Summary
The pathogenesis of Staphylococcus aureus infective endocarditis (IE) is postulated to involve invasion and damage of endothelial cells (ECs). However, the precise relationships between S. aureus – EC interactions in vitro and IE virulence and treatment outcomes in vivo are poorly defined. Ten methicillin-resistant S. aureus (MRSA) clinical isolates previously tested for their virulence and vancomycin responsiveness in an experimental IE model were assessed in vitro for their hemolytic activity, protease production, and capacity to invade and damage ECs. There was a significant positive correlation between the in vitro EC damage caused by these MRSA strains and their virulence during experimental IE (in terms of bacterial densities in target tissues; P < 0.02). Importantly, higher EC damage was also significantly correlated with poor microbiologic response to vancomycin in the IE model (P < 0.001). Interestingly, the extent of EC damage was unrelated to a strain's ability to invade ECs, hemolytic activity and protease production, or β-toxin gene transcription. Inactivation of the agr locus in two MRSA strains caused ∼20% less damage as compared to the corresponding parental strains, indicating that a functional agr is required for maximal EC damage induction. Thus, MRSA-induced EC damage in vitro is a unique virulence phenotype that is independent of many other prototypical MRSA virulence factors, and may be a key biomarker for predicting MRSA virulence potential and antibiotic outcomes during endovascular infections.
Introduction
Throughout the industrialized world, Staphylococcus aureus is the leading cause of endovascular infections including vascular catheter sepsis and infective endocarditis (IE) (Fowler et al., 2005, Miro et al., 2005). The increased incidence of infections due to methicillin-resistant S. aureus (MRSA) and the high rates of vancomycin clinical failures have further complicated the management of patients who are infected with these strains (Chang et al., 2003, Fowler et al., 2004, Moise et al., 2007, Moise et al., 2000).
S. aureus expresses a large number of virulence factors that contribute to the establishment, course and ultimate outcome of endovascular infections such as IE (reviewed in (Moreillon et al., 2004, Moreillon et al., 2002)). Upon entering the blood stream, S. aureus must avoid being cleared by host defense mechanisms in order to cause disease. In addition to persisting in the bloodstream, the organism must adhere to damaged cardiac valve endothelium to initiate IE. In order to persistently infect such sites, S. aureus must subsequently invade the cardiac endothelial cells. The ability of the organism to persist, invade, and proliferate at these initial infection sites is critical to the development of IE. Importantly, intracellular elaboration of exoenzymes by the organism may cause host cell lysis, and likely facilitates hematogenous spread to other target organs (e.g., kidneys and spleen). Recent studies suggest that EC damage plays a crucial role in the pathogenesis of valvular heart disease (reviewed in (Chorianopoulos et al., 2009)]). Lysis of the endothelium harboring S. aureus would expose procoagulant subendothelial collagen, resulting in platelet adherence and promotion of vegetation formation. Moreover, induction of EC damage might play an important role in IE pathogenesis by providing a route for dissemination of S. aureus to distant sites.
In order to injure ECs, S. aureus must adhere to and invade these cells. The major adhesins involved in S. aureus invasion by ECs are thought to be fibronectin-binding proteins (FnBPs) (Peacock et al., 1999, Piroth et al., 2008, Que et al., 2005, Sinha et al., 2000). These proteins bind to fibronectin, which acts as a bridging molecule between S. aureus FnBPs and α5β1 integrin on the EC surface (Jaffe et al., 1978, Sinha et al., 1999). The binding of FnBPs to α5β1 integrin induces the rearrangement of the EC cytoskeleton, and subsequent endocytotis of the pathogen (reviewed in (Sinha et al., 2010, Sinha et al., 2005)). However, adherence to and subsequent invasion of ECs are required, but not sufficient for S. aureus to induce EC damage (Grundmeier et al., 2010, Haslinger-Löffler et al., 2005). Many factors have been suggested to mediate EC damage including bacterial toxins and immune response cytokines (reviewed in (Chorianopoulos et al., 2009)).
At present, little is known about the specific role of S. aureus – EC interactions, especially EC damage, in the pathogenesis and antibiotic responsiveness of endovascular infectious syndromes. In order to address this fundamental void in knowledge, we studied the relationship between EC invasion and damage, and their correlations with in vivo virulence and antibiotic responsiveness in an experimental IE model, using ten recent MRSA clinical strains. Our results indicate that S. aureus - induced EC damage, rather than invasion, is crucial for innate virulence and vancomycin responsiveness in experimental IE model. EC damage might thus serve as a ‘biomarker’ for certain aspects of host - pathogen interactions in vivo related to S. aureus.
Results
Effects of inoculum and incubation time on EC damage
EC damage was assessed by a 51Cr release assay in which ECs are loaded with 51Cr. In this assay, 51Cr is taken up by the ECs and binds to proteins in the cytosol. When the cells are damaged (due to necrosis and/or apoptosis), there is membrane leakage and the 51Cr is released into the medium where it can be detected. Because the 51Cr release assay has not been previously used to study S. aureus - induced EC damage, it was essential to identify an optimal multiplicity-of-infection (MOI; organism:EC ratio). We, therefore, determined the effect of MOIs ranging from 5 to 500 on the extent of EC damage caused by S. aureus strain 6850. This strain caused relatively low levels of damage at the 3h invasion time point at all three MOIs tested (Figure 1). However, strain 6850 caused significantly more EC damage after 24 h, inducing a specific chromium release of ∼50% at a MOI of 50. As expected (Tuchscherr et al., 2010), the small colony variant JB-1 induced significantly less EC damage than did its parental strain 6850 at 24h (Figure 2 A). Based on these data, this MOI was employed for all further EC damage assays, as a specific chromium release of ∼50% enabled us to reliably discriminate among the capacity of the different strains to induce EC damage (see below).
Figure 1. Endothelial cell injury by strain 6850 as determined by 51Cr release.
Staphylococcus aureus strain 6850 induces EC damage in an inoculum-dependent manner. Data are shown as means + SD of three independent experiments performed in triplicate.
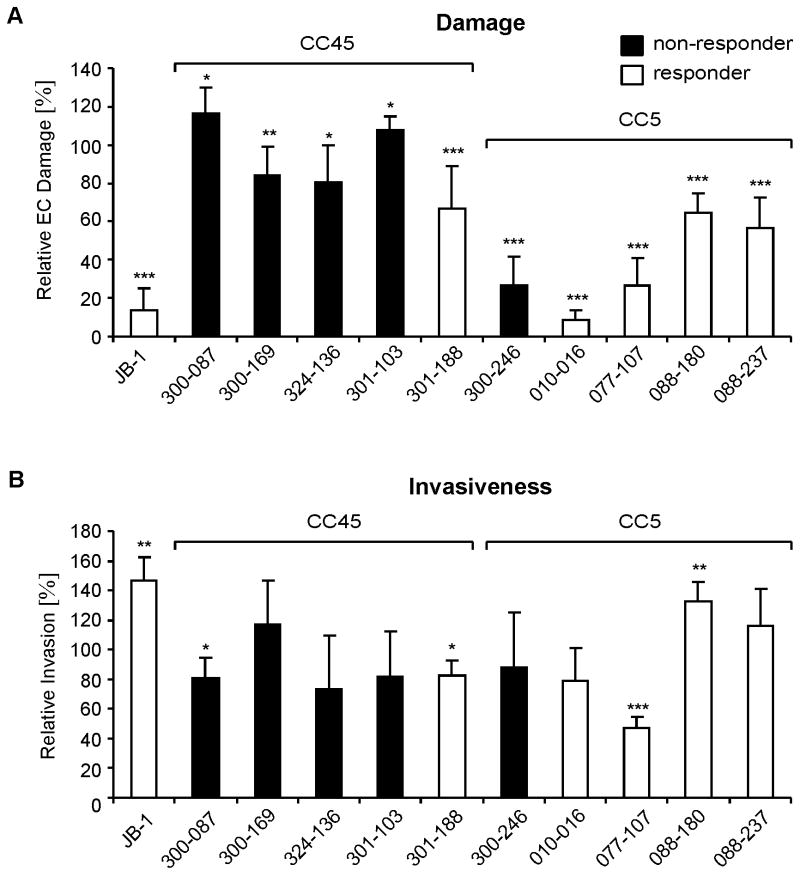
Figure 2. Endothelial cell invasion and damage.
A, Endothelial cell damage as determined by specific 51Cr release 24h after infection. Data are shown as means + SD of three independent experiments performed in triplicate and expressed as the percentage of damage caused by strain 6850 (set up as 100%). B, Corrected invasiveness shown as means + SD of three independent experiments performed in duplicate and expressed as the percentage of invasiveness of strain 6850 (set up as 100%). Responder: MRSA strains that responded to vancomycin treatment in the experimental IE model; non-responder: MRSA strains that did not respond to vancomycin treatment in the IE model (see Table 1 for detailed descriptions). *; P < 0.05; ** P < 0.01; ***, P < 0.001 as compared to strain 6850.
After 3h, all MRSA study strains caused little EC damage, and the amount of damage that was induced was similar to that induced by strain 6850 (data not shown). After 24 h there was a significant difference in the capacity of the various strains to induce EC damage (Figure 2 A). At this time point, isolates from the CC45 genetic background induced significantly more damage as compared to isolates from the CC5 background (Table 1).
Table 1.
Correlation of S. aureus - EC interactions and genetic background of MRSA strains.
| Mean ± SD | |||
|---|---|---|---|
| CC45 | CC5 | P | |
| Relative Invasion (%)a | 86 ± 31 | 93 ± 39 | 0.48 |
| Relative EC damage (%)b | 86 ± 25 | 34 ± 24 | < 0.0001 |
Corrected invasiveness as expressed as the percentage of invasiveness of strain 6850 (set up as 100%).
S. aureus induced EC damage as determined by specific 51Cr release at 24h after infection. Data are expressed as damage induced by strain 6850 (set up as 100%).
The capacity of MRSA strains to invade ECs is independent of their capacity to induce EC damage
S. aureus must adhere to and invade EC in order to damage these cells (Haslinger-Löffler et al., 2005). The adherence of the ten clinical MRSA strains to ECs has been previously reported (Seidl et al., 2011). We found no correlation between adherence to ECs and virulence or vancomycin responses in terms of MRSA colony counts in target tissues in the IE model (P > 0.1 for all comparisons). We, thus, focused here on the subsequent step in S. aureus - EC interactions, i.e., the capacity of the various strains to invade ECs. The invasiveness of these strains was analyzed under the same in vitro conditions as in the damage assay, but at a MOI of 1. This latter MOI caused no detectable EC damage which would have prevented accurate quantification of invasiveness. As expected (Tuchscherr et al., 2010), the small colony variant, JB-1, was significantly more invasive than its parental strain 6850 (Figure 2 B). Most of the MRSA clinical isolates invaded ECs similarly to control strain 6850. However, two CC45 and one CC5 strains invaded ECs significantly less than strain 6850 (Figure 2 B). There were no overall differences in the invasiveness of isolates from the two different genetic backgrounds (Table 1). Of interest, there was no relationship between the capacity of the different strains to invade ECs and their ability to induce EC damage (P = 0.58). These results are in accordance with previous findings (Grundmeier et al., 2010, Haslinger-Löffler et al., 2005), and indicate that S. aureus invasion of ECs and induction of EC damage are distinct processes.
EC damage, but not invasion, is positively correlated with virulence and reduced vancomycin responsiveness in an experimental IE model
The 10 clinical MRSA isolates were susceptible to vancomycin, with MICs of 0.5 or 1.0 μg/ml; none exhibited vancomycin tolerance based on in vitro kill-curve kinetics (Seidl et al., submitted; Antimicrob Agents Chemother). Population analyses revealed no evidence of vancomycin-heteroresistant subpopulations (Seidl et al., submitted). These 10 isolates were originally selected for the study because they belonged to two distinct genetic backgrounds (CC45 and CC5) that have been associated with enhanced virulence in humans (Fusco et al., 2009, McCalla et al., 2008, Sakoulas et al., 2002, van Leeuwen et al., 2000). We recently analyzed these 10 strains in an experimental rabbit IE model for their comparative fitness and therapeutic response profiles to vancomyin (Table 2, Seidl et al., submitted; Antimicrob Agents Chemother). In this model, vancomycin treatment of IE caused by five of the ten strains (four CC45 strains and one CC5 strain; ‘non-responders’) was relatively ineffective, resulting in < 1.5 log10cfu/gram reductions in the bacterial load in the three target tissues (vegetations; kidneys, spleen). IE caused by the other five MRSA strains (one CC45 and four CC5 strains) responded well to vancomycin treatment (‘responders’), with ≥ 5 log10cfu/g reductions in the number of CFUs in the vegetations, and ≥ 3 log10cfu/g reduction in bacterial counts in the kidney and spleen (Table 2, Seidl et al., submitted; Antimicrob Agents Chemother).
Table 2. Staphylococcus aureus strains used in this study.
| Strain | Description | α-hem a | δ-hem a | VAN responseb | Reference |
|---|---|---|---|---|---|
| Clinical MRSA isolates | |||||
| 300-087 | agr-I, SCCmec IV, CC45 | + | ++ | No | (Fowler et al., 2006, Seidl et al., 2011) |
| 324-136 | agr-I, SCCmec IV, CC45 | + | ++ | No | as above |
| 300-169 | agr-I, SCCmec IV, CC45 | + | ++ | No | as above |
| 300-103 | agr-I, SCCmec IV, CC45 | + | +/- | No | as above |
| 301-188 | agr-I, SCCmec IV, CC45 | + | +/- | Yes | as above |
| 300-246 | agr-II, SCCmec I, CC5 | +/- | ++ | No | as above |
| 010-016 | agr-II, SCCmec II, CC5 | + | +/- | Yes | as above |
| 077-107 | agr-II, SCCmec II, CC5 | + | +/- | Yes | as above |
| 088-180 | agr-II, SCCmec II, CC5 | + | +/- | Yes | as above |
| 088-237 | agr-II, SCCmec II, CC5 | - | - | Yes | as above |
| Mutants | |||||
| 324-136Δagr | 324-136 agr∷tet(M), Tcr | +/- | - | No | (Seidl et al, submitted; Antimicrob Agents Chemother) |
| 300-169Δagr | 300-169 agr∷tet(M), Tcr | +/- | - | No | (Seidl et al, submitted; Antimicrob Agents Chemother) |
| Laboratory strains (controls) | |||||
| 6850 | Wild type isolate from osteomyelytis | (Balwit et al., 1994) | |||
| JB-1 | Menadione auxotroph SCV from strain 6850 | (Balwit et al., 1994) | |||
| COL | Homogeneous methicillin-resistant strain, hlb+ | (Tomasz et al., 1989) | |||
| N315 | Clinical methicillin-resistant isolate, θN315, hlb- | (Kuroda et al., 2001) | |||
Presence of α- and δ-hemolysin toxins as previously determined (Seidl et al, submitted). Results are listed semiquantitatively in 4 categories: -, not present; +/-, borderline; +, present, ++, strong activity.
Vancomycin response in an experimental IE model as previously determined (Seidl et al., submitted). Yes: ≥ 5 log10 cfu reduction per g of vegetations, and ≥ 3 log10 cfu/g reductions per g of kidneys and spleen due to vancomycin treatment. No: < 1.5 log10 cfu reduction per g of vegetations, kidneys and spleen due to vancomycin treatment.
Tcr, tetracyclin resistance.
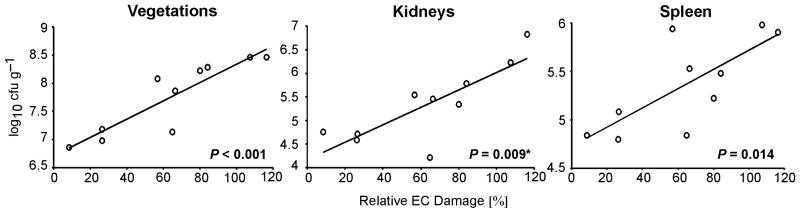
The extent of EC damage induced by the 10 MRSA isolates was directly correlated with their bacterial counts in target tissues in the IE model (Figure 3). Among the three target tissues, the relationship between EC damage and tissue bacterial burden was the strongest for vegetations, and weakest for spleen. Furthermore, as a group, MRSA strains with a good response to vancomycin therapy in the experimental IE model induced significantly less EC damage than did MRSA strains that did not respond to vancomycin treatment (Table 3). In addition, CC45 strains inducing the greatest EC damage in vitro responded the least to vancomycin in vivo (Figure 2 A). Therefore, the capacity of CC45 isolates to damage ECs may in some manner enable it to avoid being killed by vancomycin in vivo.
Figure 3. Relationship between EC injury and virulence in experimental IE.
EC damage induced by ten clinical MRSA isolates was plotted against average bacterial densities in vegetations, kidneys, and spleen. Simple linear regression and Spearman rank correlation (*) showed a direct relationship, whereby higher EC damage was associated with higher bacterial density in all target tissues. The results do not change significantly when two agr mutant strains are included in the analysis. EC damage is expressed as the percentage of damage induced by strain 6850.
Table 3.
Correlation of S. aureus - EC interactions and response to vancomycin treatment in an experimental IE model.
| Mean ± SD | |||
|---|---|---|---|
| Respondera | Non-respondera | P | |
| Relative Invasion (%)b | 81 ± 35 | 87 ± 35 | 0.62 |
| Relative EC damage (%)c | 45 ± 29 | 77 ± 36 | < 0.0001 |
Responder: MRSA strains that responded to vancomycin treatment in the experimental IE model; non-responder: MRSA strains that did not respond to vancomycin treatment in the IE model (see Table 1 for detailed descriptions).
Corrected invasiveness as expressed as the percentage of invasiveness of strain 6850 (set up as 100%).
S. aureus induced EC damage as determined by specific 51Cr release at 24h after infection. Data are expressed as damage induced by strain 6850 (set up as 100%).
Of interest, there was no significant association between the capacity of the study strains to invade ECs in vitro and their virulence during IE, as determined by respective bacterial densities in the infected target tissues (P > 0.1 for all comparisons). Moreover, the EC invasiveness of the strains that responded well to vancomycin in the IE model was similar to that of the strains that responded poorly to this antibiotic (Table 3). Thus, almost all of the study strains invaded ECs well, suggesting that this phenotype is required for subsequent pathogenetic steps in endovascular infections.
The presence of a functional agr locus is dispensable for EC invasion, but is necessary for greater EC damage
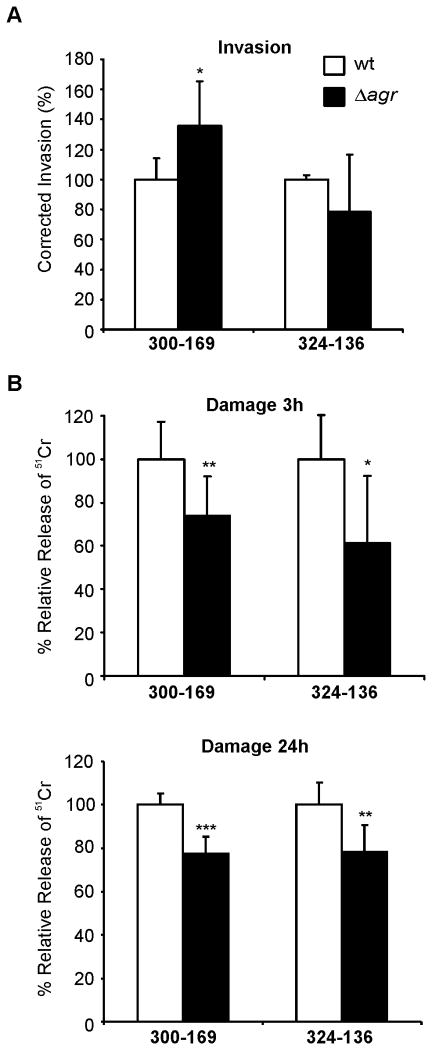
The accessory gene regulator (agr) locus is a principal global regulator within the overall staphylococcal virulon, regulating many adhesins and secreted virulence factors, such as proteases and toxins (e.g., α-toxin). The agr effector, RNAIII, also encodes the cytolysin, δ-toxin (reviewed in (Verdon et al., 2009)). Because several previous studies have suggested that the agr locus plays a key role in S. aureus - induced host cell death (Giese et al., 2011, Haslinger-Löffler et al., 2005), we investigated the EC interactions of two engineered agr mutant strains. The two agr null mutants were constructed only in the CC45 backgrounds as we were not able to introduce the mutation into the CC5 background. The mutants produced no δ-toxin and had reduced α-toxin gene (hla) transcription as compared to their wild-type parent strains (Table 2). The EC invasion of strain 300-169Δagr was significantly higher than that of its parental strain (Figure 4 A). In contrast, the EC invasion of strain 324-136Δagr was slightly lower than that of its parental strain, without reaching statistical significance. Although relatively modest in extent, each mutant strain induced significantly less EC damage than did its respective parental strain, at both 3h and 24h (Figure 4 B). Collectively, these results indicate that agr is dispensable for MRSA invasion of ECs, although necessary for maximal induction of EC damage.
Figure 4. Influence of agr on EC invasion and damage.
(A) Relative EC invasiveness of and (B) damage induced by two clinical MRSA isolates and their corresponding agr mutants. Data for the mutants are expressed as percentage of the corresponding parental strains. Data represent the mean of three independent experiments carried out in duplicate and triplicate for invasion and damage, respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Hemolysin and protease production did not correlate with EC damage
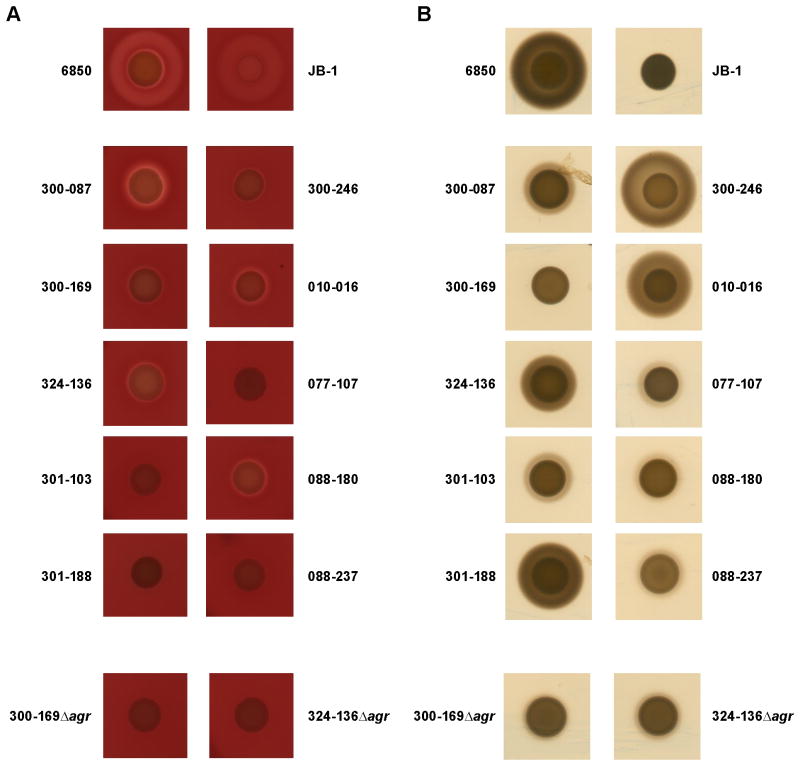
There was no significant correlation between the hemolytic activity of the individual strains and their capacity to induce EC damage (Figure 5 A). For instance, strain 088-237 was non-hemolytic, but was nevertheless able to induce more EC damage than strain 010-016, which was strongly hemolytic (Figures 2 A and Figure 5 A). Similarly, there was no apparent relationship between extracellular protease production and EC damage (Figure 2 A and Figure 5 B). Thus, strain 301-103, which induced greater EC damage than did strain 6850, exhibited very low protease activity. In contrast, strain 010-016, which caused the least EC damage, demonstrated strong protease production (Figures 2 A and Figure 5 B).
Figure 5. Hemolytic activity and protease production.
A, Hemolytic activity was assessed on sheep blood agar. B, Protease production was assessed on caseinate agar (Ismail et al., 2000).
β-toxin is not required for induction of EC damage
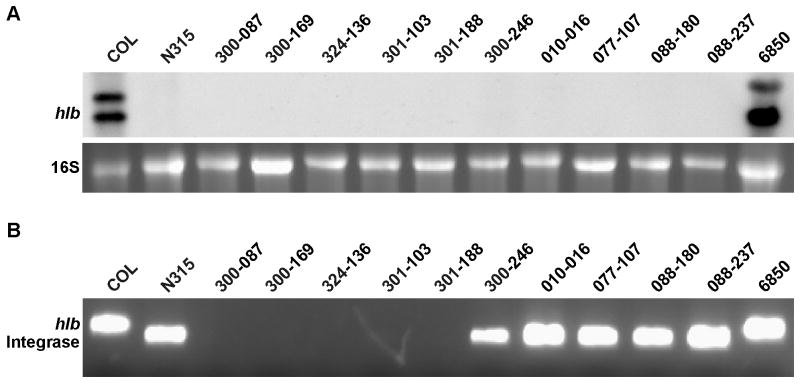
It was recently reported that the combined elaboration of β-toxin and δ-hemolysin is required for S. aureus to escape from phago-endosomes and induce EC damage (Giese et al., 2011). β-toxin is variably produced by S. aureus strains, depending on the presence or absence of β-toxin converting phages whose insertion inactivates the β-toxin gene hlb. While strains COL and 6850 exhibited hlb transcripts, none of the ten clinical MRSA strains used in this study expressed the hlb gene in vitro (Figure 6 A). To determine if the lack of hlb transcript in these latter strains was due to the presence of hlb-converting phages, we tested for their presence by multiplex PCR. The control strains, COL and 6850, both possessed an intact hlb-gene. Like control strain N315, all five CC5 strains had an integrated hlb-disrupting phage (Figure 6 B). No PCR product was obtained for any of the CC45 strains; the reason for their lack of hlb transcription remains unclear. However, these data demonstrate that EC damage in this strain cohort is unambiguously independent of β-toxin elaboration.
Figure 6. hlb expression and detection of hlb-converting phages.
A, Northern blot analysis of hlb transcripts at late exponential growth phase (4h). B, Multiplex PCR for the detection of the intact hlb and phage integrase.
Discussion
Although S. aureus has been principally considered an extracellular pathogen, recent data have confirmed that this organism is internalized by a variety of non-professional phagocytes, such as ECs. While the interactions between vascular ECs and circulating bacteria, as noted above, are considered to be important in the initial phases of S. aureus IE pathogenesis (i.e., tissue infection), little is known about the specific impact of the S. aureus:EC interactions upon the virulence and response to antibiotic therapy in endovascular infections. It has been recognized that internalized S. aureus can avoid host immune defenses, as well as the bactericidal effects of many antimicrobial agents, thus fostering persistent and/or relapsing infections (Reviewed in (Garzoni et al., 2009)]). Moreover, the intra-EC milieu appears to foster small colony variant morphotypes, an additional intracellular persistence strategy for S. aureus (Schröder et al., 2006, Sendi et al., 2009, Vesga et al., 1996). On the other hand, S. aureus - infected ECs could initiate a brisk inflammatory response, prompting production of key cytokines, chemokines and adhesion molecules. Such responses might either assist in efficiently eliminating invading bacteria, or in contrast, promote further disease pathogenesis at the infection site (Golias et al., 2007). Therefore, in the present study, we defined S. aureus - EC interactions, focusing on two key post-EC binding events: i) invasion (internalization); and ii) the ability to induce EC damage. Importantly, the effects of these two interaction metrics on virulence and antibiotic treatment outcome was defined in a relevant model of endovascular infection.
Our present study demonstrated several pivotal findings. First, the extent of EC damage by different MRSA strains predicted their virulence in terms of achievable bacterial densities in IE target tissues. Those MRSA strains which induced less EC damage were also significantly less virulent, as compared to MRSA strains that caused greater EC damage. Second, there was a significant inverse correlation between MRSA-induced EC damage and vancomycin therapeutic responses in the IE model. Thus, with one exception (strain 300-246), higher EC damage correlated with worse response to vancomycin therapy in the IE model. This differential vancomycin outcome among strains was not linked to several standard in vitro vancomycin susceptibility yardsticks, including MICs, killing kinetics, tolerance or population analyses profiles. The ability of individual MRSA strains to induce EC damage was significantly related to their genetic background in terms of CC types: i.e., CC45 isolates caused significantly more EC damage than did the CC5 isolates. Furthermore, these CC45 isolates were significantly more virulent than the CC5 strains in the IE model (Seidl et al., submitted; Antimicrob Agents Chemother). It has been shown that CC45 MRSA strains can cause severe human infectious syndromes (Fusco et al., 2009). Collectively, these data suggest that induction of EC damage is a key event during endovascular infections, and that this process is not only associated with the innate virulence of an individual strain, but also its capacity to persist despite vancomycin therapy.
The relationship between the capacity of an organism to damage ECs in vitro and virulence in experimental animal models of infection has also been reported for Candida albicans and Aspergillus fumigatus (Chiang et al., 2008, Sanchez et al., 2004). However, a unique finding in the current study was that except for one strain (300-246), MRSA isolates with the greatest capacity to induce EC damage also induced infections that had the poorest response to vancomycin therapy. Although the exact cause of this relationship remains to be determined, we speculate that the strains that cause the greatest host cell damage may produce the largest areas of necrosis within the host. Such necrosis may also stimulate cytokine-mediated and/or tissue factor-induced procoagulant activity at these sites (Lopes-Bezerra et al., 2003, Mattsson et al., 2008, Matussek et al., 2005, Veltrop et al., 1999), leading to exuberant platelet-fibrin vegetation formation. The net result of these events may well render the organism better able to survive within such areas of necrosis-vegetation formation (e.g., due to reduced vancomycin penetrations).
A number of different assays have been utilized to assess S. aureus - induced cytotoxicity (Park et al., 2008, Strober, 2001). In the present study, we used a 51Cr release assay, which has been used to detect damage in endothelial and epithelial cells induced by Candida albicans (Filler et al., 1995, Park et al., 2009, Phan et al., 2000) and Aspergillus fumigatus (Bezerra et al., 2004, Kamai et al., 2006). To our knowledge, this assay has not been previously used to assess S. aureus - induced EC damage. We determined that the extent of EC damage induced by the control S. aureus strain 6850 was inoculum-dependent, similar to the finding of Haslinger-Loeffler et al. (Haslinger-Löffler et al., 2005). In parallel, confirming other investigations, very little EC damage was induced by strain JB-1, a stable small colony variant of strain 6850 (Sendi et al., 2009, Tuchscherr et al., 2010). These results demonstrate that the 51Cr release assay is a convenient, reliable, and reproducible method for assessing the capacity of S. aureus strains to induce EC damage. Our finding that the extent of EC damage measured using this assay directly correlated with virulence during IE and poor response to vancomycin argues that this assay is an excellent tool for elucidating key aspects of the host-pathogen interaction during MRSA IE.
Another interesting finding was that the capacity of the MRSA study isolates to invade ECs did not correlate with their ability to induce EC damage. This is in accordance with previous findings, which suggest that even though invasiveness is a prerequisite for S. aureus-induced cytotoxicity, it does not necessarily lead to EC activation and/or cytotoxicity (Grundmeier et al., 2010, Haslinger-Löffler et al., 2005, Tuchscherr et al., 2010). These results strongly suggest that EC invasion and damage are distinct and separate processes, which are likely governed by different MRSA genetic networks and phenotypic effectors.
Other investigators have reported that many factors are associated with S. aureus - induced EC damage, including the elaboration of α-, β-, and δ-toxins. The RNAIII effector molecule of the agr locus, which encodes δ-toxin, has been reported to be a key factor in S. aureus-induced cytotoxicity (Shompole et al., 2003). δ-toxin has been shown to possess cytolytic properties (reviewed in (Verdon et al., 2009)), and has been suggested to mediate phagosomal escape by S. aureus (Giese et al., 2011, Shompole et al., 2003), a proposed prerequisite for induction of host cell death (Klein et al., 2006). We found that agr null mutants caused approximately 20% less EC damage than did their respective parental strains. Thus, although agr-induced δ-toxin production plays a modest role in inducing EC damage, other factors must also contribute to this process.
A recent investigation demonstrated that β-toxin in combination with δ-toxin was required for phago-endosomal escape from ECs; the authors speculated that this process induced EC death (Giese et al., 2011). β-toxin is variably produced by S. aureus strains, depending on the presence or absence of hlb-converting phages, whose insertion inactivates hlb. We found that none of our 10 MRSA clinical isolates expressed detectable hlb mRNA. In five of the ten isolates, the absence of hlb mRNA was due to the presence of a β-toxin converting phage. Because the majority of these isolates still induced significant EC damage, β-toxin production appears to be dispensable for this process. Therefore, phago-endosomal escape may not necessarily cause cell death. Alternatively, the necessity of β-toxin might be host cell-dependent.
The role of S. aureus α-toxin in cytotoxicity is not clear, with results from previous investigations being rather inconsistent (Reviewed in (Sinha et al., 2010)). We found no relationship between α-toxin expression and EC damage among the MRSA strains tested. For instance, strain 088-237 did not produce α-toxin, but still induced significant EC damage. Taken together, our findings suggest that individually, α-, β-, and/or δ-toxins play a modest role in the induction of EC damage by MRSA. On the other hand, it is also possible that the effects of deletion/absence of a single toxin gene are masked by the increased expression of other toxin genes. Additional experiments using strains that lack multiple toxin genes are necessary to evaluate this possibility.
In summary, S. aureus - induced EC damage significantly contributes to both virulence and responsiveness to antimicrobial (vancomycin) therapy in experimental IE. The 51Cr release assay used to quantify S. aureus - induced EC damage provides an excellent technique to study biologically relevant host-pathogen interactions under controlled experimental conditions. We are currently using this assay to test additional MRSA strains with different genetic backgrounds and with distinct gene knockouts to better understand the MRSA - EC interactions in IE pathogenesis and antibiotic treatment outcomes.
Experimental procedures
Reagents
M199 medium, streptomycin, penicillin and HBSS were obtained from Gibco (Grand Island, NY, USA); tissue culture plastics were from Costar (Cambridge, MA, USA). Fetal bovine serum and bovine calf serum were purchased from Gemini Bioproducts (West Sacramento, CA, USA). Trypsin was purchased from Irvine Scientific (Santa Ana, CA, USA). Human albumin and fibronectin from human serum, collagenase, lysostaphin and casein sodium salt from bovine milk were from Sigma-Aldrich (St. Louis, MO, USA). Agar plates contained 5% Sheep in Tryptic Soy Agar Base (Hardy Diagnostics, Santa Maria, CA, USA).
Bacterial strains and growth conditions
The bacterial strains used in this study are listed in Table 1. The ten MRSA strains studied here originated from a multi-national S. aureus bacteremia clinical trial collection, conducted between 2002 and 2005 (Fowler et al., 2006). All isolates had similar in vitro growth kinetics. When not otherwise specified, bacteria were grown overnight in Bacto BHI broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at 37°C. In addition to the above ten strains, agr deletion mutants of two isolates were used (Seidl et al., submitted; Antimicrob Agents Chemother).
In the EC damage assays (described below), the well-characterized S. aureus strains 6850 and JB-1 (kindly provided by R. Proctor; University of Wisconsin) were included as controls. Strain 6850, a clinical isolate from a patient with invasive infection (osteomyelitis), was used as a high damage-inducing strain, because it was previously shown to induce apoptosis in human ECs (Haslinger-Löffler et al., 2005, Tuchscherr et al., 2010). Strain JB-1, a stable gentamicin-induced small colony variant (SCV) of 6850 was used as a negative control because it is known to invade ECs without causing substantial EC damage (Balwit et al., 1994).
Endothelial cell (EC) damage
ECs were harvested from human umbilical cord veins by the method of Jaffe et al. (Jaffe et al., 1973) and maintained as previously described (Filler et al., 1995). For use in the damage assays, confluent ECs (second or third passage) in 24-well tissue culture plates were incubated overnight in complete M-199 medium (with 10% fetal bovine serum, 10% bovine calf serum plus penicillin, 100IU/ml; streptomycin, 100 μg/ml) containing Na251CrO4 (6 μCi per well; MP Biomedicals, Solon, Oh). Prior to inoculation, unincorporated 51Cr was aspirated from the ECs and the wells were rinsed twice with warm HBSS. The various bacterial strains were grown overnight, washed twice and added to the ECs in invasion medium (1% human albumin and 25 mM HEPES, pH 7.3 in M199 without serum or antibiotics, (Haslinger-Löffler et al., 2005, Tuchscherr et al., 2010)) at the indicated multiplicities of infection (MOIs). After 3 h incubation at 37°C in 5% CO2, the medium was aspirated and collected (3 h wash), wells were washed with HBSS and 500 μl of fresh complete M-199 medium containing 10 μg/ml lysostaphin was added (Cheung et al., 2007, Vann et al., 1987). After a total incubation time of 24 h at 37°C in 5% CO2, 0.25 ml of medium was gently aspirated from each well, after which the endothelial cells were lysed by the addition of 0.5 ml of 6 N NaOH. The lysed cells were aspirated and collected, and wells were rinsed twice with Radiac Wash (Atomic Products, Inc. Shirley, NY). These rinses were added to the lysed cells, and the 51Cr activity of the medium and the cell lysates was determined. Uninfected control wells which underwent the same washes were processed in parallel to measure the spontaneous 51Cr release. After corrections were made for the differences in the incorporation of 51Cr in each well, the specific release of 51Cr at the 3 h and 24 h time points were calculated as previously described (Filler et al., 1995).
EC invasion
The capacity of the various strains to invade ECs was determined by the lysostaphin protection assay (Cheung et al., 2007, Suzuki et al., 2011) using conditions similar to those used for damage assays (see above) at an MOI of 1 (105 bacteria per well). Briefly, invasion was allowed to proceed for 3h before extracellular bacteria were lysed with 10μg/ml lysostaphin in complete M-199 medium, after which the ECs were lysed and the number of internalized organisms was determined by quantitative culture as described (Cheung et al., 2007). Previously published equations were used to determine relative invasiveness (Suzuki et al.): percent invasion = (number of bacteria internalized/number of bacteria initially added) × 100; Growth index = total number of bacteria at 3h/number of bacteria initially added; corrected invasion = percent invasion/growth index.
Assessment of hemolytic and protease activity
To investigate the hemolytic and proteolytic capacities of the study strains, the cells were grown overnight on sheep blood agar and resuspended in physiological NaCl solution to a density of 0.5 McFarland units (∼108 cells/ml). Next, 2 μl of the suspension was spotted onto sheep blood agar (for hemolytic activity) and onto 1% caseinate agar (for proteolytic activity) (Ismail et al., 2000) and incubated at 37°C for 24 h. Zone sizes (in mm) around each colony were measured. Each strain was tested at least twice.
Northern blot and multiplex PCR analyses for β-hemolysin
Both Northern blotting and multiplex PCR were used to detect hlb transcription and the presence of an intact β-toxin gene. For Northern blotting, RNA from bacterial cultures grown to late exponential (4h) growth phase was isolated using the RNAeasy kit (Qiagen, Valencia, CA). A total of 5 μg of total RNA from each sample were separated through a 1.5% agarose-20 mM guanidine thiocyanate gel in 1× Tris-borate-EDTA running buffer (McCallum et al., 2006). Digoxigenin-labeled DNA probes, produced by using the PCR DIG Probe synthesis kit (Roche, Basel, Switzerland), were used for the detection hlb transcripts by Northern hybridization, according to the instructions of the manufacturer using previously published primers (Said-Salim et al., 2003). The Northern blot analyses were performed using two independent RNA samples.
Multiplex PCR to detect the presence of hlb-converting phages was carried out using primers hlb-2, hlb-527 and θN315int-for as previously published (Goerke et al., 2006). Primers hlb-2 and hlb-527 span the attB-site, and therefore can be generated only in the absence of the phage, an hlb-specific amplicon. The integrase gene of the phage is detected by primers θN315int-for and hlb-527, thus only the hlb-integrated phage is detected. Strain COL, that has an intact hlb gene and N315 that has an integrated phage were used as control strains.
Statistical analysis
Means, medians, and standard deviations of continuous variables were calculated for all strains. For the analysis of the relationships of continuous variables to genotype, EC damage, and vancomycin responses, univariate analyses were performed using the student's t-test or the Wilcoxon rank sum test. For the analysis of the relationship between continuous variables and virulence in terms of bacterial density in target tissues, univariate analyses were performed with simple linear regression or Spearman rank-correlation, as appropriate.
Acknowledgments
This work was supported by the Swiss National Science Foundation (PBZHP3-123284 to K. S.), the American Heart Association (SDG 0630219N and GIA 09GRNT2180065 to Y.Q.X.), and the U.S. National Institutes of Health (R01AI-39108 to A.S.B.) and (R01AI054928 and R01AI073829 to S.G.F.). The endothelial cells used in these studies were isolated from human umbilical cords, which were collected by the General Clinical Research Center in the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (NIH NCRR MO1-RR00425). J.M. appreciates the logistic support of the GCRC at Harbor-UCLA Medical Center (NIH NCRR MO1-RR00425). We thank Vance Fowler (Duke University Medical Center, Durham, North Carolina) for kindly providing us the ten MRSA clinical isolates. We also thank Norma Solis and Trang Q. Phan at Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center for sharing their expertise in working with HUVECs.
References
- Balwit J, van Langevelde P, Vann J, Proctor R. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170:1033–1037. doi: 10.1093/infdis/170.4.1033. [DOI] [PubMed] [Google Scholar]
- Bezerra LML, Filler SG. Interactions of Aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood. 2004;103:2143–2149. doi: 10.1182/blood-2003-06-2186. [DOI] [PubMed] [Google Scholar]
- Chang FY, MacDonald BB, Peacock JE, Jr, Musher DM, Triplett P, Mylotte JM, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003;82:322–332. doi: 10.1097/01.md.0000091185.93122.40. [DOI] [PubMed] [Google Scholar]
- Cheung AL, Bayles KW. Tissue culture assays used to analyze invasion by Staphylococcus aureus. Curr Protoc Microbiol. 2007 doi: 10.1002/9780471729259.mc09c04s4. 9C.4.1. [DOI] [PubMed] [Google Scholar]
- Chiang LY, Sheppard DC, Gravelat FN, Patterson TF, Filler SG. Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease. Infect Immun. 2008;76:3429–3438. doi: 10.1128/IAI.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorianopoulos E, Bea F, Katus H, Frey N. The role of endothelial cell biology in endocarditis. Cell Tissue Res. 2009;335:153–163. doi: 10.1007/s00441-008-0687-4. [DOI] [PubMed] [Google Scholar]
- Filler SG, Swerdloff JN, Hobbs C, Luckett PM. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler VG, Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- Fowler VG, Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190:1140–1149. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- Fusco DN, Alexander EL, Weisenberg SA, Mediavilla JR, Kreiswirth BN, Schuetz AN, et al. Clinical failure of vancomycin in a dialysis patient with methicillin-susceptible vancomycin-heteroresistant S. aureus. Diagn Microbiol Infect Dis. 2009;65:180–183. doi: 10.1016/j.diagmicrobio.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 2009;17:59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Giese B, Glowinski F, Paprotka K, Dittmann S, Steiner T, Sinha B, Fraunholz MJ. Expression of δ-toxin by Staphylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of β-toxin. Cell Microbiol. 2011;13:316–329. doi: 10.1111/j.1462-5822.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- Goerke C, Wirtz C, Flückiger U, Wolz C. Extensive phage dynamics in Staphylococcus aureus contributes to adaptation to the human host during infection. Mol Microbiol. 2006;61:1673–1685. doi: 10.1111/j.1365-2958.2006.05354.x. [DOI] [PubMed] [Google Scholar]
- Golias CH, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo. 2007;21:757–769. [PubMed] [Google Scholar]
- Grundmeier M, Tuchscherr L, Brueck M, Viemann D, Roth J, Willscher E, et al. Staphylococcal strains vary greatly in their ability to induce an inflammatory response in endothelial cells. J Infect Dis. 2010;201:871–880. doi: 10.1086/651023. [DOI] [PubMed] [Google Scholar]
- Haslinger-Löffler B, Kahl BC, Grundmeier M, Strangfeld K, Wagner B, Fischer U, et al. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol. 2005;7:1087–1097. doi: 10.1111/j.1462-5822.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- Ismail SA, Deak T, Abd El-Rahman HA, Yassien MAM, Beuchat LR. Presence and changes in populations of yeasts on raw and processed poultry products stored at refrigeration temperature. Int J Food Microbiol. 2000;62:113–121. doi: 10.1016/s0168-1605(00)00414-1. [DOI] [PubMed] [Google Scholar]
- Jaffe E, Mosher DF. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978;147:1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins: Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai Y, Chiang LY, Lopes Bezerra LM, Doedt T, Lossinsky AS, Sheppard DC, Filler SG. Interactions of Aspergillus fumigatus with vascular endothelial cells. Medical Mycology. 2006;44:115–117. doi: 10.1080/13693780600897989. [DOI] [PubMed] [Google Scholar]
- Klein M, Krönke M, Krut O. Expression of lysostaphin in HeLa cells protects from host cell killing by intracellular Staphylococcus aureus. Med Microbiol Immunol. 2006;195:159–163. doi: 10.1007/s00430-006-0014-1. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- Lopes-Bezerra LM, Filler SG. Endothelial cells, tissue factor and infectious diseases. Braz J Med Biol Res. 2003;36:987–991. doi: 10.1590/s0100-879x2003000800004. [DOI] [PubMed] [Google Scholar]
- Mattsson E, Heying R, Van De Gevel JS, Hartung T, Beekhuizen H. Staphylococcal peptidoglycan initiates an inflammatory response and procoagulant activity in human vascular endothelial cells: a comparison with highly purified lipoteichoic acid and TSST-1. FEMS Immunology & Medical Microbiology. 2008;52:110–117. doi: 10.1111/j.1574-695X.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- Matussek A, Strindhall J, Stark L, Rohde M, Geffers R, Buer J, et al. Infection of human endothelial cells with Staphylococcus aureus induces transcription of genes encoding an innate immunity response. Scand J Immunol. 2005;61:536–544. doi: 10.1111/j.1365-3083.2005.01597.x. [DOI] [PubMed] [Google Scholar]
- McCalla C, Smyth DS, Robinson DA, Steenbergen J, Luperchio SA, Moise PA, et al. Microbiological and genotypic analysis of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:3441–3443. doi: 10.1128/AAC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum N, Karauzum H, Getzmann R, Bischoff M, Majcherczyk P, Berger-Bachi B, Landmann R. In vivo survival of teicoplanin-resistant Staphylococcus aureus and fitness cost of teicoplanin resistance. Antimicrob Agents Chemother. 2006;50:2352–2360. doi: 10.1128/AAC.00073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–514. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51:2582–2586. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise PA, Schentag JJ. Vancomycin treatment failures in Staphylococcus aureus lower respiratory tract infections. Int J Antimicrob Agents. 2000;16:S31–S34. doi: 10.1016/s0924-8579(00)00303-4. [DOI] [PubMed] [Google Scholar]
- Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363:139–149. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- Moreillon P, Que YA, Bayer AS. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect Dis Clin North Am. 2002;16:297–318. doi: 10.1016/s0891-5520(01)00009-5. [DOI] [PubMed] [Google Scholar]
- Park H, Liu Y, Solis N, Spotkov J, Hamaker J, Blankenship JR, et al. Transcriptional responses of Candida albicans to epithelial and endothelial cells. Eukaryot Cell. 2009;8:1498–1510. doi: 10.1128/EC.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WB, Lee JH, Jeon JH, Lee SJ, Kim SH, Kim NJ, et al. Effect of tumor necrosis factor-α on intracellular Staphylococcus aureus in vascular endothelial cells. FEMS Immunol Med Microbiol. 2008;52:247–252. doi: 10.1111/j.1574-695X.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- Peacock SJ, Foster TJ, Cameron BJ, Berendt AR. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiol. 1999;145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- Phan QT, Belanger PH, Filler SG. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun. 2000;68:3485–3490. doi: 10.1128/iai.68.6.3485-3490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroth L, Que YA, Widmer E, Panchaud A, Piu S, Entenza JM, Moreillon P. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein A synergistically promote endothelial invasion and experimental endocarditis. Infect Immun. 2008;76:3824–3831. doi: 10.1128/IAI.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que YA, Haefliger JA, Piroth L, François P, Widmer E, Entenza JM, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med. 2005;201:1627–1635. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, McNamara PJ, et al. Global regulation of Staphylococcus aureus genes by rot. J Bacteriol. 2003;185:610–619. doi: 10.1128/JB.185.2.610-619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoulas G, Eliopoulos GM, Moellering RC, Jr, Wennersten C, Venkataraman L, Novick RP, Gold HS. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46:1492–1502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AA, Johnston DA, Myers C, Edwards JE, Jr, Mitchell AP, Filler SG. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect Immun. 2004;72:598–601. doi: 10.1128/IAI.72.1.598-601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder A, Kland R, Peschel A, von Eiff C, Aepfelbacher M. Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: small colony variants are able to survive in lysosomes. Med Microbiol Immunol. 2006;195:185–194. doi: 10.1007/s00430-006-0015-0. [DOI] [PubMed] [Google Scholar]
- Seidl K, Bayer AS, Fowler VG, Jr, McKinnell JA, Hady WA, Sakoulas G, et al. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother. 2011;55:575–582. doi: 10.1128/AAC.01028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendi P, Proctor RA. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol. 2009;17:54–58. doi: 10.1016/j.tim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Shompole S, Henon KT, Liou LE, Dziewanowska K, Bohach GA, Bayles KW. Biphasic intracellular expression of Staphylococcus aureus virulence factors and evidence for Agr-mediated diffusion sensing. Mol Microbiol. 2003;49:919–927. doi: 10.1046/j.1365-2958.2003.03618.x. [DOI] [PubMed] [Google Scholar]
- Sinha B, Francois P, Que YA, Hussain M, Heilmann C, Moreillon P, et al. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect Immun. 2000;68:6871–6878. doi: 10.1128/iai.68.12.6871-6878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, François PP, Nüße O, Foti M, Hartford OM, Vaudaux P, et al. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- Sinha B, Fraunholz M. Staphylococcus aureus host cell invasion and post-invasion events. Int J Med Microbiol. 2010;300:170–175. doi: 10.1016/j.ijmm.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Sinha B, Herrmann M. Mechanisms and consequences of invasion of endothelial cells by Staphylococus aureus. Thromb Haemost. 2005;94:266–277. doi: 10.1160/TH05-04-0235. [DOI] [PubMed] [Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. John Wiley & Sons, Inc.; 2001. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Swoboda JG, Campbell J, Walker S, Gilmore MS. In vitro antimicrobial activity of wall teichoic acid biosynthesis inhibitors against Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:767–774. doi: 10.1128/AAC.00879-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A, Drugeon HB, de Lencastre HM, Jabes D, McDougall L, Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989;33:1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, et al. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. 2010;202:1031–1040. doi: 10.1086/656047. [DOI] [PubMed] [Google Scholar]
- van Leeuwen W, van Nieuwenhuizen W, Gijzen C, Verbrugh H, van Belkum A. Population studies of methicillin-resistant and -sensitive Staphylococcus aureus strains reveal a lack of variability in the agrD gene, encoding a staphylococcal autoinducer peptide. J Bacteriol. 2000;182:5721–5729. doi: 10.1128/jb.182.20.5721-5729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann JM, Proctor RA. Ingestion of Staphylococcus aureus by bovine endothelial cells results in time- and inoculum-dependent damage to endothelial cell monolayers. Infect Immun. 1987;55:2155–2163. doi: 10.1128/iai.55.9.2155-2163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltrop MHAM, Beekhuizen H, Thompson J. Bacterial species- and strain-dependent induction of tissue factor in human vascular endothelial cells. Infect Immun. 1999;67:6130–6138. doi: 10.1128/iai.67.11.6130-6138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon J, Girardin N, Lacombe C, Berjeaud JM, Héchard Y. δ-hemolysin, an update on a membrane-interacting peptide. Peptides. 2009;30:817–823. doi: 10.1016/j.peptides.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Vesga O, Groeschel M, Otten M, Brar D, Vann J, Proctor R. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J Infect Dis. 1996;173:739–742. doi: 10.1093/infdis/173.3.739. [DOI] [PubMed] [Google Scholar]