Abstract
Background
Localized Amyloidosis (AL) may rarely involve oral mucosa. This is the first known reported case describing the development of tongue AL in a 30-year-old patient with Neurofibromatosis (NF) type-2.
Case
A female patient presented with a painless, well-circumscribed nodule of the tongue. Her medical history included NF type-2 with chromosome-22 abnormal karyotype (mosaicism), multiple intracranial and spinal meningiomas/schwannomas and unilateral blindness/deafness. The biopsy of the excised lesion of the tongue revealed subepithelial accumulation of an amorphous, nodular, fibrillar material positive for Congo red. Blood examination showed increased Thyroxine-T4 due to thyroid multinodular colloid goiter, but excluded any other hematological/immunological disorder or organ dysfunction. No recurrence was observed after a six-month follow-up.
Conclusion
This case highlights the possibility of oral manifestations as the only sign of AL and reveals the unexpected co-existence of AL and NF 2, for the first time.
Keywords: Oral amyloidosis, Neurofibromatosis type II
Introduction
Amyloidosis results from the continuous, excessive production and accumulation of an extracellular, insoluble, fibrillar proteinaceous material, called amyloid [1]. This disease can be acquired or hereditary, limited in a tissue/organ with benign prognosis (localized form) or may affect multiple tissues/organs (kidneys, heart, liver, gastrointestinal tract, bladder etc.) leading to significant morbidity and at times mortality (systemic form) [2–4].
The main subtypes of systemic amyloidosis are AL (amyloid light chain-AL) and AA (amyloid A-AA). AL as the most common form can present with no underlying cause or can be related to plasma cell dyscrasias/multiple myeloma form. AA (amyloid A-AA) is the secondary form of amyloidosis associated with chronic infectious or inflammatory diseases such as rheumatoid arthritis, connective tissue diseases, Crohns or other inflammatory bowel disease [5, 6]. There is also the β2 microglobulin amyloidosis in chronic renal failure/hemodialysis patients and the autosomal-dominant hereditary/familial form including Mediterranean fever (AA-type) and familial amyloidosis accompanied by polyneuropathy with transthyretin/light chain-related amyloid (ATTR) depositions [4, 7, 8].
The secondary form of amyloidosis can affect the head and neck area including orbit, sinuses, salivary glands, pharynx/larynx and oral cavity in 10–40% of the cases. Potential sites of oral lesions are buccal, palatal and gingival mucosa and tongue [6, 8–12].
The aim of this article is to describe, for the first time, a case of localized tongue amyloidosis in a patient with Neurofibromatosis type II (NF2) a genetic disorder related to chromosome 22 alterations, characterized by bilateral vestibular schwannomas, meningiomas, gliomas, and schwannomas of cranial/spinal nerves with related consequences [13].
Case Report
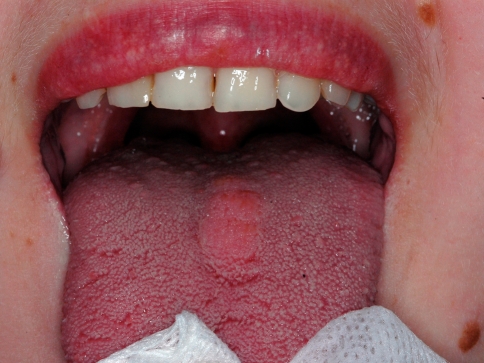
A 30-year-old female with mild mental retardation presented to the Department of Oral Medicine/Pathology, School of Dentistry of Aristotle University of Thessaloniki, Greece with a painless, nodular mass on the midline dorsal tongue, covered by normal mucosa (Fig. 1).
Fig. 1.
Nodular lesion on the dorsum surface of the tongue, covered by normal mucosa
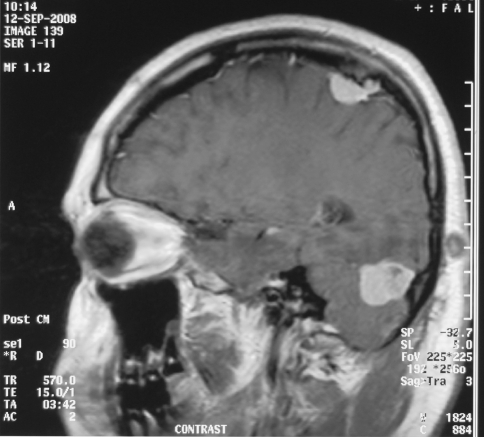
The onset/duration of the tongue lesion was unclear. Medical history reported no weight loss, fever, chest pain, nausea, vomiting, or arthritis, but 10 years previously a diagnosis of NF2 with characteristic karyotype including the existence of ring-type or the absence of chromosome 22 (mosaicism), multiple cranial (cerebellum/parietal) (Fig. 2) and spinal (thoracic) meningiomas and schwannomas (orbital/inner ear), as confirmed by imaging and microscopy of the excised lesions. These lesions caused mild imbalance, right eye blindness and left ear hearing loss.
Fig. 2.
Multiple cranial meningiomas and schwannoma of the ear
Laboratory tests revealed elevated thyroxine T4 which was due to a thyroid multinodular colloid goiter. Serum and urine protein electrophoresis did not reveal any monoclonal immunoglobulin proteins. Furthermore, renal, heart, liver and spleen morphology and function were normal based on laboratory tests and imaging analysis.
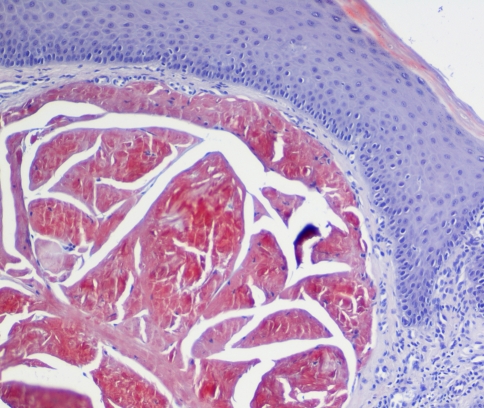
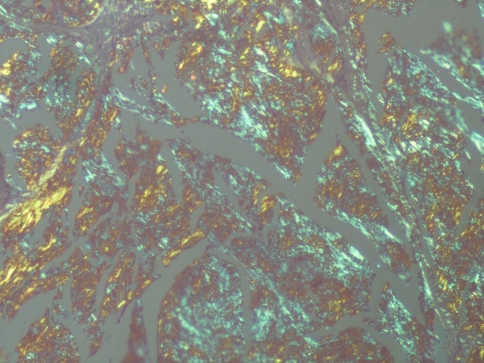
The histological examination of the excised tongue lesion showed a subepithelial, multinodular amorphous, fibrillar accumulation. The amorphous material had positive staining for Congo-Red, exhibiting a reddish color under light microscopy (Fig. 3) and apple green birefringence under polarized light (Fig. 4). Correlating the pathologic, laboratory and imaging findings with the medical history, a diagnosis of a localized form of amyloidosis without systemic involvement was established. In a 6-month follow-up no recurrence or systemic complication was detected but afterwards the patient was lost to follow up.
Fig. 3.
Fibrillar depositions positive for Congo-Red stain as observed by light microscopy (magnification ×100)
Fig. 4.
The amyloid depositions presented as apple green birefringence, under polarized light (magnification ×100)
Discussion
In contrast to Neurofibromatosis type 1 (NF1), a genetic disorder, with gene mutations in chromosomes 17 and multiple cutaneous and CNS neurofibromas and pigmentation, NF2 is a dominantly inherited, tumor prone disorder with pathogenic mutations or mosaicism in the NF2 gene on chromosome 22 characterized by the development of multiple schwannomas, meningiomas, ependymomas, and only infrequently, neurofibromas [13]. Its prevalence is estimated approximately at 1:60,000 [13] or less (1:87,410) [14]. The gold standard for diagnosis is the development of bilateral or unilateral vestibular schwannomas accompanied by hearing loss, tinnitus, and/or imbalance. Other important tumor features are schwannomas of cranial, spinal and peripheral nerves, intracranial and intraspinal meningiomas, ependymomas and gliomas [15, 16].
In our case, the laboratory, imaging and clinical examination revealed a single, localized lesion in the middle of the tongue dorsal surface without any signs of systemic amyloidosis. In fact, based on the patient’s medical history of Neurofibromatosis type II (NF2), the nodular lesion was clinically suspicious for a “neurogenic” tumor. Cases of neurofibromas have been described in oral mucosa in NF-1 [17] and perineuriomas in patients with NF2-gene abnormalities [18], but so far nothing is known of oral manifestations as part of NF2 disorder, except for a case of a nodular lesion of the tongue but without histological confirmation, as described by Barsley and Cottone [19] in 1992.
As aforementioned, our patient’s genetic analysis revealed ring-shape chromosome 22 or even its loss (mosaicism) and according to Ruggieri and Huson [20] this phenomenon may explain the development of ipsilateral (left ear) but not bilateral vestibular schwannomas. Noteworthy, imaging and/or histological investigation of the excised lesion revealed multiple intracranial meningiomas, including an optic nerve meningioma (right) leading to unilateral blindness as well as multiple meningiomas in the thoracic spine. Instead, our patient had not cutaneous nodules resembling neurofibromas of NF-1.
Not only neurofibroma or schwannoma, but also other entities such as fibroma, granular cell tumor, lipoma, leiomyoma, thyroglossal duct cyst, salivary gland neoplasm and median rhomboid glossitis were also included in the clinical differential diagnosis. On microscopic examination of the excised lesion, detection of eosinophilic amorphous material positive for Congo-Red in association with the clinical and laboratory analysis confirmed the diagnosis of localized amyloidosis without systemic involvement.
Amyloidosis represents a rare heterogenous group of conditions characterized by extracellular proteinaceous depositions of amyloid which may cause organ abnormalities [3, 8]. The mechanism, of amyloid formation, includes amyloid (pro)precursor genes activation, under the influence of cytokines, clonal plasma cells, abnormal proteolysis and the production of several precursor pools of different amyloidogenic molecules, which in turn form fibrils of amyloid after their enrichment with proteoglycans, glycosaminoglycans, inorganic ions and serum amyloid P [3, 21, 22].
The incidence of amyloidosis is approximately 5-12 patients per million per year [23, 24], with a peak age in 50 years and male to female ratio 2:1, without racial bias. Disease severity ranges from asymptomatic to severe, life-threatening [4, 7, 25]. Beyond the initial symptoms like fatigue and weight loss, organs such as heart [26], kidney [27], gastrointestinal, liver, spleen [28] and nervous system (central and peripheral) [29] may involved with severe consequences.
Prognosis depends on the severity of the disease at the time of diagnosis and the rate of amyloid accumulation. Median survival, after diagnosis of AL amyloidosis is 1–2 years, whereas 10-year survival rate is seen only in less than 5% of patients. The poor prognosis is associated with cardiac and liver involvement, neuropathy, or lack of control of any possible underlying plasma-cell disease (i.e. multiple myeloma). In contrast, the prognosis of localized amyloidosis is much better, but as in our case a careful follow-up is needed for the possible demonstration of a latent hematological disorder or organ involvement, in the future [4].
Oral manifestations of amyloidosis are quite uncommon, and may be presented early in the course of the disease as mucosal papules, nodules or ulcers, macroglossia, petechiae/ecchymoses or even hemorrhagic bullae [12]. Although the tongue is the most common oral site [6, 10, 30–32], cases of amyloid deposition in the palate [11, 33, 34], maxillary vestibule [35], gingival [9] and floor of the mouth [36] have been mentioned as well. Rarely, minor or major salivary glands infiltration and related hypofuction-xerostomia can be seen [37–39]. Noteworthy, oral involvement is extremely rare as the localized solitary manifestation of amyloidosis, but when present the tongue is primarily affected [6, 8–12].
In summary, this case report describes the existence of localized amyloidosis in a patient with NF-2. Although a familial “neuro”-related type of amyloidosis with peripheral neuropathy caused by ATTR amyloid accumulations has been described, so far nothing is known of an association between Amyloidosis and Neurofibromatosis. Thus, the synchronous presence of these two distinctive pathological entities in our patient at this time suggests a random co-existence, rather than a direct relationship.
References
- 1.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–586. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 2.Hierschfield GM. Amyloidosis: a clinic-pathophysiological synopsis. Semin Cell Develop Biol. 2004;15:39–44. doi: 10.1016/j.semcdb.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Westermark P, Benson MD, Buxbaum JN, et al. A primer of amyloid nomeclature. Amyloid. 2007;14:179–183. doi: 10.1080/13506120701460923. [DOI] [PubMed] [Google Scholar]
- 4.Petersson T, Konttinen YT. Amyloidosis-recent developments. Semin Arthritis Rheum. 2010;39(5):356–358. doi: 10.1016/j.semarthrit.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Merlini G, Westermark P. The systemic amyloidoses: clearer understanding of the molecular mechanisms offers hope for more effective therapies. J Intern Med. 2004;255(2):159–178. doi: 10.1046/j.1365-2796.2003.01262.x. [DOI] [PubMed] [Google Scholar]
- 6.Penner CR, Muller S. Head and neck amyloidosis: a clinicopathologic study of 15 cases. Oral Oncol. 2006;42(4):421–429. doi: 10.1016/j.oraloncology.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Kasper DS, Braunwald E, Fauci AS, Houser SL, Longo DL, Jameson JL. Harrison’s principles of internal medicine. 6th ed. Mac Graw-Hill, Medical Publishing Division 2005;2024.
- 8.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 3rd ed. Saunders Elsevier 2009;822.
- 9.Stoopler ET, Sollecito TP, Chen SY. Amyloid deposition in the oral cavity: a retrospective study and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(6):674–680. doi: 10.1067/moe.2003.136. [DOI] [PubMed] [Google Scholar]
- 10.Serdar A, Basak D, Sercan G, et al. Solitary amyloid tumor of the tongue base. Int J Otolaryngol. 2009;2009:515068-71. [DOI] [PMC free article] [PubMed]
- 11.Henley E, Houghton N, Bucknall R, et al. Localized amyloidosis of the palate. Clin Exp Dermatol. 2008;33(1):100–101. doi: 10.1111/j.1365-2230.2007.02528.x. [DOI] [PubMed] [Google Scholar]
- 12.Laskaris G. Pocket atlas of oral diseases. 2nd ed. Georg Thieme Verlag 2006;290.
- 13.Antinheimo J, Sankila R, Carpen O, et al. Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology. 2000;54(1):71–76. doi: 10.1212/wnl.54.1.71. [DOI] [PubMed] [Google Scholar]
- 14.Mautner VF, Lindenau M, Baser ME, et al. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurg. 1996;38:880–885. doi: 10.1097/00006123-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kanter WR, Eldridge R, Fabricant A, et al. Central neurofibromatosis with bilateral with bilateral acoustic neuroma. Genetic clinical and biochemical distinctions from peripheral neurofibromatosis. Neurology. 1980;30:851–859. doi: 10.1212/wnl.30.8.851. [DOI] [PubMed] [Google Scholar]
- 16.Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433–1443. doi: 10.1212/wnl.56.11.1433. [DOI] [PubMed] [Google Scholar]
- 17.Bongiorno MR, Pistone G, Aricò M. Manifestations of the tongue in neurofibromatosis type 1. Oral Dis. 2006;12(2):125–129. doi: 10.1111/j.1601-0825.2005.01168.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang J, Khang SK, Choi J, et al. Intraneural perineurioma in the tongue. Korean J Pathol. 2007;41:51–54. [Google Scholar]
- 19.Barsley RE, Cottone JA. Oral manifestations of neurofibromatosis types I and II. Oral Surg Oral Med Oral Pathol. 1992;73:376–382. doi: 10.1016/0030-4220(92)90139-H. [DOI] [PubMed] [Google Scholar]
- 20.Mautner VF, Lindenau M, Baser ME, Kluwe L, Gottschalk J. Skin abnormalities in neurofibromatosis 2. Arch Dermatol. 1997;133(12):1539–1543. doi: 10.1001/archderm.133.12.1539. [DOI] [PubMed] [Google Scholar]
- 21.Khan MF, Falk RH. Amyloidosis. Postgrad Med J. 2001;77(913):686–693. doi: 10.1136/pmj.77.913.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steciuk A, Dompmartin A, Troussard X, et al. Cutaneous amyloidosis and possible association with systemic amyloidosis. Int J Dermatol. 2002;41(3):127–134. doi: 10.1046/j.1365-4362.2002.01411.x. [DOI] [PubMed] [Google Scholar]
- 23.Waal RI, Scheur MR, Huijgens PC, et al. Amyloidosis of the tongue as a paraneoplastic marker of plasma cell dyscrasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(4):444–447. doi: 10.1067/moe.2002.126342. [DOI] [PubMed] [Google Scholar]
- 24.Sanchorawala V, Blanchard E, Seldin DC, et al. AL amyloidosis associated with B-cell lymphoproliferative disorders: frequency and treatment outcomes. Am J Hematol. 2006;81(9):692–695. doi: 10.1002/ajh.20635. [DOI] [PubMed] [Google Scholar]
- 25.Falk RH, Skinner M. The systemic amyloidoses: an overview. Adv Intern Med. 2000;45:107–137. [PubMed] [Google Scholar]
- 26.Rivera RJ, Vicenty S. Cardiac manifestations of amyloid disease. Bol Asoc Med P R. 2008;100(4):60–70. [PubMed] [Google Scholar]
- 27.Nishi S, Alchi B, Imai N. New advances in renal amyloidosis. Clin Exp Nephrol. 2008;12(2):93–101. doi: 10.1007/s10157-007-0008-3. [DOI] [PubMed] [Google Scholar]
- 28.Petre S, Shah IA, Gilani N. Review article: gastrointestinal amyloidosis—clinical features, diagnosis and therapy. Aliment Pharmacol Ther. 2008;27(11):1006–1016. doi: 10.1111/j.1365-2036.2008.03682.x. [DOI] [PubMed] [Google Scholar]
- 29.Simmons Z, Specht CS. The neuromuscular manifestations of amyloidosis. J Clin Neuromuscul Dis. 2010;11(3):145–157. doi: 10.1097/CND.0b013e3181d05994. [DOI] [PubMed] [Google Scholar]
- 30.Kerner MM, Wang MB, Angier G, et al. Amyloidosis of the head and neck. A clinicopathologic study of the UCLA experience, 1955–1991. Arch Otolaryngol Head Neck Surg. 1995;121(7):778–782. doi: 10.1001/archotol.1995.01890070064014. [DOI] [PubMed] [Google Scholar]
- 31.Angiero F, Seramondi R, Magistro S, et al. Amyloid deposition in the tongue: clinical and histopathological profile. Anticancer Res. 2010;30(7):3009–3014. [PubMed] [Google Scholar]
- 32.Fahrner KS, Black CC, Gosselin BJ. Localized amyloidosis of the tongue: a review. Am J Otolaryngol. 2004;25(3):186–189. doi: 10.1016/j.amjoto.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Pentenero M, Davico Bonino L. Localized oral amyloidosis of the palate. Amyloid. 2006;13(1):42–46. doi: 10.1080/13506120500537343. [DOI] [PubMed] [Google Scholar]
- 34.Aono J, Yamagata K, Yoshida H. Local amyloidosis in the hard palate: a case report. Oral Maxillofac Surg. 2009;13(2):119–122. doi: 10.1007/s10006-009-0158-4. [DOI] [PubMed] [Google Scholar]
- 35.Koloktronis A, Chatzigiannis I, Paloukidou N. Oral involvement in a case of AA amyloidosis. Oral Dis. 2003;9(5):269–272. doi: 10.1034/j.1601-0825.2003.03940.x. [DOI] [PubMed] [Google Scholar]
- 36.Kokong DD, Ibekwe TS, Okolo CA, et al. Amyloid angiopathy of the floor of the mouth: a case report and review of the literature. J Med Case Reports. 2007;1:117–120. doi: 10.1186/1752-1947-1-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myssiorek D, Alvi A, Bhuiya T. Primary salivary gland amyloidosis causing sicca syndrome. Ann Otol Rhinol Laryngol. 1992;101(6):487–490. doi: 10.1177/000348949210100607. [DOI] [PubMed] [Google Scholar]
- 38.Caporali R, Bonacci E, Epis O, et al. Safety and usefulness of minor salivary gland biopsy: retrospective analysis of 502 procedures performed at a single center. Arthritis Rheum. 2008;59(5):714–720. doi: 10.1002/art.23579. [DOI] [PubMed] [Google Scholar]
- 39.Finkel KJ, Kolansky DM, Giorgadze T, et al. Amyloid infiltration of the salivary glands in the setting of primary systemic amyloidosis without multiple myeloma. Otolaryngol Head Neck Surg. 2006;135(3):471–472. doi: 10.1016/j.otohns.2005.03.013. [DOI] [PubMed] [Google Scholar]