Abstract
Background
Local tolerance of topical glaucoma treatment is important to achieve a good compliance. The aim of this study was to evaluate the consequences of local anti-glaucoma drug side effects on the vision-related quality of life (QoL).
Methods
A mail survey was sent to 20,000 homes of a panel representative of the French population asking whether one member of the family was suffering from ocular hypertension (OHT) or glaucoma. (POAG) A computer-assisted telephone interviewing system was used to confirm self-reported glaucoma, to describe the disease and its treatment, and medical item consumption. Vision-related QoL was assessed with the NEI-VFQ-25 (National Eye Institute Visual – Function Questionnaire) and local tolerance with the COMTOL (Comparison of Ophthalmic Medications for TOLerability).
Results
13,352 homes (66.7%) answered the mail. 581 people declared they were suffering from glaucoma or OHT. Prevalence was 1.8%, increasing with age. 204 patients were selected at random Their NEI-VFQ-25 global score showed an overall good QoL. Two domain scores showed some deterioration: general health and driving. 62.4% of the patients cited at least one local side effect. 25.4% had burning, 20.8% blurred vision and 20.2% tearing. Poor vision related QoL was associated with the presence of local side effects leading to poor perceived treatment satisfaction. Dissatisfied patients visited their ophthalmologist more frequently.
Conclusion
Based on a representative French sample, poor vision related QoL was associated with topical drug side effects that also impact patient satisfaction and compliance. Longitudinal data collection should be performed to confirm our findings.
Keywords: Glaucoma, topical treatment, side effects, quality of life
Background
Primary open-angle Glaucoma (POAG) is characterised by a progressive alteration of the optic nerve. Glaucoma is one of the three leading causes of blindness in developed countries along with cataract and age-related macular degeneration [1]. Prevalence varies between 1% and 4% in people over 40 and they increase with age [2-12]. Risk factor analyses found a higher intra-ocular pressure (IOP) [13-18] although all patients with ocular hypertension (OHT) will not develop POAG [19].
Control of IOP is one of the critical items of glaucoma prevention in the >40 old population. Several medical strategies have been developed to reach this objective including drugs, laser and surgery. Choice of glaucoma therapy involves patient characteristics, clinical history, and co-morbidity [20,21] but also the likely effectiveness of an agent, the side effect profile [22], comfort upon administration, and the daily frequency of administration.
Topical treatment aimed at decreasing IOP is for the whole life of patients [23,24]. Compliance is of major importance [25] to get the full potential protective effect against visual field defect. Therefore, tolerance of topical treatment is a critical issue.
Odberg [23,24] demonstrated that the QoL of patients was affected at the time of the diagnosis announce, although no strong relationship was found between visual field disability and vision related QoL. This correlation was also confirmed in the Collaborative Initial Glaucoma Treatment [25,26] and other studies [27-31]. Most of QoL evaluation in glaucoma was measured in clinical trial setting [32-38] showing small differences between the alternatives that reached very rarely the statistical significance.
The link between QoL and patient-reported side effect of topical treatment has been very rarely studied. Wandell [39] found that beta-blockers with or without miotic agent had no negative impact on health related quality of life using a generic quality of life instrument. Barber [40,41] developed a dedicated instrument (The COMTOL: Comparison of ophthalmic medications for tolerability) aimed at measuring the QoL consequences of topical adverse events.
The aim of this study was to evaluate the relationship between vision-related quality of life and local side effects on a representative French sample of glaucoma patients treated with a topical anti-glaucoma drug who were contacted outside of any medical environment.
Methods
The survey was performed according to the French Law. The aim of the study was explained to the subjects before starting the questionnaire and they were asked whether they would accept to participate. The Sofres panel was expected to meet the objective of being representative of the French population and therefore by questioning on their medical treatment to get a sample representative of French patients treated for OHT / POAG. This panel is made up of 20,000 households (53,000 people) stratified by geographic area, city size, resident age, social and professional clusters and number of people within the household. Quotas were determined according to the results of the national census survey [42] with an average pick-up rate of 1/1000 done at two levels: 69 strata for the households and 114 strata for the individuals. Selected households were expected to stay in the panel for 4 years. Household replacements followed the same quota rules. Yearly renewal rate was 27%. For the last 10 years, the response rate with medical questionnaire used to be between 70 to 80% [43-46].
A mail survey was sent to all the households of the panel to identify those in which one resident was suffering from OHT or glaucoma (diagnosed by a practitioner). A Computer Assisted Telephone Interviewing (CATI) system was used to collect information on a random sample (patient Id generated by computer) of households having answered positively to the previous question. This system allowed remote data entry while querying and the performance of on-line data consistency checks.
With 200 patients we had 95% chance to capture at least one patient-reported side effects whose incidence was higher than 1.5%. Lastly, according to Mangione [47], alpha being fixed at 5% and beta at 20%, 69 patients per group were sufficient to show a difference of 10 points on the global score.
Socio-demographics and glaucoma history were recorded. The National Eye Institute Visual Function Questionnaire [48,49] was selected since its 51-item release demonstrated to be a reliable and valid tool for group-level comparisons of vision-targeted, health-related quality of life in clinical research. The short-form (NEI-VFQ-25) [50] was selected since its reliability and validity was found comparable to the 51-items. A French validated [51] of the NEI-VFQ-25 administered by phone interview was used. Anti-glaucoma treatments were identified based on the citation of a Rx brand name sold in France [52]: the list of all brand names available on the French market were cited to the patients. Since anti-glaucoma generics representing less than 2% of the market, the INN (International Non-Proprietary Name) list were not cited [53]. Special attention was given to the current treatment (name, dosage, combination, duration, etc...) but also to the previous treatment, if any, and reasons for change. The COM-TOL [40,41] was used to assess the local side effects of the current topical treatment. At the conclusion of the interview, global treatment satisfaction (a 6-level answer from very satisfied to very unsatisfied) was assessed. Compliance was assessed with the following question: "In the two last weeks, how many times have you missed your eye drops ? Possible answers were: never, rarely, some time, rather often, often, almost always, always missed. Other medical items included the number of visits to ophthalmologists, dedicated to glaucoma exams and workdays lost.
Analysis was performed using SAS Software (release 6.12) and SPAD (release 5.0). The analysis was conducted on the subset of patients treated with a topical anti-glaucoma treatment. NEI-VFQ-25 scores were calculated according to the instrument author's algorithm.
A multi-factorial correspondence analysis [54-56] was performed to study the relationship between vision-related quality of life, patient-reported side effects, compliance and treatment satisfaction. The Burt table was made of the 25 questions of NEI-VFQ-25. Patient-reported side effects, compliance and treatment satisfaction were considered as supplementary variable. Missing data were considered as a specific modality for each item. Chi-square was the used distance.
Comparisons between patients with and without side effects were performed using an analysis of variance. In case of variables that could confound the QoL results, adjustment was made using linear model according to the least square method. Confidence intervals were calculated, two-sided, at 95%. Since several statistical tests were performed when analysing the links between QoL and patient-reported side effects, a test was considered as statistically significant if p < 0.001 to keep the global type I error at 5%.
Results
In December 2000, the survey was sent to 20.000 homes belonging to the Sofres panel. 13,352 homes answered the mail (66.7%) corresponding to 32,685 people. 581 patients indicated they had a glaucoma or an OHT diagnosed by a practitioner, 564 being older than 40. This leads to a glaucoma / OHT prevalence of 1.8%. This rate increased with age from 1.1% (40 to 49 years old) to 8.4% (>80 years old). No statistical difference was found between geographical areas of France (from 1.4% to 2.3%).
In February 2001, a sample of 204 people was picked up at random (random patient Id was generated by computer) and queried by phone. The questionnaire required 20 to 30 minutes to be completed. 31 patients were not queried since they declared they did not take a topical treatment the day of the interview. 173 patients indicated they were taking at least one topical treatment at the time of the interview. 48.5% were males and the mean age was 70 years. 20.2% lived in the countryside, 28.3% in cities over 100.000 inhabitants and 16.8% in the Paris area. Median monthly household revenue was around EUR 2000 and 15% had a paid professional activity.
Glaucoma / OHT was diagnosed 9.4 years before the interview on average. Mean current topical treatment duration was 5.9 years and 47% had already changed their treatment at least once, mainly due to lack of efficacy (54.9%) or intolerance (35.2%). 74.6% of the patients declared that they never forgot their dose. A topical combination was prescribed for 28.9%. 69.4% of the patients had a beta-blocker, 5.2% a miotic, 6.4% an alpha2 agonist, 20.2% a carbonic anhydrase inhibitor, 19.7% a prostaglandin, and 10.4% a treatment from another class. 89.9% of the patients declared they were satisfied or very satisfied with the treatment. 92.4% of the patients declared they were "satisfied" or "very satisfied" with the way their disease was managed. The average number of visits to the ophthalmologist to care for a glaucoma / OHT patient was 2.35 per year.
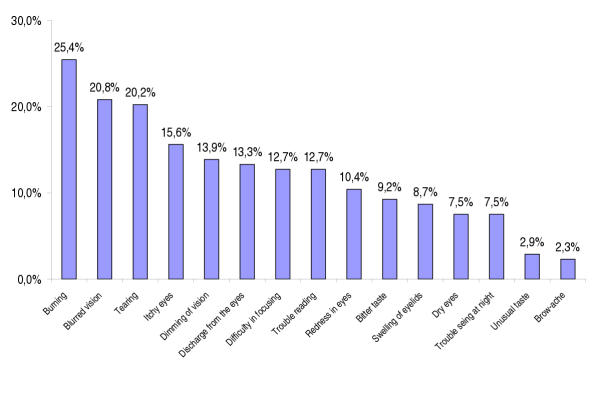
Figure 1 illustrates the prevalence of each individual side effect as reported by the COM-TOL. 37.6% had no side effects, 24.9% had one, 11.6% had 2, 7.0% had 3, and 18.9% had 4 and more. Three side effects were reported by more than 20% of the patients: burning (25.4%), blurred vision (20.8%) and tearing (20.2%) were mentioned most often. Patients with and without patient-reported side effects were found comparable on all socio-demographics and glaucoma history parameters with the exception of gender: females declared more patient-reported side effects (70.2% versus 55.1%; P < 0.04).
Figure 1.
Side effect prevalence according to the COM-TOL checklist.
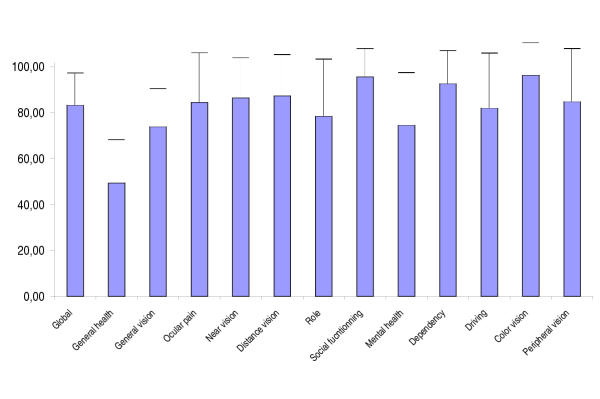
Figure 2 shows the average (+/- standard deviation) scores on each domain of the NEI-VFQ-25. All scores were higher than 50 except the general health score. All vision related scores were higher than 70 and the general vision score was the lowest of the vision-related scores. Some scores were found statistically higher in men: global, vision, near vision, distance vision, mental, role and driving.
Figure 2.
NEI-VFQ-25 average score (standard deviation). 0: worst quality of life; 100 highest quality of life.
Table 1 (see Additional file: 1) shows QoL scores difference (with 95% confidence limit) between patients with and without an patient-reported side effect (adjusted for gender).
Before adjustment for test multiplicity, four dimensions of the NEI-VFQ-25 were strongly associated with side effects: Ocular pain (11 events), Mental health (10 events), Global (10 events) and Dependency (8 events). Six side effects were associated with more than half of the 13 dimensions of NEI-VFQ-25: Trouble seeing at night (11 scores), Dimming of vision (10), Dry eyes (9), Reading trouble (8), Burning / stinging in the eyes (7) and Difficulty in focusing from near to far (7). The decrease in quality of life varied from 5.6 (tearing) to 31.2% (troubles seeing at night side effect on driving score).
After adjustment for test multiplicity, the global score was affected by Burning, Dimming of vision, Reading and Trouble seeing at night. Ocular pain was associated with Burning, Itchy eyes and Redness. Near vision was associated with Dimming of vision, Mental health with Burning, Dimming of vision, Focusing and Troubles seeing at night. Lastly, Dependency and Driving were affected by Troubles seeing at night
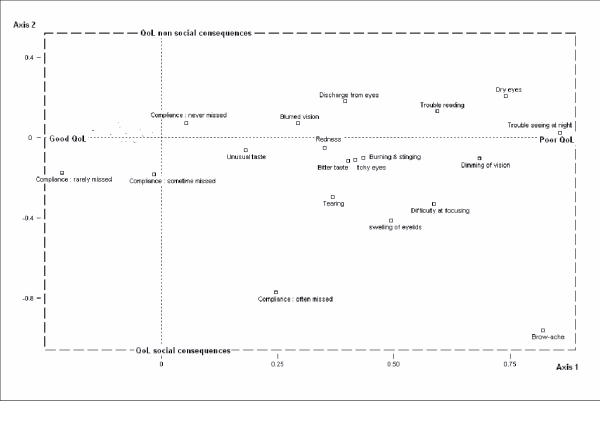
Figure 3 describes the results of the multiple factorial correspondence analysis. Only the graph with axes 1 and 2 was presented since it supports the biggest part of the information. Missing values did not contribute to the QoL information. Axis one involved vision-related QoL: the more positive the value, the greater the negative consequences on vision related quality of life. Axis 2 involved QoL social consequences: the lower the number the greater the QoL social consequences.
Figure 3.
Multiple Factorial Correspondence Analysis. Distance used: Chi-square. Axis 1 (QOL global score) by Axis 2 (Social Consequences). Active variables (the one that determined the structure and the identification of the axes) were the 25 items of the NEI-VFQ-25; they were not presented in this graph to improve readability. Illustrative variables (the one that allowed to identify patient sub-groups) consisted in side effect list of COM-TOL, compliance. Axis 1 estimated the intensity of QoL a global unique score: negative values were associated with 'Good QoL' while positive values were associated with 'Poor QoL'. Negative values on Axis 2 were associated with 'Bad social QoL'. Points, which were close, were highly correlated while points, which were far, were independent.
Brow-ache, Dimming of vision, Trouble seeing at night, Dry eyes, Dimming of vision, Trouble reading and Difficulty at focusing were the side effects that decreased the most on vision related QoL while unusual taste was the item having the least consequence. Brow-ache was the item perceived as having consequences on Social QoL in opposition to dry eye. It appeared that there was a strong relationship between QoL and treatment satisfaction: "rather unsatisfied" patients and "unsatisfied" patients had worse vision related QoL than the "satisfied" patients. Patients having bad QoL (axis 1) and social consequences (axis 2) were those who were the least compliant and unsatisfied towards their treatment.
Patient satisfaction was linked to number of visits to the ophthalmologist (P < 0.01): patients who were "rather satisfied" or more visited an ophthalmologist 2.27 per year against 3.50 for the unsatisfied patients.
Discussion
The use of the Sofres panel allowed us to reach one of our major objectives: to get a representative sample of patients being treated with a glaucoma drug. We had the expected response rate (66.7% is close to the usual 70% observed with our panel) to the mail sent to the households. The socio-demographics characteristics of responders were not different from the non-responders. Moreover, these characteristics were close to the nation-wide INSEE (Institut National de la Statistique et des Etudes Economiques) survey [42], suggesting no major selection bias. The way we captured patients was based on the diagnosis made by a practitioner, which is the most obvious information for a patient treated for a chronic condition. The prescription frequencies of anti-glaucomatous drugs estimated in our study were close to those observed in IMS (International Medical Statistics) data [53] and prevalence figures were close to the literature [2-12]. In no way does this study allow one to conclude that French patients were correctly treated on a national basis but resources dedicated to glaucoma treatment / prevention are in accordance to international literature prevalence estimates.
This was a cross-sectional survey and this study was limited to the link between patient-reported side effects and vision-related quality of life. Longitudinal data collection should be performed to confirm the causality of the relationship.
The use of patient phone interview was chosen in order to avoid medical interactions and to collect information as perceived by the patients. Although we did not cross check the collected information with medical records, it appeared that regardless of treatment duration, two-thirds of the patients had side effects. Of course, systematic queries might lead to overestimating side effect rates resulting in an observational bias. In any case, according to this survey, these side effects were associated with lower patient vision related QoL. Being in a non-medical environment, we were not able to collect hard medical information. Therefore, we were not able to confirm the disease (POAG versus OHT) and QoL could not be adjusted for co-morbidity (for example cataracts, macular degeneration). Neither we had a confirmation of medical therapy or compliance. However, we found no differences between patients with and without patient-reported side effects on all the collected variables with the exception of gender. Of course, we were not able to check the group comparability on the medical parameters and therefore we were not able to adjust for any potential bias Therefore, we decided to adjust our QoL estimates on gender. Moreover, since we focused mainly on local side effects and vision related QoL, the effect of co-morbidity on vision-specific QoL should be less than if QoL data were collected with a generic instrument. Lastly, the use of a vision-specific QoL instrument, which should be more sensitive than a generic one, allowed us to find some relationships with side effects, somewhat in contradiction with Wandell [39].
Some association was found between patient satisfaction and quality of life. This could, at least partially, be explained by factors we were not able to collect. Moreover, the reasons of the visits to the ophthalmologist were not documented and we don't know whether it was associated with poor medication control or vision function loss, etc ...
We did not calculate the QoL score from the COMTOL since, as a very glaucoma-specific QoL instrument (i.e. dedicated to side effects in glaucoma / OHT treatment), it does not allow to estimate the usual dimensions of QoL, like mental, social, general health, etc.. This is why we used the COMTOL only to list local side effects and the NEI-VFQ-25 to get their effects on QoL.
Trouble seeing at night, Dimming of vision, Trouble reading and Difficulty at focusing from near to far were consistently associated with poor vision related QoL. The differences observed between patients with and without these patient-reported side effects were comparable to the ones described by Mangione between patients with glaucoma and control case [49]. Drawing a conclusion from Brow-ache and Dry eye is difficult due to the too few patients having experienced these events.
The quality of life of patients was globally high (>70) on most of the dimensions, excepted general health. This might reflect the QoL of an old population living in the community with only few of them presenting severe visual field defect, although the absence of a control group in our survey does not allow a direct measure of the medical significance of our findings. Therefore, avoiding topical drug side effects should be a major concern to maintain this high QoL level. However, our general health domain score is lower than the one in Mangione [48,49]. In the latter, QoL data were collected in the front of an ophthalmologist, leading to some observational bias. Our population was older. Lastly, their sample was not taken at random. Unfortunately, in our study, declarative co-morbidity and concomitant medications were not collected since we did not want the phone interview duration to exceed 30 minutes per subject.
Questioning patient on compliance during a visit to an ophthalmologist is often a very difficult issue, since there is no objective way to measure it. We found 4 patient-reported side effects that were associated with poor vision-related QoL: trouble seeing at night, trouble reading, dimming of vision and difficulty at focusing. We also found an association between compliance (patients often missed their drop) and poor quality of life. Our findings suggest that patients presenting with one of these 4 patient-reported side effects should be queried into details in order to capture lack of compliance and, if requested, to switch to a drug which is more tolerable.
Conclusion
In our survey, vision related QoL of French patients with topical anti-glaucomatous drug side effects has been found lower, with poor treatment satisfaction and additional visits to the ophthalmologist. Further longitudinal data collection should be collected to confirm our findings.
Authors' contributions
JPN was in charge of the medical expertise and contributed to the writing of the paper.
NA and SR participated in the design of the study and carried out data collection.
GB conceived the study and participated in its design and co-ordination.
All authors read and approved the final manuscript.
Supplementary Material
Acknowledgments
Acknowledgement
The survey was supported by an unrestricted grant from Alcon Laboratories SA, Rueil-Malmaison, France, was conducted according to the local laws, and was contracted to Taylor Nelson Sofrès Santé, Montrouge, France.
Contributor Information
Jean-Philippe Nordmann, Email: j.p.nordmann@quinze-vingts.fr.
Nadia Auzanneau, Email: nadia.auzanneau@tnsofres.com.
Séverine Ricard, Email: severine.ricard@tnsofres.com.
Gilles Berdeaux, Email: gilles.berdeaux@alconlabs.com.
References
- Goldstein H. Magnitude and causes of blindness : Sources and limitations of data. In: Freemann JS, editor. In clinical ophthalmology. Vol. 5. Lippincott Company, Philadelphia, The USA; 1990. pp. 1–10. [Google Scholar]
- Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains eye study. Ophthalmology. 1996;103:1661–9. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Sponsel WE, Franke T, Cantor LB, Martone J, Menage MJ. Prevalence of glaucoma. The Beaver Dam Eye study, Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- Ekstrom C. Prevalence of open-angle glaucoma in central Sweden. The Tierp glaucoma survey. Acta Ophthalmologica Scandinavica. 1996;74:107–12. doi: 10.1111/j.1600-0420.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Bengtsonn B. The prevalence of glaucoma. British Journal ofOphthalmology. 1981;65:46–9. doi: 10.1136/bjo.65.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn HA, Leibowitz HM, Ganley JP, Kini MM, Colton T, Nickerson RS, Dawber TR. The Framingham eye study. I. Outline and major prevalence findings. II. Associations of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. American Journal of Epidemiology. 1977;106:33–41. doi: 10.1093/oxfordjournals.aje.a112429. [DOI] [PubMed] [Google Scholar]
- Kahn HA, Milton RC. Alternative definitions of open angle glaucoma. Effects on prevalence and associations in the Framingham Eye Study. Archives of Ophthalmology. 1980;98:2172–7. doi: 10.1001/archopht.1980.01020041024003. [DOI] [PubMed] [Google Scholar]
- Kahn HA, Milton RC. Revised Framingham eye study prevalence of glaucoma and diabetic retinopathy. American Journal of Epidemiology. 1980;111:769–76. doi: 10.1093/oxfordjournals.aje.a112955. [DOI] [PubMed] [Google Scholar]
- Martinez GS, Campbell AJ, Reinken J, Allan BC. Prevalence of ocular disease in population 65 years old and older. American Journal of Ophthalmology. 1982;94:181–189. doi: 10.1016/0002-9394(82)90073-3. [DOI] [PubMed] [Google Scholar]
- Podgor MJ, Leske MC, Ederer F. Incidence estimates for lens changes, macular changes, open-angle glaucoma and diabetic retinopathy. American Journal of Epidemiology. 1983;118:206–212. doi: 10.1093/oxfordjournals.aje.a113628. [DOI] [PubMed] [Google Scholar]
- Tuck MW, Crick RP. The age distribution of primary open angle glaucoma. Ophthalmic Epidemiology. 1998;5:173–183. doi: 10.1076/opep.5.4.173.4192. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Investigative Ophthalmology and Visual Science. 1997;38:83–91. [PubMed] [Google Scholar]
- Armaly MF. Ocular pressure and visual fields : A ten-year follow-up study. Arch Ophthalmol. 1969;81:25–44. doi: 10.1001/archopht.1969.00990010027005. [DOI] [PubMed] [Google Scholar]
- Rota-Bartelink AM, Pitt A, Story I. Influence of diurnal variation on the intraocular pressure measurement of treated primary open-angle glaucoma during office hours. J Glaucoma. 1996;5:410–5. [PubMed] [Google Scholar]
- Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–42. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Gonzalez I, Pablo LE, Pueyo M, Ferrer E, Melcon B, Abecia E, Honrubia FM. Assessment of diurnal tensional curve in early glaucoma damage. Int Ophthalmol. 1997;20:113–5. doi: 10.1007/BF00212956. [DOI] [PubMed] [Google Scholar]
- Zeimer RC, Wilensky JT, Gieser DK, Viana MAG. Association between intra-ocular pressure peaks and progression of visual field loss. Ophthalmology. 1991;98:64–69. doi: 10.1016/s0161-6420(91)32340-6. [DOI] [PubMed] [Google Scholar]
- The AGIS investigators The advanced glaucoma intervention study (AGIS) : 7. The relationship between control of intra-ocular pressure and visual field deterioration. American Journal of Ophthalmology. 2000;130:429–440. doi: 10.1016/S0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Horie T, Aoki S. Untreated ocular hypertension. A long-term prospective study. Arch Ophthalmol. 1977;95:1180–1189. doi: 10.1001/archopht.1977.04450070078004. [DOI] [PubMed] [Google Scholar]
- Anderson DR. The management of elevated intraocular pressure with normal optic discs and visual fields. Surv Ophthalmol. 1977;21:479–89. doi: 10.1016/s0039-6257(77)80004-0. [DOI] [PubMed] [Google Scholar]
- Fingeret M. Glaucoma medications, glaucoma therapy, and the evolving paradigm. J Am Optom Assoc. 1998;69:115–21. [PubMed] [Google Scholar]
- Reyes E, Izquierdo NJ, Blasini M. Adverse drugs reactions associated with glaucoma medications. Bol Asoc Med P R. 1997;89:51–5. [PubMed] [Google Scholar]
- Odberg T, Jakobsen JE, Hultgren SJ, Halseide R. The impact of glaucoma on the quality of life of patients in Norway. II. Patient response correlated to objective data. Acta Ophthalmologica Scandinavica. 2001;79:121–4. doi: 10.1034/j.1600-0420.2001.079002121.x. [DOI] [PubMed] [Google Scholar]
- Odberg T, Jakobsen JE, Hultgren SJ, Halseide R. The impact of glaucoma on the quality of life of patients in Norway. I. Results from a self-administered questionnaire. Acta Ophthalmologica Scandinavica. 2001;79:116–20. doi: 10.1034/j.1600-0420.2001.079002116.x. [DOI] [PubMed] [Google Scholar]
- Mills RP, Janz NK, Wren PA, Guire KE. Correlation of visual field with quality-of-life measures at diagnosis in the Collaborative Initial Glaucoma Treatment Study (CIGTS) Journal of Glaucoma. 2001;10:192–198. doi: 10.1097/00061198-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE. Quality of life in newly diagnosed glaucoma patients : The collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2001;108:887–97. doi: 10.1016/S0161-6420(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Sherwood MB, Garcia-Siekavizza A, Meltzer MI, Hebert A, Burns AF, McGorray S. Glaucoma's impact on quality of life and its relation to clinical indicators. A pilot study. Ophthalmology. 1998;105:561–6. doi: 10.1016/S0161-6420(98)93043-3. [DOI] [PubMed] [Google Scholar]
- Mills RP. Correlation of quality of life with clinical symptoms and signs at the time of glaucoma diagnosis. Transactions of the American Ophthalmological Society. 1998;96:753–812. [PMC free article] [PubMed] [Google Scholar]
- Parrish RK, Gedde SJ, Scott IU, Feuer WJ, Schiffman JC, Mangione CM, Montenegro-Piniella A. Visual function and quality of life among patients with glaucoma. Archives of Ophthalmology. 1997;115:1447–55. doi: 10.1001/archopht.1997.01100160617016. [DOI] [PubMed] [Google Scholar]
- Parrish RK. Visual impairment, visual functioning, and quality of life assessments in patients with glaucoma. Transactions of the American Ophthalmological Society. 1996;94:919–1028. [PMC free article] [PubMed] [Google Scholar]
- Wilson MR, Coleman AL, Yu F, Bing EG, Sasaki IF, Berlin K, Win J, Lai A. Functional status and well being in patients with glaucoma as measured by the Medical Outcomes Study Short Form-36 questionnaire. Ophthalmology. 1998;105:2112–6. doi: 10.1016/S0161-6420(98)91135-6. [DOI] [PubMed] [Google Scholar]
- Marchetti A, Magar R, An P, Nichol M. Clinical and economic impact of new trends in glaucoma treatment. MedGenMed [serial online] 2001;3:6. [PubMed] [Google Scholar]
- Cantor LB, Hoop J, Katz LJ, Flartey K, Alphagan/Betaxol Clinical Study Group Comparison of the clinical success and quality-of-life impact of brimonidine 0.2% and betaxolol 0.25 % suspension in patients with elevated intraocular pressure. Clin Ther. 2001;23:1032–9. doi: 10.1016/S0149-2918(01)80089-8. [DOI] [PubMed] [Google Scholar]
- Javitt JC, Schiffman RM. Clinical success and quality of life with brimonidine 0.2% or timolol 0.5% used twice daily in glaucoma or ocular hypertension: a randomized clinical trial. Brimonidine Outcomes Study Group I. Journal of Glaucoma. 2000;9:224–34. doi: 10.1097/00061198-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Javitt J, Goldberg I. Comparison of the clinical success rates and quality of life effects of brimonidine tartrate 0.2% and betaxolol 0.25% suspension in patients with open-angle glaucoma and ocular hypertension. Brimonidine Outcomes Study Group II. Journal of Glaucoma. 2000;9:398–408. doi: 10.1097/00061198-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Lee DA, Gornbein JA. Effectiveness and safety of brimonidine as adjunctive therapy for patients with elevated intraocular pressure in a large, open-label community trial. Journal of Glaucoma. 2001;10:220–6. doi: 10.1097/00061198-200106000-00014. [DOI] [PubMed] [Google Scholar]
- Strohmaier K, Snyder E, Adamsons I. A multicenter study comparing dorzolamide and pilocarpine as adjunctive therapy to timolol: patient preference and impact on daily life. Journal of the American Optometric Association. 1998;69:441–51. [PubMed] [Google Scholar]
- Gutierrez P, Wilson MR, Johnson C, Gordon M, Cioffi GA, Ritch R, Sherwood M, Meng K, Mangione CM. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997;115:777–84. doi: 10.1001/archopht.1997.01100150779014. [DOI] [PubMed] [Google Scholar]
- Wandell PE, Lundstrom M, Brorsson B, Aberg H. Quality of life among patients with glaucoma in Sweden. Acta Ophthalmol Scand. 1997;75:584–8. doi: 10.1111/j.1600-0420.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Barber BL, Strahlman ER, Laibovitz R, Guess HA, Reines SA. Validation of a questionnaire for comparing the tolerability of ophthalmic medications. Ophthalmology. 1997;104:334–42. doi: 10.1016/s0161-6420(97)30314-5. [DOI] [PubMed] [Google Scholar]
- Barber BL, Santanello NC. Relating spontaneous adverse experience reports to scores on a questionnaire querying tolerability. International Journal of Clinical Pharmacology and Therapeutics. 1995;33:598–604. [PubMed] [Google Scholar]
- INSEE. 2000. http://www.insee.fr/
- Rouillon F, Blachier C, Dreyfus JP, Bouhassira M, Allicar MP. Etude pharmaco-épidémiologique de la consommation des antidépresseurs en population générale. L'Encéphale. 1996;Sp I.:39–48. [PubMed] [Google Scholar]
- Frexinos J, Denis P, Allemand H, Allouche S, Los F, Bonnelye G. Etude descriptive des symptômes fonctionnels digestifs dans la population générale française. Gastroenterol Clin Biol. 1998;22:785–91. [PubMed] [Google Scholar]
- Antona D, Guérin N. Couverture vaccinale rougeole – rubéole – oreillons en France en 1998 : première et deuxième doses. BEH. 1999;19:74–5. [Google Scholar]
- Le Pen C, Levy E. Socio-économie de la maladie veineuse : les enseignements d'une enquête sur les consommateurs de médicaments phlébotoniques en France. Phlébologie. 1993;46:47–63. [PubMed] [Google Scholar]
- Mangione CM. The National Eye Institute 25-item Visual Function, Questionnaire (VFQ-25) – Scoring algorithm. 2000. pp. 1–15.
- Mangione CM, Berry S, Spritzer K, Kanz NK, Klein R, Owsley C, LPP Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Archives of Ophthalmology. 1998;116:227–33. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Archives of Ophthalmology. 1998;116:1496–504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RI, National Eye Institute Visual Function Questionnaire Field Test Investigators Development of the 25-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- Nordmann JP, Sullivan K, Viala M, Arnoult B, Berdeaux G. Psychometric validation of the NEI-VFQ 25 French version in a population of patients treated for ocular hypertension and glaucoma. PharmacoEconomics. [DOI] [PubMed]
- Vidal. Ed du Vidal, Paris. 2001.
- International Medical Statistics, Paris. 2001.
- Guttman L. The qualification of a class of attributes: a theory and method of a scale construction. In: Horst P, editor. The Prediction of Personal Adjustment. New York: SSCR; 1941. pp. 251–264. [Google Scholar]
- Burt C. The factorial analysis of qualitative data. British Journal of Statistical Psychology. 1950;3:166–185. [Google Scholar]
- Hayashi C. Theory and examples of quantification (II) Proceedings of the Institute of Statistics and Mathematics. 1956;4:19–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.