Abstract
Intradermal (ID) vaccination can offer improved immunity and simpler logistics of delivery, but its use in medicine is limited by the need for simple, reliable methods of ID delivery. ID injection by the Mantoux technique requires special training and may not reliably target skin, but is nonetheless used currently for BCG and rabies vaccination. Scarification using a bifurcated needle was extensively used for smallpox eradication, but provides variable and inefficient delivery into the skin. Recently, ID vaccination has been simplified by introduction of a simple-to-use hollow microneedle that has been approved for ID injection of influenza vaccine in Europe. Various designs of hollow microneedles have been studied preclinically and in humans. Vaccines can also be injected into skin using needle-free devices, such as jet injection, which is receiving renewed clinical attention for ID vaccination. Projectile delivery using powder and gold particles (i.e., gene gun) have also been used clinically for ID vaccination. Building off the scarification approach, a number of preclinical studies have examined solid microneedle patches for use with vaccine coated onto metal microneedles, encapsulated within dissolving microneedles or added topically to skin after microneedle pretreatment, as well as adapting tattoo guns for ID vaccination. Finally, technologies designed to increase skin permeability in combination with a vaccine patch have been studied through the use of skin abrasion, ultrasound, electroporation, chemical enhancers, and thermal ablation. The prospects for bringing ID vaccination into more widespread clinical practice are encouraging, given the large number of technologies for ID delivery under development.
Keywords: Stratum Corneum, Influenza Vaccine, Inactivate Poliovirus Vaccine, Microneedle Patch, Chemical Enhancer
Introduction
Immunologic Motivation for Intradermal Delivery
The skin contains high concentrations of antigen-presenting cells, and is thus a site capable of inducing potent immune responses. The skin is composed of multiple layers, each with characteristic resident and transient immune cell subsets. Outermost is the thin layer of the epidermis (0.05–0.2 mm), which is primarily made up of epithelial cells as well as Langerhans cells, melanocytes, and Merkel cells. Beneath the epidermis, the dermis is a thicker layer (1.5–3 mm) consisting of a network of collagen fibers. Cells of the adaptive and innate system reside in or circulate through the dermis, including macrophages, mast cells, Langerhans cells, and dermal dendritic cells. Antigen-presenting cells in the skin perform an essential role in processing incoming antigens, resulting in immune system activation or immune tolerance of self or harmless antigens (Nicolas and Guy 2008). For these reasons, it is possible that delivery of vaccines to the epidermis or dermis may result in superior immune responses compared to other anatomical sites (Glenn and Kenney 2006; Lambert and Laurent 2008; Nicolas and Guy 2008). Alternatively, an equivalent immune response could be stimulated by delivery of a smaller quantity of vaccine antigen to the skin. Either of these mechanisms could be beneficial for developing vaccines against new disease targets, improving immune responses in hard-to-treat groups, or lowering the cost of vaccine antigens, and may be particularly valuable for improving access to vaccines in low-resource settings.
While a substantial number of clinical studies evaluating intradermal (ID) delivery of vaccines have been performed, the majority of studies have not been designed to evaluate whether ID delivery is immunologically superior to other routes. In most cases, to simplify administration, a reduced dose (10 or 20%) delivered ID was compared to the full dose delivered either subcutaneously (SC) or intramuscularly (IM). Only a few studies have compared delivery of the same dose of vaccine ID and SC/IM. Further research will be needed to establish whether the potential for dose-sparing is unique to ID delivery (PATH 2009). However, some ID delivery devices in development offer additional desirable features such as needle-free delivery or improved ease of administration, which may be drivers for further adoption of ID vaccine delivery even if there is no net immunologic benefit.
Current Intradermal Vaccines
Smallpox
Vaccines for smallpox have been delivered to the skin dating back to Edward Jenner’s first experiments in 1796 demonstrating that exposure to cowpox could protect against smallpox infection. A variety of scarification techniques and devices have been used to allow virus introduction, including knives, needles, scalpels, and rotary lancets. During the global smallpox eradication campaign, both multi-dose nozzle jet injectors and bifurcated needles were used for ID vaccinia virus inoculation (Henderson et al. 2008).
BCG
Bacille Calmette-Guérin (BCG) vaccine for tuberculosis is globally the most widely delivered ID vaccine. ID injection by needle and syringe is the most commonly used method, but in some areas BCG is also delivered to the skin using a multipuncture device. New versions of BCG are under development in an effort to improve immune protection, and are also delivered ID (Hoft et al. 2008).
Rabies
Rabies vaccines are conventionally delivered IM, but due to the high cost of cell-culture-derived vaccines and the pressing need for affordable vaccination regimens in endemic regions, ID delivery has been extensively studied. Both post-exposure prophylaxis and pre-exposure prophylaxis ID regimens induce protective titers, and WHO has recommended ID delivery of reduced doses of rabies vaccines since 1991 (WHO 2005; 2007). Given equivalent doses of antigen, delivery to the dermis appears to be either superior or equivalent to IM/SC (Bernard et al. 1982; Bernard et al. 1987; Fishbein et al. 1987; Phanuphak et al. 1990). A detailed review on ID rabies vaccination can be found elsewhere in this special volume on ID immunization (Warrell 2011).
Clinical Studies on other Intradermal Vaccines
Influenza
Multiple studies of reduced-dose delivery of influenza vaccines have been conducted, providing some of the most informative clinical data on the potential for dose-sparing through ID delivery. One study found that ID delivery of 6 μg HA per influenza strain was comparably immunogenic as the standard IM dose of 15 μg HA per strain (Belshe et al. 2004). A later comparison of 3, 6, and 9 μg delivered both ID and IM found equivalent responses for the two delivery routes for each dose (Belshe et al. 2007). Trials have also been conducted with influenza using novel microneedle devices to aid accurate ID delivery, as discussed in Sect. 3.2.
Hepatitis B
ID delivery of reduced doses of hepatitis B vaccine has been evaluated in healthy infant, child, and adult populations as well as in immuno-compromised patient groups. Meta-analyses have concluded that seroconversion rates are lower than full-dose IM delivery, although responses are higher in children and females (Chen and Gluud 2005; Sangare et al. 2009). When the same dose of hepatitis B antigen has been delivered ID and IM, immune responses were equivalent for both routes (Ayoola 1984; Milne et al. 1986; Heijtink et al. 1989; Coberly et al. 1994; Rahman et al. 2000).
Hepatitis A
Hepatitis A vaccines have also been proposed as a possible target for reduced-dose ID delivery. Two studies found that reduced doses delivered ID produced comparable immune responses to IM delivery, while a third indicated that the ID route was inferior (Brindle et al. 1994; Carlsson et al. 1996; Pancharoen et al. 2005). Local reactogenicity was observed for alum-adjuvanted formulations.
Polio
In a few countries, ID was originally the standard route of delivery for inactivated poliovirus vaccine, but injection depth was later shifted to IM (Weniger and Papania 2008). Studies have found that ID delivery of reduced doses is capable of inducing seroconversion, which may help make this vaccine more affordable for use in developing countries (Samuel et al. 1991; Samuel et al. 1992; Nirmal et al. 1998). More recently, the WHO Global Polio Eradication Initiative has worked to determine the potential for this mode of delivery to be used in post-eradication settings after phase-out of oral polio vaccine.
Measles
Several studies have been conducted evaluating ID delivery of measles vaccine, with mixed results (Burland 1969; Kok et al. 1983; Whittle et al. 1984; de Moraes et al. 1994). However, the vaccine dose and method used to deliver the vaccine varied, and it is possible that trials using older generation delivery technology did not deliver vaccine reliably to the dermis (PATH 2009). Transcutaneous immunization of measles vaccine on a coated patch has also been attempted. Although a salivary sIgA response was observed, the key marker of immunity, an increase in neutralizing serum IgG, was not detected (Etchart et al. 2007).
Yellow Fever
Studies were conducted delivering the 17D attenuated yellow fever virus vaccine by scarification, but this delivery mode was abandoned as efficacy was low. More recently, a clinical trial compared full dose SC delivery of a 17D vaccine to 1/5 dose delivered ID by Mantoux injection, which found equivalent seroprotection between the two routes (Roukens et al. 2008). A more extensive description on ID vaccination against yellow fever is provided elsewhere in this special volume on ID immunization (Roukens et al. 2011).
Others
A number of other vaccines have been considered for ID delivery. Research has shown that a reduced ID dose of vaccines for diphtheria-tetanus-pertussis, tetanus toxoid, and tick-borne encephalitis can generate a comparable immune response to the standard dose and way of injection (Stanfield et al. 1972; Zoulek et al. 1984; Zoulek et al. 1986; Dimache et al. 1990). ID delivery is also under investigation for a number of vaccines in development, including vaccines for tuberculosis, enterotoxigenic E. coli, and pandemic influenza, as well as DNA vaccines.
Difficulties to Make Intradermal Delivery More Widespread
The traditional methods used for ID delivery of vaccines have limitations which may hinder adoption of ID delivery. Bifurcated needles and multipuncture devices have been used successfully for delivery of smallpox and BCG vaccines, but do not deliver reproducible quantities of vaccine antigen to the dermis and are therefore unlikely to be appropriate delivery devices for new vaccines (Lambert and Laurent 2008). The Mantoux method of inserting a needle at a shallow angle into the skin can also be inconsistent, and requires additional training and skill to perform correctly (Flynn et al. 1994). The perceived difficulty of performing an ID injection using this method may prevent development of vaccines for ID delivery. New generations of devices, such as those discussed in the rest of this article, may improve the reliability of ID delivery and enable adoption of the ID route for more vaccines.
Injecting into the Skin
Most vaccines are administered IM or SC using a hypodermic needle. To achieve ID vaccination, conventional hypodermic needles can be used by employing the Mantoux technique to inject into the skin. Simpler and more reliable ID injection is being pursued through adaptations of hypodermic needle technology, as well as novel hollow microneedle devices produced by microfabrication (Prausnitz et al. 2009).
Hypodermic Needles: Mantoux Intradermal Injection
The Mantoux technique is an ID injection method characterized by a needle inserted at a 5–15 degree angle, approximately 1 mm deep into the dermis, to inject a vaccine or drug (Fig. 1a). This method was developed by Charles Mantoux in the early 20th century, and it has been used to identify tuberculosis infection by the ID injection of tuberculin (Mantoux 1909). However, this technique requires training and is often considered an inconsistent delivery method, thus preventing vaccine makers or medical practitioners from using ID injection as a common immunization method (Lambert and Laurent 2008). Also, age or elasticity-related skin conditions have a significant effect on adequate placement of the needle in the dermis for the traditional ID injection technique (Dean et al. 2005; Laurent et al. 2007), thus leading to inadequate vaccination. Other disadvantages of Mantoux technique injection include inaccurately delivered dosage of vaccine, vaccine wastage in dead space of the needle, and variable injection success when using different gauge needles (Flynn et al. 1994). Moreover, success rate of ID injection by untrained personnel was found to be 80–90% (Howard et al. 1997). In an effort to reduce training requirements and to improve the reliability of the Mantoux injection technique, an intradermal adapter is under development by PATH (Seattle, WA, USA), a nonprofit, international health agency that develops and advances health technologies for low resource settings, and SID Technologies. This device fits over a conventional hypodermic needle and syringe and limits the angle and depth of penetration of the needle into the skin in order to facilitate delivery to the dermis.
Fig. 1.
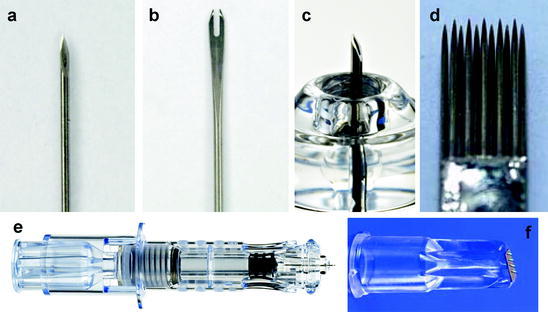
Needles used for ID vaccination. a 32 gauge hypodermic needle with ID bevel used for Mantoux technique injections. b Bifurcated needles used for smallpox vaccination by scarification. c Hollow microneedle developed for reliable ID injection, currently used for ID influenza vaccination (Courtesy of BD). d Mag-11 tattoo needle. e Microneedle injection system, consisting of a single-use syringe coupled to a microneedle shown in part c (Courtesy of BD). f Microneedle injection system containing of a row of four microneedles (Courtesy of NanoPass Technologies)
Single Hollow Microneedles
To overcome these limitations of conventional ID injection, Becton–Dickinson (BD) has developed a micro-sized needle that can be inserted into skin vertically, unlike the angled injection of the Mantoux method. This novel microneedle device has been studied in animals and human subjects, and is currently used in approved influenza vaccines (INTANZA® and IDflu®). BD’s microneedle device (called SoluviaTM) uses a 30 gauge microneedle that extends 1.5 mm beyond an insertion depth-limiting tip, which is connected to a prefilled syringe (Figs. 1c and 1e). The microneedle system was evaluated versus the conventional Mantoux technique to compare delivery efficiency and safety in human subjects. Using ultrasound echography analysis, the distribution of fluid delivered by the microneedle was seen to be larger than the Mantoux injection control. In addition, the microneedle system had a high ID administration success rate (95%) and, in a study of patient compliance and safety, the microneedle device showed promising results. This system also caused fewer occurrences of injuries to the papillary dermis, lesser pain than Mantoux injection and was administered easily by untrained personnel (Laurent et al. 2007).
Preclinical Studies
An earlier prototype of the BD microneedle using a 1 mm, 34 gauge needle has been tested in rats for delivery of influenza vaccines which showed dose sparing effects compared to an IM control. ID microneedle administration of a low dose (0.01 μg) of inactivated virus vaccine induced similar serum antibody response as IM injection of a dose 100 times larger (1 μg). Additionally, using microneedles for ID immunization with split-viron vaccine (seasonal H1N1 strain) showed approximately ten-fold dose-sparing compared to IM immunization. In the same study, ID immunization using plasmid DNA vaccine encoding the hemagglutinin protein of influenza A virus showed similar dose-sparing effects after multiple immunizations (Alarcon et al. 2007). The BD microneedle was also used to deliver a live attenuated vaccine against Japanese encephalitis (ChimeriVaxTM–JE) in non-human primates. In this study, ID microneedle injection was compared to SC injection and transcutaneous microabrasion (see Sect. 6.1). The microneedle ID injection provided the best and most consistent immune responses (i.e., neutralizing antibodies) of the three types of immunizations (Dean et al. 2005).
A further study compared anthrax vaccine delivery using ID microneedle immunization, IM injection, intranasal delivery, and epidermal delivery by microabrasion (Mikszta et al. 2005) into mice (10 μg dose) and rabbits (50 μg dose). Microneedle ID vaccination showed slightly better response in the murine model than the other routes used, while all treatments in the rabbit had similar responses. A follow-up ID immunization was performed to compare ID injection with IM injection over a range of doses in a rabbit model: 10, 0.2, and 0.08 μg of anthrax vaccine (Mikszta et al. 2006). After prime immunization, ID injection showed significantly higher immunogenicity than IM injection when using 10 and 0.2 μg dosages. Interestingly, ID injection with 0.2 μg showed a statistically equivalent response to IM administration of the 10 μg dose. After administration of a booster immunization, this dose-sparing phenomenon continued. Furthermore, an aerosol lethal challenge with anthrax spores showed that a 10 μg ID injection completely protected the immunized rabbits, whereas IM injection of the same dose protected only 71% of the rabbits.
Clinical Trials
An early prototype of the BD microneedle system was first tested in a clinical study examining influenza vaccine delivery in healthy adults (18–60 yrs) and elderly adults (>60 yrs) (Belshe et al. 2004). In this study, 6 μg of hemagglutinin was delivered by ID injection and compared to a full dose (15 μg) delivered by IM immunization. It was found that in younger participants, ID immunization was not significantly different from immunization by IM, as shown by geometric mean hemagglutination inhibition (HAI) titers. However, ID administration showed lower HAI titers than IM in elderly patients. Further evaluation of ID microneedle vaccination against influenza was performed in clinical studies of healthy adults (18–57 yrs) (Leroux-Roels et al. 2008; Beran et al. 2009) and elderly persons (>60 yrs) (Holland et al. 2008; Arnou et al. 2009). For healthy adults, 9 μg of hemagglutinin (H1, H3, and B strains) was delivered by ID injection and was compared to a 15 μg IM immunization. This study confirmed previous results that reduced-dose ID injection was equally immunogenic as full-dose IM injection (Leroux-Roels et al. 2008).
In a Phase II clinical trial, the effects of lower dose ID immunization was investigated using 3, 6, and 9 μg of hemagglutinin (ID) and 15 μg of hemagglutinin (IM). ID immunization using 3 and 6 μg of hemagglutinin induced inferior immune response as shown by HAI titer, but a dose of 9 μg showed comparable response compared to full-dose (15 μg) IM vaccination (Beran et al. 2009). For elderly subjects (>60 years old), a booster vaccine (15 μg) was administered due to the inferior immune system generally found in the elderly compared to younger adults (Goodwin et al. 2006). Therefore, 15 μg of hemagglutinin was administered twice by either ID or IM routes in elderly subjects. In this phase II clinical trial, ID immunization showed significantly better immune response as determined by post-immunization GMT (geometric mean titer), seroprotection (% participants with HAI titers ≥40), GMTR (geometric mean ratio of post-immunization titer to pre-immunization titer), and rate of seroconversion (post-immunization titer in participants with a pre-immunization titer <10). Therefore, ID microneedle vaccination provided superior immunogenicity in a high priority population for protection from influenza due to high vulnerability (Holland et al. 2008). These findings were further confirmed in a phase III clinical trial for elderly persons (>60 years old), where ID immunization showed superior seroprotection, GMTR, and rate of seroconversion compared to IM after prime immunization. After administration of two booster immunizations, ID immunization induced consistently higher seroprotection rates than IM immunization (Arnou et al. 2009).
As a final note, ID immunization caused more local inflammatory-like reactions than IM immunization. It is possible that because ID delivery occurs in the skin, inflammatory or immunologic reactions are more easily visible than those that may occur after IM immunization, which presents the antigen deep into the muscle layer where an inflammatory reaction would not be visible to the eye (Belshe et al. 2004; Holland et al. 2008; Arnou et al. 2009; Beran et al. 2009; Van Damme et al. 2009).
Arrays of Hollow Microneedles
Hollow microneedles have also been developed as multi-needle arrays, which have involved shorter needles (<<1 mm) produced by novel microfabrication techniques, including laser micromachining (Davis et al. 2005), silicon-based MEMS technique using deep reactive-ion etching (Gardeniers et al. 2003; Roxhed et al. 2007), integrated lithographic molding technique (Luttge et al. 2007), deep X-ray photolithography (Perennes et al. 2006), photolithography with micromolding technique (Wang et al. 2009), drawing lithography with viscoelastic polymer (Lee et al. 2010) and others. In addition, glass hollow microneedles have been fabricated by drawn glass micropipette techniques (Wang et al. 2006). A recently developed hollow microneedle array (MicronJet from NanoPass Technologies) was used in a human clinical trial involving healthy adults (Van Damme et al. 2009). This device consists of a row of four hollow silicon microneedles that are 450 μm in length (Fig. 1f). In this study, ID injection with the array using 20 and 40% of the IM dose (15 μg) induced similar immune response as measured by GMT increase, seroconversion rate, and seroprotection rate.
Shooting into the Skin
ID delivery can also be achieved via jet injection or particle injection routes, which are needle-free methods of vaccine and drug delivery. There have been decades of clinical experience with jet injection, and more recent studies are being conducted with newer innovations in this technology.
Jet Injector
Needle-free jet injectors create a fine stream of pressurized liquid that penetrates the skin. The depth of delivery—ID, SC, or IM—is largely determined by design variables such as the injection stream coherence, quality, and pressure; orifice size, skin and tissue thickness, and the angle of the injection relative to the skin (Schramm-Baxter and Mitragotri 2004; Weniger and Papania 2008). Vaccines that have been shown to achieve immunity when administered via jet injection to conventional depths (i.e., ID, SC, or IM, depending on the vaccine) include typhoid, cholera, BCG, tetanus-diphtheria for adults, whole cell diphtheria-tetanus-pertussis (DTP), measles, meningococcal A and C, smallpox, yellow fever, hepatitis A, hepatitis B, influenza, plague, polio, and tetanus (Weniger and Papania 2008).
History
Historically, multi-use nozzle jet injector (MUNJI) devices with reusable nozzles were used successfully worldwide in the latter half of the 20th century to deliver countless millions, or by some estimates billions of doses of vaccines to both adults and children over the course of several decades (Weniger and Papania 2008). In response to the risks of disease transmission due to cross contamination from reuse of injection devices, a new generation of jet injector designs were developed starting in the late 1980s to address this safety concern. These new jet injectors utilize a sterile, disposable cartridge or syringe for each patient injection and a reusable hand-piece that relies on a power source, such as a manually powered spring or gas canister. A number of disposable-syringe jet injectors (DSJIs) have been developed and approved by national regulatory authorities for a variety of applications and uses, including vaccine delivery. Some of these are low-cost, manually powered DSJI technologies, developed specifically for application to developing countries’ immunization requirements and needs, which include design features to prevent reuse (‘auto-disable’) of the needle-free syringes. DSJIs in clinical development for ID delivery include the Biojector® 2000 and Zetajet® (Bioject), and PharmaJet® (PharmaJet Inc.).
There is a long history of ID delivery via the jet injector route through the use of modified syringe orifice nozzles that can either have direct contact to the skin or can involve a setback feature or ‘spacer’ intended to introduce a gap between the nozzle orifice and the injection site, thereby weakening the injection stream and limiting deposition to the dermal space (Weniger and Papania 2008) (Figs. 2a, b). MUNJI devices provided millions of ID smallpox doses during the implementation of the smallpox eradication program (Millar and Foege 1969; Weniger and Papania 2008). Jet injectors have also been utilized historically for ID vaccination of rabies (Bernard et al. 1982; Bernard et al. 1987), hepatitis A (Williams et al. 2000), BCG (Paul et al. 1978; Parker 1984), DTP combination vaccine (Stanfield et al. 1972), measles (Burland 1969; Kok et al. 1983), and influenza vaccine (Weniger and Papania 2008).
Fig. 2.
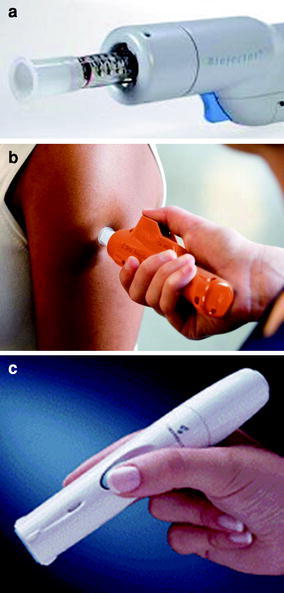
Liquid jet and solid projectile injectors. a Jet injector (Biojector 2000) with ID spacer (white portion at end of syringe), used for investigational use only (Courtesy of BioJect). b Jet injector applied to the skin for injection (Courtesy of PharmaJet). c Epidermal powder immunization device for ID projectile injection (Courtesy of PowderMed)
Recent Intradermal Vaccination Clinical Studies
A number of studies have been or will soon be implemented to address the application of DSJI ID delivery to vaccines of importance to global public health. For example, the US Centers for Disease Control and Prevention is leading a study on seasonal influenza vaccine delivered ID via a DSJI technology in children of 6–24 months of age. This study compares full and fractional dose IM with ID vaccination. Results-to-date indicate that injections were generally tolerable with few study-related adverse events. Initial blinded assay results demonstrate comparable immune response rates. Final study results and analysis can be found in Gomez et al. (2010).
The WHO Global Polio Eradication Initiative has worked to determine the potential for DSJI ID delivery of inactivated poliovirus vaccine (IPV) to be used in post-eradication settings after phasing out the use of oral polio vaccine. Studies have been conducted in Oman, Cuba, and India to evaluate reduced (‘fractional’) dose of IPV delivered with two different DSJI devices. Compared to IM, inferior seroconversion rates were found when ID doses were delivered at 6, 10, and 14 weeks of age, but non-inferior rates of protection (>95%) were seen using a later 2, 4, and 6 month schedule. When IPV was used as a booster to oral polio vaccine, inferior seroconversion rates were observed for ID compared to IM delivery (Sutter 2009; Mohammed et al. 2010; Resik et al. 2010).
DSJI technology has also been used for the delivery of DNA vaccines for malaria in young adults (Epstein et al. 2002; Wang et al. 2006) and an HIV-vaccine candidate (PATH 2009). A pilot study assessment of human papillomavirus vaccine has also recently occurred (PATH 2009). PATH is also working to implement a new study of purified Vero cell rabies vaccine for ID post-exposure prophylaxis using a DSJI technology in India. Results of this study are anticipated in 2012. Other vaccine trials of ID vaccine delivery are planned for other applications including BCG, IPV, varicella zoster virus, H1N1 and yellow fever (PATH 2009).
Projectile Delivery
Epidermal powder immunization (EPI) and particle-mediated epidermal delivery (PMED) utilize helium gas to deliver powdered proteins, polysaccharides, inactivated pathogens, or DNA-coated particles into the epidermis at supersonic speeds (Weniger and Papania 2008) (Fig. 2c). Companies involved in developing this technology include Powderject, PowderMed (acquired by Pfizer in 2006), and Iaculor Injection. It is not known if this device technology class is still in active development (PATH 2009). Conventional protein antigens must be specially formulated for delivery by EPI, and are spray dried into powders of suitable density and size (20–70 μm). A clinical trial has been conducted evaluating delivery of a powdered inactivated influenza vaccine by EPI injection, which found that immunogenicity was comparable to standard delivery by IM needle and syringe (Dean and Chen 2004). EPI has also shown efficacy in preclinical studies with hepatitis B and HIV vaccines (Chen et al. 2002; Osorio et al. 2003).
In PMED, gold beads 1–3 μm in diameter are coated with vaccine and delivered by needle-free jet injection into the epidermis. This approach may be particularly suited to DNA vaccines, as deposition of coated particles into the stratum corneum and epidermis may encourage DNA uptake and expression by resident antigen-presenting cells. DNA vaccines for hepatitis B delivered by PMED have induced protective antibodies (Roy et al. 2000; Roberts et al. 2005). Clinical studies have also been conducted with DNA vaccines for seasonal influenza to evaluate the feasibility of this approach. Results have been promising, but immune responses are not yet equivalent to standard vaccine delivery methods (Drape et al. 2006; Jones et al. 2009). EPI and PMED delivery of DNA vaccines for a variety of other diseases have also shown immunogenicity preclinically, including malaria, avian influenza, herpes simplex virus, HIV, non-small cell lung cancer, Eurasian encephalitic viruses, hantaviruses, SARS coronavirus, and smallpox (Weniger and Papania 2008).
Piercing into the Skin
For more than 200 years, various sharp instruments have been used for vaccination by creating small holes in the skin that allow vaccine to penetrate into the body (Weniger and Papania 2008). Although most vaccine administration is currently performed by hypodermic needle injection, sharp tools such as bifurcated needles have historically been used for smallpox (Frey et al. 2002) and BCG (Darmanger et al. 1977) vaccination and remain in use to this day. Over the past decade, new skin piercing technologies for ID drug transport have been developed, and include techniques such as microneedles (Prausnitz 2004) and tattooing (Bins et al. 2005). Recently these methods, especially microneedles, have shown promise for delivering vaccines to the skin, thereby enabling improved immunogenicity and simpler patient administration.
Bifurcated Needles
The bifurcated needle (Fig. 1b) was invented by Benjamin Rubin in 1961 for smallpox vaccination. It consists of two sharp prongs which hold vaccine fluid by capillary action between the two tines. The use of this device is simple and does not require trained personnel (Baxby 2002; Weniger and Papania 2008). The needles are dipped into vaccine and then punctured perpendicularly into skin repeatedly over an area of about 5 mm diameter by a process called scarification (WHO 2010). Although this method was effective for the smallpox eradication program, poorly controlled dosing, inefficient use of vaccine and needle-stick injuries were significant shortcomings that have limited the use of bifurcated needles for other vaccines.
Solid Microneedles
In addition to hollow microneedles discussed in Sect. 2, solid microneedles can be used to pierce the skin and thereby deposit vaccine in the epidermal and/or dermal space (Prausnitz et al. 2009). Techniques for vaccination using solid microneedles include the use of microneedles that penetrate the skin to make a hole through which vaccine can be transported. Vaccine formulations may be placed on the skin after microneedle penetration, coated onto microneedles or embedded within microneedles and released into the skin after insertion. Solid microneedles can be prepared as patches that can be easily applied to the skin, perhaps by self administration.
Coated Microneedles
Coated microneedles have been the most extensively studied technique for ID microneedle vaccination (Figs. 3a, b). Using this approach, vaccine forms a solid-state coating on the surface of solid microneedles that dissolves off within the skin upon application. Typically, this method provides a bolus delivery of a sub-milligram dose of antigen within minutes of application, which is often suitable for delivery of vaccines. An effective microneedle coating process typically involves dip-coating metal microneedles in a coating solution containing the vaccine, a surfactant to promote wetting of the microneedle surface, and a viscosity enhancer to increase coating thickness (Gill and Prausnitz 2007b; Gill and Prausnitz 2007a). Using this technique, compounds over a large range of sizes including small molecules, proteins, DNA, and virus particles have been coated onto microneedles. Novel coated microneedle designs for improved delivery have been demonstrated, such as the three-dimensional grooves-embedded microneedle (Han et al. 2009) and the pocketed microneedle (Gill and Prausnitz 2008). The first ID vaccination using coated microneedles delivered ovalbumin as a model protein antigen to hairless guinea pigs (Matriano et al. 2002; Widera et al. 2006). In these studies, ID microneedle vaccination showed a better immune response than an equivalent SC or IM injection at low dose. The investigators also found that immune response by microneedle vaccination was dose-dependent.
Fig. 3.
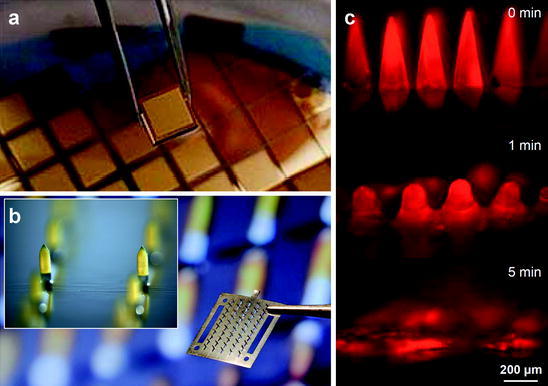
Solid microneedle patches. a Arrays of solid silicon microneedles coated with gold. (Courtesy of University of Queensland). b Array of solid stainless steel microneedles coated with yellow dye. Each 12 mm by 12 mm device contains 50 microneedles measuring 700 μm tall. Inset shows magnified view of two coated microneedles (Courtesy of Georgia Institute of Technology). c Dissolving microneedles shown intact before insertion into skin, partially dissolved 1 min after insertion into skin and fully dissolved 5 min after insertion into skin (Reproduced from (Sullivan et al. 2010); Courtesy of Georgia Institute of Technology)
Among the various vaccine candidates, influenza vaccine has received the most attention by ID immunization using small arrays of coated microneedles measuring approximately 700 μm in length (Zhu et al. 2009). Microneedles coated with 10 μg of seasonal influenza H1N1 inactivated virus vaccine induced complete protection against lethal virus infection in mice. However, subsequent studies showed that influenza vaccine lost more than 95% of its antigenicity during the coating process (Kim et al. 2010b). In order to maintain antigenicity, the disaccharide trehalose was added to the coating formulation to serve as a stabilizer. This enabled successful immunizations requiring smaller doses of vaccine (0.4 μg) as compared to immunizations with similar immune responses by conventional IM immunization (Kim et al. 2009; Kim et al. 2011). Coated microneedles also showed improved thermal stability of vaccine compared to the liquid form of vaccine (Kim et al. 2010b). More detailed studies showed that coated microneedle vaccination with inactivated influence virus vaccine induced similar antibody IgG response, HAI titer, and neutralizing activity as conventional IM immunization in mice (Kim et al. 2009; Kim et al. 2010b). To account for antigenic changes to the vaccine during the coating process, vaccine coated on microneedles was dissolved off the needles and then delivered IM by injection. In this case, vaccination using microneedles showed a better primary immune response than corresponding IM immunization using the same antigen formulation (Quan et al. 2009).
Vaccination by coated microneedles induced robust immunity to influenza after challenge in a mouse model (Kim et al. 2011). Notably, microneedle-immunized mice were shown to have undetectable levels of influenza virus titer in their lungs after challenge, unlike IM immunized mice, which had virus titers at least 100-fold higher. Additional assays for immune response from corresponding lung samples such as lung cytokine and lung IgG also consistently showed microneedle immunization to be superior to IM. As evidence for microneedle-enhanced immune system memory response, the microneedle immunized group was found to have significantly higher levels of total IgG and isotypes IgG1 and IgG2a post-challenge than pre-challenge, but antibody levels in IM immunized mice were lower post-challenge than pre-challenge (Kim et al. 2011). In addition to improved humoral immunity, coated microneedles also induced cellular recall response such as MHC II-associated CD4+ T helper cell response (Kim et al. 2009). Finally, microneedle immunization performed using a different strain of influenza (H3N2) virus vaccine induced similar complete protection against lethal challenge (Koutsonanos et al. 2009). Studies using virus-like particle (VLP) vaccine coated on microneedles were also performed. The VLP dose was controlled using a coating formulation including antigen concentration and a number of coating dips (Kim et al. 2010a). When a 0.35 μg dose of VLP was delivered, microneedle vaccination induced a stronger immune response than IM, as measured by IgG, IgG subtype (IgG1, IgG2a, IgG2b), HAI, neutralizing activity, lung IgG, lung cytokine, and more suppression of lung virus infection. Microneedle immunization by VLP showed complete protection from a lethal viral challenge without major body weight loss, unlike IM after the same dose, which partially protected mice from lethal viral infection (40%) and caused significant body weight loss (Quan et al. 2010).
A novel approach to coated microneedles involved the use of polyphosphazene (PCPP), which served as both an effective coating excipient and an immune adjuvant (Andrianov et al. 2009). ID microneedle immunization with hepatitis B surface antigen (HBsAg) in pigs using the PCPP coating formulation was superior in inducing antigen-specific IgG compared to ID injection by hypodermic needle with or without PCPP. Another study demonstrated effective generation of cellular immune responses to a hepatitis C DNA vaccine administered to mice using coated microneedles (Gill et al. 2010). Other studies have sought to specifically target delivery to antigen-presenting Langerhans cells using extremely short (~100 μm) needles that penetrate only into the epidermis. These short needles were coated using a novel coating process involving gas-jet drying (Chen et al. 2009). In an initial study, vaccination with ovalbumin-coated needles induced similar immune response to IM immunization. In a follow-up study, microneedles coated with a low dose of hemagglutinin-based influenza vaccine generated a similar immune response as IM vaccination at a 100-times larger dose. The authors proposed that these short, densely packed microneedles could deliver more than half of the antigen directly to antigen-presenting cells such as epidermal Langerhans cells and dermal dendritic cells (Fernando et al. 2010).
Methods for long-term vaccine storage without significant immunogenicity loss, especially without refrigeration, are important for vaccination campaigns. Microneedles are coated with vaccine in the solid state, which is expected to confer thermal stability. In a stability study of microneedles coated with inactivated influenza vaccine, mice immunized with coated microneedles stored at room temperature for 1 month produced similar IgG responses to those of mice immunized by microneedles stored for 1 day. Furthermore, both groups were completely protected from lethal challenge after viral infection. In vitro assay of the microneedles, however, showed a decrease in antigenicity by about 80% (Kim et al. 2010c).
Dissolving Microneedles
As an improvement over coated microneedles, dissolving microneedles have been developed in order to eliminate sharp, biohazardous waste after vaccination (Fig. 3c). Unlike non-dissolving (e.g., metal) microneedles coated with a vaccine formulation, dissolving microneedles are made solely of material such as polymers or sugars that will safely dissolve in the skin after insertion, which leaves behind only the microneedle patch backing. Typically, the vaccine is incorporated into the matrix of the microneedle and is released into the skin upon microneedle dissolution. Dissolving microneedles have been made using a number of different materials, including polyvinylpyrrolidone (Sullivan et al. 2008), maltose (Kolli and Banga 2008), carboxymethylcellulose (Lee et al. 2008), polylactic and/or polyglycolic acid (Park et al. 2005; Park et al. 2006) and dextrin (Ito et al. 2006). In a recent study, dissolving microneedles were prepared by encapsulating inactivated influenza vaccine in a polyvinylpyrrolidone matrix and used to immunize mice. The vaccine was gently encapsulated without significant damage to immunogenicity and was shown to generate similar antibody and cellular immune responses compared to IM injection of the same dose and provided complete protection against lethal challenge. Compared to IM injection, dissolving microneedle vaccination resulted in more efficient lung virus clearance and enhanced cellular recall responses after challenge (Sullivan et al. 2010). TheraJect has also developed biodegradable microneedles using carboxymethylcellulose containing various biomolecules including influenza vaccine (Oh et al. 2006).
Pretreatment with Solid Microneedles
As a simpler, albeit probably less efficient, method, microneedles can be used to pierce the skin to make it more permeable and thereby enable entry of topically applied vaccines. This method is attractive because the micro-scale pores made by microneedle insertion are generally too small for penetration of microorganisms (Donnelly et al. 2009), yet large enough for delivery of sub-unit and possibly viral vaccines. After insertion and removal of the microneedles, vaccine can be applied using a patch or other topical formulation for slow delivery by diffusion through long-lived pores (Kalluri and Banga 2011). This approach was investigated for transcutaneous vaccination using diphtheria toxoid and influenza vaccine (Ding et al. 2009a; Ding et al. 2009b). When diphtheria toxoid was applied to microneedle-pretreated skin in combination with cholera toxin adjuvant, a similar immune response was induced compared to SC injection. However, microneedle pretreatment did not enhance immune response for influenza vaccine. This vaccination approach has also been studied in an ex vivo human skin model to investigate skin immune cell responses (Ng et al. 2009). Using a related approach, blunt-tipped microneedles were used to scrape the skin, thereby making micro-troughs in the skin through which a DNA vaccine encoding HBsAg was administered (Mikszta et al. 2002). This approach generated stronger humoral and cellular immune responses than IM or ID injection.
Tattoo Vaccination
Tattoo guns use high-frequency oscillating needles to make thousands of punctures in the skin, which is conventionally used to deposit tattoo ink in the dermis, but has been adapted to deliver ID vaccines (Fig. 1d). In one study, hemagglutinin-expressing DNA vaccine was administered to pigs and derived humoral and protective immunity as shown by methods including HAI titer and improved virus clearance from nasal swabbing (Eriksson et al. 1998). To overcome the slow processing of an immune response induced by DNA vaccination, DNA tattooing was suggested for short-interval DNA vaccination (Bins et al. 2005). In this study, it was shown that short-interval ID DNA tattoo immunization generated fast and stable T cell responses to human papillomavirus and complete protection from influenza virus challenge. When compared to the IM route, DNA tattoo vaccination elicited much stronger and quicker humoral and cellular immune responses. In addition, studies indicated that even IM immunization with adjuvant was inferior to DNA tattoo immunization (Pokorna et al. 2009). To determine the effect of the tattooing process on DNA vaccine stability, the DNA topology change was evaluated, including critical factors for antigen expression and immune response (Quaak et al. 2009). It was found that the DNA tattooing tool had negligible effect on DNA structure and activity. Other vaccines including an adenoviral vector vaccine against respiratory syncytial virus (Potthoff et al. 2009) and a peptide vaccine against human papillomavirus (Pokorna et al. 2009) were administrated by ID tattooing. In the case of the adenoviral vector vaccine, tattooing showed similar performance to ID injection. Tattooing of the peptide vaccine with CpG motifs adjuvant showed better response than IM vaccination with adjuvant.
DNA tattooing was evaluated in non-human primates, which have previously shown poor DNA vaccine immunization effect, but showed remarkable enhancement of immune response by this method administering an HIV vaccine (Verstrepen et al. 2008). In order to advance this technique to human clinical trials, a human ex vivo skin model was tested, which showed that DNA concentration was the most critical factor for effective DNA vaccination by tattooing (van den Berg et al. 2009). A human clinical trial for treating melanoma is planned (Quaak et al. 2008). A comprehensive review on DNA tattooing can be found in one of the accompanying papers in this special volume on ID immunization (Oosterhuis et al. 2010).
Permeabilizing the Skin
Most of the ID vaccination methods described so far involve minimally invasive needle-based methods or non-invasive jet-based methods that actively deposit vaccine within the skin. Another set of approaches involve mostly non-invasive methods that increase skin permeability to enable vaccine transport into the skin in a transiently permeabilized state. The key to success using these approaches is disruption of skin’s outer layer, called stratum corneum. Although the stratum corneum is only 10–20 μm thick, it provides a highly effective barrier to the permeation of xenogens, including topically applied vaccine formulations (Scheuplein and Blank 1971). A number of methods to increase skin permeability have been developed, largely for drug delivery applications, many of which have been tested for vaccination (Mitragotri 2005; Prausnitz and Langer 2008).
Abrasion
A number of studies have demonstrated that the skin barrier can be broken by abrasion. A variety of abrasion methods including rough surfaces (Frerichs et al. 2008), tape-stripping (Takigawa et al. 2001; Peachman et al. 2003; Inoue and Aramaki 2007; Vandermeulen et al. 2009), and microdermabrasion devices (Gill et al. 2009) have been shown to induce adequate removal of the stratum corneum. Repeated peeling by tape (for example, Scotch® tape) effectively removes the stratum corneum. Application of tumor epitope peptides on tape-stripped mouse skin primed tumor-specific cytotoxic T cells in the lymph nodes and the spleen, protected mice against a subsequent challenge with the corresponding tumor cells, and also suppressed the growth of established tumors (Takigawa et al. 2001). Skin abrasion using a razor and a toothbrush followed by application of adenoviral vectors has yielded promising results in humans (Van Kampen et al. 2005).
Skin abrasion using an abrasive paper is perhaps the most commonly used method of disrupting the stratum corneum for immunization. For example, abrasion with emery paper, after skin hydration, has been shown to induce adequate penetration of anthrax vaccine (Matyas et al. 2004) and influenza virus vaccine (Guebre-Xabier et al. 2003), among others. This has led to the development of a Skin Prep System (SPS) to provide a controlled method of stratum corneum disruption for transcutaneous immunization currently under development by Intercell (Frerichs et al. 2008) (Fig. 4a). This technique has been shown to be effective in humans. Specifically, the skin was prepared by use of two mild strokes with the skin preparation device containing a mild abrasive affixed to a pressure-controlled device. The device was a single-use, disposable system and was discarded immediately after use. Following skin preparation, the patch containing vaccine against traveler’s diarrhea (LT patch) was applied within the marked area and worn for 6 h at each vaccination, then removed and discarded by the participant. 59 LT-patch recipients were protected against moderate-to-severe diarrhea (protective efficacy of 75%) and severe diarrhea (protective efficacy of 84%). LT-patch recipients who became ill had shorter episodes of diarrhea (0.5 vs 2.1 days) with fewer loose stools than placebo (Frech et al. 2008). In another study, a similar technique was used to boost response against influenza vaccine. In this case, prior to application, the patch area was lightly abraded with ECG-grade emery paper on skin wetted with 10% glycerol/70% alcohol to disrupt the stratum corneum. In weeks following vaccination, hemagglutination inhibition (HAI) responses in LT immunostimulatory patch recipients showed improvement over those receiving vaccine alone (Frech et al. 2005).
Fig. 4.
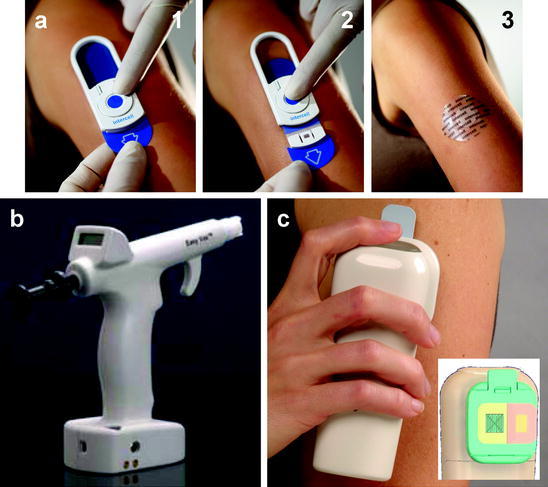
Skin permeabilization methods. a Skin abrasion device, in which a sandpaper device is placed on the skin (1), scraped across the skin in a controlled fashion (2) and then a vaccine patch is applied to the abraded skin (3) (Courtesy of Intecell). b Hand-held skin electroporation device, which uses microneedles as electrodes to cause highly localized electroporation in the skin to facilitate DNA vaccine delivery into skin cells (Courtesy of Cyto Pulse Sciences). c Heat-based device for thermal ablation of the skin. The microheater array (left side of inset) is used to ablate the skin and then a vaccine patch (right side of inset) is applied to the ablated skin (Courtesy of Altea Therapeutics)
Microdermabrasion is a common cosmetic procedure that has been adapted to remove superficial skin layers by sandblasting and thereby enable selective removal of the stratum corneum barrier. This approach has been shown to increase skin permeability and thereby enable topical application of live attenuated vaccinia virus on microdermabraded skin to generate virus-specific antibodies in the blood (Gill et al. 2009). As mentioned in Sect. 4.2.3, a microneedle-based abrasion method has also been successfully used for vaccination.
Ultrasound
Ultrasound, especially at low frequencies, is very effective in permeabilizing the skin (Tezel et al. 2001). It is now understood that acoustic cavitation, which is formation, pulsation, and collapse of gaseous bubbles under the oscillating pressure field of ultrasound, is the principal mediator for ultrasound-induced enhanced skin permeability. Several studies have shown that during ultrasound exposure, transient cavitation is predominantly induced in the coupling medium (the liquid present between the ultrasound transducer and the skin) and is primarily responsible for skin permeabilization (Tang et al. 2002; Tezel et al. 2002; Tezel and Mitragotri 2003). An estimated 10 bubble collapses/s/cm2 in the form of symmetric collapses (generating shock waves) or asymmetric collapses (producing microjets) near the surface of the skin are sufficient to explain the experimentally-observed skin permeability enhancements by ultrasound-induced skin permeabilization. Ultrasound has been shown to enhance the delivery of vaccines into skin (Tezel et al. 2005). Studies performed in mice have shown that the immune response generated by ultrasonically delivered vaccine was about 10-fold greater compared with SC injection per unit dose of the vaccine that entered the skin (about 1% of the topically applied dose entered the skin) (Tezel et al. 2005). Compared to simple topical administration, ultrasound pretreatment showed increased vaccine delivery, thereby enabling sufficient vaccine to enter the skin to activate the immune response. Furthermore, application of ultrasound resulted in activation of Langerhans cells, the reasons behind which are not clear. In another study, it was shown that application of tetanus toxoid to skin pretreated with ultrasound generated anti-tetanus toxoid IgG and neutralizing antibody titers (Dahlan et al. 2009). Several parameters, including concentration of co-applied sodium dodecyl sulfate and ultrasound duty cycle, impacted the magnitude of antibody titers. The authors concluded that the main mechanism of ultrasound-assisted skin immunization involved factors in addition to enhancement of skin permeability to topically applied antigen.
Electroporation
Electroporation involves the application of high-voltage, short-duration electric pulses to transiently disrupt lipid barriers in the body. For vaccination, electroporation has been used to increase stratum corneum permeability and thereby enable vaccine entry into the skin. Electroporation has also been used to permeabilize cells within the skin and thereby drive, for example, DNA vaccines into epidermal and dermal cells (Fig. 4b). Electroporation has been well established as a tool for delivering molecules across the stratum corneum (Prausnitz et al. 1993) or across the cell membranes (Bilitewski et al. 2003). Many studies have focused on the use of electroporation for DNA vaccination. This is not surprising given the long history of use of electroporation for delivery of DNA into cells in vitro. However, many electroporation studies involve insertion of electrode needles into the skin. Some studies have demonstrated the use of electroporation for topical vaccine delivery (Zhao et al. 2006). In one study, electroporation has been found to stimulate the exodus of Langerhans cells from the skin, which is likely to have an adjuvant-like effect (Zhao et al. 2006). In this study, the efficacy of peptide delivery was found to be comparable to that of ID injected with Freund’s complete adjuvant. Further, the peptide-specific CTL response to the vaccine delivered by electroporation was equivalent to that delivered by ID injection.
Electroporation has been shown to induce an effective immune response after delivery of DNA vaccines (Peachman et al. 2003; Foldvari et al. 2006; Medi and Singh 2008; Vandermeulen et al. 2009). For example, studies in pigs have shown the ability of electroporation to deliver HBsAg gene using a single-needle or a six-needle electrode (Babiuk et al. 2002). Studies have demonstrated that in vivo skin electroporation may be used to increase transgene expression relative to naked DNA injection (Drabick et al. 2001). Transfected cells were principally located in dermis and included adipocytes, fibroblasts, endothelial cells, and numerous mononuclear cells with dendritic processes in a porcine model. Transfected cells were also observed in lymph nodes draining electropermeabilized sites. A HBsAg-coding plasmid was used to test skin electroporation-mediated nucleic acid vaccination in a murine model. Applications for these findings include modulation of immune responses to pathogens, allergens, and tumor-associated antigens and the modification of tolerance. In another study, in vivo electroporation has shown protection against avian influenza in non-human primates (Laddy et al. 2009). A number of human clinical trials testing vaccination enhanced by electroporation are currently under way.
Chemical Enhancers
Several chemicals are known to interact with the skin and disrupt the highly ordered lipid bilayer structure in the stratum corneum. This observation led to the study of chemical agents to enhance transport across skin. More than 300 chemicals have been studied for their ability to increase skin permeability (Karande et al. 2004). Chemical permeation enhancers are relatively inexpensive and easy to formulate, they offer flexibility in their design, are simple in application and allow the freedom of self-administration to the patient. Chemical enhancers comprise a wide variety of different chemical functional groups and facilitate drug transport across the skin by a variety of complex mechanisms. They can directly exert their effect on skin structure by acting on intercellular lipids or corneocytes. Chemical enhancers can extract lipids from the skin thereby creating diffusion pathways for transdermal permeation. Alternatively, they can partition themselves into the lipid bilayers thereby disrupting the highly ordered lipid lamellae and causing their fluidization. Chemical enhancers can also significantly increase skin transport of a drug by enhancing its thermodynamic activity in the formulation (Karande et al. 2005).
Recently, chemical enhancers have been shown to possess the ability to deliver antigens and generate immune responses. This was achieved by designing formulations that possess the ability to enhance skin permeability as well as exhibit high adjuvanticity. The rational design of such multi-functional formulations from first principles requires in-depth knowledge of interactions between chemical enhancers and skin, which exist for a very limited pool of chemicals. Hence, combinatorial libraries of chemical mixtures were screened. Studies have shown that in a randomly selected population of chemical formulations, certain binary mixtures of chemicals are far more potent in permeabilizing the skin as compared to single chemicals (Karande et al. 2004). In vaccination studies, a third chemical was added with the goal of enhancing the ability to offer adjuvanticity. The lead chemical formulations were tested in mice using the model antigen ovalbumin. The formulations that exhibited high permeation and adjuvanticity potential in in vitro screening also induced high IgG titers in mice (Karande et al. 2009). In another study, penetration enhancers and immunomodulators oleic acid and retinoic acid were used to enhance transcutaneous immunization with inactivated influenza virus across tape-stripped skin (Skountzou et al. 2006). Pretreatment of mouse skin with oleic acid elicited increased levels of influenza virus-specific binding and neutralizing antibodies to levels equivalent to those induced by intra-nasal immunization with inactivated influenza virus. Oleic acid and retinoic acid treatments differentially affected the pattern of cytokine production upon stimulation with influenza viral antigen and provided enhanced protection.
Thermal Ablation
Thermal poration of skin has been used to deliver vaccines into skin. Microporation systems are designed to porate the skin and are being developed by a number of companies. In this method, an array of micropores is created in the skin by removal of stratum corneum by the application of focused thermal energy based on resistive heating via the contact of electrically heated small-diameter wires to the skin surface (Bramson et al. 2003) (Fig. 4c) or other methods based on radiofrequency or laser-based approaches. In this study, the microporation tip was comprised of a set of 80 μm diameter tungsten wires with control circuitry allowing for precise control of the electrical current pulses that were passed through each wire. The software user interface was designed to enable the control of various microporation parameters including micropore density, resistive element temperature, current pulse width, number of pulses, pulses pacing, and contact pressure. The temperature of the tip that was placed in contact with the skin was calibrated by an optical calibrator device. The study showed that microporation significantly increased the penetration of topically delivered vaccine. Microporation enhanced expression of luciferase upon placement of adenovirus vectors by 100–300-fold. The same procedure led to increased CTL response and increased IFN-γ secreting cells. In a related study, the same technology has been shown to deliver influenza vaccine into mouse skin. Eighty micropores were created in 1 cm2 area and the vaccine was placed on the porated skin. This procedure generated adequate protective response in mice (Garg et al. 2007).
Discussion
Immunologic Advantages of Intradermal Vaccination
ID vaccination offers potential immunologic advantages to public health. The skin is known to be a site rich in antigen-presenting cells, some of which are specific to the skin, including epidermal Langerhans cells and dermal dendritic cells (Glenn and Kenney 2006). In addition, antigen may be taken up directly by lymphatic vessels for transport to antigen-presenting cells in the lymph nodes. At a minimum, the ID route of vaccination appears to follow different pathways to immunity compared to IM or SC routes. However, there is evidence that the ID route is not only different, but is also beneficial (Glenn and Kenney 2006; Lambert and Laurent 2008; Nicolas and Guy 2008).
The possibility of dose sparing enabled by ID vaccination has been suggested by previous preclinical and clinical studies; however, the successful application of this approach has yet to be definitively confirmed for many vaccines (Glenn and Kenney 2006; Lambert and Laurent 2008; Nicolas and Guy 2008). Although the conclusions vary between different studies and different vaccines, there is an indication that dose sparing may be possible. However, it is not currently clear under what conditions the skin’s unique immune environment can be harnessed for optimal effect. In addition to dose sparing, there is preclinical study evidence of other beneficial differences of ID vaccination. Studies with microneedles showed improved influenza virus clearance from the lungs and enhanced memory responses compared to IM vaccination (Kim et al. 2011). Studies with EPI showed a specific role for Langerhans cells to generate robust antibody responses (Chen et al. 2004). Studies with ultrasound-mediated vaccination suggested an adjuvant effect on the skin (Dahlan et al. 2009).
Logistical Advantages of Intradermal Vaccination
ID vaccination offers potential value to public health also in terms of possible logistical advantages. For comparison, IM and SC vaccination can only be carried out by hypodermic needle injection with few other options beside jet injection. ID vaccination opens the door to many other technologies because the skin is readily accessible at the surface of the body. As a result, ID injection may enable vaccination methods that generate no biohazardous sharp waste, can be administered by personnel with minimal training, and simplify transportation and storage logistics (Table 1).
Table 1.
Capabilities of intradermal vaccination systems
| ID delivery method | Ease of usea | Vaccine utilizationb | Biohazardous sharp wastec | Technology developmentd | Vaccine reformulatione | Device costf |
|---|---|---|---|---|---|---|
| Mantoux injection | ++ | +++ | + | +++ | ++ | +++ |
| Single hollow microneedle | ++ | +++ | ++ | ++ | ++ | ++ |
| Array of hollow microneedles | ++ | +++ | ++ | ++ | ++ | ++ |
| Jet injection | + | +++ | +++ | +++ | ++ | + |
| Powder/gene gun | + | +++ | +++ | ++ | + | + |
| Bifurcated needle | +++ | + | + | +++ | ++ | +++ |
| Coated microneedles | +++ | ++ | ++ | + | + | ++ |
| Dissolving microneedles | +++ | ++ | +++ | + | + | ++ |
| Pretreatment with microneedles | +++ | + | ++ | + | + | ++ |
| Tattoo gun | + | + | + | + | ++ | + |
| Skin abrasion | +++ | + | +++ | ++ | + | +++ |
| Ultrasound | + | + | +++ | + | + | + |
| Electroporation | + | + | +++ | + | + | + |
| Chemical enhancer | +++ | + | +++ | + | + | +++ |
| Thermal ablation | +++ | + | +++ | + | + | ++ |
a +++ requires little or no personnel training, ++ requires personnel training, + requires personnel training and maintenance of a dedicated device
b +++ almost 100% in skin, ++ >50% in skin, + <50% in skin
c +++ no biohazardous sharp waste, ++ microscopic biohazardous sharp waste, + macroscopic biohazardous sharp waste
d +++ in widespread clinical practice, ++ published vaccination data in humans, + preclinical
e +++ no reformulation required, ++ possible new liquid formulation required, + reformulation required to produce solid-state vaccine
f +++ inexpensive disposable device, ++ specialty disposable device, + reusable device. Per-injection cost of reusable devices will depend on the number of times the device can be used and the cost of any disposable components
Mantoux technique injection requires specialized training by clinical personnel. Microneedle systems and patch-based delivery (accompanied by skin permeabilization technologies) offer the promise of simplified vaccination methods that require minimal training and may permit self-vaccination by patients in certain scenarios. This not only benefits routine vaccination scenarios, but is especially important to mass vaccination campaigns associated with disease eradication programs or pandemic emergencies. In contrast, some of the novel ID delivery methods, such as projectile delivery and tattoo guns, introduce new, sophisticated devices that require additional training of clinical personnel. Assuming the injection is done properly, the Mantoux technique can administer essentially all of the vaccine into the skin. Hollow microneedles and projectile delivery can be similarly efficient. However, solid microneedles typically retain some vaccine on the device and patch-based skin permeabilization methods are extremely inefficient, such that most vaccine typically remains on the skin surface. The efficiency of vaccine utilization will be of critical importance for new, costly vaccines, as well as in developing countries where vaccine cost can be a significant barrier to access.
Eliminating the hypodermic needle from vaccination is a major objective of public health, given that close to one million people die each year from disease transmission from contaminated needles (Miller and Pisani 1999; Kermode 2004). Microneedles are a step in the right direction, but still generate biohazardous sharp waste, with the exception of dissolving microneedles. Projectile delivery and patch-based methods eliminate needles and therefore offer an improved safety profile. However, some ID delivery methods can cause added tissue trauma to the skin (Bremseth and Pass 2001). Most of the new methods of ID vaccination require significant technology development. While jet injection is already in widespread clinical use, many other technologies are only in the preclinical stage of development for vaccination. That being said, many of those technologies are in much later stage of development or use for non-vaccine applications, which will facilitate their adaptation to ID vaccination.
Most of the new ID vaccination technologies also require vaccine reformulation. Hollow microneedle, jet and tattoo-based methods may use standard, currently available liquid formulation, but in some cases will need to be concentrated or otherwise modified. The other methods mostly use a solid-state vaccine formulation, which offers likely advantages in terms of vaccine stability during storage, but, however, requires significant reformulation, with associated research, regulatory, and manufacturing hurdles. Finally, device cost is a significant consideration, given that a hypodermic needle and syringe are extremely inexpensive, disposable devices. Microneedle systems and some of the patch-based methods are expected to have low manufacturing cost in mass production. However, many of the other technologies require multiple device components, which may be engineered into disposable devices with added cost or reusable devices with disposable components that require an initial investment that can be amortized over many patients.
Future Outlook
ID vaccination has already made significant impact on public health as the primary means of immunization during smallpox eradication and continues to play a role in BCG and rabies vaccination in current clinical practice (Plotkin et al. 2008). However, as discussed in this article, there are many more opportunities for ID vaccination to potentially improve immunogenicity and simplify logistics of the administration of other vaccines. A number of new ID vaccination technologies have been successful in human clinical trials. ID vaccination using the BD hollow microneedle was approved in Europe in 2009 for ID administration of the Sanofi Pasteur seasonal influenza vaccine and was introduced in Australia and New Zealand during the 2010 influenza season (Holland et al. 2008; Beran et al. 2009). This microneedle device may be adapted for use to administer other vaccines as well. Jet injectors have a long history of use for vaccination and are receiving renewed attention for ID delivery of vaccines in clinical trials, especially to address developing countries’ needs, through support from WHO and US Centers for Disease Control and Prevention (see Sect. 3.1.2.). Skin abrasion as a pretreatment before applying a vaccine patch is also in clinical trials for prevention of influenza and traveler’s diarrhea (Frech et al. 2005; Frech et al. 2008). Projectile based delivery by EPI and PMED have been studied in a number of human clinical trials for both DNA and protein-based vaccines (Dean and Chen 2004; Jones et al. 2009), although it is unclear as to what extent this technology is under continued commercial development.
Other ID delivery devices are under advanced preclinical study. Solid coated microneedles have been the subject of numerous vaccination studies in mice and larger animals to administer influenza and other vaccines (see Sect. 5.2), and have been used in a Phase II clinical trial of a drug, parathyroid hormone (Cosman et al. 2009). Likewise, skin electroporation, in some cases in combination with microneedles, has been studied in animals for skin vaccination. As evidence for clinical feasibility, electroporation of skin for targeted delivery of chemotherapeutic agents to skin tumors is approved and used in Europe (Gehl 2008). Tattooing is of course in widespread human use, and its application to vaccination has been studied preclinically. Other methods to increase skin permeability, such as ultrasound, chemical enhancers and heat, are also in clinical use or trials for transdermal drug delivery applications (Prausnitz and Langer 2008), which compliment preclinical studies of their use for vaccination. Given the large number of technologies for ID vaccination under development, and the advanced clinical status of many of them, the future outlook for bringing ID vaccination into more widespread clinical practice appears encouraging. The optimal delivery method will depend on the specific application and other factors, such as immunologic response, logistical needs, and financial constraints.
Contributor Information
Marcel B.M. Teunissen, Phone: +31 20 5664812, Email: m.b.teunissen@amc.uva.nl
M. R. Prausnitz, Email: prausnitz@gatech.edu
References
- Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14:375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, Babiuk LA, Townsend H, Mutwiri G. Poly [di (carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci USA. 2009;106:18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Ayoola EA. Hepatitis B vaccine in developing countries: problems and prospects. IARC Sci Publ. 1984;63:297–305. [PubMed] [Google Scholar]
- Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, Babiuk LA. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- Baxby D. Smallpox vaccination techniques; from knives and forks to needles and pins. Vaccine. 2002;20:2140–2149. doi: 10.1016/s0264-410x(02)00028-2. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. New Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, Frey SE. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–6763. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, Demanet E, Heijmans S, Van Belle P, Weber F, Salamand C. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7:13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard KW, Roberts MA, Sumner J, Winkler WG, Mallonee J, Baer GM, Chaney R. Human diploid cell rabies vaccine. Effectiveness of immunization with small intradermal or subcutaneous doses. JAMA. 1982;247:1138–1142. doi: 10.1001/jama.247.8.1138. [DOI] [PubMed] [Google Scholar]
- Bernard KW, Mallonee J, Wright JC, Reid FL, Makintubee S, Parker RA, Dwyer DM, Winkler WG. Preexposure immunization with intradermal human diploid cell rabies vaccine. Risks and benefits of primary and booster vaccination. JAMA. 1987;257:1059–1063. [PubMed] [Google Scholar]
- Bilitewski U, Genrich M, Kadow S, Mersal G. Biochemical analysis with microfluidic systems. Anal Bioanal Chem. 2003;377:556–569. doi: 10.1007/s00216-003-2179-4. [DOI] [PubMed] [Google Scholar]
- Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TNM, Haanen JBAG. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med. 2005;11:899–904. doi: 10.1038/nm1264. [DOI] [PubMed] [Google Scholar]
- Bramson J, Dayball K, Evelegh C, Wan YH, Page D, Smith A. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10:251–260. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- Bremseth DL, Pass F. Delivery of insulin by jet injection: recent observations. Diabetes Technol Ther. 2001;3:225–232. doi: 10.1089/152091501300209598. [DOI] [PubMed] [Google Scholar]
- Brindle RJ, Morris CA, Berger R, Kurtz JB. Inadequate response to intradermal hepatitis A vaccine. Vaccine. 1994;12:483–484. doi: 10.1016/0264-410x(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Burland WL. Measles vaccination by the intradermal route. Postgrad Med J. 1969;45:323–326. doi: 10.1136/pgmj.45.523.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson U, Brudin L, Eliasson I, Hansson BG. Hepatitis A vaccination by intracutaneous low dose administration: a less expensive alternative. Scand J Infect Dis. 1996;28:435–438. doi: 10.3109/00365549609037934. [DOI] [PubMed] [Google Scholar]
- Chen W, Gluud C (2005) Vaccines for preventing hepatitis B in health-care workers. Cochrane Database Syst Rev, Issue 4. Art. No.: CD000100. doi:10.1002/14651858.CD000100.pub3 [DOI] [PubMed]
- Chen D, Zuleger C, Chu Q, Maa YF, Osorio J, Payne LG. Epidermal powder immunization with a recombinant HIV gp120 targets Langerhans cells and induces enhanced immune responses. AIDS Res Hum Retroviruses. 2002;18:715–722. doi: 10.1089/088922202760072348. [DOI] [PubMed] [Google Scholar]
- Chen D, Burger M, Chu Q, Endres R, Zuleger C, Dean H, Payne LG. Epidermal powder immunization: cellular and molecular mechanisms for enhancing vaccine immunogenicity. Virus Res. 2004;103:147–153. doi: 10.1016/j.virusres.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Chen XF, Prow TW, Crichton ML, Jenkins DWK, Roberts MS, Frazer IH, Fernando GJP, Kendall MAF. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139:212–220. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Coberly JS, Townsend T, Repke J, Fields H, Margolis H, Halsey NA. Suboptimal response following intradermal hepatitis B vaccine in infants. Vaccine. 1994;12:984–987. doi: 10.1016/0264-410x(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Cosman F, Lane NE, Bolognese MA, Zanchetta JR, Garcia-Hernandez PA, Sees K, Matriano JA, Gaumer K, Daddona PE. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2009;95:151–158. doi: 10.1210/jc.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlan A, Alpar HO, Stickings P, Sesardic D, Murdan S. Transcutaneous immunisation assisted by low-frequency ultrasound. Int J Pharm. 2009;368:123–128. doi: 10.1016/j.ijpharm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Darmanger AM, Nekzad SM, Kuis M, Tendam HG. BCG vaccination by bifurcated needle in a pilot vaccination program. Bull WHO. 1977;55:49–61. [PMC free article] [PubMed] [Google Scholar]
- Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52:909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- de Moraes JC, Leon ME, Souza VA, Pannuti C, Travisanello C, Halsey NA, de Quadros CA. Intradermal administration of measles vaccines. Bull Pan Am Health Organ. 1994;28:250–255. [PubMed] [Google Scholar]
- Dean HJ, Chen D. Epidermal powder immunization against influenza. Vaccine. 2004;23:681–686. doi: 10.1016/j.vaccine.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Dean CH, Alarcon JB, Waterston AM, Draper K, Early R, Guirakhoo F, Monath TP, Mikszta JA. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccine. 2005;1:106–111. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- Dimache G, Stoean C, Durbaca S, Croitoru M, Ionescu M, Nedelcu IN, Corbu I. Study of specific immune response to unadsorbed concentrated tetanus vaccine administered by intradermal route to non-immunized persons in the last ten years. Arch Roum Pathol Exp Microbiol. 1990;49:51–62. [PubMed] [Google Scholar]
- Ding Z, Van Riet E, Romeijn S, Kersten GFA, Jiskoot W, Bouwstra JA. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm Res. 2009;26:1635–1643. doi: 10.1007/s11095-009-9874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Verbaan FJ, Bivas-Benita M, Bungener L, Huckriede A, van den Berg DJ, Kersten G, Bouwstra JA. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release. 2009;136:71–78. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Donnelly RF, Singh TRR, Tunney MM, Morrow DIJ, McCarron PA, O’Mahony C, Woolfson AD. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26:2513–2522. doi: 10.1007/s11095-009-9967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabick JJ, Glasspool-Malone J, King A, Malone RW. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther. 2001;3:249–255. doi: 10.1006/mthe.2000.0257. [DOI] [PubMed] [Google Scholar]
- Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006;24:4475–4481. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Epstein JE, Gorak EJ, Charoenvit Y, Wang R, Freydberg N, Osinowo O, Richie TL, Stoltz EL, Trespalacios F, Nerges J, Ng J, Fallarme-Majam V, Abot E, Goh L, Parker S, Kumar S, Hedstrom RC, Norman J, Stout R, Hoffman SL. Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum Gene Ther. 2002;13:1551–1560. doi: 10.1089/10430340260201644. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Yao F, Svensjo T, Winkler T, Slama J, Macklin MD, Andree C, McGregor M, Hinshaw V, Swain WF. In vivo gene transfer to skin and wound by microseeding. J Surg Res. 1998;78:85–91. doi: 10.1006/jsre.1998.5325. [DOI] [PubMed] [Google Scholar]
- Etchart N, Hennino A, Friede M, Dahel K, Dupouy M, Goujon-Henry C, Nicolas JF, Kaiserlian D. Safety and efficacy of transcutaneous vaccination using a patch with the live-attenuated measles vaccine in humans. Vaccine. 2007;25:6891–6899. doi: 10.1016/j.vaccine.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Fernando GJP, Chen XF, Prow TW, Crichton ML, Fairmaid EJ, Roberts MS, Frazer IH, Brown LE, Kendall MAF. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One. 2010;5:e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DB, Pacer RE, Holmes DF, Ley AB, Yager P, Tong TC. Rabies preexposure prophylaxis with human diploid cell rabies vaccine: a dose-response study. J Infect Dis. 1987;156:50–55. doi: 10.1093/infdis/156.1.50. [DOI] [PubMed] [Google Scholar]
- Flynn PM, Shenep JL, Mao L, Crawford R, Williams BF, Williams BG. Influence of needle gauge in Mantoux skin testing. Chest. 1994;106:1463–1465. doi: 10.1378/chest.106.5.1463. [DOI] [PubMed] [Google Scholar]
- Foldvari M, Babiuk S, Badea I. DNA delivery for vaccination and therapeutics through the skin. Curr Drug Deliv. 2006;3:17–28. doi: 10.2174/156720106775197493. [DOI] [PubMed] [Google Scholar]
- Frech SA, Kenney RT, Spyr CA, Lazar H, Viret JF, Herzog C, Gluck R, Glenn GM. Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch. Vaccine. 2005;23:946–950. doi: 10.1016/j.vaccine.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, Okhuysen PC, Guerrero NH, Martinez-Sandoval FG, Melendez-Romero JH, Jiang ZD, Asturias EJ, Halpern J, Torres OR, Hoffman AS, Villar CP, Kassem RN, Flyer DC, Andersen BH, Kazempour K, Breisch SA, Glenn GM. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371:2019–2025. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- Frerichs DM, Ellingsworth LR, Frech SA, Flyer DC, Villar CP, Yu J, Glenn GM. Controlled, single-step, stratum corneum disruption as a pretreatment for immunization via a patch. Vaccine. 2008;26:2782–2787. doi: 10.1016/j.vaccine.2008.02.070. [DOI] [PubMed] [Google Scholar]
- Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, Newman FK, Atmar RL, Edelman R, Nolan CM, Belshe RB. Clinical responses to undiluted and diluted smallpox vaccine. New Engl J Med. 2002;346:1265–1274. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- Gardeniers HJGE, Luttge R, Berenschot EJW, de Boer MJ, Yeshurun SY, Hefetz M, van’t Oever R, van den Berg A. Silicon micromachined hollow microneedles for transdermal liquid transport. J MEMS. 2003;12:855–862. [Google Scholar]
- Garg S, Hoelscher M, Belser JA, Wang C, Jayashankar L, Guo Z, Durland RH, Katz JM, Sambhara S. Needle-free skin patch delivery of a vaccine for a potentially pandemic influenza virus provides protection against lethal challenge in mice. Clin Vaccine Immunol. 2007;14:926–928. doi: 10.1128/CVI.00450-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehl J. Electroporation for drug and gene delivery in the clinic: doctors go electric. Methods Mol Biol. 2008;423:351–359. doi: 10.1007/978-1-59745-194-9_27. [DOI] [PubMed] [Google Scholar]
- Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- Gill HS, Prausnitz MR. Pocketed microneedles for drug delivery to the skin. J Phys Chem Solids. 2008;69:1537–1541. doi: 10.1016/j.jpcs.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Andrews SN, Sakthivel SK, Fedanov A, Williams IR, Garber DA, Priddy FH, Yellin S, Feinberg MB, Staprans SI, Prausnitz MR. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur J Pharm Sci. 2009;38:95–103. doi: 10.1016/j.ejps.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Sõderholm J, Prausnitz MR, Sällberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–268. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- Gomez V, Palomeque FS, Feris J et al. (2010) Still-blinded safety and immunogenicity data from a trial of reduced-dose, intradermal, influenza vaccination by needle-free jet injection. 13th Annual Conference on Vaccine Research, Bethesda, MD
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Guebre-Xabier M, Hammond SA, Epperson DE, Yu J, Ellingsworth L, Glenn GM. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J Virol. 2003;77:5218–5225. doi: 10.1128/JVI.77.9.5218-5225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Kim DK, Kang SH, Yoon HR, Kim BY, Lee SS, Kim KD, Lee HG. Improvement in antigen-delivery using fabrication of a grooves-embedded microneedle array. Sens Act B. 2009;137:274–280. [Google Scholar]
- Heijtink RA, Knol RM, Schalm SW. Low-dose (2 micrograms) hepatitis B vaccination in medical students: comparable immunogenicity for intramuscular and intradermal routes. J Med Virol. 1989;27:151–154. doi: 10.1002/jmv.1890270216. [DOI] [PubMed] [Google Scholar]
- Henderson DA, Borio BL, Grabenstein JD. Smallpox and vaccinia. In: Plotkin S, Orenstein WA, Offit PA, editors. Vaccines. Amsterdam: Elsevier; 2008. pp. 773–803. [Google Scholar]
- Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC, Horwitz MA. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008;198:1491–1501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, Weber F, Saville M. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- Howard A, Mercer P, Nataraj HC, Kang BC. Bevel-down superior to bevel-up in intradermal skin testing. Ann Allergy Asthma Immunol. 1997;78:594–596. doi: 10.1016/s1081-1206(10)63222-x. [DOI] [PubMed] [Google Scholar]
- Inoue J, Aramaki Y. Toll-like receptor-9 expression induced by tape-stripping triggers on effective immune response with CpG-oligodeoxynucleotides. Vaccine. 2007;25:1007–1013. doi: 10.1016/j.vaccine.2006.09.075. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29:82–88. doi: 10.1016/j.ejps.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Jones S, Evans K, McElwaine-Johnn H, Sharpe M, Oxford J, Lambkin-Williams R, Mant T, Nolan A, Zambon M, Ellis J, Beadle J, Loudon PT. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine. 2009;27:2506–2512. doi: 10.1016/j.vaccine.2009.02.061. [DOI] [PubMed] [Google Scholar]
- Kalluri H, Banga AK. Formation and closure of microchannels in skin following microporation. Pharm Res. 2011;28:82–94. doi: 10.1007/s11095-010-0122-x. [DOI] [PubMed] [Google Scholar]
- Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22:192–197. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci USA. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karande P, Arora A, Pham TK, Stevens D, Wojicki A, Mitragotri S. Transcutaneous immunization using common chemicals. J Control Release. 2009;138:134–140. doi: 10.1016/j.jconrel.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated-microneedles. Vaccine. 2009;27:6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Fomulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11:1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm Res. 2011;28:135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok PW, Kenya PR, Ensering H. Measles immunization with further attenuated heat-stable measles vaccine using five different methods of administration. Trans Roy Soc Trop Med Hyg. 1983;77:171–176. doi: 10.1016/0035-9203(83)90059-7. [DOI] [PubMed] [Google Scholar]
- Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25:104–113. doi: 10.1007/s11095-007-9350-0. [DOI] [PubMed] [Google Scholar]
- Koutsonanos DG, Martin MdP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddy DJ, Yan J, Khan AS, Andersen H, Cohn A, Greenhouse J, Lewis M, Manischewitz J, King LR, Golding H, Draghia-Akli R, Weiner DB. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol. 2009;83:4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Lee HC, Lee DS, Jung H. Drawing lithography: three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv Mat. 2010;22:483–486. doi: 10.1002/adma.200902418. [DOI] [PubMed] [Google Scholar]
- Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, Leroux-Roels G. Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–6619. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- Luttge R, Berenschot EJW, de Boer MJ, Altpeter DM, Vrouwe EX, van den Berg A, Elwenspoek M. Integrated lithographic molding for microneedle-based devices. J MEMS. 2007;16:872–884. [Google Scholar]
- Mantoux C. Tuberculin intradermo reactions in the treatment of tuberculosis: intradermi-tuberculisation. C R Hebd Seances Acad Sci. 1909;148:996–998. [Google Scholar]
- Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. Macroflux® microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- Matyas GR, Friedlander AM, Glenn GM, Little S, Yu J, Alving CR. Needle-free skin patch vaccination method for anthrax. Infect Immun. 2004;72:1181–1183. doi: 10.1128/IAI.72.2.1181-1183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medi BM, Singh J. Delivery of DNA into skin via electroporation. Methods Mol Biol. 2008;423:225–232. doi: 10.1007/978-1-59745-194-9_16. [DOI] [PubMed] [Google Scholar]
- Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- Mikszta JA, Sullivan VJ, Dean C, Waterston AM, Alarcon JB, Dekker JP, Brittingham JM, Huang J, Hwang CR, Ferriter M, Jiang G, Mar K, Saikh KU, Stiles BG, Roy CJ, Ulrich RG, Harvey NG. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191:278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- Mikszta JA, Dekker JP, Harvey NG, Dean CH, Brittingham JM, Huang J, Sullivan VJ, Dyas B, Roy CJ, Ulrich RG. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006;74:6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JD, Foege WH. Status of eradication of smallpox (and control of measles) in West and Central Africa. J Infect Dis. 1969;120:725–732. doi: 10.1093/infdis/120.6.725. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pisani E. The cost of unsafe injections. Bull WHO. 1999;77:808–811. [PMC free article] [PubMed] [Google Scholar]
- Milne A, Allwood GK, Pearce NE, Lucas CR, Krugman S. Low dose hepatitis B vaccination in children. New Zeal Med J. 1986;99:47–49. [PubMed] [Google Scholar]
- Mitragotri S. Immunization without needles. Nat Rev Immun. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- Mohammed AJ, AlAwaidy S, Bawikar S, Kurup PJ, Elamir E, Shaban MMA, Sharif SM, van der Avoort HGAM, Pallansch MA, Malankar P, Burton A, Sreevatsava M, Sutter RW. Fractional doses of inactivated poliovirus vaccine in Oman. New Engl J Med. 2010;362:2351–2359. doi: 10.1056/NEJMoa0909383. [DOI] [PubMed] [Google Scholar]
- Ng KW, Pearton M, Coulman S, Anstey A, Gateley C, Morrissey A, Allender C, Birchall J. Development of an ex vivo human skin model for intradermal vaccination: tissue viability and Langerhans cell behaviour. Vaccine. 2009;27:5948–5955. doi: 10.1016/j.vaccine.2009.07.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7:1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- Nirmal S, Cherian T, Samuel BU, Rajasingh J, Raghupathy P, John TJ. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine. 1998;16:928–931. doi: 10.1016/s0264-410x(97)00293-4. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Harrison T, D’Andrea A, Kwon SY, Burkoth TL, Burke R. Intradermal influenza vaccine delivery using skin-penetrating dissolvable vaccine microneedles. AAPS Annual Meeting. TX: San Antonio; 2006. [Google Scholar]
- Oosterhuis K, van den Berg JH, Schumacher TN, Haanen JBAG. DNA vaccines and intradermal vaccination by DNA tattooing. Curr Top Microbiol Immunol. 2010;351:221–250. doi: 10.1007/82_2010_117. [DOI] [PubMed] [Google Scholar]
- Osorio JE, Zuleger CL, Burger M, Chu Q, Payne LG, Chen D. Immune responses to hepatitis B surface antigen following epidermal powder immunization. Immunol Cell Biol. 2003;81:52–58. doi: 10.1046/j.1440-1711.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- Pancharoen C, Mekmullica J, Thisyakorn U, Kasempimolporn S, Wilde H, Herzog C. Reduced-dose intradermal vaccination against hepatitis A with an aluminum-free vaccine is immunogenic and can lower costs. Clin Infect Dis. 2005;41:1537–1540. doi: 10.1086/497266. [DOI] [PubMed] [Google Scholar]
- Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23:1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- Parker V. Jet gun or syringe? A trial of alternative methods of BCG vaccination. Public Health. 1984;98:315–320. doi: 10.1016/s0033-3506(84)80023-2. [DOI] [PubMed] [Google Scholar]
- PATH (2009) Intradermal delivery of vaccines: a review of the literature and the potential for development for use in low- and middle-income countries. PATH/WHO, Ferney Voltaire, France. http://www.path.org/publications/detail.php?i=1746 14 Sept 2010.
- Paul SS, Raveendra NK, Chhabra AK, Verma M. Comparison of BCG inoculation by conventional intradermal and jet methods. Indian Pediatr. 1978;15:341–347. [PubMed] [Google Scholar]
- Peachman KK, Rao M, Alving CR. Immunization with DNA through the skin. Methods. 2003;31:232–242. doi: 10.1016/s1046-2023(03)00137-3. [DOI] [PubMed] [Google Scholar]
- Perennes F, Marmiroli B, Matteucci M, Tormen M, Vaccari L, Di Fabrizio E. Sharp beveled tip hollow microneedle arrays fabricated by LIGA and 3D soft lithography with polyvinyl alcohol. J Micromech Microeng. 2006;16:473–479. [Google Scholar]
- Phanuphak P, Khaoplod P, Benjavongkulchai M, Chutivongse S, Wilde H. What happens if intradermal injections of rabies vaccine are partially or entirely injected subcutaneously? Bull WHO. 1990;68:83–85. [PMC free article] [PubMed] [Google Scholar]
- Plotkin S, Orenstein W, Offit P, editors. Vaccines. Philadelphia: Saunders; 2008. [Google Scholar]
- Pokorna D, Polakova I, Kindlova M, Duskova M, Ludvikova V, Gabriel P, Kutinova L, Muller M, Smahel M. Vaccination with human papillomavirus type 16-derived peptides using a tattoo device. Vaccine. 2009;27:3519–3529. doi: 10.1016/j.vaccine.2009.03.073. [DOI] [PubMed] [Google Scholar]
- Potthoff A, Schwannecke S, Nabi G, Hoffmann D, Grunwald T, Wildner O, Brockmeyer NH, Uberla K, Tenbusch M. Immunogenicity and efficacy of intradermal tattoo immunization with adenoviral vector vaccines. Vaccine. 2009;27:2768–2774. doi: 10.1016/j.vaccine.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz MR, Bose VG, Langer R, Weaver JC. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci USA. 1993;90:10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaak SGL, van den Berg JH, Toebes M, Schumacher TNM, Haanen J, Beijnen JH, Nuijen B. GMP production of pDERMATT for vaccination against melanoma in a phase I clinical trial. Eur J Pharm Biopharm. 2008;70:429–438. doi: 10.1016/j.ejpb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Quaak SGL, den Berg JHv, Oosterhuis K, Beijnen JH, Haanen JBAG, Nuijen B. DNA tattoo vaccination: effect on plasmid purity and transfection efficiency of different topoisoforms. J Control Release. 2009;139:153–159. doi: 10.1016/j.jconrel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. Intradermal vaccination with influenza VLPs using microneedles induces superior protection to intramuscular immunization. J Virol. 2010;84:7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman F, Dahmen A, Herzog-Hauff S, Bocher WO, Galle PR, Lohr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–527. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- Resik S, Tejeda A, Lago PM, Diaz M, Carmenates A, Sarmiento L, Alemani N, Galindo B, Burton A, Friede M, Landaverde M, Sutter RW. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis. 2010;201:1344–1352. doi: 10.1086/651611. [DOI] [PubMed] [Google Scholar]
- Roberts LK, Barr LJ, Fuller DH, McMahon CW, Leese PT, Jones S. Clinical safety and efficacy of a powdered hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine. 2005;23:4867–4878. doi: 10.1016/j.vaccine.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Roukens AH, Vossen AC, Bredenbeek PJ, van Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non-inferiority trial. PLoS One. 2008;3:e1993. doi: 10.1371/journal.pone.0001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roukens AHE, Gelinck LBS, Visser LG. Intradermal vaccination to protect against yellow fever and influenza. Curr Top Microbiol Immunol. 2011;351:159–179. doi: 10.1007/82_2011_124. [DOI] [PubMed] [Google Scholar]
- Roxhed N, Gasser TC, Griss P, Holzapfel GA, Stemme G. Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery. J MEMS. 2007;16:1429–1440. [Google Scholar]
- Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S, Culp J, Burkholder JK, Swain WF, Dixon RM, Widera G, Vessey R, King A, Ogg G, Gallimore A, Haynes JR, Heydenburg Fuller D. Induction of antigen-specific CD8 + T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19:764–778. doi: 10.1016/s0264-410x(00)00302-9. [DOI] [PubMed] [Google Scholar]
- Samuel BU, Cherian T, Sridharan G, Mukundan P, John TJ. Immune response to intradermally injected inactivated poliovirus vaccine. Lancet. 1991;338:343–344. doi: 10.1016/0140-6736(91)90480-d. [DOI] [PubMed] [Google Scholar]
- Samuel BU, Cherian T, Rajasingh J, Raghupathy P, John TJ. Immune response of infants to inactivated poliovirus vaccine injected intradermally. Vaccine. 1992;10:135. doi: 10.1016/0264-410x(92)90039-m. [DOI] [PubMed] [Google Scholar]
- Sangare L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine. 2009;27:1777–1786. doi: 10.1016/j.vaccine.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Scheuplein RJ, Blank IH. Permeability of the skin. Physiol Rev. 1971;51:702–747. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]
- Schramm-Baxter J, Mitragotri S. Needle-free jet injections: dependence of jet penetration and dispersion in the skin on jet power. J Control Release. 2004;97:527–535. doi: 10.1016/j.jconrel.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24:6110–6119. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Stanfield JP, Bracken PM, Waddell KM, Gall D. Diphtheria–tetanus–pertussis immunization by intradermal jet injection. Brit Med J. 1972;2:197–199. doi: 10.1136/bmj.2.5807.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SP, Murthy N, Prausnitz MR. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv Mat. 2008;20:933–938. doi: 10.1002/adma.200701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, Murthy N, Compans RW, Skountzou I, Prausnitz MR. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R. Status of GPEI research and product development. Geneva: Manufacturer’s Meeting; 2009. [Google Scholar]
- Takigawa M, Tokura Y, Hashizume H, Yagi H, Seo N. Percutaneous peptide immunization via corneum barrier-disrupted murine skin for experimental tumor immunoprophylaxis. Ann N Y Acad Sci. 2001;941:139–146. doi: 10.1111/j.1749-6632.2001.tb03717.x. [DOI] [PubMed] [Google Scholar]
- Tang H, Wang CC, Blankschtein D, Langer R. An investigation of the role of cavitation in low-frequency ultrasound-mediated transdermal drug transport. Pharm Res. 2002;19:1160–1169. doi: 10.1023/a:1019898109793. [DOI] [PubMed] [Google Scholar]
- Tezel A, Mitragotri S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys J. 2003;85:3502–3512. doi: 10.1016/S0006-3495(03)74770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel A, Sens A, Tuchscherer J, Mitragotri S. Frequency dependence of sonophoresis. Pharm Res. 2001;18:1694–1700. doi: 10.1023/a:1013366328457. [DOI] [PubMed] [Google Scholar]
- Tezel A, Sens A, Mitragotri S. Investigations of the role of cavitation in low-frequency sonophoresis using acoustic spectroscopy. J Pharm Sci. 2002;91:444–453. doi: 10.1002/jps.10024. [DOI] [PubMed] [Google Scholar]
- Tezel A, Paliwal S, Shen Z, Mitragotri S. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine. 2005;23:3800–3807. doi: 10.1016/j.vaccine.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Van den Berg JH, Nuijen B, Beijnen JH, Vincent A, Van Tinteren H, Kluge J, Woerdeman LAE, Hennink WE, Storm G, Schumacher TN, Haanen J. Optimization of intradermal vaccination by DNA tattooing in human skin. Human Gene Ther. 2009;20:181–189. doi: 10.1089/hum.2008.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, Marks D, Elmets CA, Tang DC. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Vandermeulen G, Daugimont L, Richiardi H, Vanderhaeghen ML, Lecouturier N, Ucakar B, Preat V. Effect of tape stripping and adjuvants on immune response after intradermal DNA electroporation. Pharm Res. 2009;26:1745–1751. doi: 10.1007/s11095-009-9885-3. [DOI] [PubMed] [Google Scholar]
- Verstrepen BE, Bins AD, Rollier CS, Mooij P, Koopman G, Sheppard NC, Sattentau Q, Wagner R, Wolf H, Schumacher TNM, Heeney JL, Haanen JBAG. Improved HIV-1 specific T-cell responses by short-interval DNA tattooing as compared to intramuscular immunization in non-human primates. Vaccine. 2008;26:3346–3351. doi: 10.1016/j.vaccine.2008.03.091. [DOI] [PubMed] [Google Scholar]
- Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- Wang PC, Wester BA, Rajaraman S, Paik SJ, Kim SH, Allen MG (2009) Hollow polymer microneedle array fabricated by photolithography process combined with micromolding technique. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, pp 7026–7029 [DOI] [PMC free article] [PubMed]
- Warrell MJ. Intradermal rabies vaccination: the evolution and future of pre- and post-exposure prophylaxis. Curr Top Microbiol Immunol. 2011;351:139–157. doi: 10.1007/82_2010_121. [DOI] [PubMed] [Google Scholar]
- Weniger BG, Papania MJ. Alternative vaccine delivery methods. In: Plotkin SA, Orenstein WA, Offit P, editors. Vaccines. Philadelphia: Saunders Elsevier; 2008. pp. 1357–1392. [Google Scholar]
- Whittle HC, Rowland MG, Mann GF, Lamb WH, Lewis RA. Immunisation of 4–6 month old Gambian infants with Edmonston–Zagreb measles vaccine. Lancet. 1984;2:834–837. doi: 10.1016/s0140-6736(84)90873-0. [DOI] [PubMed] [Google Scholar]
- WHO WHO expert consultation on rabies. WHO Tech Rep Ser. 2005;931:1–88. [PubMed] [Google Scholar]
- WHO Rabies vaccines. WHO position paper. Wkly Epidemiol Rec. 2007;82:425–435. [PubMed] [Google Scholar]
- WHO (2010) WHO fact sheet: smallpox. WHO, Geneva. http://www.who.int/mediacentre/factsheets/smallpox/en/ 14 Sept 2010
- Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Williams J, Fox-Leyva L, Christensen C, Fisher D, Schlicting E, Snowball M, Negus S, Mayers J, Koller R, Stout R. Hepatitis A vaccine administration: comparison between jet-injector and needle injection. Vaccine. 2000;18:1939–1943. doi: 10.1016/s0264-410x(99)00446-6. [DOI] [PubMed] [Google Scholar]
- Zhao YL, Murthy SN, Manjili MH, Guan LJ, Sen A, Hui SW. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24:1282–1290. doi: 10.1016/j.vaccine.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Zhu QY, Zarnitsyn VG, Ye L, Wen ZY, Gao YL, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang CL, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulek G, Roggendorf M, Deinhardt F. Immune response to single dose, multisite, intradermal and to intramuscular administration of vaccine against tick-borne encephalitis virus. Lancet. 1984;2:584. doi: 10.1016/s0140-6736(84)90809-2. [DOI] [PubMed] [Google Scholar]
- Zoulek G, Roggendorf M, Deinhardt F, Kunz C. Different immune responses after intradermal and intramuscular administration of vaccine against tick-borne encephalitis virus. J Med Virol. 1986;19:55–61. doi: 10.1002/jmv.1890190109. [DOI] [PubMed] [Google Scholar]


