Abstract
Interleukin-10 (IL-10) is a potent suppressor of the immune system, commonly produced by CD4+ T cells to limit ongoing inflammatory responses minimizing host damage. Many autoimmune diseases are marked by large populations of activated CD4+ T cells within the setting of chronic inflammation; therefore, drugs capable of inducing IL-10 production in CD4+ T cells would be of great therapeutic value. Previous reports have shown that the small molecule G-1, an agonist of the membrane-bound G-protein-coupled estrogen receptor GPER, attenuates disease in an animal model of autoimmune encephalomyelitis. However, the direct effects of G-1 on CD4+ T-cell populations remain unknown. Using ex vivo cultures of purified CD4+ T cells, we show that G-1 elicits IL-10 expression in T helper type 17 (Th17) -polarized cells, increasing the number of IL-10+ and IL-10+ IL-17A+ cells via de novo induction of IL-10. T-cell cultures differentiated in the presence of G-1 secreted threefold more IL-10, with no change in IL-17A, tumour necrosis factor-α, or interferon-γ. Moreover, inhibition of extracellular signal-regulated kinase (but not p38 or Jun N-terminal kinase) signalling blocked the response, while analysis of Foxp3 and RORγt expression demonstrated increased numbers of IL-10+ cells in both the Th17 (RORγt+) and Foxp3+ RORγt+ hybrid T-cell compartments. Our findings translated in vivo as systemic treatment of male mice with G-1 led to increased IL-10 secretion from splenocytes following T-cell receptor cross-linking. These results demonstrate that G-1 acts directly on CD4+ T cells, and to our knowledge provide the first example of a synthetic small molecule capable of eliciting IL-10 expression in Th17 or hybrid T-cell populations.
Keywords: estrogen receptor, G-protein-coupled estrogen receptor 1, GPR30, hybrid T cell, interleukin-10, T helper type 17, regulatory T cells
Introduction
CD4+ helper T lymphocytes orchestrate adaptive immune responses to invading pathogens, and are critical to the pathogenesis of numerous disease processes, including autoimmunity and cancer. They are an attractive drug target because of their central role in immunity, and their implication in a wide variety of diseases. There are several distinct lineages of CD4+ helper T cells, each specialized in enhancing specific branches of the immune system. The original paradigm described by Mossman and Coffmann1 divided CD4+ helper T lymphocytes into the T helper type 1 (Th1) and Th2 populations, with Th1 cells producing interferon-γ (IFN-γ) and coordinating cellular immunity responses and Th2 cells secreting humoral immunity mediators such as interleukin-4 (IL-4), IL-5 and IL-13. In 2005, the Th1–Th2 paradigm was expanded as the Th17 population emerged as a third class of helper/effector T cell. Th17 cells are characterized by expression of the transcription factor RORγt,2,3 and secrete pro-inflammatory cytokines including IL-214 and IL-17A/F. These cells are important to controlling infections by extracellular pathogens, but also appear to play a deleterious role in human health by contributing to the pathogenesis of numerous autoimmune diseases.5 In mice, Th17 differentiation depends on transforming growth factor-β (TGF-β) and IL-6- or IL-21-mediated signal transducer and activator of transcription 3 (STAT3) activation,5 while IL-23 signalling plays a critical role in stabilizing the Th17 phenotype.6 Although Th1, Th2 and Th17 effector T cells coordinate a robust and diverse arsenal of adaptive immune responses necessary for the maintenance of human health, mechanisms of restraint must limit effector responses to protect the host from immune-mediated damage.
A major breakthrough in elucidating the mechanisms of adaptive immune regulation emerged with the identification of an array of regulatory T (Treg) -cell populations. The best defined class of Treg cells expresses the forkhead transcription factor Foxp3 and suppresses numerous animal models of autoimmune disease,7 whereas loss of Foxp3 function in humans and mice precipitates a fatal multi-organ autoimmune condition marked by the inability to control T-cell responses.8,9 The T reg cells function to dampen immune responses through a variety of approaches, including contact-mediated inhibition, secretion of perforin and granzyme A/B, sequestration of key growth factors such as IL-2, and secretion of suppressive cytokines including TGF-β, IL-10 and IL-35.7 Interleukin-10 in particular plays an important role in immune homeostasis, both in mice10 and humans,11 suggesting that it has several non-redundant roles in regulating inflammatory responses. Many cell types in addition to Foxp3+ cells12 can produce IL-10, most notably several lineages of CD4+ T cells,13 including Th1,14–16 Th214,17 and Th1718–20 cells, as well as various types of Treg cells.21 In a feed-forward mechanism, IL-10 can drive its own expression through the induction of an IL-10-producing Treg-cell population termed Tr1 cells.22,23 Conversely, IL-10 can also be induced independently of IL-10 signalling in both Foxp3+ and Foxp3− Treg-cell populations.24 Given its potent anti-inflammatory effects, various strategies are being explored to target IL-10 for therapeutic intervention.25
The intimate interplay between the critical factors in development of Treg and Th17 cells, along with the dual reliance on TGF-β signalling for their differentiation,26 has led to conceptualization of a Treg–Th17 axis. From a therapeutics perspective, the identification of drugs that promote pro-inflammatory or anti-inflammatory responses by influencing differentiation along this axis has gained momentum as examples of T-cell plasticity continue to be characterized,27 in particular within the Treg-cell and Th17-cell populations.28 Moreover, several reports have characterized ‘hybrid’ T-cell populations where Foxp3 is expressed in various effector T-cell populations,29 and IL-10 can be produced by Th1, Th2 and Th17 cells.12 These results suggest that it may be possible to treat disease by shifting the balance along the Treg–Th17 axis in situ during ongoing immune responses. For example, one mechanism to dampen inflammation would be to induce IL-10 expression within Th17 cells participating in pathological inflammation. To that end, targeting non-cytokine signalling pathways may be a viable option. For example, ATP,30 sphinogosine-1-phosphate31 and vitamin D32 can modulate Th17 development, whereas antigen-presenting cell (APC)-derived indolamine 2,3-dioxygenase33 and retinoic acid34 can promote Treg-cell populations, highlighting the importance of non-cytokine signalling pathways to this paradigm.
Estrogen is a well-documented modulator of immune function in humans and mice, capable of increasing the expression of Foxp335 and IL-10.36 These effects translate to human disease wherein patients with multiple sclerosis experience a decrease in symptoms during pregnancy,37 and to murine models of autoimmune disease where estrogen inhibits development of and reverses experimental autoimmune encephalomyelitis (EAE),38 an animal model of multiple sclerosis. Although the effects of estrogen are presumed to be mediated by the classical estrogen receptors, ERα and ERβ, recent studies have pointed to the newly described G protein-coupled estrogen receptor GPR30/GPER as contributing to many of these responses. We and others have recently shown that, like estradiol (E2), the GPER-selective agonist G-1 can attenuate EAE.38,39 In the current work we show that G-1 can evoke IL-10 expression and secretion from CD4+ T cells differentiated under Th17-polarizing conditions. G-1-mediated IL-10 expression was blocked by the GPER-directed antagonist G15,40 and was dependent on extracellular signal-regulated kinase (ERK) signalling, consistent with known mechanisms of IL-10 production within effector T-cell populations.12 Analysis of IL-17A, Foxp3 and RORγt expression demonstrated that these responses occurred in cells expressing both IL-17A and RORγt, as well as in a population of Foxp3+ RORγt+ hybrid T cells. Taken together, our results demonstrate a novel immunomodulatory property for G-1. In addition, these data suggest that the family of GPER-directed small molecules may serve as model compounds for a new class of T-cell-targeted pharmaceuticals in the treatment of autoimmune disease and cancer.
Materials and methods
Mice
Male (7–11 weeks old) C57BL/6 and Foxp3egfp mice were used for this study. Mice were purchased from Jackson Laboratory (Bar Harbor, ME), and subsequently housed, bred and cared for according to the institutional guidelines in the Animal Resource Facility at the University of New Mexico. Foxp3-IRES-GFP (Foxp3egfp) transgenic mice, which contain egfp under the control of an internal ribosomal entry site (IRES) inserted downstream of the foxp3 coding region, have been previously described.41
Purification of T-cell populations
T cells were obtained from single cell suspensions following homogenization of spleens and lymph nodes by mechanical disruption and passage through a 70-μm nylon filter. Suspensions were stained with anti-CD4, anti-CD62 ligand (CD62L) and anti-CD44 antibodies (Biolegend, San Diego, CA). Enriched populations of CD4+ CD62Lhi and CD4+ CD44lo CD62Lhi naive T cells were collected by flow cytometric cell sorting on a MoFlo cell sorter (Cytomation, Carpinteria, CA). Purity was regularly > 96%. In most cases, experiments were repeated with both types of sorted naive T cells, and no differences were noted. Alternatively, CD4+ cells were collected from the single cell suspensions by magnetic bead sorting, using CD4 microbeads (Miltenyi, Bergisch Gladbach, Germany) and positive selection on an AutoMACS (Miltenyi). This yielded populations with a purity > 90%.
Culture conditions
All experiments and cell purification were carried out in RPMI-1640 medium supplemented with fetal bovine serum (FBS), penicillin/streptomycin, l-glutamine, HEPES, sodium pyruvate and 2-mercaptoethanol. Phenol red-free buffers and charcoal-stripped FBS were used to minimize exposure to estrogens or phyto/xenoestrogens that could have confounded our results. Cells were stimulated in culture with soluble anti-CD3ε (1·0 μg/ml) and anti-CD28 (2·5 μg/ml) antibodies (Biolegend), and supplemented with various combinations of TGF-β (0·5–10 ng/ml), IL-6 (20 ng/ml) and IL-23 (20 ng/ml) as described (Biolegend and eBiosciences, San Diego, CA). G-1 and DMSO were added concurrently with the stimulatory antibodies and cytokines. Non-polarizing conditions (Th0) contained no exogenous cytokines. Th17 conditions contained TGF-β + IL-6 ± IL-23. Experiments were carried out using 96-well plates with 2 × 105 cells per well (106 cells/ml). For experiments using GPER and mitogen-activated protein (MAP) kinase inhibitors, cells were pre-incubated for 60–90 min with 25 μm PD98059 [MAP kinase kinase (MEK) inhibitor], 250 nm Jun N-terminal kinase (JNK) II inhibitor, 100 nm SB203580 (p38 inhibitor), or 500 nm G15 (GPER antagonist,40 provided by Dr Jeffrey Arterburn at New Mexico State University) where indicated, before the addition of stimulatory antibodies or cytokines. All compounds used in the study were dissolved in DMSO. All cultures were incubated at 37° (+ 5% CO2).
Intracellular cytokine staining and analysis
Following 4 days in culture, cells were washed with medium and ‘rested’ for 60–90 min at 37° (+ 5% CO2). Cultures were then treated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4–5 hr in the presence of Brefeldin A (Biolegend) followed by fixation in Fixation Buffer (Biolegend). Samples were then washed and stained for intracellular proteins in Permeabilization Wash buffer (Biolegend) for 2 hr at room temperature, and washed with excess Permeabilization Wash buffer for 15 min at room temperature before centrifugation and analysis. Immediately after staining, data were collected on a FACScalibur (Becton Dickinson, Franklin Lakes, NJ). Data analysis was performed using FlowJo software (TreeStar, Ashland, OR). Antibodies for staining included anti-IL-10-allophycocyanin, anti-IL-10-phycoerythrin, anti-IL-17A-phycoerythrin, and IL-17A-peridinin chlorophyll protein and anti-IFN-γ-allophycocyanin all from Biolegend, as well as anti-RORγt-phycoerythrin from eBiosciences.
Proliferation studies
For analysis of proliferation, freshly sorted T cells were stained with 2·5 μm eFluor670 according to the manufacturer's protocols (eBiosciences). Cells were then cultured, stained and analysed as indicated above. Geometric mean fluorescence intensity (GMFI) of eFluor670 was determined using FlowJo software (TreeStar), and unstimulated controls were used to differentiate between proliferating and non-proliferating cells.
Luminex multiplex assays
Following 4 days in culture, T cells were washed with cold medium to remove any cytokines in solution, resuspended in fresh medium, and counted. For cultures of splenocytes, single cells suspensions were obtained following homogenization of spleens by mechanical disruption and passage through a 70-μm nylon filter. Cells were then plated in a 96-well plate with 2 × 105 cells per well (106 cells/ml), allowed to incubate for 60–90 min at 37° (+5% CO2), and re-stimulated with soluble anti-CD3ε (2·5 μg/ml) antibody. Following the indicated stimulation time, culture medium was collected and spun down to remove any residual cells. The concentration of IL-4, IL-6, IL-10, IL-17A, IFN-γ and tumour necrosis factor-α (TNF-α) in the cell-free culture medium was analysed using custom bead arrays from Millipore, and quantified on a Luminex 100 system (Austin, TX) with the Luminex XY plate handling platform. Assays were performed according to the manufacturer's protocols. Duplicate wells were assayed for each sample, and data are representative of the average median value for each sample. Analysis was performed using is 2.3 software (Luminex).
In vivo treatment with G-1
A vehicle consisting of 90% emulsion solution (PBS + 0·9% Tween-20 + 0·9% BSA) and 10% ethanol was used. For delivery of compounds, E2 or G-1 was dissolved in ethanol and added at appropriate concentrations such that 100 μl per animal per injection was used. The compound was added to each injection as part of the 10% ethanol found in the vehicle, so it was diluted such that < 10 μl per animal per injection was required. Injections were administered in the afternoon, and to limit stress from the long series of injections inherent in this study, animals were sedated using isofluorane before injection. Compound was delivered subcutaneously on the dorsum adjacent to the hind limb, and the side of the injection was alternated every 2 days.
Results
G-1 elicits IL-10 production in CD4+ T cells under Th17 polarizing conditions
To investigate the direct effects of G-1 on CD4+ T cells, we chose to use purified cultures of naive T cells activated by polyclonal stimulation with anti-CD3ε and anti-CD28 antibody. This eliminated secondary effects caused by the activity of G-1 on APCs within the culture. Furthermore, primary cells from male mice were used throughout the study to avoid potential confounding effects of either; (i) varying estrogen levels in female mice, or (ii) the inflammatory effects of ovariectomy. We have also determined that CD4+ CD44loCD62Lhi naive T-cell and CD4+ Foxp3+ T-reg cell populations express the G-1 target GPER (R. L. Brunsing and E. R. Prossnitz, manuscript in preparation).
Given that G-1 can protect mice from EAE38,39 and the importance of the the Th17 lineage to this model,3 we began by determining the effects of G-1 on naive T-cell differentiation under Th17-polarizing conditions (TGF-β/IL-6 ± IL-23). Hence, naive T cells from 7- to 11-week-old male C57BL/6 mice were collected by FACS and stimulated for 4 days ex vivo, supplemented with combinations of TGF-β, IL-6 and IL-23. Following 4 days of stimulation, cells were analysed for expression of IFN-γ, IL-17A and IL-10 by intracellular cytokine staining. Expression of IL-10 was present exclusively in cultures treated with IL-6 (Fig. 1a), consistent with previous findings using ex vivo culture systems where treatment with TGF-β alone blocks IL-10 expression in differentiating CD4+ T cells,13 and efficient induction of IL-10 secretion from effector T-cell populations requires one of the STAT activating cytokines (for example, IL-6).19 As expected, IL-17A expression was also largely dependent on Th17-polarizing conditions (i.e. treatment with both TGF-β and IL-6; Fig. 1a), although a small number of IL-17A+ cells was observed in the TGF-β-treated cultures (data not shown), and was enhanced by the addition of IL-23. G-1 treatment resulted in an increase in the percentage of IL-10+ cells within Th 17 cell-polarized cultures (Fig. 1b), including within cultures supplemented with IL-23 (Fig. 1c), which is known to be important in stabilizing the phenotype of Th17 populations.6 This G-1-mediated IL-10 expression was specific as no increase in the prevalence IL-17A+ cells was observed in either of the Th17-polarizing conditions (Fig. 1b,c). In addition, G-1 treatment had no effect on IFN-γ expression in cultures stimulated with CD3/28 alone (Fig. 1d); however, few IFN-γ+ cells were detected in the other culture conditions tested (see Supplementary material, Fig. S1).
Figure 1.
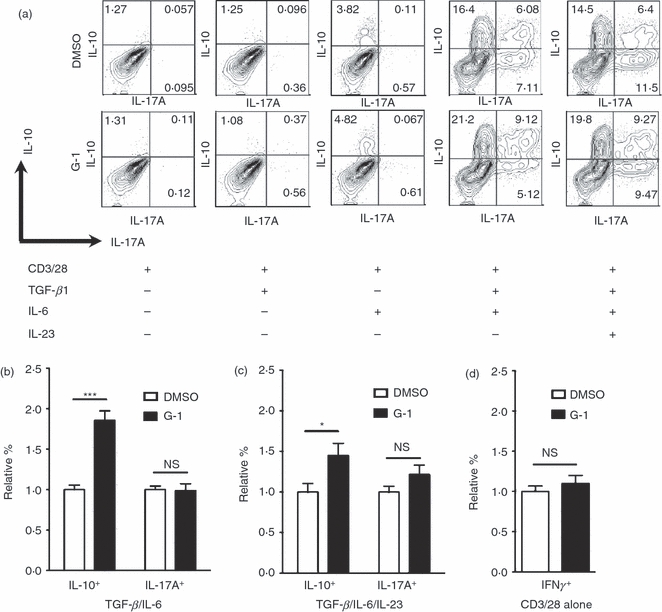
The G-protein-coupled estrogen receptor (GPER) -directed agonist G-1 induces interleukin-10 (IL-10) production from CD4+ T cells. CD4+ CD44lo CD62Lhi naive CD4+ T cells were collected by FACS and cultured for 4 days ex vivo with various combinations of transforming growth factor-β (TGF-β), IL-6 and IL-23, and supplemented with 100 nm G-1 or vehicle (DMSO, control). Cells were subsequently stained for intracellular IFN-γ, IL-17A and IL-10, then analysed by flow cytometry. (a) Representative plots from the various conditions showing intracellular IL-17A and IL-10. (b) Quantification of data from five to seven independent experiments showing relative number of total IL-10+ cells and total IL-17A+ cells in cultures treated with TGF-β + IL-6. (c) Quantification of data from four to seven independent experiments showing relative prevalence of total IL-10+ cells and total IL-17A+ cells in cultures treated with TGF-β + IL-6 + IL-23. (d) Quantification of IFN-γ+ cells in cultures stimulated with anti-CD3/28 in non-polarizing conditions (i.e. without the addition of any cytokines). P-values determined by Student's t-test; *P < 0·05; ***P < 0·0005. Error bars = SEM; NS, not significant.
To determine whether the induction of IL-10+ cells translated into a specific increase in the secretion of IL-10 from G-1-treated cultures, naive T cells were collected and stimulated as above, in the presence of TGF-β and IL-6. After 4 days of differentiation, DMSO-treated and G-1-treated cells were collected, washed with medium to remove any cytokines released over the course of differentiation, and re-plated at 106 cells/ml. Cells were then re-stimulated with anti-CD3ε antibody for 24 hr, after which culture medium was analysed for the presence of newly secreted IL-6, IL-10, IL-17A, TNF-α and IFN-γ by Luminex multiplex assay. Cells differentiated in the presence of G-1 produced approximately threefold more IL-10 than control cultures (Fig. 2a), consistent with our observation that G-1 induced an IL-10-producing population. No difference in the secretion of IL-6, IL-17A, TNF-α or IFN-γ was detected (Fig. 2b–e), again suggesting that G-1 was specifically driving the production of the anti-inflammatory cytokine IL-10, and not pro-inflammatory mediators such as TNF-α and IFN-γ. Taken together, these data show that G-1 can specifically drive IL-10 expression within, and secretion from, CD4+ T-cell populations.
Figure 2.
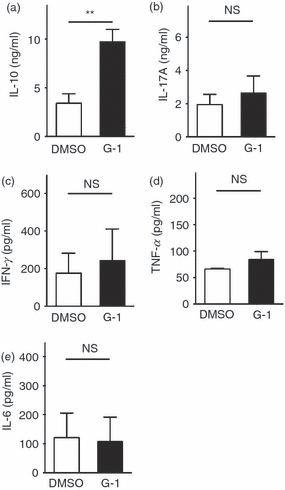
Cytokine secretion following ex vivo treatment with G-1. CD4+ CD62Lhi naive CD4+ T cells were collected by FACS and cultured for 4 days ex vivo with anti-CD3/28 + interleukin-6 (IL-6) + transforming growth factor-β (TGF-β) in the presence of 100 nm G-1 (black bars) or DMSO (white bars). Cells were washed on day 4 and re-stimulated with anti-CD3ɛ alone. Culture medium was collected after 24 hr and analysed for the presence of secreted (a) IL-10, (b) IL-17A, (c) interferon-γ (IFN-γ), (d) tumour necrosis factor-α (TNF-α), and (e) IL-6 by Luminex multiplex assay. Data are the means from three independent experiments done in triplicate. P-values determined by Student's t-test; *P < 0·02. Error bars = SEM; NS, not significant.
Induction of an IL-10+ IL-17A+ and IL-10+ IL-17A− population by G-1
As G-1-induced IL-10 expression was dependent on Th17-polarizing conditions, we sought to determine the relationship between G-1-induced IL-10+ cells and those expressing the characteristic Th17 cytokine IL-17A. Hence, naive T cells were again collected by FACS and polyclonally stimulated in the presence of TGF-β and IL-6. Cells were cultured with increasing doses of G-1 (1–500 nm) and analysed for IL-17A and IL-10 by intracellular cytokine staining (Fig. 3a). Our data reveal a dose-dependent increase in IL-10+ IL-17A− (Fig. 3a,b) and IL-10+ IL-17A+ cells (Fig. 3a,c) within G-1-treated cultures. A similar trend was observed under IL-23 polarizing conditions (Fig. 1a and data not shown). In addition, G-1-mediated IL-10 expression was blocked by the recently described GPER antagonist G15.40 The induction of a population of IL-10+ IL-17A+ cells suggests that G-1 can elicit IL-10 expression within cells that have differentiated to the Th17 lineage. Taken together, these data show that G-1 can elicit IL-10 production within the Th17 compartment, a response that is blocked by the GPER-selective antagonist G15.
Figure 3.
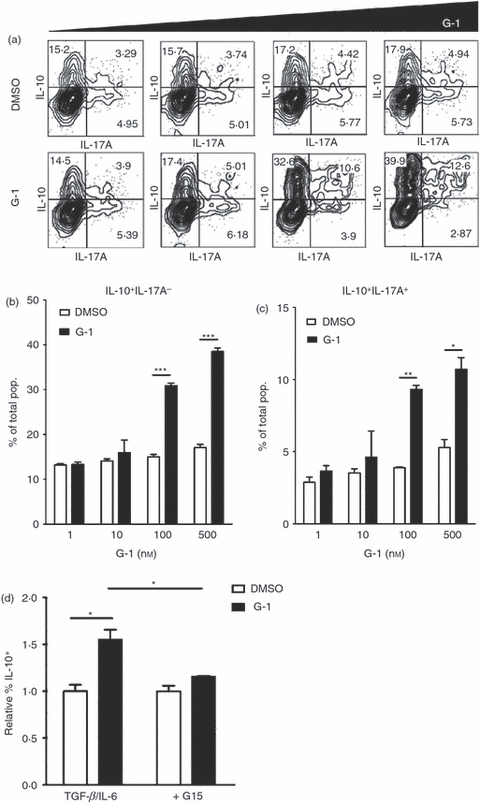
The G-protein-coupled estrogen receptor (GPER) -directed agonist G-1 induces interleukin-10 (IL-10) production in IL-17A+ cells. CD4+ CD44lo CD62Lhi or CD4+ CD62Lhi naive CD4+ T cells were collected by FACS and cultured for 4 days ex vivo with anti-CD3/28 + IL-6 + transforming growth factor-β (TGF-β). Increasing doses of G-1 (1–500 nm, black bars) or equivalent amounts of vehicle (DMSO, white bars) were added, or cultures were pre-treated with the GPER antagonist G15. Cells were subsequently stained for intracellular IL-17A and IL-10, and analysed by flow cytometry. (a) Representative plots from the various conditions showing intracellular IL-17A and IL-10. (b–c) Quantification of data from one of two independent experiments showing the percentage of cells that are (b) IL-10+ IL-17A− and (c) IL-10+ IL-17A+ for the given conditions. (d) Summary of data from two independent experiments showing that the GPER-directed antagonist G15 (500 nm) can block G-1 (100 nm)-mediated IL-10 induction. P-values determined by Student's t-test; *P < 0·01; **P < 0·001; ***P < 0·0001. Error bars = SD (b,c) or SEM (d).
ERK signalling is critical for G-1-mediated IL-10 expression
Interleukin-10 production within Th populations has been shown to be dependent on signalling through extracellular signal-regulated kinases ERK1/2,12,13 one of three MAP kinase cascades, the others comprising JNK1/2 and p38. GPER has been shown to activate the ERK pathway, although predominantly in cancer cells.42 To test whether G-1-mediated induction of IL-10 was dependent on MAP kinase signalling, naive T cells were treated with either PD98059, an inhibitor of the ERK pathway, SB203580, an inhibitor of the p38 pathway, or the JNK II inhibitor, and stimulated under Th17-polarizing conditions as before. Consistent with other published reports,13 we found that inhibition of p38 had no effect on IL-10 expression in Th17-polarized cells. Similarly, JNK signalling appeared not to be required for G-1-mediated induction of IL-10 (Fig. 4a). In contrast, there was no difference in the percentage of IL-10+ cells observed between control and G-1-treated cultures when cells were cultured with the ERK inhibitor PD98059 (Fig. 4a,b), consistent with a role for ERK signalling specifically in G-1-mediated IL-10 induction. These data suggest that G-1 mediates IL-10 expression by activating ERK signalling in CD4+ T cells.
Figure 4.
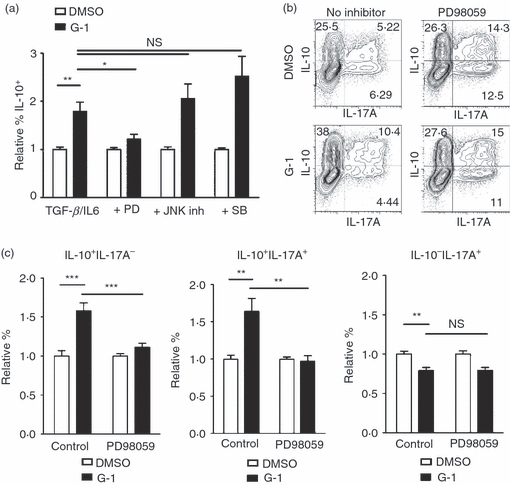
G-1-induced interleukin-10 (IL-10) production is extracellular signal regulated kinase (ERK) dependent. CD4+ CD44lo CD62Lhi naive CD4+ T cells were collected by FACS and cultured for 4 days ex vivo with anti-CD3/28 + IL-6 + transforming growth factor-β (TGF-β) in the presence of 100 nm G-1 (black bars) or DMSO (white bars). Cultures were supplemented with inhibitors of the ERK (PD98059), Jun N-terminal kinase (JNK II inhibitor), or p38 (SB203580) signalling cascades. Following culture, cells were collected and stained for intracellular IL-10 and IL-17A, and analysed by flow cytometry. (a) Graphs represent summary of data from three independent experiments. (b) Representative plots from one of four independent experiments with PD98059. (c) Summary of data from four PD98059 experiments analysing induction within populations expressing combinations of IL-10 and IL-17A. P-values determined by Student's t-test; *P < 0·05; **P < 0·005; ***P < 0·0005. Error bars = SEM; NS, not significant.
The ERK pathway is known to be a potent activator of cell proliferation. To determine if G-1-mediated increases in IL-10 were the result of increased proliferation of cells expressing IL-10 rather than induction of IL-10 de novo, naive T cells were stained with the proliferation dye eFluor670 before stimulation in culture. We were unable to detect any significant difference in the proportion of dividing cells following G-1 treatment. The observation that G-1-treated cultures demonstrate attenuated dilution of the eFluor dye compared with the DMSO-treated cultures (Fig. 5) indicates that the increase in IL-10+ cells following G-1 treatment is not the result of an increase in cell proliferation, and in fact shows that proliferating cells are going through fewer divisions when treated with G-1, perhaps because of the action of IL-10. In addition, the dramatic increase in the number of non-dividing cells expressing IL-10 in G-1-treated cultures (as indicated in the upper right quadrant in Fig. 5b) suggests that G-1 can specifically drive expression of IL-10 independent of cell division. Taken together, these data show that G-1 stimulates de novo IL-10 expression within differentiating Th17 cells through direct action on T cells via an ERK-dependent mechanism.
Figure 5.
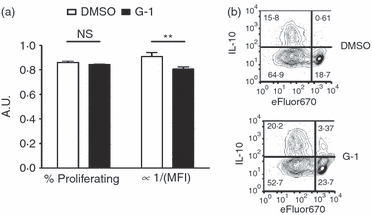
G-1 effects are not dependent on proliferation. CD4+ CD62Lhi naive CD4+ T cells were collected by FACS and stained with the proliferation dye eFluor670 before culture. Following differentiation for 4 days ex vivo in culture with anti-CD3/28 + interleukin-6 (IL-6) + transforming growth factor-β (TGF-β) in the presence of 100 nm G-1 (black bars) or DMSO (white bars), cells were stained for intracellular IL-10. (a) Percentage of cells proliferating and the inverse of geometric mean fluorescence intensity (GMFI), a measure of total proliferation. (b) Sample plots showing IL-10 expression and eFluor670 staining. The upper right quadrant shows cells expressing IL-10 without evidence of proliferation. Data show one of two independent experiments. P-values determined by Student's t-test; **P < 0·02. Error bars = SD; NS, not significant, A.U., arbitrary units.
IL-10 induction occurs within a hybrid T-cell population
An emerging paradigm in T-cell biology is the induction of ‘hybrid’ T-cell populations that express one of the canonical effector T-cell transcription factors (for example T-bet from the Th1 lineage) as well as Foxp3.29 These cells appear to play a role in the regulation of specific types of inflammatory responses, where the expression of Foxp3 imparts a suppressive phenotype, and the expression of the lineage-specific factor such as T-bet leads to a repertoire of gene products (e.g. chemokine receptors) that allow for targeting to sites of inflammation. Presumably, this provides a mechanism for the recruitment of regulatory T cells to sites on ongoing inflammatory responses. To investigate the expression of Foxp3 together with RORγt, naive T cells were collected from Foxp3egfp transgenic mice.41 Cells were stimulated for 4 days in the presence of TGF-β and IL-6 with or without G-1 added to the culture. Following differentiation, IL-10, IL-17A, RORγt and Foxp3 were analysed by intracellular cytokine staining or detection of endogenous GFP expression by flow cytometry. G-1 was equally effective at inducing IL-10 production within Foxp3− RORγt+ Th17 cells as in Foxp3+ RORγt+ hybrid T cells (Fig. 6). The Th17 (i.e. RORγt+) subset yielded an increase in both IL-10+ IL-17A+ and IL-10+ IL-17A− cells, while only IL-10+ IL-17A− cells were detected in the hybrid T-cell population. In fact no IL-17A+ cells were present in the Foxp3+ population (data not shown). These data demonstrate the ability of G-1 to induce IL-10 within the recently described hybrid Th17 population in addition to conventional (Foxp3− RORγt+) Th17 cells.
Figure 6.
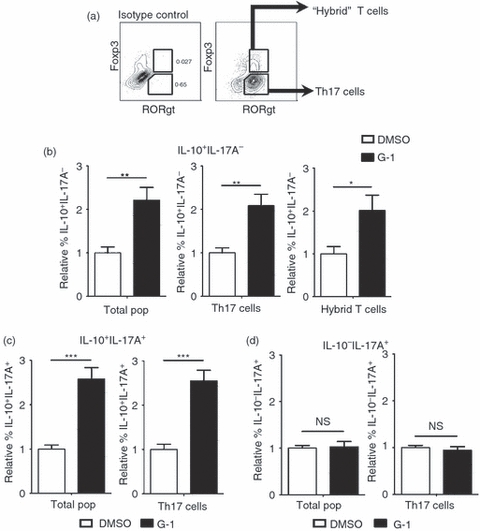
G-1 induces interleukin-10 (IL-10) production within the hybrid T-cell population. CD4+ CD62Lhi naive CD4+ T cells were collected by FACS from Foxp3egfp mice and cultured for 4 days ex vivo with anti-CD3/28 + IL-6 + transforming growth factor-β (TGF-β) in the presence of 100 nm G-1 (black bars) or DMSO (white bars). Cells were collected and stained for intracelluar IL-10, IL-17A, and RORγt, and analysed by flow cytometry. Cells that were Foxp3+ RORγt+ were designated as hybrid T cells, whereas those that were Foxp3− RORγt+ were designated as T helper type 17 (Th17) cells. (a) Gating logic to determine hybrid T-cell and Th17 populations. (b–d) Graphs represent summary of data from three independent experiments showing the relative percentage of (b) IL-10+ IL-17A−, (c) IL-10+ IL-17A+, and (d) IL-10− IL-17A+ populations. P-values determined by Student's t-test; *P < 0·05; **P < 0·005; ***P < 0·0005. Error bars = SEM; NS, not significant.
In vivo treatment with G-1 leads to increased IL-10 secretion from splenocytes
Our results show that treatment of naive T cells with G-1 in culture can lead to increased IL-10 expression and secretion. To determine if these findings translated in vivo, wild-type mice were injected subcutaneously with G-1 for 7 consecutive days, after which isolated splenocytes were stimulated in culture with anti-CD3ε and anti-CD28 antibodies. Samples of supernatant were collected 24, 48 and 72 hr after stimulation and analysed for secreted IL-6, IL-10, IL-17A, IFN-γ and TNF-α by Luminex multiplex assay. No trends were observed for any of the analytes following 24 hr of stimulation (Fig. 7). As postulated, following 72 hr of stimulation cells from the G-1 treated mice produced significantly more IL-10 (Fig. 7a), in agreement with our results with cultured naive T cells. Moreover, there was a statistically significant difference between the time–course of IL-10 secretion for the cells from G-1-treated mice compared with those from vehicle-treated animals, as determined by analysis of variance (Fig. 7a).
Figure 7.
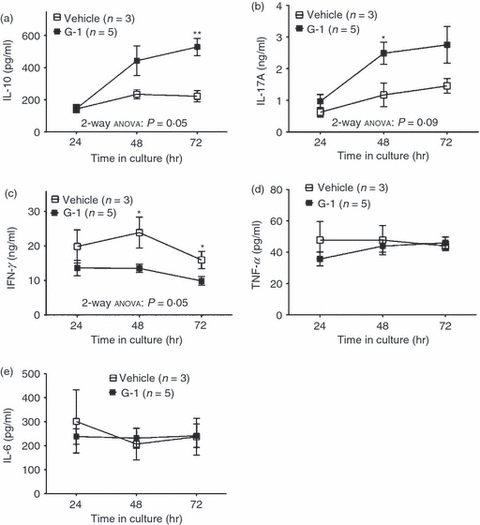
Cytokine secretion following in vivo treatment with G-1. Seven- to eleven-week-old male wild-type C57BL/6 mice were injected with G-1 (5 μg/day) or vehicle for 7 consecutive days. One day following the last injection, splenocytes were collected and cultured in the presence of anti-CD3ε (1·0 μg/ml) and anti-CD28 (2·5 μg/ml) antibody. Culture medium was collected after 24, 48, and 72 hr and analysed for the presence of secreted (a) IL-10, (b) IL-17A, (c) interferon-γ (IFN-γ), (d) tumour necrosis factor-α (TNF-α) and (e) interleukin-6 (IL-6) by Luminex multiplex assay. Graphs are mean data with three to five mice per group. P-values comparing G-1 and vehicle at a given time-point were determined by Student's t-test; *P ≤ 0·05, **P ≤ 0·01. P-values comparing G-1 and vehicle curves were determined by two-way anova as indicated. Error bars = SEM.
Some unexpected results where obtained as well. We observed that G-1-treated splenocytes demonstrated a statistically significant increase in the secretion of IL-17A at 48 hr (Fig. 7b). This differed from our findings in Fig. 1, where no change in the prevalence IL-17A+ cells was observed, and Fig. 2 where naive T cells cultured with G-1 produced similar levels of IL-17A compared with control cells. Additionally, splenocytes from G-1-treated mice produced decreased levels of IFN-γ relative to those that were treated with vehicle alone (Fig. 7c), suggesting that in addition to driving production of IL-10 and IL-17A, G-1 may act systemically to reduce the levels of IFN-γ. This result differed from those shown in Figs 1 and 2 as well, where no changes in IFN-γ expression were noted. These observations probably reflect the complex nature of the in vivo environment, with secondary effects resulting from activity on other immune populations. No changes in the secretion of TNF-α (Fig. 7d) or IL-6 (Fig. 7e) were detected, in agreement with our findings from Fig. 2. Collectively, these data suggest that pharmacological stimulation of GPER in vivo leads to an increase in the production of the cytokines IL-10 and IL-17A, and decreased production of the pro-inflammatory cytokine IFN-γ following T-cell activation, yielding an overall anti-inflammatory environment.
Discussion
It is known that CD4+ T cells play a critical role in the pathogenesis of many of the most prominent diseases of the Western world, including cancer, autoimmunity and infectious diseases. The cytokine IL-10 is a potent suppressor of immune responses, capable of acting on a multitude of cell types to dampen inflammatory responses to and limit host damage by infection and autoimmune disease. In this study, we demonstrated that the GPER-directed agonist G-1 can drive IL-10 production from Th17-polarized CD4+ T-cell populations. We observed an increase in the number of cells expressing IL-10 within, and increased IL-10 secretion from, the G-1-treated cultures. This response was not the result of global changes in cytokine production as G-1 had no effect on the expression of IL-17A under Th17-polarizing conditions, or in the induction of IFN-γ in non-polarizing (Th0) conditions. We also observed no significant change in the secretion of IL-6, IL-17A, TNF-α or IFN-γ from G-1-treated cultures, demonstrating high selectivity for the mechanism of G-1-mediated IL-10 induction.
We did occasionally detect fewer cells in G-1-treated cultures relative to those treated with DMSO (RLB and ERP, unpublished observation), but this was not a consistent finding. This observation may reflect variability in the temporal dynamics of IL-10 induction between different experiments or G-1-mediated induction of regulatory T-cell populations. As noted above, we observed a slight but significant decrease in proliferation of G-1-treated cultures (Fig. 5). Additionally, we noted a small but significant increase in expression of the apoptotic/cell death marker Annexin V in G-1-treated cultures (see Supplementary material, Fig. S2). Either of these effects may be contributing to a decrease in cell number in G-1-treated cultures. Further studies will be required to determine whether these findings reflect the direct effects of G-1 on cellular proliferation and viability, or are secondary to the induction of regulatory T-cell populations.
Systemic delivery of G-1 drove IL-10 production from splenocytes following T-cell activation in culture. It is notable that this effect does not require overt in vivo antigen recognition. This result may reflect that G-1-mediated signalling in naive T cells leads to an alteration in their resting state, perhaps through transcriptional mechanisms. Another possibility is that there is carryover of G-1 during purification of splenocytes before culture, where antigen presentation is mimicked using stimulatory antibodies, or that the effects are the result of the low levels of T-cell activation inherent in naive mice. Along those lines, we have consistently found a small population of memory cells within the spleen of untreated mice, suggesting low levels of immune activation in ‘naive’ animals (data not shown). It is also possible that pre-existing memory T cells are responsible for G-1's effect in this setting, as G-1 can drive IL-10 secretion from this population (unpublished observation). In agreement with our observations from cultured T cells (Fig. 2), systemic administration of G-1 had no effect on IL-6 or TNF-α secretion. Conversely, we did detect increased secretion of IL-17A following in vivo treatment with G-1, while also observing a decrease in the production of IFN-γ. These differences from results with purified T-cell cultures may reflect the effects of G-1 on other immune populations following in vivo treatment. Such populations may also be contributing to the observed IL-10 secretion, directly or indirectly. Another possibility includes G-1-mediated IL-10 production during the week-long injections of G-1, leading to inactivation of splenic APCs and a decrease in the secretion of Th1-polarizing cytokines like IL-12, and hence to lower IFN-γ production.
Th17 cells are localized in high numbers to sites of autoimmune inflammation. Our data suggest that it may be possible to induce IL-10 in situ where large numbers of Th17 cells persist, through systemic treatment with G-1. The feasibility of this therapeutic approach is suggested by experiments in which IL-10+ Th17 cells differentiated with TGF-β and IL-6 alone inhibited the development of EAE following adoptive transfer of neuropeptide-reactive Th17 cells.19 This effect was dependent on IL-10 production19 and suggests that such cells can inhibit fully differentiated pathogenic T-cell populations through the secretion of IL-10 in situ, as would likely be required in the case of a viable therapeutic intervention based on the results of our study. While our finding that systemic G-1 could increase IL-17A secretion from murine splenocytes warrants further attention, it must be noted that IL-17A has been shown to exhibit immunosuppressive properties in several settings, including in the development of atherosclerosis43–45 and the induction of T-cell-mediated colitis.46 Moreover, the IL-10+ Th17 cells discussed above that were shown to exhibit bystander suppressive effects in EAE also produced high levels of IL-17A.19 Consequently, the induction of IL-17A is reconcilable with its ability to attenuate EAE, despite the established importance of Th17 cells to EAE induction,3,47,48 and the fact that systemic neutralization of IL-17A/F attenuates clinical symptoms in this model.49 However, there is also clear evidence that IL-17A can contribute to pathogenic inflammation.5 Future studies aimed at determining the context in which G-1 or any related compounds elicit critical Th17 factors like IL-17A/F, IL-21, IL-22, IL-23 and the aryl hydrocarbon receptor will be critical to determining the setting(s) in which G-1 has therapeutic potential.
The observation of G-1-induced IL-17A secretion may offer some insight into autoimmune pathophysiology. There is a longstanding debate about how the apparent immunosuppressive activities of E2 can be reconciled with the higher prevalence of autoimmune disease in women. It is possible that E2-mediated activation of GPER may drive increased IL-17A production under specific circumstances, and that this contributes to augmented autoimmune pathogenesis in women. Future studies aimed at investigating this possibility should be directed at delineating the specific conditions in which GPER activation leads to IL-17A, and perhaps IL-17F, production. It would be interesting to correlate these findings with studies investigating the expression of ERα, ERβ and GPER, which may vary over time. An explanation for the sexual dimorphism in the prevalence of autoimmune disease may reside in identifying a setting where GPER plays a predominant role in estrogen signalling, perhaps as the result of down-regulation of ERα and ERβ within specific cell populations, under conditions where GPER activation leads to production of IL-17A or even IL-17F.
If these properties can be definitively described, there is also the possibility that G-1 may serve a role in T-cell-based tumour vaccine strategies. Evidence suggests that polarization of tumour-specific T-cells towards a Th17 phenotype before adoptive transfer can enhance tumour eradication.50 G-1 or a related compound may serve as a cost-effective and safe alternative to recombinant cytokines during T-cell culture, or even as a systemic adjuvant treatment to help stabilize the cells following adoptive transfer, especially given the fact that we observed increased IL-17A production following in vivo G-1 treatments. Moreover, further delineating the role of GPER in polarization along the Treg–Th17 axis, may uncover other pharmacological approaches, such as the use of G15, that can elicit anti-tumour responses by driving conversion of Treg cells into Th17 populations. This strategy was validated in principle through the use of indoleamine 2,3-dioxygenase inhibitors in the B16 melanoma model.33
Our findings also suggest that GPER-mediated induction of IL-10 may play a role in estrogen's ability to suppress autoimmune diseases. Two previous reports demonstrated that G-1 can suppress EAE.38,39 In one study, the authors found that G-1's protective effects correlated with increased programmed death-1 (PD-1) expression on Foxp3+ Treg cells, and were dependent on PD-1 expression as PD-1 knockout mice were not protected from disease by G-1.38 Notably, the authors also observed increased IL-10 production from G-1-treated splenocytes collected from diseased animals compared with placebo controls, an effect lost in the PD-1 knockout mice.38 This correlates well with our results in Fig. 7, as we observed increased IL-10 production from splenocytes of G-1-treated mice. Notably, IL-10 production in CD4+ T cells can inhibit the development of EAE,18 a disease whose pathogenesis is dependent on RORγt expression.3 The fact that we demonstrated G-1 leads to an increase in IL-10 within RORγt+ cells, and that IL-10 induction occurs even in the presence of IL-23, leads to the hypothesis that G-1 suppressed EAE through the induction of IL-10 production from RORγt+ cells specifically within the central nervous system via a PD-1-dependent mechanism. It has also been recently shown that estrogen can protect mice from EAE in a Foxp3-indpendent manner.51 Again an increase in IL-10 was noted, though it is not known what cells were responsible for this effect. Additionally, other studies have shown that: (i) E2 can increase IL-10 production in vivo in a GPER-dependent manner,36 and (ii) the in vitro suppressive activity of Treg cells from PD-1 knockout mice was enhanced following in vivo treatment with E2, without changing the expression levels of Foxp3.52 One hypothesis to explain these results may be that E2 signalling through classical estrogen receptors substitutes for PD-1-mediated signalling in the induction of IL-10 from effector populations when E2 is used in lieu of G-1. Further studies using conditional knockouts of IL-10 within the CD4+ compartment, and analysis of GPER, ERα, and ERβ signalling in Foxp3+ and Foxp3− populations, including the specific requirement of PD-1 expression, will be needed to definitively address these questions.
G-1 has been characterized as a selective agonist for the G protein-coupled estrogen receptor GPER,53 a recently identified non-classical member of the estrogen receptor family.54 Consistent with this mechanism of action, G-1-mediated IL-10 expression was inhibited by the addition of the GPER-directed antagonist G15.40 Our results are also supported by observations that G-1-mediated inhibition of EAE is dependent on GPER expression.38 Although small molecules can be subject to off-target activity, it is unlikely that both G-1 and G15 would exhibit off-target profiles that mimic their established activities towards GPER. Nevertheless, further investigation into the G-1 target(s) in T cells is warranted. Although GPER−/− mice exist, they exhibit higher levels of apoptosis in double-negative thymocytes and lack the E2-mediated increase in this population following systemic estrogen administration.55 Therefore studies aimed at verifying GPER as the target of G-1 within the T-cell population will need to employ inducible knockout strategies or retroviral RNAi targeting of GPER to avoid the confounding effects of aberrant thymic T-cell development observed in GPER−/− mice.
Our results have begun to elucidate the mechanisms by which G-1 induces IL-10 expression and production. Addition of the MEK1 inhibitor PD98059 blocked G-1-mediated IL-10 induction, whereas addition of inhibitors of the p38 and JNK pathways was without effect. These findings are consistent with reports that ERK signalling is necessary for the induction of IL-10 in Th1 and Th2 cells, and contributes to IL-10 expression in Th17 populations, with no detectable difference when p38 signalling is blocked.13 Why addition of PD98059 led to a mild increase in the number of IL-10+ cells within control (DMSO) cultures is unclear (Fig. 4b). This stands in contrast to the previous reports discussed above,12,13 yet we consistently observed this effect. Interestingly, in the work by Saraiva et al.13 blockade of ERK signalling only led to a partial inhibition of IL-10 induction from Th17 cultures. This suggests there are two pathways of IL-10 induction in Th17 cells, the ‘ERK-dependent pathway’ described above, and an alternative pathway. One hypothesis to explain the discrepancy between our findings and previous reports would be that this alternative pathway: (i) is inhibited by ERK signalling (an ‘ERK-sensitive pathway’), and (ii) is the predominant pathway for IL-10 induction in culture conditions using charcoal-stripped FBS in lieu of normal FBS, as we have done here. Given that ERK signalling is implicated in IL-10 expression within Th1 and Th2 cells, it will be interesting to determine whether G-1 can drive IL-10 production under Th1- or Th2-polarizing conditions. The lack of IL-10 expression in unpolarized (Th0) cells is not unexpected. Interleukin-10 production in Th populations requires STAT activation via IL-4, IL-6, IL-12, IL-21 and IL-27.18,20 However, these cytokines are produced by APCs and differentiated T-cell populations and are likely to be in limited supply in the pure cultures of naive T cells that we employed. We observed that G-1 was unable to induce IL-10 production in differentiating naive T cells without the addition of both TGF-β and IL-6 to the culture medium, suggesting that G-1 cannot replace any of the critical signals necessary to induce IL-10 in Th17 cells. It appears that the function of TGF-β in Th17 development is to block the differentiation of Th1 and Th2 cells.56 Hence our observation that G-1 treatment with IL-6 alone does not consistently elicit IL-10 production despite detectable levels of IL-10+ cells perhaps reflects a dependence on Th17 differentiation. Future studies will need to address this question.
Finally, the IL-10+ IL-17A+ cells we identified appear to be part of the autoregulatory pathway,21 as they express RORγt but not Foxp3. In fact we detected virtually no IL-17A+ cells within the Foxp3+ population. While not completely unexpected, because Foxp3 can inhibit some of the transcriptional activity of RORγt,57 Foxp3+ IL-17A+ cells have been previously reported.58 Our observation that G-1 induces IL-10 expression in Foxp3+ RORγt+ hybrid T cells suggests that, in addition to generating IL-10 production in populations already localized at the site of inflammation, G-1 may also enhance the suppressive function of Treg populations drawn in from the circulation. Such a response would not be unprecedented as T-bet-induced CXCR3 expression in Foxp3+ cells has been shown to play a role in targeting Treg cells to sites of Th1-type inflammation.59 If IL-10 can be stably induced in hybrid T-cell populations following in vivo G-1 treatment, their suppressive activity may be enhanced as they are recruited to sites of ongoing inflammation.
Numerous attempts have been made to harness the immunosuppressive properties of IL-10 for therapeutic benefit, many of which have been based on the use of biologics.25 To our knowledge, this is the first evidence that a synthetic small molecule can shift the balance along the Treg–Th17 axis in favour of IL-10 production, in this case by acting directly on T-cell populations. These data build on previous results demonstrating that dexamethasone and retinoic acid can elicit IL-10 from polyclonally stimulated naive T cells when IL-4, IL-12 and IFN are neutralized.60 Also worth noting is the fact that it is becoming increasingly clear that GPER probably plays a smaller role in the majority of classical estrogen responses, such as uterine imbibition, as compared with its better known counterpart ERα.40 Hence G-1 may be associated with a more tolerable adverse effect profile.
Our findings suggest that the membrane-permeable small molecule G-1 may serve as a novel T-cell-targeted immunosuppressive agent in settings where large populations of Th17 cells exist, for example in rheumatoid arthritis, inflammatory bowel disease, or psoriasis. G-1 may also prove useful for in vitro generation of IL-10-producing cells for adoptive immunotherapy. Future studies delineating the specific signalling mechanisms and targets of G-1 and other related compounds will be seminal to the continued development of this new class of immunoregulatory estrogenic small molecules. The selectivity of G-139,53 and its attractive pharmacological properties38 make this compound a strong candidate for pharmaceutical development, paving the way for the development of novel T-cell targeted immunotherapeutics.
Acknowledgments
This work was supported by National Institutes of Health grants R01 CA116662, CA118743 and CA127731 (E.R.P.). Data were generated in the Flow Cytometry Shared Resource Center supported by the University of New Mexico Health Sciences Center and the University of New Mexico Cancer Center. The authors would like to thank Drs Rick Lyons and Mary Lipscomb for insightful discussions, Kristin Owens and Lori Deihl for their technical assistance, and Dr Helen Hathaway for her expertise in the care and use of mice.
Authorship contributions and disclosure of conflict of interest
R.L.B. and E.R.P. designed and interpreted experiments and wrote the manuscript. R.L.B. carried out experiments and E.R.P. holds a U.S. patent on G-1 and G15.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. G-1 does not alter IFNγ expression.
Figure S2. G-1 increases Annexin V expression.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than about missing material) should be directed to the corresponding author for the article.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–10. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torchinsky MB, Blander JM. T helper 17 cells: discovery, function, and physiological trigger. Cell Mol Life Sci. 2010;67:1407–21. doi: 10.1007/s00018-009-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–24. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patey-Mariaud de Serre N, Canioni D, Ganousse S, Rieux-Laucat F, Goulet O, Ruemmele F, Brousse N. Digestive histopathological presentation of IPEX syndrome. Mod Pathol. 2009;22:95–102. doi: 10.1038/modpathol.2008.161. [DOI] [PubMed] [Google Scholar]
- 9.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–54. [PubMed] [Google Scholar]
- 10.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 11.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Eng J Med. 2009;361:2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 13.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–19. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 15.Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–82. [PubMed] [Google Scholar]
- 16.Jankovic D, Kullberg MC, Feng CG, et al. Conventional T-bet+Foxp3– Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–83. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 19.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 20.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 21.Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–33. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–7. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 24.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–41. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 25.O'Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–31. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Bluestone JA, Mackay CR, O'Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev. 2009;9:811–6. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–80. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Barnes MJ, Powrie F. Hybrid Treg cells: steel frames and plastic exteriors. Nat Immunol. 2009;10:563–4. doi: 10.1038/ni0609-563. [DOI] [PubMed] [Google Scholar]
- 30.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 31.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–8. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 32.Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, Hazes JM, van Leeuwen JP, Lubberts E. 1,25-Dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–42. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 33.Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–11. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 35.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Eng J Med. 1998;339:285–91. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, Offner H. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol. 2009;182:3294–303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blasko E, Haskell CA, Leung S, et al. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennis MK, Burai R, Ramesh C, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–7. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–72. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 42.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 43.Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–87. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taleb S, Romain M, Ramkhelawon B, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–77. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taleb S, Tedgui A, Mallat Z. Interleukin-17: friend or foe in atherosclerosis? Curr Opin Lipidol. 2010;21:404–8. doi: 10.1097/MOL.0b013e32833dc7f9. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–9. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 48.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–30. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–7. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19:337–43. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 53.Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–12. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 54.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–90. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Dehghani B, Magrisso IJ, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–48. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das J, Ren G, Zhang L, et al. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–16. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–67. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


