Abstract
Background
Inflammatory responses can include recruitment of cells of hematopoietic origin to the tunica muscularis. These cells can secrete a variety of factors which can reset the gain of smooth muscle cells (SMC) and influence motor patterns. Histamine, a major mediator in inflammation, is released by mast cells and exerts diverse effects in SMC by binding to histamine (H) receptors. The profiles of H receptor expression in animal models used to study inflammatory diseases are unknown.
Methods
H receptor expression and electro-mechanical responses to histamine were tested in simian and murine colonic smooth muscle using qualitative and quantitative PCR, isometric force measurements, microelectrode recordings and patch clamp techniques.
Key results
H1, H2 and H4 receptor transcripts were expressed at similar levels in simian colonic tissue whereas only the H2 receptor transcript was detected in murine colonic tissue. Stimulation of simian colonic muscles with histamine caused depolarization and contraction in the presence of TTX. Histamine activated non-selective cation channels in simian SMC. In contrast, histamine caused hyperpolarization and inhibited contractions of murine colon. The hyperpolarization was inhibited by the KATP channel blocker, glibenclamide. Histamine-activated K+ currents were inhibited by glibenclamide in murine colonic SMC.
Conclusions & Inferences
H receptor expression in simian SMC was similar to that reported in humans. However, H receptor profile and responses to histamine were considerably different in mice. Thus, monkey colon may be a more suitable model to study how inflammatory mediators affect the gain of smooth muscle excitability.
Keywords: histamine receptors, colonic excitability, contractility
Introduction
Colonic motility results from contractions of smooth muscle cells (SMC) that are regulated by intrinsic excitability and Ca2+ sensitivity mechanisms and inputs from a variety of higher-order control systems (e.g. interstitial cells of Cajal, enteric motor neurons, hormones, and paracrine substances). The responses of colonic muscles to regulatory signals depends upon the gain on excitability that is set in individual SMC. In inflammatory diseases new regulatory influences are imposed due to the release of bioactive substances from activated, resident immune cells and cells of hematopoietic origin that are recruited to the muscularis as part of the inflammatory response. Among these cells are mast cells that can be found in abundance in the tunica muscularis in response to inflammation.1 Mast cells release a variety of bioactive substances, including histamine, cytokines and interleukins.2
Histamine, a major mediator in inflammatory and allergic reactions,3 is a biogenic amine synthesized from the basic amino acid histidine. Many stimuli, including allergens, neuropeptides, and stress can activate mast cells and cause release of histamine.4 Histamine, acting as a paracrine, may be able to significantly reset the excitability of colonic SMC. In the guinea pig enteric nervous system, histamine suppresses synaptic transmission presynaptically, whereas in human submucous plexus, histamine excites enteric neurons suggesting histamine effects can differ depending on species.5,6 There are four histamine (H) receptors, H1, H2, H3 and H4, through which histamine can exert it effects. H1 receptors are widely expressed in enterocytes, muscle layer, blood vessels, immune cells and ganglion cells of the myenteric plexus in the human GI tract.7 H2 receptors are located on parietal cells in the fundic mucosa, intestinal epithelium, immune cells and myenteric ganglia.7,8 Although H3 agonists had pronounced excitatory effects on submucosal neurons from human small and large intestine, which suggests expression of the receptor in human bowel,6 minimal expression levels of H3 receptor mRNA were detected in the human GI tract (5 out of 66 samples).7 Héron et al. found that H3 receptor was expressed in the mucosa of the rat GI tract.9 Transcriptional expression of the H4 receptor is lower than H1 and H2 in human stomach, small intestine and colon and is mainly expressed in leucocytes in mucosa and submucosal blood vessels in the colon.7,10,11
Surprisingly, few studies have examined the effect of histamine on GI smooth muscle excitability. One study reported that histamine induced a dominant contractile response in human colon tissue strips, and these effects were not affected by hyoscine (a ganglion blocker) or anti-adrenergic drugs, suggesting that the effects of histamine were not dependent upon nervous activity.12 Bolton et al showed that histamine depolarized longitudinal SMC and increased action potential discharge in guinea-pig ileum.13 In the submucosal plexus of the human colon, local microinjection of H receptor-specific agonists resulted in excitation of enteric neurons.6 Many inflammatory studies are performed on rodents without an appreciation for the cellular responses of inflammatory mediators. Here we have evaluated the profile of histamine receptors in mice and in a non-human primate model (Macaca fascicularis) and characterized the electromechanical responses to histamine and a variety of H receptor-specific agonists.
Materials and methods
Animals
Male BALB/c mice (10-12 weeks old) were used for electro-mechanical experiments in this study. These mice were killed by inhalation of isoflurane (Baxter Healthcare, Deerfield, IL, USA) followed by cervical dislocation. Colons were removed following abdominal incision and rinsed with Krebs–Ringer bicarbonate buffer (KRB, see solutions and drugs section). Cynomolgus simians of either sex (13 simians, 2.5–7 years of age) were donated by Charles River Laboratories (Preclinical Services, Sparks, NV, USA). The animals used were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All experiments performed and protocols were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno.
Expression Studies
Following dispersion of simian SMC from the longitudinal muscle layer (see Isolation of SMC section), SMC were collected by applying suction to the pipette resulting in aspiration of the cells into the pipette. Total RNA isolation, cDNA preparation and amplification of simian and murine colonic longitudinal muscle strips (mucosa and submucosa removed) and simian colonic longitudinal SMC were performed as previously reported.14 Briefly, RNA was prepared using a SNAP Total RNA isolation kit (Invitrogen, San Diego, CA) as per the manufacturer's instructions. RNA was treated with Rnase-free Dnase I (2 units) at 37°C (New England Biolabs) prior to cDNA preparation. First strand cDNA was synthesized from each RNA using Superscript II Reverse Transcriptase with 500 μg μl-1 of oligo dT primers cDNA. To investigate the expression of H receptors, the following PCR primers designed against murine sequences were used (genebank accession number is given in parenthesis for the reference nucleotide sequence used): H1 (NM_008285), H2 (NM_008286), H3 (NM_133849), H4 (NM_153087). Similarly, primers were designed against the following simian sequences: H1 (XM_001088286), H2 (XM_001082927), H3 (XM_001032898), H4 (XM_001096657). The relative expression levels of H receptors in simian colons was determined by real-time quantitative PCR performed on a ABI PrismM 7000 sequence detector using SYBR® Green chemistry (Applied Biosystems, CA). Standard curves were generated for each receptor and the constitutively expressed GAPDH from regression analysis of the mean values of RT-PCRs for the log10 diluted cDNA. Unknown quantities relative to the standard curve for the H receptor primers were calculated, yielding transcriptional quantization of H receptor cDNA relative to the endogenous standard GAPDH. Each cDNA sample was tested in triplicate and cDNA was obtained from 4 different simian colons. The reproducibility of the assay was tested by analysis of variance comparing repeat runs of samples, and the mean values generated at individual time points were compared by Student's t test.
Isometric Force Measurements
Standard organ bath techniques were employed to measure the changes in force generated by murine and simian longitudinal smooth muscle strips. One end of a smooth muscle strip was attached to a fixed mount and the opposite end to an isometric strain gauge (Fort 10, WPI, Sarasota, FL, USA) in oxygenated KRB solution maintained at 37.5±0.5°C. A resting force of 500 mg (murine colon) and 1g (simian colon) was applied to set the muscles at optimum length, and the muscles were allowed to equilibrate for 1-2 h with constant perfusion with KRB solution. Mechanical responses were recorded on a computer running Axoscope (Axon instrument, Foster City, CA, USA) and measurements of the area under the curve (AUC) obtained. The AUC was determined as the integral values above the baseline of selected area for 5 min recordings (mNxmin). The AUC for the tissues exposed to tested drugs were compared to the AUC for tissues under control conditions, during an equivalent period of time. Bathing solutions were exchanged by switching the perfusion to the drug-containing solution.
Intracellular Microelectrode Recordings
After preparing the longitudinal smooth muscle tissue, impalements of cells were made with glass microelectrodes having resistances of 80–120 MΩ. Transmembrane potentials were recorded with a standard electrometer (Duo 773; WPI, Sarasota, FL, USA). Data were recorded using Axoscope (Axon instrument, Foster City, CA, USA).
Isolation of SMC
Simian colon segments were placed in KRB solution. Each segment was opened and pinned to the base of a dissecting dish coated with Sylgard elastomer (Dow Corning Corp., Miland, MI, USA) and the adhering mucosa, submucosa and circular muscle layer were removed. Freshly dispersed colonic SMC were prepared from colonic longitudinal muscle strips using Ca2+-free Hank's solution containing (mmol L-1): 125 NaCl, 5.36 KCl, 15.5 NaOH, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose and 11 Hepes, adjusted to pH 7.4 with Tris. Pieces of muscle were incubated for 50-55 minutes at 37°C in a Ca2+-free solution (2 ml) containing collagenase (4 mg mL-1, Worthington Biochemical, Lakewood, NJ), trypsin inhibitor (8 mg mL-1), fatty acid-free bovine serum albumin (8 mg mL-1), papain (2 mg mL-1), and L-dithiothreitol (LDTT, 0.3 mg mL-1, Sigma-Aldrich, MO, USA). Tissue pieces were washed with Ca2+-free solution and then gently agitated to create a cell suspension. Dispersed SMC were stored at 4°C in Ca2+-free solution. Drops of the cell suspensions were placed on the bottom of a 300 μl chamber mounted on an inverted microscope and allowed to adhere to the bottom of the chamber for 5 minutes before recording.
Colons from BALB/c mice were dissected in the same fashion as simian colons. Pieces of longitudinal muscle were incubated for 35-40 minutes at 37°C in a Ca2+-free solution (2 ml) containing collagenase (2 mg mL-1, Worthington Biochemical, Lakewood, NJ), trypsin inhibitor (4 mg mL-1), fatty acid-free bovine serum albumin (4 mg mL-1), papain (1 mg mL-1) and L-DTT (0.3 mg mL-1).
Patch Clamp Experiments
The whole-cell voltage clamp technique was used to record membrane currents from dissociated murine colonic SMC. Currents were amplified with an Axopatch 200B (Axon Instruments). Data were digitized with 16-bit analogue to digital converter (Digidata 1322A, Axon instruments, Foster City, CA, USA). Data were stored directly and digitized online using pClamp software (version 9.0, Axon instrument, Foster City, CA, USA). The data were sampled at 5 KHz with low pass filtered at 2 KHz using an eight-pole Bessel filter. Conventional and perforated whole cell patch-clamp techniques were used for recording ionic currents under voltage clamp. For perforated patches, amphotericin B (60 mg mL-1) was dissolved in DMSO, sonicated, and diluted in the pipette solution to give a final concentration of 270 μg mL-1. Experiments were performed at room temperature (between 22 and 25°C).
Solutions and Drugs
In intracellular microelectrode recordings and mechanical experiments, the tissue chamber housing longitudinal muscles was constantly perfused with oxygenated KRB solution of the following composition (in mmol L-1): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.4. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37.0 ± 0.5°C. In order to measure inward currents, colonic SMC were bathed in a Ca2+-containing physiological salt solution (CaPSS) containing (in mmol L-1): 135 NaCl, 5 KCl, 2 CaCl2, 1.2 MgCl2, 10 glucose, 10 HEPES adjusted to pH 7.4 with Tris. The pipette solution for the study of inward currents in simian colonic SMC contained (in mmol L-1): 30 CsCl, 110 Cs-aspartate, 0.1 EGTA, 0.1 Na2GTP, 3 MgATP, 10 glucose, 2.5 creatine phosphate disodium and 10 HEPES. This solution was adjusted to pH 7.2 with Tris. In order to increase the driving force for K+ influx in murine colonic SMC, cells were perfused in High K+-containing solution (135 mmol L-1 Na+ was replaced with equimolar K+) and the pipette solution contained (in mmol L-1): 135 KCl, 10 BAPTA, 0.1 Na2GTP, 3 MgATP, 10 glucose, 2.5 creatine phosphate disodium and 10 HEPES and was adjusted to pH 7.2 with Tris. H1 agonist (histamine-trifluoromethyl-toluidine, HTMT), H2 agonist (Dimaprit), H3 agonist ((R)-α-methylhistamine dihydrobromide), H4 agonist (4-methylhistamine dihydrochloride, MHDC) and H2 antagonist (Zolantidine dimaleate) were purchased from Tocris (Park Ellisville, MO, USA). Histamine, glibenclamide (GBC) and tetrodotoxin (TTX) were obtained from Sigma Chemical Co (St Louis, MO, USA).
Statistical Analysis
Data were expressed as means ± S.E.M. The Student's t-test was used where appropriate to evaluate differences in the data. P-values less than 0.05 were taken as statistically significant differences. n values refer to the number of recordings from muscle strips in electro-mechanical experiments or from cells in patch clamp experiments.
Results
Transcriptional expression of histamine receptors in murine and simian colonic muscles
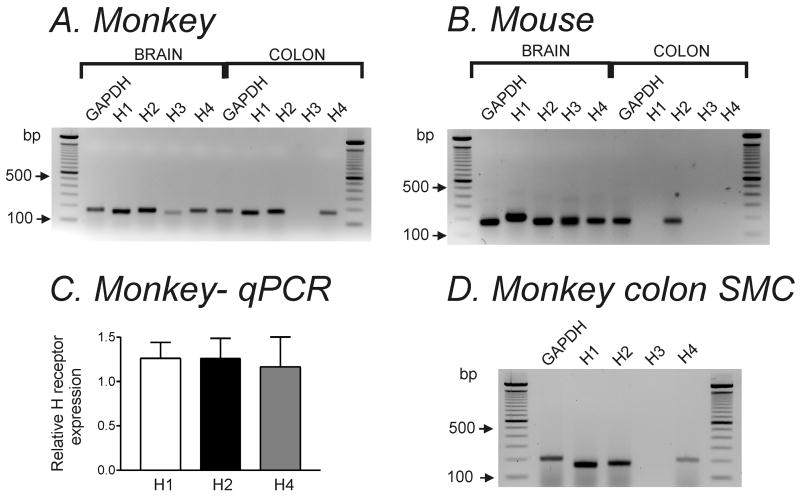
We investigated gene expression of H receptor isoforms by performing RT-PCR on colonic longitudinal muscles from monkey and mouse. Detectable amplicons for H1, H2 and H4 were revealed in simian colonic muscles (Fig. 1A). Only H2 receptor expression was detected in murine colonic longitudinal muscles (Fig. 1B). Quantitative analysis of H1, H2 and H4 receptors in simian colonic muscle revealed no significant difference in expression levels of these receptors (Fig. 1C). Since transcripts at the tissue level could include genes expressed in a variety of cell types (e.g. neurons, fibroblasts, etc), we also examined transcript expression in freshly dispersed SMC collected by suction into the pipette (∼100 cells per sample). H1, H2 and H4 receptors were expressed in SMC samples collected from 6 monkeys. These SMC samples were negative to c-Kit (interstitial cell of Cajal marker) and PGP9.5 (neuronal cell marker).
Figure 1. H receptor expression was different in simian and murine colonic longitudinal muscle.
Representative agarose gels of RT-PCR products of H1- H4 receptors in (A) simian and (B) murine colonic tissue. RT-PCR was performed with H receptor isoform specific primers. GAPDH was used for control. (C) Summary graph of real-time quantitative PCR (qPCR) analysis of H1, H2 and H4 receptors in simian colonic tissue. Expression of each H receptor isoform was normalized relative to GAPDH expression. (D) Representative agarose gel of H1-H4 receptor transcripts in simian colonic SMC (n=6).
Histamine had different effects on contractility in simian and murine colonic smooth muscles
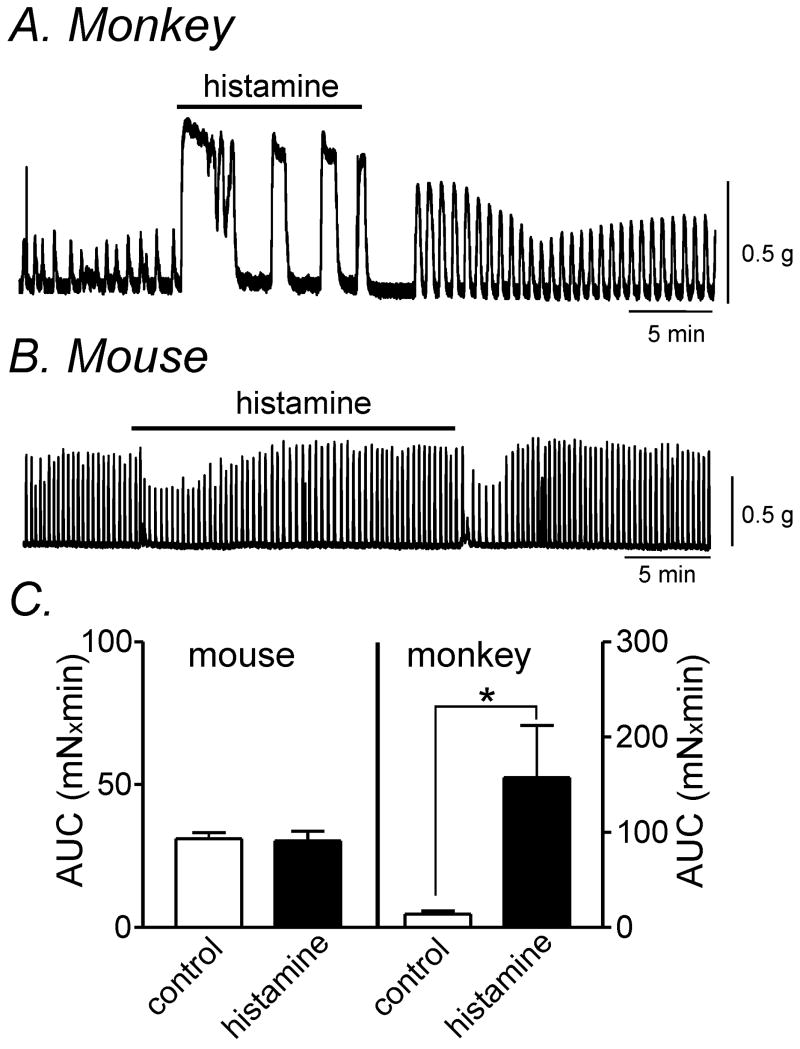
We compared the effects of histamine on contractions in simian and murine colons because the expression of H receptors was markedly different in the 2 species. Contractions were quantified by calculating the averaged areas under the contraction curves (AUC) for 5-min recording periods (see materials and methods section). Histamine (10 μmol L-1) increased AUC from 14.0±3.4 to 157.4±54.8 mNxmin in simian muscles (Fig 2A & C, n=4, P<0.05). In contrast, histamine (10 μmol L-1) had inconsistent effects in murine colonic muscles. In 8 of 15 tissues, histamine (10 μmol L-1) transiently decreased AUC from 27.7±2.3 to 21±2.2 mNxmin (P<0.01) (Fig. 2B). However, in the other 7 murine tissues tested, there was no significant change in AUC. Analysis of all 15 tissue experiments found that the AUC for the initial 5min period after histamine application was not significantly different (31.0±2.1 to 30.3±3.4 mNxmin) (Fig. 2C; n=15).
Figure 2. Histamine altered contractility in simian and murine colonic longitudinal smooth muscle.
(A) Representative mechanical trace illustrating that histamine (10 μmol L-1) caused an increase in contractile force in simian colonic muscle. (B) Application of histamine (10 μmol L-1) resulted in a transient decrease in the force of contractions in murine colonic smooth muscle in 8 out of 15 tissues tested. (C) Summary data of area under the curve (AUC) for 5 min recording before and after histamine treatment from murine (n=15) and simian colonic smooth muscle (n=4). * denotes P<0.05.
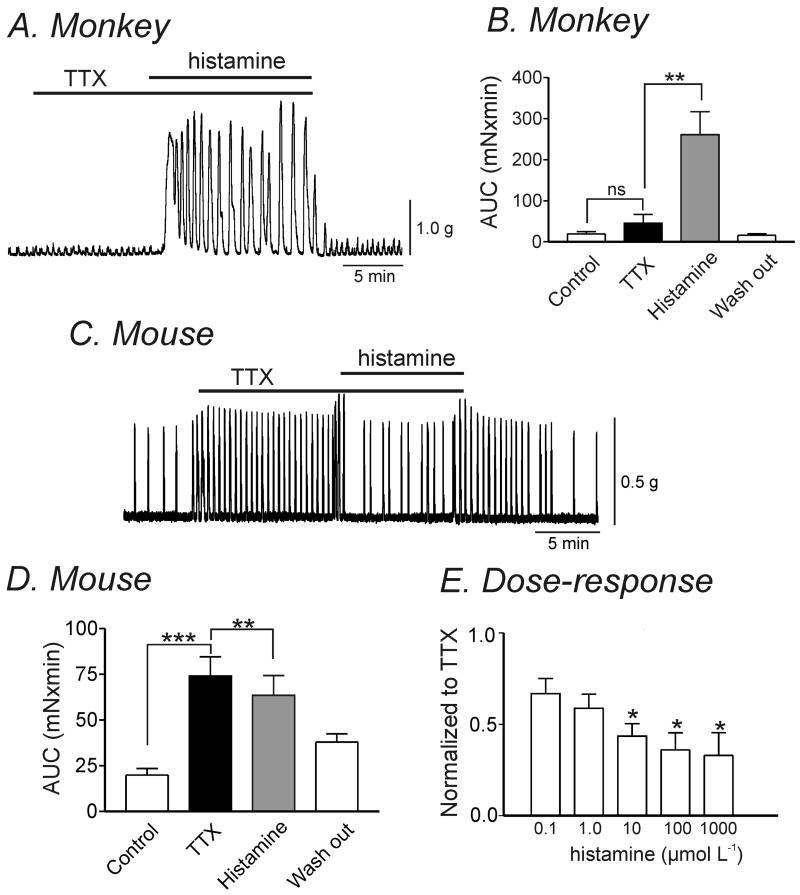
Neuronal cells also express H receptors, so the effects of histamine could also be due to stimulation or inhibition of enteric motor neurons. Therefore, we repeated contractile responses in the presence of tetrodotoxin (TTX, 1 μmol L-1). As previously reported, TTX application resulted in a significant increase in contractile force in murine colonic muscles reflecting a dominant inhibitory influence of neurons on spontaneous contractile activity (Fig. 3C & 3D).15 In comparison, TTX had a minor effect on contractile force in simian colonic muscles suggesting a minor neuronal influence on basal spontaneous contractility. Histamine (10 μmol L-1) caused a significant increase in contractile force in simian muscles (AUC from 45.2±21.3 to 260.9±56 mNxmin) in the presence of TTX (Fig. 3A & 3B; n=9, P<0.005). In murine colonic muscles, a sustained decrease in contractile force and frequency of spontaneous contractions in the presence of TTX was observed (AUC from 74.1±10.4 to 63.5±10.8 mNxmin; Fig. 3C & 3D; n=17, P<0.005). In the presence of TTX, there was a dose-dependent reduction on AUC in murine colonic muscles by increasing concentrations of histamine (Fig. 3E; n=4, P<0.05).
Figure 3. Histamine-evoked changes in contractility in simian and murine colonic longitudinal smooth muscle in the presence of TTX.
(A) Representative mechanical trace showing that in the presence of TTX (1 μmol L-1), exposure to histamine (10 μmol L-1) increased contractile amplitude in simian colonic smooth muscle. (B) Graph summarizing the significant increase in AUC by histamine in the presence of TTX in simian colonic tissue (n=9, **P<0.005). (C) In murine colonic smooth muscle, histamine caused a sustained decrease in contractile force in the presence of TTX (1 μmol L-1). (D) Graph summarizing the significant decrease in AUC by histamine in the presence of TTX in murine colonic muscle (n=17, **P<0.005, ***P<0.001). (E) Summary graph illustrating dose-dependent effects of histamine on murine colonic AUC normalized to control (TTX presence) (n=4, * P<0.05).
Effects of histamine on membrane potential in simian and murine colonic muscle
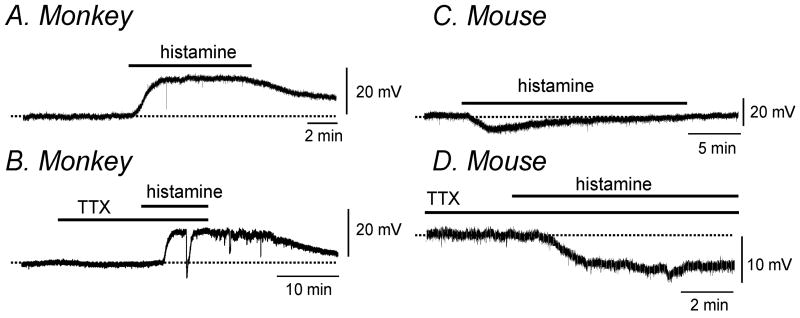
We performed intracellular microelectrode recordings from simian and murine colonic longitudinal muscles to examine the effects of histamine on membrane potential. Histamine (10 μmol L-1) caused sustained depolarization of simian colonic muscle that was reversible upon washout (Fig. 4A, n=5, 16±4mV; P<0.01). TTX (1 μmol L-1) did not alter this effect (Fig. 4B). In contrast, histamine (10 μmol L-1) caused a transient hyperpolarization of murine colonic muscles from -45.5±0.6 mV to -50.6±1.8mV (Fig. 4 C, n=9; P<0.05). Furthermore hyperpolarization induced by histamine was sustained in the majority of cases (9 of 11) following pretreatment with TTX (1 μmol L-1) (ΔmV=- 4.8±0.6mV, P<0.005, Fig. 4D).
Figure 4. Histamine altered membrane potential in simian and murine colonic longitudinal smooth muscle.
Representative traces illustrating that histamine (10 μmol L-1) caused depolarization in the absence (A) and presence (B) of TTX (1 μmol L-1) in simian colonic smooth muscle. In contrast, in murine colonic smooth muscle, representative traces show that histamine (10 μmol L-1) induced transient hyperpolarization in the absence (C) and sustained hyperpolarization in the presence (D) of TTX (1 μmol L-1).
Effects of histamine receptor agonists on simian colonic electrical and mechanical activity
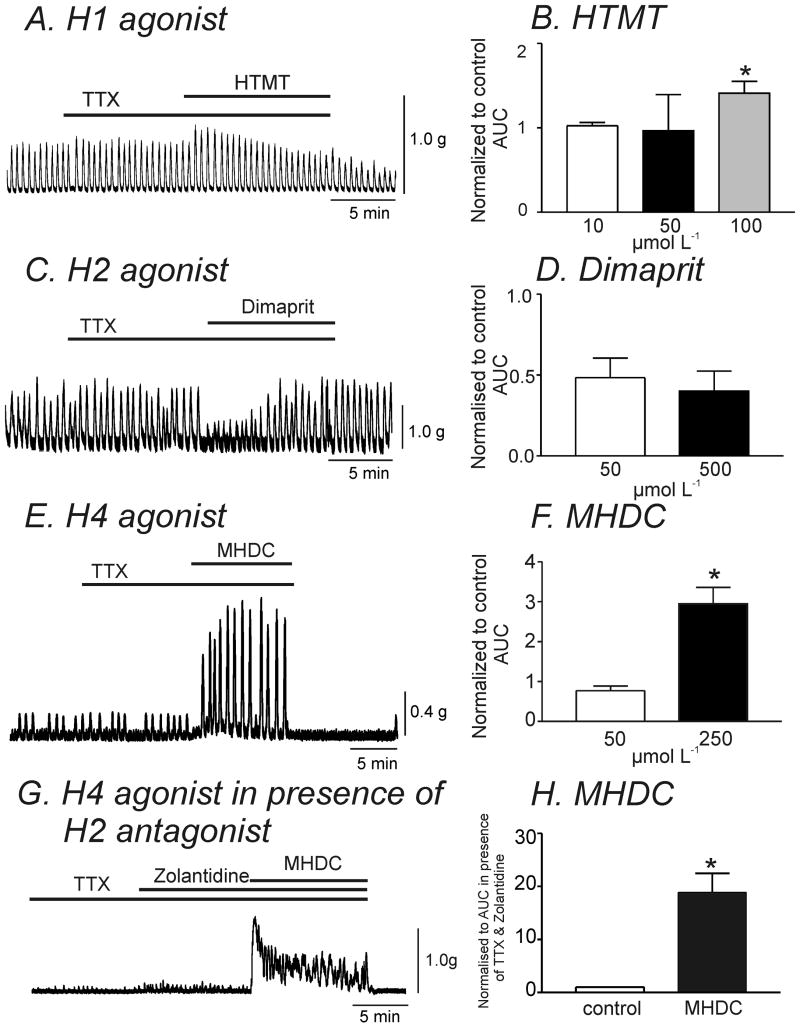
H1, H2 and H4 receptor transcripts were found in simian colonic muscle and SMC (see Fig.1A & 1D). Therefore, we investigated the effects of specific H receptor agonists on contractions. The H1 agonist, HTMT (10-50 μmol L-1), had only minor effects on contractility. However, higher concentrations of HTMT (100 μmol L-1) increased contractile force (41±14%) in the presence of TTX (1 μmol L-1) (Fig. 5A & B, n=4, P<0.05). The H2 receptor agonist, dimaprit (50 and 500 μmol L-1), transiently decreased the force of contractions (-52±12%, and -42±15%, respectively (Fig. 5C & D, n=4). The H4 agonist, MHDC, at lower concentrations (50 μmol L-1) had no significant effect on contractility (P=0.1, n=7). However, a higher concentration of MHDC (250 μmol L-1) caused a dramatic increase in contractile force (195±41%, n=4, P<0.05), an effect similar to that seen with application of histamine (Fig. 5E & F). H4 agonist (MHDC) can also activate H2 receptors.16 Therefore to exclude possible effects of the H4 agonist on H2 receptors, we performed experiments in the presence of the H2 inhibitor, Zolantidine (10 μmol L-1). Zolantidine pretreatment augmented the response to MHDC (250 μmol L-1, 19-fold increase in AUC, Fig. 5G & H) compared to without H2 antagonist (3-fold increase in AUC, Fig. 5F).
Figure 5. Specific H receptor agonists had different effects on contractility in simian colonic longitudinal smooth muscle.
(A & B) Representative trace illustrating that application of the H1 agonist, HTMT (100 μmol L-1), caused a transient increase in contractile force. (B) AUC during application of HTMT was normalized to the control AUC in the presence of TTX (1 μmol L-1). HTMT (100 μmol L-1) transiently increased AUC (n=4). (C & D) The H2 agonist (dimaprit, 50 μmol L-1) transiently decreased the amplitude of spontaneous contractions but not significantly (n=4). (E & F) Representative trace illustrating that the H4 agonist, MHDC (250 μmol L-1) caused a dramatic increase in contractile force (n=4). (G) In the presence of the H2 antagonist, Zolantidine (10 μmol L-1), the contractile response to MHDC (250 μmol L-1) was augmented. (H) Summarized data showing the effect of MHDC (250 μmol L-1) normalized to control AUC (in presence of TTX and Zolantidine) (n=4). * denotes P<0.05.
In order to confirm the absence of H3 receptors in simian colonic tissue (see gel in Fig. 1A), we examined the effects of the H3 agonist, (R)-α-methylhistamine (1μM), on contractility. These experiments revealed no significant change in contractile force upon application of this agonist (data not shown, n=4).
Histamine activated KATP whole-cell currents in murine colonic SMC
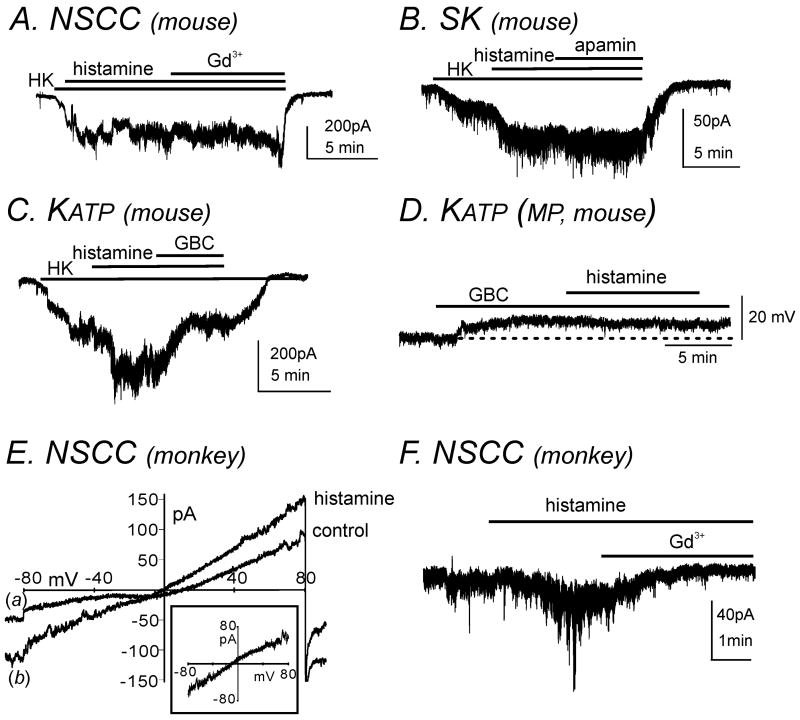
Histamine induced hyperpolarization in murine colonic smooth muscle, which could be due to activation of K+ conductance(s) and/or inhibition of non-selective cation channels (NSCC). The only H receptor expressed in colonic muscles couples through Gs to activate production of cAMP, which has been linked to activation of several K+ conductances in canine colonic circular smooth muscle.17 Therefore, we performed perforated whole-cell patch clamp experiments to resolve the mechanism of hyperpolarization in murine colonic muscles. Isolated cells were held at -80mV and the external solution (CaPSS) was replaced with high K+ solution (HK; 140 mmol L-1 K+, see Materials and methods section). Under these conditions histamine (10 μmol L-1) activated inward currents. We tested whether Gd3+ (10 μmol L-1), a blocker of NSCC, blocked currents activated by histamine. Gd3+ had no effect on the inward currents activated by histamine (Fig. 6A, n=4). These data suggested the conductance activated by histamine was likely to be a K+ conductance. Therefore, we tested blockers of small conductance K+ (SK) channels (apamin) and KATP channels (glibenclamide) on the conductance activated by histamine-activated by histamine. Apamin (300 nmol L-1) did not affect the conductance activated by histamine (Fig. 6B, n=4), but glibenclamide (10 μmol L-1) reduced histamine-activated currents (Fig. 6C, n=4).
Figure 6. Histamine activated KATP currents in murine colonic longitudinal SMC and NSCC in simian colonic longitudinal SMC.
(A) Murine colonic SMC was held at a holding potential of -80mV under perforated whole-cell configuration. CaPSS was replaced with high K+ solution (HK; 140 mmol L-1 K+, externally). Application of histamine (10 μmol L-1) activated inward currents which were not inhibited by the NSCC blocker, Gd3+ (10 μmol L-1). (B) Addition of the small-conductance Ca2+-activated K+ (SK) channel blocker, apamin (300 nmol L-1), did not affect histamine- activated inward currents. (C) The KATP channel blocker, glibenclamide (GBC, 10 μmol L-1), inhibited histamine-activated inward currents. (D) Application of histamine (10 μmol L-1) had no significant effect on membrane potential in the presence of GBC (10 μmol L-1) in murine colonic smooth muscle using intracellular microelectrode recordings. Dotted line denotes the resting membrane potential. (E) Simian colonic SMC was held at -80mV under whole-cell configuration. Histamine (10 μmol L-1) activated inward currents during ramp depolarization from -80 to +80 mV (see line (b) compared with control, line (a)). Subtracted (a-b) histamine-sensitive currents reversed at approximately 0mV (subtracted current in inset, ECl= -40mV). (F) Histamine-activated currents were inhibited by Gd3+ (10 μmol L-1).
Our cellular experiments suggest that the hyperpolarization of murine colonic muscles (Fig.4C and 4D) by histamine might be mediated by activation of KATP channels. Thus, we tested the effects of histamine in muscles pretreated with glibenclamide. Addition of the KATP blocker caused depolarization (indicating basal activation of KATP channels as previously reported in murine colonic smooth muscle).18 Addition of histamine in the presence of glibenclamide had no effect on membrane potential (Fig. 6D). These data suggest that the hyperpolarization and reduced contractility in murine colonic smooth muscle may be through activation of the H2/Gs pathway which increases protein kinase A (PKA) levels and activates KATP channels.
Histamine activated NSCC whole-cell currents in simian colonic SMC
Unlike murine smooth muscle, histamine induced depolarization and increased contractility in simian colonic smooth muscle which may be the result of activation of NSCC and/or Cl- currents. Therefore, we performed whole-cell patch clamp experiments to determine the mechanism of depolarization in simian colonic muscles. The internal Cl- concentration was 30 mmol L-1 in Cs+-rich solution (see Materials and Methods section) and the bath solution was CaPSS. Therefore, ECl was set at -40mV. At a holding potential of -80mV, perfusion of histamine (10 μmol L-1) activated inward currents (n=4). Subtraction of currents evoked by a single ramp depolarization from -80 to +80mV before (a) and after (b) histamine application, revealed a histamine-sensitive current that reversed at 0mV (Fig. 6E). Histamine-activated currents were inhibited by the NSCC non-specific blocker, Gd3+ (10 μmol L-1) (Fig. 6F, n=4). Therefore, histamine-induced depolarization is due to activation of NSCC but not Cl- conductance.
Discussion
In the present study we examined the effect of the major inflammatory mediator histamine, on electro-mechanical responses in colonic smooth muscle. In particular, we compared histamine receptor expression and effects of histamine receptor activation in murine and simian colonic muscles and identified several important differences between these two species. In murine colonic tissue, only the H2 receptor was expressed and histamine resulted in hyperpolarization and decreased spontaneous contractility. Conversely, simian colonic tissue expressed H1, H2 and H4 receptors and application of histamine or H1 and H4 specific agonists resulted in contraction. Histamine activated NSCC in simian colonic SMC which may underlie the depolarization seen at the tissue level.
In inflammatory bowel disease (IBD), alterations in colonic excitability have been frequently attributed to dysfunction of the enteric and/or central nervous system.19-21 Inflammation results in marked mast cell accumulation in rat rectal mucosa and human colon tissue.22-25 Furthermore, the cellular contents of mast cells, including tumor necrosis factor-α and histamine, dramatically increase in patients with ulcerative colitis.23-25 In human colitis, mast cell infiltration progresses transmurally to the smooth muscle layers of the colon, thus mast cell mediators may have direct effects on smooth muscle excitability.
H1, H2, H4 and minimal levels of H3 receptors are expressed in the human GI tract, though little is known about the functional role of H receptor subtypes in the colon.7 There is no report if the expression of H receptors in simian and murine colon are similar to expression patterns in humans therefore justifying the extrapolation of data from these models to human GI pathophysiology. Therefore, we examined the expression of H receptors in murine and simian colon using molecular studies and found there were different expression patterns. In simian colonic longitudinal smooth muscle, H1, H2 and H4 receptors were expressed in a similar pattern to human muscles. H3 receptor expression was not detected in simian tissue and SMC. This could be due to the extremely low expression of this receptor. In human colonic tissue, H3 receptor was expressed infrequently and at low levels compared with other H receptors.7 In murine colonic longitudinal tissue, only the H2 receptor was found to be expressed. Thus, from the perspective of histamine receptors and anticipated effects, the simian is potentially, but the mouse is not, a suitable model to study histaminergic influences in human colonic muscles.
Histamine can have diverse effects in different tissues due to expression of specific H receptors and activation of distinct intracellular pathways.26 H1 receptors are coupled to Gq/11 proteins which activates phospholipase C and the phosphatidylinositol 4,5-bisphosphate signaling pathway.27 They are also linked to Gi/o proteins which results in decreased production of cAMP.28 H4 receptors are coupled to Gi/o proteins that inhibit adenylate cyclase activity and therefore decrease cAMP production.4 In the gastrointestinal tract, synergistic stimulation of muscarinic receptors coupled to Gi/o and Gq/11 proteins is required to activate NSCC, resulting in membrane depolarization. Subsequent activation of voltage-dependent Ca2+ channels and an increase in Ca2+ influx results in smooth muscle contraction thus promoting intestinal motility.29 A similar phenomenon could underlie histamine-induced depolarization and contraction in simian colonic smooth muscle since H1 and H4 receptors are coupled to Gq/11 and Gi/o respectively. In equine tracheal SMC, intracellular Ca2+ release through stimulation of the H1/Gq/11 pathway was necessary for histamine-induced activation of NSCC. More importantly, H1 coupled Gi/o proteins were essential for activation of these NSCC as dialysis of anti-Gαi/Gαo antibodies blocked the effect of histamine.28 In the present study, when the effects of H1 and H4 agonists are compared in simian colonic smooth muscle, the contractile response to the H4 agonist, MHDC, closely resembles that induced by histamine. These data suggest that stimulation of the H4/ Gi/o pathway and subsequent activation of specific NSCC linked with these G-proteins underlie the major effects of histamine in simian colonic excitability. Furthermore it has been reported that H4 receptors have a higher affinity for histamine than the H1 receptor.30 Molecular identification of the NSCC targeted by histamine in simian colonic muscle will require further investigation and efforts will lie in examination of members of the Transient Receptor Potential Canonical (TRPC) family since some are reported to be regulated by Gi/o and Gq/11 pathways.31
In murine colon, histamine application induced transient hyperpolarization. We hypothesized that activation of the H2 receptor could result in activation of K+ channels to account for this effect. H2 receptors are positively coupled to adenylate cyclase via Gs, which is a potent stimulant of cAMP production and results in activation of PKA.32 Perforated patch clamp experiments revealed that histamine activated KATP-sensitive currents. In addition, pretreatment with the KATP blocker, glibenclamide, prevented histamine-induced hyperpolarization in colonic smooth muscle. Therefore, histamine exerts its effects in murine colonic smooth muscle via stimulating the H2/Gs/cAMP pathway resulting in activation of KATP channels through increases in PKA.
In the present study, electrical and contractile responses to histamine were different in murine and simian colonic tissue. Histamine caused a transient decrease in the force of contractions in murine colon and furthermore this transient decrease became sustained following inhibition of neuronal influences by pretreatment with TTX. Although TTX does not block all neural responses, in particular those at nerve varicosities, these data suggest indirectly that there is a histamine-sensitive neuronal component that contributes to the contractile response to histamine in murine colonic smooth muscle under physiological conditions. Wood and colleagues have documented multiple actions of histamine on the intestinal myenteric and submucosal plexuses in several different animal species including a suppressing effect of histamine on sympathetic inhibitory input to submucosal secretomotor neurons via presynaptic H3 receptors in guinea pig small intestine.33, 34 Furthermore, in human small intestine and colon, histamine causes excitation of submucosal plexus neurons.6 In contrast, the excitatory effects of histamine on simian colons in the presence and absence of TTX were not different suggesting that these responses may be due to stimulation of cells other than neurons (such as SMC, interstitial cells of Cajal etc.). H1 and H4 agonists increased simian colonic contractile force whereas the H2 agonist had the opposite effect. There was no significant difference in expression levels of H1, H2 and H4 receptors in simian colonic longitudinal tissue suggesting that the contractile effects caused by co-stimulation of H1 and H4 receptors by histamine appear to dominate the inhibitory effects of H2 activation.
In conclusion, our data demonstrate that the expression of H receptors and responses to histamine in relation to colonic contractility and membrane potential are different between mouse and monkey. Since H receptor expression in simian colon is closer to that reported in human7, we feel that it is a more suitable model to predict the effects of the inflammatory mediator, histamine, on colonic motility in humans.
Acknowledgments
This work was supported by NIH/NIDDK PO1- 41315 for KMS and SDK. We would like to thank Charles River Laboratories for the generous donation of monkey proximal colon samples.
Abbreviations
- AUC
area under the curve
- DSS
dextran sulphate sodium
- GBC
glibenclamide
- GI
gastrointestinal
- H
histamine
- HTMT
histamine-trifluoromethyl-toluidine
- IBD
inflammatory bowel disease
- MHDC
4-methylhistamine dihydrochloride
- NSCC
non-selective cation channels
- PKA
protein kinase A
- SK
small conductance K+ channel
- SMC
smooth muscle cells
- TTX
tetrodotoxin
Footnotes
Author contributions: H.K. and J.H.S. performed the intracellular and contractile recordings. J.B. performed contractile recordings. F.E.M.C, S.D.K. and L.D. performed the patch clamp experiments. F.B. and L.P. carried out the molecular studies. S.D.K. and K.M.S. were involved in conception and design of experiments. S.D.K., K.M.S. and L.D. contributed to interpretation of data and drafting of the manuscript.
Competing interests: The authors have no competing interests to disclose.
References
- 1.Gelbmann CM, Mestermann S, Gross V, et al. Structures in Crohn's disease are characterized by an accumulation of mast cells colocalised with laminin but not fibronectin or vitronectin. Gut. 1999;45:210–7. doi: 10.1136/gut.45.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy. Implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Therapeut. 2007;116:207–35. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Dy M, Schneider E. Histamine-cytokine connection in immunity and hematopoesis. Cytokine Growth Factor Rev. 2004;15:393–410. doi: 10.1016/j.cytogfr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood JD. Histamine, mast cells, and the enteric nervous system in the irritable bowel syndrome, enteritis, and food allergies. Gut. 2006;55:445–447. doi: 10.1136/gut.2005.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breunig E, Michel K, Zeller F, et al. Histamine excites neurons in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol. 2007;583.2:731–742. doi: 10.1113/jphysiol.2007.139352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sander LE, Lorentz A, Sellge G, et al. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut. 2006;55:498–504. doi: 10.1136/gut.2004.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz J, Vizuete ML, Traiffort E, et al. Localization of the histamine H2 receptor and gene transcripts in rat stomach: back to parietal cells. Biochem Biophys Res Commun. 1994;198:1195–1202. doi: 10.1006/bbrc.1994.1169. [DOI] [PubMed] [Google Scholar]
- 9.Héron A, Rouleau A, Cochois V, et al. Expression analysis of the histamine H(3) receptor in developing rat tissues. Mech Dev. 2001;105:167–73. doi: 10.1016/s0925-4773(01)00389-6. [DOI] [PubMed] [Google Scholar]
- 10.Oda T, Morikawa N, Saito Y, et al. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–86. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Wilson SJ, Kuei C, et al. Comparison of human, mouse and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther. 2001;299:121–30. [PubMed] [Google Scholar]
- 12.Bennett A, Whitney B. A pharmacological study of the motility of the human gastrointestinal tract. Gut. 1966;7:307–16. doi: 10.1136/gut.7.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolton TB, Clark JP, Kitamura, et al. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981;320:363–79. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amberg GC, Koh SD, Perrino BA, et al. Regulation of A-type potassium channels in murine colonic myocytes by phosphatase activity. Am J Physiol Cell Physiol. 2001;281:C2020–C2028. doi: 10.1152/ajpcell.2001.281.6.C2020. [DOI] [PubMed] [Google Scholar]
- 15.Dwyer L, Kim HJ, Koh BH, et al. Phospholipase C-independent effects of 3M3FBS in murine colon. Eur J Pharmacol. 2010;628:187–94. doi: 10.1016/j.ejphar.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim HD, van Rijn RM, Ling P, et al. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–21. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- 17.Koh SD, Sanders KM, Carl A. Regulation of smooth muscle delayed rectifier K+ channels by protein kinase A. Plugers Arch. 1996;432:401–12. doi: 10.1007/s004240050151. [DOI] [PubMed] [Google Scholar]
- 18.Koh SD, Bradley KK, Rae MG, et al. Basal Activation of ATP-sensitive Potassium Channels in Murine Colonic Smooth Muscle Cell. Biophysical Journal. 1998;75:1793–800. doi: 10.1016/S0006-3495(98)77621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermillion DL, Huizinga JD, Riddell RH, et al. Altered small intestinal smooth muscle function in Crohn's disease. Gastroenterol. 1993;104:1692–699. doi: 10.1016/0016-5085(93)90647-u. [DOI] [PubMed] [Google Scholar]
- 20.Galeazzi F, Haapala EM, Rooijen NV, et al. Inflammation-induced impairment of enteric nerve function in nematode-infected mice is macrophage dependent. Am J Physiol Liver Physiol. 2000;278:G259–65. doi: 10.1152/ajpgi.2000.278.2.G259. [DOI] [PubMed] [Google Scholar]
- 21.Vrees MD, Pricolo VE, Potenti FM, et al. Abnormal Motility in Patients With Ulcerative Colitis. Arch Surg. 2002;137:439–45. doi: 10.1001/archsurg.137.4.439. [DOI] [PubMed] [Google Scholar]
- 22.Iba Y, Sugimoto Y, Kamei C, et al. Possible role of mucosal mast cells in the recovery process of colitis induced by dextran sulfate sodium in rats. International Immunopharmacol. 2003;3:485–91. doi: 10.1016/S1567-5769(02)00299-0. [DOI] [PubMed] [Google Scholar]
- 23.Lilja I, Gustafson-Svard C, Franzen L, et al. Digestion. 2000;61:68–76. doi: 10.1159/000007737. [DOI] [PubMed] [Google Scholar]
- 24.Middel P, Reich K, Polzien F, et al. Interleukin 16 expression and phenotype of interleukin 16 producing cells in Crohn's disease. Gut. 2001;49:795–803. doi: 10.1136/gut.49.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002;104:185–92. doi: 10.1078/0065-1281-00641. [DOI] [PubMed] [Google Scholar]
- 26.Boer K, Helinger E, Helinger A, et al. Decreased expression of histamine H1 and H4 receptors suggests disturbance of local regulation in human colorectal tumors by histamine. Eur J Cell Biol. 2008;87:227–36. doi: 10.1016/j.ejcb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Bakker RA, Schoonus SBJ, Smit MJ, et al. Histamine H1-Receptor Activation of Nuclear Factor-kB: Roles for Gβγ- and Gαq/11-Subunits in Constitutive and Agonist-Mediated Signaling. Mol Pharmacol. 2001;60:1133–42. doi: 10.1124/mol.60.5.1133. [DOI] [PubMed] [Google Scholar]
- 28.Wang YX, Kotlikoff MI. Signalling pathway for histamine activation of non-selective cation channels in equine tracheal myocytes. J Physiol. 2000;523.1:131–38. doi: 10.1111/j.1469-7793.2000.t01-3-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zholos AV. Regulation of TRP-like muscarinic cation current in gastrointestinal smooth muscle with special reference to PLC/InsP3/Ca2+ system. Acta Pharmacol. 2006;27:833–42. doi: 10.1111/j.1745-7254.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 30.Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. B J Pharmacol. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsvilovskyy VV, Zholos AV, Aberle T, et al. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterol. 2009;137:1415–24. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 33.Wood JD. Enteric Neuroimmunophysiology and Pathophysiology. Gastroenterol. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Xia Y, Hu H, et al. Histamine H3 receptor-mediated suppression of inhibitory synaptic transmission in the submucous plexus of guinea-pig small intestine. Eur J Pharmacol. 2000;397:49–54. doi: 10.1016/s0014-2999(00)00228-4. [DOI] [PubMed] [Google Scholar]