Abstract
Background
Myosin light chain kinase (MLCK) and phosphatase (MLCP) govern myosin light chain (LC20) phosphorylation and smooth muscle contraction. Rho kinase (ROK) inhibits MLCP, resulting in greater LC20 phosphorylation and force generation at a given [Ca2+]i. Here, we investigate the role of ROK in regulating LC20 phosphorylation and spontaneous contractions of gastric fundus, gastric antrum, and proximal colon smooth muscles.
Methods
Protein and phosphorylation levels were determined by western blotting. The effects of Y27632, nicardipine, and GF109203X on phosphorylation levels and contraction were measured.
Key results
γ-Actin expression is similar in all three smooth muscles. LC20 and pS19 are highest, but ROK1 and ROK2 are lowest, in antrum and proximal colon smooth muscles. LZ+/− MYPT1, CPI-17, and pT696, pT853, and pT38 are highest in fundus and proximal colon smooth muscles. M-RIP expression is lowest in fundus, and highest in antrum and proximal colon smooth muscles. Y27632 reduced pT853 in each smooth muscle, but reduced pT696 only in fundus smooth muscles. Nicardipine had no effect on pT38 in each smooth muscle, while GF109203X reduced pT38 in proximal colon and fundus smooth muscles. Y27632 or nicardipine reduced pS19 in proximal colon and fundus smooth muscles. Y27632 or nicardipine inhibited antrum and proximal colon smooth muscle spontaneous contractions, but only Y27632 reduced fundus smooth muscle tone. Zero external Ca2+ relaxed each smooth muscle and abolished LC20 phosphorylation.
Conclusions and Inferences
Organ-specific mechanisms involving the MLCP interacting proteins LZ+/− MYPT1, M-RIP, and CPI-17 are critical to regulating basal LC20 phosphorylation in gastrointestinal (GI) smooth muscles.
Keywords: myosin regulatory light chain, MYPT1, Rho-associated kinase
External signals trigger GI smooth muscle contraction primarily by activating Ca2+ influx through voltage-gated Ca2+ channels [1]. Ca2+/calmodulin activates MLCK, which phosphorylates LC20, triggering cross-bridge cycling and contraction [2]. Following termination of the contractile stimulus, MLCK activity is decreased and MLCP dephosphorylates LC20, resulting in cross-bridge detachment and force reduction [2]. However, additional regulatory pathways contribute to force generation in GI smooth muscles [3]. Typically, a given [Ca2+]i increase in response to muscarinic receptor stimulation causes more forceful contractions than an equivalent [Ca2+]i increase caused by dihydropyridine-sensitive depolarization [1]. Receptor-induced activation of the small G protein RhoA results in MLCP inhibition by ROK-mediated phosphorylation of MYPT1 and/or CPI-17, favoring LC20 phosphorylation and greater force generation at a given [Ca2+]i [4]. ROK plays an important role in the agonist-induced and depolarization-induced contractions of GI smooth muscles [5;6]. However, it is apparent that the organs of the GI tract utilize different mechanism to generate spontaneous tension or contractile activity in accordance with their function [4]. In several GI smooth muscles, dihydropyridines greatly reduce spontaneous or basal circular smooth muscle contractions [7–10]. In contrast, sphincters of the GI tract which generate myogenic tone are sensitive to ROK inhibition and have higher levels of ROK and CPI-17, but lower MYPT1 levels, than predominantly phasic rectal smooth muscles [11]. Further complexity arises from alternative splicing of MYPT1, resulting in isoforms with different roles in Ca2+ sensitization, and additional leucine-zipper positive or negative isoforms involved in PKG-dependent relaxation, or RhoA targeting [12;13]. In addition, altered expression levels of proteins involved in Ca2+ sensitization pathways have also been linked to several pathological states of smooth muscles [14–17].
These findings indicate that ROK, MYPT1 isoforms, M-RIP, and CPI-17 expression levels are relevant factors in determining the importance of Ca2+ sensitization pathways to contractile responses of GI smooth muscles [1;4]. In this study, we examined the extent of MYPT1, CPI-17, and LC20 phosphorylation during spontaneous contractile activity of gastric fundus and antrum smooth muscles, and the functionally and regionally distinct proximal colon smooth muscles. We examined the effects of Y27632, nicardipine, or GF109203X on the basal phosphorylation levels of these proteins. We compared the expression levels of γ-actin, LC20, ROK-1, ROK-2, MYPT1, and CPI-17, in these three smooth muscles. We determined the expression levels of the LZ+ and LZ− MYPT1 isoforms, and the MLCP-anchoring protein M-RIP, for the first time in GI smooth muscles. The corresponding effects of Y27632 and nicardipine on basal tension and contraction were also measured. We found differences in expression, phosphorylation, and sensitivity to Y27632, nicardipine, and GF109203X, from gastric antrum, gastric fundus, and proximal colon smooth muscles. To expand the number of strategies available for treatment of GI motility disorders, it is necessary to investigate GI smooth muscle regulation in greater detail. These findings may facilitate studies aimed at further understanding the role of RhoA/ROK pathways in the pathophysiology of GI motility disorders associated with smooth muscle dysfunction.
MATERIALS & METHODS
Tissue Preparation
Mice were maintained and experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the University of Nevada, Reno, Institutional Animal Care and Use Committee. Male C57Bl/6 mice (6-to 8-weeks old, Charles River Laboratories, Wilmington, MA, USA) were anaesthetized with isofluorane and euthanized by decapitation. The stomachs and colons were removed, pinned to a Sylgard-lined dish containing 4°C oxygenated Krebs solution, the gastric antrum, gastric fundus, and proximal colons were identified and acquired, and the mucosa and submucosa removed by sharp dissection [18–20]. Smooth muscles were equilibrated in oxygenated Krebs at 37°C for 45 min. As indicated, drugs and inhibitors were added for an additional 20 min of incubation. All preparations contained 0.3 μM L−1 tetrodotoxin.
SDS-PAGE and western blotting
Following the equilibration and treatment periods, the smooth muscles were incubated in ice cold acetone/10 mmol L−1 dithiothreitol (DTT)/10% (w/v) trichloroacetic acid (TCA) for 2 min, snap-freezing in liquid N2, and storing at −80°C [21] . The tissues were thawed on ice for 5 min, followed by three 1 min washes in ice cold acetone/10 mmol L−1 DTT, and a 2 min wash in ice cold lysis buffer (mmol L−1; 50 Tris HCl pH 8.0, 60 beta-glycerophosphate, 100 NaF, 2 EGTA, 25 Na-pyrophosphate, 1 DTT, 1 fasudil, with 0.5% NP-40, 0.2% SDS, and protease inhibitor tablet, 5 mg mL−1) [21]. Each tissue was homogenized in 0.15 mL lysis buffer, centrifuged at 3000 × g at 4°C for 10 min, and the supernatants aliquotted and stored at −80°C. The supernatants were analyzed by SDS-PAGE and western blotting with anti γ-actin, LC20, ROK-1, ROK-2, MYPT1, LZ+, LZ−, M-RIP, and CPI-17 antibodies. Phosphorylation levels were determined by western blot analyses using anti pS19-LC20, pT696-MYPT1, pT853-MYPT1, and pT38-CPI-17 antibodies. Immunostained protein bands were detected using horseradish peroxidase-conjugated secondary antibodies and ECL Advantage (GE HealthCare Biosciences, Piscataway, NJ, USA), and visualized with a CCD camera-based detection system equipped with Visionworks software (Epi Chem II, UVP Laboratory Products, Upland, CA, USA). The tiff images were inverted, and adjusted to auto levels and resolution with Adobe Photoshop (CS2, V9.0.2, Adobe Systems, San Jose, CA, USA) for densitometry.
Mechanical responses
Contractile activity was measured using standard myobath techniques, with each strip attached to a Fort 10 isometric strain gauge (WPI, Sarasota, FL, USA) in parallel with the circular muscles [20]. A resting force of 0.6g was applied and tissues were equilibrated for 45 min prior to addition of drugs and inhibitors. Responses were recorded using Acqknowledge 3.2.7 software (BIOPAC Systems, Santa Barbara, CA, USA).
Materials
Protease inhibitor tablets were purchased from Roche Diagnostics (Indianapolis, IA, USA). Western blot materials were purchased from BioRad (Hercules, CA, USA). PVDF membranes were purchased from Millipore (Billerica, MA, USA). The sheep anti-γ-actin, goat anti-LC20, mouse anti-CPI-17, and rabbit anti-p-T38CPI-17, anti-pS19LC20, anti-MYPT1, anti-p-T696MYPT1, anti-p-T853MYPT1, anti M-RIP, anti-ROK1, and anti-ROK2 antibodies, and GF109203X were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The rabbit anti- LZ+, and LZ− MYPT1 antibodies were provided by Steven A. Fisher [22]. Horseradish peroxidase-conjugated secondary antibodies were purchased from Millipore (Chemicon) Billerica, MA, USA). Nicardipine, tetrodotoxin, and Y27632 were purchased from Calbiochem (La Jolla, CA, USA). Fasudil was purchased from Tocris Biosciences (Ellisville, Missouri, USA).
Statistical analysis
Protein concentrations were determined with the Bradford assay [23]. The sample lysates were boiled in SDS sample buffer containing 2% SDS [21]. Equal protein amounts were loaded for separation by SDS-PAGE. The densitometry values (pixel intensity units) of the immunostained protein bands were obtained using VisionWorks software. The densitometry values of total MYPT1, the LZ+, LZ− isoforms, pT696, pT853, CPI-17, and pT38 were obtained from the protein band doublets. The densitometry values were divided by the total μg protein loaded to obtain a relative expression level per μg of homogenate protein, and were analyzed for significance by t-test and are expressed as averages ± SD. Contractile responses were measured by comparing the area under curve (AUC; gram-sec) during stable 20 sec recordings of drug treated regions and control regions preceding the drugs. Contractile data was analyzed by one-way anova and tested for significance with t-test. Inhibition of contraction is expressed as the percent inhibition compared to the 100% relaxation of each smooth muscle obtained with 1 μmol L−1 nicardipine and 10 μmol L−1 SNP. Data are expressed as averages ± SD. P < 0.05 is considered significant.
RESULTS
γ-Actin, LC20, ROK-1, ROK2, total MYPT1, LZ+, LZ−, M-RIP, and CPI-17 expression
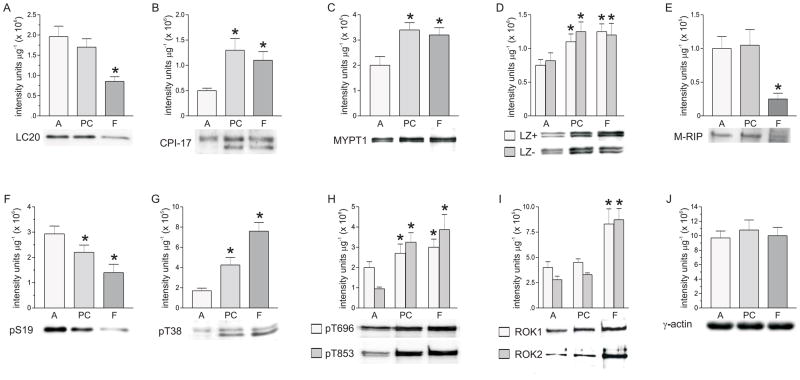
Figure 1 shows that LC20 (Fig.1A), CPI-17 (Fig.1B), total MYPT1 (Fig.1C), LZ+, LZ−, MYPT1 (Fig.1D), M-RIP (Fig.1E), and ROK1, and ROK2 (Fig. 1I) expression is different in these three smooth muscles. Fundus has the lowest LC20 expression, but the highest MYPT1, CPI-17, ROK1, and ROK2 expression. Based on the ratio calculations (not shown), total MYPT1 is composed of approximately equal amounts of the LZ+ and LZ− splice variants in all three smooth muscles. In contrast, M-RIP expression is lowest in fundus, and highest in antrum and proximal colon smooth muscles. LC20 expression in fundus smooth muscles is ~two-fold lower than the levels found in antrum and proximal colon smooth muscles. ROK1 and ROK2 expression is 1.5- to 2-fold higher in fundus smooth muscles than in antrum and proximal colon smooth muscles. Both MYPT1 and CPI-17 expression are 1.5–2.5 –fold higher in fundus smooth muscles than in antrum smooth muscles. M-RIP expression is about 4-fold higher in antrum and proximal colon than in fundus smooth muscles. Antrum has the highest LC20 expression, but the lowest ROK1, ROK2, MYPT1, and CPI-17 expression. Proximal colon is similar to antrum in having high LC20 expression and low ROK1 and ROK2 expression, but is similar to fundus in having high MYPT1 and CPI-17 expression. γ-Actin levels are similar in antrum, proximal colon, and fundus smooth muscles (Fig.1J). γ-Actin was measured rather than α-actin because γ-actin is the predominant isoform present in GI smooth muscle contractile filaments [24].
Figure 1.
Expression levels of γ-actin, LC20, pS19, ROK1, ROK2, total MYPT-1, LZ+, LZ− MYPT1, M-RIP, pT696, pT853, CPI-17, and pT38 in antrum, proximal colon, and fundus smooth muscles. Upper panels, cumulative data of average density values (+/− SD) of each protein in each smooth muscle tissue. Lower panels, representative western blots. A. LC20, I. ROK1, ROK2, E. M-RIP * average density significantly different from antrum and proximal colon (P<0.01); antrum and proximal colon average densities not significantly different from each other. B. CPI-17, C. total MYPT1, D. LZ+, LZ− MYPT1, H. pT696, pT853 * average densities significantly different from antrum (P<0.01); proximal colon and fundus average densities not significantly different from each other. F. pS19, G. pT38 * average densities significantly different from antrum (P<0.01); proximal colon and fundus average densities significantly different from each other (P<0.01). J. γ-actin average densities not significantly different from each other. γ-actin, 10 μg protein/lane; M-RIP, 25 μg protein/lane; all other proteins, 20μg protein/lane. Antrum (A), proximal colon (PC), and fundus (F) smooth muscle homogenates from at least 7 mice were analyzed by densitometry of duplicate or triplicate western blots.
pS19, pT696, pT853, and pT38 levels
Based on their different expression levels, basal phosphorylation levels of each protein were examined. Basal phosphorylation levels corresponded to the expression levels of each respective protein, and the ratios of pS19, pT696, pT853, and pT38 to their respective total proteins are not significantly different between the three smooth muscle tissues. The basal pS19 level in antrum smooth muscle is 1.5- to 2-fold higher than the pS19 levels in proximal colon and fundus smooth muscles (Fig.1F), in correlation with the LC20 levels in each smooth muscle (Fig. 1A). The pT696 and pT853 (Fig.1H), and pT38 (Fig.1G) levels are lowest in antrum smooth muscles, and highest in proximal colon and fundus smooth muscles, following the MYPT1 and CPI-17 levels in these tissues (Fig. 1C, 1B). The pT853 level in antrum smooth muscle is 3- to 4-fold lower than the levels in proximal colon and fundus, while pT696 in antrum is no more than 1.5-fold lower than the levels in proximal colon and fundus smooth muscles. The pT38 level in antrum smooth muscle is 2- to 4-fold lower than the pT38 levels in proximal colon and fundus smooth muscles.
Effects of the ROK inhibitor Y27632 on basal pT696, pT853, pT38, and pS19 levels
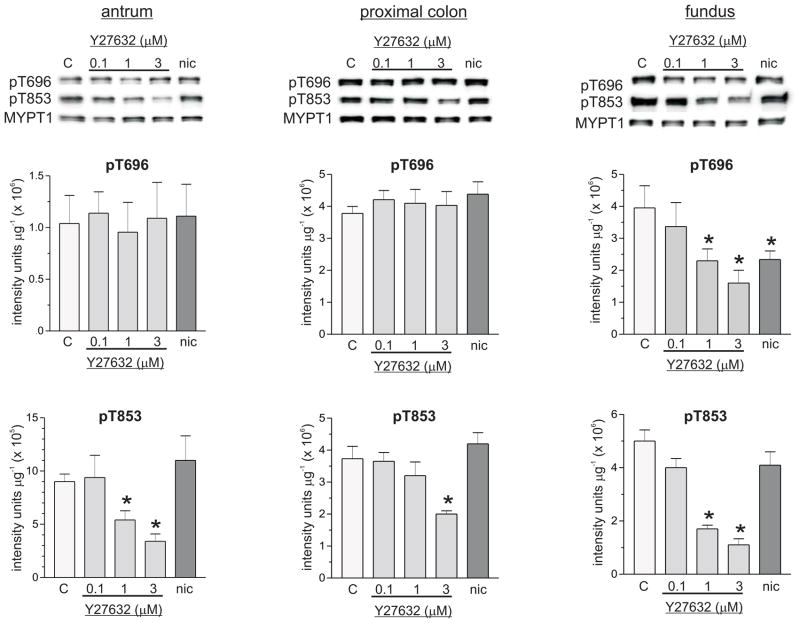
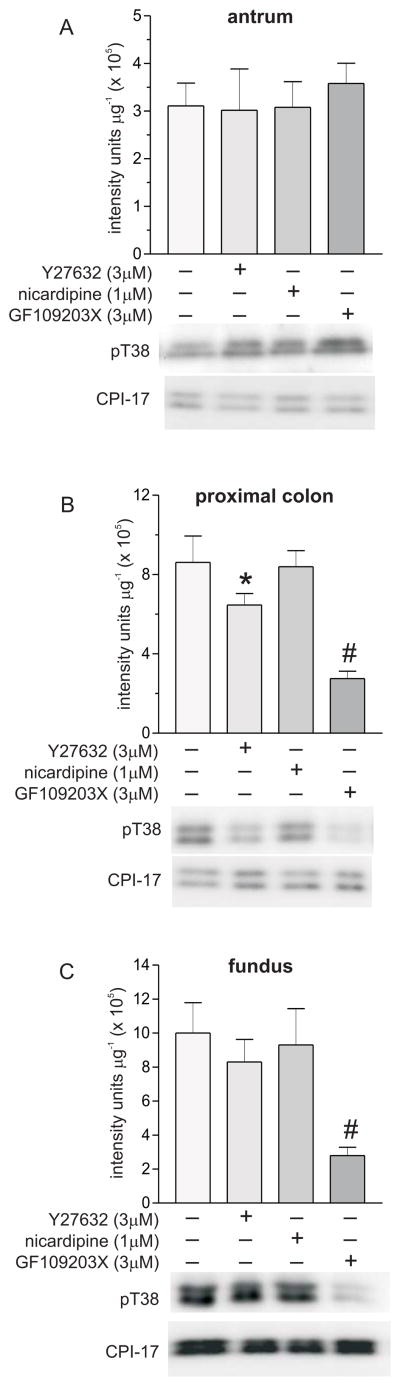
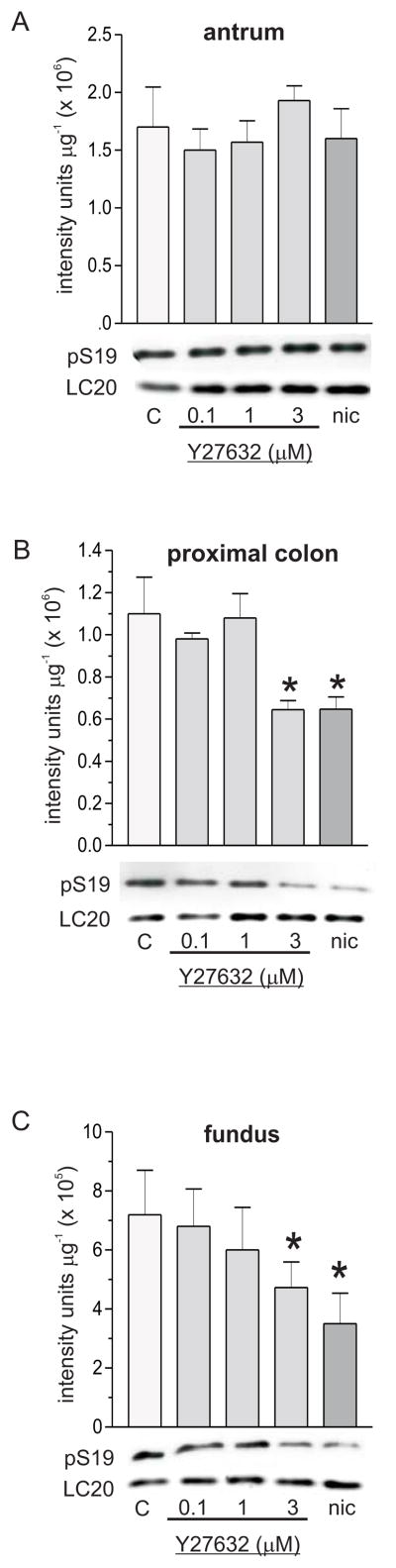
Figure 2 shows that Y27632 had no effect on pT696 in antrum or proximal colon smooth muscles, but dose-dependently decreased pT696 in fundus smooth muscles. Concentrations of Y27632 less than 10 μmol L−1 have been shown to be selective ROK inhibitors [19;22;26]. One μmol L−1 and 3 μmol L−1 Y27632 reduced the average values of the pT696 levels by 45% and 62% relative to controls, respectively, in fundus smooth muscles. Different Y27632 concentrations reduced pT853 levels to different extents in each smooth muscle tissue, with the largest decreases in fundus smooth muscle at each concentration. In antrum and proximal colon smooth muscles, 0.1 μmol L−1 Y27632 had no effect on pT853, but reduced the average value of pT853 in fundus smooth muscles by 20%. In fundus, antrum, and proximal colon smooth muscles, respectively, 1 μmol L−1 Y27632 decreased the average pT853 values by 70%, 44%, and 18%, while 3 μmol L−1 Y27632 decreased the average pT853 values by 80%, 67%, and 47% relative to controls. These findings suggest that ROK contributes to the maintenance of basal pT696 in fundus, and basal pT853 in all three smooth muscle tissues. Figure 3 shows that Y27632 (3 μmol L−1) had no effect on pT38 in antrum and fundus (Fig. 3A, 3C), but reduced the average value of the pT38 level in proximal colon smooth muscles by 25% relative to controls (Fig.3B), suggesting that ROK activity contributes to the maintenance of basal pT38 in proximal colon, but not in antrum and fundus smooth muscles. Figure 4 shows that Y27632 had no effect on basal pS19 in antrum (Fig.4A), but decreased basal pS19 in proximal colon and fundus smooth muscles. In proximal colon smooth muscles, 0.1 μmol L−1 and 1 μmol L−1 Y27632 had no effect, but 3 μmol L−1 Y27632 reduced the pS19 average value by 50% (Fig.4B). The average values of the pS19 level in fundus smooth muscles were decreased 15% and 37% relative to controls, by 1 μmol L−1 and 3 μmol L−1 Y27632, respectively, while 0.1 μmol L−1 Y27632 had no effect (Fig.4C). These findings suggest that MLCP inhibition by ROK contributes to basal LC20 phosphorylation in fundus and proximal colon, but not in antrum smooth muscles.
Figure 2.
Effects of Y27632 and nicardipine on basal pT696, and pT853 in antrum, proximal colon, and fundus smooth muscles. Top panels, representative western blots, 20 μg protein/lane. Middle panels, pT696, cumulative data of average density values (+/− SD). Bottom panels, pT853, cumulative data of average density values (+/− SD). C, control; nic, nicardipine (1μM). * average density values significantly different from control values (P<0.05). At least 4 antrum, fundus, and proximal colon smooth muscle homogenates from each treatment were analyzed by densitometry of duplicate or triplicate western blots.
Figure 3.
Effects of Y27632, GF109203X, and nicardipine on basal pT38 in antrum, proximal colon, and fundus smooth muscles. Upper panels, cumulative data of average density values (+/− SD) of pT38 in each smooth muscle tissue. Lower panels, representative western blots, 20 μg protein/lane. *P<0.05, #P<0.01, average density values significantly different from control values (no drugs present). At least 4 antrum, fundus, and proximal colon smooth muscle homogenates from each treatment were analyzed by densitometry of duplicate or triplicate western blots.
Figure 4.
Effects of Y27632 and nicardipine on basal pS19 in antrum, proximal colon, and fundus smooth muscles. Upper panels, cumulative data of average density values (+/−SD) of pS19 in each smooth muscle tissue. Lower panels, representative western blots, 20 μg protein/lane. C, control; nic, nicardipine (1μM). * average density values significantly different from control values (P<0.05). At least 4 antrum, fundus, and proximal colon smooth muscle homogenates from each treatment were analyzed by densitometry of duplicate or triplicate western blots.
Effects of nicardipine on basal pT696, pT853, pT38, and pS19
The role of Ca2+ influx through L-type Ca2+ channels in maintaining basal LC20 phosphorylation was assessed with nicardipine. Nicardipine (1 μmol L−1) had no effect on pS19 in antrum (Fig.4A), but decreased the average value of the pS19 level ~50% in proximal colon and fundus smooth muscles (Fig. 4B, 4C). These results suggest that Ca2+ influx through L-type Ca2+ channels contributes to basal LC20 phosphorylation in fundus and proximal colon, but not in antrum smooth muscles. Nicardipine had no effect on pT853 in all three smooth muscles, but decreased the average value of the pT696 level in fundus smooth muscles by ~45% (Fig.2). These findings suggest that in fundus, voltage-dependent Ca2+ entry contributes to T696 phosphorylation, and may regulate MLCP activity. Ca2+ influx can also activate PKC, resulting in CPI-17 T38 phosphorylation and MLCP inhibition [11]. However, nicardipine had no effect on pT38 in antrum, proximal colon, and fundus smooth muscles (Fig.3). The pan-PKC inhibitor GF109203X (3 μmol L−1) significantly reduced pT38 in proximal colon and fundus, but not in antrum smooth muscles (Fig.3). These results suggest that voltage-dependent Ca2+ influx does not maintain basal pT38 levels in all three tissues, and that Ca2+ independent PKC isozymes may phosphorylate CPI-17 in proximal colon and fundus, but not antrum, smooth muscles.
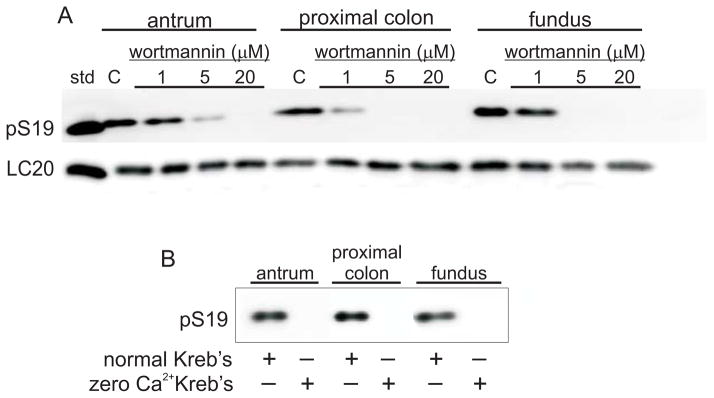
Because pS19 was still evident after nicardipine treatment, each smooth muscle tissue was incubated with the MLCK inhibitor wortmannin, or incubated in Ca2+-free Kreb’s containing 1mmol L−1 EGTA. Figure 5 shows that wortmannin treatment (Fig.5A), or incubation in a Ca2+-free solution (Fig.5B) resulted in undetectable pS19 levels in all three smooth muscle tissues. These findings suggest that MLCK activation by external Ca2+ entry is required to maintain basal pLC20 levels in each smooth muscle.
Figure 5.
Effects of wortmannin or zero Ca2+ Kreb’s on basal pS19 in antrum, proximal colon, and fundus smooth muscles. std, 20kDa protein marker; C, control. At least 3 antrum, fundus, and proximal colon smooth muscle homogenates from each treatment were analyzed by densitometry of duplicate western blots.
Effects of Y27632 or nicardipine on spontaneous contractions
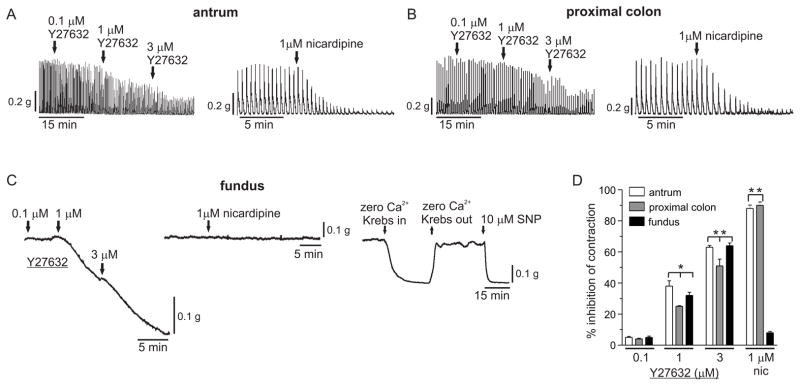
Y27632 dose-dependently reduced the spontaneous phasic activities of antrum (Fig.6A) and proximal colon smooth muscles (Fig.6B), and the resting tension of fundus smooth muscles (Fig.6C). 0.1 μmol L−1 Y27632 had no effect, while 1 μmol L− Y27632 reduced the average AUC values of antrum and proximal colon phasic contractions by ~40% and ~25%, respectively, and reduced the average AUC value of fundus smooth muscle resting tension by ~32%. 3 μmol L−1 Y27632 reduced the average AUC values of antrum and proximal colon phasic contractions by ~62% and ~50%, respectively, and reduced the average AUC value of fundus smooth muscle resting tension by ~65%. These findings suggest that ROK contributes to basal contractions in fundus, proximal colon, and antrum smooth muscles. Nicardipine (1 μmol L−1) almost completely abolished the spontaneous phasic activities of antrum and proximal colon smooth muscles (90% inhibition), but reduced the resting tension of fundus smooth muscles by only 10%. Incubation of fundus tissues in Ca2+ free solution abolished resting tension, as compared to the complete relaxation evoked by 10 μmol L−1 SNP. These results suggest that Ca2+ influx through L-type Ca2+ channels contributes to basal contractions in antrum and proximal colon, but not in fundus smooth muscles.
Figure 6.
Effects of Y27632 or nicardipine on basal contractions of antrum, proximal colon, and fundus smooth muscles. Representative traces of antrum (A), proximal colon (B), and fundus (C) smooth muscles, recorded as described in Materials and Methods. D. cumulative data from 5 antrum, proximal colon, and fundus smooth muscles of the average percent inhibition of basal contraction (+/− SD). The relaxation of each smooth muscle tissue by 1μmol L−1 nicardipine and 10μmol L−1 SNP together was taken as 100% percent inhibition. *P < 0.05, **P < 0.01, significantly different from the control regions preceding the drugs.
DISCUSSION
Considerable functional heterogeneity exists among the organs of the digestive tract. The stomach prepares ingested foods for delivery to the small intestine. Different stomach regions display different contractile behaviors consistent with their specific functions [25]. The proximal stomach consists of the fundus and corpus, with gastric accommodation being carried out by fundus relaxation, followed by generally sustained tonic contractions to deliver food to the corpus and antrum. Strong phasic contractile activity of the antrum churns and mixes the material for proper delivery to the small intestine. The colon exhibits both propulsive and accommodation behavior to receive and further process material from the small intestine for delivery to the rectum and elimination [25;26]. RhoA/ROK-mediated Ca2+ sensitization contributes to basal and agonist-evoked contractile responses of GI smooth muscles [4]. Differences in the contribution of RhoA/ROK-mediated potentiation of contraction and force in different regions of the GI tract have been reported [11;27]. In the present study we examined the contribution of MYPT-1 and CPI-17 to basal LC20 phosphorylation and the spontaneous contractile activities of gastric antrum, fundus, and proximal colon smooth muscles.
Similar to other smooth muscles, we found substantial levels of basal T696 and T853 phosphorylation in unstimulated antrum, proximal colon, and fundus smooth muscles [11;21;28–31]. Our findings that Y27632 decreased pT853 demonstrate basal ROK activity in spontaneously contracting smooth muscles in which neuronal activity is blocked with tetrodotoxin. ROK is activated by lipids, androgens, stimulation of GPCRs coupled to Gαq or Gα12/13, or by depolarization induced Ca2+ influx [32–34]. In the absence of receptor stimulation, membrane depolarization by slow wave activity might be a likely contributor to basal ROK activity in antrum and proximal colon smooth muscles. Slow waves change the membrane potential from a low open probability state for voltage-dependent Ca2+ channels to potentials where channel opening probability is elevated, resulting in periodic Ca2+ entry, which could provide a mechanism to maintain basal ROK activity [35]. Slow waves are not present in fundus smooth muscles [36]. Fundus smooth muscles are characterized by an ongoing discharge of small-amplitude unitary potentials, resulting in a more depolarized basal membrane potential that falls within the window-current range for voltage-dependent Ca2+ channels [36;37]. Ongoing Ca2+ entry via this mechanism could similarly provide a mechanism to generate basal ROK activity. Y27632 had little to no effect on pT696 in antrum and proximal colon smooth muscles, but decreased pT696 in fundus smooth muscles, suggesting that ROK is involved in maintaining pT696 in fundus, but not in antrum and proximal colon smooth muscles. Our findings suggest that ROK phosphorylates MYPT1 in a tissue-specific manner. These findings are consistent with reports that ROK preferentially phosphorylates T853, and that additional kinases can phosphorylate T696 in vivo, including MYPT1 kinase, integrin-linked kinase, myotonic dystrophy protein kinase, p21-activated protein kinase, and Raf-1 [21;28;38;39]. The kinase(s) responsible for basal T696 phosphorylation in antrum, proximal colon, and fundus smooth muscles have not been identified.
We found different MYPT1 expression levels in fundus, antrum, and proximal colon smooth muscles. MYPT1 expression was lowest in antrum, and highest in fundus and proximal colon smooth muscles. In contrast, M-RIP expression was lowest in fundus, and highest in antrum and proximal colon smooth muscles. M-RIP targets MLCP to the contractile apparatus to dephosphorylate LC20 [40]. M-RIP also targets GTP-RhoA to LZ+ MYPT1 for activation of MYPT1-bound ROK2 and MYPT1 phosphorylation and MLCP inhibition [40;41]. Because the MYPT1 and M-RIP expression pattern is different in these smooth muscle tissues, M-RIP regulation of MYPT1 could be different. The phasic smooth muscles from antrum and proximal colon are characterized by low ROK1 and ROK2 expression, but high M-RIP and LC20 expression. In the case where LC20 expression is high relative to ROK 1 and ROK2, M-RIP expression could also be high to maximize GTP-RhoA targeting to MYPT1, and facilitate more efficient ROK2 activation, MYPT1 phosphorylation, and MLCP inhibition. However, antrum and proximal colon smooth muscles differ in their relative MYPT1 expression; low in antrum but high in proximal colon. In antrum, there would be more M-RIP relative to MYPT1, potentially resulting in greater ROK2 activation and MLCP inhibition; while in proximal colon smooth muscles, there is less M-RIP relative to MYPT1, possibly resulting in less MLCP inhibition. Fundus tonic smooth muscles are characterized by high ROK1 and ROK2 expression and high MYPT1 expression, but low M-RIP and LC20 expression. In this case where ROK1, ROK2, and MYPT1 expression are high relative to LC20 expression, localization of GTP-RhoA to MYPT1 by M-RIP may not be as important for MYPT1 phosphorylation and MLCP inhibition. In a tonic smooth muscle, rapid changes in MLCP activity may not be as important as they might be in phasic smooth muscles.
The MYPT1 protein bands appear as doublets in the western blots, suggesting that the two isoforms produced by alternative splicing of a central exon are expressed [42]. Total MYPT1 also consists of approximately equal amounts of LZ+ and LZ− splice variants in each smooth muscle tissue. Although ROK2 can bind to and phosphorylate LZ+ and LZ− MYPT1 isoforms, both isoforms can also be phosphorylated by ROK1, indicating that binding to MYPT1 is not necessary for phosphorylation by Rho kinases [40]. However, M-RIP only binds to LZ+ MYPT1 [41]. Thus, GTP-RhoA is co-localized with ROK2 only on LZ+ MYPT1, facilitating MLCP inhibition by LZ+ MYPT1 phosphorylation [13]. These findings suggest that the kinetics of LZ+ MYPT1 phosphorylation by bound ROK2 could be faster than ROK2 bound to LZ− MYPT1 because of the targeting of GTP-RhoA by M-RIP mediated by the LZ motif. LZ+ and LZ− MYPT1 isoforms are usually associated with distinct smooth muscle types [43]. The presence of LZ+ and LZ− MYPT1 in fundus, antrum and proximal colon smooth muscles raises the question of the physiological significance of the expression of both isoforms in these smooth muscles. Our western blot analyses were obtained from tissues containing both the longitudinal and circular smooth muscle layers, which are distinguished by different contractile properties, receptors, innervation, and intracellular signaling pathways [44]. Separation of the longitudinal and circular muscle layers will be necessary to determine whether LZ+ and LZ− MYPT1 are characterized by distinct expression patterns in each muscle layer.
In addition, MLCP complexes consisting of either LZ+ or LZ− MYPT1 could be present within smooth muscle cells, regulating both LC20 phosphorylation and force, and the actin cytoskeleton [40]. In other smooth muscles RhoA inactivation inhibits contraction by depolymerizing the actin cytoskeleton, with only minor decreases in LC20 phosphorylation [45;46]. We found that nicardipine decreased LC20 phosphorylation, but not CPI-17 phosphorylation in fundus smooth muscles, suggesting that nicardipine decreased LC20 phosphorylation by inhibiting MLCK, and not by relieving MLCP inhibition. Although LC20 phosphorylation was reduced, nicardipine had little effect on fundus smooth muscle tone as reported previously [47;48]. Y27632 decreased both T853 and LC20 phosphorylation, and relaxed the fundus. Because Y27632 decreased pT853, ROK activity was likely decreased; suggesting that depolymerization of the actin cytoskeleton plays a role in fundus smooth muscle relaxation. However, when LC20 is completely dephosphorylated by zero external Ca2+, fundus relaxation occurs, suggesting that cross-bridge formation and polymerized actin are both important for maintenance of basal fundus tone. These findings also support the concept that other plasma membrane or intracellular cation channels, in addition to L-type Ca2+ channels, are involved in maintaining basal fundus tone and LC20 phosphorylation [47].
Different findings were obtained with the phasic antrum and proximal colon smooth muscles. In proximal colon, nicardipine did not decrease CPI-17 phosphorylation, but decreased LC20 phosphorylation and inhibited contractions. Thus similar to fundus, nicardipine likely decreased LC20 phosphorylation by inhibiting MLCK. Y27632 decreased MYPT1T853, CPI-17, and LC20 phosphorylation, suggesting Y27632 decreased LC20 phosphorylation by inhibiting ROK and activating MLCP. Y27632 also inhibited proximal colon smooth muscle phasic contractions. These results suggest that proximal colon spontaneous phasic contractions are more sensitive to LC20 dephosphorylation than the basal tone of fundus smooth muscles, but does not exclude a role for actin depolymerization [46]. In contrast, although nicardipine or Y27632 inhibited antrum contractions, neither compound decreased LC20 phosphorylation. Recently, similar results were found in rabbit urethra smooth muscles [49]. High external KCl or phenylephrine contracted rabbit urethra smooth muscles, without increasing LC20 phosphorylation [49]. Conversely, ROK inhibitors relaxed rabbit urethra smooth muscles, without decreasing LC20 phosphorylation [49]. Interestingly, we found that similar to rabbit urethra, murine gastric antrum smooth muscle has a high pS19:actin ratio [49]. How high LC20 expression and phosphorylation levels might affect the regulation of the spontaneous phasic contractile activity of gastric antrum smooth muscles is unclear. Antrum smooth muscle has the lowest ROK and MYPT1 and CPI-17 expression, but highest LC20 expression and phosphorylation levels, suggesting that MLCP inhibition may not be so effective or critical for achieving optimal pS19 levels for force generation. Conversely, MLCP activation may not be so effective in reducing pS19 levels and inhibiting the spontaneous contractile activity. Alternatively, small changes in MLCP activity might not be sufficient to dephosphorylate LC20 to a level detectable by western blotting. Basal MLCK activity may be the critical determinant in the maintenance of basal pS19 and contraction in antrum smooth muscles. This conclusion is supported by the finding that although Y27632 reduced pT853, basal pS19 levels were unchanged, suggesting that partial activation of MLCP is not sufficient to effectively change LC20 phosphorylation. Thus, ongoing regulation of MLCP by MYPT1 and CPI-17 phosphorylation appears to be less important for the maintenance of basal LC20 phosphorylation in antrum compared to proximal colon and fundus smooth muscles. Furthermore, zero external Ca2+ completely abolished LC20 phosphorylation and antrum phasic contractions (data not shown), suggesting that a basal level of MLCK activity maintains LC20 phosphorylation. However, the source of activating Ca2+ does not appear to be due to nicardipine-sensitive Ca2+ channels.
In summary, we found differences in the expression and basal phosphorylation levels of LC20, ROK-1, ROK-2, total MYPT1 and LZ+ and LZ− isoforms, M-RIP, and CPI-17 in gastric antrum, gastric fundus, and proximal colon smooth muscles. Y27632 reduced pT853 in each smooth muscle, but only reduced pT696 in fundus. The mechanisms regulating constitutive ROK activity in fundus, antrum, and proximal colon smooth muscles are unknown and are likely tissue specific. Y27632 reduced pT38 levels only in proximal colon smooth muscles. Nicardipine had no effect on pT38 in all three smooth muscles, while GF109203X reduced pT38 levels in fundus and proximal colon smooth muscles. Y27632 and nicardipine reduced pS19 levels in fundus and proximal colon smooth muscles, but had no effect on pS19 in antrum smooth muscles. Y27632 or nicardipine inhibited the spontaneous phasic contractions of antrum and proximal colon smooth muscles, but only Y27632 reduced fundus smooth muscle tone. In fundus and antrum, but not proximal colon smooth muscles, the level of LC20 phosphorylation does not appear to correlate with the spontaneous contractile activity. Additional studies of the role of actin polymerization in the spontaneous contractions of these tonic and phasic GI smooth muscles will be required to clarify the interrelationships between the actin cytoskeleton and the contractile apparatus. Overall, these findings suggest that the mechanisms by which RhoA regulates contraction differs significantly between these three different GI smooth muscles.
Acknowledgments
This work was supported by NIH grants RR08751 (BAP), and R01HL66171 (SAF). We acknowledge the many helpful discussions from Kent Sanders, Kathy D. Keef, and Michael P. Walsh (Univ. of Calgary, Canada). We thank Kathy Keef for the use of the static myobath. We thank Grant Hennig and Violeta-Mutofova-Yambolieva for their critical comments on the manuscript.
Footnotes
Author contributions: BPB performed the experiments, analyzed and interpreted the data, and contributed to the design of the experiments and the writing of the manuscript; CLA performed the M-RIP western blot analyses; SAF provided the LZ+ and LZ− MYPT1 isoform antibodies for Figure 1E; BAP analyzed and interpreted the data, designed the experiments, and wrote the manuscript.
Competing Interests: The authors have no competing interests.
References
- 1.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterology & Motility. 2008;20:39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008;12:2165–80. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–74. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 4.Rattan S, Phillips BR, Maxwell PJ. RhoA/Rho-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology. 2010;138:13–8. doi: 10.1053/j.gastro.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochemical Journal. 2003;374:145–55. doi: 10.1042/BJ20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somlyo AP, Somlyo AV. Ca2+ Sensitivity of Smooth Muscle and Nonmuscle Myosin II: Modulated by G Proteins, Kinases, and Myosin Phosphatase. Physiological Reviews. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 7.Cobine CA, Fong M, Hamilton R, Keef KD. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol Motil. 2007;19:937–45. doi: 10.1111/j.1365-2982.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 8.Kovac JR, Preiksaitis HG, Sims SM. Functional and molecular analysis of L-type calcium channels in human esophagus and lower esophageal sphincter smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2005;289:G998–1006. doi: 10.1152/ajpgi.00529.2004. [DOI] [PubMed] [Google Scholar]
- 9.Rich A, Kenyon JL, Hume JR, Overturf K, Horowitz B, Sanders KM. Dihydropyridine-sensitive calcium channels expressed in canine colonic smooth muscle cells. Am J Physiol. 1993;264:C745–C754. doi: 10.1152/ajpcell.1993.264.3.C745. [DOI] [PubMed] [Google Scholar]
- 10.Wegener JW, Schulla V, Koller A, Klugbauer N, Feil R, Hofmann F. Control of intestinal motility by the Ca(v)1. 2 L-type calcium channel in mice. The FASEB Journal. 2006;20:1260–2. doi: 10.1096/fj.05-5292fje. [DOI] [PubMed] [Google Scholar]
- 11.Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. AJP - Gastrointestinal and Liver Physiology. 2006;291:G830–G837. doi: 10.1152/ajpgi.00130.2006. [DOI] [PubMed] [Google Scholar]
- 12.Khromov A, Choudhury N, Stevenson AS, SOMLYO AV, Eto M. Phosphorylation-dependent Autoinhibition of Myosin Light Chain Phosphatase Accounts for Ca2+ Sensitization Force of Smooth Muscle Contraction. Journal of Biological Chemistry. 2009;284:21569–79. doi: 10.1074/jbc.M109.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddick N, Ohtani Ki, Surks HK. Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. Journal of Cellular Biochemistry. 2008;103:1158–70. doi: 10.1002/jcb.21488. [DOI] [PubMed] [Google Scholar]
- 14.Depoortere I, Thijs T, Janssen S, De Smet B, Tack J. Colitis affects the smooth muscle and neural response to motilin in the rabbit antrum. British Journal of Pharmacology. 2010;159:384–93. doi: 10.1111/j.1476-5381.2009.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gervaz P, Morel P, Vozenin-Brotons MC. Molecular aspects of intestinal radiation-induced fibrosis. Curr Mol Med. 2009;9:273–80. doi: 10.2174/156652409787847164. [DOI] [PubMed] [Google Scholar]
- 16.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic Treatment with Interleukin-1{beta} Attenuates Contractions by Decreasing the Activities of CPI-17 and MYPT-1 in Intestinal Smooth Muscle. Journal of Biological Chemistry. 2003;278:48794–804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Ohkura S, Kitahara Y, et al. Involvement of CPI-17 downregulation in the dysmotility of the colon from dextran sodium sulphate-induced experimental colitis in a mouse model. Neurogastroenterol Motil. 2007;19:504–14. doi: 10.1111/j.1365-2982.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Han IS, Koh SD, Perrino BA. Roles of CaM kinase II and phospholamban in SNP-induced relaxation of murine gastric fundus smooth muscle. AJP - Cell Physiology. 2006;291:C337–C447. doi: 10.1152/ajpcell.00397.2005. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Hennig GW, Park K, et al. Modulation of murine gastric antrum smooth muscle STOC activity and excitability by phospholamban. J Physiol. 2008;586:4977–91. doi: 10.1113/jphysiol.2008.156836. PMC Journal - In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qureshi S, Song J, Lee HT, Koh SD, Hennig GW, Perrino BA. CaM kinase II in colonic smooth muscle contributes to dysmotility in murine DSS-colitis. Neurogastroenterol Motil. 2010;22:186–95. e64. doi: 10.1111/j.1365-2982.2009.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol. 2009;587:2537–53. doi: 10.1113/jphysiol.2008.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne MC, Zhang HY, Shirasawa Y, et al. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. American Journal of Physiology - Heart and Circulatory Physiology. 2004;286:H1801–H1810. doi: 10.1152/ajpheart.00696.2003. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz JM, Riddervold MH, Beckett EAH, Baker SA, Perrino BA. Differential autophosphorylation of Ca2+/calmodulin-dependent protein kinase II from phasic and tonic smooth muscle tissues. Am J Physiol. 2002;283:C1399–C1413. doi: 10.1152/ajpcell.00020.2002. [DOI] [PubMed] [Google Scholar]
- 24.Morgan KG, Gangopadhyay SS. Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953–62. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 25.Wood JD. Gastrointestinal Physiology. In: Rhoades RA, Tanner GA, editors. Medical Physiology. Baltimore: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 26.Dickson EJ, Spencer NJ, Hennig GW, et al. An Enteric Occult Reflex Underlies Accommodation and Slow Transit in the Distal Large Bowel. Gastroenterology. 2007;132:1912–24. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Rattan S, Patel CA. Selectivity of ROCK inhibitors in the spontaneously tonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2008;294:G687–G693. doi: 10.1152/ajpgi.00501.2007. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez SM, Miner AS, Browne BM, Ratz PH. Failure of Bay K 8644 to induce RhoA kinase-dependent calcium sensitization in rabbit blood vessels. British Journal of Pharmacology. 2010;160:1326–37. doi: 10.1111/j.1476-5381.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, Macdonald JA. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009;75:1031–41. doi: 10.1124/mol.108.049858. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. AJP - Cell Physiology. 2008;295:C358–C364. doi: 10.1152/ajpcell.90645.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T, Kendig DM, Smolock EM, Moreland RS. Carbachol-induced rabbit bladder smooth muscle contraction: roles of protein kinase C and Rho kinase. AJP - Renal Physiology. 2009;297:F1534–F1542. doi: 10.1152/ajprenal.00095.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Montelongo MC, Marin R, Gomez T, Marrero-Alonso J, Diaz M. Androgens induce nongenomic stimulation of colonic contractile activity through induction of calcium sensitization and phosphorylation of LC20 and CPI-17. Mol Endocrinol. 2010;24:1007–23. doi: 10.1210/me.2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakurada S, Takuwa N, Sugimoto N, et al. Ca2+-Dependent Activation of Rho and Rho Kinase in Membrane Depolarization-Induced and Receptor Stimulation-Induced Vascular Smooth Muscle Contraction. Circulation Research. 2003;93:548–56. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 34.Wen W, Liu W, Yan J, Zhang M. Structure Basis and Unconventional Lipid Membrane Binding Properties of the PH-C1 Tandem of Rho Kinases. Journal of Biological Chemistry. 2008;283:26263–73. doi: 10.1074/jbc.M803417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders KM, Koh SD, Ward SM. Interstitial Cells of Cajal as Pacemakers in the Gastrointestianl Tract. Annual Review of Physiology. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 36.Kito Y, Sanders KM, Ward SM, Suzuki H. Interstitial cells of Cajal generate spontaneous transient depolarizations in the rat gastric fundus. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2009;297:G814–G824. doi: 10.1152/ajpgi.00118.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muranyi A, Derkach D, Erdodi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett. 2005;579:6611–5. doi: 10.1016/j.febslet.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 39.Yoneda A, Multhaupt HAB, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. The Journal of Cell Biology. 2005;170:443–53. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Zheng XR, Riddick N, et al. ROCK Isoform Regulation of Myosin Phosphatase and Contractility in Vascular Smooth Muscle Cells. Circulation Research. 2009;104:531–40. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surks HK, Mendelsohn ME. Dimerization of cGMP-dependent protein kinase 1[alpha] and the myosin-binding subunit of myosin phosphatase: role of leucine zipper domains. Cellular Signalling. 2003;15:937–44. doi: 10.1016/s0898-6568(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 42.Richards CT, Ogut O, Brozovich FV. Agonist-induced Force Enhancement: the role of isoforms and phosphorylation of the myosin-targeting subunit of myosin light chain phosphatase. Journal of Biological Chemistry. 2002;277:4422–7. doi: 10.1074/jbc.M111047200. [DOI] [PubMed] [Google Scholar]
- 43.Payne MC, Zhang HY, Prosdocimo T, et al. Myosin phosphatase isoform switching in vascular smooth muscle development. Journal of Molecular and Cellular Cardiology. 2006;40:274–82. doi: 10.1016/j.yjmcc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Murthy KS. Signal transduction in gastrointestinal smooth muscle. Cellular Signaling. 1997;9:269–76. doi: 10.1016/s0898-6568(96)00180-5. [DOI] [PubMed] [Google Scholar]
- 45.Corteling RL, Brett SE, Yin H, Zheng XL, Walsh MP, Welsh DG. The functional consequence of RhoA knockdown by RNA interference in rat cerebral arteries. American Journal of Physiology -Heart and Circulatory Physiology. 2007;293:H440–H447. doi: 10.1152/ajpheart.01374.2006. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Du L, Gunst SJ. The effects of the small GTPase RhoA on the muscarinic contraction of airway smooth muscle result from its role in regulating actin polymerization. American Journal of Physiology - Cell Physiology. 2010;299:C298–C306. doi: 10.1152/ajpcell.00118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayguinov O, Sanders KM. Dissociation between electrical and mechanical responses to nitrergic stimulation in the canine gastric fundus. J Physiol. 1998;509 ( Pt 2):437–48. doi: 10.1111/j.1469-7793.1998.437bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petkov GV, Boev K. The role of sarcoplasmic reticulum and sarcoplasmic reticulum Ca2+-ATPase in the smooth muscle tone of the cat gastric fundus. Pflügers Archive. 1996;431:928–35. doi: 10.1007/s004240050087. [DOI] [PubMed] [Google Scholar]
- 49.Walsh MP, Thornbury K, Cole WC, Sergeant G, Hollywood M, McHale N. Rho-associated kinase plays a role in rabbit urethral smooth muscle contraction, but not via enhanced myosin light chain phosphorylation. American Journal of Physiology - Renal Physiology. 2011;300:F73–F85. doi: 10.1152/ajprenal.00011.2010. [DOI] [PubMed] [Google Scholar]