neoadjuvant chemotherapy (1.82% vs. 6.13%, p<0.001).
Multivariable analysis demonstrated a significant relationship between advancing age, marital status, increasing stage, increasing comorbidity score and 30-day mortality (all p<0.05) among women admitted electively (Table 4). Adjusting for marital status, stage, histology, comorbidity score and year of diagnosis, women age 80-84 had at least a 2-fold increase in 30-day mortality (RR 2.10; 95%CI 1.36-3.24), and women 85 and older had almost a 5-fold increase in 30-day mortality (RR 4.77; 95%CI 3.07-7.42) compared to women ages 65-69. Higher stage, non-married status and advancing comorbidity score were significantly associated with increased short term mortality among women admitted emergently (all p<0.05). Hospital volume, provider volume and surgeon specialty were not significantly associated with 30-day mortality in either elective or emergent admissions after controlling for patient related variables. Neoadjuvant chemotherapy remained significantly associated with a lower 30-day mortality in the adjusted model among women admitted electively (RR 0.37; 95%CI 0.17-0.83). When all women were considered in a combined multivariable model adjusting for patient characteristics and hospital admission type (emergent vs elective), women with emergent admissions had almost a 3 fold increase in 30-day mortality compared to women admitted electively (RR 2.77, 95%CI 2.25-3.41).
Table 4.
Multivariable Analysis of 30-day Mortality Stratified by Admission Type
| Multivariable Incident Rate Ratio (95 % Confidence Interval) | ||||
|---|---|---|---|---|
| Model 1 Patient Variables | Model 2 Patient & Treatment Variables | |||
| Elective Admission (model n=4399) |
Emergency Admission (model n=803) |
Elective Admission (model n=3333) |
Emergency Admission (model n=554) |
|
| Age (years) | p<0.001 | p=0.181 | p<0.001 | p=0.053 |
|
| ||||
| 65-69 | ref | ref | Ref | Ref |
| 70-74 | 1.19 (0.80-1.78) | 1.09 (0.65-1.85) | 1.00 (0.59-1.69) | 2.35 (0.92-6.05) |
| 75-79 | 1.81 (1.18-2.76) | 1.44 (0.90-2.32) | 1.85 (1.13-3.03) | 2.91 (1.19-7.09) |
| 80-84 | 2.10 (1.36-3.24) | 1.61 (0.97-2.67) | 1.77 (1.02-3.10) | 3.77 (1.49-9.54) |
| 85+ | 4.77 (3.07-7.42) | 1.61 (0.93-2.81) | 4.31 (2.46-7.57) | 2.80 (1.05-7.48) |
|
| ||||
| Marital Status | p=0.031 | p=0.035 | p=0.036 | p=0.032 |
|
| ||||
| Married | ref | ref | Ref | Ref |
| Not Married | 1.34 (1.03-1.75) | 1.46 (1.03-2.09) | 1.45 (1.02-2.06) | 1.64 (1.04-2.59) |
|
| ||||
| Stage | p=0.002 | p=0.009 | p=0.242 | p=0.007 |
|
| ||||
| III | ref | ref | Ref | Ref |
| IV | 1.44 (1.14-1.82) | 1.47 (1.14-1.91) | 1.28 (0.95-1.74) | 1.61 (1.16-2.24) |
| “Distant” NOS | 2.34 (1.01-5.41) | 0.95 (0.45-2.01) | 1.56 (0.38-6.45) | 0.44 (0.08-2.34) |
|
| ||||
| Histology | p=0.387 | p=0.006 | p=0.505 | p=0.015 |
|
| ||||
| Serous/Adenocarcinoma | ref | ref | Ref | Ref |
| Mucinous | 1.51 (0.92-2.47) | 1.78 (1.19-2.67) | 1.53 (0.83-2.83) | 2.04 (1.23-3.37) |
| Endometroid | 1.14 (0.71-1.83) | 0.47 (0.18-1.22) | 1.31 (0.76-2.28) | 0.48 (0.15-1.50) |
| Clear Cell | 0.80 (0.25-2.56) | 1.48 (0.76-2.88) | 0.96 (0.22-4.13) | 1.17 (0.24-5.66) |
| Other Epithelial | 0.67 (0.44-1.03) | 0.98 (0.65-1.48) | 0.69 (0.39-1.24) | 0.74 (0.41-1.36) |
|
| ||||
| Comorbidity Score | p=0.001 | p<0.001 | p=0.215 | p=0.146 |
|
| ||||
| 0 | ref | ref | Ref | Ref |
| 1 | 1.35 (1.00-1.80) | 1.04 (0.73-1.49) | 1.14 (0.77-1.68) | 0.82 (0.49-1.36) |
| 2 | 1.84 (1.19-2.84) | 1.28 (0.87-1.87) | 1.74 (1.00-3.05) | 1.00 (0.62-1.60) |
| 3+ | 2.11 (1.2910.44) | 2.22 (1.53-3.23) | 1.53 (0.71-3.30) | 1.75 (0.99-3.07) |
|
| ||||
| Hospital Volume | p=0.434 | p=0.198 | ||
|
| ||||
| Low (1-12 cases) | Ref | Ref | ||
| Intermediate (13-28 cases) | 1.16 (0.74-1.81) | 1.13 (0.74-1.75) | ||
| High (>28 cases) | 0.89 (0.58-1.36) | 0.71 (0.42-1.21) | ||
|
| ||||
| Surgeon Specialty | p=0.535 | p=0.980 | ||
|
| ||||
| Gynecological Oncologist | Ref | Ref | ||
| Gynecologist | 0.87 (0.55-1.37) | 1.06 (0.59-1.89) | ||
| Surgeon | 1.18 (0.66-2.09) | 1.03 (0.56-1.90) | ||
|
| ||||
| Provider Volume | p=0.874 | p=0.140 | ||
|
| ||||
| Low (1-4 cases) | Ref | Ref | ||
| Intermediate (5-25 cases) | 0.97 (0.61-1.55) | 0.67 (0.38-1.17) | ||
| High (>25 cases) | 1.08 (0.64-1.84) | 1.07 (0.56-2.07) | ||
|
| ||||
| Neoadjuvant Chemotherapy | p=0.013 | p=0.727 | ||
|
| ||||
| No | Ref | Ref | ||
| Yes | 0.36 (0.16-0.80) | 0.80 (0.23-2.79) | ||
Adjusted for variables shown and year of diagnosis. p values represent wald chi2 test result for the trend
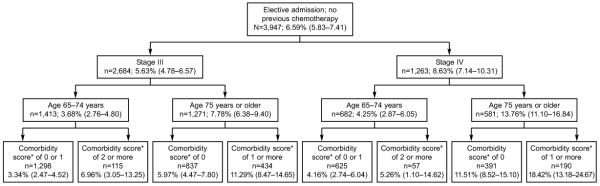
Figure 1 illustrates 30-day mortality for women admitted electively, who had not previously received neoadjuvant chemotherapy. Age, stage and comorbidity score were used to stratify the risk of 30 day mortality into low (<5%), intermediate (5-10%) and high (>10%) risk groups. The high risk group includes:
All women age 75+ with stage IV disease
Women age 75+ with stage III disease and a comorbidity score of 1+
This group represented 25.7% of the patients and had a 30-day average mortality rate of 12.71% (95%CI 10.72%-14.92%), representing almost 50% of the deaths in the cohort. The low risk group includes women age 65-74, with either stage III or IV disease and a comorbidity score of 1 or less. This group makes up 48.7% of the population and had an average 30-day mortality of 3.64% (95%CI 2.85%-4.58%). The remaining patients constitute the intermediate risk group, with an average 30-day mortality of 6.05%. (95%CI 4.66%-7.70%).
Figure 1.
30-day mortality for elderly women electively admitted for surgery for Stage III or Stage IV ovarian cancer without a history of neoadjuvant chemotherapy. N represents patients; percentage shows 30-day mortality rate, with 95% confidence intervals in parentheses. *Comorbidity score(19): 1 point each given for dementia, congestive heart failure, coronary artery disease (heart attack, angina or revascularization), diabetes, hypertension, peripheral vascular disease, pulmonary disease, renal disease or stroke; 2 points given for previous malignancy; 3 points given for hepatic disease.
Discussion
This population based study of surgical outcomes among elderly women in the US after surgery for advanced ovarian cancer reports an overall 30-day mortality rate of 8.22% among all women. When stratified by admission severity, we found a 30-day mortality of 5.56% for women admitted electively and 20.12% for women admitted emergently. After correcting for patient characteristics, emergent admission was associated with almost a 3 fold increase in the risk of 30-day mortlity. Among women admitted electively (~85% of cohort) age, stage and comorbidity score were associated with 30-day mortality. These parameters were combined to identify a high risk group of women aged 75 or older with stage IV disease and women aged 75 or older with stage III disease and a comorbidity score of 1 or more with an observed 30-day mortality of 12.7%.
Our findings are consistent with previous publications, reporting higher 30-day mortality rates for population based studies compared to single institution reports(7). Our overall 30-day mortality rate of 8.22 % is considerably higher than previous population based reports(9, 12). However this may be accounted for by the older age and advanced stage of the patients in our cohort. Increasing age has been strongly associated with an increase in operative mortality. A Netherlands population-based report for women with all stages of ovarian cancer reported post operative mortality of 6.6% for women 70-79 and 9.8% for women 80 and older(7). A previous single institution report of outcomes among women over the age of 80 with advanced ovarian cancer having surgery revealed 13% had in hospital mortality and 20% died within 60 days of surgery(28). The observed high mortality among older women in this study are consistent with the findings of our analysis.
Our primary study objective was to report post operative mortality and identify risk factors independently associated with 30-day mortality in both elective and emergently admitted elderly women having surgery for advanced ovarian cancer A secondary objective was to characterize a sub-group of women at very high risk of 30-day mortality who may benefit from an alternative primary treatment approach. In trying to characterize this subpopulation we performed another analysis limited to patients who had not already received neoadjuvant chemotherapy. Women admitted emergently were also excluded, as we hypothesized that these patients had a high probability of having severe symptoms and bowel obstructions that would favor a primary surgical approach for symptom management. In the remaining women, age, stage and comorbidity were three factors that allowed the group to be stratified into risk groups with 25.7% of patients in the highest risk group. These findings were similar to those reported in a series of 567 patients operated on at 4 US centers(29). In that analysis a subgroup of women 75 and older with poor performance or nutritional status and disseminated disease represented 6.6% of the cohort had a 90 day mortality rate of 18.4%.
In addition to exploring patient related factors associated with post operative mortality we also examined the relationship between the treatment environment and short term mortality. The relationship between surgeon and hospital volume and short term outcomes in advanced ovarian cancer has been uncertain. A Canadian population based trial of over 3800 women with all stages of ovarian cancer did not find a significant relationship between hospital or surgeon volume and 30-day post operative mortality(30). Conversely, Bristow et al. in a US population based reported a significant relationship between surgeon but not hospital volume, with high volume surgeons having 69% lower in hospital mortality rates than low volume surgeons(13). In our analysis we did not observe a significant relationship between hospital or surgeon volume and 30 day mortality after adjusting for patient characteristics. In our analysis we were able to adjust for urgency of admission, a comorbidity score that was related to treatment in the one year prior (as opposed to those billed for during the surgical admission only), and tumor specific characteristics such as stage. These differences in the methods of adjustment for covariates may account for the observed differences in findings between these analyses. A previous report utilizing a surgical complexity score failed to demonstrate an association between 3 month mortality and increasing surgical complexity(31). Our results were consistent with this study, with no association seen between either performance of upper abdominal procedures or large bowel resections and the 30-day surgical mortality. The small numbers of upper abdominal procedures performed in this cohort may also have limited our power to detect an associated between upper abdominal procedures and 30-day mortality.
There are several important limitations of this analysis. SEER-Medicare data lacks important information on labs values (such as albumin), performance status and American Society of Anesthesiologist score (ASA), all of which have been previously correlated with per-operative morbidity and mortality(31). The use of median household income as an estimation of socioeconomic status may inadequately classify patients and result in an inability to determine an association between SES and 30-day mortality. No information was available on the completeness of the surgery. The use of claims data to identify treatment and co-morbidities is likely to result in some under ascertainment of both of these variables due to inaccurate coding and alternative payment sources. Medicare data and chart review have been shown to have a high level of agreement in the identification of surgery and chemotherapy, but the accuracy of diagnostic codes is lower for co-morbid conditions and treatment complications (20, 25, 26). The ability to accurately assess provider or hospital volume from Medicare data only may be inaccurate. However, this method has been previously validated and shown to correlate well with total volume(21, 22).
We report post-operative 30-day mortality rates for women 65 and older that are substantial and increase sharply with increasing age for both elective and emergent ovarian cancer admissions. We identified a subpopulation of women previously untreated women admitted electively age 75 and older with stage IV disease, or with stage III disease and a comorbidity score of 1 or higher, who in our cohort, are at a high risk of death after surgery for advanced ovarian cancer. The completeness of the surgical resection and thus the proportion of women optimally debulked is not known from these data and its resulting impact on short and long term mortality would be critical in informing clinicians faced with decision making. These findings need to be replicated prospectively and correlated with completeness of surgical debulking. If validated, this subpopulation may benefit from better risk counseling and may be considered for less risky alterative treatment strategies.
Acknowledgments
Supported by the Marsha Rivkin Center for Ovarian Cancer Research and the National Cancer Institute (NCI) at the National Institutes of Health. Dr. Thrall is the recipient of an NCI-funded postdoctoral fellowship (T32-CA009515-26).
The authors thank the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. [cited 2010 July 6];Cancer Facts and Figures - 2010. SEER Cancer Statistics Review 1975-2007. 2010 Available from: http://seer.cancer.gov/csr/1975_2007/results_single/sect_01_table.01.pdf.
- 2.NCCN [cited 2010 July 8];Practice guidelines in Oncology v.2.2010 Ovarian Fallopian Tube and Primary Peritoneal Carcinomas. 2010 Available from: http://www.nccn.org/professionals/physician gls/PDF/ovarian.pdf.
- 3.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006 Dec;103(3):1070–6. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 4.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009 Mar 15;115(6):1234–44. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 5.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009 Jul;114(1):26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006 Jan;107(1):77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 7.Gerestein CG, Damhuis RA, Burger CW, Kooi GS. Postoperative mortality after primary cytoreductive surgery for advanced stage epithelial ovarian cancer: a systematic review. Gynecol Oncol. 2009 Sep;114(3):523–7. doi: 10.1016/j.ygyno.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Marx C, Bendixen A, Hogdall C, Ottosen C, Kehlet H, Ottesen B. Organisation and quality of primary surgical intervention for ovarian cancer in Denmark. Acta Obstet Gynecol Scand. 2007;86(12):1496–502. doi: 10.1080/00016340701622294. [DOI] [PubMed] [Google Scholar]
- 9.Engelen MJ, Kos HE, Willemse PH, Aalders JG, de Vries EG, Schaapveld M, et al. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006 Feb 1;106(3):589–98. doi: 10.1002/cncr.21616. [DOI] [PubMed] [Google Scholar]
- 10.Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, Institute NC, editors. SEER Cancer Statistics Review, 1975-2007. Bethesda, MD: 2010. [Google Scholar]
- 11.Massarweh NN, Legner VJ, Symons RG, McCormick WC, Flum DR. Impact of advancing age on abdominal surgical outcomes. Arch Surg. 2009 Dec;144(12):1108–14. doi: 10.1001/archsurg.2009.204. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Montes TP, Zahurak ML, Giuntoli RL, 2nd, Gardner GJ, Gordon TA, Armstrong DK, et al. Surgical care of elderly women with ovarian cancer: a population-based perspective. Gynecol Oncol. 2005 Nov;99(2):352–7. doi: 10.1016/j.ygyno.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009 Dec;115(3):334–8. doi: 10.1016/j.ygyno.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010 Sep 2;363(10):943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 15.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian cancer in the Medicare population. Gynecol Oncol. 2011 Jul;122(1):100–6. doi: 10.1016/j.ygyno.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 17.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995 Dec 1;76(11):2343–50. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002 Aug;40(8 Suppl):IV-26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 21.Schrag D, Earle C, Xu F, Panageas KS, Yabroff KR, Bristow RE, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006 Feb 1;98(3):163–71. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 22.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001 Jul 19;345(3):181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 23.Pollack LA, Adamache W, Eheman CR, Ryerson AB, Richardson LC. Enhancement of identifying cancer specialists through the linkage of Medicare claims to additional sources of physician specialty. Health Serv Res. 2009 Apr;44(2 Pt 1):562–76. doi: 10.1111/j.1475-6773.2008.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006 Feb 1;98(3):172–80. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 25.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002 Aug;40(8 Suppl):IV-43–8. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 26.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEERMedicare data to identify chemotherapy use. Med Care. 2002 Aug;40(8 Suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 27.Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003 Oct 21;139(8):658–65. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- 28.Moore KN, Reid MS, Fong DN, Myers TK, Landrum LM, Moxley KM, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol. 2008 Aug;110(2):133–9. doi: 10.1016/j.ygyno.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol. 2011 Jan;120(1):23–8. doi: 10.1016/j.ygyno.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Elit L, Bondy SJ, Paszat L, Przybysz R, Levine M. Outcomes in surgery for ovarian cancer. Gynecol Oncol. 2002 Dec;87(3):260–7. doi: 10.1006/gyno.2002.6834. [DOI] [PubMed] [Google Scholar]
- 31.Aletti GD, Santillan A, Eisenhauer EL, Hu J, Aletti G, Podratz KC, et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol Oncol. 2007 Oct;107(1):99–106. doi: 10.1016/j.ygyno.2007.05.032. [DOI] [PubMed] [Google Scholar]