Abstract
Background
Suspected or complicated intra-abdominal infections are common in young infants and lead to significant morbidity and mortality. Meropenem is a broad-spectrum antimicrobial agent with excellent activity against pathogens associated with intra-abdominal infections in this population. The purpose of this study was to determine the pharmacokinetics (PK) of meropenem in young infants as a basis for optimizing dosing and minimizing adverse events.
Methods
Premature and term infants <91 days of age hospitalized in 24 neonatal intensive care units were studied. Limited PK sampling was performed following single and multiple doses of meropenem 20–30 mg/kg of body weight every 8–12 hours based on postnatal and gestational age at birth. Population and individual patient (Bayesian) PK parameters were estimated using NONMEM®.
Results
Two hundred infants were enrolled and received study drug. One hundred eighty-eight infants with 780 plasma meropenem concentrations were analyzed. Their median (range) gestational age at birth and postnatal age at PK evaluation were 28 (23–40) weeks and 21 (1–92) days, respectively. In the final PK model, meropenem clearance (CL) was strongly associated with serum creatinine (SCR) and postmenstrual age (PMA) (CL [L/h/kg] = 0.12*[(0.5/SCR)**0.27]*[(PMA/32.7)**1.46]). Meropenem concentrations remained >4 μg/mL for 50% of the dose interval and >2 μg/mL for 75% of the dose interval in 96% and 92% of patients, respectively. The estimated penetration of meropenem into the cerebrospinal fluid was 70% (5–148).
Conclusions
Meropenem dosing strategies based on postnatal and gestational age achieved therapeutic drug exposure in almost all infants.
Keywords: enterocolitis, necrotizing, infant, premature, cerebrospinal fluid
INTRODUCTION
Intra-abdominal infections in young (<91 days) infants are common and often fatal. Approximately 20% of infants with necrotizing enterocolitis die, and survivors suffer from severe neurodevelopmental impairment (1–3). Most infants with suspected or confirmed intra-abdominal infections are treated with empirical antimicrobial therapy. Given the polymicrobial nature of these infections, broad-spectrum or combination antimicrobial agents are most often prescribed.
Meropenem, a carbapenem antibiotic, possesses 1 of the broadest spectra of antimicrobial activity available, including most of the bacterial pathogens responsible for producing serious, life-threatening infections occurring in young infants. Meropenem is stable against hydrolysis by most extended-spectrum beta-lactamases and AmpC chromosomal beta-lactamases, increasing the drug's activity against many antibiotic-resistant Gram-positive (e.g., penicillin-resistant S. pneumoniae) and Gram-negative (e.g., P. aeruginosa) bacteria.
United States Food and Drug Administration (FDA)-approved product labeling for meropenem includes recommendations for pediatric patients ≥3 months of age as monotherapy for bacterial meningitis and complicated intra-abdominal infections. There is substantial off-label use of meropenem in infants <3 months of age. This off-label use occurs despite the lack of adequate data regarding meropenem pharmacokinetics (PK) in this population, age-specific dosing guidelines, tolerability, and safety in this vulnerable group. Meropenem dosing in young infants is often based on PK data extrapolated from adults or older children. In adults, meropenem is primarily cleared through the kidneys; approximately 70% of the intravenous dose is recovered unchanged in the urine (4). In patients with normal renal function, the elimination half-life of meropenem is approximately 1 hour (4), and plasma protein binding is approximately 2%. In contrast, the limited meropenem PK data currently available in young infants suggest that drug disposition differs substantially from that in adults (5, 6). This prompted the FDA, under the provisions of the Best Pharmaceuticals for Children Act (BPCA; Section 505A of the Federal Food, Drug, and Cosmetic Act), to issue a formal Written Request as the mechanism to require needed PK and safety/tolerability studies of meropenem in neonates and young infants. The results from a multicenter study of meropenem PK in young infants and neonates are reported herein.
METHODS
Study Design
This was a 24-center, prospective, multi-dose PK study of meropenem in infants. Inclusion criteria included: age <91 days; likely survival >48 hours after enrollment; sufficient intravascular access (either peripheral or central) to receive study drug; and suspected or confirmed intra-abdominal infection, possible necrotizing enterocolitis, or otherwise receiving meropenem per local standard of care. Participants were excluded for the following conditions: renal dysfunction evidenced by urine output <0.5 mL/hr/kg or serum creatinine >1.7 mg/dL; history of clinical seizures or electroencephalogram-confirmed seizures; and concomitant treatment with another carbapenem (ertapenem or imipenem) at the time of informed consent. The Institutional Review Board at each participating center approved this study. The trial was registered in clinicaltrials.gov (NCT00621192). All study participants were enrolled after obtaining written permission (informed consent) from the parent or legal guardian.
Administration of Study Drug and Procedures
Patients were stratified into 1 of 4 categories based on gestational age at birth (GA, <32 weeks and ≥32 weeks) and postnatal age (PNA) at time of study drug administration (<14 days and ≥14 days) to receive multiple doses of intravenous meropenem according to the following dosing scheme: Group 1 (GA <32 weeks, PNA <14 days) 20 mg/kg body weight every 12 hours; Groups 2 (GA <32 weeks, PNA ≥14 days) and 3 (GA ≥32 weeks, PNA <14 days) 20 mg/kg body weight every 8 hours; and Group 4 (GA ≥32 weeks, PNA ≥2 weeks) 30 mg/kg body weight every 8 hours. The rationale for dose selection was based on simulation studies performed from prior small meropenem PK studies in older infants (5, 6). The simulation studies explored the likelihood of achieving a predefined therapeutic target by gestational and postnatal age. The pharmacodynamic (PD) target exposure level for each infant was defined as plasma meropenem concentrations above >4 μg/mL for 50% of the dose interval and >2 μg/mL for at least 75% of the dosing interval. Dose escalation was planned if interim PK analyses showed that <90% of infants enrolled in a cohort achieved the PD target. Meropenem was administered via constant-rate infusion over 30 minutes to each patient for at least 3 days and no more than 21 days. The protocol recommended that aminoglycoside therapy be administered concomitantly with meropenem, and administration of other antimicrobial agents was allowed per local standard of care. All antimicrobial agents and medications received during the 72 hours prior to study drug administration and for 72 hours following last dose of study drug were documented. Appropriate blood, urine, or cerebrospinal fluid (CSF) cultures were obtained as part of standard of care.
Pharmacokinetic Sampling and Assay
The PK sampling schedule around the first study dose was divided in 2 groups according to infant birthday. The first group included infants with birthdays on an odd date (e.g., 1st, 3rd, 5th) and who had blood (0.1 mL) collected any time in the 24 hours prior to the first dose and 0.5–1, 3–4, and 7–8 (Q8 hour dosing) or 10–12 (Q12 hour dosing) hours after completion of the first dose. The second group included infants with birthdays on an even date (e.g., 2nd, 4th, 6th) and who were sampled any time in the 24 hours prior to the first dose and 1–2, 4–6, and 7–8 (Q8 hour dosing) or 10–12 (Q12 hour dosing) hours after completion of the first dose. PK sampling at steady state (after the fourth dose) was performed any time in the 3 hours prior to the dose and 0.25–2.5 and 4–12 hours after completion of the dose. A minimum of 12 infants with at least 3 PK samples around the first dose and 2 PK samples at steady state were enrolled in each cohort (48 infants total), and 152 additional infants were enrolled for ongoing safety evaluation and further collection of steady-state PK data. For the CSF PK analysis, 50–200 μL of additional CSF were collected from infants when CSF was obtained per standard of care while an infant was receiving meropenem.
Plasma and CSF samples were analyzed using a validated liquid chromatography method with tandem mass spectrometric detection (LC/MS/MS). Meropenem and the internal standard (IS) (20 μg/mL, pheniramine) were extracted from the samples by protein precipitation. After the dilution of the supernatant with 0.01 mM ammonium acetate in water, the mixed solution was directly injected and analyzed using LC/MS/MS. The peaks were identified on a Varian 1200L Liquid Chromatograph Mass Spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with electrospray operating in positive ion mode. The transition masses monitored were 384–141 for meropenem and 241–169 for pheniramine (IS). Peak area ratios (meropenem/IS) were fitted to a weighted (1/concentration) least-squares quadratic regression analysis to calculate the line of best fit from the data. The equations of the calibration curves were then used to calculate the concentrations of meropenem in the samples from the measured peak area ratios. The method had a limit of quantitation for meropenem of 0.20 μg/mL. The calibration range was 0.20–100 μg/mL. This LC/MS/MS method for the determination of meropenem was validated and met the requirements for specificity, sensitivity, linearity, recovery, precision, accuracy, and dilution integrity. The average time between sample collection and analysis ranged from 3–6 months. Samples were stored at <70° C until the time of analysis. Meropenem was stable in plasma for 1 year when stored at <70° C.
Pharmacokinetic and Statistical Analysis
The plasma concentration-time data were modeled using NONMEM® version VI (ICONUS, Ellicott City, MD) software in conjunction with WINGS for NONMEM version 6.13 (Auckland, NZ). NONMEM VI was installed under the NMQUAL version 6.2.0 (Metrum Institute, Augusta, ME). Diagnostic plots were executed in SlideWrite Plus 7.0 (Advanced Graphics Software, Encinitas, CA) and SAS 9.2 (Cary, NC). The model qualification was performed with a bootstrap procedure using WINGS for NONMEM, and 1000 sample sets were generated (7). The first-order conditional estimation subroutine was used with an ETA-EPS interaction. Structurally, a 1-compartment model with first-order elimination (ADVAN1 TRANS2) was chosen, and PK parameters were scaled by patient weight before evaluation of other potential covariates. The base model was developed and used to screen for extreme outlier concentrations. Plasma meropenem concentrations with >10-fold difference from the predicted concentration (indicating likely contamination or dose administration error) were excluded from the analysis.
The parameters were assumed to have an exponential normal distribution using the full variance-covariance matrix to estimate for intersubject variability. A proportional residual error was used in the analysis. A univariate covariates screen was performed for potential associations with PK parameters. The following potential covariates were included in this analysis: serum creatinine (SCR), PNA, GA, postmenstrual age (PMA), albumin (ALB), sex, urine output (UOP), and dopamine and epinephrine (DOPA) use. For patients without available ALB concentrations, the ALB values were set to the overall median value (2.3 g/dL), while missing SCR values were imputed based on a linear regression model of SCR and postmenstrual age derived from the data. During the model-building process, potential covariates that reduced the objective function by more than 3.84 (P<~0.05) were included in the subsequent multivariable analysis. A forward inclusion approach with backwards elimination was used during the multivariable step, and a reduction of 7.88 (P<~0.005) was required for retention of a covariate in the final model. Empirical Bayesian estimates of individual patient PK parameters were generated from the final model using the post-hoc subroutine.
Assessment of the meropenem penetration into the CSF was evaluated by the CSF:plasma meropenem concentration ratio. Because plasma meropenem concentrations were not obtained concurrently with CSF sample collection, plasma concentrations at the time of CSF sample collection were predicted via simulation based on individual patient PK parameters and dosing history. CSF samples collected more than 48 hours after the last dose of meropenem were excluded from the analysis.
RESULTS
A total of 200 patients were enrolled in the study (see Table, Supplemental Digital Content 1, which displays patient demographics by study cohort); 188 had 780 evaluable plasma meropenem concentrations. A total of 27/780 (3.5%) outlier concentrations were excluded from the analysis. Ten of these samples had no measureable drug concentrations. The other 17 samples had concentrations that deviated from predicted concentrations by approximately 10-fold. The exclusion of these samples resulted in 753 concentrations used in the modeling process. The median time of PK sampling was 4.75 hours (range, 0.02–12.12 hours) after dose, and the median concentration was 15.3 μg/mL (range, 0.15–101.7 μg/mL).
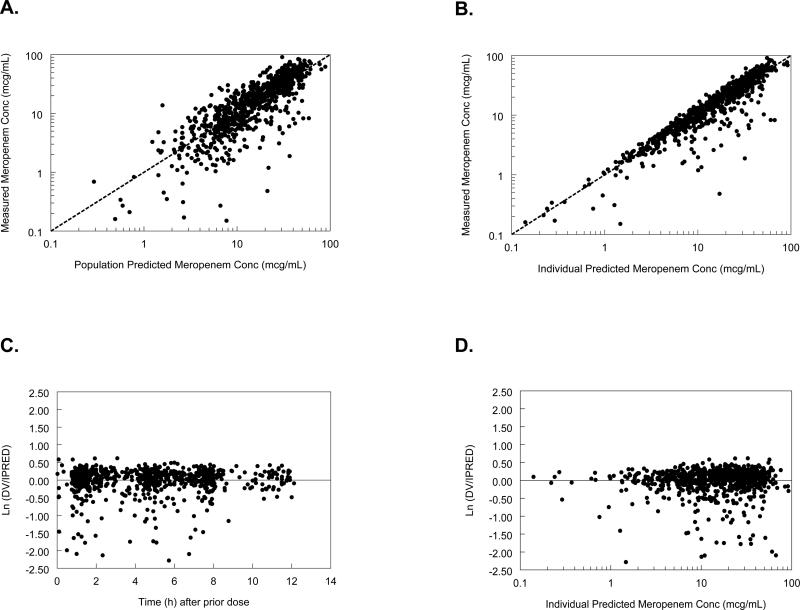
In the initial stage of population PK model development, maturation was found to be an influential factor on meropenem clearance (CL). Because maturation itself is a function of several collinear measures, combinations of GA and PNA, linear, non-linear, and categorical approaches were used to assess various approaches to maturation during the univariate screen. The univariate screen for potentially significant covariate-meropenem PK parameter associations identified relationships between meropenem CL and PMA, SCR, GA, PNA, ALB, and DOPA. Meropenem volume of distribution (V) was associated with ALB and DOPA. However, in the final multivariable evaluation, only PMA and SCR were retained as significant for CL and ALB for V (Table 1). Overall, the model described the data well (Fig. 1). These figures demonstrate that the model lacks significant bias.
FIGURE 1.
Goodness-of-fit figures: A) and B), measured versus population predicted (PRED) and individual predicted (IPRED) concentrations in the final model; C) and D), weighted residuals plot for the final population PK model versus time after previous dose and IPRED, respectively. LN(DV/IPRED) indicates natural log of the ratio of the observed concentration (DV) divided by the predicted individual concentrations (IPRED); DV, dependent variable (observed meropenem concentrations).
The median empirical Bayesian post-hoc parameter estimate for CL, 0.119 L/h/kg, was nearly identical to the typical population model estimates of 0.122 L/h/kg at the median PMA and SCR (Table 2). The median empirical Bayesian post-hoc parameter estimate for V, 0.468 L/kg, was also similar to the typical population model estimate of 0.460 L/kg. Individual patient post-hoc clearance estimates appeared to increase with PMA throughout the range of PMA values in the study (see Figure, Supplemental Digital Content 2, which shows scatter plots of meropenem weight-adjusted clearance and half-life versus PMA). The elimination half-life decreased with increasing PMA as would be expected with the increasing CL (Supplemental Digital Content 2). No relationships were observed between UOP, sex, DOPA and meropenem CL, V, or elimination half-life. Using the individual patients’ Bayesian post-hoc parameter estimates, the steady-state trough concentrations were simulated for each study patient. Despite the large range of CL and elimination half-life seen across the study population, the differential doses used for the different cohorts achieved relatively similar AUC0–24. Maximum (Cmax[ss]) and minimum (Cmin[ss]) meropenem concentrations at steady state were similar among cohorts 1–3, while cohort 4 had higher Cmax(ss) and lower Cmin(ss) as expected for the higher CL and lower V in cohort 4 (Table 2). The overall meropenem concentration PD targets were >4 μg/mL for 50% of the dose interval and >2 μg/mL for 75% of the dose interval. These targets were achieved in 96.3% (181/188) and 92.0% (173/188) of patients, respectively. The Cmin(ss) for the first 3 cohorts also exceeded 2 μg/mL in 85% (160/188) or more of patients. Based on the population PK model and an interim PK analysis of concentrations from the first 12 infants enrolled in each cohort, the dosing strategy met the overall goals for exposure, and thus, the need for dose escalation to achieve a pharmacodynamic optima was obviated.
The model was validated using a 1000-set bootstrap analysis and the program WINGS for NONMEM. Approximately 99.8% of bootstrap datasets converged to >3 significant digits. The median of bootstrap parameter estimates was within 2% of population estimates from the original dataset for all parameters. The 95% confidence interval of the bootstrap SCR (THETA3), PMA (THETA4), and ALB (THETA5) covariates excluded a null effect (0 or 1), affirming significance.
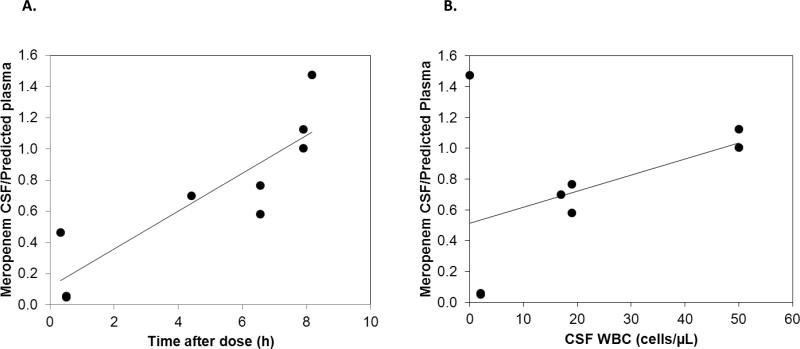
Nine CSF meropenem concentrations (3 duplicates) were available from 6 patients and ranged from 0.7–34.6 μg/mL. Measured CSF concentrations, predicted simultaneous plasma concentrations, and ratio of measured CSF/predicted plasma concentrations are listed in Table 3. Meropenem CSF penetration was highly variable. A trend between CSF collection time and CSF/plasma concentration ratio was seen, suggesting slower elimination from the CSF than the systemic circulation (Fig. 2). Another modest trend was observed between CSF white blood cell count (WBC) and the CSF/plasma concentration ratio, suggesting a potential role of meningeal inflammation in meropenem CSF concentrations (Fig. 2).
FIGURE 2.
Meropenem measured cerebrospinal fluid (CSF)/predicted plasma concentration ratio: A) measured CSF/predicted plasma concentration ratio versus time of CSF collection (9 CSF sample data points; duplicate CSF samples collected 0.5 hours after dose overlap); B) measured CSF/predicted plasma concentration ratio versus white blood cell count (WBC) in the CSF (8 CSF sample data points with documented CSF WBC; duplicate CSF samples collected 0.5 hours after dose overlap).
DISCUSSION
Meropenem is often used to treat infections in the neonatal intensive care unit; however, the drug is not FDA-approved for use in infants <3 months of age, and studies evaluating the PK of meropenem in young infants are lacking. Consequently, the most appropriate dose for this population has not previously been defined.
The 1-compartment population PK model evaluated for meropenem disposition in young infants in this study described the data well. While a sparse sampling approach was used in the study and may have limited the ability to reveal multi-compartment PK, the 1-compartment approach appeared to be sufficient as there were no apparent prediction biases based on sample collection time. The mean ETAs for V and CL were not significantly different from 0, and all parameters were nearly identical to the median bootstrap values with narrow 95% confidence intervals for CL and V (the 2.5th percentile was within 0.8 times the 95th percentile).
Maturational changes in meropenem CL were included through covariates of PMA and CR in the final model. Creatinine was retained as a significant covariate despite the exclusion of patients with elevated creatinine (>1.7 mg/dL). These associations were expected as a significant proportion of meropenem is excreted in the urine and developmental changes in renal function can be characterized by PMA (8, 9). There were no apparent age-related effects on the V for meropenem. However, meropenem V was negatively associated with ALB. While meropenem is not significantly bound to plasma proteins, albumin concentrations may reflect changes in fluid status that affect meropenem V.
The PK of meropenem in the current study was consistent with prior meropenem investigations. In 37 infants (GA 23–41 weeks) who received single doses of meropenem (10–20 mg/kg body weight), serum creatinine and PMA were the best overall predictors of meropenem CL (10). In addition, PK parameter estimates were similar to the values reported in this study; the average population CL was 0.104 L/h/kg, while the V was 0.4 L/kg (10). Monte Carlo simulations performed by these authors showed that meropenem doses of 20 mg/kg every 8 hours achieved the desired therapeutic target (time above minimum inhibitory concentration [MIC] of 4 μg/mL for >60% of dose interval for Pseudomonas aeruginosa) in 95% of preterm infants and 91% of term infants during the first 2 weeks of life. However, the number of infants <32 weeks included in this prior study was low, which may explain why the same target was achieved in the present study with every 12-hour dosing. Moreover, an 8-hour dosing interval likely yields excessive exposures for the most premature infants. Another study of meropenem in 23 preterm (GA 29–36 weeks) and 15 full-term (GA 37–42 weeks) infants found that a meropenem dose of 20 mg/kg body weight every 8 hours achieved the desired therapeutic target in the majority of infants; however, this study did not evaluate the impact of PNA on meropenem CL (5). The PK of meropenem in the current study differed from that reported in adults. The typical population CL value for infants included in this study was about 30–40% lower than the average reported CL in adults (4). These PK differences are likely related to developmental changes associated with renal function and body composition characteristic of young infants.
Meropenem exhibits time-dependent killing of Gram-negative and Gram-positive pathogens. Therefore, the goal of therapy is to keep plasma concentrations above the meropenem MIC for 40–50% of the dosage interval in adult patients (11). Because immunocompetence is reduced in preterm infants, a therapeutic target of time above MIC for >75% of the dosage interval was chosen for this trial. The Clinical and Laboratory Standards Institute (CLSI)-recommended MIC breakpoint of meropenem for Pseudomonas aeruginosa is ≤4 μg/ml, and over 80% of isolates are susceptible at an MIC of 2 μg/mL (12). Because trough meropenem concentrations exceeded this therapeutic target (2 μg/mL) in >80% of infants enrolled in this trial, meropenem doses as outlined in this trial should be sufficient for a clinical and microbiological cure across GA and PNA for infants <91 days of age.
In the present study, a small number of infants had evaluable CSF PK samples. Although meropenem CSF concentrations were highly variable, they were all therapeutic above the 4 μg/mL threshold. In addition, a trend towards increasing meropenem concentrations was observed with increasing CSF WBCs, which suggests a potential role of meningeal inflammation in the CSF penetration of meropenem. These findings are consistent with meropenem CSF concentrations collected from adults who received 20–40 mg/kg of meropenem. CSF meropenem concentrations in these adults ranged between 0.1–6.5 μg/mL (1–52% CSF penetration), and CSF concentrations tended to increase with increasing CSF WBC (13).
A dosing strategy of 20 mg/kg every 12 hours in infants <32 weeks GA and PNA <14 days; 20 mg/kg every 8 hours in infants <32 weeks GA and PNA ≥14 days and in infants ≥32 weeks GA and PNA <14 days; and 30 mg/kg every 8 hours in infants ≥32 weeks GA and PNA ≥14 days will achieve therapeutic meropenem concentrations in almost all infants <91 days of age with suspected intra-abdominal infections. Meropenem CSF concentration data were limited by the low number of CSF samples available in this trial and should be pursued further.
Supplementary Material
LIST OF SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1.doc: Patient demographics by study cohort.
Supplemental Digital Content 2.ppt: Scatter plots of meropenem weight-adjusted clearance and half-life versus PMA
ACKNOWLEDGMENTS
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators and site coordinators, in addition to those listed as authors, participated in this study:
Akron Children's Hospital: Judy Ohlinger, RN
Albany Medical Center: Mike Horgan, MD; Susan Boynton, RN, BSN
Baylor College of Medicine: Eric C. Eichenwald, MD; Karen Jones, RN, CCRC
Children's Hospital of Oakland: David J. Durand, MD; Jeanette Asselin, RRT, MS
Children's Hospital of Orange County: Kathy Shea, BA
Children's Hospital of Philadelphia: Tonia Morrison, BA, CCRC
Children's Hospital of Pittsburgh of UPMC: Robyn Baker, BSN, NNP
Children's Hospital at Vanderbilt: Millie Nannie, RN, BA, BS, CCRN, CLNC
Children's Medical Center of Dallas: Shirley Montanye, RN, ADN, CCRC
Children's National Medical Center: Elaine Williams, RN, MSN
Duke University: Sandy Grimes, RN, BSN
Evanston Northwestern Healthcare: William MacKendrick, MD; Sue Wolf, RNC-NIC, CCRP
Indiana University, James W. Riley Hospital for Children: Leslie Dawn Wilson, BSN, CCRC
Kansas City Children's Mercy Hospital: Ann Harris, RN, BSN, MBA
Kapiolani Medical Center for Women and Children: Venkataraman Balaraman, MD; Robyn Morse, BA, CCRC
Sharp Mary Birch Hospital for Women: Kathy Arnell, RNC
SUNY Downstate Medical Center: Sara Higgerson, BA
University Hospitals (Cleveland, OH): Arlene Zadell, RN, BSN
University of Alabama at Birmingham: Claire M. Roane, RN, MSN
University of California–San Diego: Wade Rich, BSHS, RRT, CCRC
University of Florida: Cindy Miller, RN
University of Louisville and Kosair Children's Hospital: Gwendolyn Pierce, RN, CCRC
University of Michigan–Ann Arbor: Ron Dechert, DPH, MS, RRT, FAARC
University of Utah, Primary Children's Medical Center: JoAnn Narus, MS, JD, MD
Yale University: Mathew Bizzaro, MD; Monica Konstantino, RN, BSN
Supported by: Dr. Smith received support from NICHD 1K23HD060040-01, DHHS-1R18AE000028-01, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp); Dr. Cohen-Wolkowiez receives support from the U.S. government for his work in pediatric clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin), from NICHD 1K23HD064814-01, and from the non-profit organization Thrasher Research Foundation; Dr. Benjamin receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C), the nonprofit organization Thrasher Research Foundation for his work in neonatal candidiasis, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp); Dr. Capparelli receives support from the U.S. government for his work in pediatric pharmacology (Government Contract HHSN267200700051C, PI: Benjamin); Dr. Kearns receives support from the National Institute of Child Health and Human Development (1U10HD31313-16S1) for his work in pediatric clinical pharmacology; Dr. Sullivan receives support from the U.S. government for her work in pediatric pharmacology (U10 HD045934 05) and from industry for neonatal and pediatric drug development; Dr. Weitkamp receives support from the National Institute of Child Health and Human Development (K08HD061607) for his work on the development of intestinal immune regulation in human infants; Dr. Blumer receives support from the U.S. government for his work in pediatric pharmacology (Government Contract HHSN267200700051C, PI: Benjamin)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632–1639. doi: 10.1016/s0002-9378(98)70037-7. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Nilsson-Ehle I. Pharmacokinetics of meropenem and its metabolite in young and elderly healthy men. Antimicrob Agents Chemother. 1992;36:1437–1440. doi: 10.1128/aac.36.7.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Anker JN, Pokorna P, Kinzig-Schippers M, et al. Meropenem pharmacokinetics in the newborn. Antimicrob Agents Chemother. 2009;53:3871–3879. doi: 10.1128/AAC.00351-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Enk JG, Touw DJ, Lafeber HN. Pharmacokinetics of meropenem in preterm neonates. Ther Drug Monit. 2001;23:198–201. doi: 10.1097/00007691-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed. 1999;59:19–29. doi: 10.1016/s0169-2607(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 8.Capparelli E, Hochwald C, Rasmussen M, Parham A, Bradley J, Moya F. Population pharmacokinetics of cefepime in the neonate. Antimicrob Agents Chemother. 2005;49:2760–2766. doi: 10.1128/AAC.49.7.2760-2766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 10.Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J. 2008;27:794–799. doi: 10.1097/INF.0b013e318170f8d2. [DOI] [PubMed] [Google Scholar]
- 11.Lomaestro BM, Drusano GL. Pharmacodynamic evaluation of extending the administration time of meropenem using a Monte Carlo simulation. Antimicrob Agents Chemother. 2005;49:461–463. doi: 10.1128/AAC.49.1.461-463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, Jones RN. A review of the in vitro activity of meropenem and comparative antimicrobial agents tested against 30,254 aerobic and anaerobic pathogens isolated world wide. Diagn Microbiol Infect Dis. 1997;28:157–163. doi: 10.1016/s0732-8893(97)00065-5. [DOI] [PubMed] [Google Scholar]
- 13.Dagan R, Velghe L, Rodda JL, Klugman KP. Penetration of meropenem into the cerebrospinal fluid of patients with inflamed meninges. J Antimicrob Chemother. 1994;34:175–179. doi: 10.1093/jac/34.1.175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LIST OF SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1.doc: Patient demographics by study cohort.
Supplemental Digital Content 2.ppt: Scatter plots of meropenem weight-adjusted clearance and half-life versus PMA