Abstract
Interleukin-23 (IL-23) is important for T helper type 17 (Th17) responses and strategies to regulate IL-23 in human dendritic cells (DC) are limited. This study describes a novel means to control IL-23 secretion by conditioning DC with a phosphatidyl inositol 3-kinase inhibitor Wortmannin (WM). Treatment of monocyte-derived DC with WM increased Toll-like receptor (TLR) -dependent IL-23 secretion 10-fold and IL-12p70 twofold, but IL-27 was unaffected. The effect of WM was restricted to TLR3/4 pathways, did not occur through TLR2, TLR7/8 or Dectin-1, and was characterized by increased p19, p35 and p40 transcription. These responses were not solely dependent on phosphatidyl inositol 3-kinase as the alternative inhibitor LY294002 did not modulate IL-23 production. The normal patterns of activation of mitogen-activated protein kinase pathways were unaffected by WM-conditioning but IL-23 secretion required p38, ERK and JNK pathways. Importantly, this effect was manifest in populations of blood DC. Conditioning freshly isolated myeloid DC with WM before TLR3 or TLR4 triggering resulted in high levels of IL-23 secretion and an absence of IL-12p70. These WM-conditioned myeloid DC were highly effective at priming Th17 responses from naive CD4+ T cells. Our findings provide a novel means to generate IL-23-rich environments and Th17 responses and suggest as yet unidentified regulatory factors, identification of which will provide new approaches to control IL-23-dependent immunity in infectious disease, autoimmunity and malignancy.
Keywords: dendritic cell, interleukin-23, myeloid, T helper type 17, Wortmannin
Introduction
Interleukin-23 (IL-23), a key regulator of T helper type 17 (Th17) cells, is secreted by dendritic cells (DC) in response to immune danger. However, relatively little is known regarding factors that regulate its secretion. Dendritic cells are the key antigen-presenting cells for priming naive T cells and link innate and adaptive responses by communicating with natural killer cells and neutrophils.1 Immature DC assess the antigenic environment and differentially mature when they encounter immune triggers such as viral double-stranded RNA or bacterial lipopolysaccharide (LPS). Mature DC up-regulate chemokine receptors, co-stimulatory and MHC molecules and migrate to draining lymph nodes for antigen-specific T-cell activation.2 The cytokines released by DC in part determine the polarization of Th-responses. Interleukin-12p70 drives differentiation of interferon-γ (IFN-γ) -secreting Th1 cells which activate cytotoxic T lymphocytes, natural killer cells and macrophages.3 In contrast, IL-10 differentiates Th2 responses important for B-lymphocyte activation while IL-1 and IL-6 drive inflammatory Th17 responses.4,5 The Th17 response is characterized by secretion of IL-17A, IL-17F, IL-21, IL-22, tumour necrosis factor (TNF) and IL-66,7 and play key roles in the pathology of organ-specific autoimmunity and chronic inflammatory diseases,8 such as multiple sclerosis, rheumatoid arthritis9 and colitis,7 as well as in the experimental autoimmune encephalitis and collagen-induced arthritis models. Furthermore, there is a body of evidence showing a protective role for IL-17 against bacterial infections.10–12 In cancer, the role of Th17 cells is less clear.13 On the one hand, IL-17 promotes angiogenesis leading to tumour progression14 and can play a role in tumour growth and metastasis.15 However, IL-17 can also play a part in tumour rejection and currently remains an interesting candidate for regulating anti-tumour immunity.16
The Th17 cells are regulated by IL-23, a heterodimer comprising p19 and the p40 subunit shared with IL-12p70.17 Both IL-12 and IL-23 play key roles in immunity against infection, e.g. Salmonella.18 Moreover, IL-23 may contribute to the differentiation of macrophages.19 Therefore, like IL-12, IL-23 plays a pivotal role in bridging innate and adaptive immune responses but comparatively little is known regarding its regulation. The two best-characterized activating signals for IL-23 bind to Toll-like receptor 4 (TLR4) and TLR2/Dectin1. The TLR4-dependent signalling events involved in IL-12 family gene activation are well understood.20 Both LPS and lipoteichoic acid activate mitogen-activated protein kinase (MAPK) and nuclear factor-κB signalling cascades21 leading to differential activation of p44/42 extracellular signal-regulated kinase (Erk) 1/2, p38 MAPK and Jun N-terminal kinase (JNK) and IL-12 gene transcription.22–24 The p38 MAPK is broadly accepted to play a positive role for IL-12p40 and IL-12p35 transcription whereas Erk serves a regulatory role.22 Moreover, enhanced phosphorylation of Erk1/2 and p38 MAPK lead to a synergistic increase in IL-23 production induced by LPS and IL-12 in cord-blood-derived DC.24 However, Utsugi et al.25 showed that pharmacological inhibition of p38 enhanced IL-12p19 transcription by LPS-treated macrophages. Moreover, inhibition of JNK increased IL-23p19 mRNA levels. There is growing interest in the role played by intracellular signalling pathways of DC, particularly as a means of altering immune responses in vaccination or therapeutic settings.
Interleukin-23 is important in immunity against fungal infection26 and Zymosan, the beta-glucan-containing a preparation of yeast cell wall, induces production of IL-6, IL-10, transforming growth factor-β and IL-23 by DC.27 Zymosan acts through receptors Dectin-1 and TLR228 and signals through spleen tyrosine kinase (Syk),29,30 caspase recruitment domain family member 9 (CARD9)26 and nuclear factor-κB.26,31 Additionally, a role has been described for phosphatidyl inositol 3-kinase (PI3K) in expression of the IL-12 family.32,33 Butchar et al.34 demonstrated that Francisella tularensis induced IL-23 in human monocytes, and pharmacological inhibition of PI3K led to its down-regulation. Furthermore, exposure of DC to Giardia lamblia reduced IL-12 secretion and this could be recovered by wortmannin (WM) treatment.35 The PI3K inhibitors WM and LY294002 give optimal target specificity for PI3K at 0·1 μm and 10 μm, respectively.36 Despite this, many studies have employed WM at concentrations up to 20 μm where WM can have off-target effects.36–38 These alternative targets include smooth muscle myosin light-chain kinase (IC50 = 0·26 μm),36 Na/K-ATPase (inhibition at 0·5 μm),39 mTOR, DNA-dependent protein kinase (inhibition at 5–20 μm),38 ataxia-telangectasia mutated (ATM), and PI4-kinase α and β.40
In a recent study of the effect of MAPK inhibitors on DC function we revealed preliminary evidence of IL-23 secretion following treatment with a PI3K inhibitor.41 The present study focuses on this initial observation and shows that IL-23 super-induction is not a direct result of PI3K inhibition alone but is probably attributable to other regulatory elements. We show that WM causes a marked and selective up-regulation of p19 and p35 and p40 genes and that this effect is restricted to TLR3 and TLR4 agonists because it does not operate when DC are triggered through TLR2/Dectin1 or TLR7/8. Importantly, we demonstrate the physiological potential of this process ex vivo in freshly isolated myeloid DC (MyDC). When treated with WM and TLR3 and TLR4 agonists MyDC show exacerbated IL-23 levels and increased capacity to prime Th17 responses. The discovery of a means to generate high-levels of IL-23 from MyDC, and subsequent Th17 responses has direct relevance for infectious disease, vaccine development, cancer and autoimmune conditions.18,42–44
Materials and methods
Reagents
Endotoxin-free reagents were used throughout. Recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ), IL-4 and IFN-γ (R&D Systems Europe, Oxford, UK) and Ultrapure TLR-agonists (Salmonella minnesota LPS, poly I:C, R848) were obtained from InvivoGen (San Diego, CA). Wortmannin, Ly294002, MAPK p38 inhibitor SB203580 and JNK inhibitor II were purchased from Merk (Calbiochem, San Diego, CA) and the MAP/ERK kinase (MEK) inhibitor U0126 was from Cell Signalling Technologies (Danvers, MA). Zymosan was purchased from Sigma-Aldrich (Gillingham, Dorset, UK). The following antibodies were used to detect members in MAPK signalling cascade: rabbit anti-human, anti-phospho-p38, anti-phospho p44/p42 (Thr202/Tyr204) (Cell Signalling Technology), anti-human phospho-Akt antibody (Dako, Capinteria, CA), IRDye 800cw donkey anti-rabbit IgG(H+L) and IRDye 680 donkey anti-mouse IgG (H+L) were purchased from LI-COR Biosiences (Cambridge, UK). Mouse anti-human β-actin antibody was obtained from Sigma. Peripheral blood was obtained with the approval of the relevant ethical review boards.
Monocyte-derived and myeloid DC
Whole blood (Buffy coat) was purchased from National Blood Service, Sheffield, UK and fractionated using Ficoll 1.077 (Histopaque 1077; Sigma) and CD14+ cells were isolated using magnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). CD14+ monocytes were cultured in RPMI-1640 medium (10% fetal calf serum, 1% sodium pyruvate) with recombinant human GM-CSF (1000 U/ml) and IL-4 (1000 U/ml) for 5 days and extra medium was added on day 3.
Myeloid DC were isolated from fresh peripheral blood using the Miltenyi CD1c microbead kit as per the manufacturer's instructions. Briefly, CD14+ monocytes were incubated with Fc-blocking beads, anti-CD19 microbeads and anti-CD1c-biotin beads and passed through a negative depletion column to remove CD19+ cells. Unselected cells were positively selected for CD1c. Purity was routinely > 95% by FACS analysis with phycoerythrin-conjugated CD1c antibodies (Miltenyi Biotec) and matched isotype control. Myeloid DC were used after resting cells for 2 hr at 37° following isolation and cultured in DC media containing 1000 U/ml recombinant human GM-CSF (at 104/well, 96-well plate, 200 μl).
Secreted cytokine determination
Immature DC and drug pre-incubated immatureDC were stimulated with LPS (500 ng/ml) and IFN-γ (1000 U/ml). Supernatant was collected after 24 hr and the secretion of IL-23p40/p19 and IL-12p70 was determined by commercial human IL-23 Ready set-go ELISA (eBioscience, San Diego, CA) and human IL-12p70 ELISA kit (BD Biosciences, Oxford, UK). Assays did not significantly react with other proteins and the sensitivities were 15 pg/ml and 7·8 pg/ml, respectively. Interleukin-6 and TNF-α were measured with assays from ImmunoTools (Friesoythe, Germany). The sensitivity of these assays was 9 pg/ml. Interleukin-1β and IL-27 were measured with IL-1β and IL-27 DuoSet (R&D Systems) and assay sensitivity was 3·9 pg/ml and 161·3 pg/ml, respectively. Absorption was measured at 450 nm using a spectrophotometer.
Quantification of mRNA levels of p19, p35 and p40 with real-time reverse transcription PCR
The RNA was isolated using the Nucleospin® RNA II Extraction kits (Macherey-Nagel, Düren, Germany). cDNA was prepared using the MessageSensor™ Reverse Transcription kit (Ambion®, Austin, TX). Real-time PCR was performed using a Taqman PCR Core Reagents kit (Applied Biosciences) and a Stratagene MXP 3000. Primers and probes for housekeeping gene Top1 were from the Applied Biosystems (Carlsbad, CA). The following oligonucleotide sequences were used as sense primers, antisense primers and probes, respectively:
5′-TACTGGGCCTCAGCCAACT-3′, 5′-GAAGGATTTTGAAGCGGAGAA-3′, and 5′-(6-Fam) CCTCAGTCCCAGCCAGCCATG(Tamra)(phosphate)-3′ for p19; 5′-CTCCTGGACCACCTCAGTTTG-3′, 3′-GGTGAAGGCATGGGAACATT-5′, 5′-(6-Fam) CCAGAAACCTCCCCGTGGCCA(Tamra)(phosphate)-3′ for p35; 5′-CGGTCATCTGCCGCAAA-3′, 5′-TGCCCATTCGCTCCAAGA-3′, and 5′-(6-Fam) CGGGCCCAGGACCGCTACTATAGCT(Tamra)(phosphate)-3′ for p40.
Primers and probes were used at 15 pmol/μl. Reactions (20 μl) were performed in triplicate and standardized to a Top1 control for each sample. The IL-23p19 and p40 mRNA levels were expressed relative to the maximal levels following LPS stimulation using Ct levels and normalized according to Top1 levels as previously described.38 The relative levels were calculated according to the formula relative value = 2 − (Ct IL-23 subunit stimulated − Ct Top1 stimulated)/2 − (Ct IL-23 subunit baseline − Ct Top1 baseline).
Pharmacological dissection of intracellular signalling
Specific kinase inhibitors (dissolved in DMSO) were used to dissect the role of signalling pathways. Immature DC were plated in 96-well tissue culture plates and exposed to inhibitors for 1 hr before stimulation via TLR4. The PI3K inhibitor Ly294002 was used at 10 μm. The MEK1/2 inhibitor U0126, p38 MAPK inhibitor SB203580 and JNK inhibitor JNK inhibitor II, were used at 10 μm.
Cell viability
All drugs were used at concentrations in agreement with established in vitro biological systems. Nevertheless, treatment of cells with various PI3K inhibitors (with the exception of 30 μm Ly294002), MAPK inhibitors and TLR agonists was repeatedly confirmed to have no deleterious effect on cell viability as determined by dye-exclusion.
Western blotting
Cell lysates were prepared in RIPA buffer (20 mm Tris–HCl, 150 mm NaCl, 1% Nonidet P-40, 0·5% sodium deoxycholate, 1 mm EDTA, 0·1% SDS) containing protease inhibitors (2 mm 4-(2-aminoethyl)benzenesulfonyl fluoride, 130 μm Bestatin, 14 μm E-64, 1 μm Leupeptin, 0·3 μm Aprotinin, Sigma) and phosphatase inhibitor cocktails 1 and 2 (Sigma). The protein content of cleared lysates was determined using a modified Bradford assay (B-acid : copper sulphate at 50 : 1, Sigma). Proteins (20 μg) were resolved on a 10% SDS–PAGE gel using Tris : glycine buffer. Following electrophoresis, proteins were transferred to nitrocellulose membrane and blocked by incubation in PBS-T (PBS + 0·1% Tween-20) containing milk (5% milk solution). For two-colour infrared imaging the membrane was incubated with primary antibody and loading control antibody anti-β-actin, the membrane was washed in PBS-T. The blot was then incubated with both IRDye 800 cw donkey anti-rabbit IgG (H+L) and IRDye 680 donkey anti-mouse IgG (H+L) antibodies. The image was analysed by the software Li-COR®odyssey-version 3.0 (Cambridge, UK).
Generation of Th17 responses
The DC were treated as previously with WM for 12 hr before the supernatant was discarded and replaced with fresh medium to prevent WM affecting T cells. We have previously shown that IL-23 is not produced until > 12 hr after TLR stimulation.41 After 48 hr, DC were harvested and, in the presence of 50% DC-conditioned media, used to stimulate autologous naive CD4+ T cells isolated using standard Miltenyi two-step procedures as previously described.23 After 5 days, T cells were harvested and rested before re-stimulating with anti-CD3 anti-CD28 for 48 hr. The concentration of IL-17A was determined using ELISA (R&D Systems).
Statistical analysis
Statistical analysis was performed using Student's t-test using Microsoft® Excel® software. (*0·05 > P value ≥ 0·01, **0·01 > P value ≥ 0·001, ***P value < 0·001). Results are presented as the mean ± standard deviation (SD), unless otherwise stated.
Results
Elevated production of IL-23 in TLR4-triggered WM-conditioned DC
In this study we dissected the IL-23 response of human DC to conditioning with WM at a concentration sufficient to inhibit PI3K (‘on-target’) (0·1 μm) and higher ‘off-target’ (1 μm) concentrations. The DC conditioned with < 0·1 μm WM secreted similar levels of IL-23 to TLR4-triggered DC alone (Fig. 1a). However, when conditioned with > 0·1 μm WM, secretion of IL-23 was significantly elevated (P < 0·05). At 1 μm WM DC typically produced 10-fold higher levels of IL-23 than in response to LPS alone. DC were also conditioned with the alternative, more selective, PI3K antagonist, LY294002. As shown in Fig. 1(b), inhibiting PI3K with LY294002 did not enhance the IL-23 response to the TLR4 agonist. At concentrations of LY294002 > 10 μm IL-23 was not secreted, but this was probably because of observed increases in cell death45 (Table 1). Importantly, cell viability was not affected by the addition of WM at any concentration tested (Table 1).
Figure 1.
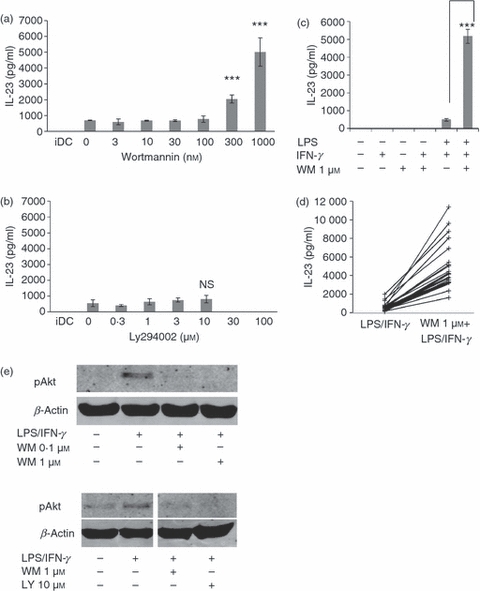
Induction of interleukin-23 (IL-23) secretion by Wortmannin (WM) -conditioned monocyte-derived dendritic cells (mdDC). (a) Dose-dependent induction of IL-23 secretion by DC conditioned with WM and activated with lipopolysaccharide/interferon-γ (LPS/IFN-γ). The mdDC were treated with the indicated dose of WM before activation by LPS/IFN-γ and IL-23 secretion was determined at 24 hr by ELISA. Note the ‘on-target’ concentration of WM is broadly accepted to be 0.1 μm. (b) The effect of the alternative phosphatidyl inositol 3-kinase (PI3K) inhibitor, Ly294002, on IL-23 production. Note the ‘on-target’ concentration of Ly294002 is 10 μm. (c) The effect of WM on IL-23 secretion by DC stimulated by various routes. The DC were activated as indicated and IL-23 secretion was measured by ELISA. (d) WM-dependent IL-23 induction by mdDC generated from 20 independent donors and activated with either LPS/IFN-γ alone or in the presence of 1 μm WM before measuring IL-23 secretion 24 hr later. Each donor is represented by an individual line. (e) Control blot showing that the effect of WM used (0.1 and 1 μm) and LY294002 on LPS-dependent activation and phosphorylation of Akt. The DC were activated by LPS/IFN-γ for 1 hr after WM treatment and the phosphorylation of Akt was determined by two-colour Western blot. Error bars indicate the SD of triplicate experiments and the results shown are taken from at least three independent donors.
Table 1.
Effect of phosphatidyl inositol 3-kinase inhibitors on viability of monocyte-derived dendritic cells
| Condition | Cell viability (%) |
|---|---|
| Immature dendritic cells | 100 |
| Mature dendritic cells | 100 |
| Wortmannin 0·03 μm | 100 |
| Wortmannin 0·1 μm | 100 |
| Wortmannin 0·3 μm | 100 |
| Wortmannin 1 μm | 100 |
| LY294002 1 μm | 100 |
| LY294002 3 μm | 100 |
| LY294002 10 μm | 100 |
| LY294002 30 μm | 47 |
Monocyte-derived DC were treated with the indicated concentration of phosphatidyl inositol 3-kinase inhibitors for 24 hr and their viability was determined by dye-exclusion. Results shown are % viability from a representative donor and viability was monitored in all experiments.
The effect of WM was TLR-dependent as WM alone or with IFN-γ did not result in IL-23 secretion (Fig. 1c). As shown in Fig. 1(d) all donors studied (n = 20) showed a marked increase in IL-23 secretion with WM. Production of IL-23 was significantly increased from 591·6 ± 487·1 pg/ml to 5127·4 ± 2569·8 pg/ml (P < 0·00001, n = 20) after conditioning with 1 μm WM. Western blotting (Fig. 1e) confirmed that phosphorylation of Akt triggered by LPS/IFN-γ was blocked by both 0·1 μm and 1 μm of WM, and also by LY294002 (10 μm), but at 0·1 μm WM or 10 μm LY294002 did not induce a high level of IL-23. As expected the alternative PI3K antagonist LY294002 also inhibited Akt phosphorylation. Therefore, although high concentrations of WM conditioned DC to produce high levels of IL-23, studies using an alternative PI3K inhibitor support the conclusion that PI3K inhibition alone was not sufficient to generate high IL-23 levels and additional signalling components may play a role.
Effect of WM on other cytokines: IL-1 β, IL-6, IL-12, IL-27, TNF-α
We determined whether WM-conditioning extended to cytokines other than IL-23. Wortmannin treatment consistently affected secretion of TNF-α, IL-23 and IL-27 in all donors studied. However, whereas a twofold increase in IL-6 and IL-12 was observed in four of six donors (Fig. 2a, Table 2) the remaining donors showed a greater increase. Interleukin-1β showed a median fourfold increase (six donors) following WM-conditioning, the levels were low and in contrast to IL-23 this was not statistically significant (Table 3).
Figure 2.
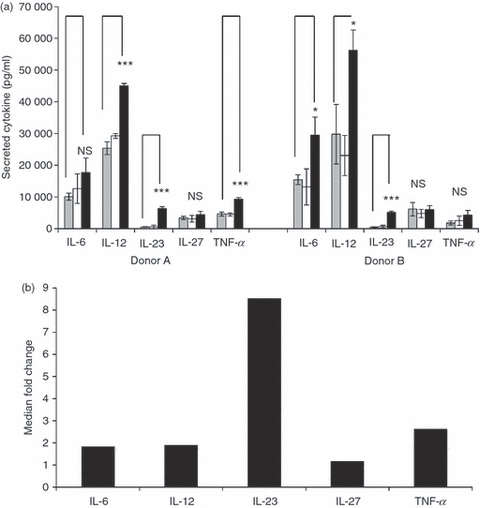
Wortmannin (WM) conditioning of monocyte-derived dendritic cells (mdDC) selectively up-regulates cytokine secretion. (a) The expression of interleukin-6 (IL-6), IL-12, IL-23, IL-27 and tumour necrosis factor-α (TNF-α) was measured by ELISA following activation of DC for 24 hr with lipopolysaccharide/interferon-γ (LPS/IFN-γ). The DC were untreated before activation (grey), or pre-treated for 1 hr with WM at 0.1 μm (white bar) or 1 μm (black bar). Note immature DC did not secrete detectable levels of any of these cytokines. Data are shown for a representative two of six donors and error bars indicate SD. (b) Summary plot showing the median fold-increase above Toll-like receptor 4 (TLR4) activation alone in cytokine secretion from DC of six healthy donors following WM conditioning.
Table 2.
Effect of Wortmannin (WM) on secretion of cytokines from mature monocyte-derived dendritic cells
| Cytokine | 0 μm WM (pg/ml ± SD) | 1 μm WM (pg/ml ± SD) | P value |
|---|---|---|---|
| Interleukin-6 | 10608 ± 6870 | 25650 ± 8160 | < 0·01 |
| Interleukin-12 | 23426 ± 17328 | 49950 ± 23017 | < 0·1 |
| Interleukin-23 | 533 ± 119 | 4603 ± 1493 | < 0·0001 |
| Tumour necrosis factor-α | 12296 ± 13574 | 34287 ± 40166 | 0·26 |
| Interleukin-27 | 6000 ± 2761 | 7306 ± 3012 | 0·54 |
Monocyte-derived dendritic cells were generated from six donors and their cytokine secretion in response to lipopolysaccharide was determined in the absence or presence of Wortmannin. Shown is the cytokine secretion at 24 hr as determined by ELISA ± SD
Table 3.
Effect of Wortmannin (WM) on secretion of interleukin-1β and interleukin-23 from mature monocyte-derived dendritic cells
| Cytokine | 0 μm WM (pg/ml ± SD) | 1 μm WM (pg/ml ± SD) | P value |
|---|---|---|---|
| Interleukin-1β | 12 ± 7 | 65 ± 60 | 0·054 |
| Interleukin-23 | 1337 ± 700 | 17374 ± 8404 | < 0·01 |
Monocyte-derived dendritic cells were generated from an additional six donors and their secretion of interleukin-1β and interleukin-23 in response to lipopolysaccharide was determined in the absence or presence of WM. Shown is the cytokine secretion at 24 hr as determined by ELISA ± SD
Wortmannin (1 μm) conditioning affected TLR4-dependent cytokine secretion as shown in Table 2. In contrast, secretion of these cytokines did not increase when DC were conditioned with 0·1 μm WM (not shown). Because the maximum absolute levels of IL-23 were less than for other cytokines studied, the median fold-increase in cytokine secretion for all six donors treated with 1 μm WM compared with LPS/IFN-γ alone is shown (Fig. 2b). It was apparent that the most pronounced and consistent effect of WM was on IL-23 secretion, whereas IL-1β, IL-6, IL-12 and TNF-α were considerably less affected (approximately twofold induction). The production of IL-27 was unaffected by any concentration of WM.
Wortmannin-dependent IL-23 induction is TLR-specific
Triggering DC through alternative TLR differentially affected IL-23 production. Zymosan alone yielded the most IL-23 and this increased twofold with the addition of IFN-γ (Fig. 3a,b). Treating with LPS, Poly I:C or R848 yielded considerably lower levels of IL-23 than with Zymosan while PamCys did not induce IL-23 production (not shown). Therefore, we determined whether the effect of WM was restricted to TLR4 or applied to a range of DC danger-receptors. Conditioning with WM only caused pronounced increases in IL-23 secretion with TLR3 and TLR4 agonists (Fig. 3a). The magnitude of this effect was increased by the addition of IFN-γ for TLR4 only (Fig. 3b) and under these conditions the level of cytokine secretion was greater than the maximum achieved with Zymosan. In contrast, WM did not markedly affect IL-23 secretion when cells were activated by TLR2 alone, TLR2/Dectin1 or TLR7/8 ligands.
Figure 3.
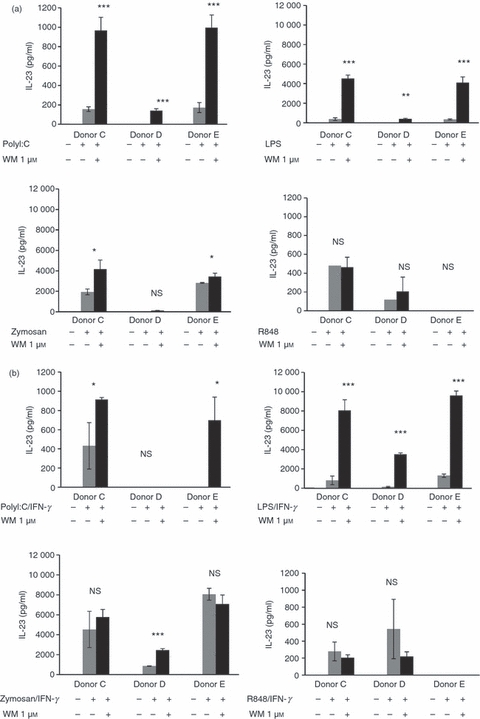
Wortmannin (WM) -dependent induction of interleukin-23 (IL-23) occurs with selected Toll-like receptor (TLR) agonists. The response of WM-conditioned (1 μm) monocyte-derived dendritic cells (mdDC) from three representative donors (out of five) to Zymosan (TLR2/Dectin1), lipopolysaccharide (LPS; TLR4), Poly I:C (TLR3) or R848 (TLR7/8) was studied. Secretion of IL-23 was determined in (a) the absence or (b) the presence of interferon-γ (IFN-γ) (1000 U/ml). Error bars indicate the SD of triplicate experiments. Missing bars indicate where cytokine secretion was below the limits of sensitivity for the ELISA.
WM increases transcription of the p19, p35 and p40 genes
When DC conditioned with WM (1 μm) and activated with LPS were compared with cells treated with LPS alone, or conditioned with 0·1 μm WM/LPS, there was a marked difference in gene transcription. Wortmannin (1 μm) pre-treatment resulted in a 15-fold increase in p19 mRNA compared with the peak-transcription observed with LPS alone (Fig. 4a) [It should be noted that the effect of WM (1 μm) when compared with resting levels of gene transcription were in the order of 100 s of fold induction]. Transcription of p19 was maximum at 12 hr but returned to baseline levels by 24 hr. Transcription of the p35 and p40 genes also increased in WM (1 μm) -treated DC. Increased IL-12 secretion associated with prominent increased p40 transcription (Fig. 4b). In contrast to the amount of mRNA produced, the kinetics of transcription of p19, p35 and p40 genes were unaffected by WM conditioning, suggesting a direct effect on the IL-23 genes.
Figure 4.
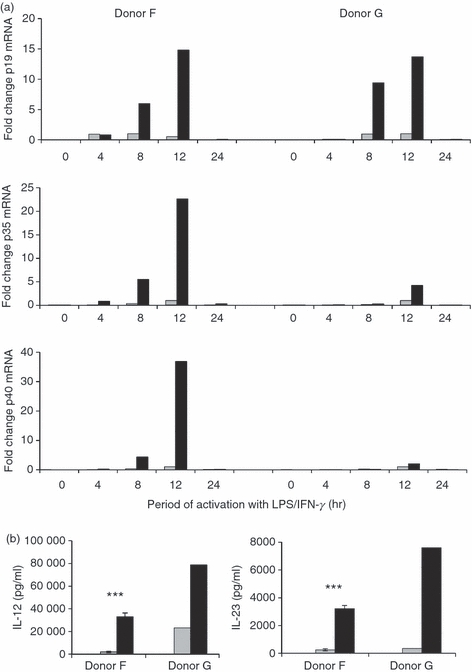
Wortmannin (WM) -mediated interleukin-23 (IL-23) induction is transcriptionally r egulated. (a) Quantitative PCR (TaqMan) was undertaken to determine the relative abundance of p19, p35 and p40 transcripts in monocyte-derived dendritic cells (mdDC) conditioned with 1 μm WM (black bar) or left untreated (grey bar) before lipopolysaccharide/interferon-γ (LPS/IFN-γ) activation. Data shown show the fold-induction above the maximal level obtained with LPS/IFN-γ alone (grey). Gene expression is normalized to TOP1 expression, a housekeeping gene previously shown by us to be stably expressed in human DC.23 Data shown are from two independent donors. PCR experiments were conducted in triplicate. (b) Corresponding IL-12 and IL-23 release by DC from the same donors following WM 1 μm treatment.
Role of MAPK pathways in super-induction of IL-23
The MAPK pathways play essential roles in TLR4-dependent signalling.23 Therefore, we determined if there was a role for MAPK in the super-induction of IL-23 by WM. The p38 MAPK was critical for IL-23 production as inhibition of p38 MAPK function with SB203580 completely abolished IL-23 secretion (Fig. 5a). Inhibition of MEK1/2 and JNK also significantly (P < 0·0001 and P < 0·00001, respectively) reduced, but did not entirely ablate, the effect of WM on IL-23 secretion. The resting levels of p38 and Erk1/2 phosphorylation were unaffected by WM treatment (Fig. 5b) and both MAPK were activated within 30 min following addition of LPS. Wortmannin conditioning at either concentration studied did not alter the kinetics of phosphorylation of p38 or Erk1/2 MAPK. This observation was consistent in the three donors studied (not shown).
Figure 5.
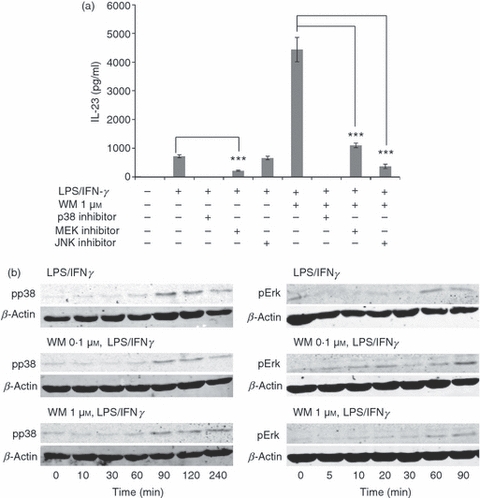
The role of mitogen-activated protein kinase (MAPK) signalling pathways in Wortmannin (WM) -dependent interleukin-23 (IL-23) induction. (a) Monocyte-derived dendritic cells (mdDC) were treated with the indicated MAPK inhibitors (10 μm) for 1 hr before treatment with WM (1 μm) and subsequent activation with lipopolysaccharide/interferon-γ (LPS/IFN-γ). IL-23 secretion was measured by ELISA at 24 hr. (b) The effect of conditioning with WM on the magnitude and kinetics of activation of p38 or Erk MAPK was studied by 2-colour Western blot. Data represents one experiment from at least two independent donors. Error bars indicate SD and statistical significance is shown: ***P < 0.001.
WM-conditioned MyDC secrete IL-23 and prime Th17 responses
We examined the response of blood MyDC ex vivo to WM conditioning and TLR-activation. Freshly purified peripheral blood MyDC (Fig. 6a,b) did not secrete IL-23 in the absence of TLR ligands (Fig. 6c,d). Even with the addition of single TLR ligands, two of three donors did not produce detectable levels of IL-23. However, following conditioning with WM (1 μm) all donors studied secreted high levels of IL-23 comparable with those obtained with monocyte-derived DC (mdDC) on a per-cell basis. In contrast to our findings with mdDC, MyDC secreted similar levels of IL-23 when trigger through TLR3 or TLR4 in the presence of WM. Despite the marked induction of IL-23 secretion in WM-conditioned MyDC there was no detectable IL-12p70 produced with either TLR agonist (not shown). Therefore, physiological antigen-presenting cells exhibited a markedly biased IL-23 response when conditioned ex vivo with WM raising the prospect of future immunomodulation of IL-23 following treatment with appropriate signalling inhibitors.
Figure 6.
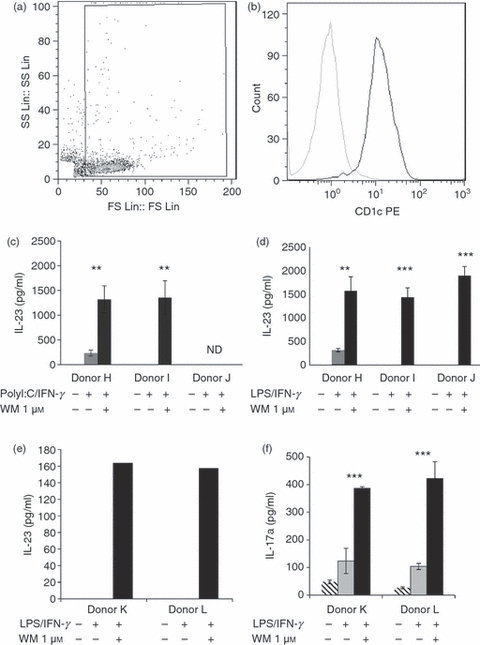
Wortmannin (WM) -conditioned myeloid dendritic cells (MyDC) prime the T helper type 17 (Th17) response. Myeloid DC were purified from healthy donor peripheral blood by magnetic cell sorting. Representative flow cytometric profile of purified MyDC for one donor is shown (a, b). Flow-cytometric demonstration of MyDC purity as shown by forward and side-scatter profile (a), and CD1c positivity (b). Dark histogram shows CD1c and grey shows isotype control. Myeloid DC were conditioned with WM (1 μm) as indicated (black bar) or left untreated (grey bar). DC were subsequently activated with either Poly I:C (c) or lipopolysaccharide (LPS) (d) in the presence of interferon-γ (IFN-γ) for 24 hr and interleukin-23 (IL-23) was secretion determined by ELISA. (e) IL-23 production by MyDC from two representative donors. (f) IL-17A production from autologous CD4+ CD45RA+ T cells primed for 5 days by the above DC in the presence of 50% DC supernatant (1 : 1 DC supernatant : T-cell medium) from the respective donor DC. T cells were re-stimulated with anti-CD3/CD28 before analysis of IL-17A secretion. Error bars show SD, ND – not determined.
Last, we examined the capacity of WM-conditioned MyDC to polarize naive CD4+ T cells into Th17 cells. As shown in Fig. 6(f), WM-conditioned MyDC primed prominent Th17 responses characterized by high levels of IL-17A secretion. In contrast MyDC matured in the absence of WM primed considerably lower levels of Th17 response than their WM-conditioned counterparts. The generation of Th17 responses from naive CD4+ T cells correlated with the secretion of IL-23 by the relevant DC populations (Fig. 6e). It is important to note that the Th17 polarization is independent of any differential effects of WM-conditioned DC on proliferation as IL-17A secretion was determined in an antibody-mediated re-stimulation of a fixed number of cells.
Discussion
Novel strategies to regulate IL-23 production and subsequent Th17 responses would have important immune and therapeutic ramifications. In this study we identified a means to markedly increase IL-23 release and Th17 priming by human DC. Importantly, MyDC responded in a similar way to mdDC with increased IL-23 release following WM-conditioning, showing the potential physiological relevance of WM-mediated super-induction. Although IL-12p70 was also affected by WM, this was restricted to mdDC and was much less. The effect of WM was selective and did not alter production of IL-27. The effect of WM was most pronounced in MyDC as they only secreted IL-23 and did not secrete IL-12, suggesting more distinct polarization towards support of Th17 immunity.
Interleukin-23, IL-12 and IL-27 are members of the IL-6/IL-12 cytokine superfamily46 and our results showed that WM differentially regulated several members of this family. Marked up-regulation in IL-23 and increased IL-6 and IL-12 secretion suggest that these cytokines may share similar regulatory mechanisms. Control of IL-23 is broadly understood to involve the PI3K/Akt,34 MAPK47 and Syk-CARD926,28,48 intracellular signalling pathways. The signalling mechanism for IL-23 super-induction by WM is unlikely to be caused by PI3K inhibition alone as the alternative PI3K antagonist Ly294002 did not affect IL-23. Furthermore, the IL-23 response to Dectin-1/TLR2 ligand was not super-induced with WM conditioning. This suggests that WM preferentially affects TLR3 and TLR4 pathways but does not interact with regulators of the Syk-CARD9, TLR2 or TLR7/8 pathways. Future work will examine the interaction with other pathways including the TLR5 ligand Flagellin and Dectin-1 ligand β-glucan49 and the effect on simultaneous triggering of multiple TLR pathways, already shown to co-operate.50 What distinguishes TLR3/4 regarding the observed WM effect remains unknown but clues may lie in discrete aspects of their signalling pathways. Signalling through TLR3 exclusively employs the adaptor molecule Toll-IL-1 receptor resistance (TIR) domain-containing adaptor inducing IFN-β (TRIF) whereas the TRIF-related adaptor molecule is used by TLR4 to recruit TRIF.51 There are a number of negative regulation checkpoints involved in TLR-signalling pathways.52 The TRIF-dependent activation of transcription factors, such as IFN regulatory factor 3, is limited by the sterile alpha and TIR motif containing protein (SARM) which does not affect MyD88-dependent processes.53 Therefore one possible mechanism of action is that WM impedes regulatory adaptors such as SARM and so increases TRIF-dependent signalling to the IL-23 genes. In this regard, induction of IL-12, IL-18 and IL-23 in DC is impaired in the absence of functional TRIF.54 However, little is currently known regarding a role for SARM in IL-23 regulation. Murine knock-out models would provide an appropriate platform in which to test this hypothesis but our studies in several strains of mice have shown only a twofold effect of WM on IL-23 release by DC (unpublished results). Future studies will use small interfering RNA in human DC.
The MAPK cascades are central signalling pathways for TLR-dependent activation.55 A number of studies, including our own, have highlighted the critical role for p38 MAPK in IL-12 family cytokine secretion. Jang et al.24 showed that enhanced phosphorylation of Erk1/2 and p38 MAPK synergistically increased IL-23 production by human cord-blood-derived DC and we and others showed that p38 MAPK is required for both IL-12p40 and IL-12p35 expression.22 Our results confirm that p38 MAPK plays a ‘switch like’ role as WM-treated DC did not secrete IL-23 when p38 was inhibited. Therefore WM-conditioning does not bypass the p38 MAPK ‘switch’ and the Erk and JNK pathways are also important for IL-23 super-induction.
Although WM is a selective PI3K inhibitor (50–200 nm range), at higher concentrations it also inhibits other targets at higher concentrations. These include smooth muscle myosin light chain kinase (IC50 = 260 nm), the catalytic subunit of DNA-dependent protein kinases and PI3K-related kinases.36,40,56 However, our study suggests that the effect of WM on IL-23 secretion may not be mediated through PI3K inhibition alone as the alternative inhibitor Ly294002 exerted no effect on IL-23.36 Therefore, IL-23 induction by WM is probably mediated by signalling mediators other than, or in addition to, PI3K and their identity remains to be discovered. In this regard, experiments are currently underway to precisely define the target through which WM super-induces IL-23. These include mapping changes in the phospho-proteome57 with tandem mass-spectrometry and this series of experiments will occupy the next 18 months. Other parallel studies are examining the role played by nuclear factor-κB58 and the other signalling proteins previously described to be affected by WM. A number of other signalling molecules including Rac1, B cell leukemia 3 (Bcl3) and protein phosphatase 2A (PP2A)59 have previously been described as playing regulatory roles for IL-23 but there is no evidence to suggest that these are affected by WM.
Identification of the stimulatory or regulatory targets of 1 μm WM would provide important translational opportunities. On the one hand, IL-23 clearly enhances aspects of immunity, notably Th17 responses,19 and has anti-tumour60 and anti-metastastic activity.61 In contrast, IL-23 and associated IL-17 production are key mediators of autoimmune62 and inflammatory pathology.63 Specifically addressing the target of WM would offer potential for the treatment of diseases, including inflammatory bowel disease,43,64 Crohn's disease,44 rheumatoid arthritis9 and multiple sclerosis.42 The key to these lies in the role(s) played by Th17 cells in health and disease and in this regard we are presently exploring the role of WM-conditioned DC and subsequent Th17 polarization in pre-clinical models of vaccine efficacy and cancer immunity.
Acknowledgments
Q.W. is in receipt of a University of Nottingham IRES fellowship. H.F. is currently funded by an MRC CRTF grant.
Disclosures
There are no conflicts of interest of any authors.
References
- 1.Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr Top Microbiol Immunol. 2006;298:157–74. doi: 10.1007/3-540-27743-9_8. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–25. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 4.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–8. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 5.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–55. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–49. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Mulcahy LA, Mohammed RA, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HR, Kim HS, Park MK, Cho ML, Lee SH, Kim HY. The clinical role of IL-23p19 in patients with rheumatoid arthritis. Scand J Rheumatol. 2007;36:259–64. doi: 10.1080/03009740701286813. [DOI] [PubMed] [Google Scholar]
- 10.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Happel KI, Zheng M, Young E, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–6. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji Y, Zhang W. Th17 cells: positive or negative role in tumor? Cancer Immunol Immunother. 2010;59:979–87. doi: 10.1007/s00262-010-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 15.Alexandrakis MG, Pappa CA, Miyakis S, Sfiridaki A, Kafousi M, Alegakis A, Stathopoulos EN. Serum interleukin-17 and its relationship to angiogenic factors in multiple myeloma. Eur J Intern Med. 2006;17:412–6. doi: 10.1016/j.ejim.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirhonen J, Matikainen S, Julkunen I. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J Immunol. 2002;169:5673–8. doi: 10.4049/jimmunol.169.10.5673. [DOI] [PubMed] [Google Scholar]
- 18.MacLennan C, Fieschi C, Lammas DA, et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190:1755–7. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 19.Hao JS, Shan BE. Immune enhancement and anti-tumour activity of IL-23. Cancer Immunol Immunother. 2006;55:1426–31. doi: 10.1007/s00262-006-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107:2432–9. doi: 10.1182/blood-2005-06-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 22.Goodridge HS, Harnett W, Liew FY, Harnett MM. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology. 2003;109:415–25. doi: 10.1046/j.1365-2567.2003.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson AM, Mulcahy LA, Zhu XW, O'Donnell D, Patel PM. Tumour-mediated disruption of dendritic cell function: inhibiting the MEK1/2-p44/42 axis restores IL-12 production and Th1-generation. Int J Cancer. 2008;123:623–32. doi: 10.1002/ijc.23530. [DOI] [PubMed] [Google Scholar]
- 24.Jang MS, Son YM, Kim GR, Lee YJ, Lee WK, Cha SH, Han SH, Yun CH. Synergistic production of interleukin-23 by dendritic cells derived from cord blood in response to costimulation with LPS and IL-12. J Leukoc Biol. 2009;86:691–9. doi: 10.1189/JLB.1008639. [DOI] [PubMed] [Google Scholar]
- 25.Utsugi M, Dobashi K, Ishizuka T, Kawata T, Hisada T, Shimizu Y, Ono A, Mori M. Rac1 negatively regulates lipopolysaccharide-induced IL-23 p19 expression in human macrophages and dendritic cells and NF-kappaB p65 trans activation plays a novel role. J Immunol. 2006;177:4550–7. doi: 10.4049/jimmunol.177.7.4550. [DOI] [PubMed] [Google Scholar]
- 26.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 27.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 28.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–80. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 29.Robinson MJ, Osorio F, Rosas M, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–51. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slack EC, Robinson MJ, Hernanz-Falcon P, Brown GD, Williams DL, Schweighoffer E, Tybulewicz VL, Reis e Sousa C. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur J Immunol. 2007;37:1600–12. doi: 10.1002/eji.200636830. [DOI] [PubMed] [Google Scholar]
- 31.Dillon S, Agrawal S, Banerjee K, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–28. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shumilina E, Zahir N, Xuan NT, Lang F. Phosphoinositide 3-kinase dependent regulation of Kv channels in dendritic cells. Cell Physiol Biochem. 2007;20:801–8. doi: 10.1159/000110440. [DOI] [PubMed] [Google Scholar]
- 33.Bonifazi P, D'Angelo C, Zagarella S, et al. Intranasally delivered siRNA targeting PI3K/Akt/mTOR inflammatory pathways protects from aspergillosis. Mucosal Immunol. 2009;3:193–205. doi: 10.1038/mi.2009.130. [DOI] [PubMed] [Google Scholar]
- 34.Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, Tridandapani S. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–54. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 35.Kamda JD, Singer SM. Phosphoinositide 3-kinase-dependent inhibition of dendritic cell interleukin-12 production by Giardia lamblia. Infect Immun. 2009;77:685–93. doi: 10.1128/IAI.00718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carnero A, Lacal JC. Wortmannin, an inhibitor of phosphatidyl-inositol 3-kinase, induces oocyte maturation through a MPF-MAPK-dependent pathway. FEBS Lett. 1998;422:155–9. doi: 10.1016/s0014-5793(97)01619-0. [DOI] [PubMed] [Google Scholar]
- 38.Boulton S, Kyle S, Yalcintepe L, Durkacz BW. Wortmannin is a potent inhibitor of DNA double strand break but not single strand break repair in Chinese hamster ovary cells. Carcinogenesis. 1996;17:2285–90. doi: 10.1093/carcin/17.11.2285. [DOI] [PubMed] [Google Scholar]
- 39.Ecay TW, Dickson JL, Conner TD. Wortmannin inhibition of forskolin-stimulated chloride secretion by T84 cells. Biochim Biophys Acta. 2000;1467:54–64. doi: 10.1016/s0005-2736(00)00204-2. [DOI] [PubMed] [Google Scholar]
- 40.Ethier MF, Madison JM. LY294002, but not wortmannin, increases intracellular calcium and inhibits calcium transients in bovine and human airway smooth muscle cells. Cell Calcium. 2002;32:31–8. doi: 10.1016/s0143-4160(02)00111-2. [DOI] [PubMed] [Google Scholar]
- 41.Jackson AM, Mulcahy LA, Porte J, et al. Role of mitogen activated protein kinase and PI3K pathways in regulation of IL-12 family cytokines in dendritic cells and the generation of TH-responses. Eur Cytokine Netw. 2010;21:319–28. doi: 10.1684/ecn.2010.0219. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Inducible IL-23p19 expression in human microglia via p38 MAPK and NF-kappaB signal pathways. Exp Mol Pathol. 2008;84:1–8. doi: 10.1016/j.yexmp.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2009;60:97–110. doi: 10.1146/annurev.med.60.051407.123757. [DOI] [PubMed] [Google Scholar]
- 44.Neurath MF. IL-23: a master regulator in Crohn disease. Nat Med. 2007;13:26–8. doi: 10.1038/nm0107-26. [DOI] [PubMed] [Google Scholar]
- 45.Guo M, Joiakim A, Reiners JJ., Jr Suppression of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated aryl hydrocarbon receptor transformation and CYP1A1 induction by the phosphatidylinositol 3-kinase inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1- benzopyran-4-one (LY294002) Biochem Pharmacol. 2000;60:635–42. doi: 10.1016/s0006-2952(00)00379-8. [DOI] [PubMed] [Google Scholar]
- 46.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175:2191–200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 47.Kim HR, Cho ML, Kim KW, et al. Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF-kappaB- and p38 MAPK-dependent signalling pathways. Rheumatology (Oxford) 2007;46:57–64. doi: 10.1093/rheumatology/kel159. [DOI] [PubMed] [Google Scholar]
- 48.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–81. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–31. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerosa F, Baldani-Guerra B, Lyakh LA, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–61. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGettrick AF, Brint EK, Palsson-McDermott EM, Rowe DC, Golenbock DT, Gay NJ, Fitzgerald KA, O'Neill LA. Trif-related adapter molecule is phosphorylated by PKC-epsilon during Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2006;103:9196–201. doi: 10.1073/pnas.0600462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Hu Y, Deng WW, Sun B. Negative regulation of Toll-like receptor signaling pathway. Microbes Infect. 2009;11:321–7. doi: 10.1016/j.micinf.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–81. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 54.Weighardt H, Jusek G, Mages J, Lang R, Hoebe K, Beutler B, Holzmann B. Identification of a TLR4- and TRIF-dependent activation program of dendritic cells. Eur J Immunol. 2004;34:558–64. doi: 10.1002/eji.200324714. [DOI] [PubMed] [Google Scholar]
- 55.Goodridge HS, Underhill DM. Fungal recognition by TLR2 and Dectin-1. Handb Exp Pharmacol. 2008;183:87–109. doi: 10.1007/978-3-540-72167-3_5. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol. 2005;12:99–107. doi: 10.1016/j.chembiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 57.Boateng JS, Auffret AD, Matthews KH, Humphrey MJ, Stevens HN, Eccleston GM. Characterisation of freeze-dried wafers and solvent evaporated films as potential drug delivery systems to mucosal surfaces. Int J Pharm. 2010;389:24–31. doi: 10.1016/j.ijpharm.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Breckpot K, Escors D. Dendritic cells for active anti-cancer immunotherapy: targeting activation pathways through genetic modification. Endocr Metab Immune Disord Drug Targets. 2009;9:328–43. doi: 10.2174/187153009789839156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang J, Voorhees TJ, Liu Y, Zhao Y, Chang CH. Interleukin-23 production in dendritic cells is negatively regulated by protein phosphatase 2A. Proc Natl Acad Sci U S A. 2010;107:8340–5. doi: 10.1073/pnas.0914703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shan BE, Hao JS, Li QX, Tagawa M. Antitumor activity and immune enhancement of murine interleukin-23 expressed in murine colon carcinoma cells. Cell Mol Immunol. 2006;3:47–52. [PubMed] [Google Scholar]
- 61.Lo CH, Lee SC, Wu PY, et al. Antitumor and antimetastatic activity of IL-23. J Immunol. 2003;171:600–7. doi: 10.4049/jimmunol.171.2.600. [DOI] [PubMed] [Google Scholar]
- 62.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]


