Abstract
Objective
We performed an open label Phase I/II trial to evaluate the safety and tolerability of vagus nerve stimulation (VNS) in patients with treatment-resistant fibromyalgia (FM) as well as to determine preliminary measures of efficacy in these patients.
Methods
Of 14 patients implanted with the VNS stimulator, 12 completed the initial 3 month study of VNS; 11 returned for follow-up visits 5, 8 and 11 months after start of stimulation. Therapeutic efficacy was assessed with a composite measure requiring improvement in pain, overall wellness, and physical function. Loss of both pain and tenderness criteria for the diagnosis of FM was added as a secondary outcome measure because of results found at the end of 3 months of stimulation.
Results
Side effects were similar to those reported in patients treated with VNS for epilepsy or depression and, in addition, dry mouth and fatigue were reported. Two patients did not tolerate stimulation. At 3 months, five participants had attained efficacy criteria; of these, two no longer met widespread pain or tenderness criteria for the diagnosis of FM. The therapeutic effect seemed to increase over time in that additional participants attained both criteria at 11 months.
Conclusions
Side effects and tolerability were similar to those found in disorders currently treated with VNS. Preliminary outcome measures suggested that VNS may be a useful adjunct treatment for FM patients resistant to conventional therapeutic management but further research is required to better understand its actual role in the treatment of FM.
Fibromyalgia (FM) affects 3.4% of women and 0.5% men in North America (1). Despite this prevalence, only three medications are currently approved for its use. Anecdotal data among FM practitioners suggest that many patients, however, continue to suffer pain which interferes substantially with their physical function and quality of life.
We evaluated the possibility that periodic stimulation of the left vagus nerve by Vagus Nerve Stimulation [VNS] throughout the 24 hr day might be a safe, tolerable, and useful adjunct treatment for patients reporting continued severe pain despite receiving current best medical management. Three observations guided the reasoning for undertaking this trial: first, experimental studies suggested that afferent vagal stimulation may modulate descending serotonergic and noradrenergic neurons to reduce pain (2); second, VNS has FDA approval for treatment resistant epilepsy and depression – disorders which have been treated by similar medicines as those used to treat FM (3;4) and third, VNS appeared to decrease pain perception in patients with treatment-resistant depression (5). To test our hypothesis, we initiated a Phase I/II safety and tolerability trial of VNS in a cohort of FM persons with continued substantial pain complaints despite medical treatment. While the primary purpose of this “proof of concept” trial was to assess the safety and tolerability of VNS in FM, we also collected preliminary data assessing potential treatment efficacy.
METHODS
Study participants
This was an open label, longitudinal, single-center study using VNS in a group of FM patients refractory to conventional pharmacological treatment. To be eligible, patients had to have FM, diagnosed by a physician, for at least two years, be between 18 to 60 years of age, and attain at least average scores on the vocabulary subscale of the Wechsler Adult Intelligence Scale–III (WAIS-III (6)). In addition, FM patients had to provide physician-documented evidence that the following medications had been tried to treat FM pain but either did not provide sufficient relief or were tolerated poorly: non-steroidal anti-inflammatory drugs, tricyclic antidepressants or duloxetine (an SNRI), any one anti-convulsant drug, and tramadol. Patients had to be on a stable medication regimen for at least six weeks prior to study entry and were asked to maintain this regimen throughout the initial or acute phase of the study. While reductions in dosage did not affect continuing eligibility, dosage increases or the introduction of additional drugs were not allowed during the acute phase of the study; thereafter, there were no restrictions.
Exclusions included other medical illness that could cause widespread body pain; use of antipsychotic drugs or any non-pharmacological treatment for FM within three months of enrollment; vagotomy; being in litigation at time of enrollment; reporting the onset of FM following physical trauma; positive history of psychotic depression, bipolar disorder, psychotic disorders, substance abuse/dependence within 10 years prior to study intake on diagnostic psychiatric interview [MINI (7)]; patients with non-psychotic depression were not excluded.
One hundred and twelve individuals were recruited and screened between November 2006 and May 2008. Of these, 14 women fulfilled entry criteria and provided informed consent to undergo VNS implantation, activation, current intensity ramp up, and fixed stimulation for three months. Eleven of the 14 implanted patients participated in a longitudinal study lasting an additional 8 months.
Study procedures
Timeline
After signing an IRB approved screening consent, participants visited the Pain & Fatigue Study Center on two occasions to be evaluated for study eligibility. The evaluation included a careful medical history, physical examination, and blood tests (CBC with differential, sedimentation rate, SMA-18, TSH/T4/T3 uptake, CPK, ANA, Rheumatoid factor, C6-Lyme Elisa) to rule out other possible causes of widespread pain and to allow collection of baseline data. On each of these visits, eligibility was ascertained by confirming the diagnosis of FM using ACR criteria (8). Those criteria required (a) the presence of chronic widespread pain defined as ≥ three months of pain in at least three bodily quadrants plus pain in the axial skeletal area and (b) the report of pain upon pressure of at least 11 of 18 points with a pressure of 4 kg. To determine whether a point was tender or not, we used a standardized and validated examination (9) in which a “positive” tender point was defined as a patient rating of 2 or more on a 0 to 10 pain scale (0 was none, 5 moderate and 10 worst pain imaginable) upon palpitation with 4 kg pressure. At baseline, participants were required to wear a watch-type electronic diary (Actiwatch, Respironics, Inc, Portland, OR) that polled pain intensity five times a day over 9 days (at least 37 readings required for inclusion). Eligible participants had to have a median pain intensity score of at least 5 (where 0 indicated No Pain and 10 Worst Pain Imaginable). Once eligibility was confirmed, patients signed an implantation enrollment consent approved by the IRB of UMDNJ-NJMS.
Baseline data collected on the screening visits included the SF-36 (10), the Margolis Pain Drawing (11), and reports of usual FM pain intensity during the past week, scaled the same as the electronic diary. Quantitative sensory testing (QST) assessing heat pain was conducted using the TSA 2001 apparatus (Medoc, Ltd, Ramat Yishai, Israel). Participants rated the pain intensity and unpleasantness of seven stimulus intensities ranging from 43° C to 49° C in 1° increments, each presented twice and ordered at random, on a 10 point magnitude rating scale.
After a median of 60 days following enrollment, study participants were implanted with the VNS device. After two weeks for surgical recovery, participants began a two-week stimulation adjustment period during which VNS intensity was increased to deliver as high a current as could be comfortably tolerated [target range: 1 to 2 mA] while holding all other stimulation parameters constant [pulse width = 250 µsec; frequency = 20 Hz; duty cycle = 30 sec on, 5 min off – i.e., the parameters used by Cyberonics Inc, the device manufacturer, for previous trials of VNS]. Stimulus parameters were then held constant over the next 12 weeks, referred to as the “Acute Study,” although reductions in VNS current intensity due to side effects were allowed. During this phase of the study, participants could decrease medications but could neither increase dose or frequency of existing medications nor add new medications. During their 9 return visits, study participants provided data on side effects of VNS, FM and medication status, as well as usual pain ratings since their last visit; they also completed QST and self-report questionnaires. Participants who continued in the follow up study after 5, 8 and 11 months of stimulation provided these same data; they could now also request upward adjustment of current intensity due to lessening of side effects over time or diminishing pain relief after the acute study.
Assessment of safety and tolerability
Primary safety endpoints included (a) the number of participants who tolerated implantation of the VNS device, its activation and ramp up through the end of the acute study and (b) the range of VNS output current tolerated at the end of the acute study and at the 5, 8, and 11 month follow-up visits. We aimed to determine whether: a) types of adverse events were similar to those reported in patients with refractory epilepsy and treatment resistant depression and b) rates of occurrence of adverse events were similar.
Efficacy outcomes
We assessed participants for clinical improvement at each of the planned study visits. Our primary outcome measure was whether participants attained a minimal clinically important difference (MCID+) following VNS; this criterion has been previously employed in a 3-month drug trial in FM (12). To become MCID+, participants had to show improvement on three separate measures: a 30% improvement from baseline ‘usual pain ratings in the last week’ AND a Patient Global Impression of Change score rated as markedly or moderately improved [1–2 on a 7 point scale] AND an improvement of at least 6 points [0.6 SD] on the Physical Function subscale of the SF-36.
The secondary outcome measure presented in this paper – i.e., loss of FM caseness – was not an a priori hypothesis since no data exist to support the notion that this variable might change with treatment. However, we added FM caseness as a post hoc outcome measure, because of the unexpected results seen at the end of the acute study period. We defined loss of FM caseness as a patient’s no longer fulfilling both 1990 ACR FM criteria -- widespread pain and at least 11 tender points on palpation. We operationalized the definition of widespread pain by considering it present if patients had pain in at least 3 bodily quadrants plus having axial pain (score of ≥ 4). Thus scores ≤ 3 no longer fulfilled the widespread pain criterion. Patients having less than 11 tender points no longer fulfilled the tender point criterion.
We report safety and tolerability data for all 14 implanted participants; outcome is reported using an intent to treat analysis.
RESULTS
Participants were all women with ages ranging from 35 to 54. Four had major depressive disorder [MDD] on entry; three were disabled. Of the remaining 10 without MDD, four were disabled.
Safety and Tolerability
All 14 study participants tolerated implantation of the VNS device, its activation and the subsequent ramp up of VNS output current. Ultimately, 100% of the study sample tolerated implantation well, while 93% tolerated ramp up and fixed stimulation during the acute study (see below).
There were 4 unanticipated/serious adverse events occurring in 3 patients. The first was not device related: participant #118 was non-compliant with the protocol requirement for not changing medication during the 16-week acute study and was hospitalized for opiate overdose. The second was device related: Participant #115 experienced a device failure necessitating surgical revision. The third, also occurring in participant #115, was not device related: Following device re-activation and ramp up of stimulus intensity, she reported such marked dyspepsia that she asked that the stimulator be turned off. Dyspepsia continued despite cessation of VNS. Data from these two participants – both of whom came into the study positive for current MDD – are not included in the preliminary efficacy analysis of the acute study. The last adverse event was classified as possibly stimulation related: Participant #121 reported stimulus-bound electric-like sensations across her chest and into her left arm that were reduced by lowering VNS intensity; this side effect of stimulation is persisting but has been well tolerated.
At the end of the acute study, current intensity ranged from 0.75 to 2 mA [median = 1.5 mA]. Thereafter, participants were free to adjust their output current, but median output current remained stable at 1.5 mA: ranges of output current at 5, 8 and 11 month stimulation follow-up visits respectively were 1.0–2.5 mA; 0.5–2.25 mA, and 1.0–2.5 mA. Despite objective evidence of improvement at the end of the acute study, one patient [#105] with MDD perceived VNS as not beneficial for her widespread pain and felt it exacerbated her pre-existing headache disorder; she requested that the stimulator be turned off and elected to have it explanted subsequent to completing the acute study.
Frequencies of observed adverse events (AEs) related to surgery, the device, or stimulation for all 14 implanted participants are listed in Table 1. Most adverse events were similar to those reported in patients with refractory epilepsy and treatment resistant depression (13); they were self limited and decreased in severity over time.
Table 1.
Study participant adverse event profile
| Observed Surgery Related AEs (N=14) | Mild | Moderate | Severe |
|---|---|---|---|
| Dyspnea | 3 | ||
| Voice Alteration | 1 | 1 | |
| Infection/Fever | 1 | ||
| Incision pain | 1 | 1 | |
| Skin irritation | 1 | 1 | |
| Nausea | 2 | ||
| Neck pain | 1 | ||
| Sleep difficulties/Insomnia | 2 | ||
| Surgery-related complications such as upper respiratory infection | 1 | ||
| Observed Device or Stimulation Related AEs (N=14) | Mild | Moderate | Severe |
| Agitation/anxiety/panic | 1 | ||
| Chest pain | 1 | ||
| Device migration | 2 | ||
| Decreased appetite/weight loss | 1 | ||
| Dyspepsia | 1 | 2 | |
| Dysphagia | 2 | ||
| Dyspnea | 3 | 4 | |
| Ear pain | 1 | 2 | |
| Facial pain | 1 | 3 | 1 |
| Gastritis | 1 | ||
| Headache | 2 | 1 | |
| Increased coughing | 1 | ||
| Mania, hypomania, and related symptoms | 1 | ||
| Nausea and vomiting | 2 | 3 | |
| Neck/throat pain | 1 | 3 | 3 |
| Sleep disturbances/difficulties, including worsening of pre-existing obstructive sleep apnea, insomnia | 4 | ||
| Tinnitus | 1 | ||
| Tooth pain | 1 | ||
| Voice alteration | 5 | 4 | |
| AEs potentially specifically related to Fibromyalgia (N=14) | Mild | Moderate | Severe |
| Surgery related | |||
| Dry mouth | 1 | ||
| Fatigue | 1 | ||
| Headache | 1 | ||
| Neck numbness | 1 | ||
| Device and stimulation related | |||
| Abdominal pain | 1 | ||
| Depression worsening | 1 | 1 | |
| Dry mouth | 1 | 2 | |
| Excessive production of saliva | 1 | ||
| Fatigue | 1 | 2 | |
| Nasal congestion | 1 | ||
| Neck numbness | 1 | ||
| Photophobia | 1 |
Surgery and stimulation related adverse events not reported previously, but observed here included mild (n=1) to moderate (n=2) dry mouth and moderate (n=1) to severe (n=2) increases in fatigue. While rates of occurrence for voice alteration for FM patients were similar to those of patients with treatment resistant MDD and epilepsy (64% versus 58 and 54%, respectively), rates of neck/facial pain, headaches, and dyspnea were greater in the FM sample (50%, 21%, and 50%, respectively versus 13–16%, less than 5%, and 14–16%, respectively). These observations in this small sample suggest that individuals with treatment resistant FM, a chronic pain disorder, may be more sensitive to pain related to vagus nerve stimulation. However, this increased sensitivity did not result in termination of stimulation.
Efficacy
A priori outcome measure
Table 2 indicates the time points when individual participants became MCID+ [light grey shading]. At the end of the acute study, five [36%] of the 14 implanted participants had become MCID+, and in the follow up study, two, eight, and seven of the 14 implanted participants [14%, 57%, and 50% respectively] had become MCID+ at the 5, 8 and 11 month stimulation visits. Two participants were MCID+ across all 4 assessment times; one study patient was MCID+ at the end of the acute study and then again at the 8 and 11 month stimulation follow up visits; three patients became MCID+ at the 8 month and 11 month time points – suggestive of progressive improvement over time. Less successful outcomes included one participant who was MCID+ only at the end of the acute study and a second one who was MCID+ only at the end of the acute study and at the 8 month assessment.
Table 2.
Efficacy outcome measures: Tender points (# quadrants of Pain) Across the Study
| ID | Baseline Average |
Acute Study End |
After 5 Months of Stim. |
After 8 Months of Stim. |
After 11 Months of Stim. |
|---|---|---|---|---|---|
| 102 | 12 (5) | 9 (5) | 5 (4) | 10 (5) | 14 (5) |
| 104 | 18 (5) | 2 (4) | 4 (5) | 1 (1) | 8 (2) |
| 105 | 14.5 (5) | 6 (4) | STOP | STOP | EXPLANT |
| 106 | 18 (5) | 11 (5) | 8 (0) | 5 (2) | 7 (3) |
| 107 | 16 (5) | 12 (5) | 8 (5) | 6 (3) | 3 (3) |
| 108 | 17 (5) | 4 (1) | 4 (3) | 7 (0) | 6 (0) |
| 111 | 15.5 (5) | 0 (1) |
8 (5) | 9 (5) | 9 (4) |
| 114 | 18 (5) | 16 (5) | 16 (5) | 8 (4) | 13 (5) |
| 115 | 18 (5) | DEVICE PROBLEM THEN SIDE EFFECTS→ STOP | |||
| 117 | 18 (5) | 18 (5) | 5 (0) | 2 (2) | 5 (2) |
| 118 | 13.5 (5) | STUDY VIOLATION; EXCLUDED | |||
| 119 | 18 (5) | 18 (5) | 18 (5) | 18 (5) | 18 (5) |
| 121 | 18 (5) | 18 (4) | 18 (5) | 18 (3) | 18 (5) |
| 124 | 15 (5) | 15 (4) | 14 (4) | 11 (4) | 15 (4) |
![]()
Light grey filled cell indicates MCID+
Thick black outlined cell indicates no longer fulfilling EITHER widespread pain OR tenderness criteria for FM
Dark grey filled cell indicates patient who at last visit did not fulfill tenderness criterion for FM
A posteriori outcome measure
At baseline, each of the 14 women had tenderness in 4 bodily quadrants as well as in axial skeletal areas [noted in Table 2 as 5 within parentheses], and tender point counts ranged from 12 to 18 [median = 17.5]. Table 2 also shows the points in time when individual participants ceased fulfilling both criteria for FM caseness – that is, when participants had three or fewer quadrants of pain and had fewer than 11 tender points [thick outlined boxes]. These numbers increased over the course of the study from two at the end of the acute study to five at the end of the follow-up study. There was an association between MCID status and FM status: participants no longer fulfilled criteria for FM at 14 of the 22 time points [63.6%] where participants were MCID+, while only one no longer fulfilled criteria for FM at the 23 time points [4.3%] where participants were MCID- [Fisher’s test = 0.001, 2-tailed]. Concordance between the two outcome measures seemed to improve over time. While only two of the five MCID+ participants no longer fulfilled the two criteria for FM at the end of the acute study, five of the seven MCID+ participants no longer fulfilled both criteria for FM at the end of the follow-up study.
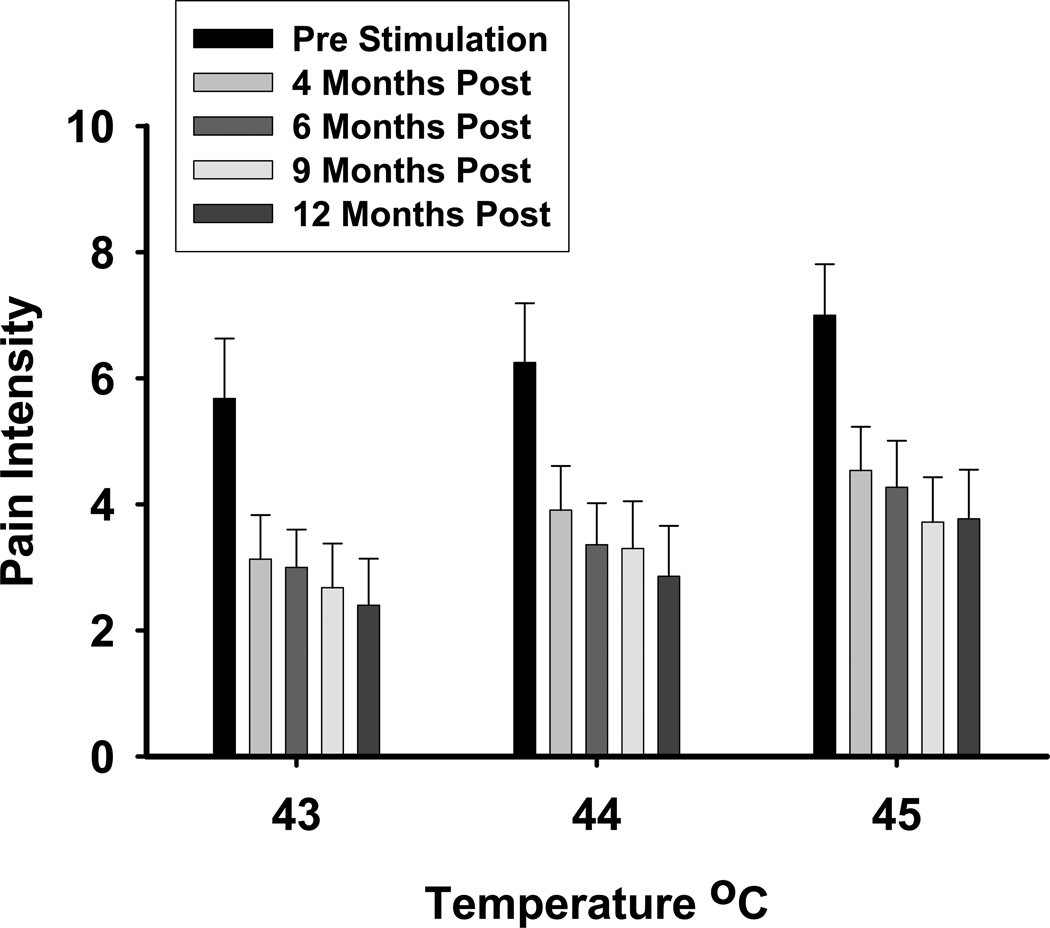
The overall decrease in pain sensitivity is supported by the QST results obtained prior to device activation and at each of the subsequent study visits for the 11 patients completing the follow up trial [see Figure 1]. As expected, reported pain intensity increased as the actual temperature of the probe increased, ANOVA for repeated measures F2,26 = 14.1, p = 0.001. Results also showed that there was a significant and progressive decrease in pain intensity reported to each of the three temperatures over the course of the study, ANOVA for repeated measures F5,77 = 9.3, p = 0.001.
Figure 1.
Mean pain intensity ratings (± SEM) across the duration of the study. Data are plotted in months following surgical implantation of VNS.
DISCUSSION
In general, FM patients had the same types of side effects to VNS as those reported in patients with treatment-resistant epilepsy and depression – most often stimulus-bound voice alteration, neck pain, nausea, and dyspnea; these side effects tended to dissipate with time. Dry mouth and increased fatigue were two AEs not previously reported and present in this study population. While implantation surgery was tolerated well, two patients did not complete the acute study [one due to problems tolerating stimulation and the other due to study violations]; a third patient requested device explantation due to treatment inefficacy. This non-completion rate does not differ from that reported at the end of the one year trial of VNS for major depression [270 completers of 295 implanted (14); fishers test NS]. Eleven women completed the follow up study. No late emerging AEs were observed.
While the primary purpose of this study was to assess the safety and tolerability of VNS in FM, a secondary goal was to do a preliminary evaluation of its efficacy. We assessed the MCID and another measure added at the end of the acute study phase – the existence of the diagnosis of FM consistent with 1990 ACR FM criteria (2), i.e., FM caseness. We had not considered loss of FM caseness (2) as a possible outcome measure when we designed the study because no published treatment had been efficacious enough to affect diagnosis, but since we found this to occur in certain VNS-treated patients, we realized that using loss of FM caseness as an outcome variable might be useful for clinicians in judging the potential efficacy of VNS.
Both outcome measures showed substantial improvement over time. At the end of the acute study, five of the 14 participants became MCID+ and two no longer fulfilled both diagnostic criteria for the 1990 ACR FM case definition (2). In contrast to studies using reduction in pain alone to indicate the therapeutic efficacy of a drug in treating FM, only this and one other published trial used the more demanding MCID to determine a positive therapeutic effect (12). Importantly, no study has ever reported sufficient improvement in pain that treated patients no longer fulfill criteria for the diagnosis of FM (2).
This therapeutic effect seemed to increase beyond the acute trial. At the end of the 11 month study, seven patients were MCID+ and parallel improvement was seen in terms of FM caseness (2): five patients no longer fulfilled either the widespread pain criterion or the tender point criterion for the diagnosis of FM (2), and a sixth patient continued to have wide spread pain but had fewer than 11 tender points (dark grey shading in Table 2). We were surprised by the robustness and ubiquity of response to the VNS treatment. While it is true that “improvement in tender point threshold appears to be a difficult outcome to achieve” (4), our results suggest that an FM treatment can reduce tender point threshold to the degree that the point tested is no longer tender.
However, tender point count was not a reliable predictor of continued therapeutic success over time as can be seen with #102 as an example (see Table 2). She had widespread pain throughout the trial and had as few as five tender points at one visit; but, then, number of tender points increased thereafter. The best predictor of outcome seemed to be reduction in painful quadrants to three or lower. For every patient except #121 at her 8 month visit, this reduction in bodily pain boded well for continued clinical improvement.
The reduction in QST/psychophysical response to heat pain stimuli suggests that VNS had an effect on the sensitivity of the nociceptive system. Patients reported large decreases in pain ratings from the pre-stimulation baseline to the end of the acute study phase, and these changes persisted throughout the remaining study visits. These data suggest that VNS may tune down the pathophysiological processes responsible for central sensitization, thus providing a potential mechanism as to how VNS can reduce widespread musculoskeletal pain in FM. The results of the entire QST battery are currently being prepared as a separate manuscript.
Since this is an uncontrolled pilot study, an obvious question is whether this positive therapeutic effect is specific to VNS itself or is a placebo effect secondary to extraneous factors related to being in a treatment trial necessitating surgery, feeling a sensory stimulus throughout the day, and having high hopes for a good therapeutic outcome. Some data do exist to show that non-specific [i.e., placebo] effects can last for many months in trials requiring surgery. But studies reporting that outcome were for episodic events – syncope (15) or angina (16) – very different conditions from one with chronic pain. One trial on Parkinsonian patients has been cited as showing a long-lived placebo effect, but not one which improved patients’ neurological impairment or their objective function (17); another with sham surgery for knee pain did produce a 10% reduction in pain over one year (18). Thus, published data indicating a prolonged effect of nonspecific factors in reducing chronic symptoms are sparse.
Some evidence for an initial non-specific effect may be seen from the data of one participant, subject #124, who became MCID+ at the end of the acute study but at no time point thereafter. However, the continued improvement over time shown by some patients and the fact that more patients attained outcome criteria over time argues against a non-specific or placebo explanation for the therapeutic benefit; such an incrementing response has been reported for VNS treatment of refractory epilepsy (19). Nevertheless, a controlled trial is needed to determine the specificity of these effects.
Acknowledgements
This work was supported by NIH # AR-053732. A patent application for the use of VNS in FM is pending for GL under US Patent Application No. 12/322,741. We thank Dr. Daniel Clauw, a physician expert in FM and FM treatment trials, and Dr. Sandra Helmers, an expert in the use of VNS in epilepsy, for their help in various aspects of this study. We acknowledge the help of Dr. Adam Perlman in patient recruitment and medical decision making and the help of Ms. Kristin Thorsen, editor of the Fibromyalgia Network News, for publicizing the existence of this clinical “proof of concept” trial to the FM community at large and thus aiding in recruitment of participants.
Reference List
- 1.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res Brain Res Rev. 1992;17:77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE, Jr., et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336–1344. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 4.Russell IJ, Mease PJ, Smith TR, Kajdasz DK, Wohlreich MM, Detke MJ, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136:432–444. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Borckardt JJ, Kozel FA, Anderson B, Walker A, George MS. Vagus nerve stimulation affects pain perception in depressed adults. Pain Res Manag. 2005;10:9–14. doi: 10.1155/2005/256472. [DOI] [PubMed] [Google Scholar]
- 6.Wechsler D. Wechsler Adult Intelligence Scale - Revised, Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- 7.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- 8.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 9.Okifuji A, Turk DC, Sinclair JD, Starz TW, Marcus DA. A standardized manual tender point survey .I. development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J Rheumatol. 1997;24:377–383. [PubMed] [Google Scholar]
- 10.McHorney CA, Ware JE, Raczek AE. The MOS 36-item short form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24:57–65. doi: 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30:1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Cyberonics Inc. Epilepsy Physicians' Manual, Neuro Cybernetic Prosthesis System NCP Pulse Generator, Models 100 and 101. 2002 http://us.cyberonics.com/en/vns-therapy-for-epilepsy/healthcare-professionals/vns-therapy/manuals-page. [Google Scholar]
- 14.Cyberonics Inc. For Health Care Professionals. Houston TX: 2008. Depression Information. VNS Therapy™ Pulse Generators; p. 23. [Google Scholar]
- 15.Connolly SJ, Sheldon R, Thorpe KE, Roberts RS, Ellenbogen KA, Wilkoff BL, et al. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: Second Vasovagal Pacemaker Study (VPS II): a randomized trial. JAMA. 2003;289:2224–2229. doi: 10.1001/jama.289.17.2224. [DOI] [PubMed] [Google Scholar]
- 16.Cobb LA, Thomas GI, Dillard DH, Merendno KA, Bruce RA. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med. 1959;260:1115–1118. doi: 10.1056/NEJM195905282602204. [DOI] [PubMed] [Google Scholar]
- 17.McRae C, Cherin E, Yamazaki TG, Diem G, Vo AH, Russell D, et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry. 2004;61:412–420. doi: 10.1001/archpsyc.61.4.412. [DOI] [PubMed] [Google Scholar]
- 18.Moseley JB, O'Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 19.Morris GL, III, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53:1731–1735. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]