Abstract
A case (female, 39 years of) of thyroid-like nasopharyngeal low-grade papillary adenocarcinoma with a significant spindle cell component is presented. The tumor was located on the posterior nasal septum. The spindle cells displayed nuclear features very much similar to the epithelial component and the two cell types merged imperceptibly. Immunohistochemically, the neoplastic cells (including the spindle cell component) were strongly and diffusely positive for TTF-1, cytokeratins (AE1-3), cytokeratin 19 and vimentin. C-kit immunohistochemistry showed diffuse mild to moderate membranous positivity with focal areas displaying moderate to strong immunoreactivity. EMA was strongly positive in the epithelial component with membranous and cytoplasmic reactivity whereas the spindle cell component was weakly although diffusely positive. Carcinoembryonic antigen, calcitonin, chromogranin A, S100-protein, thyroglobulin, cdx2 and p63 were negative. The proliferative activity (Mib-1/Ki-67) was low; 3–4%. In the molecular genetic study we found no mutations at position 1799 (exon 15) in the BRAF-gene, (BRAFV600E) or in exons 9 and 11 of the KIT-gene.
Keywords: Low-grade papillary adenocarcinoma, Thyroid-like, Nasopharynx, TTF-1, BRAF-gene, KIT-gene
Introduction
Low-grade papillary adenocarcinomas of the nasopharynx (LGPACNP) is a rare neoplasm that was first described and characterized by Wenig et al. in a series of nine cases in 1988 [1]. Since then one series (comprising 13 cases; from Mario Luna at MD Anderson Cancer Center in 2006) and a few case reports have been published on this neoplastic entity [2–10]. In 2005 Carrizo and Luna presented 2 cases of LGPACNP with nuclear positivity for thyroid transcription factor-1 (TTF-1) [2]. Based on this immunohistochemical feature (and the positivity for cytokeratin 19) in conjunction with the histological characteristics (papillarity, psammoma bodies, neoplastic cells with overlapping nuclei and clear chromatin), the authors coined the term “thyroid-like nasopharyngeal papillary adenocarcinoma”, which has been used by subsequent investigators. Given the similarities between LGPACNP (especially those with nuclear expression of TTF-1) and papillary thyroid carcinoma (PTC), we also undertook a molecular genetic study regarding the mutational status of the BRAF-gene. The presence of an “intervening” spindle cell component between the papillary structures in LGPACNP, has been mentioned and illustrated in one recent case report [5]. In none of the previous published papers has a prominent spindle cell component been described or illustrated. In the case presented herein, a case of LGPACNP with a prominent spindle cell component is presented which gave the initial impression of a biphasic neoplasm.
Case Report
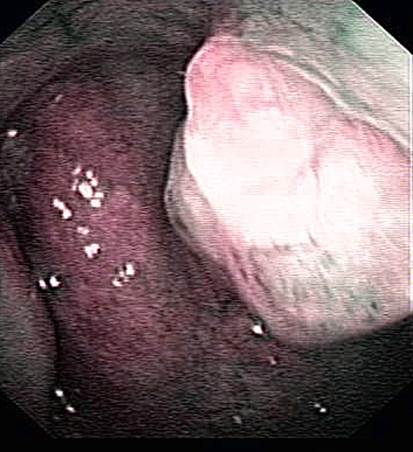
A previously healthy non-smoking 39-year-old Chinese lady with no significant family history of cancer presented with a 4-month history of right-sided epistaxis on a background of frequent blocked nose and rhinorrhoea. On clinical examination, no thyroid tumor or cervical lymphadenopathy was found. Examination with Narrow-band imaging (NBI) nasendoscopy showed a 1 cm polypoidal mass arising from the posterior edge of the bony septum (Fig. 1). There was no extension onto the inferior wall of the sphenoid sinus, the soft palate or laterally onto the choanae. A biopsy of the dominant portion of the mass on the right was performed. After the biopsy a magnetic resonance imaging (MRI) examination showed no definitive residual tumor. However, follow-up flexible nasendoscopy with NBI, revealed limited suspicious remnant neoplastic tissue on the left side of the posterior edge of the nasal septum. Endoscopic resection of this was performed. Histopathological examination failed to reveal definitive residual tumor.
Fig. 1.
On nasendoscopy, a well-defined 1 cm large, polypoidal tumor was seen located on the right aspect of the upper posterior septal edge. The tumor showed scanty vascularity as compared to the surrounding normal mucosa of the septum and nasopharynx, The tumor did not extend to adjacent sphenoid base, choana or soft palate
Materials and Methods
The tissue was fixed in formalin, embedded in paraffin and 4 μm thin sections were cut and stained with hematoxylin and eosin and Periodic acid Schiff (PAS) with diastase digestion. An immunohistochemical study was performed using commercially available antibodies to the following antigens: TTF-1, thyroglobulin, S100-protein, p63, cytokeratins (CK;AE1-3), CK19, vimentin and epithelial membrane antigen (EMA), carcinoembryonic antigen (CEA), calcitonin, chromogranin A, cdx2, surfactant B, c-kit/CD117 and Ki-67. Protocols according to the manufacturers’ recommendations were employed. For the molecular genetic study (BRAF and KIT) mutation analysis), 5 unstained sections were obtained from the tumor (biopsy) paraffin block. DNA was isolated with the QIAamp DNA MicroKit, in accordance with the manufacturer’s protocol. The PCR amplification and subsequent direct sanger sequencing of BRAF exon 15 were carried out according to published protocols [11]. Our test validation of the BRAF exon 15 detection by direct sequencing had shown 100% sensitivity and specificity, with a lower limit of detection set to 30% of mutated cells in a background of 70% of non-mutated cells [12]. For KIT exons 9 and 11 mutation analysis, polymerase chain reaction (PCR) amplification of 100 ng of genomic DNA was set up as described previously [13]. Mutation analysis of KIT exons 9 and 11 was performed by bidirectional sequence analysis on the ABI3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using the BigDye_ Terminatorv3.1 Cycle Sequencing Kit (Applied Biosystems). For the detection of SYT-SSX1/2 fusion transcripts t(X;18)(p11.2;q11.2) by RT-PCR, RNA was isolated from prepared sections of the received formalin-fixed, paraffin-embedded tissue with the high pure RNA Paraffin Kit method by Roche, according to the manufacturer’s protocol. Reverse transcription was performed using the high capacity cDNA Reverse Transcription Kit by Applied Biosystems. The PCR amplification was carried out according to published protocols [14, 15].
Detection of SYT (18q11.2) gene rearrangement was performed using Vysis LSI SYT dual color, break apart rearrangement probe. At least one hundred non-overlapped nuclei with distinct signals were scored and the interpretation of intact and split signals was based on generally accepted guidelines recommended by Vysis.
Results
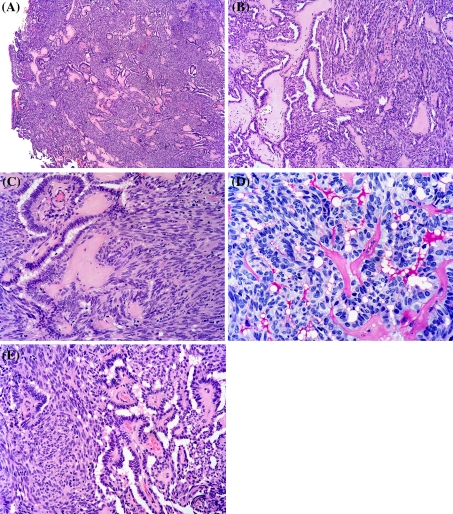
Histological examination of the biopsy (8 × 7 mm after formalin fixation) revealed a tumor which was partly lined by ciliated respiratory epithelium. (Fig. 2a) The superficial aspect of the tumor displayed papillary structures with hyalinized fibrovascular cores, which focally merged with the superficial epithelium (Fig. 2b). The papillae were lined by bland columnar epithelium with elongated slightly crowded nuclei which displayed fine chromatin and absent to inconspicuous nucleoli (Fig. 2c). The papillae were frequently compressed and formed gland-like spaces that contained PAS positive diastase resistant secretory material imparting a somewhat bubbly appearance (Fig. 2d). In large parts of the tumor, a significant spindle cell component was seen in between the compressed papillary structures (Fig. 2a–c, e). These spindle cells displayed nuclear features very much similar to the columnar epithelium and the two cell types merged imperceptibly (Fig. 2e). No necrosis or mitotic activity was discerned. No PAS-positive diastase resistant cytoplasmic zymogen granules were seen. No “Indian-file-type” infiltrative pattern or targetoid perineural invasion was identified.
Fig. 2.
Hematoxylin and eosin stained sections show a tumor which was partly lined by ciliated respiratory epithelium (a). The tumor displayed papillary structures with hyalinized fibrovascular cores and a prominent intervening spindle cell component (a–c, e). The papillae were lined by bland columnar epithelium with elongated slightly crowded nuclei which displayed fine chromatin and absent to inconspicuous nucleoli (c). The papillae were frequently compressed and formed gland-like spaces that contained PAS positive diastase resistant secretory material imparting a somewhat bubbly appearance (d). The spindle cells and the columnar epithelium merged imperceptibly (e)
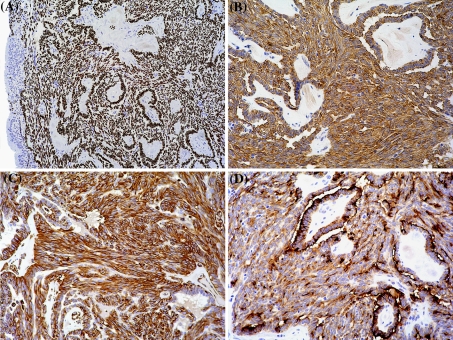
The immunohistochemical study showed that the neoplastic cells (including the spindle cell component) were strongly and diffusely positive for TTF-1, cytokeratins (AE1-3), CK19 and vimentin (Fig. 3a–c). EMA was strongly positive in the epithelial component with membranous and cytoplasmic reactivity whereas the spindle cell component was weakly although diffusely positive (Fig. 3d). There was a diffuse mild to moderate membranous positivity for c-kit with small areas displaying a moderate to strong membranous immunoreactivity (data not shown). CEA, calcitonin, chromogranin A, S100-protein, thyroglobulin, cdx2 and p63 were completely negative (data not shown). The proliferative activity (Mib-1/Ki-67) was low; 3–4% (data not shown).
Fig. 3.
Photomicrographs from the immuhohistochemical study showing strong nuclear expression of TTF-1 (a), positive reaction for CK19 (b) and vimentin (c) in both the columnar- and spindle cell component. EMA was positive in both components, but weaker in the spindle cells (d)
In the molecular genetic study we found no mutation at position 1799 (exon 15) in the BRAF-gene, (BRAFV600E). A wild-type sequence was found in the KIT gene (exons 9 and 11). Both RT-PCR and FISH assays were negative for SYT-SSX1/2 fusion transcripts t(X;18)(p11.2;q11.2) and SYT (18q11.2) gene rearrangement, respectively.
Discussion
We present a case of low-grade papillary adenocarcinoma of the nasopharynx with a prominent spindle cell component that initially gave the impression of a biphasic neoplasm. This biphasic pattern has hitherto only been mentioned in a recent case report [5], but it appears from the verbal description and the presented photomicrograph (Fig. 3c) that this was not a prominent feature of that neoplasm. In all other published studies on LGPACNP, we have neither found this feature commented on nor documented. The immunohistochemical profile of our tumor is identical to what has been published previously [1–9]. The fact that some of these neoplasms display nuclear expression of TTF-1 and some do not is intriguing. However, based on the literature, there appears to be no histological features that separate the TTF-1 positive (“thyroid-like”) from the TTF-1 negative ones. Moreover, no differences in biological/clinical behavior have been ascribed to these two subgroups of LGPACNP. The nasal septum has been involved in previous cases of LGPACNP, but this is the first case where this has been the exclusive location. Hence, this case expands the range of anatomical locations where this neoplasm may arise.
Given that some investigators have highlighted a histological similarity between LGPACNP (especially those with nuclear expression of TTF-1) and papillary thyroid carcinoma (PTC), we undertook a molecular genetic study regarding the mutational status of the BRAF-gene. At the molecular level, BRAF mutations have been estimated to occur in approximately 60–70% of conventional PTCs [16]. The BRAF V600E mutation (T to A transversion at position 1799 in exon 15 leading to a substitution of valine by glutamate at position 600 of the protein) accounts for more than 90% of the mutations in PTC [17, 18]. To the best of our knowledge, testing for the BRAF exon 15 V600E mutation has not been performed on any (“thyroid-like”) LGPACNP. Due to the immunohistochemically detected membranous positivity for c-kit, we undertook a mutational analysis of exons 9 and 11 of the KIT gene, but no mutation was detected.
From a differential diagnostic point of view, the biphasic appearance and the immunohistochemical positivity for cytokeratins and EMA of the tumor presented herein gave an initial impression of a biphasic synovial sarcoma (SS) (Fig. 1e). Reportedly, biphasic synovial sarcomas may display distinct papillary structures [19–21]. However, the bland nuclear features, lack of mitotic figures (and corresponding low proliferative activity in the immunohistochemical study) and the strong and diffuse positivity for cytokeratins in the spindle cells in the actual tumor would be highly unusual for a SS which typically displays a weak and/or patchy cytokeratin positivity in the spindle cell component. Moreover, we found no evidence of a t(X;18) in the FISH study or SYT-SSX1/SSX2 fusions transcripts in the PCR study. Interestingly, immunohistochemical positivity for TTF-1 has been reported in one synovial sarcoma [22]. However, no molecular genetic confirmation was presented; furthermore, the location of this SS was lung and the possibility of entrapped lung tissue was not commented on. The possibility of a metastatic thyroid carcinoma, either PTC or when, as in our case, there is a prominent spindle cell population, a medullary thyroid carcinoma (MTC) should be excluded. The negative immunohistochemistry for thyroglobulin and positive reaction for vimentin in conjunction with the histological features in our case are not consistent with a PTC. The combination of TTF-1- and vimentin positivity and negative reaction for thyroglobulin would be consistent with a MTC. However, the absence of immunopositivity for CEA, calcitonin and chromogranin A militate against this differential diagnostic possibility.
Regarding the histogenesis of this nosological entity, we are not convinced that this neoplasm is a carcinoma in sensu stricto. The fact that there is no recognized precursor-lesion makes it difficult to prove that this neoplasm is derived from the surface epithelium (the fact that a good number of cases—including the actual tumor—have shown surface involvement overlying—not away from—the tumor mass, raises the possibility of secondary involvement and does not proof the surface epithelium as the origin). The biphasic nature of this neoplasm associates to a group of enigmatic neoplasms that show “thymic-like or branchial pouch differentiation differentiation” [23, 24]. Such tumors include the ectopic hamartomatous thymoma (EHT), ectopic cervical thymoma (ECT), spindle epithelial tumor with thymus-like differentiation (SETTLE) and carcinoma showing thymus-like differentiation (CASTLE).
EHT is a benign neoplasm occurring predominantly in males and is typically located in the supraclavicular or suprasternal region. ECTs are rare tumors that display similar resemblance to their mediastinal counterparts in terms of morphological type, with type A, AB and micronodular types previously reported [25]. ECTs typically occur in the neck; to the best of our knowledge, there has not been a reported ECT occurring in the nasopharynx. Furthermore, although ECTs may be composed of epithelioid cells similar to their mediastinal counterparts, ECTs have not hitherto been reported to contain well-developed epithelial structures such as papillae with fibrovascular cores as seen in our case. SETTLE and CASTLE occur nearly exclusively in the thyroid. SETTLE is composed of spindled areas blending imperceptibly into areas displaying epithelial differentiation which may be in the form of glomeruloid glandular structures, sertoli-like tubules and small glands lined by cuboidal to columnar cells [26]. We have found no convincing information on the expression of TTF-1 in SETTLE in the literature and a nasal or nasopharyngeal site has not yet been reported for SETTLE to date.
Other neoplasms that need to be included in the differential work-up of a papillary neoplasm of the nasopharynx and posterior nasal cavity are papillary variants of polymorphous low-grade adenocarcinoma (PLGA-p), papillary variants of acinic cell carcinoma (ACC-p) and intestinal type of adenocarcinoma (ITAC-p), exophytic and oncocytic variants of Schneiderian papillomas, the sinonasal low-grade tubulo-papillary adenocarcinoma, a papillary variant of ectopic meningioma and a metastatic thyroid papillary carcinoma. PLGA may occur in the posterior nasal space/nasopharynx [27–29]. PLGA with a significant papillary component (formerly called low-grade papillary adenocarcinoma) has been claimed to constitute a more aggressive variant [30–32]. PLGA characteristically displays uniform cytomorphology (bland nuclear features with fine chromatin and moderate amount of eosinophilic cytoplasm), a slate-grey extracellular matrix, scarce mitotic figures and a highly variegated growth pattern within the same tumor including solid, cribriform, cystic, tubular, papillary patterns and oftentimes infiltration into the surrounding stroma with an “Indian-file” arrangement of cells and targetoid perineural invasion. Although the neoplastic cells in our tumor also had bland nuclear features in association with a minimal proliferative activity, the diversity of patterns of how lesional cells were arranged was not that of PLGA and we found no blue-grey myxoid stromal component. Moreover, even though the tumor cells in PLGA are positive for vimentin (like in our case), positivity for S100-protein frequently seen in PLGA is consistently negative in LGPACNP and positivity for TTF-1 has, to the best of our knowledge, never been reported in PLGA. Acinic cell carcinomas with a papillary component are frequently cystic (not present in our case) and display PAS-positive diastase resistant cytoplasmic zymogen granules (not present in our case). Given the range of differentiation (acinar to intercalated ducts), the immunohistochemical features of ACC is variable with cases positive for S100-protein [33–37] and even vimentin [38] are on record. Regarding papillary variants of the intestinal type of adenocarcinoma, these tumors show more nuclear atypia than our tumor and commonly display mucinous differentiation. Immunohistochemically, ITACs are positive for CK20 and express cdx-2 [39] (negative in our case). Sinonasal low-grade tubulo-papillary adenocarcinoma (“terminal tubulus adenocarcinoma” according to Kleinsasser) is a rare neoplasm characterized by Skalova et al. [40]. These tumors are composed of uniform glands and tubular structures with a papillary component containing loose (not hyalinized as in our case) fibrovascular cores. The neoplastic cells are cuboidal to columnar with bland nuclear features showing fine to clear chromatin and very low proliferative activity. Reportedly, mucinous intraepithelial microcysts (similar to those in oncocytic/columnar cell Schneiderian papillomas) were present in the series of Skalova et al. (not seen in our case) and all cases showed positivity for S100-protein in the immunohistochemical study. None of the tumors metastasized, but in 2/6 patients local recurrences were documented. The distinction between LGPACNP and all the above mentioned entities; PLGA-p, ACC-p and ITAC-p, (except the sinonasal low-grade tubulo-papillary adenocarcinoma) is critical from a prognostic point of view, since they all have a non-negligible risk of metastasis. In this context it should be mentioned that it has been claimed that the papillary variant of PLGA shows a more aggressive behaviour than the non-papillary variants [30, 41]. Schneiderian papillomas (SP) may be seen in the nasopharynx [42, 43]. SP occurring in this site display the same histological features (non-keratinizing squamous-, “transitional-type”-, and pseudostratified epithelium with oncocytoid characteristics), as seen in the sinonasal area and which differ from the hyalinized fibrovascular cores lined by the bland cuboidal to columnar cells in LGPACNP. Ectopic or extension of a primary meningeal meningioma with papillary components, intravascular papillary endothelial hyperplasia (Masson’s hemangioma), sinonasal melanomas with pseudopapillary structures and nasopharyngeal respiratory epithelial adenomatoid hamartoma (REAH) are other lesions that may be encountered in the posterior nasal space and which may show a papillary or psedopapillary architecture. However, all these lesions differ histologically and immunohistochemically from LGPACNP.
The expression of TTF-1 in some of LGPACNPs is intriguing and raises questions as to the biological basis for this phenomenon. As we have discussed previously, there are a variety of explanations that may account for this phenomenon [44]. TTF-1 is a 38kD DNA-binding protein normally present in thyroid and lung, and is currently used in routine surgical pathology as an immunohistochemical marker of primary carcinomas arising in these organs. Initially it was thought that the specificity of TTF-1 was close to 100%; with time however, an increasing body of data has shown that TTF-1 can be expressed in both primary and metastatic colonic adenocarcinomas, a significant number (5%) of invasive urothelial carcinomas of the urinary bladder as well as in prostatic, gastric and salivary gland adenocarcinomas and recently also in a thymoma [44–46]. Moreover, immunohistochemical detection of TTF-1 has been detected in all major types of gynecologic carcinomas, with a frequency of 82% reported in malignant mixed Műllerian tumors [46–49]. Interestingly, TTF-1 has also been reported to be expressed in non-neoplastic tubal and endometrial epithelium as well as in non-dysplastic gastric epithelium in patients with atrophic gastritis [49, 50]. The presence of nuclear expression of TTF-1 in various neuroendocrine neoplasms of both pulmonary and non-pulmonary origin is a well recognized phenomenon. Functionally, TTF-1 is a member of the family of the homeobox proteins, a class of transcription factors critically involved in patterning during embryological development [51]. One might therefore speculate that TTF-1 participates in development of the branchial pouch apparatus from which the primitive pharynx derives. Firm evidence for such an idea is at present lacking; in one study TTF-1 was undetectable in two ectopic hamartomatous thymomas, a mixed epithelial and stromal branchial anlage neoplasm [52]. Although not known to be expressed in the human thymus, its homolog is expressed in the mouse thymus [53]. A final explanation for this observation may be that of “aberrant” TTF-1 expression. Given the fact that a broad range of epithelial neoplasms have shown nuclear TTF-1 expression it may just be a random event reflecting the inherent genetic instability of neoplasms in general and carcinomas in particular, epitomizing the concept of “divergent differentiation”. One mechanism for strong nuclear TTF-1 expression includes genomic amplification of the TTF-1 locus, which has been previously reported in lung cancers [54]. FISH testing (which we did not have access to) will be required for definite evidence of such a mechanism in our tumor.
Our case was recently diagnosed precluding any meaningful follow-up. However, to date no case of LGPACNP has been associated with metastatic behaviour with follow-up of up to 15 years. Local recurrences may be experienced with inadequate surgical resection. Photodynamic therapy with topical 5-aminolevulinic acid has been successfully used in such an instance [55].
In summary, we present a case of “thyroid-like” nasopharyngeal low-grade papillary adenocarcinoma with a prominent spindle cell component, thus mimicking a biphasic neoplasm which was strictly located to the posterior nasal septum. Both the epithelial and spindle cell components displayed very similar nuclear features and almost identical immunohistochemical profile. No mutations at position 1799 (exon 15) in the BRAF-gene, (BRAFV600E) or in exons 9 and 11 of the KIT gene were identified.
Acknowledgments
We thank Chin Sze Yung for skillful technical assistance.
References
- 1.Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal papillary adenocarcinoma. A clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol. 1988;12:946–953. doi: 10.1097/00000478-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol. 2005;9:189–192. doi: 10.1016/j.anndiagpath.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Fu CH, Chang KP, Ueng SH, et al. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx. 2008;35:579–582. doi: 10.1016/j.anl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Nojeg MM, Jalaludin MA, Jayalakshmi P. Papillary adenocarcinoma of the nasopharynx–case report and review of the literature. Med J Malaysia. 1998;53:104–106. [PubMed] [Google Scholar]
- 5.Ohe C, Sakaida N, Tadokoro C, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of two cases. Pathol Int. 2010;60:107–111. doi: 10.1111/j.1440-1827.2009.02480.x. [DOI] [PubMed] [Google Scholar]
- 6.Pineda-Daboin K, Neto A, Ochoa-Perez V, et al. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol. 2006;10:215–221. doi: 10.1016/j.anndiagpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Sillings CN, Weathers DR, Delgaudio JM. Thyroid-like papillary adenocarcinoma of the nasopharynx: a case report in a 19-year-old male. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e25–e28. doi: 10.1016/j.tripleo.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Hasselt CA, Ng HK. Papillary adenocarcinoma of the nasopharynx. J Laryngol Otol. 1991;105:853–854. doi: 10.1017/s0022215100117542. [DOI] [PubMed] [Google Scholar]
- 9.Wu PY, Huang CC, Chen HK, et al. Adult thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with thyroid transcription factor-1 expression. Otolaryngol Head Neck Surg. 2007;137:837–838. doi: 10.1016/j.otohns.2007.06.725. [DOI] [PubMed] [Google Scholar]
- 10.Yang TH, Chen CT, Wang CP, et al. Photodynamic therapy suppresses the migration and invasion of head and neck cancer cells in vitro. Oral Oncol. 2007;43:358–365. doi: 10.1016/j.oraloncology.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 12.Pang NK, Nga ME, Chin SY, et al. KRAS and BRAF mutation analysis can be reliably performed on aspirated cytological specimens of metastatic colorectal carcinoma. Cytopathology. 2009;Epub. [DOI] [PubMed]
- 13.Salto-Tellez M, Nga ME, Han HC, et al. Tissue microarrays characterise the clinical significance of a VEGF-A protein expression signature in gastrointestinal stromal tumours. Br J Cancer. 2007;96:776–782. doi: 10.1038/sj.bjc.6603551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tvrdik D, Povysil C, Svatosova J, et al. Molecular diagnosis of synovial sarcoma: RT-PCR detection of SYT-SSX1/2 fusion transcripts in paraffin-embedded tissue. Med Sci Monit 2005;11:MT1-7. [PubMed]
- 15.Tsuji S, Hisaoka M, Morimitsu Y, et al. Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol. 1998;153:1807–1812. doi: 10.1016/S0002-9440(10)65695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 17.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 18.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 19.Chan JA, McMenamin ME, Fletcher CD. Synovial sarcoma in older patients: clinicopathological analysis of 32 cases with emphasis on unusual histological features. Histopathology. 2003;43:72–83. doi: 10.1046/j.1365-2559.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 20.Hartel PH, Fanburg-Smith JC, Frazier AA, et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol. 2007;20:760–769. doi: 10.1038/modpathol.3800795. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen GP, Shaw PA, Rosenberg AE, et al. Synovial sarcoma of the vulva: a report of two cases. Mod Pathol. 1996;9:970–974. [PubMed] [Google Scholar]
- 22.Lewis JS, Ritter JH, El-Mofty S. Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Mod Pathol. 2005;18:1471–1481. doi: 10.1038/modpathol.3800451. [DOI] [PubMed] [Google Scholar]
- 23.Chan JK, Rosai J. Tumors of the neck showing thymic or related branchial pouch differentiation: a unifying concept. Hum Pathol. 1991;22:349–367. doi: 10.1016/0046-8177(91)90083-2. [DOI] [PubMed] [Google Scholar]
- 24.Fetsch JF, Laskin WB, Michal M, et al. Ectopic hamartomatous thymoma: a clinicopathologic and immunohistochemical analysis of 21 cases with data supporting reclassification as a branchial anlage mixed tumor. Am J Surg Pathol. 2004;28:1360–1370. doi: 10.1097/01.pas.0000135518.27224.3f. [DOI] [PubMed] [Google Scholar]
- 25.Yan B, Lim D, Petersson F. Ectopic cervical thymoma: a report of two cases of a rare entity frequently misdiagnosed on fine needle aspiration cytology and frozen section. Head Neck Pathol. 2010;4:152–156. doi: 10.1007/s12105-010-0172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folpe AL, Lloyd RV, Bacchi CE, et al. Spindle epithelial tumor with thymus-like differentiation: a morphologic, immunohistochemical, and molecular genetic study of 11 cases. Am J Surg Pathol. 2009;33:1179–1186. doi: 10.1097/PAS.0b013e31819e61c8. [DOI] [PubMed] [Google Scholar]
- 27.Wenig BM, Harpaz N, DelBridge C. Polymorphous low-grade adenocarcinoma of seromucous glands of the nasopharynx. A report of a case and a discussion of the morphologic and immunohistochemical features. Am J Clin Pathol. 1989;92:104–109. doi: 10.1093/ajcp/92.1.104. [DOI] [PubMed] [Google Scholar]
- 28.Wei YC, Huang CC, Chien CY, et al. Polymorphous low-grade adenocarcinoma of the nasopharynx: a case report and brief review. J Clin Pathol. 2008;61:1124–1126. doi: 10.1136/jcp.2008.059642. [DOI] [PubMed] [Google Scholar]
- 29.Garzaro M, Pecorari G, Landolfo V, et al. Nasopharyngeal polymorphous low-grade adenocarcinoma in a patient with nonfunctioning pituitary macroadenoma. B-Ent. 2010;6:59–62. [PubMed] [Google Scholar]
- 30.Slootweg PJ, Muller H. Low-grade adenocarcinoma of the oral cavity. A comparison between the terminal duct and the papillary type. J Craniomaxillofac Surg. 1987;15:359–364. doi: 10.1016/s1010-5182(87)80083-5. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Ordonez B, Linkov I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32:521–529. doi: 10.1046/j.1365-2559.1998.t01-2-00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell DA, Eveson JW, Ord RA. Polymorphous low-grade adenocarcinoma of minor salivary glands–a report of three cases. Br J Oral Maxillofac Surg. 1989;27:494–500. doi: 10.1016/S0266-4356(89)80008-7. [DOI] [PubMed] [Google Scholar]
- 33.Hara K, Ito M, Takeuchi J, et al. Distribution of S-100b protein in normal salivary glands and salivary gland tumors. Virchows Arch A Pathol Anat Histopathol. 1983;401:237–249. doi: 10.1007/BF00692648. [DOI] [PubMed] [Google Scholar]
- 34.Kahn HJ, Baumal R, Marks A, et al. Myoepithelial cells in salivary gland tumors. An immunohistochemical study. Arch Pathol Lab Med. 1985;109:190–195. [PubMed] [Google Scholar]
- 35.Nakazato Y, Ishida Y, Takahashi K, et al. Immunohistochemical distribution of S-100 protein and glial fibrillary acidic protein in normal and neoplastic salivary glands. Virchows Arch A Pathol Anat Histopathol. 1985;405:299–310. doi: 10.1007/BF00710066. [DOI] [PubMed] [Google Scholar]
- 36.Zarbo RJ, Regezi JA, Batsakis JG. S-100 protein in salivary gland tumors: an immunohistochemical study of 129 cases. Head Neck Surg. 1986;8:268–275. doi: 10.1002/hed.2890080406. [DOI] [PubMed] [Google Scholar]
- 37.Dardick I, George D, Jeans MT, et al. Ultrastructural morphology and cellular differentiation in acinic cell carcinoma. Oral Surg Oral Med Oral Pathol. 1987;63:325–334. doi: 10.1016/0030-4220(87)90199-X. [DOI] [PubMed] [Google Scholar]
- 38.Gustafsson H, Virtanen I, Thornell LE. Expression of cytokeratins and vimentin in salivary gland carcinomas as revealed with monoclonal antibodies. Virchows Arch A Pathol Anat Histopathol. 1988;412:515–524. doi: 10.1007/BF00844287. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz-Rey JA, Alvarez C, San Miguel P, et al. Expression of CDX2, cytokeratins 7 and 20 in sinonasal intestinal-type adenocarcinoma. Appl Immunohistochem Mol Morphol. 2005;13:142–146. doi: 10.1097/01.pai.0000133058.00588.15. [DOI] [PubMed] [Google Scholar]
- 40.Skalova A, Cardesa A, Leivo I, et al. Sinonasal tubulopapillary low-grade adenocarcinoma. Histopathological, immunohistochemical and ultrastructural features of poorly recognised entity. Virchows Arch. 2003;443:152–158. doi: 10.1007/s00428-003-0844-9. [DOI] [PubMed] [Google Scholar]
- 41.Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24:1319–1328. doi: 10.1097/00000478-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Low WK, Toh ST, Lim CM, et al. Schneiderian papilloma of the nasopharynx. Ear Nose Throat J. 2002;81:336–338. [PubMed] [Google Scholar]
- 43.Sulica RL, Wenig BM, Debo RF, et al. Schneiderian papillomas of the pharynx. Ann Otol Rhinol Laryngol. 1999;108:392–397. doi: 10.1177/000348949910800413. [DOI] [PubMed] [Google Scholar]
- 44.Yan B, Seng SC, Petersson F. Thymoma with nuclear expression of thyroid transcription factor-1: a potential diagnostic pitfall on core biopsy. Appl Immunohistochem Mol Morphol. 2011;19:76–81. doi: 10.1097/PAI.0b013e3181ec8a50. [DOI] [PubMed] [Google Scholar]
- 45.Kubba LA, McCluggage WG, Liu J, et al. Thyroid transcription factor-1 expression in ovarian epithelial neoplasms. Mod Pathol. 2008;21:485–490. doi: 10.1038/modpathol.2008.4. [DOI] [PubMed] [Google Scholar]
- 46.Matoso A, Singh K, Jacob R, et al. Comparison of thyroid transcription factor-1 expression by 2 monoclonal antibodies in pulmonary and nonpulmonary primary tumors. Appl Immunohistochem Mol Morphol. 2010;18:142–149. doi: 10.1097/PAI.0b013e3181bdf4e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siami K, McCluggage WG, Ordonez NG, et al. Thyroid transcription factor-1 expression in endometrial and endocervical adenocarcinomas. Am J Surg Pathol. 2007;31:1759–1763. doi: 10.1097/PAS.0b013e3181131e21. [DOI] [PubMed] [Google Scholar]
- 48.Zhang PJ, Gao HG, Pasha TL, et al. TTF-1 expression in ovarian and uterine epithelial neoplasia and its potential significance, an immunohistochemical assessment with multiple monoclonal antibodies and different secondary detection systems. Int J Gynecol Pathol. 2009;28:10–18. doi: 10.1097/PGP.0b013e3181804bc6. [DOI] [PubMed] [Google Scholar]
- 49.Niu HL, Pasha TL, Pawel BR, et al. Thyroid transcription factor-1 expression in normal gynecologic tissues and its potential significance. Int J Gynecol Pathol. 2009;28:301–307. doi: 10.1097/PGP.0b013e31819901c6. [DOI] [PubMed] [Google Scholar]
- 50.Rau T, Dimmler A, Hafner M, et al. Aberrant expression of TTF-1 and forkhead factor HFH-4 in atrophic gastritis and ciliated metaplasia suggests gastric broncho-pulmonary transdetermination. J Pathol. 2005;206:383–387. doi: 10.1002/path.1795. [DOI] [PubMed] [Google Scholar]
- 51.Morgan R. Hox genes: a continuation of embryonic patterning? Trends Genet. 2006;22:67–69. doi: 10.1016/j.tig.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Zhang RY. Ectopic hamartomatous thymoma: a clinicopathological and immunohistochemical study of two cases. Zhonghua Bing Li Xue Za Zhi. 2005;34:397–401. [PubMed] [Google Scholar]
- 53.Farr AG, Dooley JL, Erickson M. Organization of thymic medullary epithelial heterogeneity: implications for mechanisms of epithelial differentiation. Immunol Rev. 2002;189:20–27. doi: 10.1034/j.1600-065X.2002.18903.x. [DOI] [PubMed] [Google Scholar]
- 54.Kendall J, Liu Q, Bakleh A, et al. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc Natl Acad Sci USA. 2007;104:16663–16668. doi: 10.1073/pnas.0708286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang CP, Chang YL, Chen CT, et al. Photodynamic therapy with topical 5-aminolevulinic acid as a post-operative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma: a case report. Lasers Surg Med. 2006;38:435–438. doi: 10.1002/lsm.20291. [DOI] [PubMed] [Google Scholar]