Abstract
Background:
Despite advances in traumatic wound care and management, infections remain a leading cause of mortality, morbidity and economic disruption in millions of wound patients around the world. Animal models have become standard tools for studying a wide array of external traumatic wound infections and testing new antimicrobial strategies.
Results:
Animal models of external traumatic wound infections reported by different investigators vary in animal species used, microorganism strains, the number of microorganisms applied, the size of the wounds and for burn infections, the length of time the heated object or liquid is in contact with the skin.
Methods:
This review covers experimental infections in animal models of surgical wounds, skin abrasions, burns, lacerations, excisional wounds and open fractures.
Conclusions:
As antibiotic resistance continues to increase, more new antimicrobial approaches are urgently needed. These should be tested using standard protocols for infections in external traumatic wounds in animal models.
Key words: animal models, wounds, burns, infection, bacteria, fungi
Introduction
Despite advances in trauma care and management, infections remain a leading cause of mortality, morbidity and economic disruption in millions of wound patients around the world.1–7 Challenges remain in countering microorganisms even where antimicrobials are available. Much remains to be learned about basic aspects of the host-pathogen relationship and the complexity of the immune response to infection. In order to develop new therapies, we need to have a detailed understanding of the events that are triggered in a host after infection.
Animal models have become standard tools for the study of a wide array of external traumatic wound infections.8 However, a comprehensive review of the animal models of external traumatic wound infections has never been conducted. In this review, we will discuss the animal models of infected external traumatic wounds including surgical wounds, skin abrasions, burns, lacerations, excisional wounds and open fractures.
Animal Models of Surgical Site Infections
Surgical site infections (SSIs) are considered to be “low-inoculum” infections.9 These infections develop in apparently sterile operative environments when low numbers of contaminating microorganisms interact with devitalized tissues and foreign material (e.g., hematomas, sutures, prosthetic devices) in such a way as to remain viable in the face of antimicrobials and host defenses. SSIs account for 22% of healthcare-acquired infections (HAIs) and are a leading cause of patient mortality and morbidity as well as increased health cost.5 The ideal animal model of a SSI would include the following features: low inocula of pathogenic microorganisms that predictably induce infection, the infecting process is amenable to modification by use of prophylactic antimicrobials, and the end point meets the clinical definition of infection (i.e., the presence of purulent material yielding viable bacteria).9
Superficial incision—foreign body model.
McRipley and Whitney10 developed an SSI model by creating incisions on the backs of mice followed by the inoculation of microorganisms to the incisions. Female CF-1 mice weighing 18–20 g were used. On the day of infection superficial surgical wounds were produced on the shaved backs of anesthetized mice by making a longitudinal midline incision, 2.3 ± 0.2 cm in length and extending down to the panniculus carnosus. The skin on either side of the incision was retracted, and the wound was infected by the insertion of a contaminated segment of cotton thread (suture) through the skin with a suturing needle in such a way that the suture lay diagonally across the panniculus, with the ends extending slightly from the skin. The wound eventually covered approximately 6% of the total body surface area (TBSA)11 of a mouse. Results indicated that 10 Staphylococcus aureus cells/suture were insufficient to induce an infection, whereas an inoculum of 103 or 105 cells could elicit an infection. When the wound had been infected with S. aureus or Pseudomonas aeruginosa, clinical signs of infection were present but were not striking. Suppuration was noted occasionally beneath the retracted skin. No deaths occurred in mice infected with S. aureus and infection persisted for 1 to 2 weeks; only a few deaths were recorded in mice infected with P. aeruginosa. P. aeruginosa was isolated from all internal organs cultured of mice that died, indicating generalized infection.
The model was used to study the efficacy of antimicrobials,10,12 virulence of bacteria in SSIs13 and real-time monitoring of bacterial infections in vivo using bioluminescent bacteria.14
Espersen et al.15 reported a mouse model of SSI using a catheter segment as the foreign body. Ten- to 12-week-old CF-1 female mice weighing approximately 25 g were operated under ether anesthesia. A 1 cm incision was made in the lateral abdominal wall by an aseptic technique. A catheter segment was placed in the peritoneal cavity, and the wound was closed with one suture. The sutures were removed from all mice before 18 h after the operation. The model was investigated with a range of different inocula from 2.1 × 103 to 2.5 × 109 colony-forming units (CFU). Approximately 1 h after operation, experimental infection was produced by intraperitoneal (I.P.) challenge in the lateral abdominal wall opposite to the operation wound with 1 mL of staphylococci suspension. It was reported that a challenge dose of greater than 1 × 105 CFU gave nearly 100% infection rates, while an inoculum of greater than 5 × 108 CFU resulted in mortality. The infection persisted as an intra-abdominal abscess surrounding the catheter for at least 30 d.
Another foreign-body mouse model (using male albino Webster-derived CD-1 mice) of SSI was introduced by Actor and Grappel16 by surgical implantation of a 6.35 mm filter paper disc saturated with 10% sterile carrageenan solution. The skin of the ventral abdomen was incised in the midline with the treated discs being implanted in the underlying subcutaneous tissue. Bacteroides fragilis and Escherichia coli were used to establish wound infections. The implantation of a carrageenan-saturated disc along with an appropriate inoculum of microorganism resulted in uniform infections that could be quantified. In some cases the organisms spread from the local site, and the infections progressed to lethality, especially in neutropenic mice treated with cyclophosphamide (CY).
Foreign body subcutaneous injection model.
Ford et al.17 reported the use of microcarriers as abscess promoters in the formation of S. aureus subcutaneous abscesses in mice. S. aureus mixed with dextran or gelatin microcarrier beads injected subcutaneously into CF-1 mice (20 g, male or female) resulted in the formation of reproducible, sustained abscesses with as few as 2 × 103 CFU. Without microcarrier beads, 4 × 107 CFU were required to produce an abscess. The abscesses that developed with microcarriers attained a diameter of up to 1.5 cm and persisted for several days before discharging through the skin. A similar mouse model was also reported by Bunce et al.18
Kaiser et al.9 developed a model of SSI by inoculating suspensions of S. aureus and dextran microbeads into intramuscular sites on the dorsum of Hartley guinea pigs. Hartley guinea pigs of either sex weighing 450–550 g were used. Three strains of S. aureus were evaluated with the inoculum ranging from 1 to 160 CFU. To minimize the overgrowth of pathogenic microorganisms within the gastrointestinal tract of the guinea pigs, gentamicin and polymyxin B were added to the drinking water of the guinea pigs 24 h before the day of bacterial inoculation. It was observed that the infection rate rose with increasing inoculum size. As inocula rose from 0.625 to 160 organisms, 0–100% of lesions yielded staphylococcal growth on subculture. Inspection and biopsy of the lesions 3–4 d after intermuscular inoculation of viable bacteria typically revealed an indurated, well-circumscribed mass of microbeads and inflammatory tissue ∼1 cm in diameter. These lesions usually adhered tightly to adjacent tissues and could not be removed without dissection.
Pocket wound model.
Yarboro et al.19 reported a rat model of SSI to mimic the situation encountered in orthopedic surgery. Female Sprague-Dawley retired breeder rats with weights ranging from 200 to 400 g were used. After isoflurane anesthesia, the lateral aspect of the right thigh was then shaved with electric clippers. The skin was opened with a 10 mm longitudinal incision in the lateral aspect of the thigh with use of scissors. A 2 × 2 cm pocket was formed, with blunt dissection, in the quadriceps muscle down to the femur. A 32-gauge stainless-steel suture was placed around the femur inside the wound to act as a surgical implant or foreign body to promote infection. Approximately 8.0 × 105 CFUs of gentamicin-sensitive S. aureus was pipetted into the pocket. High mortality was observed in the animals with six of nine rats dying. The model was used to evaluate the effect of prophylaxis against SSI with local or systemic antibiotics.19–21
Using a similar procedure, Bergamini et al.22 introduced a SSI model in male guinea pigs weighing approximately 750 g. Three incisions, 2 cm in length, were made on each side of the midline within the shaved area of each animal's back. The incisions were made down to but not through the deep fascia and extended laterally to create pouches approximately 2 × 2 cm square. No foreign body was applied in this model. The wounds were inoculated with 1.5 × 107 or 1 × 1010 CFU of S. aureus. The model was used to investigate the efficacy of combined topical and systemic antibiotic prophylaxis in experimental wound infection.22
Deep incision—foreign body model.
A deep SSI model using rats (male Sprague-Dawley, 450 to 550 g) was documented by Fallon et al.23 Skin preparation consisted of clipping the hair from the back followed by skin cleansing. A standard incision measuring 4 cm in length and 5 mm lateral and parallel to the vertebral column was carried through the skin. The incision was then continued to a depth of approximately 1 cm into the underlying paraspinous muscles. Sterile sand (100 mg) was introduced into each wound as an infection-potentiating foreign body and the wounds were inoculated with 100 µL of a methicillin-resistant S. aureus (MRSA) suspension containing approximately 5.0 × 107 CFU. The skin incisions were closed with surgical staples. Clinical evidence of wound infection, defined as the presence of an abscess or frank pus within the wound, was observed in 8 of 13 (62%) animals. The authors used this model to evaluate and compare the efficacy of topical versus systemic therapy with cefazolin for the treatment of S. aureus infections.
In a guinea pig model developed by Moesgaard et al.24 a 2 cm skin incision was made 1 cm to the right of the midline in the shoulder region, extending down to, but not through, the deep fascia. Two single sutures of 4/0 atraumatic dexon (polyglycolic acid) were placed in the muscular fascia. Contamination with E. coli plus B. fragilis was carried out with 0.1 mL of each of the bacterial suspensions spread evenly over the sides and the base of the wound with micro-syringes. Wound infection, defined as accumulation of pus draining spontaneously or after opening of the wound, developed in 19 out of 22 guinea pigs (86%) after intra-incisional contamination with 107 E. coli plus 108 B. fragilis before wound closure. A similar guinea pig model (with a 4 cm vertical incision injected with a 0.4 mL inoculum of S. aureus at a concentration of 103, 104 or 105 CFU/mL) was also described by Stratford et al.25 to investigate the effect of lidocaine and epinephrine on S. aureus infection.
Summary.
There are numerous studies on the use of animal models of SSIs. Species of animals used include mouse, rat and guinea pig. The approaches to inflict surgical wounds and induce infections include incisional wounds with foreign bodies, subcutaneous injection of foreign bodies and microorganisms into pocket wounds with or without foreign bodies. Foreign bodies used include sutures, paper discs, dextran microbeads, sand and catheter segments. Depth of wound ranges from superficial sites down to the panniculus carnosus (in mice) to deep sites (1–2 cm) into the muscles (in rats or guinea pigs). Surgical wounds were made on the back, abdominal, thigh or, less frequently, the shoulder region of the animals. S. aureus is the most commonly studied bacterial species in the animal models. Table 1 shows a summary of representative animal models of SSIs.
Table 1.
Representative animal models of surgical site infections (SSIs)
| Animal species | Microorganism and inoculum | Foreign body | Methods used to produce wounds and infections | End point | Refs |
| Female CF-1 mouse | S. aureus; P. aeruginosa 103 or 105 CFU | Segment of cotton thread (Suture) | A longitudinal midline incision, 2.3 ± 0.2 cm in length and extending down to the panniculus carnosus was made on the back. Each wound was infected by the insertion of a contaminated suture through the skin. |
No deaths occurred in mice infected with S. aureus, infection persisted for 1 to 2 weeks; a few deaths were recorded in mice infected with P. aeruginosa. | 10 |
| Female CF-1 mouse | S. aureus >1 × 105 CFU | Catheter segment | A 1 cm incision was made in the lateral abdominal wall. Infection was produced by I.P. challenge in the lateral abdominal wall opposite to the operation wound with S. aureus suspension. |
A challenge dose >1 × 105 CFU gave nearly 100% infection rates, while an inoculum >5 × 108 CFU resulted in mortality. The infection persisted as an intra-abdominal abscess surrounding the catheter for at least 30 days. | 15 |
| Male albino Webster-derived CD-1 mouse | B. fragilis (105–108 CFU); E. coli (105–108 CFU) | A 6.35 mm filter paper disc | Mice were made neutropenic by the I.P. challenge of cyclophosphamide. The skin of the ventral abdomen was incised in the midline. The infection was induced by the implantation of a carrageenan-saturated disc along with microorganism in the underlying subcutaneous tissue. |
In some cases the organisms spread from the local site, and the infections progressed to lethality, especially in neutropenic mice treated with cyclophosphamide. | 16 |
| Male or female CF-1 mouse | S. aureus 2 × 103 CFU | Dextran or gelatin beads | S. aureus mixed with dextran or gelatin beads were injected subcutaneously into mice. | The abscesses that developed with microcarriers attained a diameter of up to 1.5 cm and persisted for several days. | 17 |
| Guinea pig | S. aureus 160 CFU | Dextran microbeads | Inoculating suspensions of S. aureus and dextran microbeads into intramuscular sites on the dorsum of guinea pigs. | As inocula rose from 0.625 to 160 organisms, 0–100% of lesions yielded staphylococcal growth on subculture. | 9 |
| Female Sprague-Dawley rat | S. aureus 8 × 105 CFU | 32 gauge stainless steel (suture) | A 1 cm longitudinal incision was made in the lateral aspect of the thigh. A 2 × 2 cm pocket was formed in the quadriceps muscle down to the femur. S. aureus was pipetted into the pocket. | High mortality was observed in the animals with six of nine rats dying. | 19 |
| Female Sprague-Dawley rat | S. aureus 5 × 107 CFU | Sterile sand (100 mg) | A standard incision measuring 4 cm in length and 5 mm lateral and parallel to the vertebral column was carried through the skin. The incision was then continued to a depth of approximately 1 cm into the underlying paraspinous muscles. Sterile sand was introduced into each wound and the wound was inoculated with 100 µL of MRSA suspension. |
Clinical evidence of wound infection was observed in 8 of 13 (62%) animals. | 23 |
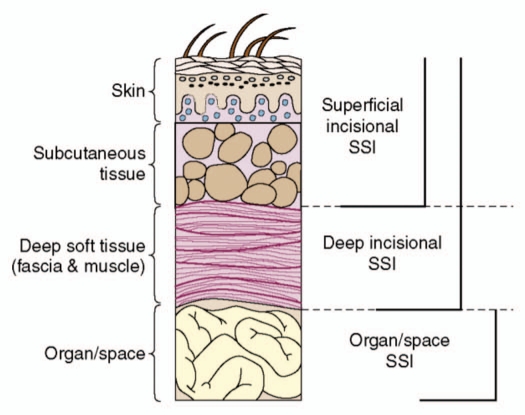
In accordance with the depth of the infection, SSIs are clinically classified into superficial incisional SSI, deep incisional SSI and organ/space SSI26 (Fig. 1). Accordingly, animal models of SSIs can also be classified into the models to mimic superficial incisional SSI,10,15–17 deep incisional SSI,9,23–25 and organ/space SSI,19,22 respectively. For superficial SSI, mouse is the commonly used species. For deep incisional SSI and organ/space SSI, larger animals (e.g., rats and guinea pigs) are usually used because the size of mice is insufficient for mimicking these lesions. According to the situation of whether mortality occurs or not, the animal models can be classified into lethal infection model and chronic infection model. For superficial wounds, lethal infections can be developed using lethal bacterial strain (e.g., P. aeruginosa)10 and/or by making the mice neutropenic.16 Chronic infection model can usually be made using less lethal bacterial strain (e.g., S. aureus).9,10,15,17 For deep wounds, S. aureus can also induce lethal infections.19 The severity of infection is microorganism inoculum-dependent.15 As SSIs develop in the sterile environment, the use of low inoculums of infecting microorganism would be relevant to clinical situations.9 The minimum inoculum to induce SSI varies significantly in different studies.9,10,15,17 This might be due to differences in bacterial strain/species, animal species, the size and depth of lesions, and also probably the different foreign bodies used.
Figure 1.
Surgical site infections (SSIs) are classified as superficial incisional SSI, deep incisional SSI and organ/space SSIs.26
Animal Models of Skin Abrasion Wound Infections
Skin abrasions (scrapes) are skin wounds that rub or tear off skin. Skin abrasions can occur on any part of the body but usually affect bony areas, such as the hands, forearms, elbows, knees or shins. Most skin abrasions are shallow and do not extend far into the skin. An abrasion is often contaminated with dirt, gravel and other foreign material. Infection is a risk, especially with crushed or dirty abrasions. Due to the feature of skin abrasion wound, skin abrasion infections are usually superficial infections localized in the epidermis and upper layer of dermis.
Needle scratch model.
Dai et al.27 developed a mouse model of skin abrasion infected with genetically engineered bioluminescent MRSA. Female BALB/c mice, 6–8-week-old and weighing 17–21 g, were used. At days 4 and 1 before the infection, mice were administered two doses of CY. The first dose, 150 mg CY per kg mouse body weight (150 mg/kg) was injected I.P. followed by the second dose of 100 mg/kg. This treatment reduced peripheral blood neutrophils to <100/mL blood, fostering a more vulnerable environment in the mice to infection. Skin abrasion wounds were made on the dorsal surfaces of mice using 28-gauge needles by creating 6 × 6 crossed scratch lines within a defined 1 × 1 cm2 area. The scratches were made in such a manner that they only damaged the stratum corneum and upper-layer of the epidermis but not the dermis. Five minutes after wounding, an aliquot of 50 µL suspension containing 108 CFU of bioluminescent MRSA was inoculated over each defined area containing the crossed scratches with a pipette tip. A stable infection was developed in the mouse wounds as characterized by bioluminescence imaging. In the non-treated wounds, the scratches were expanded during the following few days (Fig. 2A) and infections were spread over the area surrounding the scratches as indicated by bioluminescence imaging. Gram staining of the histological section of a representative skin abrasion specimen (harvested at day 3 post-infection) (Fig. 2B) showed that MRSA was localized superficially in the epidermis. The model was used to demonstrate the effectiveness of photodynamic therapy; the topical application of photosensitizers targeted to bacteria followed by illumination.
Figure 2.
(A) Wound morphology at day 4 post-infection of a representative mouse wound. (B) A Gram-stained section of a mouse skin abrasion specimen showing the biofilms formed by Gram-positive MRSA near the skin surface. Dark blue area: biofilms of MRSA. The mouse skin abrasion specimen was harvested at day 3 post-infection.
A similar model was presented by Zolfaghari et al.28 In this model using female C57 black mice of 8-week-old, 25 mm2 square-shaped wounds were created in the skin of the back by scarification using a 27-gauge needle, run ten times parallel in one direction and another ten times perpendicular to the original tracks. Ten microliters of the MRSA suspension was placed on each wound (4 × 107 CFU), and incubated for 1 h prior to treatment. This method also resulted in a reproducible MRSA wound colonization model, which persisted for up to 5 d post-inoculation.
Blade scrape model.
Kraft et al.29 reported a mouse model of superficial cutaneous bacterial infections resulting after a minor wounding process. Male CF-1 mice (24 to 28 g) were treated with 0 to 150 mg of CY per kg 4 days before infection. Mice were anesthetized before superficial wounding. One drop (0.02 mL) of the prepared culture suspension containing 0 to 106 CFU S. aureus or S. epidermidis was applied to the shaved skin, which was scraped with a no. 24 scalpel blade until a reddened area appeared (just short of drawing blood). The wound site (∼10 mm2) was occluded with a 15 mm2 piece of sterile plastic film secured with a 25 mm-wide girdle of surgical tape. At specified intervals after challenge, occlusive dressings were removed and wound sites were examined for white-gray abscesses. At a CY dose of 50 mg/kg and an inoculum of 105 CFU, 89% of the mice (96 of 108) developed large abscesses (15 mm diameter). The model was used to by the authors to compare cutaneous infection in normal and immunocompromised mice,29 and by Hahn et al.30 to investigate the effects of superficial exudates of neutrophils on prevention of Bacillus anthracis infections.
Tape stripping model.
Kugelberg et al.31 presented an animal model of superficial skin infections using a tape stripping method. Six- to 8-week-old female BALB/c mice were used for all experiments. The mice were anesthetized and the fur was stripped from the mice with an elastic adhesive bandage. An area of ca. 2 cm2 was tape stripped. In order to standardize the degree of barrier disruption elicited by the tape stripping, the transepidermal water loss (TEWL) was measured by using a Derma Lab TEWL probe. By tape stripping the back of the mice 7 to 10 times in succession, the TEWL reached approximately 70 g/m2 h. Following this procedure, the skin became visibly damaged and was characterized by reddening and glistening but no frank bleeding. Microscopically, this procedure resulted in the controlled removal of most of the epidermal layer, with only a few basal epidermal cells remaining. After stripping of the skin, a bacterial infection was initiated by placing on the skin a 5 µL droplet containing 107 S. aureus cells concentrated from an overnight bacterial culture in stationary phase. The number of CFU recoverable from the wound 4 h after application of 107 CFU of S. aureus was 7.03 ± 0.37 log10. This is evidence of the successful establishment of a staphylococcal infection in this model. The model was used with slight modifications by other investigators to evaluate a topical antibiotic for S. aureus infections32 and elucidate the mechanisms by which the organisms are cleared from the skin.33
Sand paper model.
Gaspari et al.34 developed a skin abrasion model by abrading the skin in Transgenic (Tg) mice with sand paper. A 2 × 2 cm area of abdominal skin was shaved until all fur was removed. The exposed skin was then gently abraded with fine-grade, sterile sandpaper until the skin was glistening but not bleeding. Next, 107 Candida albicans blast conidia were pipetted onto the surface of the skin in a 20 µL volume of sterile PBS. The area was occluded with a 0.5 × 0.5 cm piece of sterile gauze that was moistened with sterile PBS. This layer was further occluded with a 1 × 1 cm piece of plastic sheeting. Histological analysis showed that the infection remained localized to the stratum corneum of the epidermis, rarely invaded the viable epidermis and never invaded the dermis. The infection persisted over 5 to 7 d in the mice. The model was used by the authors to determine whether enhanced cutaneous delayed type hypersensitivity (DTH) is seen in these Tg mice in response to cutaneous fungal infections.
Dermatome model.
A very early dermatome skin abrasion model was reported by Munster et al.35 Partial thickness skin corresponding to 20% TBSA was removed from the anesthetized Sprague Dawley rats (180–200 g) by an electro-dermatome, with an aperture of 20/1,000 inches. Rats were inoculated on the wound surface with 108 CFU of P. aeruginosa. All animals died within four days, with histological evidence of invasion and widespread visceral seeding. The model was reported to be reproducible, measurable and controllable. In addition, it was simple and rapid.
In another study, a superficial abrasion model in rabbits was created by Jeray et al.36 also using an electric dermatome. New Zealand White rabbits (either females or males) of approximately the same age and size (2.8 to 3.9 kg) were used. The hair on the dorsum of the anesthetized rabbits was removed using clippers. A degreasing agent was used to remove the skin oils. Two 2 × 2 cm sites were marked with a skin marker, one caudal and one cranial. One site served as a control, with the skin intact, while the other served as the abrasion site. An electric dermatome set to a thickness of 0.0015 in created a superficial abrasion (roughly 2 × 2 cm) on the otherwise unprepared sites. Both sites on each rabbit were inoculated with 50 µL of encapsulated S. aureus. Results showed that the amount of bacteria on the normal skin (control sites) dropped significantly (p < 0.02) for all time periods (6, 12, 24, 48 h post-infection) except six hours (p < 0.20). On the abraded skin sites the bacteria flourished for all time periods.
Summary.
Based on the approaches the wounds were produced, the types of animal models of skin abrasion infection can be classified into needle scratch model, blade scarp model, tap striping model, sand paper model and dermatome model. The needle scratch model is more clinically relevant to shallow skin cuts. Animal species used include mouse, rat and rabbit. Skin abrasions are made either on the dorsal or abdominal surface of the animals. S. aureus is the most common bacterial species investigated, while P. aeruginosa and C. albicans have also been studied. Table 2 summarizes the representative animal models of skin abrasion wound infections.
Table 2.
Representative animal models of skin abrasion wound infection
| Animal species | Microorganism & inoculum | Methods used to produce wound and infections | Wound area | End point | Refs |
| Female BALB/c mouse | S. aureus 1 × 108 CFU | Mice were pre-treated with cyclophosphamide. Skin abrasion wounds were made on the dorsal surfaces of mice using needles by creating 6 × 6 crossed scratch lines within a defined 1 × 1 cm area. Bacterial suspension was inoculated over the crossed scratches with a pipette tip. |
1 × 1 cm | A stable infection, persisted for over 10 days, was developed in the mouse wounds as characterized by bioluminescence imaging. | 27 |
| Female C57 Black mouse | S. aureus 4 × 107 CFU | Twenty-five square millimeter square-shaped wounds were created in the skin of the back by scarification using a needle, run ten times parallel in one direction and another ten times perpendicular to the original tracks. Bacterial suspension was topically placed on each wound. |
25 mm2 | This method resulted in a reproducible MRSA wound colonization model, which persisted for up to 5 days post inoculation. | 28 |
| Male CF-1 mouse | S. aureus, S. epidermidis, S. saprophyticus, Micrococcus luteus. 105 CFU | Mice were pre-treated with cyclophosphamide. One drop (0.02 mL) of the prepared culture suspension of S. aureus or S. epidermidis was applied to the shaved skin, which was scraped with a scalpel. | 10 mm2 | 89% of the mice (96 of 108) developed large abscesses (15 mm diameter). | 29 |
| Female BALB/c mouse | S. aureus 107 CFU | An area of ca. 2 cm2 on the mouse back was tape stripped. Bacterial infection was initiated by topically placing on the skin a 5 µL S. aureus suspension. |
2 cm2 | The number of CFU recoverable from the wound 4 hours after bacterial application was 7.03 ± 0.37 log10. | 31 |
| B7-1 or B7-2 Tg mice | C. albicans 107 CFU | The exposed skin was gently abraded with fine-grade, sterile sandpaper. C. albicans blast conidia were pipetted onto the surface of the wound. |
2 × 2 cm | Infection remained localized to the stratum corneum of the epidermis, rarely invaded the viable epidermis, and never invaded the dermis. The infection persisted over 5 to 7 days in the mice. | 34 |
| Sprague-Dawley rat | P. aeruginosa 108 CFU | Partial thickness skin corresponding to 20% TBSA was removed from the rats by an electro-dermatome. Rats were inoculated on the wound surface with P. aeruginosa suspension. |
20% TBSA | All animals died within 4 days, with histological evidence of invasion and widespread visceral seeding. | 35 |
| Male or female New Zealand white rabbit | S. aureus 1 × 106 to 1.2 × 109 CFU | An electric dermatome set to a thickness of 0.0015 inches created a superficial abrasion (roughly 2.0 × 2.0 cm) on the shaved dorsum. Wounds were inoculated with 50 µL of encapsulated S. aureus suspension. |
2 × 2 cm | On the abraded skin sites the bacteria flourished for all time periods at 6, 12, 24 and 48 h post-infection. | 36 |
Lethal infection models could be developed by using P. aeruginosa,35 while the use of S. aureus usually induces chronic infections in animals.27–29,36 In comparison to the needle/blade/tape approaches, the use of dermatome can result in the wounds with better-controlled, more uniform depths.35,36 To confirm clinically relevant infections are induced in the animals, microscopic observations27,31 are recommended to monitor the infections (e.g., the depth of the injury and the depth of the infected area).
Animal Models of Burn Infections
In previous times serious burns occupying a large percentage of body surface area were an almost certain death sentence because of subsequent infection. A number of factors such as disruption of the skin barrier, ready availability of bacterial nutrients in the burn milieu, destruction of the vascular supply to the burned skin, lack of re-epithelialization of basal epidermal tissue and systemic disturbances leading to immune-suppression combined together to make burns particularly susceptible to infection.1,2
Boiling water burn model.
An early animal model of burn wound can be traced back to over 40 years ago in 1968. Mason and Walker37 developed a burn model of rat using boiling water to inflict the thermal injury. It was the first animal model burn for research and was used as a standard scald burn model by the US Army Surgical Research Center in San Antonio, TX for various studies.37 The template device was made of a thin metal halfcylinder with an aperture of calculated size cut from the central portion of the half-cylinder. The dimensions of the aperture were selected to provide the size of burn desired in animal of known weight (the upper limit of burn size permitting by this approach was approximately 30% of TBSA). The anesthetized animal was shaved on the back and then placed supine in the burning device. The exposed area was immersed in boiling water. It was reported that 10 sec exposure produced a full-thickness burn and 3 sec a partial-thickness burn. The burns per se did not interfere greatly with mobility and the animals ate and drank easily. This model has been widely used in the studies on burn infections, including the pathogenesis of candidiasis after thermal injury,38 bacterial translocation in burn infections,39 evaluation of antibiotics40–43 or antimicrobial peptides44,45 for eliminating bacteria in burns, gene therapy of wound infections,46,47 wound dressing for preventing and treating burn infections,48–50 the use of electrolyzed oxidized water functions as a bactericide,51 the ability of insulin to treat infections,52 relationship between prostaglandin synthesis and prostaglandin degradation,53 cardiac contractile responses of burn injury complicated by sepsis,54–56 whether sepsis exacerbates the myocardial inflammatory responses,57 etc.
A similar model using guinea pigs (male and female guinea pigs weigh 300–350 g) was created by Bjornson et al.58 Scald burns were created by placing the animal in a custom made insulated template, which exposed a 60 cm2 area on the dorsum equal to approximately 15% TBSA. The area was immersed in 99°C water for 13 sec. After 1 h, the animals were again placed in the template, and a second dorsal burn was created. Immediately after burning, the animals were injected subcutaneously under the burn wound with 5 × 105 CFU of S. aureus, P. aeruginosa or C. albicans.
Ethanol bath burn model.
Stieritz and Holder59 reported an ethanol bath burn model in mice. In this model, female CF1 mice weighing 22–24 g were used. Mice were anesthetized and shaved on the back. An asbestos board with a window accounting for approximately 30% of TBSA was pressed firmly against the back of mice. Ethanol was evenly spread over the area outline by the window, ignited and allowed to burn for 10 sec. The infections were initiated by an immediate subcutaneous injection of 100 CFU of P. aeruginosa in the burned area. The infections turned out to be rapidly fatal and the animals appeared moribund 20 h after infection. The model was used to investigate the pathogenesis of P. aeruginosa59–61 and Klebsiella pneumonia62 in burn infections as well as the local delivery of polyclonal immunoglobulin against burn infections.63
Gas flame burn model.
A mouse burn model created by using a gas flame was developed by Katakura et al.64 Thermal injury was produced by pressing a custom-made insulated mold (with a 2.5 × 3.5 cm window) firmly against the shaved back of each mouse and subsequently exposing the area to a gas flame for 9 sec. A burner equipped with a flame-dispersing cap was used as a gas flame source. A third degree burn, on approximately 15% of TBSA for a 26 g mouse, was consistently produced by this procedure. Thermally injured SCIDbg mice were exposed to MRSA at a dose of 2 × 103 CFU/mouse. This model was used in the studies on pathogenesis of MRSA,64 Enterococcus faecalis translocation65,66 in immunodeficient mice with thermal injury and the effects of a soluble polysaccharide immune-modulator on the resistance to burn infections.67
Pre-heated double brass block burn model.
Stevens et al.68 developed a mouse model of full-thickness burn by applying two pre-heated brass blocks (92–95°C) to the opposing sides of an elevated skin folder on the backs of shaved mice for 5 sec (Fig. 3). Six-week-old male CD1 mice weighing 25–30 g were used. The combined brass block area was 1.8 cm × 1.8 cm, corresponding to a 5% of TBSA. After the infliction of burns, the eschars were immediately injected intradermally with 10–106 CFU of P. aeruginosa. Survival at 10 days was 100% with burn injury alone and 60% with infected burns. P. aeruginosa (consistently 108 CFU/g tissue) were recovered from the unburned muscle by 24 hours for all the different inocula of bacteria. This mouse model were employed by Lambrechts et al.,69 Dai et al.,70 Dai et al.71 and Ragas et al.72 with slight modifications to evaluate the efficacy of antimicrobial photodynamic therapy (PDT) and chitosan acetate bandage for burn infections respectively. A similar model using rats with the same burning procedure was described by Busch et al.73 to study Gram-negative bacteremia and sepsis.
Figure 3.
Full-thickness burn was created by applying two pre-heated brass blocks (92–95°C) to the opposing sides of an elevated skin fold on the back of a shaved mouse. Square: burned area. Bacteria were applied to surface of burn as a suspension in PBS.
Pre-heated (single) metal plate/bar burn model.
A burn model using guinea pig was developed by Orenstein et al.74 In this model, a copper plate of 1 × 1 × 3 cm equipped with a 20 cm handle, heated to 150°C on a heating plate, was placed on the shaved back for 10 sec, resulting in a third degree burn. Burns were infected 15 min after the burning by the smearing of 1 mL of S. aureus suspension containing 108 cells/mL onto the burned surfaces. Twenty-four hours after infection, the bacterial count in the untreated burns reached 2 × 109 CFU/g eschar. The model with slight modifications was used in the studies of prevention,75 treatment,74 wound healing76 and pathogenesis77 of infected burns as well as shock78 and hypermetabolic response79 induced by infected burns.
A deep partial thickness burn model produced by applying cylindrical aluminum templates heated to 75°C for 5 sec to the depilated moistened skin in guinea pigs was described by Kaufman et al.80 Cylindrical aluminum templates (diameter, 3.76 cm; height, 3.78 cm; length of the handle, 24 cm; total weight, 500 g) were heated in a water bath for 2 h prior to the injury at a constant temperature of 75°C. The anesthetized guinea-pig was restrained and stretched on a metal meshed board and the midline corresponding to the spine was marked on the animal's back as well as the horizontal upper limits of both sacroiliac joints. The heated and moistened template was applied at right angles to the skin of the animals' back according to the pre-marked locations. Only minimal pressure was required to ensure a perfect contact between the template surface and the underlying dorsal skin.
For the comparison of different topical drug treatments and dressing materials in a setting that closely mimics clinical reality, a porcine model of full-thickness burn was proposed by Branski et al.81 Yorkshire pigs (40–65 kg) were used in the study. Contact burns were applied paravertebrally under aseptic conditions. This was accomplished by placing a heated aluminum bar on the dorsum of the animal. The aluminum bar was heated to 200°C with a gas burner. The heated bar was placed on the animal for 30 sec. Burn sites of approximately 40 cm2 each were made on the dorsum of the animal with 4 cm between each site or from the spine. The total burn size did not exceed 15% TBSA.
Manafi et al.82 introduced a mouse model by applying a 1 × 2 cm piece of hot metal to the thigh, producing a burn of up to 10% of TBSA and extending to all layers of skin but not involving the muscular tissue. After 24 h, 108 CFU of toxigenic strains of P. aeruginosa were inoculated subcutaneously into the burned area. A similar mouse model was described by Kumari et al.83 using a single preheated brass bar as the heater. In this model, the burns were created on the backs of mice followed by topical inoculation of K. pneumoniae culture on the burned area.
A modified model using mice was reported by Calum et al.84 Specified pathogen-free female 12-week-old C3H/HeN mice (20–25 g) were used. The mice were placed onto a sledge and covered with a heat-resistant blanket with a window (1.7 × 2.6 cm) corresponding to approximately 6% of the TBSA. Above the fire blanket a metal plate with a window (1.7 × 2.6 cm) was placed. The sledge with mice, the fire blanket and metal plate were moved into a stream of hot air with a temperature of 330°C delivered by a hot-air blower for 7 sec. The temperature was kept constant using the same time schedule. When the procedure was disrupted, the procedure was restarted following a constant time schedule controlling the temperature. This procedure resulted in a third-degree burn and was confirmed by histological examination. The mice were injected subcutaneously with 100 µL challenge solution (infection dose 107 CFU/mL) with a 27-gauge needle beneath the burn wound.
Other burn models.
A model of a partial thickness burn was developed by Bahar et al.85 using male Wistar rats weighing 250–300 g. After the animal was anesthetized, a 2.5 × 2.5 cm, 5 mm thick piece of absorbent lint cloth that had been immersed in boiling water (100°C) was placed over the dorsum. The hot cloth was applied for different time intervals (5 or 12 sec). This method produced reliable superficial and deep dermal burns, as confirmed by the histological features.
Suzuki et al.86 designed a burn model of rat based on skin contact with a glass chamber through which water circulates at a pre-determined temperature. Male Wistar rats weighing 450–550 g were used. The glass chamber (applicator) was set in a glass sheath which was clipped onto the centre of the abdominal wall to permit direct contact between the chamber base and the skin surface using a total weight of the chamber and water of 10 g/cm2. Observations were made on each rat for each combination of temperature (35–60°C) and exposure time (1 min–7 d), which amounted to 162 combinations of temperature-expose time. The advantage of this model was the possibility of varying temperature and exposure time, as required by the researcher, and also of applying higher or lower contact pressure.
Summary.
The animal models of burn infections can be categorized by the heat source used as boiling water, burning ethanol bath, gas flame, pre-heated double brass blocks, pre-heated single metal plate/bar, etc. The animal species used included mouse, rat and pig (guinea pig and Yorkshire pig). Burns were usually made on the shaved backs of animals. The size of the burns ranged from 5% TBSA to 30% TBSA. The time that the heated object or fluid in contact with animal skin mostly ranged from 3 to 30 sec, resulting in partial to full thickness burns. The microorganisms investigated included P. aeruginosa, S. aureus, K. pneumonia, A. baumannii and C. albicans. To induce infections, microorganisms were either topically applied or subcutaneously injected to the burned area. Table 3 describes representative animal models of burn wound infections.
Table 3.
Representative animal models of burn wound and infections
| Animal species | Microorganism & inoculum | Methods used to produce wounds and infections | Area of burn | Burning time | End point | Refs |
| Rat | Not infected | Boiling water. | 30% TBSA | 3 or 10 s | 37 | |
| Male Sprague Dawley rat | P. aeruginosa 105 CFU | Boiling water. Burns were topically inoculated with P. aeruginosa broth. |
20% TBSA | 10 s | Without treatment, 19 of 20 rats died. | 49 |
| Female CF1 mouse | P. aeruginosa 100 CFU | Ethanol bath. The infections were initiated by an immediate subcutaneous injection of bacterial suspension in the burned area. |
30% TBSA | 10 s | The animals appeared moribund 20 h after infection. | 59 |
| Female C57BL/6J mouse | P. aeruginosa 1 × 107 CFU | A full thickness flame burn was achieved using a Bunsen burner applied to the exposed skin. P. aeruginosa suspension was applied topically to the burn wound on day 5 after burn injury. |
35% TBSA | 10 s | Long-term survival of the infected mice was 25%. | 67 |
| Male CD1 mouse | P. aeruginosa 10 to 106 CFU | Applying two pre-heated brass blocks (92–95°C) to the opposing sides of an elevated skin folder on the backs of shaved mice. After the infliction of burns, the eschars were immediately injected intradermally with P. aeruginosa suspension. |
%5 TBSA | 5 s | Survival at 10 d was 60% with infected burns. P. aeruginosa (consistently 108 CFU/gtissue) were recovered from the unburned muscle by 24 hours for all the different inocula of bacteria. | 68 |
| Guinea pig | S. aureus 108 CFU | A copper plate heated to 150°C was placed on the shaved back. Burns were infected 15 min after the burning by the smearing of S. aureus suspension onto the burned surfaces. |
1 cm × 1 cm | 10 s | Twenty four hours after infection, the bacterial count in the untreated burns reached 2 × 109 CFU/g-eschar. | 74 |
| Yorkshire pig | Not infected | Burns were made by placing a heated aluminum bar on the dorsum of the animal. The aluminum bar was heated to 200°C with a gas burner. | <15% TBSA | 30 s | 81 | |
| Male Wistar rat | Not infected | Absorbent lint cloth that had been immersed in boiling water (100°C) was placed over the dorsum. | 2.5 × 2.5 cm | 5 or 12 s | 85 | |
| Male Wistar rat | Not infected | Based on skin contact with a glass chamber through which water circulates at a predetermined temperature (35–60°C). | 7 cm2 | 1 min–7 days | 86 |
In comparison to the use of small animals of mouse and rat, the use of guinea pig and Yorkshire pig as the models of burn infections can mimic clinical reality more closely, as the skin structures of the pigs are more similar to that of human beings than mouse and rat. However, the small animals have the advantage of cheaper cost. For the introduction of the burn infections, to apply the infecting microorganism topically64,70,71,74,83 is more clinically relevant than to inject the microorganism under the burn eschar.58,59,68,69,82 Apparently, the minimum inoculum of microorganism required to induce infection by use of subcutaneous injection in lower than that using topical application. Similar to the models SSI and skin abrasion infection, lethal infections can be developed in animals by use of lethal microorganisms (e.g., P. aeruginosa),49,59,71 while chronic infections can be produced using less lethal microorganisms (e.g., S. aureus).69,72
Animal Models of Lacerated Wound Infections
Lacerations are irregular wounds resulting from a blunt trauma (Fig. 4). Lacerations involve separation of skin or other tissues such as tendons, muscles, ligaments, nerves, blood vessels or bone. Laceration wounds are the third most commonly encountered problems in the emergency departments.87 Traumatic laceration is rarely a life-threatening event but improper management increases the chances of wound infection. Deep lacerations with devitalized tissue are more prone to infection. Infection of sutured lacerations may lead to impaired healing, pain, poor cosmesis and loss of function.87
Figure 4.
Schematic depiction of laceration wound.
Non-crushed lacerated wounds.
The first animal model of lacerated wound infection was reported by Edlich et al.88 Albino guinea pigs (weighing 300–350 g) were selected because of similar susceptibility to the infections as found in humans. Skin preparation consisted of hair clipping, depilation and skin cleaning. Two 3 cm incisions were made parallel and equidistant from the vertebral column, through the subcutaneous tissue down to the fascia, utilizing a modified non-crushing intestinal clamp and a surgical blade. Ten microliters of S. aureus suspension containing 107 CFU was introduced to each wound 5 min after the incision. At the end of the experiment (the authors did not report how long the experiment last), purulent exudates were observed in over 60% of contaminated wounds and bacterial positive cultures were found in 100% of the contaminated wounds. This model was widely used to study the care and management of contaminated lacerations.89–93
Using the guinea pig model with slight modifications, Howell et al.94 compared the effects of different scrubbing and irrigation techniques on eradicating bacteria in contaminated lacerations. Albino guinea pigs weighing 450–500 g were used in the study. Hair on the dorsum was trimmed but not shaved. Four dorsal lacerations with 3 cm length were made parallel to the spine and to deep fascia. After hemostasis with sterile gauze and pressure, 30 µL of Streptococcus pyogenes (2.6 × 109 CFU/mL) or S. aureus (3 × 109 CFU/mL) suspension was placed in each laceration. The model was also used by the same group to study the effect of closing lacerations with suture or cyanoacrylate tissue adhesive.95
A rat model of simple skin laceration was discussed by Moscati et al.96 Two wound sites were chosen on each animal, with one on either side of the thoracolumbar spine. Each site was shaved and the surface was prepped. A 3 cm full-thickness laceration was made at each site. Bleeding was controlled with sterile gauze pressure. The wounds were then inoculated with 0.4 mL of S. aureus at uniform concentrations of 1 to 4 × 108 CFU/mL and allowed to sit undisturbed for 30 min. The model was used by the authors to compare the efficacies of normal saline irrigation with tap water irrigation. No non-treated/infected wounds were used in the study. The contaminated wounds were irrigated either with tape water or normal saline at 30 min after contamination. No long-term follow up was carried out.
Crushed lacerated wounds.
A guinea pig model to mimic an animal bite wound in humans was reported by Lammers et al.97 It was reasoned that lacerated wounds in prior animal studies were made with a scalpel that left clean edges and no devitalized tissue and wounds were inoculated with a single species of microorganism, whereas bite wounds contain crushed tissues and are contaminated with multiple species. They designed an animal bite wound model in albino guinea pigs by inoculating crushed incisions with three species of bacteria. Wounds were created on the dorsum of anesthetized animals by making four paravertebral lacerations, 2 cm long and 2 cm apart, extending to deep fascia. Wound edges were clamped with hemostat along a 1.5 cm length of epidermal/dermal junction for 5 sec to create crushed, devitalized tissue within each wound; 0.4 mL of mixed bacterial inoculum containing S. aureus, B. fragilis and Pasteurella multocida at a density of 4 to 6 × 108 CFU/mL was instilled into each laceration. It was observed that 33% animals had either erythema, induration or purulence at 6 d after bacterial inoculation.
A crushed laceration wound model with necrotic tissue using albino rats was reported by Gross et al.98 to study the effectiveness of pulsating water jet lavage in treating contaminated crushed wounds. Albino rats (average weight 350 g) were anesthetized and the left preauricular area of each animal was shaved. A vertical incision through skin was made in the masseter muscle, 1.5 cm long and extending to lateral surface of the mandibular ramus. After the skin was reflected, an identical second incision was made in the muscle, 3 mm distant and parallel to the first one. The wounds were then inoculated with 0.03 mL of moist soil that contained four bacterial species—S. aureus, Proteus mirabilis, P. aeruginosa and K. pneumoniae (3 × 107 bacteria/soil sample). Pliers were inserted into two parallel incisions to the depth of the wound and squeezed firmly causing necrosis of the tissue and also forcing the inoculum into the crushed tissue. No results of non-treated control wounds were reported by the authors. For wounds lavaged with tap water, at 6 d after contamination, positive bacterial cultures were found in all wounds and 13 of 20 wounds were observed purulent.
A similar crushed contaminated wound model in male Wistar albino rats was employed by Erdur et al.99 to investigate the prophylactic efficacies of mupirocin and nitrofurazone on contaminated crush injuries. Two paravertebral linear incisions (2 cm long, 4 cm apart) reaching the deep fascial layer were made. The incision extended for the distance of four vertebral bodies. To stimulate a devitalized crush wound, 1.5 cm of segments of the wound edges were crushed with hemostats for 5 sec. Aliquots of 0.2 mL of (108 CFU/mL) S. aureus or S. pyogenes were dripped into each wound and left to absorb without spilling from the wound edges.
Summary.
Lacerated wound infection models have been developed in relative large animals, such as guinea pigs and rats, due to the depth of this type of wounds. The wounds are inflicted by making one or more incision of various lengths usually on the dorsum of the animal. This has been considered as a limitation of the available animal models, as most lacerations in humans occur in the highly vascular areas of the body, such as the face and scalp.87 In some models, the tissue was crushed and devitalized by clamping the wound edges with hemostats. The most frequently studied microorganism was S. aureus, while S. pyogenes, P. aeruginosa, K. pneumonia and P. mirabilis have also been investigated. Table 4 shows a summary of animal model of laceration wound infections.
Table 4.
Representative animal models of laceration wound infections
| Animal species | Microorganism & inoculum | Methods used to produce wounds and infections | End point | Refs |
| Guinea pig | S. aureus 107 CFU | Two 3 cm incisions were made parallel and equidistant from the vertebral column, through the subcutaneous tissue down to the fascia. Bacterial inoculum was delivered to the selected wound using an ultra-micro blowout pipette. |
Purulent exudates were observed in over 60% of contaminated wounds, and bacterial positive cultures were found in 100% of the contaminated wounds. | 88 |
| Guinea pig | S. pyogenes (7.8 × 107 CFU) S. aureus (9 × 107 CFU) | Four dorsal lacerations with 3 cm length were made parallel to the spine and to deep fascia. Bacterial inoculum was placed in each laceration by using a pipette system. |
Four days after infection, the bacterial counts were over 107 CFU/g-tissue for both bacterial species. | 94 |
| Male lab rat | S. aureus 0.4 to 1.6 × 107 CFU | Two 3 cm full-thickness lacerations were made on each animal, with one on either side of the thoracolumbar spine. The wounds were inoculated with bacterial suspension. |
No non-treated control wounds were used in the study. No long-term follow up was carried out. | 96 |
| Guinea pig | Mixed bacterial inoculum containing 1.6 to 2.4 × 108 CFU of S. aureus, B. fragilis and P. multocida. | Wounds were created on the dorsum of animals by making four paravertebral lacerations, 2 cm long, and 2 cm apart, extending to deep fascia. Wound edges were clamped with hemostat for 5 sec to create crushed, devitalized tissue. Bacterial inoculum was instilled into each laceration using a sterile pipette system. |
At 6 d after bacterial inoculation, 33% animals had either erythema, induration or purulence and the bacterial counts >105 CFU/g-tissue. | 97 |
| Albino rat | Mixed culture of S. aureus, P. mirabilis, P. aeruginosa and K. pneumoniae 3 × 107 CFU | Two parallel vertical incisions through skin were made in the masseter muscle, 1.5 cm long, 3 mm apart, and extending to lateral surface of mandibular ramus. Pliers were inserted into two parallel incisions to the depth of the wound and squeezed firmly causing necrosis of the tissue. Each wound was then contaminated with 0.03 mL of moist soil which contained equal concentrations of the four bacterial species used. |
No results of non-treated control wounds were reported by the authors. For wounds lavaged with tap water, at 6 d after contamination, positive bacterial cultures were found in all wounds, and 13 of 20 wounds were observed purulent. | 98 |
| Male Wistar albino rat | S. aureus S. pyogenes 2 × 107 CFU | Two paravertebral linear incisions (2 cm long, 4 cm apart) reaching the deep fascial layer were made. 1.5 cm of segments of the wound edges were crushed with hemostats for 5 sec. Bacterial Aliquots of 0.2 mL were dripped into each wound and left for resorption without spilling from the wound edges. |
Infections were developed in 71.4% animals. Six days after bacterial inoculation, the bacterial counts > 104 CFU/g-tissue. | 99 |
Non-crushed laceration is similar to deep surgical incisional wound. The principle difference between non-crushed lacerated wound infection and deep incisional SSI lies in the fact the latter usually occurs in sterilized operational environment and the inocula of the infecting microorganism is much lower.
Animal Models of Excisional Wound Infections
Excisional wounds can be induced by excisional surgery. Excisional wounds involve the removal of a significant volume of the target tissue and are frequently complicated by infection and prolonged healing.
Excisional wounds in mice.
Hamblin et al.100 developed a mouse model of excisional wound infections. Male BALB/c mice weighing 20–25 g were anesthetized, shaved on the back, and then depilated. Four full-thickness excisional wounds were made in a line along the dorsal surface using surgical scissors and forceps. Wounds measured 8 × 12.5 mm and had at least 5 mm of unbroken skin between them. The bottom of the wound was panniculus carnosus, with no visible bleeding. A suspension (50 µL PBS) containing 5 × 106 cells of mid-log phase bioluminescent E. coli was inoculated into each wound. The next day, infected wounds in living mice had lost, on an average, 90% of the original luminescence signal but with considerable inter-animal variability. The model was rather artificial in that it used a clean excision, a noninvasive bacterial strain and a large inoculum (5 × 106 bacteria). Nevertheless, it served to establish a proof-of-principle that antimicrobial photodynamic therapy (PDT) could destroy infection in vivo.
The model was also used by other investigators with slight modifications to investigate antimicrobial and wound healing effects of chitosan acetate bandage on infected wounds,101,102 the effects of peptide on the healing of infected wounds103 and bacterial colonization and the expression of inducible nitric oxide synthase (iNOS) in murine wounds.104
Shi et al.105 made an infection model using MRSA on the skin of diabetic db/db mice. Female mutant diabetic mice (C57BL/ksJ db/db) and heterozygous control mice (C57BL/ksJ db/+) at 8 weeks of age were used. The dorsal hair was gently clipped. Two round, full-thickness wounds were prepared on the back of each mouse in the anterior-posterior direction using a punch biopsy instrument (3 mm diameter). Each wound was covered with a sterilized transparent dressing and inoculated with 1 × 105 CFU of MRSA in 30 µL of PBS by injection into the wounds covered by the sterilized transparent dressing. Hematoxylin and eosin (H&E) staining of the wounds of revealed delayed reepithelialization, inflammatory cell infiltration and edema due to MRSA infection. Gram staining of MRSA-infected wounds showed numerous Gram-positive cocci.
A similar mouse model with punch excisional wound was reported by Schierle et al.106 to investigate the effect of Staphylococcal biofilms on wound healing. Male C57Bl6/J mice weighing 35–50 g were used. Full-thickness excisional punch wounds were created through the skin down to the panniculus carnosus. Bacterial biofilms were applied at time of wounding. Microscopic analysis of cutaneous mouse wounds inoculated with S. aureus and S. epidermidis confirmed formation of a discrete biofilm after an incubation period of approximately 3 d. The model was utilized by to Martinez et al.107 to investigate applicability of topically applied nitric oxide through nanoparticles (NO-np) to S. aureus skin infections.
Excisional wounds in rats.
A rat model of excisional wound was designed by Nayak et al.108 Healthy inbred albino Wistar strain male rats weighing 180–220 g were used. The dorsal fur of the animals was shaved with an electric clipper. A full thickness of the excision wound of circular area 400 mm2 and 2 mm depth was created using toothed forceps, a surgical blade and pointed scissors. The entire wound was left open. This model was used to evaluate the effect of helium-neon laser on the healing of the wounds. The model with slight modification was used by other investigators to study the treatment of infected wounds with the antimicrobial peptide109 or iron-based hemostatic agent110 and collagen-based wound dressing for drug delivery to infected wounds.111
In another similar model reported by Saymen et al. using female Lewis or Sprague Dawley rats weighing 200 to 300 g, a 1 in (2.54 cm) square was outlined by an incision into the skin, dorsolaterally just anterior to the pelvis. The depth of the incision extended through the full thickness of skin but did not include the underlying muscle or panniculus carnosus. The anteriomedial corner of the incised skin was lifted with a toothed forceps and carefully dissected away from the panniculus by using the index finger wrapped in a gauze pad. The resulting full-thickness surface wound provided the basic surgical preparation for all experiments. The surface of the wound was seeded with 107 CFU of P. aeruginosa by pipetting 0.1 mL of the supernatant culture described above onto the panniculus carnosus. Care was taken to prevent run-off from the wound surface.
Excisional wounds in pigs.
Fries et al.113 reported a model involving dermal excisional wound in pigs. Female specific pathogen-free domestic pigs weighing 80 lb were used. The wound sites over the dorsal trunk area were shaved. Excisional dermal wounds (n = 10; two sets of 5) were created on the back of each pig. Full-thickness sections of skin (1 × 1 in) were removed during the wounding process. Duragesic (fentanyl transdermal system) patches were placed on the pinna to alleviate pain in response to wounding. The model was used to assess the efficacy of topical oxygen treatment. A similar model using female Yorkshire pigs (diabetic) was reported by Hirsch et al.114,115 to monitor the effect of bacterial infections in diabetic wounds and analyze the effect of hBD-3 expression in infected diabetic wounds.
Excisional wounds in rabbits.
A rabbit model of excisional wound was presented by Kilpadi et al.116 to test the hypothesis that vacuum-assisted closure (VAC) therapy could restore normal vasoresponse, reduce vascular resistance and increase perfusion in tissue subjacent to the wound bed. New Zealand albino rabbits (2–3 kg) of both sexes were used. The dorsal hindquarters were shaved with a clipper and the areas surrounding the designated wound sites treated for 5 min with depilatory cream to remove remaining hair. Two matching, full-thickness, square, cutaneous wounds approximately 2 × 2 cm were then created on the right and left dorsal hindquarters. The wounds were created with a surgical scalpel, beginning with four initial incisions through the outer dermis and ligation or cauterization of the underlying blood vessels. Once hemostasis was assured, the fascial layers were excised to expose the muscle surface.
Summary.
The animal species used in excisional wound infection models include mouse, rat, pig and rabbit. The microorganisms investigated include S. aureus, P. aeruginosa and E. coli. The configurations of the wounds were either square- or round-shaped. Table 5 shows a summary of the representative animal models of excisional wound infections.
Table 5.
Representative animal models of excisional wound infections
| Animal species | Microorganism & inoculum | Methods used to produce infections | Wound area | End point | Refs |
| Male BALB/c mouse | E. coli 5 × 106 CFU | Four full-thickness excisional wounds were made in a line along the dorsal surface. Wounds measured 8 × 12.5 mm2. The bottom of the wound was panniculus carnosus. A bacterial suspension (50 µL) was inoculated into each wound using a yellow tip. |
8 × 12.5 mm | Infected wounds in living mice showed only a slight loss of bacterial luminescence over a period of 4 h. The next day, control infected wounds in living mice had lost, on an average, 90% of the original luminescence signal. | 100 |
| Female mutant diabetic mouse | S. aureus 1 × 105 CFU | Two round, full-thickness wounds were prepared on the back of each mouse using a punch biopsy instrument (3 mm diameter). Each wound was covered with a sterilized transparent dressing and inoculated with 105 CFU MRSA or MSSA in 30 µL of PBS. |
3 mm diameter | Wounding and infection caused by MRSA on the back of the diabetic mice significantly induced increased CFU on wounds compared to those of the MRSA-infected normal mice. |
105 |
| Male Wistar rat | Not infected | A full thickness of the excision wound of circular area 400 mm2 and 2 mm depth was created. | 4 cm2 | 108 | |
| Female domestic pig | Not infected | Excisional dermal wounds (n = 10; two sets of 5) were created on the back of each pig. Full-thickness sections of skin (1 × 1 in.) were removed during the wounding process. | 1 × 1 in n = 10 | 113 | |
| Female Yorkshire pig (diabetic and non-diabetic) | S. aureus 2 × 108 CFU | Fourteen full thickness excisional wounds (1.5 × 1.5 × 0.5 cm) were created using a blade. 1 mL of the bacterial suspension was injected into each chamber. |
1.5 × 1.5 cm | Diabetic wounds showed a sustained significant infection compared to non-diabetic wounds over the whole time course of the experiment. | 114 |
| New Zealand White rabbit with both sexes | Not infected | Two matching, full-thickness, square, cutaneous wounds approximately 2 cm × 2 cm were then created on the right and left dorsal hindquarters. | 2 × 2 cm | 116 |
Excisional wounds usually occur after the excisional surgery (e.g., excisional debridement of burns). As a result, excisional wound infections, similar to incisional SSIs, develop under sterile operation environment. An animal model of excisional wound infected with large number of bacteria100,105,114 is to some extent artificial. In addition, in most of animal models of excisional wound, the wounds are produced by only removing the full-thickness skin but no injury to the underlying muscle. This is not reasonably relevant to the clinical situations where large volume of tissue could be excised. Nevertheless these models of excisional wound infections are reproducible and are very useful in testing antimicrobial therapies.
Animal Models of Open Fracture Infections
Infection resulting from open fracture is a common problem in orthopedics. Break down of the tissue barrier between the fracture zone and the environment leaves the underlying bone prone to direct contact with contaminating agent. Due to improved aseptic and operative techniques, survival rate of the patients has increased. However, loss of the injured extremity due to wound infection and nonunion fracture is of major concern, especially when combined with a major vascular trauma.6,117
Models of open tibial fractures.
Open tibial fractures in rabbits. An early model of open fractures using male New Zealand White rabbits with a minimum weight of 3.5 kg was reported by Worlock et al.118 The left hind limb of each animal was shaved, cleaned and draped to provide a sterile field. The medial side of the tibia was exposed and a reproducible fracture of the mid-part of the tibial shaft was created. This was stabilized with a 3 mm diameter Kirschner wire (K-wire). The deep fascia and skin were closed in layers. The animals were inoculated with an isolate of S. aureus that had been obtained from a patient who had chronic osteomyelitis. For the inoculation of each fracture, 0.5 mL of the bacterial suspension containing 107 CFU was injected into the site of the fracture through the previously placed 23-gauge needle. Another syringe, containing 0.1 mL of a 0.9% solution of saline, was used to flush the inoculum in the needle into the site of the fracture.
Thirty-four rabbits (out of 62) were diagnosed as being infected. At the time of death, thirty-three of them had a positive culture for S. aureus as well as radiographic and histological changes that were characteristic of osteomyelitis. The remaining rabbits had no discharge and a culture of a specimen that had been taken from the medullary canal at the time of death was negative. However, the rabbit had typical radiographic and histological signs of infection and the identification of Gram-positive cocci on histological sections confirmed the presence of osteomyelitis.
The model with slight modification was used by other investigators to evaluate the efficacy of local therapy with cefazolin microspheres for the prevention of infection with contaminated open tibial fractures stabilized with internal fixation119,120 and the influence of materials and design for fixation implants on local infections.121,122
Open tibial fractures in canines. In another study conducted by Khodaparast et al.,123 canine tibiae were fractured by a penetrating captive bolt device, and an interlocking intramedullary nail was used to repair the community open fractures. The skin overlaying the proximal third of the tibia was incised. A 3.75 cm plexiglass block with a 1.2 cm hole was placed between the captive bolt device and the exposed bone to act as a spacer for the reduction of the penetration depth of the piston. To create the open fracture, the captive bolt device was fired to deliver a force of 6,800 N to the proximal tibia. The open fracture had the following characteristics: soft tissue injury, periosteal stripping and bone comminution. The blunt end of the captive bolt device protruded through the tibia and periosteum and exited posteriorly through the skin. After injury, an intramedullary nail was used for fixation of the open fracture. Proximal and distal interlocking cortical screws were placed in the medial-lateral plane. A 1 mL culture suspension containing 1 × 106 CFU of S. aureus was given to the canines via the medullary canal proximal and distal to the fracture. S. aureus was allowed to flow freely into the surrounding soft tissue. A pedicled gastrocnemius rotation flap was rotated to cover the fracture site after inoculation in the “inoculated muscle” flap group. In all groups, wound closure was carried out in a standard layered fashion using fasciocutaneous tissue, with primary skin closure in all animals. Dermal edges were freshened, and the skin was reapproximated with O-polypropylene suture in a simple, uninterrupted fashion. Before primary closure, microdialysis catheters were sutured into control and fracture sites; collections were performed for 90 min. It was found that, in the presence of S. aureus, vascular endothelial growth factor (VEGF) mRNA expression on day 7 decreased from initial day 0 levels, while in the non-infected fractures, VEGF mRNA levels increased from day 0 to day 7.
Open tibial fractures in rats. A traumatic open tibial fracture with accompanying soft-tissue damage and vascular injury, akin to a Gustilo type III wound that is commensurate with an internally stabilized open fracture was developed by Buxton et al.124 Adult male Sprague-Dawley rats weighing approximately 350 g each were used in all experiments. The left hind leg of each rat was shaved and the surgical site cleaned. A 3 in (ca. 7.6 cm) stockinette was rolled over the rat, and the left hind leg was exteriorized by cutting a small hole in the stockinette. A skin incision 2 cm in length was created on the left craniomedial tibia starting 1 cm distal to the patella by using a no. 10 surgical blade. Then, a second 2 cm longitudinal incision was made deep to the first incision by using a no. 15 surgical blade to incise the subcutaneous tissue; the musculature then was reflected to expose the periosteum of the craniomedial tibia. The periosteum was incised with the no. 15 surgical blade. A battery-powered heat cautery device was used on the subcutaneous and periosteal tissues for hemostasis.
A 1 cm longitudinal trough was created in the tibia medullary cavity by using a high-speed drill with a 1 mm carbide burr. The trough was started by burring into the anterior cortex from a proximal to distal mark. The burr was placed back into the trough proximally and the process repeated. The endosteal blood supply was disrupted with three to five passes of a high temperature loop-tipped handheld cautery device.
For contamination, S. aureus was introduced into the exposed medullary cavity at the rearmost section of the lumen (dorsal surface) by using a sterile micro-syringe (10 µL volume). When the endosteal surface was reached and resistance felt, the bacterial suspension was inoculated. At 10 min after introduction of bacteria, the troughs were curetted with a dissecting curette.
The troughs then were pulsatile-lavaged using 100 mL of Ringer's saline (23.7 lb/in2). The musculature and skin were closed by simple continuous and interrupted sutures, respectively. It was determined that the minimal inoculum of S. aureus that reproducibly infected 50% and 95% of tibiae after 24 h was 1.8 × 103 CFU and 9.2 × 103 CFU, respectively.
Open tibial fractures in ovines. An ovine model of open tibial fracture was developed by Hill et al.125 to study the outcome following intramedullary nailing of a heavily contaminated fracture. Suffolk-cross female sheep were used (mean weight 64.9 kg, range 55–80) in the study. The left hind limb was shaved and then cleaned. Under sterile conditions, the mid-shaft of the tibia was exposed through a 2 cm longitudinal incision over the subcutaneous border. A chevron osteotomy was created using a Gigli saw under saline irrigation. Soft tissue damage was minimized and the periosteum was not elevated. A standard inoculum (3 × 108 CFU) of S. aureus was introduced into the osteotomy on a piece of previously sterile bovine type I collagen (3 × 30 mm). The limb was supported for 6 h following inoculation by means of an external cast. In the non-treated control group the surgical wounds showed early signs of infection, with soft tissue swelling at the fracture site by the end of the first week and wound breakdown in the second week.
Open tibial fractures in goats. A complex musculoskeletal wound model containing tibial fracture in adult male Spanish-Boer goats was reported by Svoboda et al.126 The goat was placed supine on the operating table, and the left lower extremity was shaved and aseptically prepared. A 5 cm skin incision approximately 1 cm lateral to, and at the level of, the tubercle was made and extended distally to the level of the medial periosteum and fascia overlying the anterior and lateral leg compartments. The lateral compartment was elevated from its attachment to the lateral aspect of the tibia after the lateral compartment fascia was incised with use of electrocautery. The fascia was also elevated from the superficial surface of the anterior and lateral compartments. The medial tibial periosteum was exposed and incised longitudinally throughout the length of the skin incision and parallel to the incision in the anterior compartment fascia. This incision was measured so that a 6 mm strip of periosteum was left intact on the anteromedial aspect of the tibia. The more posterior portion of periosteum was elevated with a blunt periosteal elevator and retracted medially. A partial medial cortical injury measuring 1.2 cm in diameter was created in the tibia with use of a 3 mm drill-bit on a twist drill and a small osteotome. Three Kelly clamps were spaced evenly over a 5 cm segment of the anterior compartment muscles and were left in place for 3 min to induce a standardized crush injury to the anterior compartment muscles. Concurrently, electrocautery was used to create thermal damage to the intervening muscle between the clamps. Thus, the wound rendered was complex, involving injury to muscle, fascia, periosteum and bone (Fig. 5). The wound was inoculated with 1 mL of >108 CFU/mL of bioluminescent P. aeruginosa, which was spread evenly over the wound surfaces with a cotton-tipped applicator soaked in the same inoculum. The wound was left open for a five-minute period after which it was dressed open with a cover sponge, a rolled gauze dressing and Vetrap bandaging tape.
Figure 5.
A complex wound involving injury to muscle, fascia, periosteum and bone.126
Curtis et al.127 reported another goat model of contaminated tibial fractures. After surgical draping, the tibia was exposed through a 10 cm medial incision along the subcutaneous border. The periosteum was reflected for 3 cm to simulate soft-tissue damage and a chevron osteotomy, simulating an open tibial fracture, was then created with a power saw. Saline irrigation was used. S. aureus suspensions containing 103 CFU were placed at the fracture site on a 5 × 5 mm piece of absorbable gelatin sponge and the skin was loosely approximated with 3-0 Dexon sutures to prevent further postoperative contamination. The inoculum was placed at the fracture site after stabilization to prevent its dissemination during the surgical manipulation required to stabilize the fracture. The model was used by the authors to compare different treatment modalities of contaminated fractures.
Models of open femoral fractures.
Open femoral fractures in rats. A rat model of a blunt trauma open fracture was developed by Lindsey et al.128 using male Sprague-Dawley rats (400–450 g). The model included a blunt trauma injury, an open wound, exposure to common bacteria and definitive surgical fixation. After the rat was anesthetized, the leg was placed over the platforms of the fracture device (ventral side up) with a blunted blade placed at the mid-shaft of the femur. A weight of 0.94 kg was dropped from 15.3 cm, which impacted the blunted blade delivering a calculated force of 104.80 N. This method allowed for a reproducible mid-shaft femur fracture with associated soft tissue injury.
Following fracture, an incision was made on the dorsolateral surface of the femur from the area of the greater trochanter to the epicondyles of the femur through the gluteus superficialis. The fracture ends were exposed. S. aureus inoculum containing 102 CFU was placed directly into the wound after both ends of the fracture were exposed. The fracture was then left open for 1 h to mimic the “golden hour” of a trauma patient. Fixation was then started immediately. Using a 0.045 K-wire, the investigators drilled in a retrograde fashion starting at the fracture site and coming out in the piriformis fossa. The K-wire was removed and placed in an antegrade fashion through that hole behind the abductors, first into the proximal fragment and then into the distal fragment and partially into the epiphysis for distal fixation. The wound was closed using 4-0 polysorb sutures for myofascial closure and staples for skin closure. A reproducible rate of infection of 90–100% was displayed using this model.
The model was used by the authors to study the effect of interleukin-12 systemic therapy on open fracture infections129 and was used by other investigators with slight modifications for the evaluation of local monocyte chemoattractant protein-1 and interleukin-12 p70 therapies to prevent open fracture infections130 as well as a biodegradable scaffold as a carrier for antibiotics in infected open fractures.131
Another rat model of open femoral fracture was used by Brown et al.132 to determine the effects of earlier debridement and antibiotic administration on decreasing infection. Briefly, male Sprague-Dawley rats weighing 388.7 ± 10.68 g were used. Under aseptic conditions, a longitudinal incision was made over the left anterolateral femur and the entire femoral shaft was exposed using blunt dissection. A polyacetyl plate (length 25 mm, width 4 mm and height 4 mm) was fixed to the surface of the femur using six threaded K-wires. A 6 mm mid-diaphyseal full-thickness defect was created with a small reciprocating saw blade under continuous irrigation with sterile saline. The defects in all animals were implanted with 30 mg of type I bovine collagen ethanol sterilized and wetted with 105 CFUs of bioluminescent S. aureus suspended in 0.1 mL of sterile normal saline. The contaminated collagen was packed into the defect and the wound was closed in a layered fashion. A high resolution radiograph of each femur with stabilized defect was obtained at initial surgery to confirm appropriate placement of the implant and adequate creation of the defect. The bacteria quantity in wounds was monitored using a photo counting camera together with quantitative cultures. Purulence and pin loosening were observed in four of the 10 wounds without antibiotic treatment. Bacteria in the bone increased significantly between the 2 and 6 h time points. There was a further increase between the 6 and 24 h time points.
Open femoral fractures in hamsters. An open fracture model using Syrian hamsters was created by Merritt and Dowd.133 A skin incision was made on the lateral aspect of the left thigh of the animal. The muscle bundles were gently separated to expose the femur. The wound was deflected with retractors. An osteotomy was made with an osteotomy blade on an air saw. As the bone fractured instantly, no irrigation was used. Internal fixation chosen was an intramedullary 0.9 mm K-wire. S. aureus (104 CFU), P. mirabilis (106 CFU) or a mixture of the two organisms was injected into the muscle at the site of the wound after the osteotomy. Animals killed at 1 week had many organisms in the muscle at the site of the osteotomy indicating that tissue colonization was still clearing and osteomyelitis had not yet fully developed. The animals killed at 2 weeks rarely had organisms in the tissue, and thus the organisms isolated from the bone reflected a true infection of the bone and not contamination with adhering tissue. In addition, organisms were isolated when the rod was cultured indicating that the organisms were in the bone and causing osteomyelitis. It was also observed that that the presence of mixed organisms altered the infection rate. The presence of the Gram-positive organism markedly increases the infection rate with the Gram-negative organism. The presence of the Gram-positive organism markedly increases the infection rate with the Gram-negative organism.
Open femoral fractures in rabbits. Grewe et al.134 developed an open femoral fracture model in New Zealand white rabbits. An incision was made over the proximal third of femur, and using a sharp 2.5 mm drill, a hole was placed through both cortices of the femur. The hole was then tapped with a 3.5 mm tap. Half of the rabbits received a 3.5 mm stainless screw, and for the other half the hole was left empty. The wound was inoculated with 0.5 mL of culture medium containing 108 CFU S. aureus and closed with single running suture. No buried suture was used. Results showed that in the study group received screws, 30 out of 49 rabbits (61.2%) became infected, while in the control group without screw, 19 out of 53 (35.8%) became infected.
Summary.
The animal models of open fracture infections can be categorized as open tibial fracture infection and open femoral fracture infection. The animal species used included rat, canine, rabbit and sheep or goat. The open fractures were inflicted using saw, weight drop in combination with blunted blade or high speed drill. The most commonly investigated pathogenic microorganism is S. aureus. A summary of representative animal models of open fracture infections is depicted in Table 6.
Table 6.
Representative animal models of open fracture infections
| Animal species | Microorganism & inoculum | Methods used to produce infections | End point | Refs |
| Male New Zealand White rabbit | S. aureus 107 CFU | The medial side of the tibia was exposed and a reproducible fracture of the mid-part of the tibial shaft was created. Bacterial suspension was injected into the site of the fracture through a previously placed needle. |
Thirty-four rabbits (out of 62) were diagnosed as being infected. At the time of death, thirty-three of them had a positive culture for S. aureus. | 118 |
| Canine | S. aureus 106 CFU | Canine right tibiae were fractured with a penetrating, captive-bolt device. S. aureus suspension was given to the canines via the medullary canal proximal and distal to the fracture. |
In the presence of S. aureus, VE GF mRNA expression on day 7 decreased from initial day 0 levels. | 123 |
| Male Sprague-Dawley rat | S. aureus 1.8 × 103 CFU to 9.2 × 103 CFU | A 1 cm longitudinal trough was created in the tibia medullary cavity by using a high-speed drill. S. aureus was introduced into the exposed medullary cavity at the rearmost section of the lumen by using a micro-syringe. |
The minimal inoculum of S. aureus that reproducibly infected 50% and 95% of tibiae after 24 h was 1.8 × 103 CFU and 9.2 × 103 CFU, respectively. | 124 |
| SuKolk-cross female sheep | S. aureus 3 × 108 CFU | The mid-shaft of the tibia was exposed through a 2 cm longitudinal incision over the subcutaneous border. A chevron osteotomy was created using a Gigli saw. S. aureus was introduced into the osteotomy on a piece of sterile bovine type I collagen. |
The wounds showed early signs of infection, with soft tissue swelling at the fracture site by the end of the first week. | 125 |
| Goat | S. aureus 103 CFU | A chevron osteotomy was created with a power saw. S. aureus suspensions were placed at the fracture site on a 5 × 5 mm piece of absorbable gelatin sponge. |
There were significantly fewer and less severe infections in fractures fixed with external fixation than in those fixed with an intramedullary nail with or without reaming. | 127 |
| Male Sprague Dawley rat | S. aureus 102 CFU | Fracture device with a blunted blade placed at the mid-shaft of the femur. A weight of 0.94 kg was dropped from 15.3 cm. S. aureus inoculum was placed directly into the wound after both ends of the fracture were exposed. |
A reproducible rate of infection of 90–100% was displayed using this model. | 128 |
| Male Sprague Dawley rat | S. aureus 105 CFU | A 6 mm mid-diaphyseal full-thickness defect was created with a small reciprocating saw blade. The defects in all animals were implanted with 30 mg of type I bovine collagen and wetted with S. aureus suspension. | Purulence and pin loosening were observed in 4 of the 10 wounds. Bacteria in the bone increased significantly between the 2 and 6 h time points. There was a further increase between the 6 and 24 h time points. | 132 |
| Syrian hamsters | S. aureus (104 CFU), P. mirabilis (106 CFU) | An osteotomy was made with an osteotomy blade on an air saw. Bacterial suspension was injected into the muscle over the femur. |
Animals killed at 1 week had many organisms in the muscle at the site of the osteotomy. The animals killed at 2 weeks rarely had organisms in the tissue but only in the bone. | 133 |
| New Zealand white rabbits | S. aureus 108 CFU | Using a 2.5 mm drill, a hole was placed through both cortices of the femur. The hole was then tapped with a 3.5 mm tap. Half of the rabbits received a 3.5 mm stainless screw, and for the other half the hole was left empty. The wound was inoculated with S. aureus suspension. |
In the study group received screws, 30 out of 49 rabbits (61.2%) became infected, while in the control group without screw, 19 out of 53 (35.8%) became infected. | 134 |
To closely mimic the clinical reality, an ideal model of open fracture is the one involved with bone, soft tissue, periosteum and fascia injuries.123,124,126–128 Besides the Gram-positive S. aureus, the microorganisms commonly causing fracture infections also include Gram-negative species A. baumannii, P. aeruginosa, etc.135,136 These microorganisms should be investigated especially when evaluating the efficacy of antimicrobial therapy, because Gram-negative species are more resistant to many antimicrobials than their Gram-positive counterparts. It is reported that fractures in rabbits are more prone to infections than other animals.137 Rats are much less expensive than rabbits; however, their small size does not facilitate the testing of larger fixation devices or implants and does not provide for easy intravenous antibiotic administration.137 In addition, the infection rate, dose of organisms, and type of organisms causing infection are very different in the rat from those in human infections.133
Discussions
In brief, animal models of external traumatic wound infections are used for two major purposes: (1) to study the basic pathogenesis and virulence; (2) to study treatment modalities. Basic approaches of evaluation include clinical observation, radiography, microbiology and histology.
Animal species commonly used in external traumatic wound infection models include mouse, rat, pig, rabbit, as well as dog, goat and sheep. The mouse strains used include BALB/c, CD-1, CF-1, C57/BL, as well as mutant diabetic mouse, SCID bg mouse. The major strains of rat used are Sprague-Dawley, Wistar and Lewis. The strains of pig used include guinea pig, domestic pig and Yorkshire pig. The major strain of rabbit used is New Zealand white rabbit. The most commonly investigated pathogenic microorganisms are Gram-positive S. aureus and Gramnegative P. aeruginosa.
Except for laceration wound and open fracture infection models, the mouse is the most frequently used species in external traumatic wound infection models. Mice are an ideal organism in which to understand human infectious diseases. Despite some differences, the immune systems of mice and humans are similar and they can often be challenged with the same or similar pathogens.138,139 Another advantage of using mice is the availability of genetically modified animals or knockouts. Knockout mice, in which a specific gene has been deleted, have proved extremely useful in studying mechanisms of infectious disease.140 In addition, mouse models have the advantage of using animals that are inexpensive to purchase and maintain, thereby allowing one to perform studies with enough samples to yield statistically significant conclusions. The wounding procedure is quick, simple to perform, requires no specialized equipment and yields a highly reproducible result.141 The principal disadvantages of mouse models relate to the small size of the animals. Hence, for example, one is limited to the number of sequential blood draws that can be performed without compromising the mouse. Also, obtaining certain metabolic measurements, such as cardiac output readings, which would be of value in studies of septic shock, for example, are difficult to obtain in these small animals.141 The recent availability of genetically engineered bioluminescent pathogens and low light imaging cameras has greatly eased the non-invasive real-time monitoring of progress of infections in small animals such as mice.142–145
In contrast, larger animals (e.g., rabbit, sheep, pig, etc.,) address many of the deficiencies of small animals using as external traumatic wound inaction models. For example, the larger size allows for serial sampling of blood, other fluids and tissues. Furthermore, in contrast to rodent models, the ability to obtain vast amounts of time-dependent data for a wide variety of variables allows for great statistical power and ability to derive correlations with relatively few animals. In addition, for some traumatic wounds, like open fractures, mouse is not a suitable candidate due to the size of animal. Food and Drug Administration (FDA) guidelines suggest that all new drug applications include data from two species of animals, one of which must be a non-rodent large animal.
Despite these advantages, challenges remain in applying large animal models in the study of external traumatic wound infections. Experimental therapies for large animal models require at least 100 to 1,000 times more compound than mice because the large sizes make each experiment more expensive. The animal housing regulations for large animals is quite rigorous, detailed and expensive. Use of species more closely related to humans (non-human primates) presents extraordinary difficulties: These animals are extremely difficult to handle and the availability of these animals is very low.140 In addition, unlike the mouse, gene deletion technology is not available for larger animals and the larger animal's genome has not been fully elucidated. Both reagents and genetic information are vital for the assessment and understanding of particular phenomena such as pathology of infection, bacterial virulence and host resistance.
We have developed a method to monitor bacterial numbers and viability in real-time in living animals using genetically engineered bacteria that emit luminescence together with sensitive low-light imaging cameras. The rate of luciferase enzyme turnover in the presence of substrate allows for real-time measurements and the enzyme is active at the body temperature of mammals. This method is a significant improvement on the traditional use of survival or body fluid sampling and subsequent plating and colony counting. The first method suffers from the disadvantage of being wasteful of animals, and does not really address the question of where the bacteria are in the animal, while the second method suffers from the disadvantage that tissue sampling introduces another source of experimental error, is laborious and does not give real-time results. The in vivo bioluminescence imaging technique has now been widely used in the studies of external traumatic wound infections, especially in rodents.27,69–71,101,132,146–149
Due to reason for cost, almost universally, animal models of external traumatic wound infections use young animals with physiologic ages equivalent to humans less than 18 years old. Older wound patients are also more likely to develop infections and die from sepsis. Gender of animals may also affect the outcome of the studies. In many studies, only a single gender of animals was used.
The main determinants of the severity of external traumatic wound infections and whether the animals develop sepsis and die are as follows: the number of bacteria applied to the wound, the virulence of the particular bacterial strain, the size of the wound expressed as % of TBSA and the immune status of the animals. For burn infections, whether the bacteria are applied to the surface or injected into or beneath the burn, and the length of time the heated object or liquid is in contact with the skin also affect the infection severity. Animal models of external traumatic wound infections reported by different instigators vary significantly in the above parameters. To enable outcomes from different research sites to be compared, standard protocols for establishing animal models of external traumatic wound infections as well as basic approaches of evaluation are warranted.
Acknowledgments
Research in the Hamblin laboratory is supported by the NIH (grant RO1AI050875) and the US Air Force MFEL program (contract FA9550-04-1-0079). T.D. was partially supported by a Bullock-Wellman Fellowship Award and an Airlift Research Foundation Extremity Trauma Research Grant (grant #109421).
References
- 1.Pruitt BA, Jr, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 2.Murray CK. Infections in burns. J Trauma. 2007;62:73. doi: 10.1097/TA.0b013e318065af1a. [DOI] [PubMed] [Google Scholar]
- 3.Cheadle WG, Turina M. Infection and organ failure in the surgical patient: a tribute to seminal contributions by Polk HC Jr. Am J Surg. 2005;190:173–177. doi: 10.1016/j.amjsurg.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Sebeny PJ, Riddle MS, Petersen K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin Infect Dis. 2008;47:444–449. doi: 10.1086/590568. [DOI] [PubMed] [Google Scholar]
- 5.Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91:2–9. doi: 10.2106/JBJS.I.00549. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer T, Bayer GS, Wagner M. Open fractures and infection. Acta Chir Orthop Traumatol Cech. 2006;73:301–312. [PubMed] [Google Scholar]
- 7.Broex EC, van Asselt AD, Bruggeman CA, van Tiel FH. Surgical site infections: how high are the costs? J Hosp Infect. 2009;72:193–201. doi: 10.1016/j.jhin.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Zak O, O'Reilly T. Animal models in the evaluation of antimicrobial agents. Antimicrob Agents Chemother. 1991;35:1527–1531. doi: 10.1128/aac.35.8.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser AB, Kernodle DS, Parker RA. Low-inoculum model of surgical wound infection. J Infect Dis. 1992;166:393–399. doi: 10.1093/infdis/166.2.393. [DOI] [PubMed] [Google Scholar]
- 10.McRipley RJ, Whitney RR. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob Agents Chemother. 1976;10:38–44. doi: 10.1128/aac.10.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilpin DA. Calculation of a new Meeh constant and experimental determination of burn size. Burns. 1996;22:607–611. doi: 10.1016/s0305-4179(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 12.Rittenhouse S, Singley C, Hoover J, Page R, Payne D. Use of the surgical wound infection model to determine the efficacious dosing regimen of retapamulin, a novel topical antibiotic. Antimicrob Agents Chemother. 2006;50:3886–3888. doi: 10.1128/AAC.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rupp ME, Ulphani JS, Fey PD, Bartscht K, Mack D. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect Immun. 1999;67:2627–2632. doi: 10.1128/iai.67.5.2627-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuklin NA, Pancari GD, Tobery TW, Cope L, Jackson J, Gill C, et al. Real-time monitoring of bacterial infection in vivo: development of bioluminescent staphylococcal foreign-body and deep-thigh-wound mouse infection models. Antimicrob Agents Chemother. 2003;47:2740–2748. doi: 10.1128/AAC.47.9.2740-2748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espersen F, Frimodt-Moller N, Corneliussen L, Riber U, Rosdahl VT, Skinhoj P. Effect of treatment with methicillin and gentamicin in a new experimental mouse model of foreign body infection. Antimicrob Agents Chemother. 1994;38:2047–2053. doi: 10.1128/aac.38.9.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Actor P, Grappel SF. Efficacy of ceftizoxime and related compounds in animals models of infection. J Antimicrob Chemother. 1982;10:81–89. doi: 10.1093/jac/10.suppl_c.81. [DOI] [PubMed] [Google Scholar]
- 17.Ford CW, Hamel JC, Stapert D, Yancey RJ. Establishment of an experimental model of a Staphylococcus aureus abscess in mice by use of dextran and gelatin microcarriers. J Med Microbiol. 1989;28:259–266. doi: 10.1099/00222615-28-4-259. [DOI] [PubMed] [Google Scholar]
- 18.Bunce C, Wheeler L, Reed G, Musser J, Barg N. Murine model of cutaneous infection with gram-positive cocci. Infect Immun. 1992;60:2636–2640. doi: 10.1128/iai.60.7.2636-2640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarboro SR, Baum EJ, Dahners LE. Locally administered antibiotics for prophylaxis against surgical wound infection. An in vivo study. J Bone Joint Surg Am. 2007;89:929–933. doi: 10.2106/JBJS.F.00919. [DOI] [PubMed] [Google Scholar]
- 20.Cavanaugh DL, Berry J, Yarboro SR, Dahners LE. Better prophylaxis against surgical site infection with local as well as systemic antibiotics. An in vivo study. J Bone Joint Surg Am. 2009;91:1907–1912. doi: 10.2106/JBJS.G.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Wijngaerden E, Peetermans WE, Vandersmissen J, Van Lierde S, Bobbaers H, Van Eldere J. Foreign body infection: a new rat model for prophylaxis and treatment. J Antimicrob Chemother. 1999;44:669–674. doi: 10.1093/jac/44.5.669. [DOI] [PubMed] [Google Scholar]
- 22.Bergamini TM, Lamont PM, Cheadle WG, Polk HC., Jr Combined topical and systemic antibiotic prophylaxis in experimental wound infection. Am J Surg. 1984;147:753–756. doi: 10.1016/0002-9610(84)90193-4. [DOI] [PubMed] [Google Scholar]
- 23.Fallon MT, Shafer W, Jacob E. Use of cefazolin microspheres to treat localized methicillin-resistant Staphylococcus aureus infections in rats. J Surg Res. 1999;86:97–102. doi: 10.1006/jsre.1999.5686. [DOI] [PubMed] [Google Scholar]
- 24.Moesgaard F, Lykkegaard Nielsen MC, Justesen T. Experimental animal model of surgical wound infection applicable to antibiotic prophylaxis. Eur J Clin Microbiol. 1983;2:459–462. doi: 10.1007/BF02013904. [DOI] [PubMed] [Google Scholar]
- 25.Stratford AF, Zoutman DE, Davidson JS. Effect of lidocaine and epinephrine on Staphylococcus aureus in a guinea pig model of surgical wound infection. Plast Reconstr Surg. 2002;110:1275–1279. doi: 10.1097/01.PRS.0000025427.86301.8A. [DOI] [PubMed] [Google Scholar]
- 26.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 27.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, et al. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraft WG, Johnson PT, David BC, Morgan DR. Cutaneous infection in normal and immunocompromised mice. Infect Immun. 1986;52:707–713. doi: 10.1128/iai.52.3.707-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn BL, Bischof TS, Sohnle PG. Superficial exudates of neutrophils prevent invasion of Bacillus anthracis bacilli into abraded skin of resistant mice. Int J Exp Pathol. 2008;89:180–187. doi: 10.1111/j.1365-2613.2008.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kugelberg E, Norstrom T, Petersen TK, Duvold T, Andersson DI, Hughes D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob Agents Chemother. 2005;49:3435–3441. doi: 10.1128/AAC.49.8.3435-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y, Shamaei-Tousi A, Liu Y, Coates A. A new approach for the discovery of antibiotics by targeting non-multiplying bacteria: a novel topical antibiotic for staphylococcal infections. PLoS One. 2010;5:11818. doi: 10.1371/journal.pone.0011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onunkwo CC, Hahn BL, Sohnle PG. Clearance of experimental cutaneous Staphylococcus aureus infections in mice. Arch Dermatol Res. 2010;302:375–382. doi: 10.1007/s00403-010-1030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaspari AA, Burns R, Nasir A, Ramirez D, Barth RK, Haidaris CG. CD86 (B7-2), but not CD80 (B7-1), expression in the epidermis of transgenic mice enhances the immunogenicity of primary cutaneous Candida albicans infections. Infect Immun. 1998;66:4440–4449. doi: 10.1128/iai.66.9.4440-4449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munster AM, Leary AG, Baird B. A simple trauma model in the rat. J Trauma. 1973;13:827–828. doi: 10.1097/00005373-197309000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Jeray KJ, Banks DM, Phieffer LS, Middlebrooks ES, Frankenburg KP, Hudson MC, et al. Evaluation of standard surgical preparation performed on superficial dermal abrasions. J Orthop Trauma. 2000;14:206–211. doi: 10.1097/00005131-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Fader RC, Nunez D, Unbehagen J, Linares HA. Experimental candidiasis after thermal injury. Infect Immun. 1985;49:780–784. doi: 10.1128/iai.49.3.780-784.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones WG, 2nd, Minei JP, Barber AE, Rayburn JL, Fahey TJ, 3rd, Shires GT, 3rd, et al. Bacterial translocation and intestinal atrophy after thermal injury and burn wound sepsis. Ann Surg. 1990;211:399–405. doi: 10.1097/00000658-199004000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salisbury RE, Bevin AG, Steinkraus GE, Enterline DS. Burn wound sepsis: effect of delayed treatment with topical chemotherapy on survival. J Trauma. 1980;20:120–122. [PubMed] [Google Scholar]
- 41.Rode H, de Wet PM, Davies MR, Cywes S. An experimental evaluation of the germicidal efficacy of three topical antimicrobial agents in burns. Prog Pediatr Surg. 1981;14:189–208. [PubMed] [Google Scholar]
- 42.Nakae H, Inaba H, Endo S. Usefulness of procalcitonin in Pseudomonas burn wound sepsis model. Tohoku J Exp Med. 1999;188:271–273. doi: 10.1620/tjem.188.271. [DOI] [PubMed] [Google Scholar]
- 43.Barnea Y, Carmeli Y, Kuzmenko B, Gur E, Hammer-Munz O, Navon-Venezia S. The establishment of a Pseudomonas aeruginosa-infected burn-wound sepsis model and the effect of imipenem treatment. Ann Plast Surg. 2006;56:674–679. doi: 10.1097/01.sap.0000203984.62284.7a. [DOI] [PubMed] [Google Scholar]
- 44.Chalekson CP, Neumeister MW, Jaynes J. Improvement in burn wound infection and survival with antimicrobial peptide D2A21 (Demegel) Plast Reconstr Surg. 2002;109:1338–1343. doi: 10.1097/00006534-200204010-00020. [DOI] [PubMed] [Google Scholar]
- 45.Steinstraesser L, Klein RD, Aminlari A, Fan MH, Khilanani V, Remick DG, et al. Protegrin-1 enhances bacterial killing in thermally injured skin. Crit Care Med. 2001;29:1431–1437. doi: 10.1097/00003246-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 46.Steinstraesser L, Tack BF, Waring AJ, Hong T, Boo LM, Fan MH, et al. Activity of novispirin G10 against Pseudomonas aeruginosa in vitro and in infected burns. Antimicrob Agents Chemother. 2002;46:1837–1844. doi: 10.1128/AAC.46.6.1837-1844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobsen F, Mittler D, Hirsch T, Gerhards A, Lehnhardt M, Voss B, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 2005;12:1494–1502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 48.Ulkur E, Oncul O, Karagoz H, Celikoz B, Cavuslu S. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass) and silver sulfadiazine 1% (Silverdin) for topical antibacterial effect in Pseudomonas aeruginosa-contaminated, full-skin thickness burn wounds in rats. J Burn Care Rehabil. 2005;26:430–433. doi: 10.1097/01.bcr.0000176879.27535.09. [DOI] [PubMed] [Google Scholar]
- 49.Chu CS, McManus AT, Mason AD, Pruitt BA., Jr Topical silver treatment after escharectomy of infected full thickness burn wounds in rats. J Trauma. 2005;58:1040–1046. doi: 10.1097/01.ta.0000162993.91698.fa. [DOI] [PubMed] [Google Scholar]
- 50.Chu CS, McManus AT, Pruitt BA, Jr, Mason AD., Jr Therapeutic effects of silver nylon dressings with weak direct current on Pseudomonas aeruginosa-infected burn wounds. J Trauma. 1988;28:1488–1492. doi: 10.1097/00005373-198810000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Nakae H, Inaba H. Effectiveness of electrolyzed oxidized water irrigation in a burn-wound infection model. J Trauma. 2000;49:511–514. doi: 10.1097/00005373-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Gauglitz GG, Toliver-Kinsky TE, Williams FN, Song J, Cui W, Herndon DN, et al. Insulin increases resistance to burn wound infection-associated sepsis. Crit Care Med. 2010;38:202–208. doi: 10.1097/CCM.0b013e3181b43236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hahn EL, Tai HH, He LK, Gamelli RL. Burn injury with infection alters prostaglandin E2 synthesis and metabolism. J Trauma. 1999;47:1052–1057. doi: 10.1097/00005373-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 54.White J, Thomas J, Maass DL, Horton JW. Cardiac effects of burn injury complicated by aspiration pneumonia-induced sepsis. Am J Physiol Heart Circ Physiol. 2003;285:47–58. doi: 10.1152/ajpheart.00833.2002. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Quan J, Johnston WE, Maass DL, Horton JW, Thomas JA, et al. Age-dependent differences of interleukin-6 activity in cardiac function after burn complicated by sepsis. Burns. 2010;36:232–238. doi: 10.1016/j.burns.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White J, Maass DL, Giroir B, Horton JW. Development of an acute burn model in adult mice for studies of cardiac function and cardiomyocyte cellular function. Shock. 2001;16:122–129. doi: 10.1097/00024382-200116020-00007. [DOI] [PubMed] [Google Scholar]
- 57.Horton JW. A model of myocardial inflammation and dysfunction in burn complicated by sepsis. Shock. 2007;28:326–333. doi: 10.1097/01.shk.0000238064.54332.c8. [DOI] [PubMed] [Google Scholar]
- 58.Bjornson AB, Bjornson HS, Lincoln NA, Altemeier WA. Relative roles of burn injury, wound colonization and wound infection in induction of alterations of complement function in a guinea pig model of burn injury. J Trauma. 1984;24:106–115. doi: 10.1097/00005373-198402000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Stieritz DD, Holder IA. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975;131:688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- 60.Cryz SJ, Jr, Furer E, Germanier R. Prevention of fatal experimental burn-wound sepsis due to Klebsiella pneumoniae KP1-O by immunization with homologous capsular polysaccharide. J Infect Dis. 1984;150:817–822. doi: 10.1093/infdis/150.6.817. [DOI] [PubMed] [Google Scholar]
- 61.Cryz SJ, Jr, Furer F, Germanier R. Experimental Klebsiella pneumoniae burn wound sepsis: role of capsular polysaccharide. Infect Immun. 1984;43:440–441. doi: 10.1128/iai.43.1.440-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stover GB, Drake DR, Montie TC. Virulence of different Pseudomonas species in a burned mouse model: tissue colonization by Pseudomonas cepacia. Infect Immun. 1983;41:1099–1104. doi: 10.1128/iai.41.3.1099-1104.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Felts AG, Giridhar G, Grainger DW, Slunt JB. Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa infection in a murine burn wound model. Burns. 1999;25:415–423. doi: 10.1016/s0305-4179(99)00017-0. [DOI] [PubMed] [Google Scholar]
- 64.Katakura T, Yoshida T, Kobayashi M, Herndon DN, Suzuki F. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin Exp Immunol. 2005;142:419–425. doi: 10.1111/j.1365-2249.2005.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shigematsu K, Asai A, Kobayashi M, Herndon DN, Suzuki F. Enterococcus faecalis translocation in mice with severe burn injury: a pathogenic role of CCL2 and alternatively activated macrophages (M2aMphi and M2cMphi) J Leukoc Biol. 2009;86:999–1005. doi: 10.1189/jlb.0409235. [DOI] [PubMed] [Google Scholar]
- 66.Tsuda Y, Shigematsu K, Kobayashi M, Herndon DN, Suzuki F. Role of polymorphonuclear neutrophils on infectious complications stemming from Enterococcus faecalis oral infection in thermally injured mice. J Immunol. 2008;180:4133–4138. doi: 10.4049/jimmunol.180.6.4133. [DOI] [PubMed] [Google Scholar]
- 67.Lyuksutova OI, Murphey ED, Toliver-Kinsky TE, Lin CY, Cui W, Williams DL, et al. Glucan phosphate treatment attenuates burn-induced inflammation and improves resistance to Pseudomonas aeruginosa burn wound infection. Shock. 2005;23:224–232. [PubMed] [Google Scholar]
- 68.Stevens EJ, Ryan CM, Friedberg JS, Barnhill RL, Yarmush ML, Tompkins RG. A quantitative model of invasive Pseudomonas infection in burn injury. J Burn Care Rehabil. 1994;15:232–235. doi: 10.1097/00004630-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Lambrechts SA, Demidova TN, Aalders MC, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem Photobiol Sci. 2005;4:503–509. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–3934. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53:393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ragas X, Sanchez-Garcia D, Ruiz-Gonzalez R, Dai T, Agut M, Hamblin MR, et al. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. J Med Chem. 2010;53:7796–7803. doi: 10.1021/jm1009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Busch NA, Zanzot EM, Loiselle PM, Carter EA, Allaire JE, Yarmush ML, et al. A model of infected burn wounds using Escherichia coli O18:K1:H7 for the study of Gram-negative bacteremia and sepsis. Infect Immun. 2000;68:3349–3351. doi: 10.1128/iai.68.6.3349-3351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orenstein A, Klein D, Kopolovic J, Winkler E, Malik Z, Keller N, et al. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol Med Microbiol. 1997;19:307–314. doi: 10.1111/j.1574-695X.1997.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 75.Stinnett JD, Loose LD, Miskell P, Tenney CL, Gonce SJ, Alexander JW. Synthetic immunomodulators for prevention of fatal infections in a burned guinea pig model. Ann Surg. 1983;198:53–57. doi: 10.1097/00000658-198307000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singer AJ, McClain SA. Persistent wound infection delays epidermal maturation and increases scarring in thermal burns. Wound Repair Regen. 2002;10:372–377. doi: 10.1046/j.1524-475x.2002.10606.x. [DOI] [PubMed] [Google Scholar]
- 77.Breuing K, Kaplan S, Liu P, Onderdonk AB, Eriksson E. Wound fluid bacterial levels exceed tissue bacterial counts in controlled porcine partial-thickness burn infections. Plast Reconstr Surg. 2003;111:781–788. doi: 10.1097/01.PRS.0000041540.22057.89. [DOI] [PubMed] [Google Scholar]
- 78.Wolfe RR, Miller HI. Burn shock in untreated and saline-resuscitated guinea pigs. Development of a model. J Surg Res. 1976;21:269–276. doi: 10.1016/0022-4804(76)90037-8. [DOI] [PubMed] [Google Scholar]
- 79.Herndon DN, Wilmore DW, Mason AD., Jr Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res. 1978;25:394–403. doi: 10.1016/s0022-4804(78)80003-1. [DOI] [PubMed] [Google Scholar]
- 80.Kaufman T, Lusthaus SN, Sagher U, Wexler MR. Deep partial skin thickness burns: a reproducible animal model to study burn wound healing. Burns. 1990;16:13–16. doi: 10.1016/0305-4179(90)90199-7. [DOI] [PubMed] [Google Scholar]
- 81.Branski LK, Mittermayr R, Herndon DN, Norbury WB, Masters OE, Hofmann M, et al. A porcine model of full-thickness burn, excision and skin autografting. Burns. 2008;34:1119–1127. doi: 10.1016/j.burns.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manafi A, Kohanteb J, Mehrabani D, Japoni A, Amini M, Naghmachi M, et al. Active immunization using exotoxin A confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC Microbiol. 2009;9:23. doi: 10.1186/1471-2180-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumari S, Harjai K, Chhibber S. Topical treatment of Klebsiella pneumoniae B5055 induced burn wound infection in mice using natural products. J Infect Dev Ctries. 2010;4:367–377. [PubMed] [Google Scholar]
- 84.Calum H, Moser C, Jensen PO, Christophersen L, Maling DS, van Gennip M, et al. Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clin Exp Immunol. 2009;156:102–110. doi: 10.1111/j.1365-2249.2008.03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bahar T, Bilezikci B, Maral T, Borman H. A modified partial-thickness burn model in rats. Burns. 2007;33:52–53. [Google Scholar]
- 86.Suzuki T, Hirayama T, Aihara K, Hirohata Y. Experimental studies of moderate temperature burns. Burns. 1991;17:443–451. doi: 10.1016/0305-4179(91)90069-s. [DOI] [PubMed] [Google Scholar]
- 87.Hollander JE, Singer AJ, Valentine SM, Shofer FS. Risk factors for infection in patients with traumatic lacerations. Acad Emerg Med. 2001;8:716–720. doi: 10.1111/j.1553-2712.2001.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 88.Edlich RF, Tsung MS, Rogers W, Rogers P, Wangensteen OH. Studies in management of the contaminated wound. I. Technique of closure of such wounds together with a note on a reproducible experimental model. J Surg Res. 1968;8:585–592. doi: 10.1016/0022-4804(68)90013-9. [DOI] [PubMed] [Google Scholar]
- 89.Edlich RF, Prusak M, Panek P, Madden J, Wangensteen OH, Thul J. Studies in the management of the contaminated wound. 8. Assessment of tissue adhesives for repair of contaminated tissue. Am J Surg. 1971;122:394–397. doi: 10.1016/0002-9610(71)90266-2. [DOI] [PubMed] [Google Scholar]
- 90.Edlich RF, Madden JE, Prusak M, Panek P, Thul J, Wangensteen OH. Studies in the management of the contaminated wound. VI. The therapeutic value of gentle scrubbing in prolonging the limited period of effectiveness of antibiotics in contaminated wounds. Am J Surg. 1971;121:668–672. [PubMed] [Google Scholar]
- 91.Custer J, Edlich RF, Prusak M, Madden J, Panek P, Wangensteen OH. Studies in the management of the contaminated wound. V. An assessment of the effectiveness of pHisoHex and Betadine surgical scrub solutions. Am J Surg. 1971;121:572–575. doi: 10.1016/0002-9610(71)90142-5. [DOI] [PubMed] [Google Scholar]
- 92.Edlich RF, Custer J, Madden J, Dajani AS, Rogers W, Wangensteen OH. Studies in management of the contaminated wound. 3. Assessment of the effectiveness of irrigation with antiseptic agents. Am J Surg. 1969;118:21–30. doi: 10.1016/0002-9610(69)90089-0. [DOI] [PubMed] [Google Scholar]
- 93.Edlich RF, Rogers W, Kasper G, Kaufman D, Tsung MS, Wangensteen OH. Studies in the management of the contaminated wound. I. Optimal time for closure of contaminated open wounds. II. Comparison of resistance to infection of open and closed wounds during healing. Am J Surg. 1969;117:323–329. doi: 10.1016/0002-9610(69)90365-1. [DOI] [PubMed] [Google Scholar]
- 94.Howell JM, Dhindsa HS, Stair TO, Edwards BA. Effect of scrubbing and irrigation on staphylococcal and streptococcal counts in contaminated lacerations. Antimicrob Agents Chemother. 1993;37:2754–2755. doi: 10.1128/aac.37.12.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Howell JM, Bresnahan KA, Stair TO, Dhindsa HS, Edwards BA. Comparison of effects of suture and cyanoacrylate tissue adhesive on bacterial counts in contaminated lacerations. Antimicrob Agents Chemother. 1995;39:559–560. doi: 10.1128/aac.39.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moscati R, Mayrose J, Fincher L, Jehle D. Comparison of normal saline with tap water for wound irrigation. Am J Emerg Med. 1998;16:379–381. doi: 10.1016/s0735-6757(98)90133-4. [DOI] [PubMed] [Google Scholar]
- 97.Lammers R, Henry C, Howell J. Bacterial counts in experimental, contaminated crush wounds irrigated with various concentrations of cefazolin and penicillin. Am J Emerg Med. 2001;19:1–5. doi: 10.1053/ajem.2001.18115. [DOI] [PubMed] [Google Scholar]
- 98.Gross A, Cutright DE, Bhaskar SN. Effectiveness of pulsating water jet lavage in treatment of contaminated crushed wounds. Am J Surg. 1972;124:373–377. doi: 10.1016/0002-9610(72)90044-x. [DOI] [PubMed] [Google Scholar]
- 99.Erdur B, Ersoy G, Yilmaz O, Ozkutuk A, Sis B, Karcioglu O, et al. A comparison of the prophylactic uses of topical mupirocin and nitrofurazone in murine crush contaminated wounds. Am J Emerg Med. 2008;26:137–143. doi: 10.1016/j.ajem.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 100.Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 101.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, A PC, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burkatovskaya M, Castano AP, Demidova-Rice TN, Tegos GP, Hamblin MR. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simonetti O, Cirioni O, Ghiselli R, Goteri G, Scalise A, Orlando F, et al. RNAIII-inhibiting peptide enhances healing of wounds infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52:2205–2211. doi: 10.1128/AAC.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahoney E, Reichner J, Bostom LR, Mastrofrancesco B, Henry W, Albina J. Bacterial colonization and the expression of inducible nitric oxide synthase in murine wounds. Am J Pathol. 2002;161:2143–2152. doi: 10.1016/s0002-9440(10)64492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi CM, Nakao H, Yamazaki M, Tsuboi R, Ogawa H. Mixture of sugar and povidone-iodine stimulates healing of MRSA-infected skin ulcers on db/db mice. Arch Dermatol Res. 2007;299:449–456. doi: 10.1007/s00403-007-0776-3. [DOI] [PubMed] [Google Scholar]
- 106.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354–359. doi: 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 107.Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009;129:2463–2469. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- 108.Nayak BS, Maiya A, Kumar P. Influence of Helium-Neon laser photostimulation on excision wound healing in Wistar Rats. OnLine J Biol Sci. 2007;7:89–92. [Google Scholar]
- 109.Chalekson CP, Neumeister MW, Jaynes J. Treatment of infected wounds with the antimicrobial peptide D2A21. J Trauma. 2003;54:770–774. doi: 10.1097/01.TA.0000047047.79701.6D. [DOI] [PubMed] [Google Scholar]
- 110.Bracho DO, Barsan L, Arekapudi SR, Thompson JA, Hen J, Stern SA, et al. Antibacterial properties of an iron-based hemostatic agent in vitro and in a rat wound model. Acad Emerg Med. 2009;16:656–660. doi: 10.1111/j.1553-2712.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 111.Adhirajan N, Shanmugasundaram N, Shanmuganathan S, Babu M. Collagen-based wound dressing for doxycycline delivery: in-vivo evaluation in an infected excisional wound model in rats. J Pharm Pharmacol. 2009;61:1617–1623. doi: 10.1211/jpp/61.12.0005. [DOI] [PubMed] [Google Scholar]
- 112.Saymen DG, Nathan P, Holder IA, Hill EO, Macmillan BG. Infected surface wound: an experimental model and a method for the quantitation of bacteria in infected tissues. Appl Microbiol. 1972;23:509–514. doi: 10.1128/am.23.3.509-514.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fries RB, Wallace WA, Roy S, Kuppusamy P, Bergdall V, Gordillo GM, et al. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005;579:172–181. doi: 10.1016/j.mrfmmm.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 114.Hirsch T, Spielmann M, Zuhaili B, Koehler T, Fossum M, Steinau HU, et al. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg. 2008;8:5. doi: 10.1186/1471-2482-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hirsch T, Spielmann M, Zuhaili B, Fossum M, Metzig M, Koehler T, et al. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med. 2009;11:220–228. doi: 10.1002/jgm.1287. [DOI] [PubMed] [Google Scholar]
- 116.Kilpadi DV, Feeley TD, Kiel JW. Therapy normalizes vascular response of injured tissue in full-thickness wounds in rabbits. Ann Plast Surg. 2007;58:555–560. doi: 10.1097/01.sap.0000244991.90285.c3. [DOI] [PubMed] [Google Scholar]
- 117.Quinn RH, Macias DJ. The management of open fractures. Wilderness Environ Med. 2006;17:41–48. doi: 10.1580/pr09-05.1. [DOI] [PubMed] [Google Scholar]
- 118.Worlock P, Slack R, Harvey L, Mawhinney R. The prevention of infection in open fractures. An experimental study of the effect of antibiotic therapy. J Bone Joint Surg Am. 1988;70:1341–1347. [PubMed] [Google Scholar]
- 119.Jacob E, Cierny G, 3rd, Fallon MT, McNeill JF, Jr, Siderys GS. Evaluation of biodegradable cefazolin sodium microspheres for the prevention of infection in rabbits with experimental open tibial fractures stabilized with internal fixation. J Orthop Res. 1993;11:404–411. doi: 10.1002/jor.1100110312. [DOI] [PubMed] [Google Scholar]
- 120.Jacob E, Cierny G, 3rd, Zorn K, McNeill JF, Fallon MT. Delayed local treatment of rabbit tibial fractures with biodegradable cefazolin microspheres. Clin Orthop Relat Res. 1997:278–285. doi: 10.1097/00003086-199703000-00036. [DOI] [PubMed] [Google Scholar]
- 121.Arens S, Schlegel U, Printzen G, Ziegler WJ, Perren SM, Hansis M. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78:647–651. [PubMed] [Google Scholar]
- 122.Arens S, Eijer H, Schlegel U, Printzen G, Perren SM, Hansis M. Influence of the design for fixation implants on local infection: experimental study of dynamic compression plates versus point contact fixators in rabbits. J Orthop Trauma. 1999;13:470–476. doi: 10.1097/00005131-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 123.Khodaparast O, Coberly DM, Mathey J, Rohrich RJ, Levin LS, Brown SA. Effect of a transpositional muscle flap on VEGF mRNA expression in a canine fracture model. Plast Reconstr Surg. 2003;112:171–176. doi: 10.1097/01.PRS.0000066170.56389.27. [DOI] [PubMed] [Google Scholar]
- 124.Buxton TB, Travis MT, O'Shea KJ, McPherson JC, 3rd, Harvey SB, Plowman KM, et al. Low-dose infectivity of Staphylococcus aureus (SMH strain) in traumatized rat tibiae provides a model for studying early events in contaminated bone injuries. Comp Med. 2005;55:123–128. [PubMed] [Google Scholar]
- 125.Hill PF, Clasper JC, Parker SJ, Watkins PE. Early intramedullary nailing in an animal model of a heavily contaminated fracture of the tibia. J Orthop Res. 2002;20:648–653. doi: 10.1016/S0736-0266(01)00163-2. [DOI] [PubMed] [Google Scholar]
- 126.Svoboda SJ, Bice TG, Gooden HA, Brooks DE, Thomas DB, Wenke JC. Comparison of bulb syringe and pulsed lavage irrigation with use of a bioluminescent musculoskeletal wound model. J Bone Joint Surg Am. 2006;88:2167–2174. doi: 10.2106/JBJS.E.00248. [DOI] [PubMed] [Google Scholar]
- 127.Curtis MJ, Brown PR, Dick JD, Jinnah RH. Contaminated fractures of the tibia: a comparison of treatment modalities in an animal model. J Orthop Res. 1995;13:286–295. doi: 10.1002/jor.1100130217. [DOI] [PubMed] [Google Scholar]
- 128.Lindsey BA, Clovis NB, Smith ES, Salihu S, Hubbard DF. An animal model for open femur fracture and osteomyelitis: Part I. J Orthop Res. 2010;28:38–42. doi: 10.1002/jor.20960. [DOI] [PubMed] [Google Scholar]
- 129.Lindsey BA, Clovis NB, Smith ES, Salihu S, Hubbard DF. An animal model for open femur fracture and osteomyelitis—Part II: Immunomodulation with systemic IL-12. J Orthop Res. 2010;28:43–47. doi: 10.1002/jor.20959. [DOI] [PubMed] [Google Scholar]
- 130.Li B, Jiang B, Dietz MJ, Smith ES, Clovis NB, Rao KM. Evaluation of local MCP-1 and IL-12 nano-coatings for infection prevention in open fractures. J Orthop Res. 2010;28:48–54. doi: 10.1002/jor.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stewart RL, Cox JT, Volgas D, Stannard J, Duffy L, Waites KB, et al. The use of a biodegradable, load-bearing scaffold as a carrier for antibiotics in an infected open fracture model. J Orthop Trauma. 2010;24:587–591. doi: 10.1097/BOT.0b013e3181ed1349. [DOI] [PubMed] [Google Scholar]
- 132.Brown KV, Walker JA, Cortez DS, Murray CK, Wenke JC. Earlier debridement and antibiotic administration decrease infection. J Surg Orthop Adv. 2010;19:18–22. [PubMed] [Google Scholar]
- 133.Merritt K, Dowd JD. Role of internal fixation in infection of open fractures: studies with Staphylococcus aureus and Proteus mirabilis. J Orthop Res. 1987;5:23–28. doi: 10.1002/jor.1100050105. [DOI] [PubMed] [Google Scholar]
- 134.Grewe SR, Stephens BO, Perlino C, Riggins RS. Influence of internal fixation on wound infections. J Trauma. 1987;27:1051–1054. doi: 10.1097/00005373-198709000-00016. [DOI] [PubMed] [Google Scholar]
- 135.Mody RM, Zapor M, Hartzell JD, Robben PM, Waterman P, Wood-Morris R, et al. Infectious complications of damage control orthopedics in war trauma. J Trauma. 2009;67:758–761. doi: 10.1097/TA.0b013e3181af6aa6. [DOI] [PubMed] [Google Scholar]
- 136.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis. 2007;45:409–415. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 137.An YH, Kang QK, Arciola CR. Animal models of osteomyelitis. Int J Artif Organs. 2006;29:407–420. doi: 10.1177/039139880602900411. [DOI] [PubMed] [Google Scholar]
- 138.Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. 2002;2:688–698. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- 139.Buer J, Balling R. Mice, microbes and models of infection. Nat Rev Genet. 2003;4:195–205. doi: 10.1038/nrg1019. [DOI] [PubMed] [Google Scholar]
- 140.Zanotti-Cavazzoni SL, Goldfarb RD. Animal models of sepsis. Crit Care Clin. 2009;25:703–719. doi: 10.1016/j.ccc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 141.Neely AN, Holder IA, Warden GD. Then and now: studies using a burned mouse model reflect trends in burn research over the past 25 years. Burns. 1999;25:603–609. doi: 10.1016/s0305-4179(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 142.Rocchetta HL, Boylan CJ, Foley JW, Iversen PW, LeTourneau DL, McMillian CL, et al. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001;45:129–137. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68:3594–3600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, et al. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun. 2001;69:3350–3358. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thorn RM, Greenman J. A novel in vitro flat-bed perfusion biofilm model for determining the potential antimicrobial efficacy of topical wound treatments. J Appl Microbiol. 2009;107:2070–2079. doi: 10.1111/j.1365-2672.2009.04398.x. [DOI] [PubMed] [Google Scholar]
- 147.Jawhara S, Mordon S. Monitoring of bactericidal action of laser by in vivo imaging of bioluminescent E. coli in a cutaneous wound infection. Lasers Med Sci. 2006;21:153–159. doi: 10.1007/s10103-006-0388-8. [DOI] [PubMed] [Google Scholar]
- 148.Jawhara S, Mordon S. In vivo imaging of bioluminescent Escherichia coli in a cutaneous wound infection model for evaluation of an antibiotic therapy. Antimicrob Agents Chemother. 2004;48:3436–3441. doi: 10.1128/AAC.48.9.3436-3441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, Vecchiarelli A, et al. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun. 2009;77:4847–4858. doi: 10.1128/IAI.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]