Abstract
Natural killer (NK) cells are known as innate immune lymphocytes that respond rapidly when challenged by pathogens but little is known about adaptive immune features including memory related to NK cells from human beings. In the present study, we demonstrate for the first time that human NK cells expressing the memory-associated marker CD45RO were persistent in pleural fluid cells (PFCs) from tuberculous patients. CD45RO+ NK cells produced significantly more interferon-γ and were more cytotoxic compared with CD45RO− NK cells from PFCs when stimulated with interleukin-12 (IL-12). Consistently, IL-12 enhanced the expression of granzyme B, CD69, CD25, NKG2D, IL-12 receptors β1 and β2 on CD45RO+ NK cells from PFCs. Our experiments contribute to a better understanding of the NK cells from PFCs and indicate that human CD45RO+ NK cells from PFCs expressing a ‘memory-like’ phenotype may have an important role in defending against infection by Mycobacterium tuberculosis.
Keywords: cytokines, cytotoxicity, interleukin-12, memory, natural killer cells
Introduction
Mycobacterium tuberculosis is one of the leading causes of death among infectious diseases and tuberculous pleurisy is a good model for the study of M. tuberculosis-specific cells.1–3 Natural killer (NK) cells have been traditionally considered part of the innate immune system because of their early production of cytokines and ability to lyse target cells without previous sensitization.4 It was reported that NK cells were potent inducers of interferon-γ (IFN-γ) production and were associated with early resistance against M. tuberculosis infection.5,6 Further investigation showed that NK cells instructed cytotoxic CD8+ T cells to kill M. tuberculosis-infected monocytes. The interaction needed direct contact between NK cells and monocytes, which was strictly dependent on the production of IFN-γ by NK cells.7,8
Based on the expression of CD56 and CD16, NK cells can be divided into various sub-populations and display major function differences in their cytokine production and cytolytic activity. CD56+ CD16− NK cells display little cytotoxic activity but produce high levels of cytokines whereas CD56+/− CD16+ NK cells produce low levels of cytokines but exert strong cytolytic ability.9
It has been suggested that NK cells have the characteristics of both innate and adaptive immunity including memory. In a mouse model of contact hypersensitivity, it has been advocated that NK cells could retain a long-lived and antigen-specific adaptive immune response independent of B cells and T cells.10 In another report, Sun et al. documented that NK cells possessed nearly all the hallmarks of the adaptive immune system in a mouse model of cytomegalovirus infection. Using adoptive transfer systems, the author observed that these ‘memory’ NK cells were more protective against murine cytomegalovirus than naive NK cells.11 Recently, Cooper et al.12 illustrated that NK cells with a history of previous activation by cytokines could produce more IFN-γ after treatment with interleukin-12 (IL-12) plus IL-15 or via engagement of activating NK receptors. Another study demonstrated that hepatic NK cells could develop an adaptive immune response to structurally diverse antigens, which required NK cell-expressed CXCR6.13 However, limited information has been obtained from human memory-like NK cells.
In the present study, higher expression of the memory-associated molecule CD45RO was observed on a subset of NK cells from pleural fluid cells (PFCs) compared with NK cells from peripheral blood mononuclear cells (PBMCs). Importantly, CD45RO+ NK cells produced more IFN-γ compared with CD45RO− NK cells from PFCs in response to IL-12. Furthermore, CD45RO+ NK cells from PFCs were more cytotoxic for K562 cells with IL-12 treatment. These results demonstrated for the first time that NK cells from PFCs expressing the memory-associated marker CD45RO responded more robustly than CD45RO− NK cells from PFCs following stimulation with IL-12. Our data provide new insight with which to re-evaluate the functions of NK cells in M. tuberculosis infections, which could provide fresh evidence for the fight against tuberculosis.
Materials and methods
Subjects
Thirty-five patients infected with M. tuberculosis (25 men, 10 women, aged between 16 and 65 years) were enrolled in this study. They were newly diagnosed with tuberculous pleurisy at the Chest Hospital of Guangzhou, China. Diagnosis of patients was generally confirmed by positive cultures for M. tuberculosis and pleural fluid was obtained from the patients during diagnostic thoracentesis before starting chemotherapy. Thirty-five age-matched adult healthy donors (24 men, 11 women, aged between 18 and 55 years) were recruited from the Zhongshan School of Medicine. Adequate informed consents were obtained from both patients and healthy volunteers. Both patients and healthy donors who had been diagnosed with HIV, hepatitis B virus or hepatitis C virus infection or who had a history of autoimmune diseases were excluded from this study.
Monoclonal antibodies
The following monoclonal antibodies were used for phenotypic and intracellular cytokine analysis: phycoerythrin (PE) -labelled anti-CD3 (Cat. No. 555333), anti-CD14 (Cat. No. 554542), anti-CD19 (Cat. NO.12-0199-42), anti-CD56 (Cat. No. 555516), anti-CD45RA (Cat. No. 555489), anti-CD62L (Mat. No.555544), anti-IL-12 receptor β1 (IL-12Rβ1; Cat. No. 556065), fluorescein isothiocyanate (FITC)-labelled anti-CD3 (Cat. No. 555339), anti-CD25 (Cat. No. 555431), anti-CD45RA (Cat. No. 555488), anti-CD45RO (Cat. No. 555492), anti-granzyme B (Cat. No. 560211), PE-Cy7)-labelled anti-CD69 (Cat. No. 557745), anti-CD56 (Cat. No. 557747), allophycocyanin (APC) -labelled anti-CD3 (Cat. No. 349355) and anti-IFN-γ (Cat. No. 554702) were obtained from BD Pharmingen (San Jose, CA). The APC-labelled anti-CD27 (Cat. No. 302810), anti-CD16 (Cat. No. 302012) and FITC-labelled anti-CD16 (Cat. No. 302006) were purchased from Biolegend (San Diego, CA), and PE-labelled anti-NKG2D (Cat. No. FAB139P) and APC-labelled anti-NKG2A (Cat. No. FAB1059A) were obtained from R&D Systems (Minneapolis, MN).
Preparation of PFCs and PBMCs
Pleural fluid was collected by therapeutic thoracentesis from tuberculous patients. Pleural fluid cells and PBMCs were isolated by Ficoll–Hypaque (Tianjin Hao Yang Biological Manufacture, Tianjin, China) gradient centrifugation within 24 hr of sampling and washed twice in Hanks’ balanced salt solution. The cells were suspended at a final concentration of 2 × 106/ml in complete RPMI-1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (HyClone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine and 50 μm 2-mercaptoethanol.
Isolation of NK cells from PFCs and PBMCs
Purified NK cells were obtained by negative selection from PFCs and PBMCs using the NK cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. Briefly, after washing twice in the buffer (PBS supplemented with 2 mm EDTA and 0·5% BSA), cells were suspended and incubated for 10 min with a Biotin–Antibody Cocktail (containing anti-CD3, anti-CD4, anti-CD14, anti-CD15, anti-CD19, anti-CD36, anti-CD123 and anti-CD235a antibodies) at 4°, after which the Anti-Biotin Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were added for an additional 15 min at 4°. Cells were washed once and then passed through a magnetic column for collection of purified NK cells. The purity of NK cells assessed by FACS was > 95%.
Cell sorting
Purified NK cells from PFCs were stained with FITC-labelled anti-CD45RO for 30 min at 4° in the dark and washed twice with the buffer as described above. Cells were suspended in the buffer and sorted using a FACS Aria II flow cytometer (BD company, San Jose, CA) for the subsets of CD45RO+ or CD45RO− NK cells from PFCs. Purified NK cells from PFCs were stained with PE-labelled anti-CD56 and APC-labelled anti-CD16. Cells were collected and sorted into subsets of CD56+ CD16− and CD56+ CD16+ NK cells from PFCs. The purity of these cells analysed by FACS was > 95%.
Cell surface and intracellular staining
Cells were washed twice with PBS buffer containing 0·1% BSA and 0·05% sodium azide (FACS buffer). For surface staining, cells were incubated with the respective monoclonal antibodies at 4° for 30 min in the dark, then washed twice and fixed in 0·5% paraformaldehyde before acquisition. Cells were firstly gated on CD3− CD14− CD19− cells and NK cells were divided into three subsets of CD56+ CD16−, CD56+ CD16+ and CD56− CD16+ based on the expression of cell surface CD56 and CD16 molecules. Intracellular staining for granzyme B was performed after fixation in 4% paraformaldehyde, followed by permeabilization and staining in FACS buffer containing 0·1% saponin. These cells were collected by FACSCalibur™ (Becton Dickinson, San Jose, CA) and analysed with FlowJo software (Treestar, San Carlos, CA). For IL-12Rβ2 staining, cells were incubated with the monoclonal antibody for IL-12Rβ2 (2B6) (BD Pharmingen) for 30 min, followed by biotinylated anti-rat IgF(ab’)2 fragments (Jackson Immuno Research, West Grove, PA) for 30 min, and finally incubated with streptavidin conjugated to PE (BD Pharmingen) for 20 min. These cells were collected by FACSCalibur.
The detection of IFN-γ by FACS
Purified NK cells from PBMCs labelled with 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, OR) were mixed with purified NK cells from PFCs and stimulated with or without PMA (20 ng/ml; Sigma-Aldrich, St Louis, MO) and ionomycin (1 μg/ml; Sigma-Aldrich) for 4 hr at 37° with 5% CO2 in the presence of brefeldin A (10 μg/ml; Sigma-Aldrich). Cells were collected and stained with surface markers and intracellular APC-labelled anti-IFN-γ as described above. Total PFCs were stimulated with IL-12 (5 ng/ml; eBioscience, Santiago, Chile) for 4 hr at 37° with 5% CO2 in the presence of brefeldin A. Cells were collected and stained with PE-labelled anti-CD3, PE-Cy7-labelled anti-CD56, FITC-labelled anti-CD45RO and APC-labelled anti-IFN-γ as described above. These cells were collected by FACSCalibur and analysed with FlowJo software.
The detection of IFN-γ by ELISA
Purified NK cells from PFCs and PBMCs or freshly sorted subsets of NK cells from PFCs were suspended in complete RPMI-1640 medium and stimulated with or without PMA and ionomycin in a round-bottom 96-well plate at a concentration of 2 × 106 cells/ml in triplicate and incubated for 24 hr at 37° with 5% CO2. The supernatants were harvested and assayed for the production of IFN-γ by ELISA (detection limit, 9·4 pg/ml) according to the manufacturer's protocol (BD Pharmingen, San Diego, CA).
Cytotoxicity assays
K562 cells were labelled with CFSE and used as target cells. The PFCs were cultured with or without IL-12 for 72 hr as effector cells. Cells were washed twice before the killing assay. Target cells and effector cells were co-incubated at different effector to target ratios for 4 hr at 37° with 5% CO2. Control tubes including only target or effector cells were assayed to determine spontaneous cell death. Cells were washed twice and suspended with 200 μl FACS buffer and propidium iodide solution 50 μg/ml in PBS; Sigma, Saint Louis, MO) was added to each tube and incubated for 10–15 min at room temperature in the dark. The dead target cells were quantified immediately by FACSCalibur and analysed with FlowJo software. Purified NK cells from PFCs stimulated with or without IL-12 for 72 hr were the effector cells and the killing assay was performed as described above. Freshly sorted CD45RO+ and CD45RO− NK cells from PFCs were cultured with or without IL-12 for 36 hr as effector cells. Cells were co-incubated with CFSE-labelled K562 for 2 or 4 hr for killing assay.
Statistical analysis
Data are presented as the mean ± SD. Comparison between two groups was performed by unpaired Student's t-tests. A value of P < 0·05 was considered significant.
Results
Decreased total number of NK cells but increased subset of CD56+ CD16− NK cells from PFCs compared with PBMCs
The distribution of NK cells from PFCs and PBMCs was examined by FACS. The results showed that the mean frequency of CD3− CD14− CD19− cells from PFCs (14%) was significantly lower compared with CD3− CD14− CD19− cells from PBMCs (34%). Analysis for the sub-populations of NK cells indicated that the subset of CD56+ CD16− NK cells from PFCs (18·18% ± 9·8%) was markedly higher compared with the same subset of NK cells from PBMCs (7·23% ± 5·18%, P < 0·05). However, the subset of CD56+ CD16+ NK cells from PFCs (19·28% ± 14·25) was obviously lower compared with from PBMCs (41·34% ± 14·8%, P < 0·05). No significant difference was observed with regard to the subset of CD56− CD16+ NK cells from PFCs and PBMCs (Fig. 1a,b). These results indicate that the sub-populations of NK cells from PFCs were different from those from PBMCs.
Figure 1.
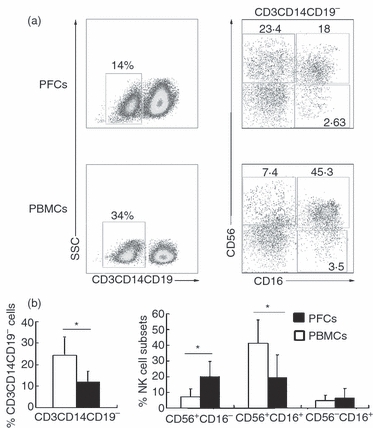
Decreased total number of natural killer (NK) cells and increased subset of CD56+ CD16− NK cells in pleural fluid from tuberculous patients compared with peripheral blood mononuclear cells (PBMCs). (a) Percentage of CD3− CD14− CD19− cells and subsets of NK cells from pleural fluid cells (n = 23) and PBMCs (n = 25) were analysed by FACS. (b) Mean value of CD3− CD14− CD19− cells and NK cell sub-populations including CD56+ CD16−, CD56+ CD16+ and CD56− CD16+ subsets from pleural fluid cells (filled histograms) and PBMCs (open histograms) were analysed by FACS (Mean ± SD). Student's t-test was used for statistical analysis. *P < 0·05 was considered significant.
NK cells from PFCs expressed memory-associated molecule CD45RO
To better characterize the subsets of NK cells, we analysed the expression of memory-associated markers such as CD45RO, CD45RA, CD62L and CD27 on three subsets of CD56+ CD16−, CD56+ CD16+ and CD56− CD16+ NK cells from PFCs or PBMCs. As shown in Fig. 2, the percentage and mean fluorescence intensity (MFI) of CD45RO on subsets of CD56+ CD16+ and CD56− CD16+ NK cells from PFCs were significantly higher compared with the same subsets of NK cells from PBMCs, which was in accordance with the lower expression of CD45RA on subsets of CD56+ CD16−, CD56+ CD16+ and CD56− CD16+ NK cells from PFCs compared with NK cells from PBMCs. In addition, the expression of CD62L and CD27 was markedly higher on subsets of CD56+ CD16+ and CD56− CD16+ NK cells from PFCs compared with NK cells from PBMCs. Taken together, these results indicate that some of the NK cells from PFCs expressed the T-cell memory-associated marker CD45RO, which was not previously associated with NK cells.
Figure 2.
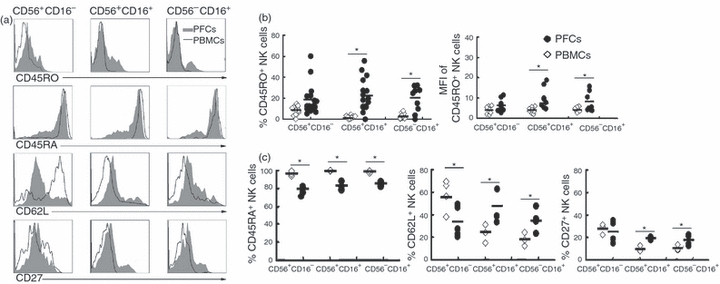
Expression of various memory-associated molecules on the subsets of natural killer (NK) cells from pleural fluid cells (PFCs) and peripheral blood mononuclear cells (PBMCs). (a) The expression of CD45RO, CD45RA, CD62L and CD27 on CD56+ CD16−, CD56+ CD16+ and CD56− CD16+ subsets of NK cells from PFCs (filled histogram) or PBMCs (open histogram). Histograms were representative of several independent experiments with similar results. (b) Statistical results of CD45RO on the subsets of NK cells both in percentage and mean fluorescence intensity (MFI) (n = 22). (c) Evaluation of the expression for CD45RA (n = 6), CD62L (n = 5) and CD27 (n = 5). The median and individual frequencies for each patient and healthy volunteer were calculated. *P < 0·05 was considered significant.
CD45RO+ but not CD45RO− NK cells from PFCs produced more IFN-γ following the stimulation with IL-12
Based on the observation that CD45RO+ NK cells were present in PFCs, we extended our study to analyse the production of IFN-γ by NK cells from PFCs and PBMCs. To guarantee the identical condition of stimulation, purified NK cells from PFCs were mixed and co-cultured with CFSE-labelled purified NK cells from PBMCs. As shown in Fig. 3(a), < 25% NK cells from PBMCs but > 50% NK cells from PFCs produced IFN-γ under stimulation with PMA plus ionomycin. Interestingly, the subsets of CD45RA− and CD45RA+ NK cells from PFCs produced similar levels of IFN-γ. The results from FACS were confirmed by ELISA: the production of IFN-γ by NK cells from PFCs was about 10-fold more than that by NK cells from PBMCs (Fig. 3b). To further ascertain which subset of NK cells from PFCs produced IFN-γ, we sorted the subsets of CD56+ CD16− and CD56+ CD16+ NK cells from PFCs. As shown in Fig. 3(c), most of the IFN-γ was produced by the subset of CD56+ CD16− NK cells from PFCs.
Figure 3.
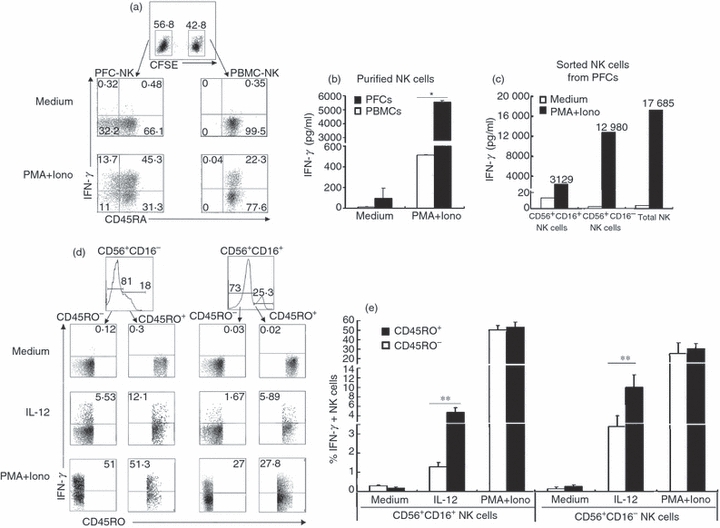
CD45RO+ natural killer (NK) cells from pleural fluid cells (PFCs) produced more interferon-γ (IFN-γ) compared with CD45RO− NK cells under stimulation with interleukin-12 (IL-12). (a) Freshly purified NK cells from peripheral blood mononuclear cells (PBMCs) labelled with CFSE were co-incubated with purified NK cells from PFCs. Cells were stimulated with or without PMA and ionomycin in the presence of brefeldin A for 4 hr. The expression of IFN-γ was analysed by FACS. Representative dot plots of three independent experiments were shown. (b) Purified NK cells from PFCs or PBMCs were incubated with or without PMA and ionomycin. The expression of IFN-γ in supernatants was detected by ELISA. Results were given as mean ± SD. *P < 0·05 was considered significant. (c) Freshly sorted CD56+ CD16− NK cells, CD56+ CD16+ NK cells and total NK cells from PFCs were stimulated with or without PMA and ionomycin. The expression of IFN-γ in supernatants was evaluated by ELISA. (d) PFCs were stimulated with or without IL-12 or PMA plus ionomycin for 4 hr in the presence of brefeldin A. The expression of IFN-γ was evaluated by FACS. Representative dot plots of three independent experiments were shown with similar results. (e) Statistical results of IFN-γ expression by CD45RO+ or CD45RO− NK cells from PFCs were shown as mean ± SD in histogram. *P < 0·05 was considered significant.
To better analyse the production of IFN-γ by CD45RO+ NK cells, PFCs were cultured with IL-12 or PMA plus ionomycin for 4 hr and the production of IFN-γ by NK cells was detected by FACS. Cells were first gated on CD3− CD14− CD19− cells. As illustrated in Fig. 3(d,e), the subset of CD56+ CD16− NK cells was also the primary population for the production of IFN-γ. More importantly, CD45RO+ NK cells produced significantly more IFN-γ (about twofold to threefold up) compared with CD45RO− NK cells from PFCs under stimulation with IL-12. However, CD45RO+ and CD45RO− NK cells from PFCs induced a similar level of IFN-γ under the polyclonal stimulation, which was consistent with Fig. 3(a). These results strongly suggested that CD45RO+ NK cells responded more robustly than CD45RO− NK cells from PFCs treated with IL-12.
CD45RO+ but not CD45RO− NK cells from PFCs displayed greater and more rapid cytotoxic ability against tumour cells in response to IL-12
To analyse the cytolytic activity of NK cells from PFCs, total PFCs or purified NK cells from PFCs were used as effector cells and CFSE-labelled K562 cells were used as target cells for the cytotoxicity assay. The results indicated that NK cells from PFCs displayed lower cytolytic capacity compared with NK cells from PBMCs in the absence of IL-12 (data not shown). However, the cytotoxic ability could be enhanced when NK cells from PFCs were incubated with IL-12 (Fig. 4a,b). We further analysed the cytotoxic ability of freshly sorted CD45RO+ and CD45RO− NK cells from PFCs. The cytolytic activity of CD45RO+ and CD45RO− NK cells from PFCs for K562 cells was no different in the absence of IL-12 (Fig. 4c). However, CD45RO+ NK cells exerted stronger cytolytic activity compared with CD45RO− NK cells from PFCs following incubation with IL-12 (Fig. 4c,d). When the time of co-incubation was shortened from 4 hr to 2 hr, CD45RO+ NK cells could also display more powerful killing ability compared with CD45RO− NK cells from PFCs (Fig. 4e,f). These results demonstrated that CD45RO+ NK cells from PFCs exerted stronger and faster cytolytic activity for K562 cells in response to IL-12.
Figure 4.
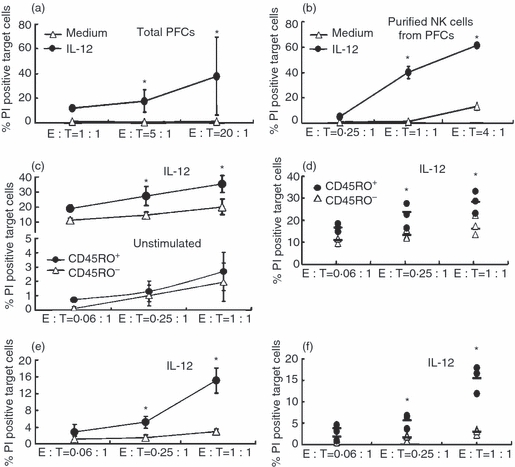
CD45RO+ natural killer (NK) cells from pleural fluid cells (PFCs) displayed stronger and more rapid cytotoxic ability compared with CD45RO− NK cells for K562 cells when the stimulated with interleukin-12 (IL-12). (a) Total PFCs were incubated in the presence or absence of IL-12 for 72 hr. These cells were co-cultured with CFSE-labelled K562 at different effector to target (E : T) ratios for 4 hr. The cytotoxicity of effector cells was estimated by FACS. Results are shown as mean ± SD. (b) The cytotoxicity of purified NK cells from PFCs was performed as total PFCs. (c) and (e) Freshly sorted CD45RO+ and CD45RO− NK cells from PFCs were cultured with or without IL-12 for 36 hr and co-incubated with CFSE-labelled K562 at different E : T ratios for 2 hr (e) or 4 hr (c). The cytotoxicity was estimated by FACS. (d) and (f) Statistical results corresponding with (c) and (e) were shown as percentage of propidium iodide-positive target cells (n = 3). The median and individual frequencies for each experiment were calculated. *P < 0·05.
Expression of phenotypic and intracellular molecules on CD45RO+ and CD45RO− NK cells from PFCs in the presence or absence of IL-12
To investigate the possible mechanisms of the strong response by CD45RO+ NK cells from PFCs in the presence of IL-12, we compared the expression of granzyme B, CD69, CD25, NKG2A, NKG2D, IL-12Rβ1 and IL-12Rβ2 between CD45RO+ and CD45RO− NK cells from PFCs following treatment with or without IL-12. As illustrated in Fig. 5, a higher percentage expression of granzyme B on CD45RO+ NK cells was observed compared with CD45RO− NK cells from PFCs. However, there was no significant difference regarding the expression of CD69 and CD25 between CD45RO+ and CD45RO− NK cells from PFCs in the absence of IL-12, but expression was greater for CD69 and CD25 as a percentage and for NKG2D in MFI on CD45RO+ NK cells compared with CD45RO− NK cells from PFCs in the presence of IL-12. Furthermore, significantly higher levels of IL-12Rβ1 and IL-12Rβ2 as a percentage and in MFI were observed on CD45RO+ NK cells compared with CD45RO− NK cells from PFCs under stimulation with IL-12 (Fig. 6).
Figure 5.
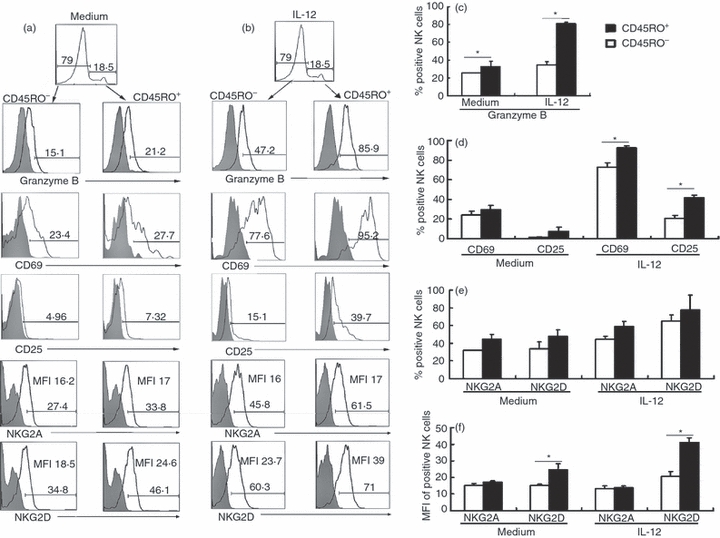
Expression of phenotypic and intracellular molecules on CD45RO+ and CD45RO− natural killer (NK) cells from pleural fluid cells (PFCs) in the presence or absence of interleukin-12 (IL-12). (a) Expression of granzyme B, CD69, CD25, NKG2A and NKG2D on CD45RO+ or CD45RO− NK cells from PFCs. Cells were gated on CD3− CD56+ CD45RO+ (right side) and CD3− CD56+ CD45RO− (left side) NK cell subsets. Isotype controls are depicted as grey filled histograms. Results are shown in histograms representative of three independent experiments with similar results. (b) Representative histograms of granzyme B, CD69, CD25, NKG2A and NKG2D expression on CD45RO+ or CD45RO− NK cells from PFCs under the stimulation of IL-12 for 36 hr. (c), (d) and (e) Statistical histograms of granzyme B (n = 5), CD69 (n = 5), CD25 (n = 5), NKG2A (n = 6) and NKG2D (n = 6) on CD45RO+ or CD45RO− NK cells from PFCs as a percentage were evaluated as mean ± SD. (f) Mean fluorescence intensity (MFI) for the expression of NKG2A and NKG2D on CD45RO+ or CD45RO− NK cells from PFCs is shown as mean ± SD in the histogram. *P < 0·05 was considered significant.
Figure 6.
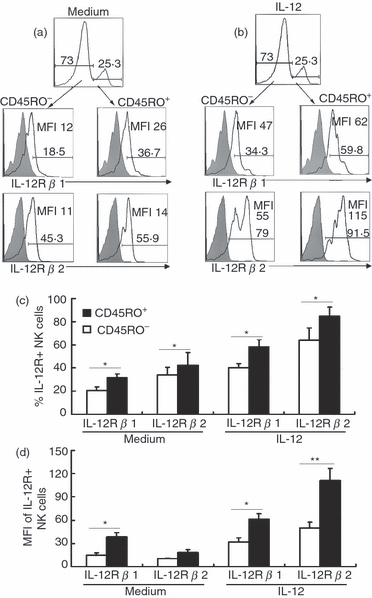
Expression of interleukin-12 receptor (IL-12R) β1 and IL-12Rβ2 on CD45RO+ or CD45RO− natural killer (NK) cells from pleural fluid cells (PFCs) with or without interleukin-12 (IL-12). (a) Expression of IL-12Rβ1 and IL-12Rβ2 on CD45RO+ or CD45RO− NK cells from PFCs were evaluated by FACS. Results were shown representative of three independent experiments with similar results. (b) Representative histograms of IL-12Rβ1 and IL-12Rβ2 on CD45RO+ or CD45RO− NK cells from PFCs when stimulated with IL-12 for 36 hr. (c) and (d) Statistical results of IL-12Rβ1 (n = 5) and IL-12Rβ2 (n = 5) on CD45RO+ or CD45RO− NK cells from PFCs both as percentage and MFI evaluated as mean ± SD. *P < 0·05 was considered significant.
Discussion
Human NK cells represent approximately 10% of all peripheral blood lymphocytes, which are associated with early resistance against intracellular pathogens and are potent producers of IFN-γ.14–16 Several lines of evidence using animal models indicated that NK cells exhibited memory-like properties. However, limited evidence proved that memory-like NK cells were present in human beings. In the current study, we evaluated NK cells in pleural fluids from tuberculous patients. Because no pleural fluids were present in healthy donors, PBMCs from healthy volunteers were used as controls to compare the tuberculous patients and healthy donors. We identified three subsets of NK cells: CD56+ CD16−, CD56+ CD16+ and CD56− CD16+.9 Interestingly, higher expression of CD45RO in parallel with lower expression of CD45RA was observed on subsets of CD56+ CD16+ and CD56− CD16+ NK cells from PFCs compared with NK cells from PBMCs. We detected expression of CD45RO on NK cells from PBMCs of tuberculous patients and the result was consistent with PBMCs from healthy donors, that a low level of CD45RO was observed on NK cells from PBMCs of tuberculous patients (data not shown). We also found that in NK cells of pleural fluids from pulmonary carcinoma, no CD45RO+ NK cells were observed on malignant tumours (data not shown). CD45RO and CD45RA represented memory and naive T cells in previous studies.17–21 Memory T cells were CD45RA+/− and CD45RO+, which could deliver a rapid antigen-specific recall response after secondary exposure, whereas naive T cells were CD45RA+ and CD45RO−, which produced a low level of cytokines when stimulated.19,21 Accordingly, our results suggested that NK cells from PFCs might display ‘memory-like’ phenotypic characterizations. Other surface markers such as adhesion molecules CD62L and the co-stimulation marker CD27 also contributed to distinguish subsets of memory T cells.19 The higher expression of CD62L and CD27 on subsets of CD56+ CD16+ and CD56− CD16+ NK cells from PFCs compared with PBMCs indicates the heterogeneity between different subsets of NK cells. Taken together, the results indicated that NK cells from PFCs displayed ‘memory-like’ phenotypes.
To better evaluate functions of CD45RO+ NK cells from PFCs, we analysed the production of IFN-γ by NK cells. In accordance with our findings, early reports proved that IL-12 displayed an important role in the production of IFN-γ-mediated anti-Mycobacterium effector functions.8,22 Interleukin-12-stimulated NK cells can activate human macrophages to inhibit the growth of M. tuberculosis. The cross-talk between NK cells and antigen-presenting cells involved Toll-like receptors 2 and 4 and mannose receptor was also necessary for M. tuberculosis-induced production of IFN-γ by NK cells from PFCs. Furthermore, we found that NK cells from PFCs produced more IFN-γ compared with NK cells from PBMCs under the stimulation of PMA plus ionomycin. This was most likely because the subset of CD56+ CD16− NK cells from PFCs was significantly increased compared with NK cells from PBMCs, which was the main population for the production of IFN-γ.9 More importantly, our experiments proved that CD45RO+ NK cells from PFCs exerted stronger responses by producing IFN-γ under the stimulation with IL-12.
Killing ability is also a crucial function of NK cells. Previous studies demonstrated that human NK cells not only lysed M. tuberculosis-infected cells but also actively restricted the growth of M. tuberculosis in an apoptosis-dependent but Fas/FasL-independent manner. The killing ability of NK cells can be further enhanced by addition of IL-2, IL-12 and glutathione.23 Similar to other studies, we found that cytotoxic granule granzyme B was significantly decreased mainly on the subsets of CD56+ CD16+ and CD56− CD16+ NK cells from PFCs, whereas the expression of CD69 was increased, which represented a functional triggering on activated NK cells (data not shown), suggesting that the effector molecule granzyme B was released after the activation of NK cells from PFCs. However, IL-12 could enhance the cytolytic activity of NK cells from PFCs. Furthermore, IL-12-stimulated CD45RO+ NK cells responded more robustly and rapidly to lyse K562 cells compared with CD45RO− NK cells from PFCs.
Analysis of the expression of surface and intracellular molecules between CD45RO+ and CD45RO− NK cells from PFCs indicated that CD45RO+ NK cells were more activated by IL-12, with percentage expression of granzyme Bhigh CD69high CD25high and expression of NKG2Dhigh, as shown by MFI, compared with CD45RO− NK cells from PFCs for a short time. This was most likely one of the reasons why CD45RO+ NK cells responded more strongly in culture with IL-12. Moreover, the biological activities of IL-12 are mediated through a specific and high-affinity receptor composed of IL-12Rβ1 and IL-12Rβ2 subunits that exist primarily on NK cells.24 The high level of expression of IL-12Rβ1 and IL-12Rβ2 on CD45RO+ NK cells from PFCs suggested that CD45RO+ NK cells were more sensitive to activation and could supply stronger protection by producing IFN-γ and lysing target cells when stimulated with IL-12.
In conclusion, despite its preliminary character, our study clearly indicates that a sub-population of NK cells from PFCs with the expression of memory associated marker CD45RO exert stronger immune responses when stimulated by IL-12. Unfortunately, we could not prove that CD45RO+ NK cells from PFCs were antigen-specific ‘memory-like’ cells nor could we find mechanisms for the formation of ‘memory-like’ NK cells from PFCs. Nevertheless, the study of memory NK cells in humans is just beginning and our data will supply fresh insight for us to understand the biology of NK cells in M. tuberculosis infection.
Acknowledgments
We thank Prof. Limin Zheng, State Key Laboratory of Oncology, Sun Yat-sen University, Guangzhou, China, for kindly providing us with K562 cells. This work was supported by the 115 grant (No. 2008ZX10003-011), National Nature Science Foundation of China (No. 30872300), National Key Basic Research Programme of China (973; No. 2007CB512404), Specialized Research Fund for the Doctoral Programme of Higher Education of China (No: 20070558213) and Medical Scientific Research Foundation of Guangdong Province, China (No: A2009161).
Glossary
Abbreviations
- APC
allophycocyanin
- CFSE
5- (and 6-) carboxyfluorescein diacetate succinimidyl ester
- IFN
interferon
- IL-12
interleukin-12
- IL-12Rβ1
interleukin-12 receptor β1
- MFI
mean fluorescence intensity
- NK cells
natural killer cells
- PBMCs
peripheral blood mononuclear cells
- PE
phycoerythrin
- PFCs
pleural fluid cells
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- 1.Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat Med. 2000;6:1327–9. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- 2.Schierloh P, Yokobori N, Aleman M, et al. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J Immunol. 2005;175:6852–60. doi: 10.4049/jimmunol.175.10.6852. [DOI] [PubMed] [Google Scholar]
- 3.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ. 1994;72:213–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Roder JC, Pross HF. The biology of the human natural killer cell. J Clin Immunol. 1982;2:249–63. doi: 10.1007/BF00915064. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez LE, Young LS. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991;146:265–70. [PubMed] [Google Scholar]
- 6.Ota T, Okubo Y, Sekiguchi M. Analysis of immunologic mechanisms of high natural killer cell activity in tuberculous pleural effusions. Am Rev Respir Dis. 1990;142:29–33. doi: 10.1164/ajrccm/142.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Vankayalapati R, Klucar P, Wizel B, Weis SE, Samten B, Safi H, Shams H, Barnes PF. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J Immunol. 2004;172:130–7. doi: 10.4049/jimmunol.172.1.130. [DOI] [PubMed] [Google Scholar]
- 8.Esin S, Batoni G, Pardini M, et al. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette–Guérin. Immunology. 2004;112:143–52. doi: 10.1111/j.1365-2567.2004.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 11.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paust S, Gill HS, Wang BZ, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–38. [PubMed] [Google Scholar]
- 15.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–52. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janeway CJ, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 17.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 18.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory and effector memory CD4+ T cells. Pathol Biol (Paris) 2003;51:64–6. doi: 10.1016/s0369-8114(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;2:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 20.Tough DF. Deciphering the relationship between central and effector memory CD8+ T cells. Trends Immunol. 2003;24:404–7. doi: 10.1016/s1471-4906(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 21.Muraro PA, Pette M, Bielekova B, McFarland HF, Martin R. Human autoreactive CD4+ T cells from naive CD45RA+ and memory CD45RO+ subsets differ with respect to epitope specificity and functional antigen avidity. J Immunol. 2000;164:5474–81. doi: 10.4049/jimmunol.164.10.5474. [DOI] [PubMed] [Google Scholar]
- 22.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–8. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millman AC, Salman M, Dayaram YK, Connell ND, Venketaraman V. Natural killer cells, glutathione, cytokines, and innate immunity against Mycobacterium tuberculosis. J Interferon Cytokine Res. 2008;28:153–65. doi: 10.1089/jir.2007.0095. [DOI] [PubMed] [Google Scholar]
- 24.Wu CY, Gadina M, Wang K, O'Shea J, Seder RA. Cytokine regulation of IL-12 receptor beta2 expression: differential effects on human T and NK cells. Eur J Immunol. 2000;30:1364–74. doi: 10.1002/(SICI)1521-4141(200005)30:5<1364::AID-IMMU1364>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]


