Abstract
Study Objectives:
To evaluate the next-morning residual effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects following bedtime dosing and a middle of the night awakening.
Design:
Single-center, randomized, double-blind, double-dummy, placebo-controlled, crossover study.
Setting:
Utrecht University, The Netherlands.
Participants:
30 healthy volunteers (15 males and 15 females).
Interventions:
a single dose of ramelteon (8 mg), zopiclone (7.5 mg), and placebo, administered at bedtime.
Measurements:
A balance test was performed at night. Other tests were performed the following morning, 8.5 h after administration. Subjects performed a 100-km highway driving test in normal traffic. Primary outcome measure was the standard deviation of the lateral position (SDLP), i.e., the weaving of the car. After driving, cognitive, memory, and psychomotor tests were performed and mood was assessed.
Results:
SDLP was significantly increased after the intake of ramelteon (+2.2 cm) and zopiclone (+2.9 cm). Ramelteon and zopiclone produced significant impairment on reaction time (P < 0.024) in the Sternberg Memory Scanning Test, slow (P < 0.007) and fast (P < 0.010) tracking, reaction speed (P < 0.015) and tracking (P < 0.001) in the Divided Attention Test, and delayed recall (P < 0.032) in the Word Learning Test. In contrast to ramelteon, zopiclone additionally impaired performance on the Digit Symbol Substitution Test (P < 0.001) and the balance test (P < 0.001).
Conclusions:
Ramelteon (8 mg) and zopiclone (7.5 mg) significantly impaired driving performance, cognitive, memory, and psychomotor performance the morning following bedtime administration. In contrast to zopiclone, ramelteon produced no balance impairments.
Clinical Trial Identifier:
Citation:
Mets MAJ; de Vries JM; de Senerpont Domis LM; Volkerts ER; Olivier B; Verster JC. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. SLEEP 2011;34(10):1327–1334.
Keywords: Driving, ramelteon, zopiclone, memory, balance, psychomotor
INTRODUCTION
The most commonly prescribed drugs in the treatment of insomnia are benzodiazepines and the “Z-drugs” (i.e., zaleplon, zopiclone, and zolpidem). These hypnotics bind to the γ-aminobutyric acid-A (GABAA) receptor complex, which mediates a large number of physiological functions including sedation, anxiolytic effects, and muscle relaxation.1 In this fashion, these drugs are able to initiate and maintain sleep. Unfortunately, they may also produce next-morning residual effects, including drowsiness and cognitive and psychomotor impairment. These unwanted residual effects are of specific concern to patients who want to drive a car the morning after having taken a sleep medication. On-the-road driving studies have shown that benzodiazepine hypnotics significantly impair driving performance.2,3 Consistent with these findings, epidemiological studies have demonstrated an increased risk of traffic accidents and related injuries in patients using benzodiazepine hypnotics.4–6 Significant driving impairment has also been found for zopiclone,7–9 comparable to that observed with benzodiazepine hypnotics. In fact, Barbone et al.6 reported a four-fold increase in traffic accident risk for patients treated with zopiclone.
The other “Z-Drugs,” zolpidem and zaleplon, do not impair driving ability when used as recommended.9–11 However, with higher dosages and shortened time between drug use and driving, zolpidem may seriously compromise traffic safety.12,13 In addition to an increased risk of traffic accidents, hypnotic drugs may also have a negative effect on postural balance,14 increasing the risk of falls and hip fractures, especially in the elderly.15 This highlights a need for the development of new hypnotics without these unwanted residual effects.
Ramelteon is the first drug in a novel class of hypnotics currently licensed for the treatment of chronic insomnia in the US, Japan, Philippines, Indonesia, and Taiwan. Ramelteon is a selective melatonin receptor agonist and exerts its effect through selectively binding to melatonin receptors 1 and 2 (MT1/2), thereby inducing sleep.16 Since the sleep-promoting effect of ramelteon does not incorporate the sedative effects associated with the GABA-benzodiazepine receptor agonists, clinically important sedative and performance-impairing effects of ramelteon are minimal.17–19 Ramelteon has minimal affinity for benzodiazepine, dopamine and opiate receptors, ion channels, and a number of receptor transporters.17 However, its active metabolite M-II has weak affinity for the serotonin 5-HT2B receptor.20 Until now, no clinically relevant next-morning residual effects on cognitive or memory functioning have been reported.18,19,21–27 Consequently, it is expected that ramelteon will not show the next-morning impairing effects that are common with benzodiazepine hypnotics. The aim of the present study was to examine the residual effects of ramelteon (8 mg) versus placebo on driving performance, memory, and cognitive and psychomotor functioning. Because of its known impairing effects on driving,2,3 zopiclone (7.5 mg) was included as positive control.
METHODS
Subjects
Thirty healthy volunteers (15 male, 15 female) were recruited by means of public advertisements on and around the campus of Utrecht University. Inclusion criteria for subjects were: age 21-55 years, body mass index 18-34 kg/m2, normal vision, and possession of a valid driver's license ≥ 3 years with a reported average annual mileage ≥ 5,000 km during the 3 years prior to inclusion in the study. To exclude subjects with any evidence of clinically relevant diseases, each subject was medically examined before inclusion. This screening comprised clinical laboratory tests (hematology, serum chemistry, and urinalysis), 12-lead electrocardiogram, urine pregnancy test (for women), urine drug screen, and an alcohol breath test. Subjects were excluded if they had abnormal findings in the laboratory tests; history or presence of any clinically significant physical or mental disease; history of alcohol or drug dependence; past or current drug use; excessive caffeine consumption (> 5 cups/day), smoking (> 10 cigarettes per day), or alcohol intake (> 21 drinks per week). On the last visit, 1 week after the last experimental session, subjects underwent a post-study medical examination. The use of concomitant medication was prohibited except for oral contraceptives and peripherally acting analgesics. Alcohol was prohibited from 24 h before each dose of study drug until the end of the test day. Smoking and caffeine were not permitted on test days. The study was conducted according to good clinical practice as laid down in the Declaration of Helsinki and its latest amendments. The medical ethics committee approved the protocol, and participants provided written informed consent prior to enrollment.
Study Design and Procedure
This was a double-blind, double-dummy, randomized, placebo-controlled, 3-way crossover study in healthy adult volunteers examining the single-dose effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo.
Subjects participated in 1 training session and 3 experimental sessions, with wash-out periods ≥ 1 week between sessions. They were habituated to the sleep facility during a sleep rehearsal night. Subsequently, subjects' eligibility to participate in the study was assessed during the training session. Participants were included if the standard deviation of the lateral position (SDLP) at the end of the training driving test did not exceed 24 cm. In addition, subjects were familiarized with the procedures and were trained on the tests (car driving, balance and psychometric test battery) to avoid learning effects in the experimental sessions.
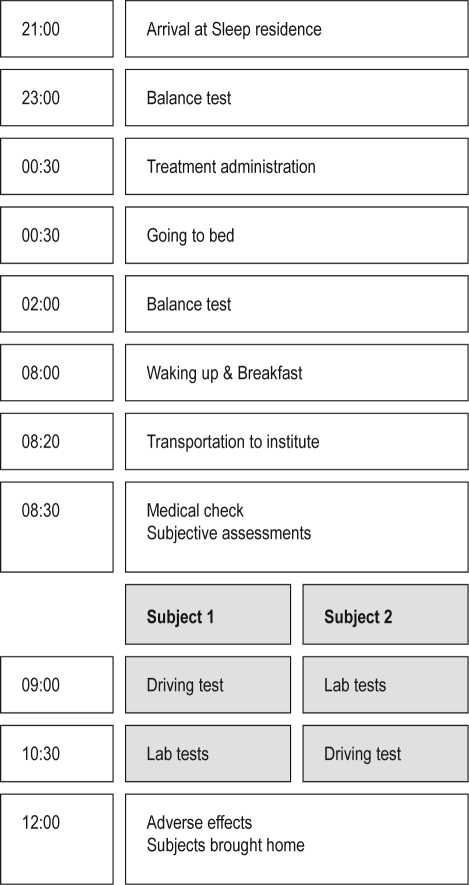
Figure 1 displays the flow chart of a regular test day. During each experimental session, 2 subjects reported to the sleep residence at 21:00. At 23:00, a balance test was performed. Immediately before bedtime and lights out, at 00:30, each subject received a single oral dose of ramelteon 8 mg, zopiclone 7.5 mg, or placebo administered with water. To ensure study blinding, treatment was administered in double-dummy fashion. Subjects received one tablet of zopiclone or matching placebo and one capsule of ramelteon or matching placebo. At 02:00, 1.5 h after drug intake and at peak plasma concentration, the subjects were woken up for the balance test. After the test, subjects went back to sleep and were woken up at 08:00. A light, standard breakfast was provided, and at 08:20 subjects were taken to the institute where they received a medical check. At 09:00, 8.5 h after treatment administration, one subject started the driving test, while the second subject started the laboratory tests. At 10:30, 10 h after treatment administration, the first subject started the laboratory test and the second subject started the driving test. At 12:00, subjects underwent a short medical check for adverse events and were brought home.
Figure 1.
Flow chart of each test day.
The On-the-Road Driving Test
A standardized method of measuring driving ability, the on-the-road driving test28 was performed on a 100 km-primary highway circuit, i.e., the primary highway (A12) between the cities of Utrecht and Arnhem. To guard safety during the test, a licensed driving instructor who had access to dual controls accompanied the subject.
Subjects were instructed to drive with a steady lateral position between the delineated boundaries of the right (slower) traffic lane while maintaining a constant speed of 95 km/h. A camera mounted on the roof of the car continuously recorded the position of the car within the traffic lane, by measuring the relative distance of the car from the left lane delineation. The speed and lateral position of the car were continuously recorded, digitally sampled at 2 Hz, and edited off-line to remove data that were disturbed by extraneous events (e.g., overtaking maneuvers, traffic jam). The standard deviation of lateral position (SDLP), i.e., the amount of weaving of the car, was the primary outcome measure. Standard deviation of speed (SDS, km/h) was the secondary outcome measure. Mean lateral position (MLP, +/− cm) to the right (+) or left (−) of the lane center, and mean speed (MS, km/h) were control variables.
Subjective Assessments
After the driving test, subjects indicated the perceived quality of their driving performance on a visual analog scale, which ranged from “I drove exceptionally poorly” to “I drove exceptionally well” around a midpoint of “I drove normally.” The level of effort they had to invest in performing the task was indicated on a 15-cm equal-interval scale.
The ARCI-49 questionnaire29 was also completed after the driving test. It comprised 5 scales that indicated mood changes accompanying different drug classes: pentobarbital-chlorpromazine-alcohol group scale (measuring sedation), Benzedrine group scale (measuring intellectual efficacy and energy), morphine-Benzedrine group scale (measuring euphoria), amphetamine scale (measuring amphetamine-like effects), and the lysergic acid diethylamide scale (measuring dysphoria and somatic symptoms).
Sleep Quality
Each morning after waking up, quality of sleep was assessed using the 14-item Groningen Sleep Quality Scale (GSQS).30 GSQS scores range from 0 to 14. The GSQS has previously been used in patients with seasonal affective disorder31 and shift-workers.32 In general, if sleep is unrestricted and undisturbed, subjects score 0 to 2 points. A higher score (6 to 7) indicates disturbed sleep.
Balance Test
At presumed peak plasma concentration,18,33 i.e., 1.5 h after bedtime, subjects were woken to perform the balance test. Body sway was measured using the AccuSway Plus platform (Advance Medical Technology, Massachusetts, USA). Subjects were instructed to stand on the balance platform with their arms at their side. Subjects' balance was recorded standing on the platform for 60 sec with their eyes open and then 60 sec with their eyes closed. Recordings took place in a quiet room, and speaking was not allowed during the recordings. The primary performance measure was the change in center of pressure (CoP). Subjects performed the test approximately 5 min after waking up and returned to bed within 15 minutes.
Psychometric Test Battery
The psychometric test battery comprised 4 different tasks: Word Learning Test, Sternberg Test, Tracking Test, and Divided Attention Test, which were selected from a larger set of tasks predefined in ERTS 3.17 (Experimental Run Time System). Different versions of the tests were used at each training session and test day. The test battery was carried out with subjects seated at a table in a sound-attenuated test room with constant luminosity during the entire study.
Word Learning Test (immediate and delayed recall)
The Word Learning Test (WLT) is the Dutch language version of the standardized, clinically validated test for verbal memory. A list of 15 monosyllabic meaningful nouns was presented on a screen at a fixed rate of 2 sec per word. As soon as the presentation stopped, the subjects were given 1 min to write down as many words as they were able to remember. The highest separate test score of 5 subsequent trials was the Immediate Recall Score (WLT-IR). Following a 30-min delay in which the other psychometric tests were performed, subjects were asked to write down as many of the previously shown words as possible within 1 minute. The number of correct responses was the Delayed Recall Score (WLT-DR). Finally, a series of words were presented on the computer screen, including the original set and 15 distracter words in random order. Subjects indicated by button press whether the given word was part of the original set. The number of correct recognitions and the average speed (ms) of correct recognitions were recorded as the Recognition Score (WLT-RS) and the Recognition Time (WLT-RT).
Sternberg Memory Scanning Test (working memory)
Subjects were asked to memorize a list of successively presented digits (i.e., 0-9). Probe digits were subsequently presented on the computer screen. By pressing a button, subjects had to indicate whether a digit was part of the memory set. The mean reaction time (RT, ms) and percentage of errors were the outcome measures.
Tracking Test (motor control)
Participants were instructed to keep an unstable moving bar in the middle of a horizontal plane by using a computer mouse. The task consisted of 2 parts: a slow moving bar and a fast moving bar. The root mean square (RMS) deviation of the mouse movements was the primary response measure.
Divided Attention Test
The objective of this task was to memorize a fixed set of digits and subsequently perform 2 tasks simultaneously. After the presentation of the memory set, the subject had to keep an unstable slow moving bar in the middle of a horizontal plane. Simultaneously, probe digits were presented on the computer screen, on which the subject had to decide whether a digit was part of the initially presented memory set. Outcome measures were reaction time (ms), percentage of errors, and RMS deviation of the mouse movements.
Digit-symbol substitution test (DSST)
Subjects were presented with a code in which the numbers 0 to 9 were matched with a simple symbol, and with a list of digits randomly arranged in rows. Subsequently, participants were asked to attempt as many correct symbol-for-digit substitutions as possible within 1.5 minutes. Outcome measures were the total number of correctly completed pairs and the percentage of errors.
Statistical Analysis
Statistical analysis was performed using SAS, Version 8.2. The treatment groups were compared using a 3-way crossover analysis of variance (ANOVA) model with treatment, sequence, and period as fixed factors, and subject within sequence as a random effect. A pairwise comparison between the active treatments and placebo was conducted. Differences from placebo were considered significant if P < 0.05.
RESULTS
A total of 31 subjects were screened. One subject voluntarily withdrew from the study. Thirty subjects completed the study. Their mean age was 25.9 (6.53 SD) years old. The mean (SD) sleep quality score was 3.96 (3.77) for placebo, 5.22 (3.67) for ramelteon, and 2.63 (1.55) for zopiclone. Neither hypnotic significantly differed from placebo (P > 0.05). No significant order effects or gender effects were observed. Results of the driving test are summarized in Table 1 and Figure 2.
Table 1.
Driving test results
| Ramelteon | Zopiclone | Placebo | |
|---|---|---|---|
| SDLP (cm) | 20.9 (0.7)* | 21.6 (0.8)* | 18.7 (0.7) |
| SDS (km/h) | 2.7 (0.2) | 2.6 (0.1) | 2.5 (0.1) |
| MLP (cm) | 26.3 (3.0) | 26.7 (3.0) | 27.2 (3.1) |
| MS (km/h) | 93.5 (0.3) | 93.8 (0.3) | 93.8 (0.2) |
Data are reported as mean (SEM).
SDLP, standard deviation of lateral position; SDS, standard deviation of speed; MLP, mean lateral position; MS, mean speed; SEM, standard error of the mean.
P < 0.05 vs placebo.
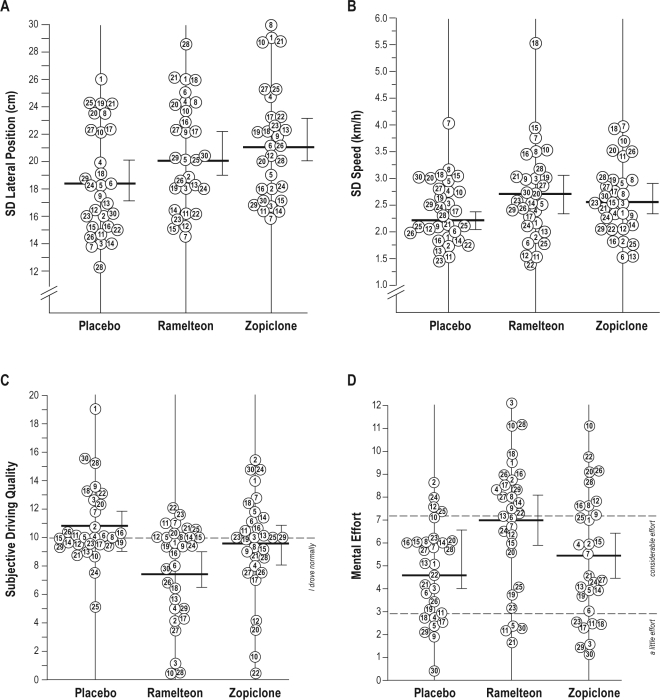
Figure 2.
Driving test results. Individual data for (A) standard deviation of lateral position (SDLP), (B) standard deviation of speed, (C) subjective driving quality, ranging from 0 meaning “I drove exceptionally poorly” to 20 “I drove exceptionally well” around a midpoint of 10 “I drove normally” and (D) mental effort to perform the test; examples of statements provided at anchorpoints along the VAS scale are displayed next to the dashed lines. Same numbers represent same subjects. Group mean and 95% confidence interval are indicated for each treatment.
All subjects completed their driving tests. Relative to placebo, ramelteon (+2.2 cm) and zopiclone (+2.9 cm) significantly increased SDLP (P < 0.001). Standard deviation of speed, mean speed, and mean lateral position did not differ significantly between treatments. When compared to placebo, subjective driving quality was rated as significantly worse after ramelteon (P < 0.001), but not after zopiclone. Also, mental effort to perform the test was significantly higher after ramelteon (P < 0.01), whereas no differences were reported between zopiclone and placebo. A summary of performance on the psychometric and balance tests are presented in Table 2.
Table 2.
Performance on the memory and psychomotor tests
| Ramelteon | Zopiclone | Placebo | |
|---|---|---|---|
| WLT-IR | 13.5 (0.4) | 13.1 (0.4) | 13.5 (0.3) |
| WLT-DR | 11.1 (0.6)* | 10.1 (0.6)* | 12.2 (0.5) |
| WLT-RS | 13.7 (0.3) | 13.6 (0.2) | 13.9 (0.2) |
| WLT-RT (ms) | 722.1 (33.2) | 767.4 (32.7) | 713.6 (31.3) |
| SMST-RT (ms) | 565.6 (22.2)* | 584.1 (19.3)* | 536.1 (20.1) |
| SMST- errors (%) | 5.2 (0.8) | 5.2 (0.9) | 4.2 (0.6) |
| Tracking-Slow | 11.1 (1.5)* | 11.0 (1.4)* | 8.6 (1.3) |
| Tracking-Fast | 26.1 (0.9)* | 25.5 (1.0)* | 23.8 (1.1) |
| DAT-tracking | 14.0 (1.5)* | 13.9 (1.5)* | 10.8 (1.5) |
| DAT- errors (%) | 4.3 (0.5) | 4.1 (0.5) | 6.7 (3.1) |
| DSST | 67.0 (2.1) | 64.3 (2.1)* | 68.5 (2.0) |
| COP-change from pre-dose (eyes open) | 1.5 (0.3) | 5.5 (1.1)* | 1.5 (0.6) |
| COP-change from pre-dose (eyes closed) | 1.2 (0.3) | 5.7 (1.5)* | 1.4 (0.6) |
Data are reported as mean (SEM).
WLT, Word Learning Test; IR, immediate recall; DR, delayed recall; RS, recognition score; RT, reaction time; SMST, Sternberg Memory Scanning Test; DAT, Divided Attention Test; DSST, digit symbol substitution test; COP, center of pressure.
P < 0.05 vs placebo.
In the word learning test, immediate word recall was not affected by any treatment. Also, no significant differences between treatments were found for word recognition. Delayed word recall was significantly impaired after ramelteon (P < 0.032) and zopiclone (P < 0.01). In the Sternberg Memory Scanning Test, mean reaction time was significantly higher after both ramelteon (P = 0.024) and zopiclone (P < 0.001) than after placebo. No differences from placebo were found in the percentage of errors in the Sternberg Memory Scanning Test. Ramelteon and zopiclone significantly impaired performance in the tracking test, in both the slow (P ≤ 0.007) and fast condition (P ≤ 0.010). In the Divided Attention Task, tracking was significantly impaired after both ramelteon and zopiclone (P < 0.001), and mean reaction speed was significantly slower after zopiclone (P = 0.007) and ramelteon (P = 0.015). There were no statistically significant differences between treatments in the percentage of errors. In the DSST, when compared to placebo, the number of correct pairs were significantly less after zopiclone (P < 0.001) but not after ramelteon (P = 0.084). On the balance test, ramelteon did not affect performance, whereas zopiclone increased postural sway in both eyes open and eyes closed conditions (P < 0.001).
Results from the ARCI-49 Questionnaire showed that when treated with ramelteon, subjects reported significantly less euphoria (P < 0.007), more dysphoric/somatic symptoms (P < 0.049), more sedation (P < 0.001), less intellectual efficiency (P < 0.0001), and less activation (P < 0.006), compared to placebo. After zopiclone treatment, subjects did not report any significant differences from placebo.
Adverse Events
No serious adverse events were reported. Somnolence, disturbance in attention, and fatigue were most frequently reported. Somnolence was reported by 20 subjects (66.7%) taking ramelteon, 22 subjects (73.3%) taking zopiclone, and 10 subjects (33.3%) taking placebo. Disturbance in attention was reported by 12 subjects (40.0%) taking ramelteon, 7 subjects (23.3%) taking zopiclone, and 6 subjects (20.0%) taking placebo. Fatigue was reported by 11 (36.7%) subjects taking ramelteon, 6 (20.0%) taking zopiclone, and 8 (36.7%) taking placebo.
DISCUSSION
Contrary to expectations, this study showed that on the morning following bedtime administration, ramelteon significantly impaired performance on driving, psychometric and memory tests and affected mood. In line with previous studies, zopiclone significantly impaired performance on most tests.
As established in studies on the effect of alcohol on driving performance, an increase in SDLP of 2.4 cm (i.e., the relative difference in weaving between placebo and the active substance) is considered to be the cut-off point for a clinically relevant difference. This SDLP increment was found previously for a blood alcohol concentration (BAC) of 0.05%, the legal limit for driving a car in many countries.34 A BAC of 0.05% is the point at which the ability to perform skills necessary for driving becomes affected and at which the risk of being involved in a traffic accident increases substantially.35–37 The mean increase in SDLP after the intake of ramelteon (+2.2 cm) was just below this limit. However, relative to placebo, one-third (10/30) of subjects receiving ramelteon had an SDLP increment of more than 2.4 cm. Zopiclone caused a mean SDLP increment of 2.9 cm, and more than half (17/30) of the subjects drove worse than the BAC 0.05% limit. The mean effect of zopiclone is comparable to that observed with a BAC between 0.05 and 0.08%.34
The clinical implication of these findings is that patients initiating treatment with ramelteon (and zopiclone) should be cautioned when they want to drive a car, because there is a potential risk that driving may be impaired the morning following bedtime administration. In this context, the VAS scales assessing subjective driving quality and mental effort to perform the driving test yielded important information. That is, whereas subjects acknowledged reduced subjective driving quality and increased mental effort to perform the driving test after using ramelteon, they did not after using zopiclone. Of concern, after using zopiclone subjects rated their driving quality as normal, whereas in fact driving was more impaired than after using ramelteon. Our findings for zopiclone were similar to those observed in previous on-the-road driving studies.38,39 In line with impairment on the driving test, both ramelteon and zopiclone produced significant impairment on psychomotor, memory, and cognitive tests. Similar to previous studies,21,23 and in contrast to benzodiazepine hypnotics,14 body balance, assessed in the middle of the night at peak plasma concentration, was not affected by ramelteon. In contrast, zopiclone significantly increased postural sway.
Significant next-day residual effects on psychomotor performance, memory, performance, and mood were found for both ramelteon and zopiclone. The effects of both hypnotics are comparable with those found in a study examining the effects of BAC 0.05%, using the same driving test and psychometric test battery.12
The unexpected results of this study are difficult to explain when compared to most previous studies as well as the kinetics of the drug. Effects of ramelteon on memory functioning have not been demonstrated in most other studies.18,21–25,27 However, a study including a simulated night shift demonstrated significant impairment on Probed Recall Memory recognition.40 Two studies using word learning tests similar to the one used here, also reported significant impairment, one in chronic insomniacs41 and one in subjects experiencing jet lag.42 The study in insomniacs demonstrated small decreases in delayed memory performance after one week of treatment with ramelteon (8 mg) and on immediate recall after three weeks of treatment. The jet lag study showed effects on immediate memory recall in all dosages (1, 4, and 8 mg) after four days of bedtime intake. In the present study, both immediate and delayed recall were tested in the morning after bedtime intake. In other studies, words were presented in the evening before ramelteon administration and delayed memory was tested in the morning. The fact that subjects were asleep between learning and retrieval may have facilitated retention,43 and may thus explain differences with the current study. One study in patients with chronic insomnia did conduct a memory test in the morning after intake, but measurements were performed not earlier than one week after treatment onset.24 It remains uncertain why pharmacodynamic studies performed during the day did not show negative effects.18,21,23
Regarding psychomotor functioning, no other study found an effect on DSST18,19,21,22,24–27,41 or on a circular lights test.21 In contrast to these studies, the present study used an elaborate test battery including more complex psychomotor tests, e.g., Sternberg memory scanning, tracking, and divided attention. There is a possibility that impairments in daytime functioning after bedtime intake of ramelteon cannot be revealed by simple tests of relative short duration. This would also explain why the night shift study, that also used an elaborate test battery, also reported significant impairment after administration of ramelteon.40 In the present study, duration of the driving test was about 1 hour, and most psychometric tests took about 8 to 10 minutes to complete. It can therefore be assumed that in the present study mental effort to perform the test battery and driving test was higher than tests performed in other clinical trials.
It is unlikely that performance of the middle-of-the night balance task (requiring a short disruption of sleep) may have influenced next-morning test performance in this cross-over design. Since the balance test was performed approximately five minutes after being woken up, sleep inertia most likely had already disappeared and did not influence results. The fact that zopiclone and ramelteon have a different mechanism of action may explain why zopiclone does impair balance and ramelteon does not.
Due to its half-life of 0.83 to 1.90 hours,19 it seems unlikely that ramelteon still had an effect in the morning. There is, however, a possibility that the active metabolite M-II, which has a half-life of 2.27 to 3.39 hours,19 caused the effects. Future studies should examine the possible causes of the residual effects of ramelteon.
Limitations
The absence of polysomnography as objective measure of sleep parameters, or any other objective measure of sleep, may be seen as a limitation of the current study. An important rationale for not conducting polysomnography was that the previous studies that specifically examined sleep found either an improvement with ramelteon relative to placebo, or no difference. In fact, in no study did ramelteon impair sleep.22,26 Thus, there was no a priori reason to expect sleep disturbance associated with ramelteon use, or much less than the degree of sleep disturbance to impair next-day performance.
In retrospect, given the surprising nature of our results, data on sleep and circadian phase would have been helpful in explaining these results. However, it must be recognized that explanations would not negate the current finding of impaired driving with ramelteon use.
In the current study, healthy volunteers were included instead of patients with insomnia. This was done on purpose. The vast majority of driving studies have been done in healthy volunteers44; similarly, on-the-road studies examining the residual effects of hypnotic drugs have been performed in healthy volunteers.2,3 These studies provide the most direct measure of drug effects, which also emphasizes the importance of our results. Studies in healthy volunteers are critical to understand the specific effects of drugs in the absence of disturbed sleep. In our study, disturbed sleep was an exclusion criterion, and all participants underwent a sleep rehearsal night. If studies in insomniacs would negate these residual effects, and if tolerance develops after daily use of ramelteon, are interesting topics for future research.
In summary, our data show that both ramelteon (8 mg) and zopiclone significantly impair next-morning driving performance, memory, cognitive, and psychomotor functioning. In contrast to zopiclone, ramelteon had significant effects on next-day mood but produced no impairment on the DSST or middle-of-the night balance test. Future studies should aim at finding explanations for the residual effects of ramelteon by assaying both sleep and circadian phase.
DISCLOSURE STATEMENT
The study was financially supported by Takeda Europe Research – Development Centre Ltd. Dr. Verster is advisor/consultant for Transcept, Red Bull GmbH, and Sepracor. Drs. Mets and Olivier have received research funding from Red Bull GmbH. The other authors have no other conflicts of interest to disclose.
Footnotes
A commentary on this article appears in this issue on page 1285.
REFERENCES
- 1.Ebert B, Wafford KA, Deacon S. Treating insomnia: Current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–29. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev. 2004;8:309–25. doi: 10.1016/j.smrv.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Verster JC, Veldhuijzen DS, Patat A, Olivier B, Volkerts ER. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63–71. doi: 10.2174/157488606775252674. [DOI] [PubMed] [Google Scholar]
- 4.Neutel CI. Risk of traffic accident injury after a prescription for a benzodiazepine. Ann Epidemiol. 1995;5:239–244. doi: 10.1016/1047-2797(94)00112-7. [DOI] [PubMed] [Google Scholar]
- 5.Hemmelgarn B, Suissa S, Huang A, Biovin JF, Pinard G. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA. 1997;278:27–31. [PubMed] [Google Scholar]
- 6.Barbone F, McMahon AD, Davey PG, et al. Association of road traffic accidents with benzodiazepine use. Lancet. 1999;352:1331–6. doi: 10.1016/s0140-6736(98)04087-2. [DOI] [PubMed] [Google Scholar]
- 7.Volkerts ER, Louwerens JW, Gloerich ABM, Brookhuis KA, O'Hanlon JF. Haren, The Netherlands: Traffic Research Centre, University of Groningen; Zopiclone's residual effect upon actual driving performance versus those of nitrazepam and flunitrazepam. (Report VK 84-10) [Google Scholar]
- 8.Vermeeren A, Danjou PE, O'Hanlon JF. Residual effects of evening and middle-of-the-night administration of zaleplon 10 and 20 mg on memory and actual driving performance. Hum Psychopharmacol Clin Exp. 1998;13:S98–S107. [Google Scholar]
- 9.Vermeeren A, Riedel W, Van Boxtel MPJ, Darwish M, Paty I, Patat A. Differential residual effects of zaleplon and zopiclone on actual driving: a comparison with a low dose of alcohol. Sleep. 2002;25:224–31. [PubMed] [Google Scholar]
- 10.Vermeeren A, O'Hanlon JF, DeClerck AC, et al. Acute effects of zolpidem and flunitrazepam on sleep, memory and driving performance, compared to those of partial sleep deprivation and placebo. Acta Ther. 1995;21:47–64. [Google Scholar]
- 11.Vermeeren A, Danjou PE, O'Hanlon JF. Residual effects of evening and middle-of-the-night administration of zaleplon 10 and 20 mg on memory and actual driving performance. Hum Psychopharmacol Clin Exp. 1998;13:S98–S107. [Google Scholar]
- 12.Verster JC, Volkerts ER, Schreuder AHCML, et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions and psychomotor performance. J Clin Psychopharmacol. 2002;22:576–83. doi: 10.1097/00004714-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Verster JC, Volkerts ER, Johnson W, Liddicoat L. Zoplidem and traffic safety – the importance of treatment compliance. Curr Drug Safety. 2007;2:220–6. doi: 10.2174/157488607781668882. [DOI] [PubMed] [Google Scholar]
- 14.Mets MAJ, Volkerts ER, Olivier B, Verster JC. Effect of hypnotic drugs on body balance and standing steadiness. Sleep Med Rev. 2010;14:259–67. doi: 10.1016/j.smrv.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Allain H, Bentué-Ferrer D, Polard E, Akwa Y, Patat A. Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly. A comparative review. Drugs Aging. 2005;22:749–65. doi: 10.2165/00002512-200522090-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301–10. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.France CP, Weltman RH, Koek W, Cruz CM, McMahon LR. Acute and chronic effects of ramelteon in rhesus monkeys (Macaca mulatta): dependence liability studies. Behav Neurosci. 2006;120:535–41. doi: 10.1037/0735-7044.120.3.535. [DOI] [PubMed] [Google Scholar]
- 18.Greenblatt DJ, Harmatz JS, Karim A. Age and gender effects on the pharmacokinetics and pharmacodynamics of ramelteon, a hypnotic agent acting via melatonin receptors MT1 and MT2. J Clin Pharmacol. 2007;47:485–96. doi: 10.1177/0091270006298602. [DOI] [PubMed] [Google Scholar]
- 19.Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006;46:140–8. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto M, Kato K, Hirai K, et al. TAK-375 and its active metabolite: lack of binding to non-ML receptor bindings sites [abstract no. 0194.C] Sleep. 2003;26(Abstr Suppl):A79. [Google Scholar]
- 21.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63:1149–57. doi: 10.1001/archpsyc.63.10.1149. [DOI] [PubMed] [Google Scholar]
- 22.Zammit G, Schwartz H, Roth T, Wang-Weigand S, Sainati S, Zhang J. The effects of ramelteon in a first-night model of transient insomnia. Sleep Med. 2009;10:55–9. doi: 10.1016/j.sleep.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Zammit G, Wang-Weigand S, Rosenthal M, Peng X. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5:34–40. [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32:351–60. doi: 10.1093/sleep/32.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28:303–7. [PubMed] [Google Scholar]
- 27.Roth T, Seiden D, Wang-Weigand S, Zhang J. A 2-night, 3-period, crossover study of ramelteon's efficacy and safety in older adults with chronic insomnia. Curr Med Res Opin. 2007;23:1005–14. doi: 10.1185/030079907x178874. [DOI] [PubMed] [Google Scholar]
- 28.O'Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Brit J Clin Pharmacol. 1984;18:121s–129s. doi: 10.1111/j.1365-2125.1984.tb02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haertzen CA, Hill HE, Belleville RE. Development of the Addiction Research Center Inventory (ARCI): Selection of items that are sensitive to the effects of various drugs. Psychopharmacologia. 1963;4:155. doi: 10.1007/BF02584088. [DOI] [PubMed] [Google Scholar]
- 30.Mulder-Hajonides van der Meulen WREH, Wijnberg JR, Hollander JJ, De Diana IPF, van den Hoofdakker RH. Measurement of subjective sleep quality. Eur Sleep Res Soc. 1980;5:98. [Google Scholar]
- 31.Meesters Y, Jansen JHC, Lambers PA, Bouhuys AL, Beersma DGM, van den Hoofdakker RH. Morning and evening light treatment of seasonal affective disorder: response, relapse and prediction. J Affect Disord. 1993;28:165–77. doi: 10.1016/0165-0327(93)90102-p. [DOI] [PubMed] [Google Scholar]
- 32.Meijman TF, Thunnissen MJ, de Vries-Griever AGH. The after-effects of a prolonged period of day-sleep on subjective sleep quality. Work Stress. 1990;4:65–70. [Google Scholar]
- 33.Villain M, Chèze M, Tracqui A, Ludes B, Kintz P. Testing for zopiclone in hair application to drug-facilitated crimes. Forensic Sci Int. 2004;145:117–21. doi: 10.1016/j.forsciint.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Louwerens JW, Gloerich ABM, De Vries G, Brookhuis KA, O'Hanlon JF. The relationship between drivers, blood alcohol concentration (BAC) and actual driving performance during high speed travel. In: Noordzij PC, Roszbach R, editors. Alcohol, Drugs and Traffic Safety - T86. Amsterdam: Excerpta Medica; 1987. pp. 183–92. [Google Scholar]
- 35.Moskowitz H, Fiorentino D. Washington, DC: US Department of Transportation, National Highway Traffic Safety Administration; 2000. A review of the literature on the effects of low doses of alcohol on driving-related skills. Report DOT HS 809 028. [Google Scholar]
- 36.Borkenstein RF, Crowther RP, Shumate RP, Zeil WW, Zylman R. Bloomington, IN: Department of Police Administration, Indiana University; 1964. The role of drinking drivers in traffic accidents. [Google Scholar]
- 37.Compton RP, Blomberg RD, Moskowitz H, Burns M, Peck RC, Fiorentino D. Crash risk of alcohol impaired driving. In: Mayhew DR, Dussault C, editors. Proceedings of alcohol, drugs and traffic safety- T 2002: 16th international conference on alcohol, drugs and traffic safety; August 4-9, 2002; Montreal, Canada: International Council on Alcohol, Drugs and Traffic Safety (ICADTS); 2002. pp. 39–44. [Google Scholar]
- 38.Volkerts ER, Louwerens JW, Gloerich ABM, Brookhuis KA, O'Hanlon JF. Groningen, The Netherlands: Traffic Research Centre; 1984. Zopiclone's residual effect upon actual driving performance versus those of nitrazepam and flunitrazepam. VSC, Report 84-10. [Google Scholar]
- 39.Leufkens TR, Lund JS, Vermeeren A. Highway driving performance and cognitive functioning the morning after bedtime and middle-of-the-night use of gaboxadol, zopiclone and zolpidem. J Sleep Res. 2009;18:387–96. doi: 10.1111/j.1365-2869.2009.00746.x. [DOI] [PubMed] [Google Scholar]
- 40.Cohen DA, Wang W, Klerman EB, Rajaratnam SMW. Ramelteon prior to a short evening nap impairs neurobehavioral performance for up to 12 hours after awakening. J Clin Sleep Med. 2010;6:565–71. [PMC free article] [PubMed] [Google Scholar]
- 41.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med. 2007;3:495–504. [PMC free article] [PubMed] [Google Scholar]
- 42.Zee PC, Wang-Weigand S, Wright KP, Peng X, Roth T. Effects of ramelteon on insomnia symptoms induced by rapid, eastward travel. Sleep Med. 2010;11:525–33. doi: 10.1016/j.sleep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Ficca G, Salzarulob P. What in sleep is for memory. Sleep Med. 2004;5:225–30. doi: 10.1016/j.sleep.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Verster JC, Roth T. Standard operation procedures for conducting the on-the-road driving test, and measurement of the standard deviation of lateral position (SDLP) Int J Gen Med. 2011;4:359–71. doi: 10.2147/IJGM.S19639. [DOI] [PMC free article] [PubMed] [Google Scholar]