Abstract
In rats with salt-induced hypertension or post myocardial infarction (MI), AT1 receptor (AT1R) densities and oxidative stress increase and neuronal nitric oxide synthase (nNOS) levels decrease in the paraventricular nucleus (PVN). The present study was designed to determine whether these changes may depend on activation of the aldosterone – “ouabain” neuromodulatory pathway. After intracerebroventricular (icv) infusion of aldosterone (20ng/h) for 14 days, blood pressure (BP) and heart rate (HR) were recorded in conscious Wistar rats, and mRNA and protein for nNOS, endothelial NOS (eNOS), AT1R and NADPH oxidase subunits were assessed in brain tissue. BP and HR were significantly increased by aldosterone. Aldosterone significantly increased mRNA and protein of AT1R, P22phox, P47phox, P67phox and Nox2, and decreased nNOS but not eNOS mRNA and protein in the PVN, and increased angiotensin converting enzyme (ACE) and AT1R binding densities in the PVN and SON. The increases in BP and HR as well as changes in mRNA, proteins and ACE and AT1R binding densities were all largely prevented by concomitant icv infusion of Digibind (to bind “ouabain”) or benzamil (to block presumably epithelial sodium channels). These data indicate that aldosterone via “ouabain” increases in the PVN ACE, AT1R and oxidative stress but decreases nNOS, and suggest that endogenous aldosterone may cause this similar pattern of changes observed in salt sensitive hypertension and heart failure post MI.
Keywords: brain aldosterone, NADPH oxidase, oxidative stress, NO, AT1R, “ouabain”, sodium channels
Introduction
Activation of the brain renin-angiotensin-aldosterone system (RAAS) plays an essential role in hyperactivity of the sympathetic nervous system and progressive cardiac dysfunction post myocardial infarction (MI) and salt-sensitive hypertension in rats (Wang et al., 2004; Huang et al., 2006a; Yu et al., 2008). Hypothalamic aldosterone content increases and angiotensin converting enzyme (ACE) and AT1 receptor (AT1R) mRNA and protein or binding, densities increase in several hypothalamic nuclei in Dahl salt-sensitive (S) rats and rats post MI (Wang et al., 2003b; Tan et al., 2004; Yu et al., 2008). In rats post MI, neuronal NO synthase (nNOS) mRNA is decreased (Guggilam et al., 2008), and NO-mediated inhibition of RSNA is blunted (Zhang et al., 2001). In the paraventricular nucleus (PVN), AT1R stimulation appears to increase neuronal oxidative stress, decrease nitric oxide (NO), and to blunt NO-mediated inhibition of sympathetic nerve activity (Campese et al., 2002; Campese et al., 2005).
General blockade of mineralocorticoid receptors (MR) in the central nervous system (CNS) by intra-cerebroventricular (icv) infusion of an MR antagonist prevents sympathetic hyperactivity in Dahl S on high salt (Gomez-Sanchez et al., 1992; Huang et al., 2009) or rats post MI (Francis et al., 2001; Huang & Leenen, 2005), and attenuates the up-regulation of AT1R and ACE and increase in oxidative stress in the hypothalamus (Yu et al., 2008). Icv infusion of aldosterone causes sympathetic hyperactivity and hypertension in rats (Wang et al., 2003a; Huang et al., 2005; Zhang et al., 2008), associated with increases in ACE and AT1R mRNA in the hypothalamus and oxidative stress in the PVN (Zhang et al., 2008). Functionally, central effects of aldosterone depend on binding to MR (Zhang et al., 2008), activation of sodium channels, presumably epithelial sodium channel (ENaC) (Wang et al., 2003a), release of endogenous ouabain-like compounds (“ouabain”) (Wang et al., 2003a) and AT1R stimulation (Zhang et al., 2008). Activation of the aldosterone – MR – ENaC pathway may increase Na+ entry into neurons or glia and thereby contribute to release of “ouabain” from magnocellular neurons containing “ouabain” in the supraoptic nucleus (SON) or PVN (Yamada et al., 1992; Yoshika et al., 2011), or increase production/release of “ouabain” by astrocytes (Kala et al., 2000). “Ouabain” can inhibit Na+/K+-ATPase (Kent et al., 2004) and thereby lower the neuronal membrane potential or increase intracellular Ca2+ (Pulina et al., 2010) which can enhance activity of angiotensinergic sympatho-excitatory pathways. “Ouabain” also contributes to the increases in hypothalamic ACE and AT1R binding densities in rats post MI (Tan et al., 2004) or by central infusion of sodium (Huang et al., 2006b), as well as to the increase in ACE mRNA and activity in Dahl S rats on high salt (Zhang et al., 2008). We postulated that 1) “ouabain” also mediates the effects of aldosterone on AT1R and oxidative stress in the PVN; and 2) aldosterone decreases activity of the NO system in the PVN, also via release of “ouabain”.
For the present study, we selected the PVN as the main nucleus of interest (Zhang et al., 2008), and the SON as a possible nucleus involved in aldosterone- induced “ouabain” release (Yoshika et al., 2011). The cortex was used as a ‘control’. We examined in Wistar rats the effects of icv infusion of aldosterone on BP and HR, changes in mRNA expression and protein of AT1R, nNOS and eNOS, and NADPH oxidase subunits P22phox, P47phox, P67phox as well as the catalytic subunit Nox2 in the hypothalamic nuclei PVN and SON as well as cortex, with and without concomitant icv infusion of benzamil to block sodium channels or Digibind to bind “ouabain”. The results indicate that aldosterone mainly through sodium channels and “ouabain” release increases ACE and AT1R as well as expression of NADPH oxidase subunits and decreases activity of the NO system in the PVN.
Methods
Male Wistar rats weighing ~200 g (Charles River, Montreal, Canada) were housed 2 per cage, in a climatized room on a 12-h light/dark cycle at constant room temperature and humidity, and given standard laboratory chow (120 μmol Na+ per gram) and tap water ad libitum. All surgeries and in vivo experiments were performed at the University of Ottawa and were approved by the University of Ottawa Animal Care Committee, and conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication NO. 85-23, revised 1996).
Experimental Protocol
After 5 days of acclimatization, under isoflurane anesthesia, a 23-gauge right-angled stainless steel cannula was implanted into the left lateral cerebral ventricle and fixed to the skull of the rat with acrylic cement (Huang et al., 2005). The upper end of cannula was connected to an osmotic minipump (Model 2ML2, rate: 5μl/h, Alza, Palo Alto, CA.) for a two-week icv infusion. Two sets of 4 groups of rats were used. In each of the 2 sets, rats were allocated to one of the following treatments for 2 weeks: 1) icv infusion of aldosterone (20ng/h); 2) icv infusion of aldosterone (20ng/h) plus Digibind ® (Glaxo Wellcome, Canada) (7μg/h); 3) icv infusion of aldosterone (20ng/h) plus benzamil (Sigma-Aldrich Ltd, Canada) (1μg/h, in 15% propylene glycol); and 4) icv vehicle (15% polyethylene glycol in aCSF). The dose of aldosterone (Sigma-Aldrich Ltd, Canada) is similar to the dose used in previous studies in rats (Gomez-Sanchez et al., 1990; Wang et al., 2003a; Huang et al., 2005), in which icv aldosterone increased brain “ouabain”, sympathetic activity or BP, but had no demonstrable effects on plasma electrolytes, renin, vasopressin, or aldosterone. The doses of Digibind and benzamil were based on previous studies (Huang et al., 2000, Wang & Leenen, 2003). At a CSF production of 6 ml/day and total CSF volume of 0.5 ml (Harnish & Samuel, 1988), icv infusion of benzamil at the rate of 1 μg/h (0.003μmol/h) will result in a concentration of ~10 μmol/l in the CSF, but steady state levels are likely a factor lower considering its distribution into brain tissue and clearance in the CNS. Icv infusion of benzamil at this rate may lead to maximal inhibition of ENaC (Kleyman & Cragoe Jr, 1988), but some inhibition of other sodium channels or transporters cannot be excluded.
At the end of the 2 week- infusion, in the first set of rats (n=6/group) the abdominal aorta was cannulated and resting BP and HR recorded in freely moving rats. Briefly, under isoflurane anesthesia a PE catheter (PE10 fused to PE50) was placed into the abdominal aorta via the right femoral artery. The catheter was filled with heparinized saline and exteriorized on the back of each rat. The following morning about 18 hours after the arterial cannulation, the arterial catheter was connected to a pressure transducer. The rat was allowed to rest for about 30 min, and BP and HR were then recorded for 5 minutes. The average of BP and HR recorded over 5 min was used as resting BP and HR. Rats were then decapitated without anesthesia and brains collected, frozen in dry-ice, and stored in −80C° for in vitro autoradiography. In the second set of rats (n=8/group), BP was not measured. Brains of each group were sub-divided into 2 subgroups (n=4/subgroup) for measurement of either mRNA or protein for AT1R, NADPH oxidase subunits, nNOS, endothelial NO synthase (eNOS) in the PVN, SON and cortex.
Micropunch of the PVN, SON and cortex
Six consecutive 100 μm-thick coronal sections were cut with a cryostat (−18°C). The PVN was punched bilaterally with a blunt needle (ID: 0.5 mm) according to the method of Palkovits and Brownstein (Palkovits & Brownstein, 1983). The punched tissue was put in 0.5 ml of TRI Reagent (MRC Inc, OH) or protein extraction buffer, and total RNA and protein were extracted. The SON was punched at the same rostrocaudal level as the PVN and the dorsal cortex to serve as anatomic control region from the same cross-section.
Real-time RT-PCR for AT1R, NADPH oxidase subunits, nNOS and eNOS mRNA
Total RNA extracted from the punched tissue was subjected to reverse transcription as described previously (Zheng et al., 2009). All gene-specific primer pairs were designed using BeconDesign 4.0 (Biorad Laboratories, CA) and the sequences of the oligonucleotide primers were published previously (Zhu et al., 2004; Sun et al., 2006; Zheng et al., 2006). Relative mRNA expression was calculated using the Pfaffl equation which relates expression of the target gene to expression of a reference gene (RPL19).
Western Blot Assay of AT1R, NADPH oxidase subunits, nNOS and eNOS protein
The punched tissues were incubated with 100 μl of lysis buffer (1 M Tris, 0.5 M EDTA, 10% SDS, Triton-X-100 and 100 mM phenylmethylsulfonyl fluoride). As described previously (Zheng et al., 2009), the protein concentration was determined using a BCA protein assay kit (Pierce, Rockford, IL). The membrane was probed with primary antibodies (rabbit anti- AT1R, nNOS, eNOS, P22phox, P47phox, P67phox, Nox2 and GAPDH antibodies, Santa Cruz, CA). In a preliminary study, AT1R protein levels in different tissues and different brain nuclei showed the expected pattern of tissue and regional differences using an AT1R antibody from Santa Cruz, CA or from Abcam, CA. The antibody from Santa Cruz is raised against a peptide mapping within an N-terminal extracellular domain of the human AT1 receptor, and the antibody from Abcam is corresponding to C terminal amino acids of the human AT1 receptor. Western blots on different tissues including brain, kidney, heart and muscle were performed, and varying amounts of the same molecular band were found in various tissues. Specific areas in the brain were examined, and the level of AT1 receptor protein varied as expected: protein levels were 10- fold lower in the SON compared to the PVN and close to undetectable in the optic tract (negative control). The signals were detected by digital image system (UVP BioImaging, Upland, CA) and the signals were quantified by Kodak 1D software (Eastman Kodak Company, Rochester, NY). The expressions of proteins were calculated as the ratio of intensity of the nNOS, eNOS, AT1R and NADPH oxidase subunits band relative to the intensity of the GAPDH band.
In vitro autoradiography for ACE and AT1R binding densities
The standard autoradiography protocol was performed as described in details previously (Huang et al., 2006b). Briefly, serial cryostat 20-μm sections were mounted onto Superfrost Plus microscope slides (VWR, West Chester, PA) and stored at −80°C. To assess AT1 - receptor binding, sections were pre-incubated in 5 mM Na2EDTA, 0.2% BSA, and 0.4 mM bacitracin (Sigma, USA) at room temperature for 15 min and then incubated in the same buffer with 0.3 μCi/ml 125I-Sar1,Ile8-ANG II (specific activity of 2176 Ci/mmol, purchased from Department of Pharmacology and Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, MS) plus PD 123319 (10−5M; Sigma), an AT2-receptor antagonist, for 1 h at room temperature. Nonspecific binding was determined in the presence of 1 μM unlabeled ANG II. For ACE autoradiography, the 10 mM phosphate incubation buffer (pH 7.4) contained 0.3 μCi/ml (30 pM) of 125I-labeled 351A and 0.2% BSA. The derivate of lisinopril, 351A, was kindly donated by Dr. Sun (University of Tennessee Health Science Center, Memphis, TN) and iodinated by the chloramine T method (Chai et al., 1987). Nonspecific binding was determined in the presence of 100 mM EDTA, which completely abolished the 125I-351A binding signal. The two ligands at these concentrations cause maximal binding for measurement of actual densities. The slides were dried and then exposed to Kodak Biomax MR film (Eastman Kodak, Rochester, NY) for 48 h, along with a set of methylacrylate 125I standards (Washington State University Peptide Radioiodination Service Center). The film was processed in a Kodak X-OMAT automatic developer. 125I-labeled Ang II (125I-Ang II) binding and ACE binding densities were quantified using a computer-assisted image analysis system (AIS/C, Imaging Research, St. Catharines, ON, Canada) and converted to femtomoles per milligram and femtomoles per gram, respectively, by comparison with the calibrated relative optical density of the 125I standards. Specific binding density was calculated as total binding minus nonspecific binding, which was <2–5% in control and experimental rats. ACE and AT1 -receptor binding densities were measured bilaterally in coronal cryostat sections of the brain containing the magnocellular and parvocellular regions of the PVN (mPVN and pPVN) and the SON. For each rat, 4-6 sections containing the nucleus of interest were quantified and presented as average density for the entire nucleus. The localization of these nuclei was defined according to the rat brain atlas of Paxinos and Watson (Paxinos & Watson, 1998).
Statistical Analysis
For comparison of the measured parameters in the 4 groups, one-way ANOVA was performed. When F values were significant, Newman-Keuls test was applied to identify which groups were significantly different. Statistical significance was defined as p < 0.05.
Results
Icv infusion of aldosterone caused significant increases in mean arterial pressure (MAP) and HR (Table 1). These increases were largely prevented by icv infusion of Digibind or benzamil. There was no significant difference in gain of body weight among treatment groups.
Table 1.
Gain of body weight and resting MAP and HR in rats treated with icv infusion of vehicle, or of aldosterone alone or combined with Digibind or benzamil for 2 weeks.
| N | Gain of body weight (g) |
MAP (mmHg) |
HR (bpm) |
|
|---|---|---|---|---|
| vehicle | 6 | 81±4 | 103±4 | 392±13 |
| aldosterone | 6 | 83±4 | 122±3* | 439±10* |
| aldosterone + Digibind | 6 | 79±3 | 107±4# | 404±18 |
| aldosterone + benzamil | 6 | 78±5 | 112±3# | 410±15 |
Data are means±SEM.
F=5.6 (p=0.003) for MAP and F=3.4 (p=0.005) for HR.
p<0.05, vs vehicle
p<0.05, vs aldosterone.
Effects of icv infusion of aldosterone and central blockades on AT1R, NADPH oxidase subunits and NOS mRNA and protein
AT1R mRNA and protein
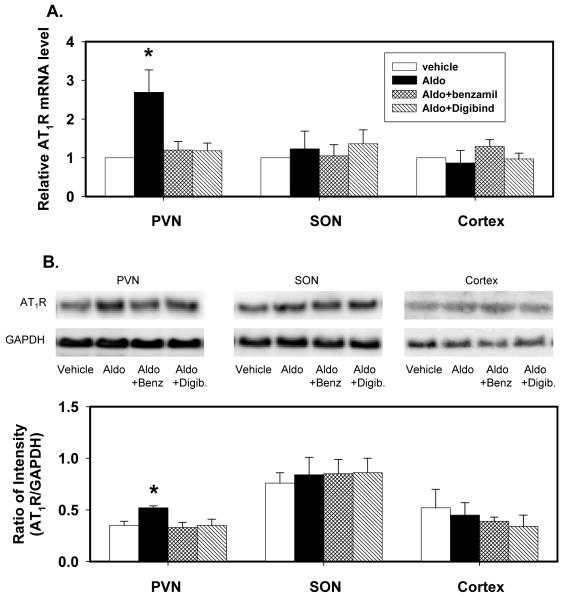
Icv infusion of aldosterone significantly increased AT1R mRNA expression by ~150% and protein by ~50% in the PVN, but not in the SON and cortex (Fig1). The increases in AT1R mRNA expression and protein in the PVN were prevented by icv infusion of either benzamil or Digibind.
Fig. 1.
AT1R mRNA expression (A) and protein (B) in the PVN, SON and cortex of rats after icv infusion of vehicle, aldosterone (Aldo), aldosterone plus benzamil (Aldo+Benz), or aldosterone plus Digibind (Aldo+Digib) for 2 weeks.
mRNA levels are expressed relative to the levels in rats treated with vehicle, assigned a value of 1.
Data are means±SEM (n= 4/group).
F=7.3 (p=0.001) for mRNA; and F=6.6 (p=0.003) for protein in the PVN.
* p<0.05, versus vehicle.
AT1R binding densities were very low in the SON compared to the mPVN and pPVN. Icv aldosterone significantly increased ACE and AT1R binding densities by 20-35% both in the PVN and SON (Table 2). These increases were prevented /attenuated by icv infusion of either benzamil or Digibind
Table 2.
ACE and AT1R binding densities measured by autoradiography in the PVN and SON in rats treated with icv infusion of vehicle, aldosterone (Aldo), aldosterone plus benzamil (Aldo+Benz), or aldosterone plus Digibind (Aldo+Digib) for 2 weeks.
| N | vehicle | Aldo | Aldo+Benz | Aldo+Digib | |
|---|---|---|---|---|---|
| AT1R densities (fmol/mg) | |||||
| mPVN | 6 | 468±16 | 635±21* | 465±17# | 508±26# |
| pPVN | 6 | 370±14 | 476±16* | 370±12# | 402±18# |
| SON | 6 | 128±7 | 170±10* | 138±7# | 146±12 |
| ACE densities (fmol/g) | |||||
| mPVN | 6 | 1740±57 | 2129±89* | 1834±59# | 1857±94# |
| pPVN | 6 | 669±39 | 883±37* | 757±60 | 788±34 |
| SON | 6 | 1057±39 | 1381±43* | 1236±74 | 1297±88* |
Data are means±SEM.
p<0.05, vs vehicle
p<0.05, vs aldosterone.
mPVN: magnocellular region of paraventricular nucleus
pPVN: parvocellular region of paraventricular nucleus
SON: supraoptic nucleus.
NADPH oxidase subunits mRNA and protein
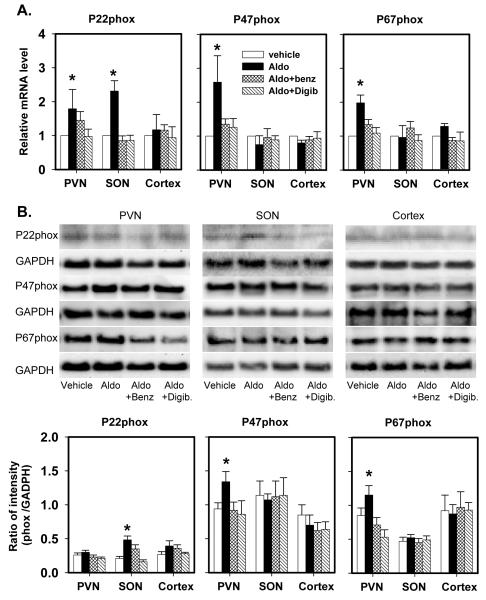
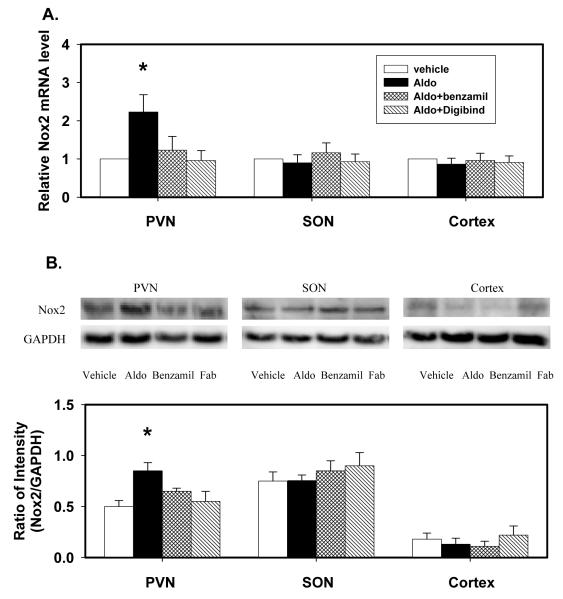
Icv infusion of aldosterone significantly increased P22phox, P47phox, P67phox and Nox2 mRNA expression in the PVN by 80-160% (Fig 2, 3). Aldosterone had no effects on P22phox protein, but increased P47phox, P67phox and Nox2 protein by 40-70% in the PVN. Aldosterone increased P22phox mRNA and protein in the SON, but had no effects on mRNA and protein of the other oxidase subunits in the SON, and no effects at all in the cortex.
Fig. 2.
P22phox, P47phox and P67phox mRNA expression (A) and protein (B) in the PVN, SON and cortex of rats after icv infusion of vehicle, aldosterone (Aldo), aldosterone plus benzamil (Aldo+Benz), or aldosterone plus Digibind (Aldo+Digib) for 2 weeks.
mRNA levels are expressed relative to the levels in rats treated with vehicle, assigned a value of 1.
Data are means±SEM (n= 4/ group).
For P22phox, F=3.2 (p=0.01) for mRNA in the PVN; and F=5.3 (p=0.004) for mRNA and F=6.4 (p=0.003) for protein in the SON.
For P47phox, F=6.2 (p=0.003) for mRNA; and F=7.3 (p=0.001) for protein in the PVN.
For P67phox, F=6.3 (p=0.003) for mRNA; and F=7.3 (p=0.001) for protein in the PVN.
* p<0.05, versus vehicle.
Fig. 3.
Nox2 mRNA expression (A) and protein (B) in the PVN, SON and cortex of rats after icv infusion of vehicle, aldosterone (Aldo), aldosterone plus benzamil (Aldo+Benz), or aldosterone plus Digibind (Aldo+Digib) for 2 weeks.
mRNA levels are expressed relative to the levels in rats treated with vehicle, assigned a value of 1.
Data are means±SEM (n= 4/group).
F=4.0 (p=0.003) for mRNA; and F=8.2 (p=0.001) for protein in the PVN. * p<0.05, versus vehicle.
The increases in mRNA of P22, P47 and P67phox and Nox2, and in protein of P47 and P67phox and Nox2 in the PVN, and in mRNA and protein of P22phox in the SON were all prevented by icv infusion of benzamil or Digibind (Fig 2, 3).
NOS mRNA and protein
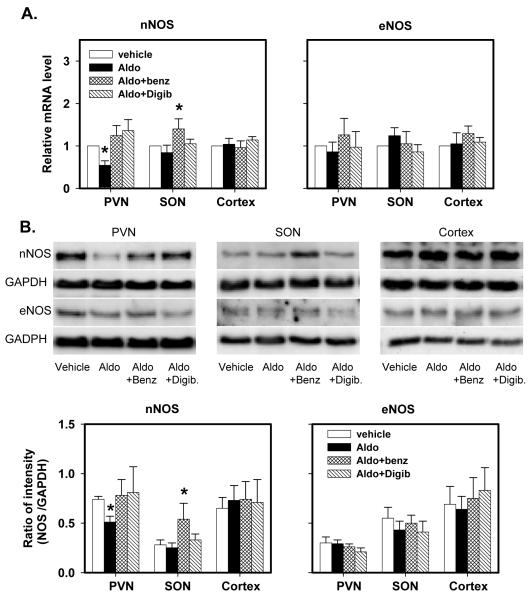
Icv infusion of aldosterone significantly decreased nNOS mRNA and protein by ~50% in the PVN but not in the SON and cortex. Aldosterone had no effects on eNOS expression and protein in these 3 regions (Fig 4).
Fig. 4.
nNOS and eNOS mRNA expression (A) and protein (B) in the PVN, SON and cortex of rats after icv infusion of vehicle, aldosterone (Aldo), aldosterone plus benzamil (Aldo+Benz), or aldosterone plus Digibind (Aldo+Digib) for 2 weeks.
mRNA levels are expressed relative to the levels in rats treated with vehicle, assigned a value of 1.
Data are means±SEM (n= 4/group).
For nNOS, F=5.3 (p=0.004) for mRNA; and F=8.7 (p=0.0003) for protein in the PVN.
F=3.2 (p=0.007) for mRNA; and F=6.4 (p=0.003) for protein in the SON.
* p<0.05, versus vehicle.
The decreases in nNOS mRNA and protein in the PVN were prevented by icv infusion of benzamil or Digibind (Fig 4). In the SON, nNOS mRNA expression and protein were not affected by icv infusion of aldosterone alone, but significantly increased by icv aldosterone together with benzamil. Aldosterone alone or combined with either blockade did not affect eNOS mRNA expression and protein in the PVN, SON or cortex. Benzamil or Digibind had no effects on any parameter in the cortex.
Discussion
The main new findings of the present study are that in rats, chronic icv infusion of aldosterone increases in the PVN mRNA or protein of AT1R and ACE and of the NADPH oxidase subunits P22, P47, P67phox and Nox2, but decreases mRNA and protein of nNOS. These effects are largely prevented by central infusion of Digibind or benzamil.
Functional studies indicate that an aldosterone – ENaC – “ouabain” neuromodulatory pathway is involved in the chronic activation of central pathways causing sympatho-excitation. Icv infusion of aldosterone increases “ouabain” in the hypothalamus, which is prevented by icv infusion of the sodium channel blocker benzamil (Wang et al., 2003a). Sympathetic hyperactivity and hypertension induced by icv infusion of aldosterone can be prevented by icv infusion of benzamil or Digibind (Wang et al., 2003a). Aldosterone induced increase in hypothalamic “ouabain” appears to enhance AT1 receptor stimulation and thereby sympathetic hyperactivity, since sympatho-excitatory and pressor effects elicited by aldosterone or ouabain infused icv or directly into the PVN can be blocked by an AT1 receptor blocker (Huang et al., 1996; Yu et al., 2008; Zhang et al., 2008; Yoshika et al., 2011). As evidence for a functional role in pathophysiology, in Dahl S rats on high salt intake (Huang & Leenen, 1998; Huang et al., 2009), Wistar rats with chronic icv infusion of Na+-rich aCSF (Huang et al., 2006b) and in rats post MI (Huang et al., 2000; Huang & Leenen, 2005; Yu et al., 2008), icv infusion of an aldosterone synthase inhibitor, MR blocker, benzamil, Digibind or AT1 receptor blocker largely prevent sympathetic hyperactivity, hypertension or cardiac remodeling. The actual neuro-anatomical pathways for this neuromodulatory pathway have not yet been established. One may speculate that aldosterone via MR activates sodium channels, presumably ENaC, possibly in magnocellular neurons containing “ouabain” in the SON and PVN (Yamada et al., 1992; Yoshika et al., 2011) leading to “ouabain” release as recently demonstrated by Yoshika et al (Yoshika et al., 2011), and thereby enhanced Ang II release and AT1R stimulation, possibly in the PVN (Huang & Leenen, 1996; Gabor & Leenen, 2009).
Felder’s group (Zhang et al., 2008) recently reported that in Sprague-Dawley rats, chronic icv infusion of aldosterone at 22.5ng/h for 1 week upregulates AT1R and ACE mRNA in hypothalamic tissue and increases oxidative stress in the PVN as assessed by dihydroethidium staining. Moreover, icv infusion of the radical scavenger tempol prevents the aldosterone-induced sympathetic hyperactivity and hypertension (Zhang et al., 2008). The present study suggests that increased activity of NADPH oxidase and NADPH oxidase-derived reactive oxidative species (ROS) in the PVN are major contributors to the aldosterone-induced increases in oxidative stress in the PVN. NADPH oxidase is a multicomponent enzyme complex that consists of the two membrane-spanning polypeptide subunits p22phox and Nox2, and three cytoplasmic polypeptide subunits p40phox, p47phox, and p67phox, contributing together to enzymatic activity and production of superoxide (DeLeo & Quinn, 1996). In the present study, aldosterone caused clear increases in mRNA and protein of p47phox, p67phox and Nox2 subunits as well as increase in mRNA of p22phox in the PVN. Enzymatic activity per se was not measured in the present study. It it is likely that this increased as well, since all relevant components such as membrane-located catalytic subunits as well as the cytosolic subunits needed to assemble the active oxidase significantly increased at both the mRNA and protein level. For P22phox only the mRNA was found increased but not the protein, possibly reflecting enhanced turnover of protein for this particular subunit.
Ang II up-regulates the gene expression of most NADPH oxidase subunits (Mollnau et al., 2002). An increase in Nox2 protein is likely a critical component for increased activity considering that icv injections of adenoviral vector expressing small interfering RNA to selectively silence Nox2 markedly attenuate Ang II-mediated ROS production and pressor actions in the CNS (Peterson et al., 2009). In the present study, icv infusion of aldosterone infusion up-regulated AT1 receptors in the PVN, which may contribute to an Ang II-induced increase in NADPH oxidase derived ROS–generating activity.
In addition, we show that aldosterone appears to decrease NO –mediated inhibition in the PVN. An increase in NO in the PVN by e.g. microinjection of sodium nitroprusside decreases renal sympathetic nerve activity, BP and HR (Zhang et al., 1997), and inhibition of NO is associated with sympatho-excitation (Zhang et al., 1997). To our knowledge, the present study is the first showing that chronic icv infusion of aldosterone decreases nNOS mRNA and protein in the PVN with no effects on eNOS mRNA and protein. The aldosterone -induced decrease in nNOS may reflect an effect of AT1 receptor mediated increase in oxidative stress on abundance of nNOS in the PVN (Campese et al., 2002, Campese et al., 2004). Acutely, Ang II can activate this NO -mediated negative feedback loop thereby attenuating the Ang II-induced sympatho-excitation (Latchford & Ferguson, 2003; Li et al., 2006). However, icv infusion of Ang II decreases nNOS mRNA in the PVN, and appears to decrease NO release and NO-mediated inhibition (Campese et al., 2002).
The present study also assessed some of the pathways/mechanisms which may mediate the effects of aldosterone on the above sympatho-excitatory and –inhibitory mechanisms. Icv infusion of benzamil or of Digibind fully prevented the changes in mRNA and protein in the PVN, consistent with the concept that sodium channels and “ouabain” mediate the effects of central aldosterone on the PVN. Regarding the action of “ouabain”, it is tempting to speculate that “ouabain” in the PVN enhances Ang II release resulting in increased AT1R stimulation and oxidative stress (Zhang et al., 2008), the latter contributing to the increase in AT1R expression (Liu et al., 2008), as well as the decrease in nNOS expression (Campese et al., 2002). Zhang et al (Zhang et al., 2008) demonstrated that icv infusion of an MR block or superoxide dismutase mimetic, but not the AT1 receptor blocker losartan attenuated or prevented the increase in hypothalamic AT1 receptor mRNA expression induced by chronic icv infusion of aldosterone. All 3 blockers/compounds attenuated aldosterone-induced superoxide production in the PVN, but the inhibitory effect was larger for an MR blocker compared with losartan (Zhang et al., 2008). Zhang et al suggested that aldosterone may increase oxidative stress and AT1 receptor mRNA expression in the PVN by both a direct effect and an indirect effect through AT1 receptor stimulation (Zhang et al., 2008). In the present study, benzamil and Digibind similarly inhibited aldosterone induced increases in mRNA and protein expression of AT1 receptor and NADPH oxidase subunits as well as increases in Ang II and ACE binding densities. Together, these findings suggest that aldosterone via MR increases “ouabain” release, presumably from magnocellular neurons (Yoshika et al., 2011), and “ouabain” mediates both direct and indirect effects of aldosterone contributing to increased oxidative stress in the PVN. The aldosterone- induced hypertension unlikely contributes to these changes in the PVN. Up-regulation of hypothalamic AT1 receptor and NADPH oxidase subunits is also observed in rats after myocardial infarction with increased hypothalamic aldosterone and without hypertension (Yu et al., 2008). The increase in BP, however, may activate the nucleus tractus solitarius (NTS) through the arterial baroreflex, and via afferent pathways from the NTS (Shioya & Tanaka, 1989) attenuate the extent of these changes in the PVN.
Effects of aldosterone were largely limited in the PVN. No changes were noted in the cortex, whereas the SON showed an increase in P22phox mRNA and protein but not the other NADPH oxidase subunits, neither a change in nNOS. The relevance of the isolated change in P22phox is not readily apparent. Aldosterone also did not increase AT1R mRNA and protein in the SON but did increase the low AT1R binding densities. This dissociation may reflect a re-distribution of the protein to the membrane. Benzamil prevented the aldosterone-induced increase in P22phox mRNA in the SON (Fig 2) but not the increases in P22phox protein and ACE binding density in the SON. Moreover, aldosterone alone did not affect nNOS in the SON, but aldosterone combined with benzamil increased both nNOS mRNA and protein. These results may reflect an involvement of other regulatory mechanisms in the SON, some being activated after blockade of sodium channels.
Limitations of the present study
The present study did not assess whether icv infusion of benzamil or Digibind alone affects mRNA and protein levels for nNOS, eNOS, AT1R and NADPH oxidase subunits in the brain of control rats. This seems unlikely, since in Wistar rats chronic icv infusion of benzamil (Wang & Leenen, 2003) at the same rate as used in the present study has no effects on resting BP and hypothalamic “ouabain” levels and icv infusion of Digibind (34) does not affect BP, HR, and AT1R and ACE binding densities in the hypothalamus. Previous studies demonstrated that central blockade of MR, ENaC or AT1R, or central infusion of tempol largely prevent sympathetic hyperactivity and hypertension caused by acute (Wang et al., 2003a) or chronic (Huang et al., 2005; Zhang et al., 2008) central infusion of aldosterone. Further studies are needed to assess to what extent the effects of aldosterone in the PVN shown in the present study indeed are mediated by AT1R stimulation and contribute to the sympatho-excitation.
Perspectives
The PVN plays a pivotal role in the increase in sympathetic activity post MI. Both an increase in excitatory (Zhang et al., 2008) and decrease in inhibitory (Zhang et al., 1997; Li et al., 2002) mechanisms contribute to enhanced output from the PVN. The same pattern can be induced by central infusion of aldosterone (Zhang et al., 2008; present study). Further studies using an aldosterone synthase inhibitor and MR blocker may determine whether post MI aldosterone produced locally in the CNS or derived from the circulation also activates the PVN and to what extent this activation is mediated by MR or other – non-genomic – actions of aldosterone.
Conclusion
The present study demonstrates that an increase in brain aldosterone up-regulates AT1R and ACE, increases oxidative stress, and decreases activity of the NO system particularly in the PVN. It is possible that aldosterone in the CNS mainly via activation of ENaC and “ouabain”, 1) increases angiotensinergic activity and thereby oxidative stress in the PVN; and 2) decreases activity of NO system in the PVN possibly as a result of increased oxidative stress. Both together may mediate aldosterone induced sympathetic hyperactivity post MI or in salt-sensitive hypertension.
ACKNOWLEDGEMENTS
Frans Leenen holds the Pfizer Chair in Hypertension Research, an endowed chair supported by Pfizer Canada, University of Ottawa Heart Institute Foundation, and Canadian Institutes of Health Research.
SOURCES OF FUNDING This study was supported by operating grant #FRN-13182 from the Canadian Institutes of Health Research (to Dr. Leenen) and operating grant #HL 62222 from the NIH (to Dr. Patel).
References
- Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2005;46:533–539. doi: 10.1161/01.HYP.0000179088.57586.26. [DOI] [PubMed] [Google Scholar]
- Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2002;39:519–524. doi: 10.1161/hy0202.102815. [DOI] [PubMed] [Google Scholar]
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol. 2004;287:H695–H703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- Chai SY, Mendelsohn FA, Paxinos G. Angiotensin converting enzyme in rat brain visualized by quantitative in vitro autoradiography. Neuroscience. 1987;20:615–627. doi: 10.1016/0306-4522(87)90114-x. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol. 2001;281:H2241–H2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- Gabor A, Leenen FHH. Mechanisms in the PVN mediating local and central sodium-induced hypertension in Wistar rats. Am J Physiol. 2009;296:R618–R630. doi: 10.1152/ajpregu.90417.2008. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Fort C, Thwaites D. Central mineralocorticoid receptor antagonism blocks hypertension in Dahl S/JR rats. Am J Physiol. 1992;262:E96–E99. doi: 10.1152/ajpendo.1992.262.1.E96. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Venkataraman MT, Thwaites D, Fort C. ICV infusion of corticosterone antagonizes ICV-aldosterone hypertension. Am J Physiol. 1990;258:E649–E653. doi: 10.1152/ajpendo.1990.258.4.E649. [DOI] [PubMed] [Google Scholar]
- Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: Cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnish PP, Samuel K. Reduced cerebrospinal fluid production in the rat and rabbit by diatrizoate. Ventriculocisternal perfusion. Invest Radiol. 1988;23:534–536. doi: 10.1097/00004424-198807000-00010. [DOI] [PubMed] [Google Scholar]
- Huang BS, Amin MS, Leenen FHH. The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol. 2006;21:295–304. doi: 10.1097/01.hco.0000231398.64362.94. [DOI] [PubMed] [Google Scholar]
- Huang BS, Cheung WJ, Wang H, Tan J, White RA, Leenen FHH. Activation of brain renin-angiotensin-aldosterone system by central sodium in Wistar rats. Am J Physiol. 2006;291:H1109–H1117. doi: 10.1152/ajpheart.00024.2006. [DOI] [PubMed] [Google Scholar]
- Huang BS, Leenen FHH. Sympathoexcitatory and pressor responses to increased brain sodium and ouabain are mediated via brain ANG II. Am J Physiol. 1996;270:H275–H280. doi: 10.1152/ajpheart.1996.270.1.H275. [DOI] [PubMed] [Google Scholar]
- Huang BS, Leenen FHH. Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension. 1998;32:1028–1033. doi: 10.1161/01.hyp.32.6.1028. [DOI] [PubMed] [Google Scholar]
- Huang BS, Leenen FHH. Blockade of brain mineralocorticoid receptors or Na+ channels prevents sympathetic hyperactivity and improves cardiac function in rats post-MI. Am J Physiol. 2005;288:H2491–H2497. doi: 10.1152/ajpheart.00840.2004. [DOI] [PubMed] [Google Scholar]
- Huang BS, Wang H, Leenen FHH. Chronic central infusion of aldosterone leads to sympathetic hyperreactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol. 2005;288:H517–H524. doi: 10.1152/ajpheart.00651.2004. [DOI] [PubMed] [Google Scholar]
- Huang BS, White RA, Jeng AY, Leenen FHH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol. 2009;296:R994–R1000. doi: 10.1152/ajpregu.90903.2008. [DOI] [PubMed] [Google Scholar]
- Huang BS, Yuan B, Leenen FHH. Chronic blockade of brain “ouabain” prevents sympathetic hyper-reactivity and impairment of acute baroreflex resetting in rats with congestive heart failure. Can J Physiol Pharmacol. 2000;78:45–53. doi: 10.1139/cjpp-78-1-45. [DOI] [PubMed] [Google Scholar]
- Kala G, Kumarathasan R, Peng L, Leenen FHH, Hertz L. Stimulation of Na+,K+-ATPase activity, increase in potassium uptake, and enhanced production of ouabain-like compounds in ammonia-treated mouse astrocytes. Neurochem Int. 2000;36:203–211. doi: 10.1016/s0197-0186(99)00117-5. [DOI] [PubMed] [Google Scholar]
- Kent MA, Huang BS, Van Huysse JW, Leenen FHH. Brain Na+,K+-ATPase isozyme activity and protein expression in ouabain-induced hypertension. Brain Res. 2004;1018:171–180. doi: 10.1016/j.brainres.2004.05.059. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Latchford KJ, Ferguson AV. Angiotensin II activates a nitric-oxide-driven inhibitory feedback in the rat paraventricular nucleus. J Neurophysiol. 2003;89:1238–1244. doi: 10.1152/jn.00914.2002. [DOI] [PubMed] [Google Scholar]
- Li YF, Roy SK, Channon KM, Zucker IH, Patel KP. Effect of in vivo gene transfer of nNOS in the PVN on renal nerve discharge in rats. Am J Physiol. 2002;282:H594–H601. doi: 10.1152/ajpheart.00503.2001. [DOI] [PubMed] [Google Scholar]
- Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol. 2006;290:R1035–R1043. doi: 10.1152/ajpregu.00338.2004. [DOI] [PubMed] [Google Scholar]
- Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res. 2008;103:186–193. doi: 10.1161/CIRCRESAHA.108.179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein M, Cuello AE. Brain Microdissection Techniques. Wiley; London: 1983. Brain microdissection techniques. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed Acadamic Press; San Diego, CA: 1998. [Google Scholar]
- Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension. 2009;54:1106–1114. doi: 10.1161/HYPERTENSIONAHA.109.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulina MV, Zulian A, Berra-Romani R, Beskina O, Mazzocco-Spezzia A, Baryshnikov SG, Papparella I, Hamlyn JM, Blaustein MP, Golovina VA. Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain-induced hypertensive rats. Am J Physiol. 2010;298:H263–H274. doi: 10.1152/ajpheart.00784.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioya M, Tanaka J. Inputs from the nucleus of the solitary tract to subfornical organ neurons projecting to the paraventricular nucleus in the rat. Brain Res. 1989;483:192–195. doi: 10.1016/0006-8993(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Sun H, Zheng H, Molacek E, Fang Q, Patel KP, Mayhan WG. Role of NAD(P)H oxidase in alcohol-induced impairment of endothelial nitric oxide synthase-dependent dilation of cerebral arterioles. Stroke. 2006;37:495–500. doi: 10.1161/01.STR.0000199033.06678.c3. [DOI] [PubMed] [Google Scholar]
- Tan J, Wang H, Leenen FHH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physio. 2004;286:H1665–H1671. doi: 10.1152/ajpheart.00858.2003. [DOI] [PubMed] [Google Scholar]
- Wang H, Huang BS, Ganten D, Leenen FHH. Prevention of sympathetic and cardiac dysfunction after myocardial infarction in transgenic rats deficient in brain angiotensinogen. Circ Res. 2004;94:843–849. doi: 10.1161/01.res.0000120864.21172.5a. [DOI] [PubMed] [Google Scholar]
- Wang H, Huang BS, Leenen FHH. Brain sodium channels and ouabainlike compounds mediate central aldosterone-induced hypertension. Am J Physiol. 2003;285:H2516–H2523. doi: 10.1152/ajpheart.00299.2003. [DOI] [PubMed] [Google Scholar]
- Wang H, Leenen FHH. Brain sodium channels and central sodium-induced increases in brain ouabain-like compound and blood pressure. J Hypertens. 2003;21:1519–1524. doi: 10.1097/00004872-200308000-00016. [DOI] [PubMed] [Google Scholar]
- Wang JM, Veerasingham SJ, Tan J, Leenen FHH. Effects of high salt intake on brain AT1 receptor densities in Dahl rats. Am J Physiol. 2003;285:H1949–H1955. doi: 10.1152/ajpheart.00744.2002. [DOI] [PubMed] [Google Scholar]
- Yamada H, Naruse M, Naruse K, Demura H, Takahashi H, Yoshimura M, Ochi J. Histological study on ouabain immunoreactivities in the mammalian hypothalamus. Neurosci Lett. 1992;141:143–146. doi: 10.1016/0304-3940(92)90881-7. [DOI] [PubMed] [Google Scholar]
- Yoshika M, Komiyama Y, Takahashi H. An ouabain-like factor is secreted from immortalized hypothalamic cells in an aldosterone-dependent manner. Neurochem Int. 2011;59:104–108. doi: 10.1016/j.neuint.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension. 2008;51:727–733. doi: 10.1161/HYPERTENSIONAHA.107.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol. 2008;294:H1067–H1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- Zhao X, White R, Huang BS, Van Huysse J, Leenen FHH. High salt intake and the brain renin--angiotensin system in Dahl salt-sensitive rats. J Hypertens. 2001;19:89–98. doi: 10.1097/00004872-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol. 2009;297:R1364–R1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Mayhan WG, Bidasee KR, Patel KP. Blunted nitric oxide-mediated inhibition of sympathetic nerve activity within the paraventricular nucleus in diabetic rats. Am J Physiol. 2006;290:R992–R1002. doi: 10.1152/ajpregu.00363.2005. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH, Wang W. AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol. 2004;287:H1828–H1835. doi: 10.1152/ajpheart.01245.2003. [DOI] [PubMed] [Google Scholar]