Abstract
We recently reported on a new wrinkle of complexity in how eukaryotic genes are regulated by providing evidence for a hitherto unknown nuclear function of the signaling kinase, Protein Kinase C-theta (PKC-θ). This chromatin-anchored complex positively regulates inducible immune genes and negatively regulates target miRNA genes. These data challenge the traditional view of mammalian signaling kinases and provides new avenues for therapeutic drug design.
Key words: chromatin, signaling kinases, transcription, inducible genes, T cell, immune system, microRNAs
Complex programs of transcriptional regulation orchestrate the carefully coordinated expression of many eukaryotic genes, whose functions are required in different tissues and at particular times during development and in mature eukaryotic organisms. The molecular mechanisms that mediate such precise and intricate control over the expression of eukaryotic genes have been extensively studied; however, the extent to which dynamic changes in chromatin structure contribute to this process remains poorly understood. We recently reported on a new wrinkle of complexity in how eukaryotic genes are regulated in order to achieve specific outcomes in a specific cell type or biological context. Our recent work demonstrates that the signaling kinase PKC-θ plays perhaps equally significant biological roles when docked in lipid rafts in the plasma membrane and when tethered to chromatin in the nucleus of human T cells.1 PKC-θ forms a chromatin-anchored complex and acts as an indirect activator of downstream transcription factors in transcriptionally activated human T cells. Notably, the active multi-subunit complex includes RNA polymerase II (RNAP II), histone kinase MSK-1, demethylase LSD1 and the adaptor molecule 14-3-3ζ. Furthermore, this complex is enriched in regulatory and 5′-transcribed regions of inducible immune response genes in activated but not resting human T cells, and in the promoter regions, but not in transcribed regions, of target microRNA (miRNA) genes. Because PKC-θ negatively regulates target miRNA genes identified to date, the latter function is novel and was entirely unanticipated. These data challenge the traditional view of mammalian signal transduction protein kinases and demands a re-examination of the scope of their intracellular function.
PKC-θ was traditionally thought to function exclusively in the cytoplasm; however, our observation that PKC-θ also plays a role in the nucleus and associates with chromatin is not without precedent. For example, it is now recognized that actin and myosin, previously thought of as cytoplasmic cytoskeletal proteins involved primarily in cell structure and motility, cooperate with all three RNA polymerases to regulate transcription of target genes in the nucleus.2–4 Similarly, in plants the metabolic enzyme nuclear hexokinase1 forms a glucose signaling complex that directly regulates transcription of target genes.5 Gomez-Ospina et al. showed that a calcium channel can act as both an ion pore and function as a transcription factor.6 Another well-known example of a protein kinase with dual roles in two different subcellular compartments is the yeast stress-induced MAP kinase Hog1, which is the homolog of mammalian p38α. Upon activation, such signal transduction kinases interact directly with specific target genes in the nucleus.7–9 In the case of Hog1, this process is critical for the response to osmotic stress and is tightly regulated; similar to PKC-θ, activated Hog1 associates with the promoter as well as downstream transcribed regions of its target genes.7,9,10
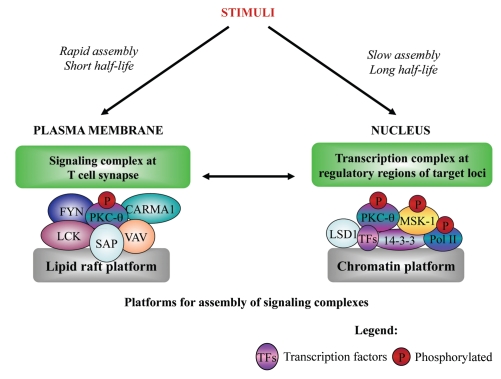
Upon stimulation of the TCR and CD28 co-receptor, activation of a signaling scheme involving PKC-θ is among the complex series of molecular events initiated.11,12 PKC-θ is a member of the Ca2+-independent novel PKC (nPKC) subfamily (which also includes PKC-δ, ε and η) and is primarily expressed in T lymphocytes, muscle cells and platelets,13–15 although a smaller isoform, PKCθII, is specifically expressed in the testis.16 Conversely, the conventional PKCs (cPKCs; α, β and γ) are activated by Ca2+ and the second messenger diacylglycerol (DAG), whilst the atypical PKCs (aPKCs; ζ and λ) are not activated by either Ca2+ or DAG.17 PKC-θ selectively migrates to the central supramolecular activation complex region of the immunological synapse at the plasma membrane via lipid rafts in activated T cells.12,18,19 Here, PKC-θ interacts with other signaling molecules (Fig. 1) to induce a cascade of events in antigen- stimulated T cells, including activation of transcription factors such as NFκB and AP-1.11 For the first time, we have shown that in addition to the PKC-θ complex that assembles on lipid rafts, a second signaling complex forms that is tethered to critical transcriptional targets on chromatin (Fig. 1). Importantly, PKC-mediated phosphoinositide signaling appears to be intact and responsive to both the plasma membrane-associated and chromatin-associated PKC-θ complexes.20–22 Not only is PKC-θ signaling necessary for T-cell activation and IL-2 expression, but this kinase also plays a pivotal part in T-cell-mediated immune disorders that are characterized by Th1/Th2 CD4+ T-cell-mediated responses.23–26 Furthermore, dysregulation of PKC-θ has been implicated in pathologies such as tumorigenesis.27–29 Indeed, our genome-wide analysis in conjunction with our functional studies support these findings, as PKC-θ appears to be coupled to processes that regulate epithelial to mesenchymal transition that is a key feature of tumor progression.1
Figure 1.
Following activation of T cells, two distinct PKC-θ signaling complexes are initiated, either at the plasma membrane or in the nucleus. The complex at the immunological synapse is formed rapidly within minutes by the transient association of numerous signaling molecules, such as those depicted in this figure, using the lipid rafts as an anchoring platform. Incontrast, an independent nuclear PKC-θ signaling complex associated with the active transcription apparatus is formed at gene-specific loci using chromatin as the docking platform. Here, it is proposed that this nuclear signaling complex is formed by the association of distinct proteins, compared to that at the plasma membrane. Different stimuli contribute to the formation of each of these signaling complexes, possibly attributing to the more delayed nuclear PKC-θ assembly kinetics that can be detected by ChIP up to 24 h post-stimulation.
To understand the duplicity of PKC-θ and the downstream events that it regulates, future studies must address many unanswered questions: how does PKC-θ enter the nucleus? How are the cytoplasmic and nuclear functions of PKC-θ integrated to efficiently drive an appropriate physiological response? Does chromatin-associated PKC-θ have distinct binding partners in different cell types? Does chromatin-associated PKC-θ exist and regulate gene expression programs in multiple T-cell subsets? When and how is the phosphorylation/active state of components of this nuclear complex regulated? Does PKC-θ recruit other histone modifying complexes or chromatin remodeling enzyme complexes such as SWI/SNF to target genes, as previously demonstrated for p38α.30 What role do protein phosphatases play in modulating the downstream effects of plasma membrane-chromatin-associated PKC-θ complexes, a fundamental question raised previously.31 Future studies will also address whether other PKC family members form chromatin-associated complexes. Consistent with this possibility, a recent study showed that PKC-β1 associates with chromatin and has a functional interaction with demethylase LSD1.32 Thus, it is possible that a plethora of signaling kinases perform roles similar to that of chromatin-associated PKC-θ in different cell types or different biological contexts.
It seems reasonable to suggest that protein kinases translocate into the nucleus and bind directly to chromatin, and that cells exploit this mechanism to enhance an efficient response to external stimuli and environmental challenges. However, the exact function and mechanism by which signaling kinases are recruited to active genes remains elusive. Pokholok et al. presented several models to explain how this may occur, and how chromatin-associated signaling kinases might generate variable occupancy patterns at specific gene subsets in different cells or in response to different cell stressors.8 One novel aspect of the chromatin-associated PKC-θ complex is its putative ability to modulate chromatin structure via the chromatin-remodeling activities of histone kinase MSK-1 and demethylase LSD1.
Three main mechanisms of chromatin remodeling have been proposed: (1) ATP-dependent chromatin remodeling driven by the energy of ATP hydrolysis; (2) enzymatically-introduced post-translational modifications (PTMs) of nucleosomal histones and (3) incorporation, depletion or exchange of histone variants.33 These highly inter-dependent mechanisms modify chromatin either transiently or on a more longterm, stable and heritable manner. We hypothesize that chromatin-associated PKC-θ is an example of an additional layer of regulation in the complex multilayered hierarchy of gene regulatory processes in eukaryotic cells. Here, chromatin-tethered PKC-θ (and other signaling kinases yet to be identified with dual roles in the cytoplasm and nucleus) could promote changes in chromatin structure and/or directly modulate gene expression. PKC-θ could be recruited to regulatory regions of inducible genes initially by specific transcription factors or by specific histone PTMs in activated T cells.34 PKC-θ might also function as a structural adaptor that recruits transcriptional machinery or chromatin remodeling complexes to specific promoters. Alternatively, PKC-θ could phosphorylate and activate transcription regulatory proteins in transcription complexes. Our data does not support a role for PKC-θ in phosphorylating histones in vivo (at least for serine-10 on histone H3), even though it has been reported that PKC-θ phosphorylates histones in vitro.35–37 However, the latter studies did not examine endogenous histone phosphorylation on specific genes. Metzger et al. have shown that PKC-β has the ability to phosphorylate H3T6 and thereby blocking the H3K4 demethylation activities of LSD1 and JMJC domain-containing demethylases, such as JARID1B in vitro.32 It remains to be determined whether PKC-θ may also phosphorylate T6 on histone H3 or other histone motifs in vivo.
We have shown that PKC is catalytically active in the nucleus, and that the catalytic core of PKC-θ appears to be essential for its association with chromatin and formation of the chromatin-tethered complex. These findings are based on the PKC-θ kinase-dead mutant being unable to associate with inducible gene promoters and, importantly, these findings cannot be attributed to lack of mutant nuclear localization.1 Thus, our results suggest that the region encompassing the KR mutation within the PKC-θ catalytic core may be crucial for tethering this kinase to chromatin. Similarly, the catalytic activity of yeast Hog1 is required for its association with gene promoters and the activated form of the kinase recruits RNAP II and engages with gene promoters via the transcription factor, Hot1p.38,39 Generally, PKC proteins are regulated by their phosphorylation status and specific cofactors, while their enzymatic activation is regulated by redistribution of the enzyme in different cellular compartments.40,41 As mentioned above, Hog1 in yeast and PKC-θ in human T cells distribute in both promoter and transcribed regions of their respective target genes. The significance of this observation is currently under investigation. It is plausible that PKC-θ enters the transcribed region by “piggybacking” with RNAP II or chromatin regulators,42 thus modulating chromatin structure during transcription. Although both RNAP II and PKC-θ appear to be essential for efficient transcription of inducible genes in activated T cells, the exact role of PKC-θ during transcription elongation remains unclear. Chow and Davis proposed that association of protein kinases with the coding region could enhance mRNA production.31 We suggest that such a complex system of gene activation could also ensure a more tightly controlled transcriptional response and that the transcribed region of inducible genes is an important hub for transcriptional activity in activated T cells.
Another important and unanticipated finding is that PKC-θ not only tethers to and positively regulates inducible immune response genes, but that it also negatively regulates miRNAs crucial for cytokine repression in activated T cells. Strikingly, PKC-θ preferentially interacts with the promoters of a subset of miRNA genes, while it preferentially interacts with the 5′-transcribed regions of its target immune response genes. The stable assembly of PKC-θ in miRNA gene promoters might promote formation of a repressive complex, functionally distinct from the chromatin-associated PKC-θ complex enriched in the 5′-transcribed regions of actively-transcribing immune response genes. Alternatively, we speculate that this differential distribution of PKC-θ is mediated by sequence-specific transcription factors bound to cognate sites in a specific cohort of gene promoters. Repressor proteins may block the entry of PKC-θ into the transcribed region of miRNA genes or activating complexes essential for this elongation process are not recruited. Future studies will be needed to determine whether chromatin-tethered enzymes tend to repress miRNA genes in other cell types, or if this phenomenon is only observed in activated T cells. It will also be interesting to determine whether other PKC isoforms repress expression of target miRNAs genes in a similar manner. The ability of chromatin-tethered PKC-θ to positively and negatively regulate transcription could be a critical mechanism by which to fine-tune gene expression in activated T cells.
One of the most exciting challenges in the field of eukaryotic transcription is the identification and characterization of the vast number of transcription regulatory protein complexes and their associated functions. By exploring the precise roles of nuclear PKC-θ, we provide novel insights into the molecular mechanisms that elicit specific and distinct transcriptional programs in activated T cells. Ultimately, these data could lead to novel or improved therapeutic strategies for T-cell-mediated disease processes. Moreover, our genome-wide analysis identified many novel PKC-θ target genes that might be implicated in Th1 cell development, differentiation, apoptosis and proliferation. Future studies will improve our understanding of how nuclear and cytoplasmic regulatory mechanisms are integrated to synergistically and precisely activate complex transcriptional programs in activated human T cells or other cell types.
Acknowledgments
We thank Miriam Sander, Ph.D. (www.pageoneeditorial.com) for professional scientific editing.
References
- 1.Sutcliffe EL, Bunting KL, He YQ, Li J, Phetsouphanh C, Seddiki N, et al. Chromatin-associated protein kinase C-theta regulates an inducible gene expression program and microRNAs in human T lymphocytes. Molecular cell. 2011;41:704–719. doi: 10.1016/j.molcel.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Grummt I. Actin and myosin as transcription factors. Curr Opin Genet Dev. 2006;16:191–196. doi: 10.1016/j.gde.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.de Lanerolle P, Johnson T, Hofmann WA. Actin and myosin I in the nucleus: what next? Nat Struct Mol Biol. 2005;12:742–746. doi: 10.1038/nsmb983. [DOI] [PubMed] [Google Scholar]
- 4.Ye J, Zhao J, Hoffmann-Rohrer U, Grummt I. Nuclear myosin I acts in concert with polymeric actin to drive RNA polymerase I transcription. Genes Dev. 2008;22:322–30. doi: 10.1101/gad.455908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho YH, Yoo SD, Sheen J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell. 2006;127:579–589. doi: 10.1016/j.cell.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual-Ahuir A, Struhl K, Proft M. Genome-wide location analysis of the stress-activated MAP kinase Hog1 in yeast. Methods. 2006;40:272–278. doi: 10.1016/j.ymeth.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- 9.Proft M, Mas G, de Nadal E, Vendrell A, Noriega N, Struhl K, et al. The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol Cell. 2006;23:241–250. doi: 10.1016/j.molcel.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Sims RJ, III, Mandal SS, Reinberg D. Recent highlights of RNA-polymerase-II-mediated transcription. Curr Opin Cell Biol. 2004;16:263–271. doi: 10.1016/j.ceb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, et al. PKC-theta is required for TCR-induced NFkappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 13.Baier G, Telford D, Giampa L, Coggeshall KM, Baier-Bitterlich G, Isakov N, et al. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J Biol Chem. 1993;268:4997–5004. [PubMed] [Google Scholar]
- 14.Chang JD, Xu Y, Raychowdhury MK, Ware JA. Molecular cloning and expression of a cDNA encoding a novel isoenzyme of protein kinase C (nPKC).A new member of the nPKC family expressed in skeletal muscle, megakaryoblastic cells and platelets. J Biol Chem. 1993;268:14208–14214. [PubMed] [Google Scholar]
- 15.Osada S, Mizuno K, Saido TC, Suzuki K, Kuroki T, Ohno S. A new member of the protein kinase C family, nPKCtheta, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niino YS, Irie T, Takaishi M, Hosono T, Huh N, Tachikawa T, et al. PKCtheta II, a new isoform of protein kinase C specifically expressed in the seminiferous tubules of mouse testis. J Biol Chem. 2001;276:36711–36717. doi: 10.1074/jbc.M104348200. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol Res. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman A, Villalba M. Protein kinase C-theta (PKCtheta): it's all about location, location, location. Immunol Rev. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 19.Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ, et al. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 20.Divecha N, Banfic H, Irvine RF. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991;10:3207–3214. doi: 10.1002/j.1460-2075.1991.tb04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzoli L, Martelli AM, Billi AM, Faenza I, Fiume R, Cocco L. Nuclear phospholipase C: involvement in signal transduction. Prog Lipid Res. 2005;44:185–206. doi: 10.1016/j.plipres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Visnjic D, Banfic H. Nuclear phospholipid signaling: phosphatidylinositol-specific phospholipase C and phosphoinositide-3-kinase. Pflugers Arch. 2007;455:19–30. doi: 10.1007/s00424-007-0288-1. [DOI] [PubMed] [Google Scholar]
- 23.Baier G, Wagner J. PKC inhibitors: potential in T cell-dependent immune diseases. Curr Opin Cell Biol. 2009;21:262–267. doi: 10.1016/j.ceb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Healy AM, Izmailova E, Fitzgerald M, Walker R, Hattersley M, Silva M, et al. PKC-theta-deficient mice are protected from Th1-dependent antigen-induced arthritis. J Immunol. 2006;177:1886–1893. doi: 10.4049/jimmunol.177.3.1886. [DOI] [PubMed] [Google Scholar]
- 25.Tan SL, Zhao J, Bi C, Chen XC, Hepburn DL, Wang J, et al. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase C theta-deficient mice. J Immunol. 2006;176:2872–2879. doi: 10.4049/jimmunol.176.5.2872. [DOI] [PubMed] [Google Scholar]
- 26.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilo JI, Garaude J, Pardo J, Villalba M, Anel A. Protein kinase C-theta is required for NK cell activation and in vivo control of tumor progression. J Immunol. 2009;182:1972–1981. doi: 10.4049/jimmunol.0801820. [DOI] [PubMed] [Google Scholar]
- 28.Belguise K, Sonenshein GE. PKCtheta promotes c-Rel-driven mammary tumorigenesis in mice and humans by repressing estrogen receptor alpha synthesis. J Clin Invest. 2007;117:4009–4021. doi: 10.1172/JCI32424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou WB, Zhu MJ, Demetri GD, Fletcher CD, Fletcher JA. Protein kinase C-theta regulates KIT expression and proliferation in gastrointestinal stromal tumors. Oncogene. 2008;27:5624–5634. doi: 10.1038/onc.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simone C, Forcales SV, Hill DA, Imbalzano AN, Latella L, Puri PL. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- 31.Chow CW, Davis RJ. Proteins kinases: chromatin-associated enzymes? Cell. 2006;127:887–890. doi: 10.1016/j.cell.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Metzger E, Imhof A, Patel D, Kahl P, Hoffmeyer K, Friedrichs N, et al. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2011;464:792–796. doi: 10.1038/nature08839. [DOI] [PubMed] [Google Scholar]
- 33.Sutcliffe EL, Parish IA, He YQ, Juelich T, Tierney ML, Rangasamy D, et al. Dynamic histone variant exchange accompanies gene induction in T cells. Mol Cell Biol. 2009;29:1972. doi: 10.1128/MCB.01590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 35.Chang ZL, Beezhold DH. Protein kinase C activation in human monocytes: regulation of PKC isoforms. Immunology. 1993;80:360–366. [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- 37.Ways K, Riddle R, Ways M, Cook P. Effect of phorbol esters on cytosolic protein kinase C content and activity in the human monoblastoid U937 cell. J Biol Chem. 1991;266:1258–1264. [PubMed] [Google Scholar]
- 38.Alepuz PM, de Nadal E, Zapater M, Ammerer G, Posas F. Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 2003;22:2433–2442. doi: 10.1093/emboj/cdg243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alepuz PM, Jovanovic A, Reiser V, Ammerer G. Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol Cell. 2001;7:767–777. doi: 10.1016/s1097-2765(01)00221-0. [DOI] [PubMed] [Google Scholar]
- 40.Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 41.Newton AC. Protein kinase C: structure, function and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 42.Edmunds JW, Mahadevan LC. Cell signaling. Protein kinases seek close encounters with active genes. Science. 2006;313:449–451. doi: 10.1126/science.1131158. [DOI] [PubMed] [Google Scholar]