Abstract
PEComas are a family of neoplastic lesions that share overlapping morphology, immunohistochemistry, and ultrastructure that include angiomyolipoma, lymphangioleiomyomatosis, clear cell “sugar” tumor of the lung as well as similar tumors occurring in a variety of visceral, cutaneous and soft tissue sites throughout the body. The defining histopathological features are epithelioid cells with a perivascular distribution containing clear to pale eosinophilic granular cytoplasm and a round-to-oval centrally located nucleus with an inconspicuous nucleolus. Immunohistochemically, coexpression of melanocytic (HMB-45 and/or Melan-A) and myoid markers are characteristic. In the present study, we describe three PEComas occurring in the head and neck (nasal cavity and larynx) and discuss the behavior of these distinctive tumors and review the literature of head and neck PEComas. The importance of recognizing this entity will ensure its consideration in the differential diagnosis of tumors of the head and neck with a similar morphology. The histogenesis of PEComas still remains elusive and additional cases with a prolonged follow up remain important to accurately determine the behavior of these distinctive tumors. Complete surgical excision still remains the treatment of choice for histologically benign PEComas.
Keywords: Perivascular epithelioid cell tumor, PEComa, Angiomyolipoma
Introduction
The term perivascular epithelioid cell tumor (PEComa), first coined by Bonetti et al. [1], is currently defined as a mesenchymal tumor composed of distinctive perivascular myoid cells. PEComas are a family of neoplastic lesions that share overlapping morphology, immunohistochemistry, and ultrastructure and includes angiomyolipoma (AML), lymphangioleiomyomatosis (LAM), clear cell “sugar” tumor (CCST) of the lung, as well as similar tumors occurring in a variety of visceral, cutaneous, and soft tissue sites throughout the body. PEComas may be associated with the tuberous sclerosis complex (TSC), particularly AML and LAM [2–4], or represent a forme fruste of the disease [1]. Notably, non-AML and non-LAM PEComas are only rarely associated with TSC. In general, PEComas are characterized by a female predominance (with a female to male ratio of 7:1) and wide age distribution. In recent decades, these tumors have been described in virtually all body sites. The defining histopathological features are epithelioid cells with a perivascular distribution containing clear to pale eosinophilic granular cytoplasm and a round-to-oval centrally located nucleus with an inconspicuous nucleolus [5]. While the perivascular distribution of tumor cells is characteristic, it may not be seen in all PEComas, which most typically have a delicate vascular stroma surrounding tumor nests. Immunohistochemically, coexpression of melanocytic (HMB-45 and/or Melan-A) and myoid markers is characteristic [6]. The growing interest in PEComas has led to an increasing number of reports demonstrating different anatomic locations of these lesions, including several case reports involving various head and neck sites. In the present study, we describe three PEComas occurring in the head and neck (nasal cavity and larynx) and discuss the behavior of these distinctive tumors, plus review previously recorded cases at this anatomic site.
Case Histories
Case 1
An 18-year-old woman presented with a mass in the left nasal cavity. The mass had been progressively increasing in size over a period of 1 year, resulting in complete obstruction of the left nasal cavity. Magnetic resonance imaging (MRI) revealed a smoothly demarcated 2.9 cm soft tissue mass located in the anterior left nasal cavity arising from the inferior turbinate. The cribriform plate was not involved and there was no intracranial extension. Imaging studies favored a vascular tumor but also included schwannoma, inverted papilloma, and paraganglioma in the differential, although the lesion was not typical for any of these latter entities. The patient underwent tumor embolization followed by surgical resection. One month after the primary excision (debulking), a complete re-excision was performed to obtain clear margins. No residual tumor was identified. Twenty-six months after primary surgery the patient was alive and asymptomatic without evidence of disease. No personal or family history of tuberous sclerosis was reported.
Microscopically, the tumor showed extensive surface ulceration and effacement of the submucosal structures with a narrow zone of subepithelial sparing in the areas with an intact surface. The tumor was predominantly composed of uniform epithelioid clear cells arranged in nests and trabeculae and intimately associated with a uniform delicate vascular stroma (Fig. 1) but no perivascular growth was identified. There was no cytological atypia, mitotic activity, or necrosis. Patchy perivascular chronic inflammation, resembling dysgerminoma/seminoma, was present toward the surface. Diastase-sensitive PAS positive cytoplasmic granules were present, consistent with glycogen. Immunohistochemical stains demonstrated cytoplasmic calponin, HMB45 and Melan-A positivity. The cells were negative for S-100 protein, smooth muscle actin (SMA), desmin, synaptophysin, cytokeratin CAM 5.2, and pancytokeratin. Ki-67 (MIB1) revealed a very low (<5%) proliferation index.
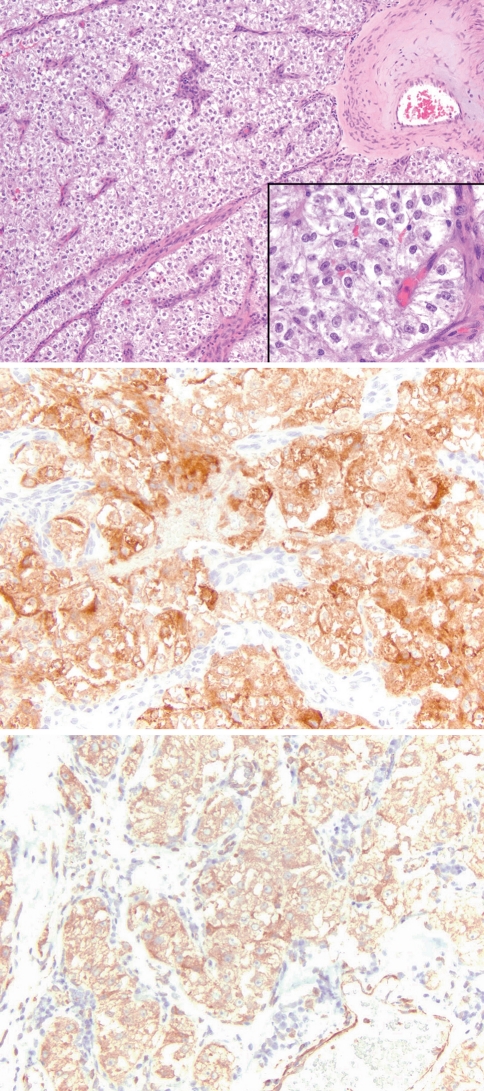
Fig. 1.
Nasal PEComa (Case 1) comprised of sheets and nests of polygonal cells associated with a prominent vascular stroma. The cells are cytologically bland with abundant clear to eosinophilic granular cytoplasm (top; H&E 100×; inset 400×). The tumor was strongly positive for Melan-A (middle; 200×) and calponin (bottom; 200×)
Case 2
A 71-year-old woman presented with a polypoid lesion of the nasal cavity of unknown duration and symptoms of nasal obstruction. No additional history or follow-up information was available. Microscopically, the lesion was composed of large polygonal cells arranged in sheets and nests and associated with a delicate vascular stroma. The tumor effaced the submucosal structures with a narrow zone of subepithelial sparing and an intact surface. The vessels were variable in size with many larger thin-walled ectatic vascular channels and scattered small capillary-sized vessels. No areas of distinct perivascular distribution of tumor cells were identified. The cells had clear to pale eosinophilic, granular to vacuolated cytoplasm with central nuclei and small nucleoli (Fig. 2). Some cells also contained a brown cytoplasmic pigment consistent with melanin. The proliferation was intimately arranged around the different types of blood vessels and there was no evidence of mitotic activity, atypia, or necrosis. A mucicarmine stain was negative and diastase-sensitive PAS positive cytoplasmic granules were present, consistent with glycogen. Immunohistochemical stains demonstrated cytoplasmic positivity for HMB45, SMA, vimentin, and focally for synaptophysin. The cells were negative for S-100 protein, pancytokeratin, and desmin.
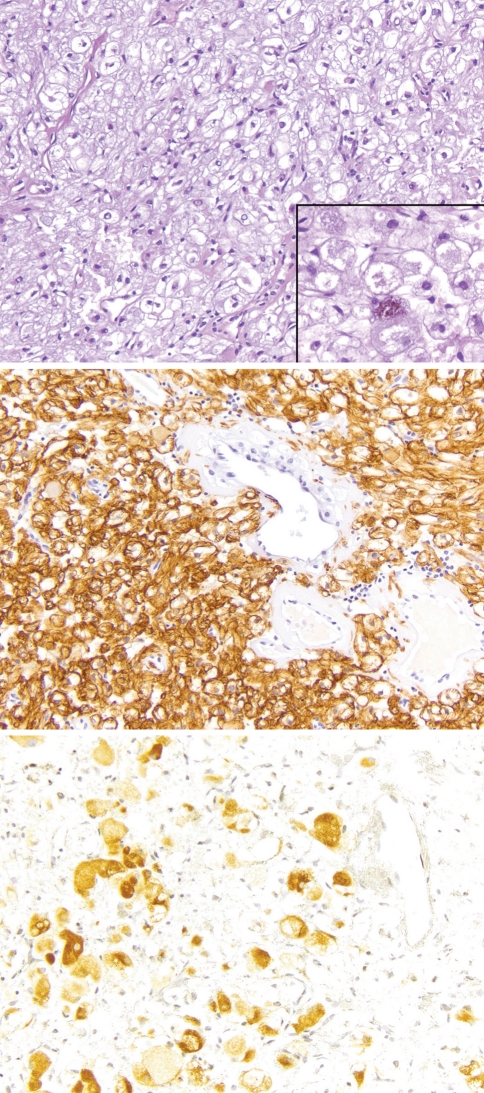
Fig. 2.
The other nasal PEComa (Case 2) had slightly larger cells with more cytoplasmic clearing and a delicate vascular stroma (top; H&E 100×). In this example, scattered cells with finely granular brown melanin pigment were present (top; inset 400×). The tumor was strongly positive for SMA (middle; 200×) and patchy HMB45 staining (bottom; 200×)
Case 3
A 26-year-old woman presented with hoarseness of unknown duration and workup revealed a mass involving the left glottis but sparing the anterior commissure. Initial biopsy of the tumor was interpreted as a paraganglioma and the patient subsequently underwent left partial vertical laryngectomy with negative margins. On further review of the morphology and immunohistochemistry, a diagnosis of PEComa was rendered. There was no evidence of a personal or family history of tuberous sclerosis. Eight years after surgery the patient was alive and disease-free. During follow up the patient has only been treated for recurrent upper respiratory tract infections.
Microscopically, the tumor was composed primarily of epithelioid clear cells arranged in nests. Like Cases 1 and 2, there was a zone of subepithelial sparing with an intact overlying surface. The periphery demonstrated separate tumor lobules (“islands”) including involvement of the vocalis muscle but no destructive infiltrative growth was identified. The tumor cells were intimately associated with a uniform delicate vascular stroma (Fig. 3) and focal areas showed a perivascular subendothelial distribution of tumor cells. There was no cytological atypia, mitotic activity, or necrosis. The cells contained abundant cytoplasmic glycogen (PAS positive, diastase sensitive). Immunohistochemical stains demonstrated cytoplasmic positivity for HMB45 and SMA, and were negative for synaptophysin, pancytokeratin, S-100 protein, and vimentin.
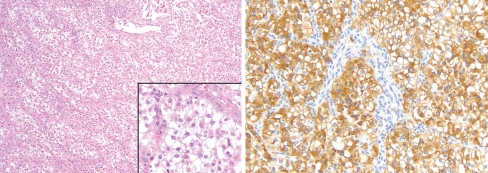
Fig. 3.
The laryngeal PEComa (Case 3) was histologically identical to Case 1, with characteristic polygonal cells and a delicate vascular stroma (inset), but showed foci with the characteristic perivascular distribution of tumor cells as seen in the upper field (left; H&E 100×; inset 400×). The tumor was strongly positive for HMB45 (right; 200×) and patchy SMA staining
Discussion
Herein, we describe three PEComas of the nasal cavity (two cases) and larynx (one case) all of which occurred in females, consistent with the sex predilection of all PEComas, who ranged from 18 to 71 years of age. Other than a mass effect, no other specific signs or symptoms were noted. PEComas are rare in the head and neck [7–17], and those arising in the nasal cavity and larynx are even more unusual [7, 12]. The common morphologic features observed in all three tumors included: large polygonal to epithelioid cells with clear or eosinophilic granular cytoplasm, centrally located nuclei with vesicular chromatin, and occasional nucleoli; a predominantly nested growth pattern associated with a delicate vascular stroma, similar to the vessels seen in clear cell renal cell carcinomas and paragangliomas; and a zone of subepithelial sparing. Except for Case 1, which showed extensive surface ulceration, the other two cases had an intact overlying surface. In Case 2, the vessels were more variable and a subpopulation of tumor cells demonstrated a faint brown cytoplasmic melanin pigment. Only Case 3 had the characteristic perivascular distribution of tumor cells, although only focally present. In addition, no case revealed significant cytological atypia, necrosis, infiltrative growth, vascular invasion, or mitotic activity. Moreover, by immunohistochemistry, all three cases reacted positively with melanocytic markers (HMB45 and/or Melan-A) and myoid markers (SMA or calponin).
The two patients with nasal PEComas were women 18 and 71 years of age, respectively. Not surprisingly, given the strong uniform expression of the melanocytic markers HMB45 and/or Melan-A, a differential of sinonasal melanoma was considered in both cases. Only two previous cases of nasal PEComas have been reported, expressing characteristic histologic and immunophenotypic features diagnostic of this tumor [7, 12]. The first case, reported in 2001 by Banerjee et al. [7], presented as a polyp in the left nasal cavity of a 34-year-old woman who complained of nasal obstruction and occasional epistaxis. There was no personal or family history of tuberous sclerosis. Twelve months after an intranasal polypectomy, the patient was well without evidence of disease. In 2009, Kuroda et al. [12] reported the second case of nasal PEComa in a 79-year-old Japanese woman who presented with a firm polypoidal mass in the nasal cavity. Notably, this was the first case of nasal PEComa expressing TFE3 protein. Follow-up information was not available for this patient, thus the prognosis could not be assessed.
In Case 3, the PEComa was originally misdiagnosed as paraganglioma and only correctly diagnosed as PEComa after subtotal laryngectomy. To our knowledge, this is the first reported case of PEComa of the larynx, although case reports of AML arising in the larynx and the epiglottis have been reported previously by Stodulski et al. [18] and Zhang et al. [19], respectively.
The descriptive term perivascular epithelioid cell (PEC) was proposed by Bonetti et al. [1, 20] to describe a novel cell type demonstrating HMB45 immunoreactivity and the presence of premelanosomes ultrastructurally, and which morphologically resembled the CCST of lung, AML of the kidney and liver, and LAM [21–26]. They hypothesized that the PEC may originate from the walls of blood vessels, given the perivascular association and the fact that there is no known normal cellular counterpart to the PEC. The PEComa family of tumors has grown to include AML (renal and extrarenal variants), LAM, CCST of the lung, clear cell myomelanocytic tumor (CCMMT) of the falciform ligament/ligamentum teres, primary extrapulmonary “sugar” tumor and clear cell tumors of diverse sites [10].
Epidemiologically, this tumor has a distinct female predominance, as observed in our three cases. Although most cases occur in middle aged patients [10], two of our three patients were under the age of 30. The role of sex hormones has been speculated in the pathogenesis of some PEComas since estrogen or progesterone receptors have been found in renal AML and LAM [5]. However, the absence of estrogen and progesterone receptor expression in other PEComas could not confirm this hypothesis [10]. Although there is an association of AML and LAM with tuberous sclerosis complex, this association has been documented in less than 10% of patients with PEComas of soft tissue and gynecologic origin [10].
Histologically, PEComas are characterized by predominantly epithelioid and/or spindled cells. Tumor cell nuclei range from round or ovoid in epithelioid cells to more elongated in the spindle cells. PEComa cells have a characteristic glycogen-rich, clear to lightly eosinophilic granular cytoplasm with vesicular nuclei and inconspicuous nucleoli. The nuclei are generally uniform with occasional cells exhibiting striking degenerative-type nuclear hyperchromasia and pleomorphism. Multinucleated giant cells may also be focally present and variable degrees of melanin pigmentation can be seen (as in Case 2). Mitotic figures are rare, though abundant and atypical mitotic figures can be seen in malignant examples. The cells have a focally nested, trabecular, or sheet-like growth pattern and may be intimately arranged around and within blood vessel walls. The vasculature ranges from a delicate capillary network to hyalinized arterioles and small arteries, best demonstrated in Case 2. At scanning magnification, the lesions may be well circumscribed or infiltrative.
Immunohistochemically, PEComas characteristically coexpress melanocytic and myoid markers [10]. Folpe et al. [10] evaluated 26 PEComas of soft tissue and gynecologic origin, and identified expression of melanocytic markers in all cases, with HMB45 being the most sensitive (92%) followed by Melan-A (72%). SMA was seen in 80% and desmin positivity in 8 (36%). Calponin was not used in this study but one case did express caldesmon. Case 1 was unusual in that there was no expression of SMA, but myoid differentiation was confirmed by immunoreactivity with calponin, another myoid marker; otherwise, this was a histologically typical PEComa with melanocytic differentiation (both HMB45 and Melan-A positive). Calponin has not been very well studied in PEComas but there are reports of calponin positive and SMA negative PEComas [11].
Based on the review by Hornick et al. [6] PEComas (excluding renal AML, LAM, and CCST of lung) most commonly involve the retroperitoneal, visceral, abdominal, and pelvic sites. The gastrointestinal tract and the uterus are the most common extrarenal organs involved with soft tissue and skin being less frequently involved. Case reports and small series have also described PEComas in unusual sites including the vulva, heart, breast, common bile duct, urinary bladder, and a variety of head and neck sites.
Based on our review of the English literature, we identified 13 cases [7–17] of primary head and neck PEComas (excluding examples described as AML). Including our three cases, this number now consists of 16 tumors (Table 1). There is less of a female predilection among this series (F: M = 10:6), compared to non-head and neck PEComas, and patients ranged in age from 7 to 80 years with both a mean and median of 48 years. The nasal cavity and ocular sites accounted for four cases each, representing half of the head and neck sites. The next most frequent sites of occurrence were scalp (three), intraoral (two), and one case each in cervical soft tissue, skull base, and larynx, respectively. Similar to PEComas at other body sites, these tumors tend to behave in an indolent fashion. Adequate follow-up was reported in 14 patients. Two patients (one skull base and one scalp) behaved in an aggressive fashion with distant or loco-regional metastases and one patient (skull base) died of disease-related causes [8, 16]. Therefore, 14% of head and neck PEComas, with available follow-up, behaved aggressively with loco-regional or distant metastases and only 7% died of disease. The patient with cervical lymph node metastasis was alive and disease-free at last follow-up. The remaining twelve patients were all reported as alive and free of disease following surgical resection.
Table 1.
Clinicopathologic findings of previously reported head and neck PEComas
| Author | Gender/age(years) | Size/location | Giant cells | Mitoses/10HPF | Necrosis | Follow-up |
|---|---|---|---|---|---|---|
| Banerjee | Female/39 | 2.0 cm/Nasal cavity | Absent | Very few | Absent | 12 months/ANED |
| Lehman | Female/49 | 5.0 × 3.8 × 3.5 cm/Skull base | Absent | 3 per HPF | Absent | Spine, lung met, DOD,3 months |
| Folpe | Male/80 | 2.0 cm/Scalp | Present | Greater than 10 per 50 HPF | Absent | Lost to follow up |
| Folpe | Female/77 | 2.6 cm/Neck soft tissue | Present | 1 per 50 HPF | Absent | 6 months/ANED |
| Iyengar | Female/9 | 1.2 × 1.0 × 0.8 cm/Orbit | Absent | Very few | Absent | 7 months/ANED |
| Koutlas | Female/46 | 4.0 × 2.0 cm/Hard palate | Absent | Absent | Present | 20 months,/ANED |
| Kuroda | Male/79 | Nasal cavity | Absent | Absent | Absent | Follow up too short |
| Furusato | Female/26 | 2.0 × 1.7 × 1.4 cm/Eyelid | Absent | Very few | Absent | 24 months/ANED |
| Furusato | Male/7 | 1.3 cm/Ciliary body | Absent | Very few | Absent | 24 months/ANED |
| Guthoff | Male/54 | 1.5 × 1.0 × 1.0 cm/Eyelid | Absent | Very few | Absent | 17 months/ANED |
| Calder | Male/76 | 1.6 × 1.3 × 1.0 cm/Scalp | Absent | 2 per 10 HPF | Absent | 5 years,Cervical node met/ANED |
| Ghazali | Female/32 | 2 cm/Cheek | Present | 2 per 10 HPF | Absent | years/ANED |
| Argani | Male/80 | 2 cm/Scalp | Absent | Absent | Absent | NA |
| Bandhlish | Female/18 | 2.9 cm/Left nasal | Absent | Absent | Absent | 26 months/ANED |
| Bandhlish | Female/71 | Nasal cavity | Absent | Absent | Absent | Lost to follow-up |
| Bandhlish | Female/26 | Glottic larynx | Absent | Absent | Absent | 8 years/ANED |
HPF high power field, met metastases, DOD dead of disease, ANED alive with no evidence of disease
The malignant potential of PEComas remains unknown. A vast majority of reported PEComas seem to behave in a benign fashion, although a malignant course with local recurrences and distant metastases has been reported [8, 10, 16, 27, 28]. Predicting the behavior of PEComas based on histology alone is challenging. However, criteria for risk classification of non-AML PEComas based on histopathologic features have recently been proposed [10]. Tumors with two or more of the following atypical features are considered malignant: size >5 cm, infiltrative growth, high nuclear grade, necrosis, mitotic rate ≥1/50 hpf and vascular invasion. Tumors with nuclear pleomorphism or multinucleated giant cells only, or those >5 cm are considered to be of uncertain malignant potential. All other tumors can be considered benign. However, this study was based on tumors of gynecologic origin and soft tissue (including two head and neck tumors), so application to all head and neck sites may not be appropriate.
Although size was known in only one of our cases (Case 1), all seemed to be much smaller than 5 cm based on review of the tumor dimensions on the glass slides (all tumor tissue was submitted in each case). None of our cases demonstrated an infiltrative growth pattern, vascular invasion, high nuclear grade, necrosis or mitotic activity. Twenty-six months (Case 1) and 8 years (Case 3) into follow-up, both patients are alive with no evidence of recurrence and/or metastases; the other patient (Case 2) was lost to follow up. These observations are consistent with the finding reported by Folpe et al. [10] regarding the benign clinical behavior of these tumors. Still, the prognostic features of these tumors are largely unpredictable since occasional cases have demonstrated metastatic potential even though malignant histopathological features of the tumor were not observed [10].
The differential diagnosis for head and neck PEComas is broad and somewhat site dependent. The differential diagnosis may include primary or metastatic melanoma, paraganglioma, clear cell sarcoma (malignant melanoma of soft parts), clear cell carcinoma, clear cell variants of salivary gland carcinomas, alveolar soft part sarcoma, granular cell tumor, clear cell oncocytoma, metastatic renal cell carcinoma and rhabdomyoma. By immunohistochemistry, negative S-100 staining and positive myoid marker expression are useful in differentiating PEComas from melanomas (primary and secondary). It must be noted, however, that some studies have shown that up to 33% of PEComas demonstrate S-100 positivity [10], although most studies indicate positivity rates of about 10%, and characterized by only focal and usually cytoplasmic (not nuclear) staining. Furthermore, these S-100 positive PEComas typically also express myoid markers, a finding not generally seen in true melanocytic lesions [10]. Spindle cell variant of melanoma may show limited SMA expression [29]. Malignant melanomas generally tend to have more significant nuclear pleomorphism and increased mitotic activity which was not seen in any of our cases. In addition, prior history of melanoma or an in situ component would support this diagnosis.
Few PEComas (13%) of the soft tissue and gynecologic origin may show focal cytokeratin expression [10] but the lack of diffuse cytokeratin expression combined with the coexpression of melanocytic and myoid markers should help to exclude the differential of clear cell carcinoma, clear cell oncocytoma, clear cell variants of salivary gland carcinoma, and metastatic renal cell carcinoma. A “Zellballen” architecture and a vascular stroma may resemble a paraganglioma, as seen in Case 3 which was initially misdiagnosed as such on incisional biopsy. But the lack of staining for chromogranin, synaptophysin, and S-100 (sustentacular distribution), as well as the expression of melanocytic and myoid markers makes this distinction straightforward. Alveolar soft part sarcoma (especially the solid type) may resemble PEComas and often show TFE3 nuclear expression. Although they may show positivity for muscle markers, they will lack expression of melanocytic markers. In addition, characteristic cytoplasmic PAS positive (diastase resistant) crystals may be identified and a recurring t(X;17) translocation resulting in a ASPL-TFE3 fusion protein can be demonstrated. However, use of TFE3 immunostaining to separate these two neoplasms may not be helpful since nearly one-third of PEComas also express nuclear TFE3 [17]. Moreover, this same study showed that five of 29 (17%) PEComas also had TFE3 gene rearrangements (four) or TFE3 amplification (one), thus limiting the utility of molecular testing in this differential diagnosis. Granular cell tumors have more abundant and granular cytoplasm coupled with characteristic positivity for S-100 and negativity for muscle markers thereby excluding this diagnosis. Clear cell sarcoma (also known as malignant melanoma of soft parts) has a nested growth pattern with clear cells and stains positively with melanocytic markers, but the cells do not express myoid markers, which can help distinguish them from PEComas. However, it may be difficult to distinguish them from the rare PEComas that are SMA negative and S-100 positive. As reported by Folpe et al. [10] not all PEComas express smooth muscle markers: 80% of PEComas of soft tissue were SMA positive in their study. Therefore, the most specific test to distinguish these two entities in challenging cases is cytogenetic and/or molecular genetic confirmation of the characteristic t(12;22) seen in clear cell sarcoma that results in EWS-ATF1 fusion protein [10]. Finally, adult rhabdomyoma can be excluded based on strong desmin reactivity, lack of melanocytic markers and characteristic large cells with, at least occasional, foci of cytoplasmic striation.
The pathogenesis of PEComas is still not completely understood and is being explored owing to their rarity. However inactivation of TSC1 and/or TSC2 genes, [30, 31] with subsequent activation of mammalian target of rapamycin (mTOR) pathway has been associated with the pathogenesis of both syndromic and sporadic PEComas [32]. Recent studies highlighting genetic events, including chromosome losses (1p, 17p, 18p, 19p) and gains (2q, 3q, 5q, 12q and X) have been indicated in the development of some PEComas [32].
The optimal treatment for this group of tumors is not well established, but surgical resection with adequate margins seems to be the gold standard. The role for adjuvant radiation is not fully appreciated but may be indicated for those tumors that qualify as histologically malignant or of uncertain malignant potential. Given the role of mTOR pathway activation, inhibitors of this pathway (such as sirolimus) may prove helpful in treating malignant PEComas, as well as unresectable, histologically benign PEComas. In our cases, surgical excision with negative margins resulted in adequate treatment in the two cases with follow-up.
Conclusion
In this study we report three PEComas arising in the larynx and the nasal cavity, which are rare sites of involvement. All with follow-up had a clinically benign course. The importance of recognizing this entity will ensure its consideration in the differential diagnosis of tumors of the head and neck with a similar morphology. The histogenesis of PEComas still remains elusive and additional cases with prolonged follow up remain important to accurately determine the behavior of these distinctive tumors. Complete surgical excision still remains the treatment of choice for histologically benign PEComas.
References
- 1.Bonetti F, Pea M, Martignoni G, et al. PEC and sugar. Am J Surg Pathol. 1992;16:307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Vang R, Kempson RL. Perivascular epithelioid cell tumor (‘PEComa’) of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002;26:1–13. doi: 10.1097/00000478-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Tazelaar HD, Batts KP, Srigley JR. Primary extrapulmonary sugar tumor (PEST): a report of four cases. Mod Pathol. 2001;14:615–622. doi: 10.1038/modpathol.3880360. [DOI] [PubMed] [Google Scholar]
- 4.Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg Pathol. 2000;24:1239–1246. doi: 10.1097/00000478-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bonetti F, Pen M, Martignoni G, et al. The perivascular epithelioid cell and related lesions. Adv Anatom Pathol. 1997;4:343–358. doi: 10.1097/00125480-199711000-00001. [DOI] [Google Scholar]
- 6.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee SS, Eyden B, Trenholm PW, et al. Monotypic angiomyolipoma of the nasal cavity: a heretofore undescribed occurrence. Int J Surg Pathol. 2001;9:309–315. doi: 10.1177/106689690100900410. [DOI] [PubMed] [Google Scholar]
- 8.Lehman NL. Malignant PEComa of the skull base. Am J Surg Pathol. 2004;28:1230–1232. doi: 10.1097/01.pas.0000128668.34934.81. [DOI] [PubMed] [Google Scholar]
- 9.Iyengar P, Deangelis DD, Greenberg M, et al. Perivascular epithelioid cell tumor of the orbit: a case report and review of the literature. Pediatr Dev Pathol. 2005;8:98–104. doi: 10.1007/s10024-004-5055-0. [DOI] [PubMed] [Google Scholar]
- 10.Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 11.Koutlas IG, Pambuccian SE, Jessurun J, et al. Perivascular epithelioid cell tumor of the oral mucosa. Arch Pathol Lab Med. 2005;129:690–693. doi: 10.5858/2005-129-0690-PECTOT. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda N, Goda M, Kazakov DV, et al. Perivascular epithelioid cell tumor of the nasal cavity with TFE3 expression. Pathol Int. 2009;59:769–770. doi: 10.1111/j.1440-1827.2009.02398.x. [DOI] [PubMed] [Google Scholar]
- 13.Furusato E, Cameron JD, Newsom RW, et al. Ocular perivascular epithelioid cell tumor: report of 2 cases with distinct clinical presentations. Hum Pathol. 2010;41:768–772. doi: 10.1016/j.humpath.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Guthoff R, Guthoff T, Mueller-Hermelink HK, Sold-Darseff J, Geissinger E. Perivascular epithelioid cell tumor of the orbit. Arch Ophthalmol. 2008;126:1009–1011. doi: 10.1001/archopht.126.7.1009. [DOI] [PubMed] [Google Scholar]
- 15.Ghazali N, Cascarini L, Norris P, et al. Perivascular epithelioid cell tumor (PEComa) of the cheek. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e26–e31. doi: 10.1016/j.tripleo.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Calder KB, Schlauder S, Morgan MB. Malignant perivascular epithelioid cell tumor (‘PEComa’): a case report and literature review of cutaneous/subcutaneous presentations. J Cutan Pathol. 2008;35:499–503. doi: 10.1111/j.1600-0560.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 17.Argani P, Aulmann S, Illei PB, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34:1395–1406. doi: 10.1097/PAS.0b013e3181f17ac0. [DOI] [PubMed] [Google Scholar]
- 18.Stodulski D, Stankiewicz C, Rzepko R, et al. Angiomyolipoma of the larynx: case report. Eur Arch Otorhinolaryngol. 2007;264:89–92. doi: 10.1007/s00405-006-0186-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LH, Sun RC, Chen Y. [A case report of epiglottic angiomyolipoma. ]. Zhonghua Bing Li Xue Za Zhi. 2005;34:645. [PubMed] [Google Scholar]
- 20.Bonetti F, Pea M, Martignoni G, et al. Cellular heterogeneity in lymphangiomyomatosis of the lung. Hum Pathol. 1991;22:727–728. doi: 10.1016/0046-8177(91)90298-4. [DOI] [PubMed] [Google Scholar]
- 21.Pea M, Bonetti F, Zamboni G, et al. Clear cell tumor and angiomyolipoma. Am J Surg Pathol. 1991;15:199–202. doi: 10.1097/00000478-199102000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Pea M, Bonetti F, Zamboni G, et al. Melanocyte-marker-HMB-45 is regularly expressed in angiomyolipoma of the kidney. Pathology. 1991;23:185–188. doi: 10.3109/00313029109063563. [DOI] [PubMed] [Google Scholar]
- 23.Weeks DA, Malott RL, Arnesen M, et al. Hepatic angiomyolipoma with striated granules and positivity with melanoma–specific antibody (HMB-45): a report of two cases. Ultrastruct Pathol. 1991;15:563–571. doi: 10.3109/01913129109016264. [DOI] [PubMed] [Google Scholar]
- 24.Bonetti F, Chiodera PL, Pea M, et al. Transbronchial biopsy in lymphangiomyomatosis of the lung. HMB45 for diagnosis. Am J Surg Pathol. 1993;17:1092–1102. doi: 10.1097/00000478-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Gaffey MJ, Mills SE, Zarbo RJ, et al. Clear cell tumor of the lung. Immunohistochemical and ultrastructural evidence of melanogenesis. Am J Surg Pathol. 1991;15:644–653. doi: 10.1097/00000478-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Gal AA, Koss MN, Hochholzer L, et al. An immunohistochemical study of benign clear cell (‘sugar’) tumor of the lung. Arch Pathol Lab Med. 1991;115:1034–1038. [PubMed] [Google Scholar]
- 27.Dimmler A, Seitz G, Hohenberger W, et al. Late pulmonary metastasis in uterine PEComa. J Clin Pathol. 2003;56:627–628. doi: 10.1136/jcp.56.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mai KT, Belanger EC. Perivascular epithelioid cell tumour (PEComa) of the soft tissue. Pathology. 2006;38:415–420. doi: 10.1080/00313020600922504. [DOI] [PubMed] [Google Scholar]
- 29.Longacre TA, Egbert BM, Rouse RV. Desmoplastic and spindle-cell malignant melanoma. An immunohistochemical study. Am J Surg Pathol. 1996;20:1489–1500. doi: 10.1097/00000478-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Henske EP, Scheithauer BW, Short MP, et al. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- 31.Pan CC, Chung MY, Ng KF, et al. Constant allelic alteration on chromosome 16p (TSC2 gene) in perivascular epithelioid cell tumour (PEComa): genetic evidence for the relationship of PEComa with angiomyolipoma. J Pathol. 2008;214:387–393. doi: 10.1002/path.2289. [DOI] [PubMed] [Google Scholar]
- 32.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1–15. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]