Abstract
Ghrelin is a gut peptide that has been studied extensively for its role in food intake and energy balance. More recent studies show that ghrelin reduces water intake in rats and some non-mammalian species. Despite the importance of the regulation of NaCl intake in body fluid homeostasis, the effects of ghrelin on saline intake have not been investigated. Accordingly, we tested the effect of ghrelin on water and 1.8% NaCl intake in two-bottle test conditions under five stimuli that increase hypertonic saline intake: central angiotensin II administration, 24 h fluid deprivation, water deprivation followed by partial rehydration, dietary sodium deficiency, and polyethylene glycol administration combined with dietary sodium deficiency. We found that ghrelin attenuated saline intake stimulated by angiotensin II, by water deprivation followed by partial rehydration, and by dietary sodium deficiency. We did not detect an effect of ghrelin on saline intake after 24 h fluid deprivation without partial rehydration or after the combination of polyethylene glycol and dietary sodium deficiency. The finding that ghrelin reduced hypertonic saline intake under some, but not all, natriorexigenic conditions mirrors the previously published findings that in one-bottle tests of drinking, ghrelin reduces water intake under only some conditions. The results provide evidence for a new role for ghrelin in the regulation of body fluid homeostasis.
Keywords: angiotensin, osmoregulation, fluid balance
Introduction
Ghrelin is a 28-amino acid peptide that is produced in the gut (Kojima et al., 1999; Date et al., 2000) and in the brain (Cowley et al., 2003). An extensive body of literature has shown that ghrelin promotes positive energy balance. Ghrelin increases feeding (Tschop et al., 2000; Wren et al., 2000) and is thought to serve as a meal initiation signal (Cummings et al., 2001). Ghrelin also promotes adiposity, independent from its effects on feeding (Tschop et al., 2000). In addition to its well-established role in feeding and energy homeostasis, a role for ghrelin in fluid balance has been recognized. A small but growing body of literature has shown that ghrelin reduces water intake in eels (Kozaka et al., 2003), chicks (Tachibana et al., 2006), and rats (Hashimoto et al., 2007; Mietlicki et al., 2009; Hashimoto et al., 2010). In rats, the anti-dipsogenic effect of ghrelin is not universal; ghrelin suppresses water intake under some dipsogenic conditions, but not others (Mietlicki et al., 2009).
Although a role for ghrelin in water intake has been established, the effects of ghrelin on salt intake remain untested. The potential effect on salt intake is important because body fluid homeostasis involves the regulation of both water and solute, especially sodium (Daniels & Fluharty, 2009). Various experimental manipulations that induce or mimic dehydration of the extracellular fluid compartment, including administration of angiotensin II (Buggy & Fisher, 1974; Avrith & Fitzsimons, 1980; Bryant et al., 1980), treatment with hyperoncotic colloids (Fitzsimons, 1961; Stricker, 1966), and fluid deprivation (Weisinger et al., 1985), stimulate intake of both water and hypertonic saline. Previous studies conducted in our laboratory and one other laboratory suggest that ghrelin attenuates the water intake induced by some of these stimuli in one-bottle tests (Mietlicki et al., 2009; Hashimoto et al., 2010), but the effect of ghrelin on hypertonic saline intake was not evaluated.
The present report describes experiments testing the hypothesis that ghrelin reduces intake of hypertonic saline under several natriorexigenic conditions. Specifically, we tested the effect of intracerebroventricular (I.C.V.) ghrelin on water and saline intake in two-bottle tests after central injection of angiotensin II (AngII), 24 h fluid deprivation, water deprivation followed by partial rehydration (WD-PR), dietary sodium deprivation, and the combination of peripheral injection of polyethylene glycol (PEG) and dietary sodium deprivation. Our results indicate that ghrelin suppresses saline intake under some, but not all, natriorexigenic conditions.
Methods
Ethical approval
All procedures were approved by the State University of New York at Buffalo Institutional Animal Care and Use Committee (IACUC) and conformed to the NIH Guide for the Care and Use of Laboratory Animals.
Animals
Adult, male Sprague Dawley rats weighing 175–199 g were purchased from Harlan Laboratories (Indianapolis, IN). Rats were housed individually in hanging wire mesh cages in a temperature- and humidity-controlled environment (21° C and approximately 35–40% humidity), under a 12-h light/12-h dark cycle (lights on 0600–1800 h). Standard rat chow (Teklad 2018, Harlan Laboratories) and tap water were available to the rats ad libitum except where noted. As described below, rats in some experiments were maintained on a sodium-deficient diet (Teklad TD.90228, Harlan Laboratories).
Surgery
Each rat was allowed to acclimate to the colony for at least one week before surgical implantation of a chronic indwelling cannula aimed at the left lateral ventricle. To this end, rats were anesthetized (70 mg kg−1 ketamine and 5 mg kg−1 xylazine, I.M.) and placed in a stereotaxic apparatus. A 26-ga guide cannula was implanted (coordinates: 0.9 mm posterior to bregma, 1.4 mm lateral to midline, 1.8 mm ventral to dura) and affixed with bone screws and dental cement. Each rat received an injection of carprofen (5 mg kg−1, S.C.) during surgery. Rats were allowed to recover from surgery for one week.
Verification of cannula placement
Correct placement of cannulae was confirmed by injecting each rat with 10 ng AngII (Bachem Bioscience Inc., King of Prussia, PA) in 1 μl autoclaved Tris-buffered saline (TBS) using a 33-ga injection cannula fabricated to extend past the guide cannula into the lateral ventricle. Rats were included in the experiments only if they drank 6 ml or more in 30 min after the injection. Experiments began approximately one week after verification.
Intake measurements
Fluid intakes were measured in graduated water bottles in Experiments 1A and 2A. For all other experiments, total fluid intake was measured by comparing the differences in weight of 237 ml bottles before and after each intake test. Measures of weight were converted to volume by dividing the fluid weight by the density of the fluid (water=0.998 g ml−1; 1.8% NaCl=1.01 g ml−1). The distribution of intake across bins was determined using a custom-built 24-channel contact lickometer (University of Pennsylvania Psychology Department Electronics Shop, Philadelphia, PA) that interfaced with a laptop computer through a USB digital I/O device (National Instruments, Austin, TX). Data were collected and initially processed in a MATLAB (MathWorks, Natick, MA) environment and further processed in Excel (Microsoft, Redmond, WA). Lickometer cages were the same stainless steel, hanging wire mesh cages used in other experiments, but were fitted with Lexan (Experiment 3) or metal (all other experiments) inserts to electrically isolate the fluid spouts from the cage and minimize contact with anything other than the rat’s tongue.
The amount of food intake was determined by comparing the weight of the available food before and after the food intake test. Spillage was collected on sheets of plastic transparency film placed under each cage and the weight of spillage was accounted for in the total food measurements. Food intake tests were concurrent with drinking tests in Experiments 1A and 2A or after a 2 h drinking test in all other experiments.
Experiment 1A: Does ghrelin differentially affect water and saline intakes in AngII-treated rats?
Rats were habituated to two-bottle access, with water and 1.8% NaCl available to drink, overnight before testing. On the morning of testing, rats received a single I.C.V. injection (2 μL total volume) containing a mixture of AngII (10 ng in 1 μl TBS) and 0.5 μg rat ghrelin (Bachem Bioscience Inc.) in 1 μl artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) or AngII (10 ng in 1 μl TBS) and 1 μl aCSF (n=5–6 rats per group). This dose of ghrelin has been shown by our laboratory to reduce AngII-induced water intake (Mietlicki et al., 2009) and has been demonstrated to have a moderate hyperphagic effect (Nakazato et al., 2001; Faulconbridge et al., 2003; Faulconbridge et al., 2005). Rats were returned to their home cages where preweighed food and graduated fluid bottles containing water and 1.8% NaCl were available. Fluid intakes were recorded at 30, 60, and 240 min post-injection; cumulative 4 h food intake also was measured as a positive control for the effectiveness of ghrelin.
Experiment 1B: Does ghrelin differentially affect water and saline intakes in AngII-treated rats in the absence of food?
Rats were habituated to two-bottle access in lickometer cages, with water and 1.8% NaCl available to drink, for 7 days prior to testing. On the morning of testing, rats were moved into clean lickometer cages before being given a single I.C.V. injection (2 μl total volume) containing AngII (10 ng in 1 μl TBS) and 0.5 μg rat ghrelin or its vehicle (1 μl aCSF; n=4–6 rats per group). After 2 h of fluid intake testing in the absence of food, hoppers containing preweighed food were returned to the cages and 2 h food intake was measured.
Experiment 2A: Does ghrelin attenuate water intake or saline intake in 24 h fluid-deprived rats?
Rats were habituated to two-bottle access, with water and 1.8% NaCl available to drink, for 6 days. After acclimation to the two-bottle paradigm, rats were deprived of fluids, but not food, for 24 h. After the fluid deprivation period, each rat received a single I.C.V. injection of ghrelin (0.5 μg) or its vehicle (1 μl aCSF; n=7 rats per group). Water and 1.8% NaCl were made available in graduated bottles. Fluid intakes were recorded at 30, 60, and 240 min. Cumulative 4 h food intake also was measured.
Experiment 2B: Does ghrelin attenuate water intake or saline intake in 24 h fluid-deprived rats in the absence of food?
Rats were habituated to two-bottle access in lickometer cages, with water and 1.8% NaCl available to drink, for 7 days. Rats then were deprived of fluids, but not food, for 24 h. After the fluid deprivation period, each rat received a single I.C.V. injection of 0.5 μg ghrelin or its vehicle (1 μl aCSF; n=6–9 rats per group). Food was removed from the cage and water and 1.8% NaCl intakes were measured over the next 2 h. After 2 h of fluid intake testing in the absence of food, hoppers containing preweighed food were returned to the cages and 2 h food intake was measured.
Experiment 3: Does ghrelin affect saline intake induced by water deprivation followed by partial rehydration (WD-PR)?
Rats were habituated to two-bottle access in lickometer cages, with water and 1.8% NaCl available to drink, for 7 days. Rats were deprived of fluids, but not food, for 24 h. After the fluid deprivation period, food was removed from the cage and rats were given access to water (not saline) for 2 h (PR phase). Rats then were given a single I.C.V. injection of ghrelin (0.5 μg) or its vehicle (1 μl aCSF; n=5–8 rats per group). Five minutes later, rats were given access to water and 1.8% NaCl and fluid intake was recorded in the absence of food. After the 2 h fluid intake test, food was returned and intake was measured for another 2 h.
Experiment 4: Does ghrelin attenuate water intake or saline intake in rats maintained on a sodium-deficient diet?
Rats were habituated to two-bottle access in lickometer cages, with water and 1.8% NaCl available to drink, for 7 days. After habituation, rats were moved into clean, standard hanging wire mesh cages with water and sodium-deficient diet available ad libitum. Returning the rats to the standard caging was important to ensure that no residual sodium was left in the cage from the standard chow or saline bottle and to prevent extinction of any learning needed to drink in the modified cages. Rats were kept in these cages and maintained on the sodium-deficient diet for 10 days.
On the morning of the 11th day, each rat was given an I.C.V. injection of 0.5 μg ghrelin or its vehicle (1 μl aCSF; n=9–10 rats per group) and placed in a clean lickometer cage. Water and 1.8% NaCl intakes were measured for 2 h in the absence of food. After the fluid intake test, sodium-deficient diet was returned and food intake was measured for another 2 h.
Experiment 5: Does ghrelin attenuate water intake or saline intake in rats maintained on sodium-deficient diet and made hypovolemic by administration of polyethylene glycol?
Rats were habituated to two-bottle access in lickometer cages, with water and 1.8% NaCl available to drink, for 7 days. After habituation, rats were moved into clean, standard hanging wire mesh cages with water and sodium-deficient diet available ad libitum. Rats were kept in these cages and maintained on the sodium-deficient diet for 4 days.
On the morning of the 5th day, food and water were removed from the cages. Each rat was given an injection of 0.1 ml lidocaine (2%, S.C.) followed immediately by a second injection of 5 ml PEG (Sigma-Aldrich, St. Louis, MO; MW 20,000; 30% w/v in autoclaved isotonic saline, S.C.). Rats were returned to their cages for 1 h without access to food or fluids to allow hypovolemia to develop. Each rat then was given an I.C.V. injection of ghrelin (0.5 μg) or its vehicle (1 μl aCSF; n=7–9 rats per group) and placed in a clean lickometer cage. Water and 1.8% NaCl intakes were measured for 2 h in the absence of food. After the fluid intake test, sodium-deficient chow was returned and food intake was measured for another 2 h.
Statistical analysis
Data were analyzed using Statistica (Version 9; StatSoft Inc., Tulsa, OK). The alpha level was set at p<0.05 for all tests. Non-cumulative fluid intake data, normalized to the duration of the shortest bin, were used to analyze the binned fluid intake data. For each experiment, separate mixed design ANOVA tests for water intake and for saline intake were used to test for between-subjects effects of Drug (ghrelin or vehicle) while accounting for the within-subjects effect of Time. The results of each repeated measures ANOVA were screened using Mauchly’s test of sphericity. When the assumption of sphericity was violated (p<0.05), we used Greenhouse-Geisser-corrected df and p-values to evaluate statistical significance. Total 2 h fluid intake was analyzed using a mixed design ANOVA, with Drug as a between-subjects factor and Fluid Type (water or 1.8% NaCl) as a within-subjects factor. Partial rehydration intake data (Experiment 3) and food intake data from all experiments were analyzed by one-way ANOVA with Drug as a between-subjects factor. Statistically significant main effects and interactions were further probed using Student-Newman-Keuls post hoc tests.
Results
Experiment 1A: Does ghrelin differentially affect water and saline intakes in AngII-treated rats?
Ghrelin attenuates the water intake stimulated by AngII in a one-bottle test (Mietlicki et al., 2009; Hashimoto et al., 2010). To test whether or not ghrelin reduces AngII-induced intake of saline and/or water when both fluids are available, rats were treated with AngII and either ghrelin or vehicle I.C.V., and subsequent intakes of water and 1.8% NaCl were measured.
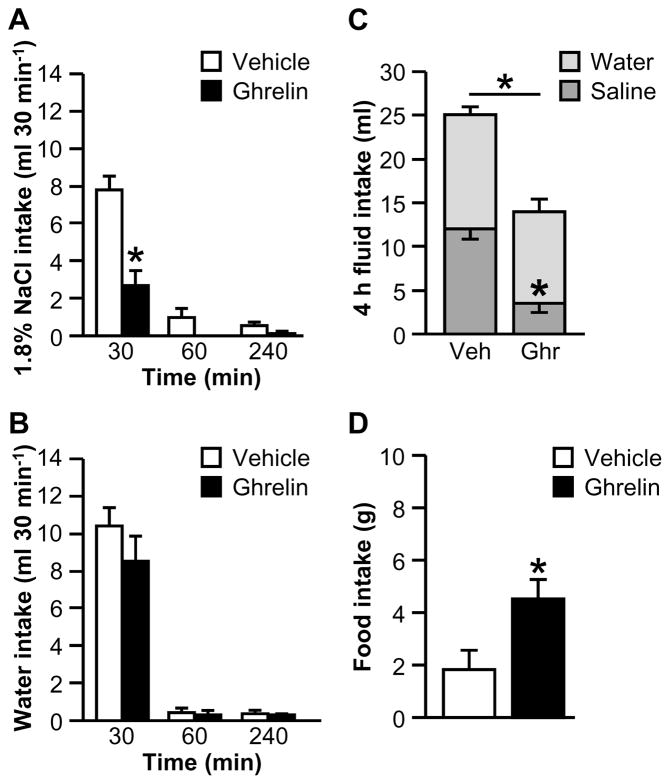
The non-cumulative, binned fluid intake data are shown in Figures 1A and 1B. The interaction between Drug and Time was statistically significant for 1.8% NaCl intake (Fig. 1A; F1.26,11.36=12.69, p<0.01). Post hoc tests revealed that ghrelin-treated rats consumed less saline in the first 30 min of testing than did vehicle-treated rats. The interaction between Drug and Time was not statistically significant for water intake (Fig. 1B; F1.08,9.74=0.96, p>0.05). Ghrelin reduced total fluid intake over the 4 h test period (Fig. 1C; main effect of Drug: F1,9=20.63, p<0.01). The two-way interaction between Fluid Type and Drug for 4 h fluid intake was statistically significant (F1,9=7.75, p<0.05) and post hoc tests revealed that 4 h saline intake was less in AngII-treated rats than in vehicle-treated rats. As shown in Fig. 1D, cumulative 4 h food intake was reliably increased by ghrelin (F1,9=6.87, p<0.05).
Figure 1.
Effect of ghrelin on water, 1.8% NaCl, and food intakes after I.C.V. administration of AngII when food was available during fluid intake testing (Experiment 1A). In a 4 h test, ghrelin reduced 30-min saline intake (A) but had no effect on non-cumulative water intake (B). Total saline intake and total fluid intake were also attenuated in ghrelin-treated rats (C). Ghrelin reliably stimulated food intake (D). In Panel C, groups of vehicle- and ghrelin-treated rats are respectively abbreviated “Veh” and “Ghr.” All data are shown as mean ± SEM (n=5–6 per treatment group) and asterisks indicate p<0.05 compared to controls.
Experiment 1B: Does ghrelin differentially affect water and saline intakes in AngII-treated rats in the absence of food?
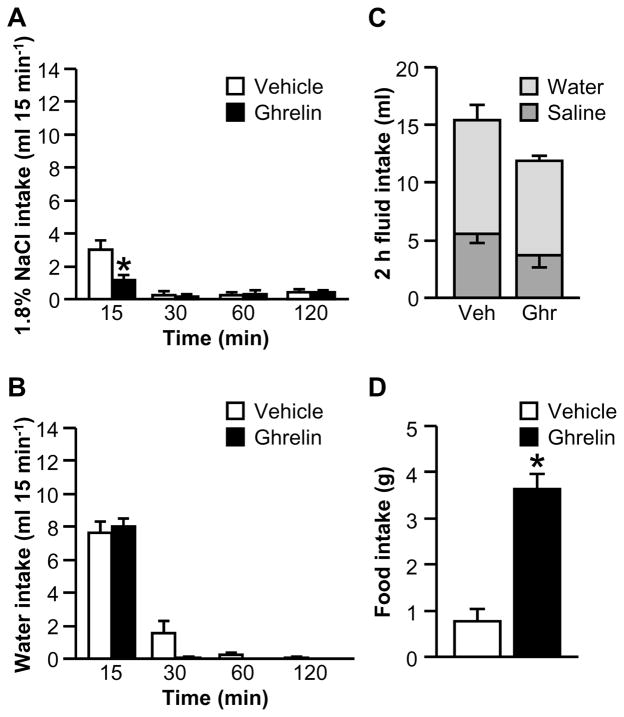
Previous experiments failed to find a systematic pattern of food intake that could account for the effect of ghrelin on water intake (Mietlicki et al., 2009). Nevertheless, to directly address the possibility that increased time spent eating caused the decreased fluid intake in Experiment 1A, the experiment was repeated without food being available during the drinking test. There was a statistically significant interaction between Drug and Time for the binned saline intake data (Fig. 2A; F3,24=6.34, p<0.01). Post hoc tests probing the effect of Drug within Time showed that ghrelin-treated rats drank less saline in the first 15 min of testing than did vehicle-treated rats. We did not find a statistically significant interaction between Drug and Time for the binned water intake data (Fig. 2B; F1.80,14.43=2.67, p>0.05). As shown in Fig. 2C, we did not find an effect of ghrelin on total fluid intake (F1,8=2.34, p>0.05) or a statistically significant interaction between Drug and Fluid Type for the 2 h test (F1,8=0.01, p>0.05). When food was returned, rats treated with ghrelin ate more than controls (Fig. 2D; F1,8=42.28, p<0.001).
Figure 2.
Effect of ghrelin on water, 1.8% NaCl, and food intakes after I.C.V. administration of AngII when food was not available during fluid intake testing (Experiment 1B). In a 2 h fluid intake test, ghrelin reduced 15-min intake of hypertonic saline (A) but had no effect on AngII-induced water intake (B). Total fluid intake was unaffected by ghrelin (C). When food was returned to the cage after the fluid intake test, ghrelin-treated rats ate more food than did vehicle-treated rats (D). In Panel C, groups of vehicle- and ghrelin-treated rats are respectively abbreviated “Veh” and “Ghr.” All data are shown as mean ± SEM (n=4–6 per treatment group) and asterisks indicate p<0.05 compared to controls.
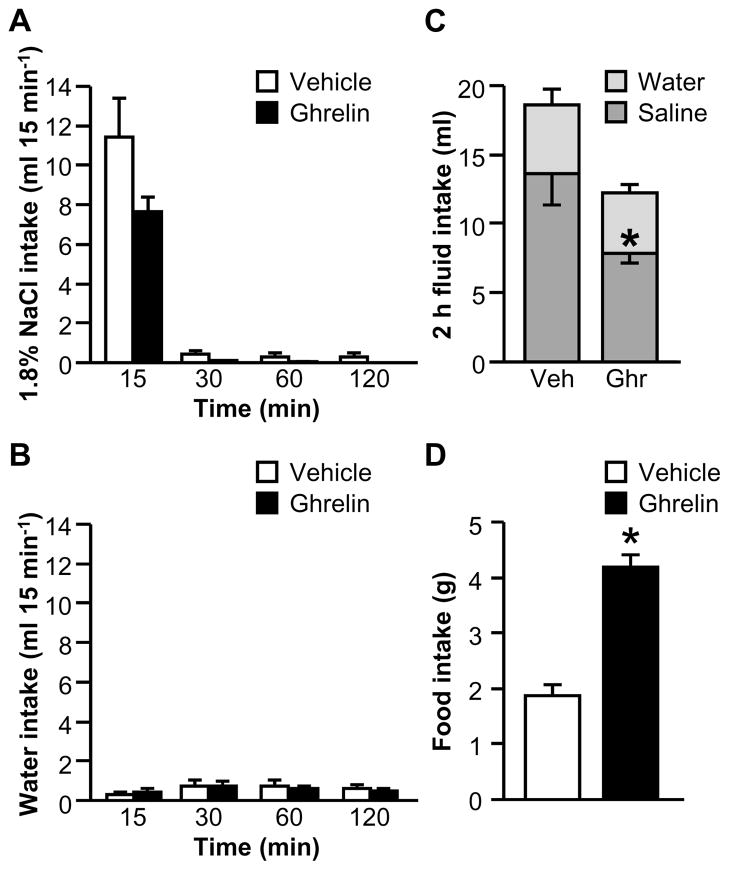
Experiment 2A: Does ghrelin attenuate water intake or saline intake in 24 h fluid-deprived rats?
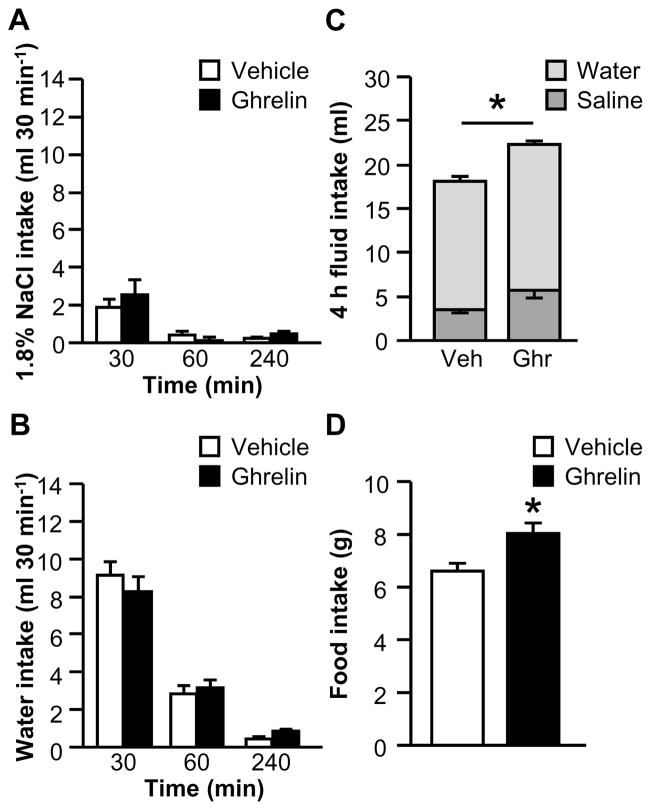
In a previous study, we failed to detect an effect of ghrelin on water intake stimulated by 24 h water deprivation (Mietlicki et al., 2009), but an effect on intake when a choice of fluids is available has not been evaluated. In a two-bottle test, no significant two-way interactions between Drug and Time were observed for the binned saline intake (Fig. 3A; F1.11,13.35=0.77, p>0.05) or binned water intake (Fig. 3B; F2,24=0.80, p>0.05) data. Figure 3C shows total 4 h fluid intake, which was modestly greater in ghrelin-treated rats (F1,12=8.76, p<0.05); however, the interaction between Drug and Fluid Type was not statistically significant (F1,12=0.02, p>0.05). Together with the results from the binned intake analysis, this suggests that there was neither an anti-natriorexigenic effect nor an anti-dipsogenic of ghrelin under this condition, but that the combination of small, non-significant effects on intake of each fluid combined to produce a modest change in total fluid intake. Cumulative food intake over the 4 h test was reliably increased by ghrelin (Fig. 3D; F1,12=8.96, p<0.05).
Figure 3.
Effect of ghrelin on water, 1.8% NaCl, and food intakes after 24 h fluid deprivation when food was available during fluid intake testing (Experiment 2A). Ghrelin had no effect on non-cumulative saline (A) or water (B) intake, but total 4 h fluid intake was increased in ghrelin-treated rats (C). Food intake was increased by ghrelin (D). In Panel C, groups of vehicle- and ghrelin-treated rats are respectively abbreviated “Veh” and “Ghr.” All data are shown as mean ± SEM (n=7 per treatment group) and asterisks indicate p<0.05 compared to controls.
Experiment 2B: Does ghrelin attenuate water intake or saline intake in 24 h fluid-deprived rats in the absence of food?
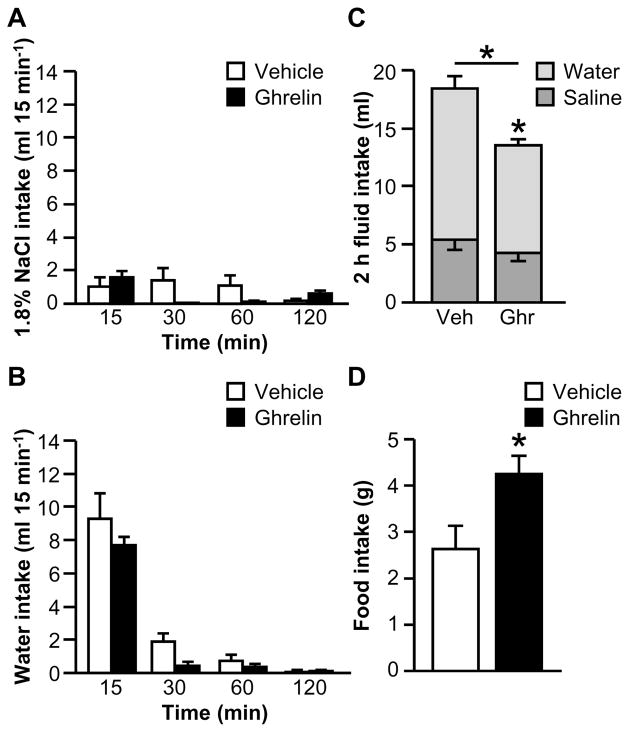
To address the possibility that the availability of food affected fluid intake in Experiment 2A, the effect of ghrelin on fluid intake stimulated by 24 h fluid deprivation was tested when food was not available. Like Experiment 2A, we found no differences between groups for the non-cumulative, binned data; the two-way interactions between Drug and Time were not statistically significant for saline intake (Fig. 4A; F3,39=2.83, p>0.05) or for water intake (Fig. 4B; F1.15,14.91=0.92, p>0.05). Ghrelin-treated rats drank less total fluid over the 2 h test than did vehicle-treated rats, as shown in Fig. 4C (F1,13=6.75, p<0.05). A statistically significant interaction between Drug and Fluid Type was observed as well (F1,13=5.91, p<0.05), but post hoc tests revealed that this difference was due to a reduction in water intake in ghrelin-treated rats, with no difference in saline intake. Accordingly, fluid intake was not affected at any particular bin, but ghrelin collectively decreased water intake during the test, without affecting saline intake. This finding is consistent with the direction, but not the magnitude, of the effect reported by Hashimoto et al. (2007). As shown in Fig. 4D, when food was returned, ghrelin-treated rats ate more than did vehicle-treated rats (F1,13=6.80, p<0.05).
Figure 4.
Effect of ghrelin on water, 1.8% NaCl, and food intakes after 24 h fluid deprivation when food is not available during fluid intake testing (Experiment 2B). Ghrelin had no effect on non-cumulative saline (A) or water (B) intake during a 2 h fluid intake test, but total 2 h fluid intake was reduced by ghrelin, primarily due to a statistically significant reduction in water intake (C). When food was made available after fluid intake testing, ghrelin reliably increased food intake (D). In Panel C, groups of vehicle- and ghrelin-treated rats are respectively abbreviated “Veh” and “Ghr.” All data are shown as mean ± SEM (n=6–9 per treatment group) and asterisks indicate p<0.05 compared to controls.
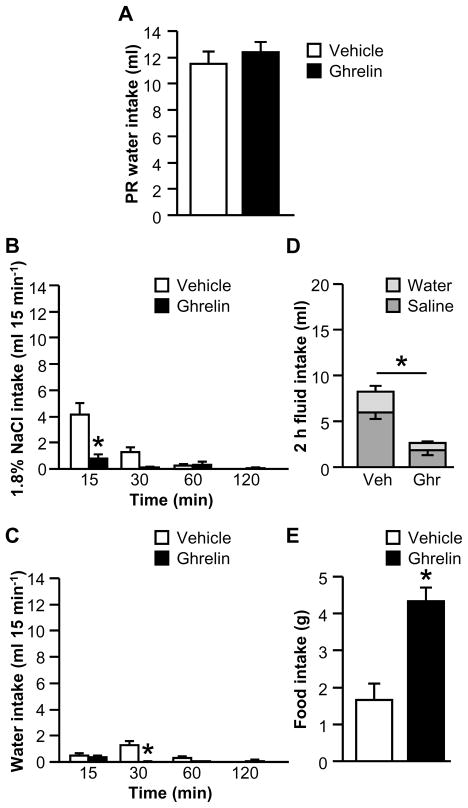
Experiment 3: Does ghrelin affect saline intake induced by water deprivation followed by partial rehydration (WD-PR)?
The previous experiments tested the effects of ghrelin after treatments that increase both saline and water intakes. To test the effect of ghrelin on fluid intake stimulated by a more selectively natriorexigenic stimulus, rats were deprived of fluids but not food for 24 h, then allowed access to water only but no food for 2 h. This water deprivation-partial rehydration (WD-PR) procedure (Sato et al., 1996) has been used numerous times to help isolate the natriorexigenic or anti-natriorexigenic components of various stimuli.
Water intake was measured during the partial rehydration phase, before injection of ghrelin or vehicle. We found no differences in water intake during this phase between rats that were assigned to receive vehicle and rats that were assigned to receive ghrelin (Fig. 5A; F1,11=0.43, p>0.05). After a single cycle of WD-PR, analysis of the binned fluid intake data revealed statistically significant interactions between Drug and Time for both saline intake (Fig. 5B; F1.28,14.03=5.65, p<0.05) and for water intake (Fig. 5C; F3,33=5.72, p<0.01). Post hoc tests probing the effects for each fluid showed that ghrelin-treated rats consumed less saline during the first 15 min of testing than did vehicle-treated rats, and also drank less water than did controls from 15–30 min. Total 2 h fluid intake was reduced by ghrelin, as shown in Fig. 5D (F1,11=23.65, p<0.01), although no statistically significant interaction was observed between Drug and Fluid Type (F1,11=4.79, p>0.05). Food intake is shown in Fig. 5E; when food was returned after the 2 h drinking test, rats treated with ghrelin ate more than did controls (F1,11=18.51, p<0.01).
Figure 5.
Effect of ghrelin on water, 1.8% NaCl, and food intakes after WD-PR (Experiment 3). There were no differences between groups in water intake during the partial rehydration phase (A). During a 2 h fluid intake test, ghrelin reduced saline intake at 15 min (B) and reduced non-cumulative water intake from 15–30 min (C). Total 2 h fluid intake was also reduced by ghrelin (D). When food was returned to the cage, ghrelin-treated rats ate more food than did vehicle-treated rats (E). In Panel D, groups of vehicle- and ghrelin-treated rats are respectively abbreviated “Veh” and “Ghr.” All data are shown as mean ± SEM (n=5–8 per treatment group) and asterisks indicate p<0.05 compared to controls.
Experiment 4: Does ghrelin attenuate water intake or saline intake in rats maintained on a sodium-deficient diet?
Maintaining rats on a sodium-deficient diet is thought to represent a more natural stimulus for sodium intake (Prakash & Norgren, 1991). Accordingly, we tested the effect of ghrelin on water and 1.8% NaCl intakes after rats had been maintained on sodium-deficient diet for 10 days. No differences in fluid intake were observed in any individual time bin and the two-way interactions between Drug and Time for each fluid type were not statistically significant (saline, Fig. 6A, F1.05,17.80=3.20, p>0.05; water, Fig. 6B, F3,51=0.13, p>0.05). Ghrelin had no effect on total 2 h fluid intake (F1,17=3.61, p>0.05; Fig. 6C), but the two-way interaction between Drug and Fluid Type was statistically significant (F1,17=10.54, p<0.01). Post hoc tests showed that ghrelin-treated rats consumed less saline than did vehicle-treated rats, but we found no effect of ghrelin on water intake, indicating a selective effect of ghrelin on saline intake. After food was returned, ghrelin-treated rats ate more than did controls (Fig. 6D; F1,17=59.30, p<0.001).
Figure 6.
Effect of ghrelin on water, 1.8% NaCl, and sodium-deficient diet intakes after dietary sodium deficiency (Experiment 4). Ghrelin had no effect on binned saline (A) or water (B) intake, but total 2 h saline intake was reduced in ghrelin-treated rats (C). When sodium-deficient diet was made available after the fluid intake test, ghrelin-treated rats consumed more food than did vehicle-treated rats (D). In Panel C, groups of vehicle- and ghrelin-treated rats are respectively abbreviated “Veh” and “Ghr.” All data are shown as mean ± SEM (n=9–10 per treatment group) and asterisks indicate p<0.05 compared to controls.
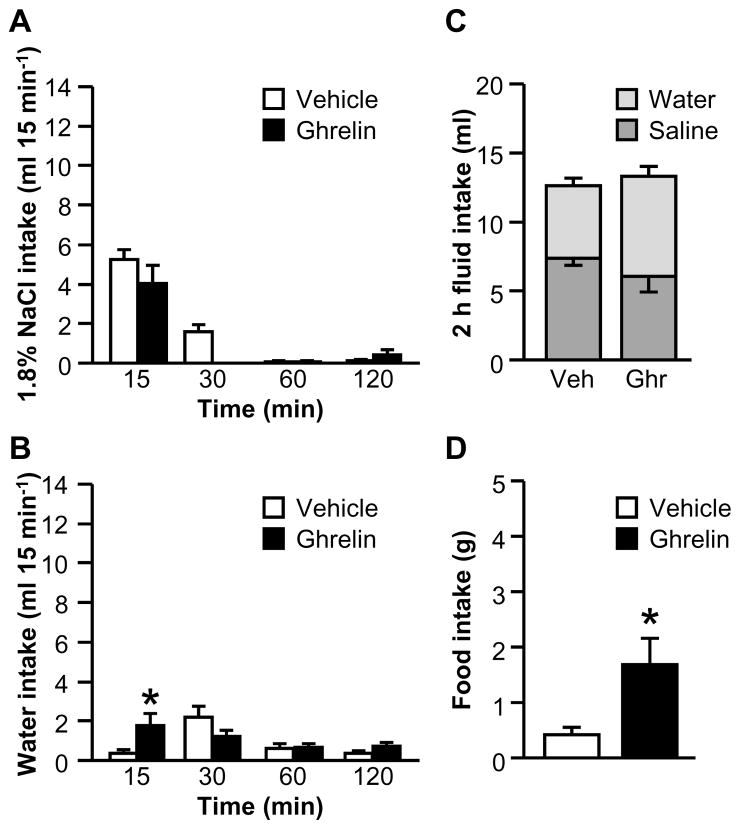
Experiment 5: Does ghrelin attenuate water intake or saline intake in rats maintained on sodium-deficient diet and made hypovolemic by administration of polyethylene glycol?
PEG administration has been widely used to study saline intake (Stricker & Wolf, 1966; Wolf & Stricker, 1967; Stricker & MacArthur, 1974; Stricker, 1981). A previous study found that ghrelin inhibited water intake in a single-bottle test after PEG treatment (Hashimoto et al., 2010), but an effect on saline intake was not evaluated. To test the effect of ghrelin on PEG-stimulated saline intake, we combined a short maintenance on sodium-deficient diet with PEG treatment. The previous maintenance on sodium-deficient diet was necessary to facilitate a rapid and consistent onset of hypertonic saline intake after PEG injection (Stricker, 1981), allowing us to administer the I.C.V. injection of ghrelin or vehicle at an appropriate time.
After PEG injection, no effect of ghrelin on binned saline intake was found; the two-way interaction between Drug and Time was not statistically significant (Fig. 7A; F1.45,20.27=2.75, p>0.05). We observed an effect of ghrelin on binned water intake, indicated by a statistically significant interaction between Drug and Time (Fig. 7B; F3,42=3.83, p<0.05). Post hoc tests probing this effect showed that ghrelin-treated rats drank more water in the first 15 min of testing than did vehicle-treated rats. As shown in Fig. 7C, we did not find an effect of ghrelin on total fluid intake over the 2 h test (F1,14=0.16, p>0.05). Although a statistically significant interaction between Drug and Fluid Type was noted (F1,14=10.37, p<0.01), post hoc tests showed no differences between vehicle-treated and ghrelin-treated rats. When food was returned after the 2 h drinking test, rats treated with ghrelin ate more than did vehicle-treated counterparts (Fig. 7D; F1,14=9.18, p<0.01).
Figure 7.
Effect of ghrelin on water, 1.8% NaCl, and sodium-deficient diet intakes after PEG treatment plus dietary sodium deficiency (Experiment 5). Ghrelin had no effect on non-cumulative intake of hypertonic saline (A), but increased non-cumulative water intake at 15 min (B). Ghrelin had no effect on total 2 h fluid intake (C). Ghrelin reliably increased food intake (D). In Panel C, groups of vehicle- and ghrelin-treated rats are respectively abbreviated “Veh” and “Ghr.” All data are shown as mean ± SEM (n=7–9 per treatment group) and asterisks indicate p<0.05 compared to controls.
Discussion
Studies on ghrelin have focused extensively on its role in energy balance, but the effects of ghrelin on body fluid homeostasis have received far less attention. Studies in non-mammalian species (Kozaka et al., 2003; Tachibana et al., 2006; Nobata & Takei, 2011) and in rats (Hashimoto et al., 2007; Mietlicki et al., 2009; Hashimoto et al., 2010) have shown that central administration of ghrelin reduces water intake under a variety of dipsogenic conditions. The behavioral regulation of fluid balance, however, involves both water intake and salt intake. To the best of our knowledge, no reported experiments have tested the role of ghrelin in regulating salt intake. To address this open question, the present experiments tested the hypothesis that ghrelin reduces hypertonic saline intake under several natriorexigenic conditions.
We tested intake of water and 1.8% NaCl in a two-bottle test after 5 different natriorexigenic stimuli: I.C.V. AngII administration, 24 h fluid deprivation, WD-PR, dietary sodium deprivation, and the combination of PEG administration and dietary sodium deprivation. Similar to our earlier findings using one-bottle water intake tests (Mietlicki et al., 2009), we found that ghrelin reduced the saline intake stimulated by some, but not all, of the natriorexigenic stimuli. Ghrelin treatment reduced 1.8% NaCl intake stimulated by central AngII administration (Experiment 1), WD-PR (Experiment 3), and maintenance on sodium-deficient diet (Experiment 4). In contrast, when fluid intake was stimulated by 24 h fluid deprivation (Experiment 2) or by sodium deprivation plus PEG (Experiment 5), we failed to find an effect of ghrelin on saline intake. Under most conditions, water intake was unaffected by ghrelin. We did, however, find a moderate suppression of water intake, without any differences in saline intake, after 24 h water deprivation, but only when food was absent during the fluid intake test. Under all conditions, ghrelin reliably stimulated food intake, whether rats were tested on sodium-deficient chow (Experiments 4 and 5) or on standard chow (Experiments 1–3), providing an important positive control for the effectiveness of ghrelin.
Experiments 1 and 2 tested the impact of food availability on the effect of ghrelin on saline intake. The time course of Experiments 1A and 2A, with food available, mirrored our previous one-bottle experiments testing the effects of ghrelin water intake (Mietlicki et al., 2009). Experiments 1B and 2B tested the effect of ghrelin on saline intake when food was not available, ensuring that the only source of sodium available to the rats was the fluid. Looking at the effects of ghrelin on AngII-induced fluid intake, when food was available (Experiment 1A), we found that ghrelin reduced 30 min saline intake. Total 4 h fluid intake was also reduced; this effect was driven primarily by a reduction in total saline intake. In Experiment 1B, when food was not available during fluid intake testing, we observed a reduction in saline intake in ghrelin-treated rats during the first 15 min of testing, but no reduction in total fluid intake or total saline intake. The results suggest that the availability of food does not change the general suppressive effect of ghrelin on saline intake, but may impact the duration of the effect, with suppression of saline intake lasting longer when food is available. The differences may also relate to the length of the fluid intake test used here (the fluid intake test was 4 h in Experiment 1A but only 2 h in Experiment 1B). The majority of fluid intake in response to AngII, however, occurs within the first 15–30 min after I.C.V. injection, and both fluid intake tests extended past this time. Nevertheless, the data support our previous conclusion that the effect of ghrelin on fluid intake is not a direct consequence of the associated hyperphagia (Mietlicki et al., 2009).
Experiment 2 addressed the effect of ghrelin on fluid intake in a two-bottle test after 24 h fluid deprivation. One-bottle experiments have produced conflicting results when the effect of ghrelin on water intake was evaluated. Our laboratory found that ghrelin had no effect on water intake after 24 h water deprivation (Mietlicki et al., 2009), but another laboratory found that ghrelin potently reduced water intake after 24 h water deprivation (Hashimoto et al., 2007). Although the focus on saline intake and the two-bottle testing in the present experiments do not directly replicate the previous experiments, water intake was measured and the present data are, therefore, potentially relevant to the existing controversy. In the present experiments, when food was not available during the fluid intake test, we found an overall decrease in water intake, without an effect on saline intake, in ghrelin-treated rats. The difference in water intake was moderate, however, and not significant at any individual bin. Nevertheless, the finding that ghrelin reduced water intake under this condition is consistent with the results of Hashimoto et al. (2007).
The differences in the effects of ghrelin on fluid intake among the various conditions tested may relate to the specific behavioral and physiological effects of each natriorexigenic condition. Although each of the conditions used here stimulates salt intake, most also increase water intake. The exception to this is WD-PR (Experiment 3), which stimulates salt intake, but produces minimal water intake during the fluid intake test (Sato et al., 1996). In spite of these similarities, the physiological context induced by each stimulus varies in a way that may relate to the differential efficacy of ghrelin. Specifically, the conditions under which we observed no effect of ghrelin on saline intake, 24 h fluid deprivation (Experiment 2) and the combination of sodium deficient diet and PEG (Experiment 5), might produce a broader range of physiological changes than the other conditions tested. Fluid deprivation for 24 h results in both intracellular and extracellular dehydration; rats become hypovolemic and the body fluid becomes more concentrated (De Luca et al., 2002). The renin-angiotensin system is activated (De Luca et al., 2002), although aldosterone does not increase (Zucker et al., 1982). In contrast, PEG administration produces hypovolemia independently of changes in tonicity. The effects of PEG on fluid intake are likely a result of renin-angiotensin system activation and increased aldosterone activity (Stricker et al., 1979) as well as baroreceptor activation in response to the hypovolemia (Kaufman, 1984). Maintaining the rats on sodium-deficient diet for 4 days prior to PEG treatment was important to facilitate a consistent onset of saline intake (Stricker, 1981), but this manipulation presents additional challenges to body fluid homeostasis, including sodium loss (Stricker, 1981). Thus, the broad range of effects of these treatments may make them less susceptible to the effects of ghrelin. It should be noted, however, that conditions involving fluid deprivation (here, 24 h water deprivation and WD-PR) also reduce body sodium (McKinley et al., 1983; Weisinger et al., 1985). The difference in the efficacy of ghrelin under the two stimuli, decreasing saline intake after WD-PR but not after 24 h water deprivation, suggests that sodium depletion alone is not sufficient to explain the differential anti-natriorexigenic effects of ghrelin.
In contrast to the broader effects of 24 h fluid deprivation or injection of PEG (with previous maintenance on sodium-deficient diet), the effects of the other natriorexigenic conditions used here have more physiological specificity. For example, maintenance on a sodium-deficient diet (Experiment 4) is more selective than the combination of this diet and treatment with PEG (Experiment 5). Likewise, WD-PR (Experiment 3) includes a partial rehydration phase that resolves the intracellular dehydration and some of the extracellular dehydration that occurs as a result of the fluid deprivation period (De Luca et al., 2002), making this stimulus more selective, physiologically, than fluid deprivation alone (Experiment 2). Although there is a range of responses to central administration of AngII (Experiment 1), the overall response could be considered less broad than the response to 24 h water deprivation or to sodium-deficient diet plus PEG treatment. Indeed, AngII is only one part of the broader physiological response to extracellular dehydration. Moreover, the central route of administration limits the responses to a subset of those observed under more natural conditions of elevated AngII. Using this method of categorizing the stimuli, into broader and more focused events, it appears that ghrelin only reduced intake under the more focused conditions. This may suggest that the mechanism underlying the relevant effects of ghrelin can be overridden when some aspect(s) of the conditions that induce more global changes is present.
Because AngII is present in all conditions (whether administered exogenously or produced endogenously as a consequence of the natriorexigenic stimulus), it is unlikely that differences in AngII can explain the differences in the effectiveness of ghrelin under the various conditions. It seems more likely that interactions between the physiological consequences of the more “challenging” stimuli prevent ghrelin from reducing saline intake. We have proposed previously that the differences in the effectiveness of ghrelin in suppressing water intake under various dipsogenic conditions may be due to differences in motivation to drink under the dipsogenic conditions (Mietlicki et al., 2009). Although within-experiment comparisons of motivation to drink under different conditions are scarce, a study by Rolls et al. suggested that 24 h water deprivation may be a more motivating stimulus for drinking than central administration of AngII as measured by a quinine tolerance test (Rolls et al., 1972). This finding is consistent with our hypothesis that motivation affects the ability of ghrelin to suppress water intake. Studies using operant responding to assess motivation to drink, however, revealed no differences between these dipsogenic conditions (Rolls et al., 1972). This highlights the difficulty in assessing motivation to drink, as do other previous publications (Miller, 1956). It is certainly possible that differences in motivation to consume fluid may underlie the differences observed in the present experiments, but to our knowledge, no publications have provided a direct comparison of motivation to consume saline under different natriorexigenic conditions. Indeed, the difficulty in accurately assessing motivation (Miller, 1956; Rolls et al., 1972) makes it challenging to properly conduct such an experiment.
The results of these experiments reveal an interesting effect of choice on the effect of ghrelin. Ghrelin reduced AngII-induced water intake when water was the only fluid available (Mietlicki et al., 2009), but when water and 1.8% NaCl were available in the present studies, ghrelin reduced saline but not water intake. The effect of ghrelin on water deprivation-induced intake is less consistent, especially between laboratories (Hashimoto et al., 2007; Mietlicki et al., 2009), but comparing our previous one-bottle results (Mietlicki et al., 2009) with the two-bottle test results here, the availability of saline seems to change the effect of ghrelin on fluid intake. This report is far from the first to show differences in intake depending on the number of bottles presented. For example, when rats are deprived of water, they drink more saline than water in separate one-bottle tests. When a two-bottle test is used, giving access to both fluids at the same time, the differences in water and saline intake are reversed and rats drink more water than saline (Rowland et al., 1995). More recent studies highlight the large differences that adding bottles can have on intake (Tordoff & Bachmanov, 2003). Thus, it should not be surprising that intake was different in the one-bottle test (Mietlicki et al., 2009) and the two-bottle tests here. Although most of our two-bottle tests suggest a more selective effect of ghrelin on saline intake than on water intake, the inhibitory effect of ghrelin on water deprivation-induced fluid intake appears to be more specific to water intake, both in one-bottle experiments (Hashimoto et al., 2007) and in Experiment 2B.
The lack of an effect of ghrelin on saline intake stimulated by 24 h fluid deprivation, however, should be interpreted with caution. Others have pointed out that the pattern of intake in a two-bottle test of water and hypertonic saline after 24 h fluid deprivation is heterogeneous and can be difficult to interpret (Sato et al., 1996). Indeed, when we used WD-PR to address more selectively the saline intake stimulated by 24 h water deprivation (Experiment 3), we found that ghrelin reliably reduced saline intake in the first 15 min, a result similar to the effect of ghrelin on AngII-induced intake in a two-bottle test (Experiment 1B). Using WD-PR as the stimulus for drinking, we also found a reduction in water intake from 15–30 min in ghrelin-treated rats. The timing of the reduction in water intake, which occurred after ghrelin-induced reductions in saline intake, suggests that the difference may be due to increased osmoregulatory drinking in the vehicle-treated rats. Specifically, control rats may have increased their water intake in the second 15 min of testing because of saline intake during the first 15 min of testing. Because ghrelin-treated rats drank less saline than did controls, the subsequent water intake was not stimulated. Therefore, the apparent suppression of water intake by ghrelin in this experiment may, instead, represent increased drinking by controls. Thus, in spite of the observed differences in water intake, the effect of ghrelin on saline intake appears more direct than any observed effect on water intake.
The present experiments use central injections of ghrelin, but it remains unclear if these injections model endogenous ghrelin of central or of peripheral origin. To the best of our knowledge, this is the first publication describing an anti-natriorexigenic effect of ghrelin and no other experiments to date have compared the effects of central and peripheral administration of ghrelin on salt intake. Water intake is suppressed by ghrelin administered to the periphery or injected directly into the brain (Hashimoto et al., 2007). This suggests the sufficiency of a central site of action, but does not rule out a peripheral contribution. There are a number of brain areas that could mediate the response to ghrelin of either central or peripheral origin. mRNA for the ghrelin receptor is found in sensory circumventricular organs such as the area postrema and subfornical organ (Pulman et al., 2006; Zigman et al., 2006) and ghrelin modulates the electrical activity of neurons in these regions (Pulman et al., 2006; Fry & Ferguson, 2009). Combined with the rich literature connecting these brain areas to thirst and salt intake, they become attractive candidate structures for the fluid intake-suppressive effects of ghrelin. These issues remain to be addressed empirically in future experiments.
In conclusion, the present data demonstrate that ghrelin reduces consumption of hypertonic saline under some, but not all, natriorexigenic conditions. This finding reveals a new role for ghrelin in body fluid homeostasis and in the general field of ingestive behavior. Further research is needed to determine the mechanism by which ghrelin suppresses saline intake.
Acknowledgments
The authors thank Leah Allen, Anikó Marshall, Naomi McKay, Kimberly Plyler, and Peter Vento for their technical assistance. The research described in this paper was supported by a grant from the American Psychological Association to EGM and National Institutes of Health awards DK-73800 and HL-91911 to DD. Some of the data were presented in preliminary form at the Annual Meeting of the Society for the Study of Ingestive Behavior in July 2010 and at the Annual Meeting of the Society for Neuroscience in November 2010.
References
- Avrith DB, Fitzsimons JT. Increased sodium appetite in the rat induced by intracranial administration of components of the renin-angiotensin system. J Physiol. 1980;301:349–364. doi: 10.1113/jphysiol.1980.sp013210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RW, Epstein AN, Fitzsimons JT, Fluharty SJ. Arousal of a specific and persistent sodium appetite in the rat with continuous intracerebroventricular infusion of angiotensin II. J Physiol. 1980;301:365–382. doi: 10.1113/jphysiol.1980.sp013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggy J, Fisher AE. Evidence for a dual central role for angiotensin in water and sodium intake. Nature. 1974;250:733–735. doi: 10.1038/250733a0. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Daniels D, Fluharty SJ. Neuroendocrinology of Body Fluid Homeostasis. In: Pfaff Dw, Arnold Ap, Etgen Am, Fahrbach Se, Rubin Rt., editors. Hormones Brain and Behavior. Vol. 1. Academic Press; San Diego: 2009. pp. 259–288. [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- De Luca LA, Jr, Xu Z, Schoorlemmer GH, Thunhorst RL, Beltz TG, Menani JV, Johnson AK. Water deprivation-induced sodium appetite: humoral and cardiovascular mediators and immediate early genes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R552–559. doi: 10.1152/ajpregu.00295.2000. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes. 2005;54:1985–1993. doi: 10.2337/diabetes.54.7.1985. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT. Drinking by rats depleted of body fluid without increase in osmotic pressure. J Physiol. 1961;159:297–309. doi: 10.1113/jphysiol.1961.sp006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Ferguson AV. Ghrelin modulates electrical activity of area postrema neurons. Am J Physiol Regul Integr Comp Physiol. 2009;296:R485–492. doi: 10.1152/ajpregu.90555.2008. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Fujihara H, Kawasaki M, Saito T, Shibata M, Otsubo H, Takei Y, Ueta Y. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology. 2007;148:1638–1647. doi: 10.1210/en.2006-0993. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Otsubo H, Fujihara H, Suzuki H, Ohbuchi T, Yokoyama T, Takei Y, Ueta Y. Centrally administered ghrelin potently inhibits water intake induced by angiotensin II and hypovolemia in rats. J Physiol Sci. 2010;60:19–25. doi: 10.1007/s12576-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S. Role of right atrial receptors in the control of drinking in the rat. J Physiol. 1984;349:389–396. doi: 10.1113/jphysiol.1984.sp015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kozaka T, Fujii Y, Ando M. Central effects of various ligands on drinking behavior in eels acclimated to seawater. J Exp Biol. 2003;206:687–692. doi: 10.1242/jeb.00146. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Nelson JF, Weisinger RS. Dehydration induces sodium depletion in rats, rabbits, and sheep. Am J Physiol. 1983;245:R287–292. doi: 10.1152/ajpregu.1983.245.2.R287. [DOI] [PubMed] [Google Scholar]
- Mietlicki EG, Nowak EL, Daniels D. The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav. 2009;96:37–43. doi: 10.1016/j.physbeh.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NE. Effects of drugs on motivation; the value of using a variety of measures. Ann N Y Acad Sci. 1956;65:318–333. doi: 10.1111/j.1749-6632.1956.tb49642.x. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nobata S, Takei Y. The area postrema in hindbrain is a central player for regulation of drinking behavior in Japanese eels. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1569–1577. doi: 10.1152/ajpregu.00056.2011. [DOI] [PubMed] [Google Scholar]
- Prakash MR, Norgren R. Comparing salt appetites: induction with intracranial hormones or dietary sodium restriction. Brain Res Bull. 1991;27:397–401. doi: 10.1016/0361-9230(91)90132-4. [DOI] [PubMed] [Google Scholar]
- Pulman KJ, Fry WM, Cottrell GT, Ferguson AV. The subfornical organ: a central target for circulating feeding signals. J Neurosci. 2006;26:2022–2030. doi: 10.1523/JNEUROSCI.3218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, Jones BP, Fallows DJ. A comparison of the motivational properties of thirst induced by intracranial angiotensin and by water deprivation. Physiol Behav. 1972;9:777–782. doi: 10.1016/0031-9384(72)90051-0. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Morian KR, Nicholson TM, Salisbury JJ. Preference for NaCl solutions in sham drinking Sprague-Dawley rats: water deprivation, sodium depletion, and angiotensin II. Physiol Behav. 1995;57:753–757. doi: 10.1016/0031-9384(94)00321-1. [DOI] [PubMed] [Google Scholar]
- Sato MA, Yada MM, De Luca LA., Jr Antagonism of the renin-angiotensin system and water deprivation-induced NaCl intake in rats. Physiol Behav. 1996;60:1099–1104. doi: 10.1016/0031-9384(96)00058-3. [DOI] [PubMed] [Google Scholar]
- Stricker EM. Extracellular fluid volume and thirst. Am J Physiol. 1966;211:232–238. doi: 10.1152/ajplegacy.1966.211.1.232. [DOI] [PubMed] [Google Scholar]
- Stricker EM. Thirst and sodium appetite after colloid treatment in rats. J Comp Physiol Psychol. 1981;95:1–25. doi: 10.1037/h0077764. [DOI] [PubMed] [Google Scholar]
- Stricker EM, MacArthur JP. Physiological bases for different effects of extravascular colloid treatments on water and NaCl solution drinking by rats. Physiol Behav. 1974;13:389–394. doi: 10.1016/0031-9384(74)90093-6. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Vagnucci AH, McDonald RH, Jr, Leenen FH. Renin and aldosterone secretions during hypovolemia in rats: relation to NaCl intake. Am J Physiol. 1979;237:R45–51. doi: 10.1152/ajpregu.1979.237.1.R45. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Wolf G. Blood volume and tonicity in relation to sodium appetite. J Comp Physiol Psychol. 1966;62:275–279. doi: 10.1037/h0023665. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Kaiya H, Denbow DM, Kangawa K, Furuse M. Central ghrelin acts as an anti-dipsogenic peptide in chicks. Neurosci Lett. 2006;405:241–245. doi: 10.1016/j.neulet.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Mouse taste preference tests: why only two bottles? Chem Senses. 2003;28:315–324. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Weisinger RS, Denton DA, McKinley MJ, Nelson JF. Dehydration-induced sodium appetite in rats. Physiol Behav. 1985;34:45–50. doi: 10.1016/0031-9384(85)90075-7. [DOI] [PubMed] [Google Scholar]
- Wolf G, Stricker EM. Sodium appetite elicited by hypovolemia in adrenalectomized rats: reevaluation of the “reservoir” hypothesis. J Comp Physiol Psychol. 1967;63:252–257. doi: 10.1037/h0024357. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker A, Gleason SD, Schneider EG. Renal and endocrine response to water deprivation in dog. Am J Physiol. 1982;242:R296–302. doi: 10.1152/ajpregu.1982.242.3.R296. [DOI] [PubMed] [Google Scholar]