Abstract
In a presynaptic nerve terminal, neurotransmitter release is largely restricted to specialized sites called active zones. Active zones consist of a complex protein network, and they organize fusion of synaptic vesicles with the presynaptic plasma membrane in response to action potentials. Rab3-interacting molecules (RIMs) are central components of active zones. In a recent series of experiments, we have systematically dissected the molecular mechanisms by which RIMs operate in synaptic vesicle release. We found that RIMs execute two critical functions of active zones by virtue of independent protein domains. They tether presyanptic Ca2+ channels to the active zone, and they activate priming of synaptic vesicles by monomerizing homodimeric, constitutively inactive Munc13. These data indicate that RIMs orchestrate synaptic vesicle release into a coherent process. In conjunction with previous studies, they suggest that RIMs form a molecular platform on which plasticity of synaptic vesicle release can operate.
Key words: synapse, neurotransmitter release, active zone, RIM, calcium, priming
Active Zones: Hot Spots for Synaptic Vesicle Release
Neurons communicate by transmitting information at synapses. Neurotransmitter release in the presynaptic nerve terminal is initiated by the opening of voltage-gated Ca2+ channels in response to the arriving action potential. Elevated Ca2+ levels then induce a membrane fusion reaction during which neurotransmitter-filled synaptic vesicles release their content into the synaptic cleft.
One exquisite property of synaptic transmission is speed.1 Most central nervous synapses transmit information from the pre- to the postsynaptic neuron within milliseconds, and they reliably follow high neuronal firing frequencies. To accomplish such ultrafast Ca2+-triggered membrane fusion, synaptic vesicles must be organized by a fusion machinery that warrants localization of release-ready vesicles close to presynaptic Ca2+ channels.2,3 At the center of the release apparatus is the active zone, forming hot spots for synaptic vesicle fusion on the presynaptic plasma membrane.4
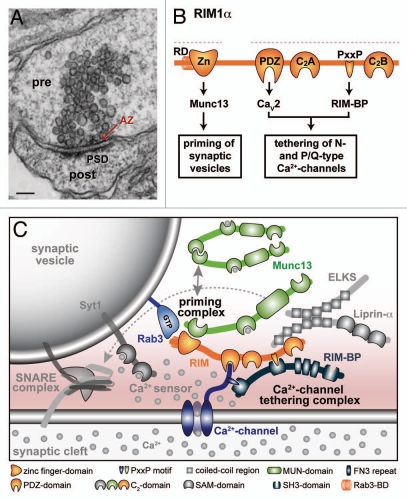
Originally, active zones were defined as sites of neurotransmitter release.5 They were later described in electronmicropscopic analyses as protein dense material that is tightly attached to the presynaptic plasma membrane,6 and exactly opposed to the postsynaptic density (Fig. 1A). This architecture allows perfect alignment of neurotransmitter release sites with the postsynaptic reception apparatus, minimizing the diffusion distance for neurotransmitters. Functionally, active zones set synaptic vesicles up for Ca2+-triggered fusion, enabling synapses to release within less than a millisecond upon arrival of an action potential. At least three processes are involved: tethering of Ca2+ channels to the active zone, docking of synaptic vesicles close to the release site, and assembly of a molecular release machinery on docked vesicles, a process we refer to as priming (Box 1).
Figure 1.
Molecular architecture and functions of presynaptic active zones. (A) Electron-micrograph showing a synaptic connection in cultured hippocampal neurons. The active zone (AZ) of the presynaptic nerve terminal (pre) is exactly opposed to the postsynaptic density (PSD) of the postsynaptic neuron (post). The electrondense material at the active zone consists of a network of proteins that is tightly attached to the presynaptic plasma membrane. Scale bar (bottom left): 100 nm. (B) Schematic of the domain structure of RIM1α depicting (from the N- to the C-terminus) the Rab3-binding α-helical domain (RD), a zinc-finger motif (Zn), the central PDZ-domain and the non-Ca2+-binding C2A and C2B domains that are separated by a linker sequence containing an SH3-domain binding proline rich region (PxxP). Dotted lines indicate fragments that were independently used for the rescue experiment described in the text. Functional implications of protein interactions with Munc13, CaV2.1 and 2.2 α-subunits of voltage gated Ca2+ channels (P/Q-type and N-type, respectively), and RIM-BPs were recently characterized,16–18 these interactions are shown below the domain structure. (C) Model of the molecular architecture of the active zone in the presyanptic nerve terminal. RIMs are central elements of active zones. They form a complex with presynaptic Ca2+ channels and RIM-BPs that localizes Ca2+ influx to the active zone close to the vesicular Ca2+ sensor synaptotagmin 1 (Syt1). In addition, they activate homodimeric, constitutively inactive Munc13 by monomerizing it through an N-terminal priming complex that includes the vesicular GTPase Rab3. The model envisions that Munc13 monomerization enables the MUN homology domain to interact with SNARE complexes (dotted arrow, SNARE complex shown together with Munc18 and complexin) to promote vesicle priming downstream of RIM. Upon Ca2+ entry, SNARE complexes drive fusion of synaptic vesicles with the presyanptic plasma membrane. ELKS and Liprin-α interact with the release machinery at the active zone, but their contributions to release are only poorly understood.
In recent years, efforts were made to determine the molecular components of active zones. Next to proteins that are required for Ca2+-triggered membrane fusion such as SNAREs and Sec1/Munc18-like (SM) proteins, they contain several protein families that are specifically associated with active zones.7 These include Munc13s, Rab3-interacting molecules (RIMs), Liprin-α's, ELKS's, RIM-binding proteins (RIM-BPs) and piccolo/bassoon (Table 1). Only now we are beginning to understand how the active zone as a complex macromolecular structure operates during synaptic transmission.
Table 1.
Active zone enriched protein families and their functions at mammalian synapses
| Protein family | Mammalian protein isoforms* | Proposed functions at mammalian active zones | Key references |
| Munc13 | Munc13-1 ubMunc13-2, bMunc13-2 Munc13-3 Munc13-4 |
synaptic vesicle docking and priming short-term synaptic plasticity |
36–38 |
| RIM | RIM1α, RIM1β RIM2α, RIM2β, RIM2γ RIM3γ RIM4γ |
synaptic vesicle docking and priming Ca2+ channel tethering short-term and long-term synaptic plasticity |
13, 14, 16–18 |
| ELKS | ELKS1A, ELKS1B ELKS2A/B and 2α/2β |
scaffolding inhibition of priming at inhibitory synapses | 29, 39, 40 |
| piccolo/bassoon | piccolo/aczonin bassoon |
active zone assembly and organization vesicle clustering, reloading of release sites |
30, 31, 41, 42 |
| Liprin-α | Liprin-α1 Liprin-α2 Liprin-α3 Liprin-α4 |
receptor anchoring? organization of active zones? |
43, 44 |
| RIM-BP | RIM-BP1 RIM-BP2 |
Ca2+ channel tethering | 16, 19 |
Active zone specific protein families.
Each line represents one gene, multiple protein isoforms expressed from the same gene are on the same line.
RIMs as Organizers of Docking, Priming and Ca2+ Channel Tethering
RIMs are multi-domain proteins at the active zone that are transcribed from four genes (Table 1).8–10 The RIM1 and RIM2 genes encode all multi-domain RIM variants at the active zone (α- and β-RIMs), whereas the RIM3 and RIM4 genes only produce small isoforms (γ-RIMs) with unknown function and localization. RIM1α, the prototypical RIM isoform produced by the RIM1 gene, contains (from the N- to the C-terminus, Fig. 1B) an α-helical region that binds to the vesicular GTPase Rab3, a zinc-finger motif that interacts with Munc13s, a central PDZ domain, and two C-terminal C2 domains that do not bind Ca2+ but are connected with a linker region that contains a RIM-BP interacting proline rich region.8,11,12 RIM2α has an identical domain structure and conserved binding activities, whereas RIM1β and RIM2β are multi-domain RIM variants that lack N-terminal sequences and are produced by alternative promoters within the RIM1 and 2 genes.9,10 γ-RIMs, consisting of a conserved single C2-domain and flanking regions, are produced by the RIM2, 3 and 4 genes and are not further considered here.
Knockout (KO) mice for RIM1α indicated critical contributions to vesicle release including modulating synaptic strength and short-term plasticity, and RIM1α was required for expression of presynaptic long-term plasticity.13–15 RIM's underlying mechanism of action, however, remained obscure because of experimental limitations in the analyses of the constitutive RIM1α KO mice. As for other active zone proteins, precise mechanistic understanding has been hindered by the large multi-domain structure of these proteins and by their the complex genetic organization.10 In a quest of understanding how RIMs operate at the active zone, we have overcome these limitations by two critical experimental approaches.
First, we generated mutant mice to conditionally delete all multi-domain RIM protein isoforms. We targeted all isoforms of the RIM1 and 2 genes by homologous recombination,10,16 allowing conditional removal of RIM1α, RIM1β, RIM2α, RIM2β and RIM2γ. To conclusively analyze synaptic transmission in the absence multi-domain RIMs, we ablated them in two types of synapses: in small hippocampal synapses of cultured neurons by postnatal application of lentiviral cre recombinase,16,17 and in a specialized excitatory synapse in the auditory brain stem, the calyx of Held, by transgenic expression of cre recombinase.18 In both preparations, removal of RIMs strongly decreased release due to a combination of a reduction in docked and primed vesicles, and impaired tethering of presynaptic Ca2+ channels in the nerve terminal.16–18
Second, we combined conditional RIM deletion with expression of rescue RIM proteins, to start dissecting functions of individual domains.16,17 In a first rescue experiment, we showed that re-expression of RIM1α reverses all phenotypes in cultured neurons. The observations that the phenotypes were entirely reversible by reintroduction of RIM1α, were largely overlapping between multiple types of synapses, and were independent of whether cre was expressed embryonically or postnatally, emphasize the critical involvement of multi-domain RIMs in neurotransmitter release at most if not all central nervous synapses. They also suggest that RIMs act similarly at all synapses, and they largely exclude that the effects we observed were due to compensatory mechanisms.
The conditional KO approach with rescue in cultured hippocampal neurons then enabled us to determine the functional significance of individual RIM domains. In a critical rescue experiment, we split RIM into two fragments, an N-terminal fragment that contains a zinc finger domain and a short α-helical sequence, and a C-terminal fragment containing numerous protein interaction domains (dotted grey lines in Fig. 1B). Intriguingly, when we expressed these two fragments separately in the RIM deficient neurons, the major RIM functions, priming and Ca2+ channel tethering, were entirely detached from each other.16,17 Each fragment rescued about 50% of the evoked synaptic currents, however, the two RIM fragments operated by distinct mechanisms (Fig. 1B). The N-terminal sequences that interact with the active zone protein Munc13 and the small vesicular GTPase Rab3 were necessary and sufficient to restore priming. In contrast, the C-terminal interaction domains had no effect on vesicle priming, but were required for Ca2+ channel tethering to the active zone.16,17 The surprising finding that RIM dependent priming and Ca2+ channel tethering were entirely independent of each other had several major implications.
First and foremost, it suggested a direct Ca2+ channel tethering function of one of RIMs C-terminal protein interaction domains. In an extensive series of biochemical, electrophysiological and Ca2+-imaging experiments, we found that the RIM PDZ domain stochiometrically binds to the C-terminus of α-subunits of N- and P/Q-type Ca2+ channels in vitro, and that this interaction localizes the channels to the presynaptic nerve terminal.16 We further showed that a tripartite complex between RIMs, Ca2+ channels and RIM-BPs (which also bind to Ca2+ channels19) is critical for extent and speed of neurotransmitter release.
Second, this split RIM experiment also suggested that RIM's short N-terminal sequences have a direct activating function on synaptic vesicle priming.17 This was unexpected, because it suggested that RIMs do not only act as classical protein scaffolds as described previously in reference 13, but that a short RIM domain autonomously initiates priming. In a series of structure-function experiments based on genetic manipulations of RIMs and Munc13s, we found that RIMs activate priming by monomerizing the constitutively homodimeric, inactive Munc13.17,20 In summary, our data suggest that RIMs connect two major active zone functions: synaptic vesicle priming and Ca2+-secretion coupling (Fig. 1C). RIMs execute these functions by virtue of independent domains. They prime vesicles by activating Munc13 via their zinc finger,17 and they tether Ca2+ channels to active zones through a direct interaction of the RIM1 and 2 PDZ domains with the C-termini of α-subunits of P/Q- and N-type Ca2+ channels.16 For fast, effective synaptic vesicle release, both functions are critical.18,21
Box 1: Synaptic terminology.
Active zone: A dense protein network that is tightly attached to the presynaptic plasma membrane in a nerve terminal. The active zone forms release sites for synaptic vesicles.
Docking: typically measured by electron microscopy, docking describes the physical contact between the synaptic vesicle membrane and the active zone of the presynaptic plasma membrane.
Priming: A process at the active zone that renders vesicles ready for release. Priming includes build-up of a molecular release machinery for a docked vesicle. The pool of primed vesicles is often referred to as the readily releasable pool.
Ca2+-secretion coupling: A requirement for fast, synchronous release is the close spatial relationship between the source of Ca2+ (voltage-gated Ca2+ channels), and the Ca2+ sensor (typically synaptotagmin1 on synaptic vesicles). A critical function of active zones is tethering Ca2+ channels to release sites.
Synaptic vesicle fusion: The final step of neurotransmitter release during which the synaptic vesicle membrane fuses with the presynaptic plasma membrane. In contrast to active zone proteins, the proteins that drive fusion (SNAREs and Sec1/Munc18-like proteins) are not specific to presynaptic release sites.
Excitatory synapse: A chemical synapse that releases an excitatory neurotransmitter to depolarize the postsynaptic target cell, thereby increasing its likelihood for action potential firing. The most common excitatory neurotransmitter is glutamate.
Inhibitory synapse: A synapse that decreases action potential firing in its target cell by hyperpolarizing the postsynaptic cell through release of inhibitory neurotransmitters. GABA is the most common inhibitory neurotransmitter in mammals.
Synaptic strength: The amplitude of change in the postsynaptic membrane potential induced by a presynaptic action potential. Activation of strong synapses has larger effects on postsynaptic firing.
Synaptic plasticity: Use dependent changes in synaptic strength that operate in a short-term (seconds to minutes) or long-term (hours) time range. Synaptic plasticity involves changes in the presynaptic nerve terminal and/or the postsynaptic compartment, and is the cellular correlate for learning and memory.
Calyx of Held: A specialized, excitatory synapse in the auditory brain stem. Presynaptic mechanisms are studied at this synapse because the large nerve terminal is accessible to direct presynaptic measurements.
Implications and Questions
Altogether, our data provide fascinating insights into mechanistic aspects of active zone functions. Together with previous studies, they suggest that four critical elements in the release machinery work in concert at the active zone to achieve the superb speed and precision required for neuronal information processing (Fig. 1C). (1) RIM forms a tethering complex with RIM-BP and presynaptic Ca2+ channels that localizes Ca2+ influx to active zones, and the RIM PDZ domain is required for this function.16,18,19 (2) With its N-terminal zinc finger domain, RIM activates Munc13 by monomerizing it through a priming complex that connects to synaptic vesicles via the vesicular GTPase Rab3.17,20,22 (3) Our model further suggests that these two RIM-dependent protein complexes localize Ca2+ channels close to the Ca2+ sensor synaptotagmin 1 on the primed synaptic vesicle.16–18 (4) Ca2+ entry then triggers fast, synchronous release of neurotransmitters into the synaptic cleft. Our data, in the context of prior observations, suggest that monomeric Munc13 enables vesicles for Ca2+-triggered release by interacting with SNARE complexes,23 which provide the force to fuse the lipid bilayers.24
Many major questions remain to be addressed for understanding how active zones control neurotransmitter release. From our work, three groups of questions arose.
First, in a narrow molecular sense, several aspects of RIM function remain poorly understood. For example, the non-Ca2+-binding C2 domains of RIMs clearly boost release but the underlying mechanism is unknown. It could be that this secretion-enhancing function operates via Liprin-α,13 via β4-subunits of Ca2+ channels,25,26 or via other known or unknown proteins. Similarly, the docking deficit we observed in hippocampal synapses and at the calyx of Held16,18 is not understood. It will be particularly interesting to investigate the components for RIM-dependent synaptic vesicle docking, and to address how docking relates to priming at a molecular level. With the availability of the conditional RIM KO mice, we can now start answering these questions.
Second, a major open question is which mechanisms are shared between different types of synapses and which are distinct. We have characterized active zone functions in excitatory and inhibitory synapses of cultured neurons16,17 and at the calyx of Held,18 a large excitatory synapse. As exemplified by these analyses, fundamental aspects of release and active zone functions are shared between these synapses. However, there are prominent functional differences between different types of synapses. Inhibitory synapses, for example, have generally a much higher release probability compared to excitatory synapses. In the long run, it will be essential to address the underlying molecular components that determine these remarkable functional differences between different types of synapses. The genetic variety of active zone protein isoforms has the potential to govern specific properties of release sites, but we are only beginning to understand isoform and synapse specific functions of RIMs,10,27 Munc13s,28 ELKS29 and bassoon.30,31 In respect to RIMs, it will be critical to determine which isoforms are present at which types of synapses, and to establish whether they may differentially control specific functional properties. Alternative splicing of protein domains, differences in binding affinities between conserved domains of specific protein isoforms and local protein concentrations may have major consequences on release.
Third, it will be fascinating to explore how molecular events at the active zone determine behavioral responses. Clearly, our data argue for multiple molecular functions of RIMs. Intriguingly, however, they also show that each function is only partially lost upon complete genetic deletion of RIMs. Presynaptic Ca2+ influx, for example, is reduced to 50%, and there is a small pool of primed vesicles (about 25%) left after removal of RIMs.16–18 This indicates that there are RIM-independent parallel mechanisms at the active zone underlying these fundamental processes during release. It also suggests that RIM-dependent priming and Ca2+ channel tethering may provide leeway for regulation of synaptic strength, supported by the notion that RIM1α is required for expression of presynaptic long-term plasticity.10,14,32,33 Correspondingly, loss of RIM1α has dramatic consequences on mouse behaviors,34,35 but the molecular mechanisms and circuits that underlie these shortcomings are not understood. This ultimately leads to the question of how neurotransmitter release sites of mammalian synapses are plastic during sustained changes in activity, and whether such changes may contribute to controlling circuit activity and behaviors. Addressing these fundamental questions will be critical to understand how the molecular machine that controls synaptic vesicle release provides a platform on which adaptations in neuronal circuits may be executed.
Acknowledgments
I would like to thank Dr. Xinran Liu for help with electron microscopy, Dr. Lunbin Deng for insightful discussions, Dr. Thomas C. Südhof for support and Dr. Yea Jin Kaeser-Woo for comments on the manuscript. I apologize to colleagues whose work I could not cite due to space restrictions, especially to researchers who made many critical contributions to active zone functions in invertebrate synapses. My recent work is supported by NIH/NIDA (DA029044).
Addendum to: Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029.; and Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014.; and Deng L, Kaeser PS, Xu W, Sudhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005.
References
- 1.Sabatini BL, Regehr WG. Timing of synaptic transmission. Ann Rev Physiol. 1999;61:521–542. doi: 10.1146/annurev.physiol.61.1.521. [DOI] [PubMed] [Google Scholar]
- 2.Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron. 2007;55:11–24. doi: 10.1016/j.neuron.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Couteaux R, Pecot-Dechavassine M. [Synaptic vesicles and pouches at the level of “active zones” of the neuromuscular junction] Comptes rendus hebdomadaires des seances de l'Academie des sciences. 1970;271:2346–2349. (Fre). [PubMed] [Google Scholar]
- 6.Akert K, Moor H, Pfenninger K. Synaptic fine structure. Adv Cytopharmacol. 1971;1:273–290. [PubMed] [Google Scholar]
- 7.Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Sudhof TC. Genomic definition of RIM proteins: evolutionary amplification of a family of synaptic regulatory proteins (small star, filled) Genomics. 2003;81:126–137. doi: 10.1016/s0888-7543(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 10.Kaeser PS, Kwon HB, Chiu CQ, Deng L, Castillo PE, Sudhof TC. RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions. J Neurosci. 2008;28:13435–13447. doi: 10.1523/JNEUROSCI.3235-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, et al. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Sugita S, Sudhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem. 2000;275:20033–20044. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- 13.Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, et al. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 14.Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 15.Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42:889–896. doi: 10.1016/j.neuron.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng L, Kaeser PS, Xu W, Sudhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca(2+) channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ, Lesage F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca(2+) channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Machius M, Dulubova I, Dai H, Sudhof TC, Tomchick DR, et al. Structural basis for a Munc13-1 homodimer to Munc13-1/RIM heterodimer switch. PLoS Biol. 2006;4:192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pernia-Andrade A, Jonas P. The multiple faces of RIM. Neuron. 2011;69:185–187. doi: 10.1016/j.neuron.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, et al. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, et al. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 24.McNew JA, Weber T, Parlati F, Johnston RJ, Melia TJ, Sollner TH, et al. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J Cell Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, et al. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat Neurosci. 2007;10:691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uriu Y, Kiyonaka S, Miki T, Yagi M, Akiyama S, Mori E, et al. Rab3-interacting molecule gamma isoforms lacking the Rab3-binding domain induce long lasting currents but block neurotransmitter vesicle anchoring in voltage-dependent P/Q-type Ca2+ channels. J Biol Chem. 2010;285:21750–21767. doi: 10.1074/jbc.M110.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, et al. Redundant functions of RIM1alpha and RIM2alpha in Ca(2+)-triggered neurotransmitter release. EMBO J. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenmund C, Sigler A, Augustin I, Reim K, Brose N, Rhee JS. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron. 2002;33:411–424. doi: 10.1016/s0896-6273(02)00568-8. [DOI] [PubMed] [Google Scholar]
- 29.Kaeser PS, Deng L, Chavez AE, Liu X, Castillo PE, Sudhof TC. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 2009;64:227–239. doi: 10.1016/j.neuron.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank T, Rutherford MA, Strenzke N, Neef A, Pangrsic T, Khimich D, et al. Bassoon and the synaptic ribbon organize Ca(2+) channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallermann S, Fejtova A, Schmidt H, Weyhersmuller A, Silver RA, Gundelfinger ED, et al. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68:710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fourcaudot E, Gambino F, Humeau Y, Casassus G, Shaban H, Poulain B, et al. cAMP/PKA signaling and RIM1alpha mediate presynaptic LTP in the lateral amygdala. Proc Natl Acad Sci USA. 2008;105:15130–15135. doi: 10.1073/pnas.0806938105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, et al. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blundell J, Kaeser PS, Sudhof TC, Powell CM. RIM1alpha and interacting proteins involved in presynaptic plasticity mediate prepulse inhibition and additional behaviors linked to schizophrenia. J Neurosci. 30:5326–5333. doi: 10.1523/JNEUROSCI.0328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 37.Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, et al. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 38.Siksou L, Varoqueaux F, Pascual O, Triller A, Brose N, Marty S. A common molecular basis for membrane docking and functional priming of synaptic vesicles. Eur J Neurosci. 2009;30:49–56. doi: 10.1111/j.1460-9568.2009.06811.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Liu X, Biederer T, Sudhof TC. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc Natl Acad Sci USA. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, et al. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. J Cell Biol. 2002;158:577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, et al. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee K, Yang X, Gerber SH, Kwon HB, Ho A, Castillo PE, et al. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc Natl Acad Sci USA. 2010;107:6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko J, Na M, Kim S, Lee JR, Kim E. Interaction of the ERC family of RIM-binding proteins with the liprin-alpha family of multidomain proteins. J Biol Chem. 2003;278:42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- 44.Serra-Pages C, Medley QG, Tang M, Hart A, Streuli M. Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J Biol Chem. 1998;273:15611–15620. doi: 10.1074/jbc.273.25.15611. [DOI] [PubMed] [Google Scholar]