Abstract
(See the editorial commentary by Hull and Montaner, on pages 1154–6.)
Background. AIDS Clinical Trials Group A5202 compared blinded abacavir/lamivudine (ABC/3TC) to tenofovir DF/emtricitabine (TDF/FTC) with efavirenz (EFV) or atazanavir/ritonavir (ATV/r) in human immunodeficiency virus (HIV)-infected treatment-naive patients, stratified by screening HIV RNA (< or ≥105 copies/mL). Due to higher virologic failure with ABC/3TC in the high HIV RNA stratum, blinded treatment was stopped in this group, but study follow-up continued for all patients.
Methods. Primary endpoints were times to virologic failure, regimen modification, and safety event.
Results. In the low HIV RNA stratum, time to virologic failure was similar for ABC/3TC vs TDF/FTC with ATV/r (hazard ratio [HR] 1.25, 95% confidence interval [CI] 0.76, 2.05) or EFV (HR 1.23, 95% CI 0.77, 1.96), with significantly shorter times to regimen modification for ABC/3TC with EFV or ATV/r and to safety events with EFV. Prior to stopping blinded treatment in the high stratum, higher virologic failure rates were seen with ABC/3TC with EFV (HR 2.46, 95% CI 1.20, 5.05) or ATV/r (HR 2.22, 95% CI 1.19, 4.14).
Conclusions. In the low HIV RNA stratum, times to virologic failure for ABC/3TC or TDF/FTC were not different with EFV or ATV/r. In the high stratum, virologic failure rate was significantly higher for ABC/3TC than for TDF/FTC when given with either EFV or ATV/r.
Recommended initial therapy for human immunodeficiency virus (HIV) type 1 infection consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) combined with a nonnucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or integrase inhibitor [1, 2]. Among NRTI combinations, abacavir/lamivudine (ABC/3TC) and tenofovir DF/emtricitabine (TDF/FTC) are currently widely used due to high antiviral potency, good tolerability, and low risk of side effects associated with mitochondrial toxicity [3, 4]. In addition, both are available in fixed-dose combinations administered as 1 pill daily.
AIDS Clinical Trials Group A5202 was a randomized equivalence study of blinded ABC/3TC or TDF/FTC with either open-label efavirenz (EFV) or atazanavir-ritonavir (ATV/r), with stratification at randomization by screening HIV RNA (low [<105 copies/mL] vs high stratum [≥105 copies/mL]). During the first interim efficacy review, an independent Data Safety Monitoring Board (DSMB) recommended stopping the NRTI comparison in patients in the high HIV RNA stratum due to a shorter time to virologic failure for ABC/3TC than TDF/FTC [5]. Of continued interest was whether ABC/3TC and TDF/FTC given with EFV or ATV/r provided comparable antiviral activity for patients from the low HIV RNA stratum. In addition, data on the comparison between NRTIs with the individual third drugs from the high HIV RNA stratum were not reported.
METHODS
Study Design
The study population and study design have been previously reported [5]. Because pretreatment resistance genotype testing was not recommended in HIV treatment guidelines until 1 December 2007, screening resistance testing was not required before study entry in all patients. The planned duration of the study was 96 weeks from enrollment of the last subject.
Patients were randomly assigned to receive fixed-dose ABC/3TC 600 mg/300 mg or TDF/FTC 300 mg/200 mg with ATV/r 300 mg/100 mg or EFV 600 mg, along with matching placebo for the other NRTI. Unblinding of the TDF/FTC and ABC/3TC components was allowed for site-investigator-suspected NRTI treatment–limiting adverse event, virologic failure, or pregnancy. Adverse events and laboratory events were assessed by the investigators and scored with the use of the Division of AIDS Table for grading the severity of adult and pediatric events, version 2004.
Randomization was stratified by screening HIV RNA level (<105 vs ≥105 copies/mL), and used a permuted-block design with dynamic balancing according to the main site. Study evaluations were completed at screening/pre-entry; entry; weeks 4, 8, 16, 24; and every 12 weeks thereafter and regardless of antiretroviral modification or study endpoint. After screening, plasma HIV RNA (Roche Amplicor Monitor assay, version 1.5; Roche Diagnostic System) was measured at a central laboratory (Department of Pathology, Johns Hopkins University).
At the time of virologic failure, genotypic resistance (baseline and failure specimens) was performed at Stanford University. Major mutations at baseline and virologic failure were defined as those listed by the International AIDS Society USA [6], as well as T69D, L74I, and G190C/E/Q/T/V for reverse transcriptase and L24I, F53L, I54V/A/T/S, G73C/S/T/A, and N88D for protease.
Statistical Analysis
The primary efficacy endpoint was time from randomization to virologic failure, defined as confirmed HIV RNA level ≥1000 copies/mL at or after 16 weeks and before 24 weeks, or ≥200 copies/mL at or after 24 weeks. The primary hypothesis for the NRTI components was that ABC/3TC was equivalent to TDF/FTC with ATV/r or EFV. The study design defined that overall, the 2 regimens were considered equivalent if the 2-sided 95% confidence interval (CI) for the hazard ratio (HR) on virologic failure was between 0.71 and 1.40. Equivalence boundaries were not specified a priori within each HIV RNA stratum.
The primary tolerability endpoint was time from initiation of randomized treatment to any regimen modification. The primary safety endpoint was time from initiation of randomized treatment to the first occurrence of a grade 3 or 4 sign, symptom, or laboratory abnormality that was at least 1 grade higher than baseline. The protocol specified that isolated unconjugated hyperbilirubinemia and creatine kinase level elevations would be excluded from the safety endpoints.
Analyses of efficacy data were intention-to-treat (ITT), including all eligible randomized subjects. Analyses of safety data were as-treated (AT) while on the initial randomized regimen. Time-to-event survival distributions were estimated with the Kaplan-Meier method, and comparisons for differences were assessed by means of 2-sided log-rank tests. HRs were estimated with Cox proportional-hazards models and overall analysis were stratified by screening HIV RNA level. Changes in CD4+ cell count and as-treated fasting lipid levels and calculated creatinine clearance at weeks 48 and 96 were compared with a Wilcoxon-Mann-Whitney test. Summaries of the high HIV RNA stratum data are restricted to the blinded study medication follow-up and were censored at the time of the DSMB recommendation implementation.
Study conduct and safety data were reviewed at 2 planned annual DSMB reviews. Early-stopping guidelines for inferiority were prespecified, with a regimen considered to be inferior if the 99.95% 2-sided CI for the HR for virologic failure did not include 1.0.
The first DSMB efficacy review on 29 January 2008 noted a higher virologic failure rate for regimens containing ABC/3TC compared with TDF/FTC. Following a DSMB-requested additional prespecified secondary factorial analysis, it was noted that the excess in virologic failure occurred within the high HIV RNA stratum. The DSMB recommend that the blinded NRTI comparison in the high HIV RNA stratum be discontinued, and that the patients be unblinded to their NRTI regimen assignment. An additional interim review was requested by the DSMB for May 2008, and the DSMB recommended continuation through planned end of follow-up.
P values and confidence intervals are 2-sided and nominal with no adjustment for interim analyses or multiple comparisons. The significance level for modification of treatment effect was prespecified at 0.10. Analyses were performed with the use of SAS software, version 9 (SAS) and with S-Plus software, version 6 (Insightful Corp.).
RESULTS
Enrollment, Disposition of Patients, and Demographics
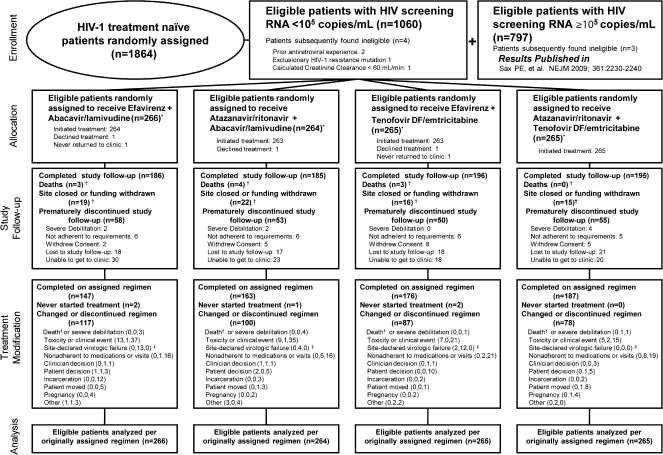
Study enrollment was from September 2005 to December 2007. Of the 1857 eligible patients enrolled, 1060 patients were randomized in the low HIV RNA stratum and followed for a median of 136 weeks (25th, 75th percentile 106, 170, respectively). Figure 1 summarizes the enrollment and disposition of patients in the low HIV RNA stratum. Demographic and baseline health characteristics are noted in Table 1.
Figure 1.
Enrollment, randomization, and disposition of patients with screening HIV RNA <105 copies/mL.
Patients were to remain in follow-up regardless of whether antiretroviral therapy was modified; therefore, study follow-up and treatment modification disposition are both presented. Reasons for treatment modification are split into (number before, number after, number without protocol-defined virologic failure) to summarize the amount of censoring of primary efficacy endpoints in as-treated analyses. * Nucleoside reverse transcriptase inhibitors were blinded through 25 February 2008 for persons with HIV-1 RNA levels of 100 000 copies/mL or more at screening and until final visits starting 1 July 2009 for those with HIV-1 RNA levels less than 100 000 copies/mL at screening. † Death was censored for premature study discontinuation and counted as a reason for treatment discontinuation if there was no previous regimen modification. Site closure was censored for premature study and treatment discontinuation. ‡ Site-declared virologic failure was by clinical determination of the site investigator, whereas protocol-defined virologic failure was determined strictly by the quantitative definition set forth in the protocol. Numbers may differ because not all patients who had protocol-defined virologic failure modified the regimen, or the drug modification may have been attributed to another reason, such as “nonadherent with medications or visits.”
Table 1.
Baseline Characteristics of Patients by Viral Load Stratum
| Low HIV RNA stratum, n = 1060 | High HIV RNA stratum, n = 797 | Total study population, n = 1857 | |
| Sex, n (%) | |||
| Male | 859 (81) | 676 (85) | 1535 (83) |
| Female | 201 (19) | 121 (15) | 322 (17) |
| Race or ethnicity, n (%)a | |||
| White non-Hispanic | 374 (35) | 372 (47) | 746 (40) |
| Black non-Hispanic | 409 (39) | 206 (26) | 615 (33) |
| Hispanic | 233 (22) | 196 (25) | 429 (23) |
| Asian, Pacific Islander | 22 (2) | 10 (1) | 32 (2) |
| Native American | 12 (1) | 2 (0) | 14 (1) |
| More than 1 race | 6 (1) | 10 (1) | 16 (1) |
| Age, median (Q1, Q3) years | 37 (30, 45) | 39 (32, 45) | 38 (31, 45) |
| HIV RNA log10 copies/mL, median(Q1, Q3)b | 4.4 (4.1, 4.7) | 5.0 (4.7, 5.6) | 4.7 (4.3, 5.0) |
| CD4+ T-cell count, median cells/mm3 (Q1, Q3)b | 266.3 (170, 358) | 144.5 (41.0, 286.3) | 229.5 (89.5, 333.8) |
| Calculated creatine clearance mL/min, median (Q1, Q3) | 116.4 (98.4, 140.0) | 113.6 (96.0,132.3) | 114.8 (97.5, 136.3) |
| Genotype before entry, n (%) | 489 (46) | 341 (43) | 830 (45) |
| History of AIDS, n (%) | 123 (12) | 189 (24) | 312 (17) |
Differences between low and high HIV RNA stratum: P < .05 for sex, race/ethnicity, age, CD4+ cell count, calculated creatinine clearance, and history of AIDS. Number missing baseline race or ethnicity category 5, and CD4+ cell count 1.
Abbreviation: HIV indicates human immunodeficiency virus.
Race or ethnicity group was self reported.
Baseline RNA is calculated as mean of pre-entry and entry log10 (copies/mL). Baseline CD4+ count is calculated as the mean of pre-entry and entry values.
Primary Virologic Outcome in Low the HIV RNA Stratum
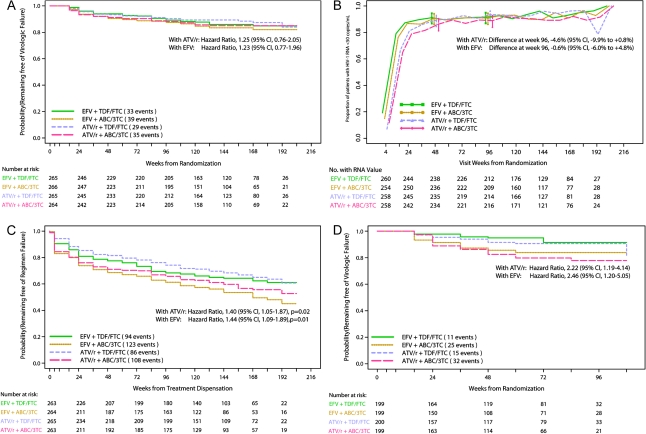
The estimated HR for virologic failure of ABC/3TC versus TDF/FTC with ATV/r was 1.25 (95% CI, 0.76, 2.05), with virologic failures occurring in 35 and 29 patients, respectively. In a post-hoc analysis, the percentages without virologic failure at week 96 for ABC/3TC and TDF/FTC, respectively, were 88.3% and 90.3% for a difference of −2.0% (95% CI, −7.5, 3.4). With EFV, the estimated HR for virologic failure of ABC/3TC versus TDF/FTC was 1.23 (95% CI, 0.77, 1.96), with virologic failures occurring in 39 and 33 patients, respectively. The percentages without virologic failure at week 96 for ABC/3TC and TDF/FTC, respectively, were 87.4% and 89.2% for a difference of −1.8% (95% CI: −7.5, 3.9). Kaplan-Meier plots of the time to confirmed virologic failure are shown in Figure 2A.
Figure 2.
A, Time to protocol-defined virologic failure in those with screening plasma HIV RNA<105 copies/mL. B, Proportion of patients in low HIV RNA stratum with HIV RNA level <50 copies/mL and 95% binomial confidence intervals at each study week where analysis includes patients with available data, regardless of whether they had previously switched therapy or met criteria for virologic failure. C, Time to regimen completion (time to the first occurrence of either confirmed virologic failure or discontinuation of initially randomized regimen) in low HIV RNA stratum. D, Time to protocol-defined virologic failure in the high HIV RNA stratum.
Sensitivity analyses that also included unconfirmed virologic failures and the first event of unconfirmed virologic failure, death, or premature discontinuation of follow-up showed results similar to the primary efficacy analysis.
Secondary Virologic Analyses in the Low HIV RNA Stratum
A cross-sectional analysis of available data evaluated the proportion of patients with HIV RNA <50 copies/mL at week 96, regardless of previous virologic failure or drug regimen change. The proportions for ATV/r were 89% with ABC/3TC and 93% with TDF/FTC for a difference of −4.6% (95% CI, −9.9, 0.8). For EFV, the proportions were 91% and 92% for ABC/3TC and TDF/FTC, respectively, for a difference of −0.6% (95% CI, −6.0, 4.8, Figure 2B).
A prespecified secondary endpoint of time to regimen failure demonstrated for the low HIV RNA stratum a significantly shorter time to regimen failure for ABC/3TC than TDF/FTC with ATV/r- (HR 1.40; 95% CI, 1.05, 1.87, P = .02) or EFV(HR 1.44; 95% CI, 1.09, 1.89; P = .01, Figure 2C).
Primary Virologic Outcomes at the Time of the DSMB Action
For the NRTI comparison in the high HIV RNA stratum with ATV/r, there was a shorter time to virologic failure with ABC/3TC (32 failures) than TDF/FTC (15 failures), HR 2.22 (95% CI, 1.19, 4.14). Similarly, there was a shorter time to virologic failure for ABC/3TC (25 failures) than TDF/FTC (11 failures), HR 2.46 (95% CI, 1.20, 5.05) with EFV, Figure 2D. There was no significant evidence that this treatment effect differed by either ATV/r or EFV (P = .82).
At the time of the DSMB action combining high and low HIV RNA strata, ATV/r with ABC/3TC versus TDF/FTC had an estimated HR for virologic failure of 1.48 (95% CI, 0.95, 2.31). Within the EFV groups, the time to virologic failure for ABC/3TC was significantly shorter than TDF/FTC, HR 1.98 (95% CI 1.22, 3.20). There was no significant interaction by ATV/r or EFV (P = .38). When the NRTI comparison was combined across ATV/r and EFV regimens (factorial analysis) for all patients (high and low HIV RNA stratum), the HR for virologic failure was 1.70 (95% CI, 1.23, 2.35).
CD4+ Cell Count Changes in the Low HIV RNA Stratum
Among those randomized to ATV/r, there was no significant difference in distribution of change from baseline CD4+ cells/mm3 between ABC/3TC and TDF/FTC at week 48 (week 96); median 170 ABC/3TC and 157 TDF/FTC (240 ABC/3TC and 241 TDF/FTC), P > .6 for both time points. Among those randomized to EFV, ABC/3TC recipients experienced significantly greater CD4+ cells/mm3 increases compared with TDF/FTC at weeks 48 and 96 (median 175 vs 147, P = .035; and 227 vs 200, P = .035, respectively).
Tolerability Endpoints in the Low HIV RNA Stratum
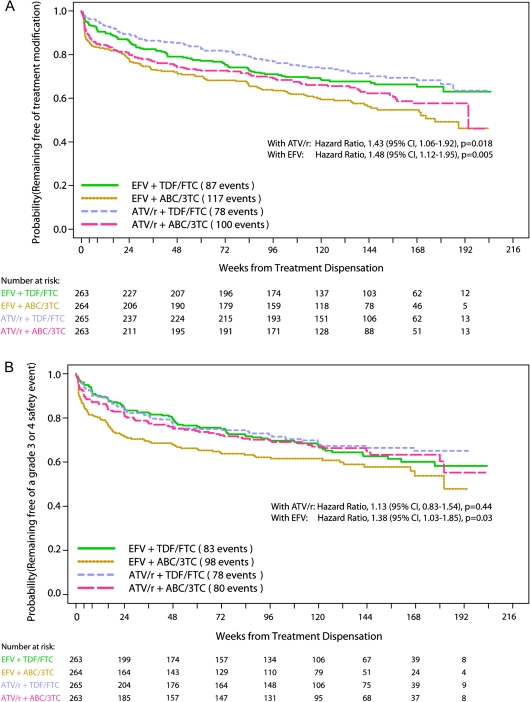
Within the ATV/r regimens, the time to first antiretroviral drug modification was significantly shorter for ABC/3TC than TDF/FTC, HR 1.43 (95% CI, 1.06, 1.92, P = .018, Figure 3A). Similarly, within the EFV regimens, the time to first antiretroviral drug modification was significantly shorter for ABC/3TC than TDF/FTC, HR 1.48 (95% CI, 1.12, 1.95, P = .005). The most common reasons for drug modification were toxicity/clinical event, virologic failure, and noncompliance with study medications, with distributions of reasons between study arms shown in Figure 1.
Figure 3.
In low HIV RNA stratum, (A) Time to tolerability endpoint, defined as first change in regimen; and (B) time to first primary safety endpoint, defined as first grade 3 or 4 sign, symptom, or laboratory abnormality while on initial randomized treatment that was at least 1 grade higher than baseline, excluding hyperbilirubinemia and elevation in the creatine kinase level.
Within the ATV/r and EFV regimens, the secondary tolerability endpoint of time to first modification of the NRTIs was significantly shorter for ABC/3TC than TDF/FTC, HR 1.57 (95% CI 1.14, 2.16, P = .006) and HR 1.84 (95% CI 1.36, 2.51, P < .0001), respectively. When this analysis was restricted to reasons other than suspected abacavir-associated hypersensitivity reaction, the time to first NRTI modification was significantly shorter for ABC/3TC than TDF/FTC with EFV, HR 1.45 (95% CI 1.05, 2.01, P = .02) but not with ATV/r, HR 1.24 (95% CI 0.89, 1.75; P = .20).
Unblinding of NRTIs in the Low HIV RNA Stratum
For ATV/r, unblinding of NRTIs for suspected drug hypersensitivity occurred in 23 and 11 patients randomized to ABC/3TC and TDF/FTC, respectively; for EFV, suspected hypersensitivity unblindings occurred in 32 patients randomized to ABC/3TC and 8 to TDF/FTC. After unblinding, 1 patient experienced a severe hypersensitivity reaction when rechallenged with TDF/FTC [7]. There were 9 unblinding requests of TDF/FTC in the low HIV RNA stratum for renal-related reasons, 4 (1.5%) randomized to ATV/r, and 5 (1.9%) to EFV.
Safety Endpoints in the Low HIV RNA Stratum
Overall, 339 patients (32%) experienced a safety event while on their initial regimen. Time to first safety event was not significantly different for ABC/3TC or TDF/FTC with ATV/r (HR 1.13; 95% CI, 0.83 to 1.54, P = .44) and was shorter for ABC/3TC than TDF/FTC when given with EFV (HR 1.38; 95% CI, 1.03, 1.85, P = .03, Figure 3B). Safety events are listed in Table 2, with most events occurring in the general body (12%) and metabolic (7%) categories.
Table 2.
Selected Events That Triggered a Safety Endpoint While Receiving Randomized Antiretroviral Drugs in Low Screening HIV RNA Stratum
| ABC (n = 263) | TDF (n = 265) | ABC (n = 264) | TDF (n = 263) | All subjects (n = 1055)a | |
| ATV/r | EFV | ||||
| Overall, n (%) | 80 (30) | 78 (29) | 98 (37) | 83 (32) | 339 (32) |
| Metabolic, n (%) | 22 (8) | 19 (7) | 24 (9) | 13 (5) | 78 (7) |
| Total cholesterol (fasting), n | 4 | 1 | 9 | 4 | … |
| LDL (fasting), n | 7 | 7 | 15 | 8 | … |
| Triglycerides (fasting), n | 8 | 3 | 5 | 0 | … |
| Glucose (nonfasting) | 2 | 5 | 0 | 1 | … |
| Gastrointestinal, n (%) | 21 (8) | 16 (6) | 12 (5) | 12 (5) | 61 (6) |
| Diarrhea/loose stool, n | 2 | 4 | 8 | 2 | … |
| ALT, n | 7 | 1 | 1 | 6 | … |
| Nausea and/or vomiting, n | 6 | 3 | 3 | 1 | … |
| Neuropsychological, n (%) | 8 (3) | 1 (<1) | 16 (6) | 14 (5) | 39 (4) |
| Depression, n | 3 | 0 | 3 | 7 | … |
| General body, n (%) | 29 (11) | 30 (11) | 42 (16) | 30 (11) | 131 (12) |
| Ache/pain/discomfort, n | 20 | 11 | 12 | 17 | … |
| Fever, n | 6 | 7 | 6 | 1 | … |
| Asthenia/fatigue, n | 3 | 3 | 7 | 3 | … |
| Rash/allergic reaction, n | 2 | 2 | 5 | 2 | … |
| Headache, n | 3 | 3 | 6 | 1 | … |
| Hematologic, n (%) | 1 (<1) | 7 (3) | 4 (2) | 7 (3) | 19 (2) |
| Neutrophil count, n | 1 | 6 | 4 | 7 | … |
Events are listed if >2% of patients had an event in a major category with frequency selected if ≥5 in any study arm.
Abbreviations: ABC indicates abacavir; TDF, tenofovir DF; ATV/r, atazanavir/ritonavir; EFV, efavirenz; LDL, low-density lipoprotein; ALT, alanine aminotransferase.
Includes patients who started study medication.
Clinical and Laboratory Events in the Low HIV RNA Stratum
There were 10 deaths in the low HIV RNA stratum, which included 4 for ATV/r with ABC/3TC (non-Hodgkin’s lymphoma, myocardial infarction, automobile accident, drug overdose/suicide) and none with TDF/FTC; 3 deaths for EFV with ABC/3TC (bladder carcinoma, hepatic carcinoma, unknown) and 3 with TDF/FTC (bacterial pneumonia, stroke, Mycobacterium avium complex). Cardiovascular events were reported in 29 for ABC/3TC, and 34 for TDF/FTC (Table 3). Bone fractures were reported in 3% receiving ABC/3TC with ATV/r, 4% TDF/FTC with ATV/r, 6% ABC/3TC with EFV, and 5% TDF/FTC with EFV.
Table 3.
Prespecified Targeted Events in Low HIV RNA Stratum (Intent-to-Treat)
| ABC (n = 264) | TDF (n = 265) | ABC (n = 266) | TDF (n = 265) | |
| ATV/r | EFV | |||
| Cardiovascular, n (%)a | 15 (6) | 15 (6) | 14 (5) | 19 (7) |
| Vascular event | 2 (<1) | 1 (<1) | 2 (<1) | 6 (2) |
| Non-AIDS malignancies, n (%) | 13 (5) | 9 (3) | 15 (6) | 10 (4) |
| Renal, n (%) | 10 (4) | 7 (3) | 10 (4) | 5 (2) |
| Bone fractures, n (%) | 7 (3) | 10 (4) | 15 (6) | 13 (5) |
Abbreviations: ABC indicates abacavir; TDF, tenofovir DF; ATV/r, atazanavir/ritonavir; EFV, efavirenz.
Defined as coronary artery disease, infarct, ischemia, angina, cerebrovascular accident, transient ischemic attack, or peripheral vascular disease.
Site-reported incidence of renal disease occurred in 4% with ABC/3TC (4% with both ATV/r and EFV) and 2% for TDF/FTC (3% with ATV/r and 2% with EFV). Data on change from baseline in calculated creatinine clearance to weeks 48 and 96 were available for the 75% and 66% of patients who started study regimen, respectively. Statistically significant improvements from baseline to weeks 48 and 96 was found within all treatment arms (all P < .018) at both time points, except for ATV/r with TDF/FTC group at week 96 (P = .14). With ATV/r, there were significant differences in the distribution of change from baseline-calculated creatinine clearance between ABC/3TC and TDF/FTC at both week 48 (median +3.3 vs −3.1 mL/min, P < .001) and week 96 (median +5.2 mL/min vs −3.1 mL/min, P < .001). For EFV with ABC/3TC vs TDF/FTC, there was no significant difference in the change from baseline in calculated creatinine clearance at week 48 (median +2.6 mL/min vs +3.3 mL/min, P = .83) or week 96 (+7.0 mL/min vs +4.5 mL/min, P = .15).
For patients on a randomized treatment regimen with fasting samples (range 154–188 patients per treatment arm), changes from baseline in lipids levels were generally greater with ABC/3TC than TDF/FTC. With ATV/r, median changes for ABC/3TC vs TDF/FTC at week 48 respectively were total cholesterol, 30 vs 8 mg/dL (P < .001); low-density lipoprotein (LDL) cholesterol, 14 vs 0 mg/dL (P < .001); high-density lipoprotein (HDL) cholesterol, 7 vs 4 mg/dL (P< .001); and triglycerides, 27 vs 14 mg/dL (P = .004). With EFV, changes in total cholesterol were 34 vs 19 mg/dL (P < .001); LDL cholesterol, 17 vs 6 mg/dL (P <.001); HDL cholesterol, 12 vs. 9 mg/dL (P = .006); and triglycerides, 12 vs 13 mg/dL (P= .49), respectively. There was no significant difference between NRTIs in the change in the total:HDL cholesterol ratio. Results were similar at week 96.
Resistance
In the low HIV RNA stratum, 136 patients had virologic failure, with resistance data available at baseline and failure in all but 2 patients (Table 4). Baseline major resistance was present in 13 (10%) patients with virologic failure. Among 122 virologic failures with no major resistance at baseline, there was no significant difference in the occurrence of major resistance mutations between ABC/3TC and TDF/FTC when given with either ATV/r or EFV.
Table 4.
Summary of Drug Resistance Mutations With Specific Major Mutations of Interest in the Low Screening HIV RNA Stratuma,b
| Variable | ABC/3TC (n = 264) | TDF/FTC (n = 265) | ABC/3TC (n = 266) | TDF/FTC (n = 265) |
| ATV/r | EFV | |||
| Virologic failure events, n (%) | 35 (13) | 29 (11) | 39 (15) | 33 (12) |
| Genotype available at failure | 35 | 29 | 38 | 33 |
| Major mutations at baseline | 1 | 2 | 6 | 4 |
| Without mutations at baseline | 34 | 27 | 32 | 29 |
| Mutations at virologic failure n (% of randomized)/(% with genotype and without baseline resistance)c | ||||
| Any major | 3 (1)/[9] | 1 (<1)/[4] | 18 (7)/[56] | 16 (6)/[55] |
| NRTI-associatedd | 2 (<1)/[6] | 1 (<1)/[4] | 8 (3)/[25] | 5 (2) [17] |
| M184I/V, N | 2 | 1 | 7 | 4 |
| L74I/V, N | 0 | 0 | 1 | 1 |
| Other, Ne | 0 | 0 | 1 | 1 |
| NNRTI-associatedd | 1 (<1)/[3] | 0 (0)/[0] | 18 (7)/[56] | 16 (6)/[55] |
| K103N, N | 0 | 0 | 15 | 13 |
| G190A/E/Q/S, N | 0 | 0 | 3 | 4 |
| Other, Ne | 1 | 0 | 6 | 3 |
| NRTI + NNRTI-associated | 0 (0)/[0] | 0 (0)/[0] | 8 (3)/[25] | 5 (2) [17] |
| Protease-associated | 0 (0)/[0] | 0 (0)/[0] | 0 (0)/[0] | 0 (0)/[0] |
Abbreviations: ABC/3TC indicates abacavir/lamivudine; TDF/FTC, tenofovir DF/emtricitabine; ATV/r, atazanavir/ritonavir; EFV, efavirenz; N, number of mutations.
Analyses were intent-to-treat with some patients having switched from originally assigned regimen prior to developing protocol-defined virologic failure.
Major mutations were defined as those listed by the International AIDS Society-USA [8], as well as T69D, L74I, and G190C/E/Q/T/V for reverse transcriptase, and L24I, F53L, I54V/A/T/S, G73C/S/T/A, and N88D for protease.
Total may not add up to 100% because some patients had >1 mutation.
Major mutations targeted but not observed in the low screening HIV RNA stratum were (1) NRTI: K65R, K70R/E, Y115F, Q151M, L210W, T215F/Y, K219E, and T69D, and (2) NNRTI: L100I, V106A/M, Y181C/I, and G190C/T/V.
Other observed targeted major mutations in the low screening HIV RNA stratum include (1) NRTI: M41L and D67N, and (2) NNRTI: V108I, Y188C/H, and P225H.
Resistance data for patients in the high HIV RNA stratum with virologic failure at the time of the DSMB review are shown in Table 5. When given with ATV/r, the emergence of major NRTI resistance mutations was not significantly different with ABC/3TC (6 of 29) or TDF/FTC (3 of 14, P = 1.0 of failures and P = .34 of randomized). With EFV, major NRTI resistance emerged in 15 of 23 and 2 of 8 randomized to ABC/3TC and TDF/FTC, respectively (P = .10 of failures and P = .002 of randomized).
Table 5.
Summary of Drug Resistance Mutations With Specific Major Mutations of Interest in the High Screening Viral Load Stratum at the Time of the Data Safety and Monitoring Board Actiona,b
| Variable | ABC/3TC (n = 199) | TDF/FTC (n = 200) | ABC/3TC (n = 199) | TDF/FTC (n = 199) |
| ATV/r | EFV | |||
| Virologic failure events, n (%) | 32 (16) | 15 (8) | 25 (13) | 11 (6) |
| Genotype available at failure | 32 | 15 | 25 | 11 |
| Major mutations at baseline | 3 | 1 | 2 | 3 |
| Without mutations at baseline | 29 | 14 | 23 | 8 |
| Mutations at virologic failure n (% of randomized)/(% with genotype and without baseline resistance)c | ||||
| Any major | 6 (3)/[21] | 3 (2)/[21] | 18 (9)/[78] | 4 (2)/[50] |
| NRTI-associatedd | 6 (3)/[21] | 3 (2)/[21] | 15 (8)/[65] | 2 (1)/[25] |
| M184I/V, N | 6 | 3 | 14 | 0 |
| K65R, N | 0 | 0 | 2 | 2 |
| L74I/V, N | 0 | 0 | 5 | 0 |
| Other, N‡ | 0 | 0 | 5 | 0 |
| NNRTI-associatedd | 0 (0)/[0] | 0 (0)/[0] | 18 (9)/[78] | 4 (2)/[50] |
| K103N, N | 0 | 0 | 12 | 2 |
| Y181C, N | 0 | 0 | 2 | 0 |
| L100I, N | 0 | 0 | 4 | 0 |
| G190A/E/S, N | 0 | 0 | 4 | 2 |
| Other, Ne | 0 | 0 | 9 | 0 |
| NRTI + NNRTI-associated | 0 (0)/[0] | 0 (0)/[0] | 15 (8)/[65] | 2 (1)/[25] |
| Protease-associated (N88N/S) | 1 (<1)/[3] | 0 (0)/[0] | 0 (0)/[0] | 0 (0)/[0] |
Abbreviations: ABC/3TC indicates abacavir/lamivudine; TDF/FTC, tenofovir DF/emtricitabine; ATV/r, atazanavir/ritonavir; EFV, efavirenz; N, number of mutations.
Analyses were intent-to-treat with some patients having switched from originally assigned regimen prior to developing protocol-defined virologic failure.
Major mutations were defined as those listed by the International AIDS Society USA [8], as well as T69D, L74I, and G190C/E/Q/T/V for reverse transcriptase, and L24I, F53L, I54V/A/T/S, G73C/S/T/A, and N88D for protease.
Total may not add up to 100% because some patients had >1 mutation.
Major mutations targeted but not observed in the high screening HIV RNA stratum at the time of the DSMB action were (1) NRTI: M41L, K70E/R, Q151M, L210W, T215F/Y, and T69D, and (2) NNRTI: Y181I, Y188C/H, and G190C/T/V.
Other observed targeted major mutations in the high screening HIV RNA stratum at the time of the DSMB action include (1) NRTI: D67N, Y115F, K219E, and (2) NNRTI: V106A/M, V108I, and P225H.
Adherence
Data on self-reported adherence were collected at weeks 8 and 24, and every 24 weeks thereafter. In the low viral load stratum, adherence was high at weeks 48 and 96, with perfect self-reported adherence over the past week in 87%–92% of patients in the ABC/3TC and TDF/FTC arms, respectively, with no significant difference between ABC/3TC and TDF/FTC with EFV or ATV/r at these time points (P ≥ .14). In the high viral load stratum, a significantly smaller proportion of patients in the ABC/3TC- than TDF/FTC-treated patients at week 48 had perfect adherence with ATV/r (87% vs 94%, P = .05); this was not significantly different with EFV (91% vs 93%, P = .63).
DISCUSSION
In this randomized, partially blinded study, the time to virologic failure for patients in the low HIV RNA stratum (<105 copies/mL) was similar between ABC/3TC and TDF/FTC when combined with ATV/r or with EFV. Time to first regimen modification was shorter for ABC/3TC than TDF/FTC with both ATV/r and EFV, and time to first safety event was shorter for ABC/3TC with EFV. The virologic failure results stand in contrast to the outcome previously reported for the high HIV RNA stratum where the difference in virologic efficacy between the NRTIs prompted an independent DSMB to recommend cessation of the comparison in this group of patients [5]. Further data presented here demonstrate that the efficacy difference in the high RNA stratum was seen when these NRTI combinations were given with either ATV/r or EFV.
There are several possible explanations, not mutually exclusive, for the difference in virologic outcomes between ABC/3TC and TDF/FTC in the 2 HIV RNA strata in this study. For the high HIV RNA stratum, a higher rate of NRTI and NNRTI mutations in those randomized to ABC/3TC than TDF/FTC with EFV was found, a difference not seen in the low HIV RNA stratum, suggesting that ABC/3TC may have a lower barrier to resistance than TDF/FTC. This finding is also consistent with the lower rate of selection of M184V/I mutations in some studies when FTC and 3TC are compared [9]. Owing to greater intrinsic antiviral activity of FTC over 3TC, the combination of TDF/FTC might be more potent than ABC/3TC [8]. Additionally, minority species of some mutations—most notably a change from methionine to valine at residue 184 in HIV reverse transcriptase—phenotypically increases resistance to ABC, but enhances susceptibility to TDF [10]. Importantly, MI84V was the most common NRTI resistance mutation observed in this study with virologic failure. Pharmacokinetic differences between ABC/3TC and TDF/FTC could also contribute, as the intracellular half-lives of both TDF and FTC are longer than ABC and 3TC [8, 11]; this difference could be particularly important in patients with suboptimal adherence [12]. While self-reported adherence was largely excellent among most of the study subjects, perfect adherence favored TDF/FTC over ABC/3TC in the high viral load stratum. Many of these factors might lead to a higher rate of virologic failure for ABC/3TC than TDF/FTC when the HIV RNA is high, but not be of a sufficient magnitude to influence virologic outcomes in patients who initiate treatment with lower viral loads. These hypotheses are being explored in resistance and pharmacokinetic studies of A5202.
When given with EFV, ABC/3TC was associated with a faster time to a safety endpoint. This difference was not observed with ATV/r. The bulk of this effect related to lipid elevations and nonspecific body aches. Two drug-specific side effects of interest with TDF and ABC are renal toxicity and cardiovascular disease, respectively. There was a significant difference in change from baseline in calculated creatinine clearance for TDF/FTC compared with ABC/3TC when given with ATV/r; these results are consistent with other studies that have shown an influence of ritonavir-boosted protease inhibitors on TDF-related changes in renal function [13, 14]. Importantly, the magnitude of this change within the TDF/FTC treatment arm was small and not statistically different from baseline, and there were few treatment modifications for renal-related toxicities. This difference between TDF/FTC and ABC/3TC in change in calculated creatinine clearance was not observed when combined with EFV. Notably, cardiovascular events were infrequent in all treatment arms.
As observed in the high HIV RNA stratum, patients in the low stratum randomized to ABC/3TC were significantly more likely to discontinue randomized NRTIs than those randomized to TDF/FTC with ATV/r and with EFV. Suspected drug hypersensitivity reactions comprised 44 of the 196 ABC/3TC discontinuations in those patients receiving ABC/3TC-containing regimens. It is notable that testing for HLA-B*5701 allele, which is associated with hypersensitivity reactions to ABC [15], was permitted but not routinely performed during this study. As HLA-B*5701 testing is now standard-of-care prior to initiating an ABC-containing regimen, it is likely that the difference in tolerability between the 2 NRTI strategies would be smaller.
A5202 is the largest randomized study comparing ABC/3TC and TDF/FTC [16, 17] Nonetheless, this study has several potential limitations. With the high HIV RNA stratum stopped early, investigators and patients in the lower stratum may have chosen to stop blinded treatment. However, there was little evidence that this influenced the low HIV RNA stratum, as few patients changed the NRTIs due to clinician or participant request after the DSMB action. Baseline genotypic resistance testing in treatment-naive patients was not standard of care when A5202 started, and hence only 45% underwent testing prior to enrollment. Finally, testing for the HLA-B*5701 allele was infrequently performed prior to beginning ABC-containing therapy, and this likely influenced some of the safety and tolerability endpoints.
In summary, this large comparative clinical trial of ABC/3TC and TDF/FTC combined with either ATV/r or EFV found little difference in virologic efficacy between the 2 NRTI strategies when the screening HIV RNA was <105 copies/mL. By contrast, in the high RNA stratum, the time to virologic failure was faster with ABC/3TC than TDF/FTC with either ATV/r or EFV; furthermore, safety and tolerability generally favored TDF/FTC over ABC/3TC. Overall, these results support recent treatment guidelines that TDF/FTC be the preferred initial NRTI combination in treatment-naive patients, with ABC/3TC being an effective alternative choice. Several factors should be considered when selecting the optimal initial NRTI combination for an individual patient, including baseline HIV RNA level, HLA-B*5701 status, coinfection with hepatitis B, renal function, and lipid parameters.
Acknowledgments
We thank the patients for their participation in this study.
In addition to the authors, the study team included the following members: Courtney Ashton, BS, MT, Frontier Science and Technology Research Foundation, Inc., Amherst, NY; Anthony Bloom, Frontier Science and Technology Research Foundation, Inc., Amherst, NY; Rob Camp, Global Community Advisory Board, Barcelona, Spain; David Currin, RN, CCRC, University of North Carolina at Chapel Hill, Chapel Hill, NC; Lisa Demeter, MD, University of Rochester Medical Center, Rochester, NY; Renard Descallar, Gilead Sciences, Inc., Foster City, CA; Bernadette Jarocki, MS, Frontier Science and Technology Research Foundation, Inc., Amherst, NY; Ana Martinez, RPh, National Institutes of Health, Division of AIDS, Bethesda, MD; Lori Mong-Kryspin, BS, MT (ASCP), Ohio State University, Columbus, OH; Gene Morse, PharmD, State University of New York at Buffalo, Buffalo, NY; Savita Pahwa, MD, University of Miami School of Medicine, Miami, FL; Keith Pappa, PharmD, GlaxoSmithKline, Inc., Research Triangle Park, NC; Sabina Pfister, RN, Gilead Sciences, Inc., Foster City, CA; Helen Patterson, LPN, The Miriam Hospital, Providence, RI; Richard Pollard, MD, University of California, Davis, Sacramento, CA; Ronald Reisler, MD, MPH, University of Maryland School of Medicine, Baltimore, MD; Nancy Shulman, MD, Roche, Palo Alto, CA; Kimberly Smith, MD, MPH, Rush-Presbyterian–St. Luke’s Medical Center, Chicago, IL;Gary Thal, MD, Bristol-Myers Squibb Company, Princeton, NJ; Joseph Timpone, MD, Georgetown University Medical Center, Washington, DC; Amanda Zadzilka, BS, Frontier Science and Technology Research Foundation, Inc., Amherst, NY.
Other investigators and contributors included the following: Sandra Navarro, MD, and Lillian Colon, RN, BSN, University of Miami (Site 901) CTU Grant #AI069477, ACTG Grant #AI27675, CFAR Grant #AI073961; Susan L. Koletar, MD, and Diane Gochnour, RN, The Ohio State University (Site 2301) CTU Grant #AI069474; Julie Hoffman, RN, and Edward Seefried, RN, UCSD (Site 701) CTU Grant #AI69432; Carl Fichtenbaum, MD, and Michelle Saemann, RN, University of Cincinnati (Site 2401) CTU Grant #AI069513; Donna Pittard, RN, and David Ragan, RN, MSN, University of North Carolina (Site 3201) CTU Grant #AI69423, CFAR Grant #AI50410, GCRC Grant #RR00046 and Grant #RR025747; Elizabeth Lindsey, RN, and Tamara James, BS, University of Alabama (Site 5801) CTU Grant # U01 AI069452-03, CCTS Grant #1UL1 RR025777-01; Graham Ray, RN, MSN, and Steven Johnson, MD, University of Colorado Health Sciences Center (Site 6101) CTU Grant #RR025780; P. Jan Geiseler, MD, and Connie A. Funk, RN, MPH, University of Southern California (Site 1201) CTU Grant #5U01 AI069428; Michael Morgan, FNP, and Brenda Jackson, RN, Vanderbilt Therapeutics CRS (Site 3652) CTU Grant #AI069439; Pablo Tebas, MD, and Aleshia Thomas, RN, University of PA, subunit of Children’s Hospital of Philadelphia (Site 6201) CTU Grant #U01 AI069467-03, CFAR Grant #5P30 AI045008-10; Ge-Youl Kim, RN, BSN, and Mark Rodriguez, RN, BSN, Washington University (Site 2101) CTU Grant #AI069495; Jorge L. Santana, MD, and Santiago Marrero, MD, University of Puerto Rico (Site 5401) CTU Grant #5U01 AI069415-03; Jane Norris, PA-C, and Sandra Valle, PA-C, Stanford University (Site 501) CTU Grant #AI69556; Gary Matthew Cox, MD, and Martha Silberman, RN, Duke University Medical Center (Site 1601) CTU Grant #5U01 AI069484-02; Sadia Shaik, MD, and Ruben Lopez, MD, Harbor-UCLA Medical Center (Site 603) CTU Grant #AI069424, GCRC Grant #M01-RR00425; Margie Vasquez, RN, and Demetre Daskalakis, MD, New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant #AI069532; Valery Hughes, NP, and Christina Megill, PA, Cornell Chelsea (Site 7804) CTU Grant #AI69419, CSTC Grant #RR024996; Jessica Shore, BSN, and BabafemiTaiwo, MBBS, Northwestern University CRS (Site 2701) CTU Grant #AI069471; Mitchell Goldman, MD, and Molly Boston, RN, Indiana University (Site 2601) CTU Grant #UO1 AI025859; Jeffrey Lennox, MD, and Carlos del Rio, MD, The Ponce de Leon Center (A5802) CTU Grant #5U01 AI069418, CFAR Grant #P30 AI050409; Timothy W. Lane, MD, and Kim Epperson, RN, Moses H. Cone Memorial Hospital (Site 3203) CTU Grant #1U01 A1069423-01; Annie Luetkemeyer, MD, and Mary Payne, RN, UCSF (Site 801) CTU Grant #1U01 AI069502-01; Barbara Gripshover, MD, and Dawn Antosh, RN, Case Western Reserve University (Site 2501) CTU Grant #AI69501; Jane Reid RN, MS, APN-BC, and Mary Adams, RN, MPh, University of Rochester (Site 1101) CTU Grant #U01 AI069511, GCRC Grant #UL1 RR024160; Sheryl S. Storey, PA-C, and Shelia B. Dunaway, MD, University of Washington (Site 1401) CTU Grant #AI069434; Ilene Wiggins, RN, and Eric Zimmerman, RN, Johns Hopkins University (Site 201) CTU Grant #AI69465, CTSA Grant #U54 RR023561; Kimberly Y. Smith, MD, MPH, and Joan A. Swiatek, RN, APN, Rush University Medical Center (Site 2702) CTU Grant #5U01 AI069471; Joseph Timpone, MD, and Princy Kumar, MD, Georgetown University (Site 1008) CTU Grant #1U01 AI069494-01; Ardis Moe, MD, and Maria Palmer PA-C, UCLA Care Center (Site 601) CTU Grant #AI069424; Jon Gothing, RN, BSN, ACRN, and Joanne Delaney, RN, BSN, Brigham and Women’s Hospital, Boston, MA (Site 107) CTU Grant #AI069472; Kim Whitely, RN, and Robert Kalayjian, MD, Metro Health Center (Site 2503) CTU Grant #AI069501; Scott M. Hammer and Michael T. Yin, HIV Prevention & Treatment (Columbia University; Site 30329) CTU Grant #5U01 AI069470, Grant #1UL1 RR024156; Mamta Jain, MD, and Tianna Petersen, MS, UT Southwestern Medical Center at Dallas (Site 3751) CTU Grant #3U01 AI046376 05S4; Roberto Corales, DO, and Christine Hurley, RN, AIDS Community Health Center (Site 1108) CTU Grant #U01 AI069511, GCRC Grant #UL1 RR024160; Keith Henry, MD, and Bette Bordenave, RN, Hennepin County Medical Center (Site 1502) Grant #N01 AI72626; Amanda Youmans, NP, and Mary Albrecht, MD, Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant #UOI A106947203; Richard B. Pollard, MD, and Abimbola Olusanya, NP, University of California, Davis Medical Center (Site 3851) Grant #AI38858; Paul R. Skolnik, MD, and Betsy Adams, RN, Boston Medical Center CRS (Site 104) CTU Grant #AI069472; Helen Patterson, LPN, The Miriam Hospital-Brown University (Partners/Harvard; Site 2951) CTU Grant #1U01 AI069472-01; Michelle Ukwu and Lauren Rogers, Peabody Health Center (Site 31443) CTU Grant #AI069471; Henry H. Balfour Jr., MD, and Kathy A. Fox, RN, MBA, University of Minnesota (Site 1501) CTU Grant #AI27661; Susan Swindells, MBBS, and Frances Van Meter, APRN, University of Nebraska Medical Center (Site 1505) CTU Grant #AI27661; University of Hawaii (Site 5201) CTU Grant #AI34853; Gregory Robbins, MD, and Nicole Burgett-Yandow, RN, BSN, Massachusetts General Hospital from the Partners/Harvard/BMC ACTU (Site 101) CTU Grant #1U01 AI069472-01; Charles E. Davis Jr., MD, and Colleen Boyce, RN, IHV Baltimore Treatment CRS (Site 4651) CTU Grant #5U01 AI069447 03; William A. O’Brien, MD, and Gerianne Casey, RN, University of Texas Medical Branch (Site 6301) CTU Grant #AI032782; Gene D. Morse, PharmD, and Chiu-Bin Hsaio, MD, SUNY- Buffalo (Site 1102) CTU Grant #5U01 A1027658; San Mateo County AIDS Program (Site 505) CTU Grant #AI27666; Jeffrey L. Meier, MD, and Jack T. Stapleton, MD, University of Iowa Healthcare (Site 1504) NIAID Grant #AI27661 and Grant #AI58740; Donna Mildvan, MD, and Manuel Revuelta, MD, Beth Israel Medical Center ACTU (Site 2851) CTU Grant #AI46370; David Currin, RN, Wake County HHS (Site 30076) CTU Grant #AI25868; Wafaa El Sadr, MD, MPH, MPA, and Avelino Loquere, RN, Harlem ACTG CRS (Site 31483) CTU Grant #5U01 AI069470-03; Nyef El-Daher, MD, and Tina Johnson, RN, McCree McCuller Wellness Center (Site 1107) CTU Grant #U01 AI069511, GCRC Grant #UL1 RR024160; Robert Gross, MD, MSCE, and Kathyrn Maffei, RN, BSN, University of Pennsylvania Health (Site 6206) CTU Grant #1U01 AI69467-01; Deborah McMahon, MD, and Barbara Rutecki, CRNP, MPH, University of Pittsburgh (Site 1001) CTU Grant #1UO1 AI069494-01; Michael Wulfsohn, MD, PhD, Andrew Cheng, MD, PhD, Michael Miller, PhD, and Norbert Bischofberger PhD, Gilead Sciences; Sara Hughes, PhD, GlaxoSmithKline, Inc.
Assurances. The human subjects committees of all sites approved the A5202 protocol and written informed consent was obtained from all participants in compliance with human experimentation guidelines of the US Department of Health and Human Services.
Financial support. This work was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), along with the previous grant number for the AIDS Clinical Trials Group (ACTG) Central Group, AI38858, and the Statistical and Data Management Center (SDMC) grant, AI68634. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH. Also supported in part by the General Clinical Research Center Units funded by the National Center for Research Resources. Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline provided the study medications.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2011. pp. 1–174. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. Accessed 28 August 2011. [Google Scholar]
- 3.DeJesus E, Herrera G, Teofilo E, et al. Abacavir versus zidovudine combined with lamivudine and efavirenz, for the treatment of antiretroviral-naive HIV-infected adults. Clin Infect Dis. 2004;39:1038–46. doi: 10.1086/424009. Epub 2004. [DOI] [PubMed] [Google Scholar]
- 4.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 5.Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV therapy. N Engl J Med. 2009;361:2230–40. doi: 10.1056/NEJMoa0906768. Epub 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV: spring 2008. Top HIV Med. 2008;16:62–8. doi: 10.1007/s11750-007-0034-z. [DOI] [PubMed] [Google Scholar]
- 7.dePerio MA, Gomez FJ, Frame PT, Fichtenbaum CJ. A Truvada hypersensitivity reaction simulating abacavir hypersensitivity. AIDS. 2007;21:3–2252. doi: 10.1097/QAD.0b013e3282f08b84. 29. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau FS, Wakeford C, Mommeja-Marin H, et al. Prospective randomized trial of emtricitabine versus lamivudine short-term monotherapy in human immunodeficiency virus–infected patients. J Infect Dis. 2003;188:1652–8. doi: 10.1086/379667. Epub 2003. [DOI] [PubMed] [Google Scholar]
- 9.Maserati R, De Silvestri A, Uglietti A, et al. Emerging mutations at virological failure of HAART combinations containing tenofovir and lamivudine or emtricitabine. AIDS. 2010;24:1013–8. doi: 10.1097/QAD.0b013e328336e962. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi V, Von Lindern J, Montes-Walters M, et al. Impact of human immunodeficiency virus type 1 reverse transcriptase inhibitor drug resistance mutation interactions on phenotypic susceptibility. AIDS Res Hum Retroviruses. 2008;24:1291–300. doi: 10.1089/aid.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins T, Veikley W, St Claire RL, 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr. 2005;39:406–11. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 12.Nelson M, Girard PM, Demasi R, et al. Suboptimal adherence to darunavir/ritonavir has minimal effect on efficacy compared with lopinavir/ritonavir in treatment-naive, HIV-infected patients: 96 week ARTEMIS data. J Antimicrob Chemother. 2010;65:1505–9. doi: 10.1093/jac/dkq150. [DOI] [PubMed] [Google Scholar]
- 13.Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor–based versus nonnucleoside reverse-transcriptase inhibitor–based therapy. J Infect Dis. 2008;197:102–8. doi: 10.1086/524061. [DOI] [PubMed] [Google Scholar]
- 14.Fux CA, Simcock M, Wolbers M, et al. Tenofovir use is associated with a reduction in calculated glomerular filtration rates in the Swiss HIV Cohort Study. Antivir Ther. 2007;12:1165–73. [PubMed] [Google Scholar]
- 15.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 16.Smith KY, Patel P, Fine D, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS. 2009;23:1547–6. doi: 10.1097/QAD.0b013e32832cbcc2. [DOI] [PubMed] [Google Scholar]
- 17.Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55:49–57. doi: 10.1097/QAI.0b013e3181dd911e. [DOI] [PubMed] [Google Scholar]