Abstract
Immunoglobulins (Ig) or antibodies are heavy plasma proteins, with sugar chains added to amino acid residues by N-linked glycosylation and occasionally by O-linked glycosylation. The versatility of antibodies is demonstrated by the various functions that they mediate such as neutralization, agglutination, fixation with activation of complement and activation of effector cells. In addition to this plethora of functions, some antibodies express enzymatic activity. Antibodies endowed with enzymatic properties have been described in human autoimmune manifestations for more than a decade in a variety of disorders such as autoimmune thyroiditis, systemic erythematosus (SLE), scleroderma, rheumatoid arthritis (RA), multiple sclerosis (MS) and acquired hemophilia (AH). Antibodies isolated from these conditions were able to specifically hydrolyze thyroglobulin, DNA, RNA, myelin basic protein (MBP), and factor VIII (FVIII) or factor IX (FIX), respectively. The therapeutic relevance of these findings is discussed.
Keywords: Autoimmunity, Autoantibodies, catalytic antibodies, abzymes
1. Introduction
Pioneering studies performed in the 20th century showed that administering an animal with almost any organic chemical could induce production of antibodies that would bind specifically to the chemical. Today, antibodies are recognized to elicit an almost unlimited range of reactivities including responses to compounds only recently synthesized in the laboratory and not previously existing in nature. In addition, molecules differing in the smallest detail could be distinguished by their reactivity with different antibodies. The highly evolved machinery of the immune system to produce structurally and functionally complex molecules like antibodies offers tremendous opportunities for chemists. Whereas chemistry provided the framework for understanding the molecular basis of biomolecular structure and function, the immune system provided a highly evolved synthetic and selective process of nature. The combination of the two was theorized and proved to generate new classes of molecules with novel functions. Indeed, one example of this productive interplay is the generation of catalytic antibodies. Linus Carl Pauling, one of the most influential chemists of the 20th century proposed that if the structure of the antigen-binding site of antibodies were to be produced in a random manner, the antigen-binding site of some of the antibodies would resemble the active site of enzymes and the latter antibodies could express enzymatic activity [1]. It took scientists many years of active research and availability of unique techniques to report finally in 1986 the first antibodies with catalytic activity [2, 3]. Antibodies expressing such an activity are termed as antibody-enzymes (abzymes) or catalytic antibodies. Following the era of artificial induction of abzymes in laboratory animals, the presence of abzymes was also documented in healthy humans (in the absence of deliberate immunization) and in several pathological conditions. The present review is a summary of our existing knowledge of catalytic antibodies in autoimmune disorders and presents the current understanding on their mechanisms at play that may hallmark their role in the pathophysiology.
2. Spontaneous occurrence of catalytic antibodies in physiology and pathology
2.1 Abzymes under physiological conditions
Catalytic antibodies are generated spontaneously by the immune system in the absence of immunization by an exogenous peptide or a protein. Catalytic IgG and IgM have been documented in the serum of healthy individuals [4–6], and antibodies with protein kinase and deoxyribonuclease activities have been detected in the milk of healthy mothers [7, 8]. In the beginning of the past decade, Wentworth et al., demonstrated that all antibodies are potentially endowed with bactericidal activity. However, this activity is also shared with other non-immunoglobulin molecules such as chicken ovalbumin and β-galactosidase [9]. Nonetheless, these observations suggested that “naturally occurring” catalytic antibodies participate directly in the elimination of the biochemical wastes released by the metabolism of the organism [10] and pointed towards an intrinsic protective role of antibodies under physiological conditions. This role is independent of the capacity of antibodies to neutralize circulating exogenous antigens, to facilitate their endocytosis by antigen-presenting cells and to participate in their elimination from the organism.
2.2 Abzymes under pathological conditions
The group of Sudhir Paul from the University of Texas (USA) reported the first example of an abzyme under pathological conditions in bronchial asthma patients, in which the antibodies were able to cleave the vasoactive intestinal peptide [11]. Since then, DNA- and RNA-hydrolyzing antibodies have been isolated from the serum of patients with different systemic autoimmune diseases: systemic lupus erythematosus, sclerodermia, rheumatoid arthritis or multiple sclerosis [12–14]. Proteolytic antibodies specific for thyroglobulin (Tg) [15] have been reported in patients with thyroiditis. Amyloid β peptide (Aβ)-hydrolyzing IgM antibodies were recently found in the sera of patients with Alzheimer’s disease (AD) [16]. Myelin basic protein (MBP)-hydrolyzing antibodies were documented in patients with multiple sclerosis [17–19]. Levels of IgG with amidase activity were higher in patients with sepsis [20]. Abzymes to coagulation factors. such as factor VIII (FVIII), in patients with hemophilia A [21–23] and to FVIII and FIX in patients with renal graft transplant [24] and acquired hemophilia [25, 26], were documented (summary in Table 1). In the present review, we will concentrate only on abzymes documented in autoimmune diseases.
Table 1.
Abzymes reported in Pathological Diseases.
| Pathology | Targeted antigen |
|---|---|
|
Inflammatory disorders
| |
| Asthma | Vasoactive intestinal peptide (VIP) |
| Sepsis | FVIII, FIX |
|
| |
|
Autoimmune disorders
| |
| Hashimoto’s Thyroïditis | Thyroglobulin |
| Systemic Lupus Erythematosus | DNA, RNA |
| Scleroderma | DNA, RNA |
| Rheumatoid Arthritis | DNA, RNA |
| Multiple Sclerosis | DNA, RNA, MBP |
| Acquired Hemophilia | FVIII, FIX |
| Alzheimer’s Disease | Aβ-peptide |
|
| |
|
Metabolic disorder
| |
| Diabetes | Grp94 |
|
| |
|
Infectious disorder
| |
| Immune thrombocytopenia associated with HIV-1 infection | Platelet GPIIIa |
|
| |
|
Neoplastic disorders
| |
| Multiple Myeloma | Prothrombin |
|
| |
|
Alloimmune disorders
| |
| Hemophilia A | FVIII |
| Renal Rejection in Transplantation | FVIII, FIX |
The presence of abzymes has been reported in many human pathological conditions. The diseases can be separated under 6 broad categories i.e., inflammatory, autoimmune, metabolic, infectious, neoplastic and alloimmune disorders. The different known target antigens to the abzymes in each disease are listed.
3. Antigen specificity and possible role of abzymes found in autoimmune conditions
3.1 DNA- and RNA- hydrolyzing abzymes
The research group of Alexander Gabibov from the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry (Moscow, Russia) first described the cleavage of antibody-mediated phosphodiester bond [12]. The results from their group described how autoantibodies purified from the sera of patients with SLE and other diseases with autoimmune manifestations caused the cleavage of phosphodiester bond [12, 27, 28]. Polyclonal IgG antibodies purified from sera of several patients with SLE and hepatitis B had RNA-hydrolyzing activities that differ from the weak RNase A-type activities found in the sera of healthy donors [13]. Kinetic studies performed using the flow linear dichroism technique of the DNA abzymes to DNA substrates showed a rather efficient binding. Not surprisingly, based on the fact that abzymes are slow catalysts when compared to the kinetic values determined for conventional DNases, the reaction turnover values for the DNA-abzymes were two-fold lower [29].
Gololobov et al., convincingly demonstrated the correlation between the presence of DNA-hydrolyzing activity of autoantibodies and the stage of development of SLE [30]. In an attempt to understand the role of DNA-hydrolyzing antibodies, Kozyr et al., [31] demonstrated the cytotoxicity of anti-DNA autoantibodies isolated from sera of SLE and chronic lymphocytic leukemia patients on a permanent cell line - L929. DNA-hydrolyzing properties of the same autoantibody preparations were analyzed in parallel. The data obtained outlined the correlation between cytotoxicity and DNA-hydrolyzing properties of the autoantibodies. In a similar line of evidence, Lee et al., [32] investigated the substrate specificity of catalytic activity of an anti-DNA monoclonal autoantibody, G1-5, which was obtained from an MRL-lpr/lpr mouse by hybridoma technology. The antibody catalyzed hydrolysis of single- and double-stranded DNA with a higher substrate specificity for thymine than adenine by either beta-glycosidic or phosphodiester bond cleavage. Treatment of human promyelocytic leukemia cells (HL60) with the antibody promoted cell death and induced the activation of caspases. The pan-caspase inhibitor inhibited the cell death induced by the antibody. Furthermore, the antibody bound to cell membrane and penetrated into the cells.
The presence and role of DNA-abzymes in conditions such as SLE is difficult to comprehend. There is no convincing data to date for the role of DNA-hydrolyzing antibodies in SLE. However, given the cytotoxic activity of DNA-abzymes towards leukemic cell lines, one could envisage their possible use in malignant conditions. DNA-hydrolyzing abzymes may be a therapeutic option to dissolve tumors. Nevertheless, the lack of understanding of different DNA-hydrolyzing antibodies and their possible intracellular mechanism of action delays implementation.
3.2 Tg-hydrolyzing abzymes
Thyroglobulin (Tg) is the precursor of thyroid hormones and is a target for autoantibodies in autoimmune thyroid diseases. Li et al., documented that co-incubation of [125 I]-labeled thyroglobulin (Tg) with anti-Tg antibodies from the plasma of patients with Hashimoto’s thyroiditis yielded cleavage of Tg. A Km of 39nM for the reaction mediated by the patient’s anti-Tg IgG indicated a strong affinity of the interaction. Interestingly, control IgG purified from the plasma of healthy blood donors did not hydrolyze Tg [15]. However, the pathophysiological role of Tg-hydrolyzing IgG in Hashimoto’s thyroiditis remains unclear. Tg functions in the thyroid and is found at only very low levels in blood under physiological conditions. Clearance of Tg in the blood by catalytic antibodies may facilitate the reduction of autoimmune reactions against this protein and be of potential benefit for patients. However, anti-Tg antibodies have been shown to access the thyroid; administration of anti-Tg antiserum and implantation of Tg-antibody-secreting hybridomas in experimental animals results in thyroid dysfunction. Moreover, B lymphocytes synthesizing anti-Tg Abs are preferentially found within the human thyroid compared with the spleen and other lymphoid organs. In this respect, entry of catalytic antibodies in the thyroid may lead to the destruction of Tg and to the depletion of the thyroid hormones T3 and T4, which are formed from Tg through iodination and peptidase processing.
3.3 Aβ peptide-hydrolyzing abzymes
Alzheimer’s disease (AD), a neurodegenerative disorder characterized by progressive dementia, judgment impairment, delusions and irritability, affects many elderly. Recent evidence classified Alzheimer’s disease as a possible autoimmune disorder that develops due to pathogen mimicry of key Alzheimer’s disease-related proteins [33]. Some consider deposition of beta-amyloid (Aβ) as an important early event in the pathogenesis of AD. The presence of increased Aβ concentrations in the AD brain may induce neurodegenerative effects. Clearance of Aβ thus represents a potential therapeutic approach, possibly with the use of monoclonal Aβ-binding IgGs. However, a monoclonal Aβ-binding IgG administered peripherally to AD patients developed magnetic resonance imaging abnormalities consistent with vasogenic edema, highlighting the deleterious effect of this therapy [34].
Taguchi et al. [16] recently reported that IgMs purified from the sera of AD patients hydrolyzed Aβ at rates superior to IgMs from age-matched humans without dementia. Interestingly, IgMs from non-elderly humans expressed the least catalytic activity. The reaction rate 4.4 × 10−3 μM Aβ/μM IgM/min was sufficient to afford appreciable degradation at physiological Aβ and IgM concentrations found in peripheral circulation. These results raise the possibility of administering catalytic IgM as therapeutic molecules for treatment of AD. We [35] reported that IgMs may also activate microglial cells via cellular Fc receptors. The use of catalytic IgM as treatment agents is not of concern if only trace amounts of the IgMs are present in the brain because of proscribed passage of peripheral IgM across the BBB and limited secretion by B cells resident in the central nervous system. In mouse AD models, clearance of amyloid plaques from the brain parenchyma induced by Aβ-binding IgGs is accompanied by Aβ deposition in the blood vessels and microhemorrhages [36]. Abzymes may exert lesser undesirable effects than conventional Aβ-binding IgGs, because the Aβ fragments generated by the abzyme reaction are less amyloidogenic than intact Aβ, and hence reduce the opportunity for amyloid re-deposition in the blood vessels. Based on these concepts, one can infer fewer deleterious effects from catalytic IgMs than from IgG class Aβ-binding classical antibodies. Catalytic IgM autoantibodies can help clear Aβ, and they open the possibility of using catalytic Abs for AD immunotherapy.
3.4 MBP-hydrolyzing abzymes
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the human CNS with heterogeneous pathophysiological and clinical manifestations and a very complicated etiology [37, 38]. B cells, plasma cells, and antibodies are commonly found in active central nervous system lesions in MS patients and are thus tightly associated with the diagnosis. It is, nevertheless, unclear whether the Ig molecules present in the lesions actively contribute to the pathogenesis or the progression of the disease. Accumulated recent evidence supports a pathogenic role for these antibodies [39].
The first description of catalytic antibodies to MBP was documented in SJL mice, with induced autoimmune encephalomyelitis [17]. Since then, hydrolytic anti-MBP antibodies were isolated from MS patients [18], in addition, the hydrolytic activity of the anti-MBP antibodies correlated with the expanded disability status [19, 40]. Six preferential sites of cleavage of the MBP molecule by abzymes isolated from patients with MS were identified [18, 41]. Interestingly, all the identified cleavage sites were located c-terminus to either arginine or lysine residues thus providing an insight into the possible of mechanism of action of abzymes (a serine protease-like activity). Surprisingly, aprotinin, a binding inhibitor of trypsin, was unable to block the abzyme-mediated hydrolysis. The mechanism of action of these abzymes and comparison to conventional serine proteases would be of considerable interest to protein biochemists studying the structure-function relationship of proteins.
Belogurov et al., [42] reported on the recognition and degradation of MBP peptides by serum autoantibodies as a novel biomarker for MS. The authors made use of a constructed MBP-derived recombinant “epitope library” that spanned the entire MBP molecule to define the epitope-binding/-cleaving activities of autoantibodies isolated from the sera of 26 MS patients and 11 healthy individuals. The levels of autoantibodies to MBP fragments as well as to whole MBP and myelin oligodendrocyte glycoprotein molecules were significantly higher in the sera of MS patients than in those of healthy donors. Patients with MS (77% of progressive and 85% of relapsing-remitting) and no healthy donors were positive for catalysis, showing pronounced epitope specificity to the encephalitogenic MBP peptide 81–103. Based on these results, anti-MBP binding and cleavage by abzymes may be regarded as a specific feature of MS as compared to healthy donors and may serve as an additional marker of disease progression.
3.5 Coagulation factor-hydrolyzing abzymes
Acquired hemophilia (AH) is a severe hemorrhagic autoimmune disorder that occurs in about 1/1,000,000 individuals each year. It is characterized by the spontaneous development of autoantibodies directed against endogenous factor VIII (FVIII), the co-factor of activated factor IX (FIX) in the coagulation cascade. Clinical features include bleeding in mucosal and soft tissues, hematuria, hematemesis or melena and prolonged post-partum or post-operative bleeding [43]. In most patients, anti-FVIII autoantibodies are idiopathic. However, the disorder is associated with other conditions in about 40–50% of cases, which mainly occur in relation to post-partum, autoimmune diseases, malignancies and drug administration [44]. Mortality in patients with AH is documented between 6.2 and 44.3 percent one year following diagnosis [45].
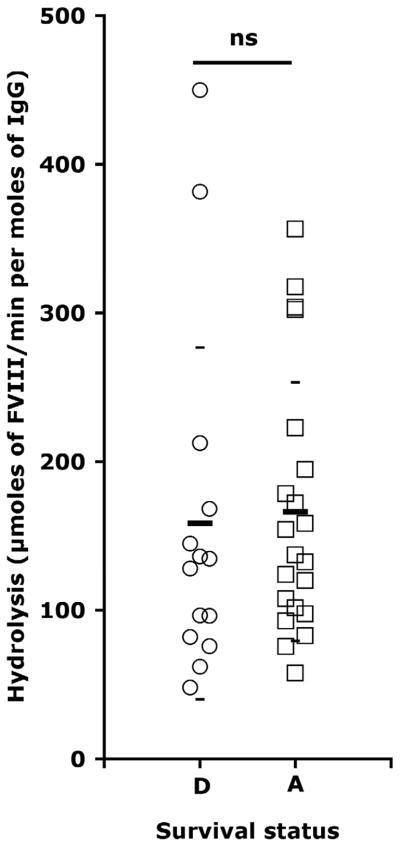
Anti-FVIII autoantibodies, also referred to as FVIII inhibitors, neutralize FVIII pro-coagulant activity by sterically preventing the interaction of FVIII with activated FIX, von Willebrand factor, phospholipids, thrombin and factor X. In a previous study, we documented that pooled IgG from healthy donors exhibited moderate FVIII-hydrolyzing activity [25]. Purified IgG from 21 of 45 patients with AH demonstrated FVIII hydrolysis rates significantly greater than control IgG. Three of four patients followed over the course of the disease had rates of FVIII hydrolysis that co-evolved with inhibitory titers in plasma, suggesting that IgG-mediated FVIII hydrolysis participates, in part, in FVIII inactivation. However, due to the complex etiology of the disease, no known clinical parameter, including the presence of FVIII-hydrolyzing IgG (Fig 1), can predict the outcome of the disease, thus arguing against a significant role for FVIII-hydrolyzing IgG in acquired hemophilia [25].
Figure 1. IgG-mediated hydrolysis of FVIII versus survival status of patients with acquired hemophilia.
The survival from acquired hemophilia was known in the case of 35 patients. Among these, fourteen patients were documented as deceased (D, open circles, 158±118.3 μmol/min/mol) and 21 patients were documented as alive (A, open squares, 166.1±87.1 μmol/min/mol), one year following diagnosis of acquired hemophilia. The significance between the IgG-mediated hydrolysis of FVIII and the survival of patients was assessed using the Mann-Whitney non-parametric test. (ns – no significance).
FIX inhibitors have occasionally been reported in patients with acquired hemophilia, alone [46–50] or in combination with FVIII inhibitors [51, 52]. We recently documented that IgG from some patients with acquired hemophilia significantly proteolyze and activate FIX [26]. The natural enzyme that activates FIX under physiological conditions in circulation is activated factor XI (FXIa). The sites of cleavage on the FIX molecule are R145 and R180. The cleavage at these two sites results in the release of an 11 kDa activation peptide. In our study, following incubation of FIX with patients’ IgG, we identified N-terminal sequences of 1Y and 181V. We do not know, however, if R145 is another cleavage site for the abzymes, but given that FIX is activated on incubation with patients’ IgG, theoretically this cleavage must be occurring. Figure 2 gives a summary of the cleavage sites on FIX by FXIa and by patients’ IgG.
Figure 2. Schematic representation of FIX.
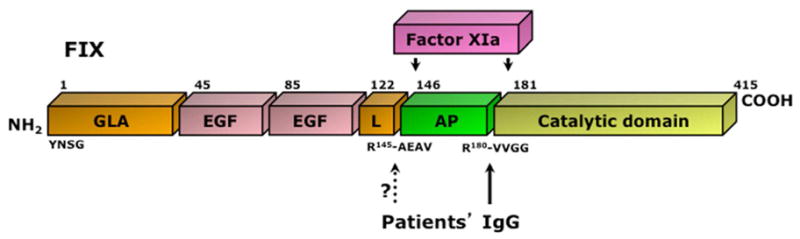
FIX is comprised of a gamma-carboxyglutamic (GLA), two epidermal growth factor (EGF)-like domains and a linker (L) domain separated from a catalytic domain by an activation peptide (AP). Activation of FIX by activated factor XI (FXIa) occurs upon cleavage at residues R145-A146 and R180-V181 and removal of the AP. The N-terminal sequences of the Gla and catalytic domains, YNSG and VVGG, respectively, are indicated. The identified and presumed cleavage sites for patients’ IgG are indicated. IgG-mediated cleavage at R145-A146 is inferred from the molecular weight of the hydrolyzed band (See Figure 2A in [26]).
Interestingly, there was no significant correlation between FVIII-hydrolyzing and FIX-hydrolyzing IgG thus suggesting that the two populations of abzymes may be different. Our results identified IgG abzymes as novel molecules able to hydrolyze and activate FIX under pathological conditions. Although the estimated enzymatic kinetics of proteolytic IgG are low compared to those of classical enzymes, this may be compensated by the long-half life and substantially higher concentration of IgG in the circulation. Interestingly, patients who survived 12 months after diagnosis statistically tended to have higher levels of FIX-activating IgG than deceased patients (P<0.1), although the difference did not reach statistical significance. Taken together, the data suggested that, in certain underlying pathologies, IgG-mediated FIX activation may be beneficial and represent an anti-hemorrhagic mechanism that compensates, at least in part, for the inhibition of endogenous FVIII by the patients’ anti-FVIII autoantibodies. In support, in vitro addition of picomolar levels of activated FIX to plasma partly restores thrombin generation, provided that residual FVIII in plasma was ≥3%. Our results raise the issue of the therapeutic relevance of the passive administration of proteolytic FIX-activating antibodies; abzymes would advantageously combine the capacity for “turn-over” that characterizes enzymatic activities, low risk for thrombotic complications owing to their low catalytic rates of FIX activation, with long half-life typical of IgG molecules.
4. The possible origin of abzymes
A major question regarding abzymes is their origin. It is unclear if antibodies endowed with catalytic activity are an intrinsic part of the physiological immune system or if the catalytic function of antibodies is gained solely after proper stimulation of the immune system by an appropriate immunogen. An important issue relates to the increased prevalence of abzymes under pathological conditions (Table 1). The presence of catalytic antibodies under pathological conditions has been described in a large number of pathologies including multiple myeloma, asthma, Hashimoto’s thyroiditis, systemic lupus erythematosus, scleroderma, rheumatoid arthritis, multiple sclerosis, HIV-related immune thrombocytopenia, hemophilia A, severe sepsis and acquired hemophilia [5, 15, 17, 18, 20, 22, 31, 40, 53–56].
The origin of disease-associated catalytic antibodies under pathological conditions is far from clear. In the absence of deliberate immunization, disease-associated catalytic antibodies may have been “induced” by the antigen implicated in the disease. Secondly, the increased occurrence of catalytic antibodies in pathology may result from the loss of repressive control over catalytic antibody-producing clones generated spontaneously under physiological conditions (as seen in the case of MLR/lpr mice that lack functional Fas signalling and hence generate higher numbers of catalytic IgG-producing B cell clones upon active immunization [57]). A third explanation for the origin of catalytic antibodies in pathological conditions is based on idiotypic network and exacerbated self-recognition in autoimmune diseases [12].
In several instances, the target antigen for catalytic antibodies is the antigen central to the pathogenesis. Thus, thyroglobulin-hydrolyzing IgG was documented in patients with Hashimoto’s thyroiditis [15] as well as FVIII-hydrolyzing IgG in patients with hemophilia A [22] and acquired hemophilia [25], myelin basic protein-hydrolyzing IgG in patients with multiple sclerosis [17–19] and Aβ-peptide hydrolyzing antibodies in patients with Alzheimer’s disease. The affinities of antibodies observed for antigen recognition were in the nanomolar range for thyroglobulin- [15] and FVIII- [22] cleaving antibodies. Since the high-affinity recognition of antigen is a known property of the V-domain affinity maturation, expression of the catalytic activity must be compatible with clonal selection under specific immunological circumstances, such as disease-associated conditions in which proteolytic antibodies have been identified.
Target antigens for disease-associated hydrolytic antibodies include prothrombin, vasoactive intestinal peptide (VIP), thyroglobulin, DNA, RNA, the anti-platelet integrin GPIIIa (β3), FVIII and FIX. In some cases, however, the target antigen is not central to the pathology in which the catalytic antibodies are detected. Thus, the presence of DNA- and RNA- hydrolyzing antibodies in patients with multiple sclerosis [14], FVIII- and FIX- hydrolyzing abzymes in patients with severe sepsis and/or renal graft transplant [20, 24] and FIX-hydrolyzing abzymes in patients with acquired hemophilia [26] argues against the hypothesis of an antigen-driven generation of catalytic antibodies. In the latter disorders, the picture is complicated by the systemic and/or the highly inflammatory nature of the disease. This suggests that the catalytic antibodies under pathological conditions may not be solely directed/linked to the antigen implicated in the disease. The catalytic antibodies may complement the general alteration of the immune response. In this respect, the pathological immune response may be directed towards different antigens, some of which are directly relevant to the disease, some of which are not, or alternatively, may be directed against a single antigen that may not be related to the disease.
5. Conclusion
The pathogenic or beneficial effect of catalytic antibodies has never been directly demonstrated by reproducing the disease in animals through passive administration of catalytic antibodies isolated from patients’ serum. Reasons include the low amounts of catalytic Ig that may be purified from the serum of patients and the absence of monoclonal IgM or IgG with serine protease activity. The availability of a monoclonal antibody with the desired qualities will aid in answering the relevance of results observed with the serum polyclonal catalytic antibodies in autoimmune pathologies. Even if monoclonal abzymes are made available, some issues will require further examination before the full potential of catalytic antibodies can be understood. Abzymes remain slow catalysts. They may exhibit slow release of products; the reported affinities in the μM range of antibodies for the antigens are capable of further improvement. More work still needs to be done to develop abzymes as irreplaceable tools of clinical application.
Finally, the authors echo the congratulations of the other contributors to this special issue highlighting the career of Professor Chella David. Chella has inspired so many students and so much work that it is nearly impossible to express our appreciation. We note that this is a special issue of the Journal of Autoimmunity/Autoimmunity Reviews in honor of a distinguished autoimmunologist. This issue is part of a special series of the journal that recognizes not only critical figures in autoimmunology but also attempts to publish special issues focusing on cutting-edge immunology that will ultimately impact the care of patients [58–79]. It is of particular interest that Chella David’s research was classified as translational immunology long before the concept of translational was recognized. Thank you, Chella.
Acknowledgments
Human recombinant FVIII were kind gifts from Bayer Healthcare (Lille, France) and CSL-Behring (Paris, France), and FIX from Baxter (Maurepas, France).
Financial support
(SLD and SVK) This work was supported by INSERM, CNRS, the Indo-French Center for Promotion of Advanced Research (CEFIPRA), Agence Nationale de la Recherche (ANR-05-MRAR-012, ANR-09-GENO-028) and from Japan Sciences and Technology Agency (JST, Tokyo, Japan). BW was the recipient of a fellowship from Laboratoire Français de Fractionnement et des Biotechnologies (LFB) while in France and is currently funded by a fellowship from the Mayo-Applebaum funds. (MR, AW, and AB) This work was also supported by grants from the National Institutes of Health (NS R01 24180, NS R01 32129), the National Multiple Sclerosis Society (CA 1011A8), the Applebaum Foundation, the Hilton Foundation, the Minnesota Partnership Award for Biotechnology and Medical Genomics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pauling L. Chemical achievement and hope for the future. American Scientist. 1948;36:51. [PubMed] [Google Scholar]
- 2.Pollack SJ, Jacobs JW, Schultz PG. Selective chemical catalysis by an antibody. Science. 1986;234:1570. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- 3.Tramontano A, Janda KD, Lerner RA. Catalytic antibodies. Science. 1986;234:1566. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- 4.Kalaga R, Li L, O’Dell JR, Paul S. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheumatoid arthritis. J Immunol. 1995;155:2695–702. [PubMed] [Google Scholar]
- 5.Paul S, Lan L, Kalaga R, O’Dell J, Dannenbring RE, Jr, Swindells S, et al. Characterization of thyroglobulin-directed and polyreactive catalytic antibodies in autoimmune disease. J Immunol. 1997;159:1530–6. [PubMed] [Google Scholar]
- 6.Planque S, Bangale Y, Song XT, Karle S, Taguchi H, Poindexter B, et al. Ontogeny of proteolytic immunity: IgM serine proteases. J Biol Chem. 2004 doi: 10.1074/jbc.M312152200. [DOI] [PubMed] [Google Scholar]
- 7.Kanyshkova TG, Semenov DV, Khlimankov D, Buneva VN, Nevinsky GA. DNA-hydrolyzing activity of the light chain of IgG antibodies from milk of healthy human mothers. FEBS Lett. 1997;416:23–6. doi: 10.1016/s0014-5793(97)01163-0. [DOI] [PubMed] [Google Scholar]
- 8.Kit Y-Y, Semenov DV, Nevinsky GA. Phosphorylation of different human milk proteins by human catalytic secretory immunoglobulin A. Biochem Molec Biol Intl. 1996;39:521–7. doi: 10.1080/15216549600201571. [DOI] [PubMed] [Google Scholar]
- 9.Wentworth JP, McDunn JE, Wentworth AD, Takeuchi C, Nieva J, Jones T, et al. Evidence of antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–9. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 10.Friboulet A, Avalle B, Debat H, Thomas D. A possible role of catalytic antibodies in metabolism. Immunol Today. 1999;20:474–5. doi: 10.1016/s0167-5699(99)01528-5. [DOI] [PubMed] [Google Scholar]
- 11.Paul S, Volle DJ, Beach CM, Johnson DR, Powell MJ, Massey RJ. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989;244:1158–62. doi: 10.1126/science.2727702. [DOI] [PubMed] [Google Scholar]
- 12.Shuster AM, Gololobov GV, Kvashuk OA, Bogomolova AE, Smirnov IV, Gabibov AG. DNA hydrolyzing autoantibodies. Science. 1992;256:665–7. doi: 10.1126/science.1585181. [DOI] [PubMed] [Google Scholar]
- 13.Vlassov A, Florentz C, Helm M, Naumov V, Buneva V, Nevinsky G, et al. Characterization and selectivity of catalytic antibodies from human serum with RNase activity. Nucleic Acids Res. 1998;26:5243–50. doi: 10.1093/nar/26.23.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baranovskii AG, Kanyshkova TG, Mogelnitskii AS, Naumov VA, Buneva VN, Gusev EI, et al. Polyclonal antibodies from blood and cerebrospinal fluid of patients with multiple sclerosis effectively hydrolyze DNA and RNA. Biochemistry (Mosc) 1998;63:1239–48. [PubMed] [Google Scholar]
- 15.Li L, Paul S, Tyutyulkova S, Kazatchkine MD, Kaveri S. Catalytic activity of anti-thyroglobulin antibodies. J Immunol. 1995;154:3328–32. [PubMed] [Google Scholar]
- 16.Taguchi H, Planque S, Nishiyama Y, Symersky J, Boivin S, Szabo P, et al. Autoantibody-catalyzed hydrolysis of amyloid beta peptide. J Biol Chem. 2008;283:4714–22. doi: 10.1074/jbc.M707983200. [DOI] [PubMed] [Google Scholar]
- 17.Ponomarenko NA, Durova OM, Vorobiev II, Aleksandrova ES, Telegin GB, Chamborant OG, et al. Catalytic antibodies in clinical and experimental pathology: human and mouse models. J Immunol Methods. 2002;269:197–211. doi: 10.1016/s0022-1759(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 18.Ponomarenko NA, Durova OM, Vorobiev II, Belogurov AA, Jr, Kurkova IN, Petrenko AG, et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc Natl Acad Sci U S A. 2006;103:281–6. doi: 10.1073/pnas.0509849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponomarenko NA, Durova OM, Vorobiev II, Belogurov AA, Telegin GB, Suchkov SV, et al. Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol Lett. 2006;103:45–50. doi: 10.1016/j.imlet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix-Desmazes S, Bayry J, Kaveri SV, Hayon-Sonsino D, Thorenoor N, Charpentier J, et al. High levels of catalytic antibodies correlate with favorable outcome in sepsis. Proc Natl Acad Sci U S A. 2005;102:4109–13. doi: 10.1073/pnas.0500586102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacroix-Desmazes S, Bayry J, Misra N, Horn MP, Villard S, Pashov A, et al. The prevalence of proteolytic antibodies against factor VIII in hemophilia A. N Engl J Med. 2002;346:662–7. doi: 10.1056/NEJMoa011979. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix-Desmazes S, Moreau A, Sooryanarayana, Bonnemain C, Stieltjes N, Pashov A, et al. Catalytic activity of antibodies against factor VIII in patients with hemophilia A. Nat Med. 1999;5:1044–7. doi: 10.1038/12483. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix-Desmazes S, Wootla B, Dasgupta S, Delignat S, Bayry J, Reinbolt J, et al. Catalytic IgG from patients with hemophilia A inactivate therapeutic factor VIII. J Immunol. 2006;177:1355–63. doi: 10.4049/jimmunol.177.2.1355. [DOI] [PubMed] [Google Scholar]
- 24.Wootla B, Nicoletti A, Patey N, Dimitrov JD, Legendre C, Christophe OD, et al. Hydrolysis of coagulation factors by circulating IgG is associated with a reduced risk for chronic allograft nephropathy in renal transplanted patients. J Immunol. 2008;180:8455–60. doi: 10.4049/jimmunol.180.12.8455. [DOI] [PubMed] [Google Scholar]
- 25.Wootla B, Dasgupta S, Dimitrov JD, Bayry J, Levesque H, Borg JY, et al. Factor VIII Hydrolysis Mediated by Anti-Factor VIII Autoantibodies in Acquired Hemophilia. J Immunol. 2008;180:7714–20. doi: 10.4049/jimmunol.180.11.7714. [DOI] [PubMed] [Google Scholar]
- 26.Wootla B, Christophe OD, Mahendra A, Dimitrov JD, Repesse Y, Ollivier V, et al. Proteolytic antibodies activate factor IX in patients with acquired hemophilia. Blood. 2010 doi: 10.1182/blood-2010-07-296103. [DOI] [PubMed] [Google Scholar]
- 27.Gabibov AG, Gololobov GV, Makarevich OI, Schourov DV, Chernova EA, Yadav RP. DNA-hydrolyzing autoantibodies. Appl Biochem Biotechnol. 1994;47:293–302. doi: 10.1007/BF02787941. discussion 3. [DOI] [PubMed] [Google Scholar]
- 28.Kozyr AV, Kolesnikov AV, Aleksandrova ES, Sashchenko LP, Gnuchev NV, Favorov PV, et al. Novel functional activities of anti-DNA autoantibodies from sera of patients with lymphoproliferative and autoimmune diseases. Appl Biochem Biotechnol. 1998;75:45–61. doi: 10.1007/BF02787708. [DOI] [PubMed] [Google Scholar]
- 29.Gololobov GV, Chernova EA, Schourov DV, Smirnov IV, Kudelina IA, Gabibov AG. Cleavage of supercoiled plasmid DNA by autoantibody Fab fragment: application of the flow linear dichroism technique. Proc Natl Acad Sci USA. 1995;92:254–7. doi: 10.1073/pnas.92.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gololobov GV, Bogomolova AE, Iadav RP, Ermolaeva MV, Belostotskaia KM, Prokaeva TB, et al. [Isolation and characteristics of catalytic antibodies to DNA in systemic lupus erythematosis] Biokhimiia. 1993;58:313–8. [PubMed] [Google Scholar]
- 31.Kozyr AV, Kolesnikov AV, Zelenova NA, Sashchenko LP, Mikhalap SV, Bulina ME, et al. Autoantibodies to nuclear antigens: correlation between cytotoxicity and DNA-hydrolyzing activity. Appl Biochem Biotechnol. 2000;83:255–68. doi: 10.1385/abab:83:1-3:255. discussion 68–9, 97–313. [DOI] [PubMed] [Google Scholar]
- 32.Lee EJ, Jang EJ, Lee E, Yu J, Chung HY, Jang YJ. Cell-penetrating autoantibody induces caspase-mediated apoptosis through catalytic hydrolysis of DNA. Bioorg Med Chem. 2007;15:2016–23. doi: 10.1016/j.bmc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Carter CJ. Alzheimer’s disease: a pathogenetic autoimmune disorder caused by herpes simplex in a gene-dependent manner. Int J Alzheimers Dis. 2010;2010:140539. doi: 10.4061/2010/140539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24:198–203. doi: 10.1097/WAD.0b013e3181c53b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howe CL, Mayoral S, Rodriguez M. Activated microglia stimulate transcriptional changes in primary oligodendrocytes via IL-1beta. Neurobiol Dis. 2006;23:731–9. doi: 10.1016/j.nbd.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Morgan D. Mechanisms of A beta plaque clearance following passive A beta immunization. Neurodegener Dis. 2005;2:261–6. doi: 10.1159/000090366. [DOI] [PubMed] [Google Scholar]
- 37.Wingerchuk DM, Lucchinetti CF, Noseworthy JH. Multiple sclerosis: current pathophysiological concepts. Lab Invest. 2001;81:263–81. doi: 10.1038/labinvest.3780235. [DOI] [PubMed] [Google Scholar]
- 38.Hafler DA. Multiple sclerosis. J Clin Invest. 2004;113:788–94. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber MS, Hemmer B, Cepok S. The role of antibodies in multiple sclerosis. Biochim Biophys Acta. 2011;1812:239–45. doi: 10.1016/j.bbadis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Polosukhina DI, Kanyshkova TG, Doronin BM, Tyshkevich OB, Buneva VN, Boiko AN, et al. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J Cell Mol Med. 2004;8:359–68. doi: 10.1111/j.1582-4934.2004.tb00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belogurov AA, Kurkova IN, Misikov VK, Suchkov SV, Telegin GB, Alekhine AI, et al. Substrate specificity of catalytic autoantibodies in neurodegenerative processes. Dokl Biochem Biophys. 2007;413:61–4. doi: 10.1134/s1607672907020068. [DOI] [PubMed] [Google Scholar]
- 42.Belogurov AA, Jr, Kurkova IN, Friboulet A, Thomas D, Misikov VK, Zakharova MY, et al. Recognition and degradation of myelin basic protein peptides by serum autoantibodies: novel biomarker for multiple sclerosis. J Immunol. 2008;180:1258–67. doi: 10.4049/jimmunol.180.2.1258. [DOI] [PubMed] [Google Scholar]
- 43.Green D, Lechner K. A survey of 215 non-hemophilic patients with inhibitors to factor VIII. Thromb Haemastos. 1981;45(3):200–3. [PubMed] [Google Scholar]
- 44.Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol. 2003;121:21–35. doi: 10.1046/j.1365-2141.2003.04162.x. [DOI] [PubMed] [Google Scholar]
- 45.Collins PW, Hirsch S, Baglin TP, Dolan G, Hanley J, Makris M, et al. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109:1870–7. doi: 10.1182/blood-2006-06-029850. [DOI] [PubMed] [Google Scholar]
- 46.Castro O, Farber LR, Clyne LP. Circulating anticoagulants against factors IX and XI in systemic lupus erythematosus. Ann Intern Med. 1972;77:543–8. doi: 10.7326/0003-4819-77-4-543. [DOI] [PubMed] [Google Scholar]
- 47.Kyriakou DS, Alexandrakis MG, Passam FH, Foundouli K, Matalliotakis E, Koutroubakis IE, et al. Acquired inhibitors to coagulation factors in patients with gastrointestinal diseases. Eur J Gastroenterol Hepatol. 2002;14:1383–7. doi: 10.1097/00042737-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Largo R, Sigg P, von Felten A, Straub PW. Acquired factor-IX inhibitor in a nonhaemophilic patient with autoimmune disease. Br J Haematol. 1974;26:129–40. doi: 10.1111/j.1365-2141.1974.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 49.Ozsoylu S, Ozer FL. Acquired factor IX deficiency. A report of two cases. Acta Haematol. 1973;50:305–14. doi: 10.1159/000208364. [DOI] [PubMed] [Google Scholar]
- 50.Roberts HR. Acquired inhibitors to factor IX. N Engl J Med. 1970;283:543–4. [PubMed] [Google Scholar]
- 51.Gouyon JB, Seiller F, Deries X, Alison M, Lorenzini JL, Devilliers E. Inhibitors of factors VIII and IX in a child with Henoch-Schonlein syndrome. Am J Pediatr Hematol Oncol. 1985;7:376–7. [PubMed] [Google Scholar]
- 52.Reussi C, Altman R, Rouvier J, Maino R. Combined factor VIII and IX inhibitors in a girl. Thromb Diath Haemorrh. 1966;16:549–58. [PubMed] [Google Scholar]
- 53.Paul S, Li L, Kalaga R, Wilkins-Stevens P, Stevens FJ, Solomon A. Natural catalytic antibodies: peptide-hydrolyzing activities of Bence Jones proteins and VL fragments. J Biol Chem. 1995;270:15257–61. doi: 10.1074/jbc.270.25.15257. [DOI] [PubMed] [Google Scholar]
- 54.Baranovskii AG, Ershova NA, Buneva VN, Kanyshkova TG, Mogelnitskii AS, Doronin BM, et al. Catalytic heterogeneity of polyclonal DNA-hydrolyzing antibodies from the sera of patients with multiple sclerosis. Immunol Lett. 2001;76:163–7. doi: 10.1016/s0165-2478(01)00185-7. [DOI] [PubMed] [Google Scholar]
- 55.Saveliev AN, Ivanen DR, Kulminskaya AA, Ershova NA, Kanyshkova TG, Buneva VN, et al. Amylolytic activity of IgM and IgG antibodies from patients with multiple sclerosis. Immunol Lett. 2003;86:291–7. doi: 10.1016/s0165-2478(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 56.Nardi M, Tomlinson S, Greco MA, Karpatkin S. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106:551–61. doi: 10.1016/s0092-8674(01)00477-9. [DOI] [PubMed] [Google Scholar]
- 57.Tawfik DS, Chap R, Green BS, Sela M, Eshhar Z. Unexpectedly high occurence of catalytic antibodies in MLR/lpr and SLJ mice immunized with a transition-state analog: Is there a linkage to autoimmunity. Proc Natl Acad Sci USA. 1995;92:2145–9. doi: 10.1073/pnas.92.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meroni PL, Shoenfeld Y. Systemic lupus erythematosus and the SLE galaxy. Autoimmun Rev. 2010;10:1–2. doi: 10.1016/j.autrev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Mozes E, Sharabi A. A novel tolerogenic peptide, hCDR1, for the specific treatment of systemic lupus erythematosus. Autoimmun Rev. 2010;10:22–26. doi: 10.1016/j.autrev.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Doria A, Zen M, Canova M, Bettio S, Bassi N, Nalotto L, Rampudda M, Ghirardello A, Iaccarino L. SLE diagnosis and treatment: When early is early. Autoimmun Rev. 2010;10:55–60. doi: 10.1016/j.autrev.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Fauchais AL, Martel C, Gondran G, Lambert M, Launay D, Jauberteau MO, Hachulla E, Vidal E, Hatron PY. Immunological profile in primary Sjogren syndrome: Clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimmun Rev. 2010;9:595–599. doi: 10.1016/j.autrev.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Alevizos I, Illei GG. MicroRNAs in Sjogren’s syndrome as a prototypic autoimmune disease. Autoimmun Rev. 2010;9:618–621. doi: 10.1016/j.autrev.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Q, Renaudineau Y, Cha S, Ilei G, Brooks WH, Selmi C, Tzioufas A, Pers J-O, Bombardieri S, Gershwin ME, Gay S, Youinou P. Epigenetics in autoimmune disorders: Highlights of the 10th Sjogren’s syndrome symposium. Autoimmun Rev. 2010;9:627–630. doi: 10.1016/j.autrev.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Youinou P, Haralampos M. Moutsopoulos: A lifetime in autoimmunity. J Autoimmun. 2010;35:171–175. doi: 10.1016/j.jaut.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Mackay IR, Leskovsek NV, Rose NR. The odd couple: A fresh look at autoimmunity and immunodeficiency. J Autoimmun. 2010;35:199–205. doi: 10.1016/j.jaut.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Kontaki E, Boumpas DT. Innate immunity in systemic lupus erythematosus: Sensing endogenous nucleic acids. J Autoimmun. 2010;35:206–211. doi: 10.1016/j.jaut.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Wiik AS, Hoier-Madsen M, Forslid J, Charles P, Meyrowitsch J. Antinuclear antibodies: A contemporary nomenclature using HEp-2 cells. J Autoimmun. 2010;35:276–290. doi: 10.1016/j.jaut.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 68.Youinou P, Pers J-O, Gershwin ME, Shoenfeld Y. Geo-epidemiology and autoimmunity. J Autoimmun. 2010;34:J163–J167. doi: 10.1016/j.jaut.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34:J168–J177. doi: 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Brooks WH, Le Dantec C, Pers J-O, Youinou P, Renaudineau Y. Epigenetics and autoimmunity. J Autoimmun. 2010;34:J207–J219. doi: 10.1016/j.jaut.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34:J234–J246. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Chang C, Gershwin ME. Drugs and autoimmunity - a contemporary review and mechanistic approach. J Autoimmun. 2010;34:J266–J275. doi: 10.1016/j.jaut.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Chen M, Daha MR, Kallenberg CGM. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276–J286. doi: 10.1016/j.jaut.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34:J314–J321. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Schilder AM. Wegener’s granulomatosis vasculitis and granuloma. Autoimmun Rev. 2010;9:483–487. doi: 10.1016/j.autrev.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ansari AA, Gershwin ME. Navigating the passage between Charybdis and Scylla: Recognizing the achievements of Noel Rose. J Autoimmun. 2009;33:165–169. doi: 10.1016/j.jaut.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Mackay IR. Clustering and commonalities among autoimmune diseases. J Autoimmun. 2009;33:170–177. doi: 10.1016/j.jaut.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Yaniv I, Ash S, Farkas DL, Askenasy N, Stein J. Considering of strategies for hematopoietic cell transplantation. J Autoimmun. 2009;33:255–259. doi: 10.1016/j.jaut.2009.09.002. [DOI] [PubMed] [Google Scholar]