Abstract
The intestinal immune system is constantly challenged by foreign antigens and commensal bacteria. Therefore, proper control of the intestinal microenvironment is required. One important arm of this regulatory network consists of regulatory T cells. In contrast to CD4+ Foxp3+ regulatory T cells, which have been well characterized, immunomodulatory CD8+ T cells that express Foxp3 are less well defined in terms of their generation and function. Failures of these regulatory mechanisms contribute to the development of inflammatory bowel disease. In this study we demonstrate that the frequency of CD8+ Foxp3+ T cells is reduced in the peripheral blood of patients with ulcerative colitis. As these cells might play a currently underestimated role in the maintenance of intestinal homeostasis, we have investigated human and murine CD8+ Foxp3+ T cells generated by stimulating naive CD8+ T cells in the presence of transforming growth factor-β and retinoic acid, mediators that are abundantly produced in the intestinal mucosa. These CD8+ Foxp3+ fully competent regulatory T cells show strong expression of regulatory molecules CD25, Gpr83 and CTLA-4 and exhibit cell–cell contact-dependent immunosuppressive activity in vitro. Our study illustrates a previously unappreciated critical role of CD8+ Foxp3+ T cells in controlling potentially dangerous T cells and in the maintenance of intestinal homeostasis.
Keywords: CD8+ Foxp3+ T cells, immune regulation, inflammatory bowel disease, retinoic acid, transforming growth factor-β
Introduction
Regulatory T cells are believed to play a crucial role in the bowel's adjustments to microbial antigens and in the modulation of tissue-damaging immune reactions; therefore, these cells are regarded as a promising new therapeutic target.1 The most prominent population of regulatory T cells is the CD4+ subset. Various populations of thymically or peripherally induced regulatory T cells, such as CD4+ CD25+ T cells,2 CD4+ CD45RBlow T cells,3 type 1 regulatory T (Treg1) cells,4 and type 3 helper T (Th3) cells,5 have been described for the control of intestinal inflammation. However, less attention has been given to the inhibitory capability of CD8+ T cells, and, although several types of CD8+ regulatory T cells with various phenotypes seem to exist in humans and in experimental animals,6–11 the nature of the primary CD8+ regulatory T cells and the mechanisms underlying their generation remain elusive. Some populations of CD8+ regulatory T cells are believed to be involved in the control of mucosal immune responses. An experimental model mimicking inflammatory bowel disease (IBD) uses the injection of CD4+ CD45RBhigh T cells into syngeneic mice deficient in the recombination activation gene 2 (Rag-2) to generate inflammation of the gut mucosa. In this model, Ménager-Marcq et al. demonstrated that CD8+ CD28− T cells, but not CD8+ CD28+ T cells, freshly isolated from the spleen or the gut efficiently prevent the development of colitis.12 In addition, Ho et al. identified a subset of CD8+ regulatory T cells characterized by CD8+ CD44−CD103high expression.13 Adoptive transfer of CD4+ T cells from mice that over-express tumour necrosis factor-α into immunodeficient Rag−/− mice induces ileitis, but co-transfer of CD8+ CD44−CD103+ T cells from wild-type mice attenuates the ileitis histology.13 Moreover, Mayer and co-workers suggested that CD8+ T cells with regulatory activity are present in the lamina propria (LP) of normal healthy persons but not in the LP of patients with IBD.14,15 Yet, whereas all of these studies clearly confer on CD8+ T cells an important role in intestinal inflammation, none of these studies has been focused on the induction of truly CD8+ regulatory T cells that express forkhead box P3 (Foxp3). In a previous study we demonstrated that the intestinal expression of a self-antigen leads to the induction of antigen-specific CD8+ Foxp3+ T cells in vivo.16 Furthermore, we have demonstrated that in vitro stimulation of antigen-specific CD8+ T cells in the presence of transforming growth factor-β (TGF-β) and retinoic acid (RA) induced a robust population of CD8+ Foxp3+ regulatory T cells.17 As the intestine is characterized by abundant production of TGF-β and RA it might therefore be prone to the induction of Foxp3+ regulatory T cells.
As these cells might play an as yet underestimated role in the maintenance of intestinal homeostasis, we have investigated CD8+ Foxp3+ T cells generated by TGF-β and RA by analysing the function and phenotype in humans and mice. Our study shows that TGF-β/RA-converted CD8+ Foxp3+ T cells share all the major features of conventional CD4+ regulatory T cells, i.e. suppressive function in vitro. Furthermore, these subsets of regulatory T cells also resemble each other at the molecular level as determined by gene expression studies. The fact that this conversion by TGF-β and RA also works with human CD8+ T cells is of particular interest because we demonstrate in this study that the frequency of CD8+ Foxp3+ T cells is reduced in the peripheral blood of patients with intestinal inflammation. Hence, our study illustrates a previously unappreciated critical role of CD8+ Foxp3+ T cells in controlling potentially dangerous T cells.
Materials and methods
Mice
Foxp3/GFP mice express both the Foxp3 and green fluorescent protein (GFP) under the endogenous regulatory sequence of the Foxp3 locus and were obtained from the Charles River Laboratories (Sulzfeld, Germany). BALB/c mice and C57BL/6 mice were obtained from Harlan Laboratories (Harlan Winkelmann GmbH, Borchen, Germany). Granzyme B (GzmB) -deficient C57BL/6 mice were kindly provided by Prof. Dr U. Dittmer (Department of Virology, University Duisburg-Essen).
Patients
Blood samples were obtained from 12 patients (five men, seven women; age range, 32–72 years) with active ulcerative colitis (UC) and from 18 healthy blood donors (eight men, ten women; age range, 22–87 years), who were used as control group. To assess disease activity, the clinical activity index (CAI) according to Rachmilewitz's criteria and the ulcerative colitis disease activity index (UCDAI) according to Sutherland's criteria, including a grading of clinical and endoscopic signs, were determined. Patients were classified as having acute UC with a CAI > 4.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells were isolated from heparin-treated blood by Bicoll density gradient centrifugation (Biochrom AG, Berlin, Germany). Isolated cells were washed with buffer and either analysed immediately or cryopreserved in medium containing 10% fetal calf serum (PAA Laboratories GmbH, Pasching, Austria) and 10% DMSO (Carl Roth GmbH, Karlsruhe, Germany).
Isolation of lymphocytes
The autoMacs separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) was used for the isolation or depletion of lymphocyte subsets according to the manufacturer's instructions. CD4+ and CD8+ T cells were negatively selected.
Antibodies and flow cytometry
All antibodies were obtained from BD Biosciences Pharmingen (Heidelberg, Germany). Staining with α-Foxp3 (eBioscience, San Diego, CA) was performed according to the manufacturer's recommendations. Flow cytometric analysis was performed with a FACSCalibur flow cytometer and CellQuest software or with an LSR II and DIVA software (both from BD Biosciences).
In vitroinduction of CD8+ Foxp3+ T cells
For the induction of Foxp3 expression in polyclonal CD8+ T cells, 2·5 × 105 CD8+ CD25− naive T cells from Foxp3/GFP transgenic mice or human CD8+ T cells isolated from peripheral blood were stimulated with 0·5 μg/ml soluble α-CD3, 2 ng/ml recombinant human TGF-β (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany) and 100 nm RA (Sigma-Aldrich, Saint Louis, MO). On day 2, 50 U/ml recombinant human interleukin-2 was added to the cultures. On day 4, Foxp3 expression in CD8+ T cells was determined by staining with α-CD8 and α-Foxp3 antibodies.
DNA microarray hybridization and analysis
Total RNA from sorted CD8+ T cells was isolated using the RNAeasy kit (Qiagen GmbH, Hilden, Germany). Quality and integrity of total RNA was controlled on an Agilent Technologies 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). Total RNA (500 ng) was used in the Cy3-labelling reaction using the one-colour Quick Amp Labeling protocol (Agilent Technologies). Labelled cRNA was hybridized to Agilent's human 4 × 44k microarrays for 16 hr at 68° and scanned using the Agilent DNA Microarray Scanner. Expression values were calculated using the software package Feature Extraction 10.5.1.1 (Agilent Technologies). Statistical analysis of the expression data was performed using the Gene Spring software package (Agilent Technologies). Clustering analysis was performed using Genesis 1.6.
Cytokine analysis
For cytokine profiling, 4 × 105 sorted CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells were re-stimulated with 10 ng/ml PMA and 1 μg/ml ionomycin (Sigma-Aldrich) for 20 h at 37°. Quantification of cytokines in cell culture supernatants was performed by using the Procarta Cytokine assay kit (Panomics, Fremont, CA) according to the manufacturer's recommendations. The assay was run with a Luminex200 instrument using Luminex IS software (Luminex Corporation, Austin, TX). For intracellular interferon-γ (IFN-γ) staining T cells were re-stimulated with 10 ng/ml PMA, 1 μg/ml ionomycin and 5 μg/ml Brefeldin A (Sigma-Aldrich) for 4 hr.
Real-time reverse transcription-PCR
Total RNA was prepared from isolated CD8+ T cells using the RNeasy kit (Qiagen) following cDNA synthesis by M-MLV Reverse Transcriptase, RNase H Minus, Point Mutation (Promega, Madison, WI) and oligo-dT mixed with random hexamers (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Real-time reverse transcription-PCR was performed in an ABI PRISM cycler (Applied Biosystems, Foster City, CA) with specific primers for GzmB. Relative mRNA levels were determined by normalization to the housekeeping gene RPS9.
Suppression assay
For human suppression assays 5 × 104 human TGF-β/RA-treated CD8+ CD25+ T cells were co-cultured with 5 × 104 freshly isolated CFSE-labelled CD4+ responder T cells from the same donor and stimulated using the Treg Suppression Inspector (Miltenyi Biotec) for 6 days. For murine T-cell suppression assays, TGF-β/RA-treated CD8+ T cells from Foxp3/GFP mice were separated into CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells by FACS on GFP expression, co-cultured with 1 × 105 freshly isolated CFSE-labelled CD4+ CD25− responder T cells in a 1 : 1 ratio and 0·5 × 105 splenic dendritic cells (DCs) from syngeneic mice, and stimulated with 0·5 μg/ml soluble α-CD3 for 3 days. When indicated, cells were separated by using a transwell system. Suppression assays in the absence of DCs were stimulated with 0·75 μg/ml plate-bound α-CD3 and 1 μg/ml soluble α-CD28 for 3 days. Proliferation of responder cells was measured by loss of CFSE dye.
Results
Decreased frequency of CD8+ Foxp3+ T cells in the blood of patients with ulcerative colitis
To analyse the relevance of CD8+ Foxp3+ T cells to intestinal homeostasis, we tested whether CD8+ Foxp3+ T cells can be detected in healthy and diseased humans with severe intestinal inflammation. Peripheral blood from patients with UC and from healthy control subjects was analysed for the expression of CD8, CD25 and Foxp3. Despite the active state of disease (Table 1), we found no difference in the percentage of CD8+ CD25+ T cells in healthy control subjects and in patients with UC (Fig. 1a). In contrast, when CD8+ CD25+ T cells were analysed for the expression of Foxp3, the percentage of these cells was significantly reduced in the peripheral blood of patients with active UC (Fig. 1b).
Table 1.
Characterization of ulcerative colitis patients
| Patient | Sex | Age | CAI | UCDAI |
|---|---|---|---|---|
| 1 | M | 48 | 6 | 6 |
| 2 | F | 38 | 9 | Missing data |
| 3 | F | 44 | 11 | 10 |
| 4 | F | 36 | 7 | 5 |
| 5 | M | 46 | 7 | 7 |
| 6 | F | 32 | 12 | 7 |
| 7 | F | 55 | 5 | 8 |
| 8 | M | 46 | 6 | 7 |
| 9 | F | 72 | 7 | 6 |
| 10 | M | 68 | 6 | 9 |
| 11 | M | 61 | 7 | 8 |
| 12 | F | 62 | 8 | 9 |
CAI, clinical activity index; F, female; M, male; UCDAI, ulcerative colitis disease activity index.
Figure 1.
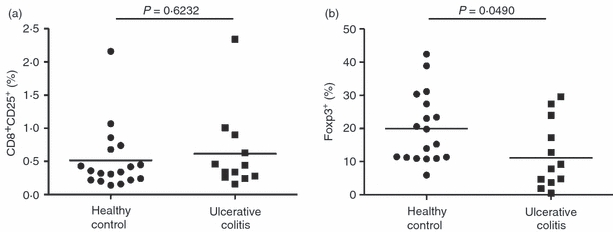
The percentage of CD8+ CD25+ Foxp3+ T cells is lower in the peripheral blood of patients with ulcerative colitis than in that of healthy blood donors. Peripheral blood mononuclear cells from healthy donors (n = 18) and from patients with ulcerative colitis (n = 12) were analysed by flow cytometry for the expression of CD8, CD25, and intracellular Foxp3. (a) Percentage of CD8+ CD25+ T cells among CD8+ T cells. Means are shown. (b) Percentage of Foxp3+ T cells among CD8+ CD25+ T cells. Means are shown. Student's t-test was used to assess the statistical significance of differences in these percentages.
In vitro induction of human CD8+ Foxp3+ suppressor T cells
Restoring the number of CD8+ regulatory T cells could be one possible mechanism for the treatment of UC. Therefore, an effective protocol for the in vitro induction of human CD8+ regulatory T cells is required. In vitro stimulation of antigen-specific CD8+ T cells in the presence of TGF-β and RA induced a robust population of CD8+ Foxp3+ regulatory T cells.17,18 To induce human CD8+ Foxp3+ T cells, we isolated naive CD8+ T cells from peripheral blood, labelled them with CFSE, and stimulated them in the presence or absence of TGF-β, RA or the combination of TGF-β and RA. As shown in Fig. 2(a) the stimulation of human CD8+ T cells with α-CD3/α-CD28 or α-CD3/α-CD28 in combination with RA induced only a slight increase in the expression of Foxp3 (3%; 7%). In contrast, stimulation in the presence of TGF-β induced a strong conversion into CD8+ Foxp3+ T cells (34%), and this conversion was further increased by the addition of RA (53%). Furthermore, these CD8+ Foxp3+ T cells showed a strong up-regulation of CD25 and CTLA-4, marker molecules characteristic for naturally occurring CD8+ regulatory T cells (Fig. 2b). To analyse the suppressive potential of induced human CD8+ Foxp3+ T cells, we sorted CD8+ CD25high T cells after stimulation in the presence of TGF-β/RA and co-cultured them with naive CFSE-labelled human CD4+ responder T cells. At day 6 after stimulation, proliferation of responder cells was measured by the loss of CFSE dye. As shown in Fig. 2(c), TGF-β/RA-treated CD8+ CD25high T cells markedly suppressed the proliferation of CD4+ responder T cells, which demonstrated the regulatory activity of human CD8+ Foxp3+ T cells in vitro.
Figure 2.
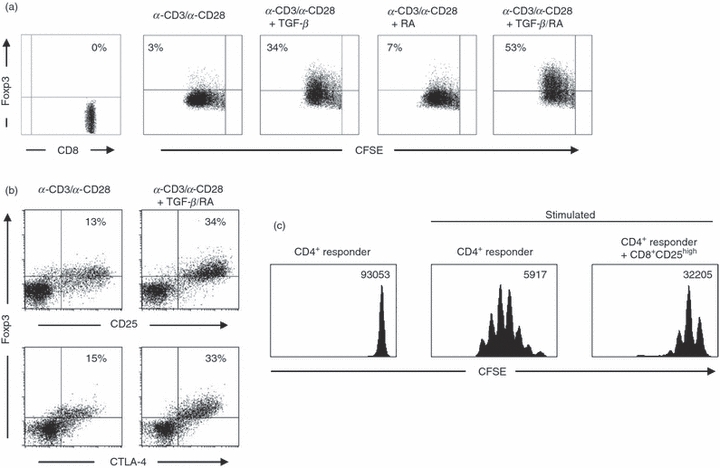
Human transforming growth factor-β (TGF-β)/retinoic acid (RA) -induced CD8+ Foxp3+ T cells express markers of regulatory T cells and exhibit suppressive activity. (a) CFSE-labelled CD8+ T cells from human peripheral blood were stimulated with soluble α-CD3 and α-CD28 for 4 days or left unstimulated. When indicated, cells were supplemented with 2 ng/ml human recombinant TGF-β or 100 nm RA. Cells were stained for the expression of CD8 and Foxp3. The use of a gating technique on proliferating CD8+ T cells demonstrates the expression of Foxp3 as compared with CFSE fluorescence intensity. The figure shows the results of one experiment representative of three independent experiments. (b) In human CD8+ T cells treated with TGF-β/RA or left untreated, Foxp3 was co-stained with either CD25 or CTLA-4. The figure shows results that are representative of those obtained from three independent experiments. (c) For in vitro T-cell suppression assays, FACS-sorted TGF-β/RA-treated CD8+ CD25high T cells were co-cultured with freshly isolated CFSE-labelled CD4+ responder T cells and polyclonal-stimulated with the Treg Suppression Inspector (Miltenyi Biotec). Histograms show the proliferation of responder T cells as determined by loss of CFSE dye. Geometric means of CFSE fluorescence intensity are shown. The figure shows results from one of three independent experiments.
Molecular and functional phenotype of TGF-β/RA-induced CD8+ Foxp3+ T cells
A prerequisite for the use of regulatory T cells in a therapeutic setting is the detailed molecular and functional characterization of these cells. To gain further insight into the biology of these CD8+ Foxp3+ T cells and to overcome the technical limitations of human cells (e.g. the lack of regulatory T-cell-specific surface molecules that can distinguish Foxp3− cells from Foxp3+ T cells), we used Foxp3/GFP transgenic reporter mice, in which GFP expression accurately identifies the Foxp3+ T-cell population. Polyclonal CD8+ Foxp3−/GFP− T cells from Foxp3/GFP mice were stimulated with α-CD3 alone or a mixture of α-CD3, TGF-β and RA. Again, only the combination of T-cell receptor stimulus plus TGF-β/RA induced a substantial conversion of CD8+ Foxp3−/GFP− cells into CD8+ Foxp3+/GFP+ T cells (Fig. 3). To define the molecular phenotype of the in vitro-induced CD8+ Foxp3+ T cells, we analysed the characteristics of these cells by using Agilent gene expression chips. CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells were FACS-sorted (Fig. 4a), and gene expression analyses were performed. A heat map generated from DNA microarray data showed that CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells cultured under the same polarizing conditions clearly exhibit distinct and specific expression profiles (Fig. 4b). To analyse whether TGF-β/RA-induced CD8+ Foxp3+ T cells share common molecular features with naturally occurring CD8+ and CD4+ regulatory T cells, we evaluated gene expression data for marker molecules specific to regulatory T cells. Interestingly, CD8+ Foxp3+/GFP+ T cells expressed a variety of genes that are known to be specific for regulatory T cells, e.g. Gpr83, CD25 and CTLA-419,20 (Fig. 4c) suggesting a regulatory phenotype of the CD8+ Foxp3+ T cells. When naive T cells are activated under the influence of RA, they acquire a gut-homing phenotype with high expression levels of CD103, α4β7 and CCR9.21 Evaluating the expression of these homing molecules on TGF-β/RA-treated CD8+ T cells revealed strong expression of CD103 and CCR9 but no difference in the expression level between CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells (Fig. 4d) demonstrating that the differential expression of Foxp3 is independent of the expression of homing molecules. To validate array-based mRNA expression levels, we confirmed the regulatory phenotype by FACS-staining. Figure 5(a) shows the specific up-regulation of CD25 and CTLA-4 molecules in CD8+ Foxp3+/GFP+ T cells. We next proceeded to characterize the proliferative properties of CD8+ Foxp3+ T cells. After re-stimulation, CD8+ Foxp3+/GFP+ T cells exhibited proliferative capability (Fig. 5b) but secreted less IFN-γ and tumour necrosis factor-αin vitro than did CD8+ Foxp3−/GFP− cells, but neither cell type expressed interleukin-10 at detectable levels (Fig. 5c).
Figure 3.

Murine transforming growth factor-β (TGF-β)/retinoic acid (RA) -induced CD8+ Foxp3+/GFP+ T cells. CD8+ CD25− splenic T cells from Foxp3/green fluorescent protein (GFP) mice were co-cultured with splenic dendritic cells from BALB/c mice and soluble α-CD3 in the presence or absence of 2 ng/ml human recombinant TGF-β and 100 nm RA for 4 days. Cells were stained for the expression of CD8 and Foxp3/GFP. The figure shows data representative of three independent experiments.
Figure 4.
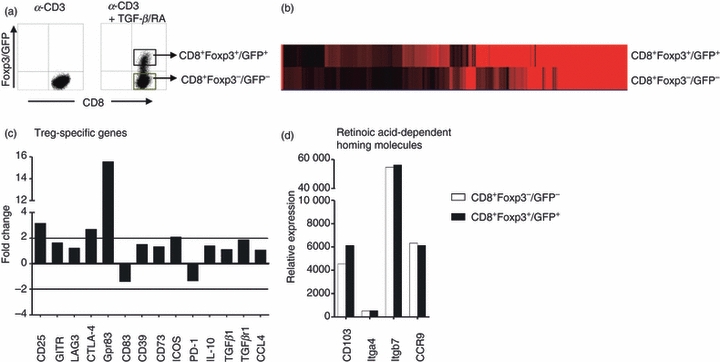
Molecular phenotype of transforming growth factor-β (TGF-β)/retinoic acid (RA) -induced CD8+ Foxp3+/GFP+ T cells. CD8+ CD25− splenic T cells from Foxp3/GFP mice were cultured as described in Fig. 3. (a) α-CD3/TGF-β/RA-treated T cells were separated into CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells by FACS on the expression of CD8 and GFP. Agilent gene expression analysis was performed. (b) Heat map reflects differential gene expression levels: black represents low expression; red represents strong expression. (c,d) The fold change of CD4+ regulatory T-cell-related genes (c) and the relative expression of gut-homing molecules (d) in CD8+ Foxp3+/GFP+ versus CD8+ Foxp3−/GFP− T cells are demonstrated as diagrams.
Figure 5.
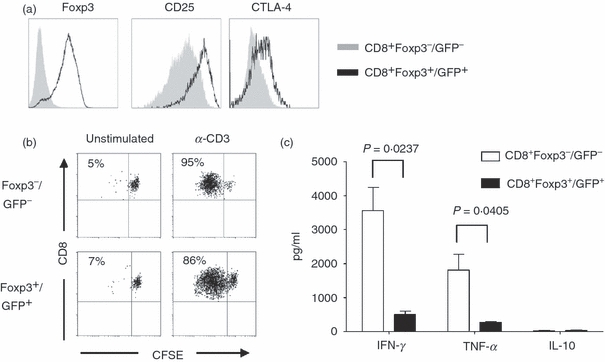
Proliferation and cytokine profile of transforming growth factor-β (TGF-β)/retinoic acid (RA) -induced CD8+ Foxp3+/GFP+ T cells. CD8+ CD25− splenic T cells from Foxp3/GFP mice were cultured as described in Fig. 3. (a) Differentiated CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells were stained for the expression of Foxp3, CD25 and CTLA-4. The figure shows data representative of three independent experiments. (b) FACS-sorted CD8+ Foxp3−/GFP− and CD8+ Foxp3+ /GFP+ T cells were labelled with CFSE and re-stimulated with soluble α-CD3 for 3 days. Proliferation was measured by loss of CFSE dye. The percentage of proliferated cells is indicated. (c) FACS-sorted CD8+ Foxp3−/GFP− and CD8+ Foxp3+ /GFP+ T cells were re-stimulated for 20 hr with PMA/ionomycin. Cell culture supernatants were analysed for several cytokines. Quantities of interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) are depicted as pg/ml. The figure shows data representative of two independent experiments.
TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells exhibit suppressor function in vitro
To study the potential of TGF-β/RA-induced CD8+ Foxp3+ T cells with regard to their immunosuppressive capability in vitro, we sorted TGF-β/RA-treated CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells and co-cultured them with naive CFSE-labelled polyclonal CD4+ CD25− responder T cells in the presence of DCs and α-CD3 stimulation. Like human CD8+ Foxp3+ T cells induced by TGF-β/RA, murine CD8+ Foxp3+/GFP+ T cells were able to suppress CD4+ T-cell proliferation in vitro (Fig. 6a). To assess the effect of TGF-β/RA-induced CD8+ Foxp3+ T cells on the effector function of CD4+ responder T cells we analysed the expression of the pro-inflammatory cytokine IFN-γ in CD4+ responder T cells (Fig. 6b). Whereas the percentage of IFN-γ-producing CD4+ responder T cells was significantly increased when co-cultured with CD8+ Foxp3−/GFP− T cells, co-culture with TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells slightly reduced the production of IFN-γ in CD4+ responder T cells. This finding suggests some suppressive function of TGF-β/RA-induced CD8+ Foxp3+ regulatory T cells in vitro.
Figure 6.
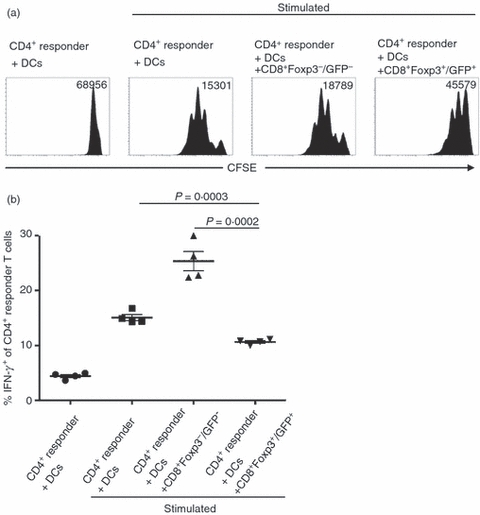
Immunosuppressive capability of transforming growth factor-β (TGF-β)/retinoic acid (RA) -induced CD8+ Foxp3+ /GFP+ T cells. CD8+ CD25− splenic T cells from Foxp3/GFP mice were cultured as described in Fig. 3. For in vitro T-cell suppression assays, FACS-sorted CD8+ Foxp3−/GFP− and CD8+ Foxp3+ /GFP+ T cells were cultured with freshly isolated CFSE-labelled CD4+ CD25− responder T cells and splenic DCs from BALB/c mice, and stimulated with soluble α-CD3 for 3 days. (a) Histograms show proliferation of responder T cells as determined by loss of CFSE dye. Geometric means of CFSE fluorescence intensity are shown. The figure shows data representative of results from five independent experiments. (b) On day 3 of in vitro T-cell suppression assays cells were re-stimulated with PMA, ionomycin and Brefeldin A for 4 hr and stained for CD4+ CD25− responder T cells and intracellular interferon-γ. The diagram shows the percentage of interferon-γ-expressing responder T cells from four independent experiments. Means are shown. Student's t-test was used to assess the statistical significance of differences in these percentages.
Cytotoxicity of TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells
Under normal inflammatory conditions CD8+ T cells exhibit cytolytic activity. Therefore, the expression of cytotoxicity-related molecules was studied. Surprisingly, granzyme B and D (GzmB and GzmD) and perforin (Prf1) were specifically up-regulated in CD8+ Foxp3+/GFP+ T cells in comparison to CD8+ Foxp3−/GFP− T cells (Fig. 7a). To validate array-based mRNA expression levels, we confirmed data by quantitative PCR. This revealed the specific up-regulation of GzmB in CD8+ Foxp3+/GFP+ T cells in comparison to Foxp3−/GFP− T cells (Fig. 7b). To further analyse whether the suppressive activity of TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells is mediated via GzmB-dependent killing of CD4+ responder T cells we studied the immunosuppressive potential of GzmB-deficient TGF-β/RA-induced CD8+ Foxp3+ T cells. For this purpose CD8+ CD25− T cells from GzmB-deficient and wild-type mice were stimulated with DCs and α-CD3 in the presence of TGF-β and RA for 4 days. The FACS-sorted CD8+ CD25high T cells from GzmB-deficient and wild-type mice expressed high levels of Foxp3 (Fig. 7c). As shown in Fig. 7(d) the inhibitory function of GzmB-deficient CD8+ CD25+ Foxp3+ T cells is comparable to the suppressive ability of wild-type CD8+ CD25+ Foxp3+ T cells, demonstrating the dispensable role of GzmB for the suppressive activity of TGF-β/RA-induced CD8+ regulatory T cells.
Figure 7.
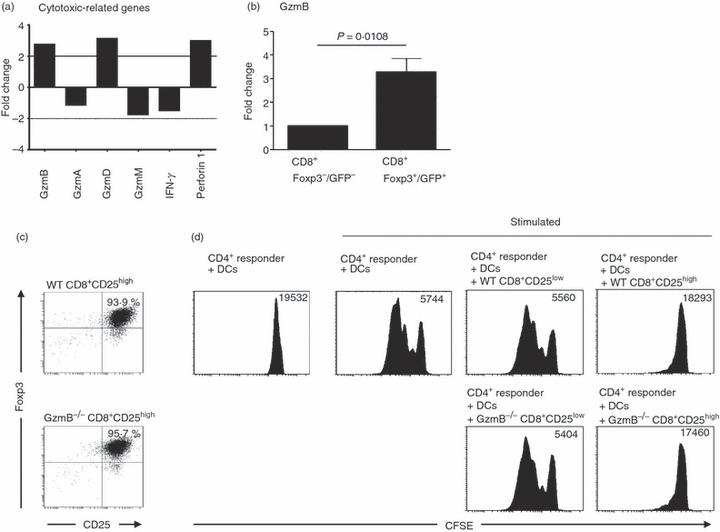
Cytotoxicity of transforming growth factor-β (TGF-β)/retinoic acid (RA) -induced CD8+ Foxp3+/GFP+ T cells. CD8+ CD25− splenic T cells from Foxp3/GFP mice were cultured as described in Fig. 3 and separated into CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells by FACS-sorting as described in Fig. 4. (a) Agilent gene expression analysis was performed. The fold changes of cytotoxic-related genes in CD8+ Foxp3+ /GFP+ versus CD8+ Foxp3−/GFP− T cells are demonstrated as diagrams. (b) Granzyme B (GzmB) expression in FACS-sorted CD8+ Foxp3−/GFP− and CD8+ Foxp3+/GFP+ T cells was assayed by quantitative PCR and was normalized relative to the expression of RPS9. The figure shows mean values from at least three independent experiments. (c) CD8+ CD25− splenic T cells from GzmB−/− and C57BL/6 mice were co-cultured with splenic dendritic cells from C57BL/6 mice and soluble α-CD3 in the presence of 2 ng/ml human recombinant TGF-β and 100 nm RA for 4 days. For in vitro T-cell suppression assays, CD8+ CD25high T cells were FACS-sorted on CD8 and CD25 expression and stained for expression of intracellular Foxp3. (d) FACS-sorted TGF-β/RA-treated CD8+ CD25high and CD8+ CD25low T cells were co-cultured with freshly isolated CFSE-labelled CD4+ CD25− responder T cells and splenic dendritic cells from C57BL/6 mice, and stimulated with soluble α-CD3. Histograms show the proliferation of responder T cells as determined by loss of CFSE dye. Geometric means of CFSE fluorescence intensity are shown. The figure shows results from one of two independent experiments.
Immunosuppressive capability of TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells is dependent on DCs
To assess whether the inhibitory function of TGF-β/RA-induced CD8+ regulatory T cells is cell–cell contact-dependent or is mediated by soluble factors, we performed inhibition assays with a transwell system, in which responder cells and regulatory T cells are separated by a semi-permeable membrane. As depicted in Fig. 8(a), cell–cell contact between CD4+ responder T cells and TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells is mandatory for the regulatory function of these cells. As a result of the cell–cell contact-dependency of TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells and the fact that modulation of antigen-presenting cells (APC) is one of several postulated mechanism of CD4+ regulatory T-cell-mediated suppression we further investigated the role of DCs in a T-cell suppression assay. Therefore, we performed inhibition assays with and without the presence of DCs. Interestingly, the suppressive activity of TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells is only detectable in the presence of DCs (Fig. 8b). This finding suggests that TGF-β/RA-induced CD8+ Foxp3+/GFP+ T cells exert their suppressive function by modulating the stimulatory function of DCs.
Figure 8.
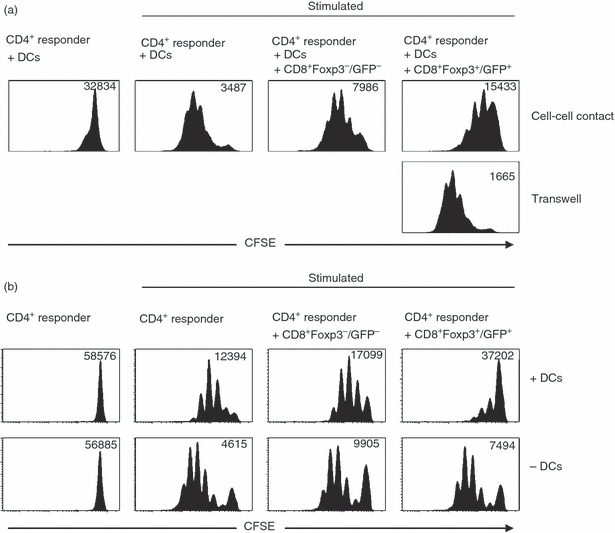
Immunosuppressive capability of transforming growth factor-β (TGF-β)/retinoic acid (RA) -induced CD8+ Foxp3+/GFP+ T cells is dependent on dendritic cells. In vitro T-cell suppression assays were performed as described in Fig. 6. Histograms show proliferation of responder T cells as determined by loss of CFSE dye. Geometric means of CFSE fluorescence intensity are shown. (a) CFSE-labelled CD4+ CD25− responder T cells were separated from CD8+ Foxp3+ /GFP+ T cells by using a transwell system. Data shown are representative of those from four independent experiments. (b) In vitro T-cell suppression assays were performed in the presence of dendritic cells and stimulated with soluble α-CD3 or in the absence of dendritic cells and stimulated with plate-bound α-CD3 and soluble α-CD28. The figure shows data representative of results from three independent experiments.
Discussion
The intestinal immune system is constantly challenged by foreign antigens and commensal bacteria. Therefore, proper control of the intestinal microenvironment is required. One important arm of this regulatory network consists of regulatory T cells. Many researchers have undertaken association studies among patients with IBD to determine whether changes in regulatory T cells can be correlated with disease severity, with a particular focus on defining the differences between circulating cells and cells from gut tissue. Most of these studies have analysed CD4+ CD25+ Foxp3+ regulatory T cells, and much less is known about the role of CD8+ regulatory T cells in IBD. Mayer and colleagues suggested that a defect of CD8+ regulatory T cells in the LP may lead to the development of IBD.14,15 These researchers demonstrated that CD8+ T cells isolated from non-inflamed mucosa display suppressive capabilities; in contrast, LP CD8+ T cells derived from patients with IBD could not suppress immune responses. They conclude that CD8+ T cells with regulatory activity are present in the LP of normal healthy persons but not in the LP of patients with IBD. In the present study we demonstrated that the peripheral blood of patients with UC contains fewer CD8+ CD25+ Foxp3+ T cells when the disease is active. These findings are in line with those of earlier studies, which demonstrated that the peripheral blood of patients with Crohn's disease and UC contains fewer CD4+ regulatory T cells during disease flares, and suggest that the severity of disease is inversely correlated with the number of regulatory T cells in the peripheral blood.22–24 Despite our limited understanding of the role of regulatory T cells in the pathogenesis of human IBD, the ability to alter regulatory pathways may be a crucial avenue for achieving long-term remission. Results from animal models suggest that the transfer of regulatory T cells may be beneficial.25 However, given that patients with IBD exhibit an increased number of regulatory T cells in the LP and a decreased number in the peripheral blood, large numbers of regulatory T cells may need to be transferred if we are to alter the balance between a regulatory and a pro-inflammatory response. Published protocols for expanding CD4+ regulatory T cells ex vivo rely on repetitive stimulation via the TCR in combination with cytokine exposure.26–28 Within the CD8+ regulatory T-cell subset, adaptive CD8+ regulatory T cells are by far the most dominant group. These cells can be induced by stimulation through the T-cell receptor under certain conditions resulting in a variety of different phenotypes. Recently, it was demonstrated that CD8+ CD25+ Foxp3+ regulatory T cells can be generated by the treatment with anti-CD3 antibody.29,30 In addition, another population of human CD8+ CD25+ Foxp3+ regulatory T cells has been described by Siegmund et al.31 Here, TGF-β and CD3/CD28 antibodies were required to expand these cells. For the CD4+ T-cell subset it was shown that TGF-β-induced conversion of CD4+ T cells into the Foxp3+ phenotype by gut-associated DCs is augmented by the key metabolite of Vitamin A, RA, in vitro.32,33 Ideally, if unwanted uncontrolled immunosuppression is to be avoided, regulatory T cells should be manipulated to express homing molecules that direct them to the tissue of interest. Most interesting in this context is the observation that the RA is synthesized in abundance by gut and gut-associated DCs21,32,33 and induces the specific gut-homing molecules CCR9 and α4β7 integrin on T cells.21 Therefore RA seems to play a predominant role in the homeostasis and homing of lymphoid populations of the gut-associated lymphoid tissue (GALT). The important role of RA in controlling Foxp3 expression in combination with TGF-β suggests that the GALT has evolved a specific system for maintaining a balanced symbiosis between the gut flora and the immune system.18,32–34 Intriguingly, in the current study we could demonstrate that the potential of TGF-β and RA to convert naive CD4+ T cells into Foxp3+ T cells is also true for both murine and human CD8+ T cells. Our work has shown that treating naive CD8+ T cells with TGF-β and RA induces murine and human CD8+ Foxp3+ T cells with suppressive activity. Although these CD8+ Foxp3+ T cells possess proliferative capability they exhibit a phenotype that is strikingly similar to that of naturally occurring CD4+ Foxp3+ regulatory T cells and TGF-β/RA-induced CD4+ regulatory T cells. Most notably, they specifically express higher levels of CD25, Gpr83 and CTLA-4 than do CD8+ Foxp3− T cells activated in vitro.
In vitro and in vivo experimental systems investigating polyclonal populations of CD8+ regulatory T cells have assumed the existence of separate subsets of CD8+ regulatory T cells on the basis of several apparently distinct mechanisms of immune regulation. Therefore it is of major interest to understand the molecular pathway through which inhibitory CD8+ T cells modulate other T-cell subsets, in particular CD4+ T cells. The increased expression of the cytolytic enzymes GzmB, GzmD and Prf1 in TGF-β/RA-induced CD8+ Foxp3+/GFP+ regulatory T cells raises the possibility that these cells may mediate suppression by killing increased numbers of responder cells or APCs. However, CD8+ Foxp3+ T cells differentiated from GzmB-deficient mice exhibited the same inhibitory capacity as CD8+ Foxp3+ T cells differentiated from wild-type mice. Additional important mechanisms for CD8+ regulatory T cell-mediated immunoregulation include the secretion of soluble factors, such as immunosuppressive cytokines, and negative signalling directly on the target cell or on APCs. CD8+ CD122+ regulatory T cells produce interleukin-10 to suppress the production of IFN-γ and the proliferation of CD8+ responder cells.35 However, immunosuppression by soluble factors is unlikely for TGF-β/RA-induced CD8+ Foxp3+/GFP+ regulatory T cells because these cells were not suppressive when separated from responders by a transwell system. In contrast, the modulation of APCs seems to be an important mechanism of TGF-β/RA-induced CD8+ Foxp3+/GFP+ regulatory T cells as the presence of APCs within the inhibition assay is mandatory for the suppressive activity of CD8+ Foxp3+/GFP+ regulatory T cells.
In conclusion, we have detected a lower number of CD8+ Foxp3+ T cells in the peripheral blood of patients with UC than in healthy persons. Therefore, the in vitro generation of CD8+ Foxp3+ regulatory T cells may provide a new strategy to modulate T-cell responses. We established a protocol for the in vitro induction of adaptive CD8+ Foxp3+ regulatory T cells that can be induced from murine and human CD8+ CD25− T cells by TCR stimulation in the presence of TGF-β and RA with the potential to suppress CD4+ T-cell proliferation in vitro in a cell–cell contact-dependent manner. Our study illustrates a previously unappreciated critical role of CD8+ Foxp3+ T cells in controlling potentially dangerous T cells in the gut and the induction of these cells in vitro may be a future perspective for the therapy of inflammatory bowel disease.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to A.M. Westendorf (WE 4472/1-1). We are grateful to Mechthild Hemmler-Roloff and Witold Bartosik for excellent technical assistance.
Disclosures
No conflicts of interest exist.
References
- 1.van Driel IR, Ang DK. Role of regulatory T cells in gastrointestinal inflammatory disease. J Gastroenterol Hepatol. 2008;23:171–7. doi: 10.1111/j.1440-1746.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- 2.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 3.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–9. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 5.Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947–54. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- 6.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 7.Joosten SA, van Meijgaarden KE, Savage ND, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA. 2007;104:8029–34. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reibke R, Garbi N, Ganss R, Hammerling GJ, Arnold B, Oelert T. CD8+ regulatory T cells generated by neonatal recognition of peripheral self-antigen. Proc Natl Acad Sci USA. 2006;103:15142–7. doi: 10.1073/pnas.0602622103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh RP, La CA, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007;178:7649–57. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–9. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van LierRA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177:2775–83. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 12.Ménager-Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. CD8+CD28− regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775–85. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573–80. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–26. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 15.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–22. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 16.Westendorf AM, Fleissner D, Deppenmeier S, Gruber AD, Bruder D, Hansen W, Liblau R, Buer J. Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8+ T cells. Gastroenterology. 2006;131:510–24. doi: 10.1053/j.gastro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Fleissner D, Hansen W, Geffers R, Buer J, Westendorf AM. Local induction of immunosuppressive CD8+ T cells in the gut-associated lymphoid tissues. PLoS ONE. 2010;5:e15373. doi: 10.1371/journal.pone.0015373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 19.Hansen W, Loser K, Westendorf AM, et al. G protein-coupled receptor 83 overexpression in naive CD4+CD25– T cells leads to the induction of Foxp3+ regulatory T cells in vivo. J Immunol. 2006;177:209–15. doi: 10.4049/jimmunol.177.1.209. [DOI] [PubMed] [Google Scholar]
- 20.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–48. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 21.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Makita S, Kanai T, Oshima S, et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–30. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 23.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25high T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 24.Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clin Immunol. 2007;125:281–90. doi: 10.1016/j.clim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–94. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 26.Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, Bluestone JA. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 28.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ablamunits V, Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8+ T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol. 2010;40:2891–901. doi: 10.1002/eji.201040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Notley CA, McCann FE, Inglis JJ, Williams RO. ANTI-CD3 therapy expands the numbers of CD4+ and CD8+ Treg cells and induces sustained amelioration of collagen-induced arthritis. Arthritis Rheum. 2010;62:171–8. doi: 10.1002/art.25058. [DOI] [PubMed] [Google Scholar]
- 31.Siegmund K, Ruckert B, Ouaked N, Burgler S, Speiser A, Akdis CA, Schmidt-Weber CB. Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells. J Immunol. 2009;182:2124–30. doi: 10.4049/jimmunol.0802271. [DOI] [PubMed] [Google Scholar]
- 32.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von BH. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737–9. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rifa'I M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–47. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]


