Abstract
Air pollution is frequently proposed as a cause of the increased incidence of allergy in industrialised countries. We investigated the impact of ozone (O3) on reactive oxygen species (ROS) and allergen content of ragweed pollen (Ambrosia artemisiifolia). Pollen was exposed to acute O3 fumigation, with analysis of pollen viability, ROS and nitric oxide (NO) content, activity of nicotinamide adenine dinucleotide phosphate (NAD[P]H) oxidase, and expression of major allergens. There was decreased pollen viability after O3 fumigation, which indicates damage to the pollen membrane system, although the ROS and NO contents were not changed or were only slightly induced, respectively. Ozone exposure induced a significant enhancement of the ROS-generating enzyme NAD(P)H oxidase. The expression of the allergen Amb a 1 was not affected by O3, determined from the mRNA levels of the major allergens. We conclude that O3 can increase ragweed pollen allergenicity through stimulation of ROS-generating NAD(P)H oxidase.
Keywords: NAD(P)H oxidase, Ozone, Pollen, Ragweed, ROS
Highlights
► O3 reduces the viability of ragweed pollen. ► ROS and allergens of ragweed pollen were not affected by O3 exposure. ► O3 enhances the activity of the ROS-generating enzyme NAD(P)H oxidase. ► O3 increases ragweed pollen allergenicity through NAD(P)H-oxidase stimulation.
This study focuses on the effects of the atmospheric pollutant ozone on ROS content and NAD(P)H oxidase activity of ragweed pollen grains.
1. Introduction
The increase in respiratory diseases arising from allergies in industrialised countries in recent years is considered to be linked to changes in certain environmental factors. One such factor relates to the higher levels of atmospheric pollution and the greater presence and distribution of allergenic taxa. Climate change has brought about large increases in the concentrations of some airborne allergens, with the resulting higher incidence and/or severity of allergic illnesses (Gilmour et al., 2006). Significant increases in allergic responses are often reported, even in individuals who have never had symptoms previously, and this leads to the belief that these allergic responses might be caused by ’new’ allergens that are released by plants used for ornamental purposes or reforestation, or by invasive plants.
In Europe the increases in pollinosis appear to be caused by pollen from birch, hornbeam and cypress trees, and above all, from ragweed, rather than by the classic allergenic pollens, such as grasses, pellitory, olive and mugwort (Corsico et al., 2000; Asero, 2004; Ridolo et al., 2006). Ambrosia artemisiifolia (short or common ragweed) is an annual, anemophilous, and extremely allergenic weed that can produce enormous amounts of pollen. As these pollen grains are small (18–22 μm), they can often be transported over long-distances (Mandrioli et al., 1998).
Ragweed started its expansion in Europe in the last decades of the 20th century, arriving from Hungary, the country where it was most abundant. Ragweed is now also found in Italy (D’Amato et al., 1998), and the two regions that have been most affected are Lombardia and Friuli Venezia-Giulia, which are located in the north-northeast of Italy (Mandrioli et al., 1998; Asero, 2002). Ragweed plants have been detected only sporadically in central-southern Italy (Pignatti, 1997). However, transboundary transport of ragweed pollen to central Italy has been demonstrated, which could have a clinical effect on atopic patients (Corsico et al., 2000; Cecchi et al., 2007). Ragweed pollen concentrations have been reported to have increased over the last decade (Oswalt and Marshall, 2008), mainly because ragweed is invasive in Europe and is an opportunistic and pioneer plant, invading field crops and open disturbed habitats or roadsides (Bassett and Crompton, 1975).
However, the increase in allergic disease might be attributed not only to greater concentrations of ragweed pollen in the atmosphere, but also to modifications to the allergenicity this pollen can promote. Although the role of atmospheric pollutants on the allergic sensitivity of airways is not yet completely clear, there is evidence to suggest that urbanisation increases allergic sensitization due to its high levels of exposure to ozone (O3), nitric oxides (NOx), sulphur oxides (SOx) and particulate matter (PM10) (D’Amato, 2002).
In addition to affecting the airways of allergic individuals, air pollutants can have direct effects on the aeroallergens in the atmosphere, which can result in changes in the antigenic characteristics of pollen. Air pollutants, and especially O3 and respirable PM10, can induce proinflammatory responses in the lung (Bernstein et al., 2004), and can have immunological adjuvant effects on IgE synthesis, as has been found with polyaromatic hydrocarbons in the particles of diesel exhaust (Nel et al., 1998).
Ozone is the main component in the so-called ’summer smog’ comprised of photochemical oxidants and appears to account for up to 90% of the total oxidant levels in cities that have a mild sunny climate (Butkovic et al., 1990). Ozone is generated at ground level by photochemical reactions that involve ultraviolet radiation of atmospheric mixtures of NO2 and hydrocarbons that can derive from vehicle emissions. These O3 trends depend not only on the substrate supply (NO2 emitted by cars), but also on the sunny weather, because of the transformation of NO2 into O3 during a photochemical smog.
Current safety standards for O3 levels are exceeded frequently in most Mediterranean countries. Indeed, the 8 h average levels of O3 for the period from 2000 to 2004 in different sites in Italy showed that the background O3 pollution exceeded the European standards (Paoletti et al., 2007) that were fixed by the European Union at 0.060 ppm (Directive 2002/3/EC, 2004).
Ozone can affect animal and plant metabolism. Its toxicity is due to the generation of reactive oxygen species (ROS), such as the superoxide anion radical (•O2−), hydrogen peroxide (H2O2), the hydroxyl radical (•OH), and singlet oxygen (1O2) (Mudd, 1997). The O3 effect on pollen grains has been considered in particular in studies of plant germination in vitro. However, opposing effects have been reported: Feder (1968, 1981) observed a reduction in Nicotiana tabacum pollen tube elongation, and similar results were reported for corn pollen by Mumford et al. (1972). In contrast, Benoit et al. (1983) reported that the ability of the pollen of Pinus strobus to germinate was not significantly reduced by O3 fumigation. It has also been reported that O3 can influence allergen release from pollens grains, and Masuch et al. (1997) found that O3 increases the content of group 5 allergenic proteins of Lolium perenne. When the pollen comes into contact with the airway mucosa, it releases not only allergenic proteins, but also lipid immuno-modulators, such as pollen-associated lipid mediators (Traidl-Hoffmann et al., 2002), and nicotinamide adenine dinucleotide phosphate (NAD[P]H) oxidases (Boldogh et al., 2005). These latter are enzymes that have an oxidase activity that can produce the •O2− ion, which is converted into H2O2 through the action of the enzyme superoxide dismutase (SOD). Therefore the NAD(P)H oxidases have fundamental roles in inflammatory processes, as they can increase the levels of ROS in the epithelium of the respiratory apparatus, and promote the flow of neutrophils towards the respiratory apparatus (Boldogh et al., 2005).
On the basis of the concomitance of episodes of high O3 levels (Gabusi and Volta, 2005) and the high concentrations of ragweed pollen in the atmosphere during the summer months (Mandrioli et al., 1998), the aim of the present study was to determine whether O3 affects mature ragweed pollen grains after their dispersal. In particular, we determine first whether O3 can increase the allergy potency of ragweed pollen by stimulating allergen and NAD(P)H oxidase release, and secondly whether O3 can induce physiological alterations that affect pollen viability, which is the ability of the pollen to complete post-pollination events and to achieve fertilisation.
For this purpose, under laboratory conditions, we exposed ragweed pollen to a high O3 concentration (100 nL L−1) during the day. This concentration corresponds to peak O3 levels measured during the summer in central Italy (http//www.arpa.umbria.it). We specifically evaluated: (i) pollen ROS and NO content, as NO is considered to be a mediator of inflammatory responses (Moilanen and Vapaatal, 1995); (ii) activity of the NAD(P)H oxidase, which can generate ROS; (iii) expression of the major ragweed pollen allergens; and (iv) pollen viability.
2. Materials and methods
2.1. Pollen
Ragweed (Ambrosia artemisiifolia) pollen was purchased from Greer Laboratories (Lenoir, NC, USA). The pollen was aliquoted into 2 mL, sterile microcentrifuge tubes, and stored dry at ≤4 °C until use.
2.2. Ozone treatment
The O3 treatment was performed by exposure of 0.5 g pollen in Petri dishes to 100 nL L−1 O3 for 5 h (08:00 h to 13:00 h) per day for 7 consecutive days in a plexiglass chamber (0.32 m3) under light with a photosynthetic photon fluence of 120 μmol m−2 s−1, as previously described (Pasqualini et al., 2009). A non-fumigated (−O3) pollen sample was maintained in a filtered-air plexiglass chamber under the same experimental conditions. After each daily O3 fumigation, the pollen samples were all left in a growth chamber under controlled conditions (14 h photoperiod, photosynthetic photon fluence rate of 120 μmol m−2 s−1, day/night air temperature 25 °C/20 °C, and relative humidity 60%–75%) until the next treatment. After the 7 days of this O3 fumigation, the pollen was either immediately analysed for viability and ROS, H2O2 and NO content, or frozen under liquid N2 and stored at −80 °C for protein quantification, NAD(P)H oxidase activity assay, and RNA analysis. The O3 treatment was replicated three times.
2.3. Pollen viability
Pollen viability was estimated using the fluorescein diacetate stain (FDA; Sigma–Aldrich, St. Louis, MO, USA), as reported by Heslop-Harrison and Heslop-Harrison (1970). Pollen grains (2 mg) were hydrated for 30 min in 2 mL 0.4 M sucrose. Then 20 μl of these suspensions were placed on a microscope slide and stained with 4 μM FDA. The total number of pollen grains was visually counted using bright-field microscopy, while the fluorescent pollen grains in the same field of view were counted using a UV epifluorescence microscope (DMLB; Leica, Leica Microsystems, Wetzlar, Germany) with a 450 nm excitation filter and a 535 nm emission filter. Pollen viability was determined as the percentage of fluorescing pollens relative to the total pollen grains. For each sample (control and O3 treated), ten slides were prepared, and for each slide, at least 100 pollen grains were counted.
2.4. Determination of intracellular nitric oxide
Intracellular NO was visualised as according to the method of Bright et al. (2009) with slight modifications using the fluorescent NO indicator dye 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate; Molecular Probes, Invitrogen, Carlsbad, CA, USA) (Kojima et al., 1998). The pollen (1 mg) was first incubated for 30 min at 4 °C in 1 mL MES–KCl buffer containing 8% sucrose, 10 mM MES, 5 mM KCl, 50 mM CaCl2 (pH 6.8), in the absence and presence of 200 μM of the NO-scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO; Sigma–Aldrich). DAF-FM diacetate (10 μM) was then added, and the samples were incubated in the dark for 20 min, to allow the dye to enter the pollen. The pollen suspensions were then centrifuged at 6500 × g for 1 min, and the resulting supernatant was discarded. Fresh MES–KCl buffer was then added to the pellets, which were resuspended and left at room temperature for 20 min. Finally, 50 μl pollen suspension were placed onto a glass slide and covered with a glass coverslip before being examined with a UV epifluorescence microscope with a 450 nm excitation filter and a 535 nm emission filter. Intracellular NO was determined as the percentage of fluorescing pollens relative to the total pollen grains. For each sample (control and O3 treated), six slides were prepared, and for each slide, at least 100 pollen grains were counted.
2.5. Detection of the reactive oxygen species
ROS detection was performed using the fluorescent ROS indicator dye 2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA; Molecular Probes) (Setsukinai et al., 2003). Pollen grains (2 mg aliquots) were hydrated at 4 °C in 2 mL 100 mM phosphate-buffered saline (PBS) for 30 min, and then transferred to 2 mL microcentrifuge tubes and centrifuged at 2000 × g for 30 s. After centrifugation, the supernatant was discarded and fresh PBS containing 2.5 μM DCFH2-DA was added to the pellet. After an incubation in the dark at 25 °C for 15 min, it was centrifuged at 2000 × g for 30 s. After centrifugation, the supernatant was discarded and the pellet was immediately resuspended in fresh PBS, gently shaken, and centrifuged as before. This step was repeated three times to remove excess dye. Finally, the pellet was resuspended in PBS, and 50 μl of the pollen suspension was placed onto glass slides, covered with glass coverslips, and examined using a UV epifluorescence microscope with a 450 nm excitation filter and a 535 nm emission filter. Content of ROS was determined as the percentage of fluorescing pollens relative to the total pollen grains. For each sample (control and O3 treated), six slides were prepared, and for each slide, at least 100 pollen grains were counted.
2.6. Preparation of pollen extracts
Pollen grains (100 mg) were hydrated in 1 mL PBS for 25 min at room temperature, with gentle shaking. The pollen suspensions were then centrifuged at 14000 × g for 10 min, and the supernatants were recovered (S25 supernatants). The pollen pellets were resuspended with 1 mL PBS, shaken for 25 min and then centrifuged again at 14000 × g for 10 min, to obtain the second supernatants (S50 supernatants). This procedure was repeated once again to obtain the third supernatants (S75 supernatants). The pellets were then resuspended in 0.5 mL PBS, sonicated, centrifuged at 14000 × g for 10 min, and the supernatants were recovered (Sson supernatants). The protein content and NAD(P)H oxidase activity were assayed for all of the supernatants.
2.7. Protein quantification
The soluble protein concentrations in the supernatants were measured using the dye-binding method of Bradford (1976), with bovine serum albumin as the standard.
2.8. NAD(P)H oxidase enzymatic activity
The NAD(P)H oxidase activity was measured in the samples of these four supernatants obtained as above (S25, S50, S75 and Sson) using the nitroblue tetrazolium (NBT) assay (Bacsi et al., 2005). This activity was only detectable in the S25 supernatants. Here, 25 μg protein was used for each assay and mixed with 2 mM NBT without or with 100 μM reduced nicotinamide adenine dinucleotide (NADH), or 100 μM reduced NAD phosphate (NADPH), and without or with the NAD(P)H oxidase inhibitor diphenyleneiodonium (DPI, 100 μM) or the •O2−-scavenging enzyme SOD (100 U mL−1). These mixtures were incubated at 37 °C for 15 min. NBT was completely removed by repeated washing with fresh PBS, and the formazan precipitate was dissolved in 100% methanol. Absorbance was determined at 530 nm by spectrophotometry.
2.9. Hydrogen peroxide content
To determine the H2O2 content, 100 mg pollen grain aliquots were hydrated for 30 min at 4 °C in 1 mL sterile distilled water; they were then centrifuged at 14000 × g for 10 min. The supernatants were recovered and immediately used for H2O2 quantification using the xylenol orange method (Jiang et al., 1990), based on the peroxidase-mediated oxidation of Fe2+ followed by the reaction of Fe3+ with xylenol orange o-cresolsulfonephtalein 3′-3′-bis[methylimino] diacetic acid, sodium salt. H2O2 was determined by adding 250 μL of distilled H2O to 250 μL of supernatant and 500 μL of assay reagent (500 μM ammonium ferrous sulphate, 50 mM H2SO4, 200 μM xylenol orange, and 200 mM sorbitol). Absorbance of the Fe3+-xylenol orange complex (A560) was detected after 45 min. The specificity for H2O2 was tested by eliminating H2O2 in the reaction mixture with catalase. Standard H2O2 curves were obtained by adding increasing amounts of H2O2 from 0 to 100 nmoles. Data were expressed as μmoles g−1 pollen fresh weight (FW).
2.10. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis and immunoblotting
Pollen grains (130 mg) were incubated in 1 mL sterile distilled water and shaken gently for 30 min at 4 °C. The pollen suspensions were then centrifuged at 14000 × g for 10 min, and the soluble protein was measured as reported above. Crude ragweed pollen extract (10 μg total protein) and 6 μg natural Amb a 1 (nAmb a 1), kindly provided by Prof. T. P. King (Rockefeller University, NY, USA), were separated by denaturing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and visualised by staining with Coomassie brilliant blue R-250 (Laemmli, 1970). For immunoblotting analysis, the protein was electroblotted onto nitrocellulose membranes (Protan, Schleicher & Schuell, Dassel, Germany) and then incubated with mouse monoclonal anti-Amb a 1 antibodies for 2 h. These mouse monoclonal anti-Amb a 1 antibodies (clone E10) were obtained by immunisation of mice with the purified recombinant Amb a 1 protein (rAmb a 1), as described by Wopfner et al. (2007). After washing, the membranes were incubated with alkaline-phosphatase-conjugated anti-mouse IgG (1/1000; Sigma–Aldrich), for 1 h at room temperature. Labelling was detected using the NBT/bromo-chloro-indolyl phosphate substrate (Sigma–Aldrich). For quantification, membranes were scanned and band intensities were determined with the image analysis software ImageJ (http://rsb.info.nih.gov/ij).
2.11. Reverse transcription-polymerase chain reaction analysis
The expression of the Amb a 1.1, Amb a 1.4, Amb a 2, Profilin 1 and Profilin 2 allergens and of the house-keeping 18S rRNA were analysed by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted from the control and O3-treated pollen grains using NucleoSpin RNA Plant (Macherey–Nagel, Düren, Germany), with pollen samples (30 mg) ground in a mortar under liquid nitrogen, and processed according to the manufacturer instructions. Both the quality and concentration of the total RNA were assessed by non-denaturing agarose gel electrophoresis and by measuring A260/A280. The RNA was immediately processed for RT-PCR or stored at −80 °C. A given amount of total RNA (1 μg) was reverse transcribed, as described in Pasqualini et al. (2009). Images of the RT-PCR ethidium bromide-stained agarose gels were acquired with a digital Kodak camera (Eastman Kodak Company, Rochester, NY, USA) and quantification of the bands was performed with Kodak 1D image analysis software.
2.12. Statistical analysis
The O3-fumigation experiment was replicated 3 times, and for each experiment, three replicates were used. One or two-way analysis of variance (ANOVA) was applied to the data reported in the Figures, as detailed in the Figure legends. The means were compared using Duncan’s multiple range tests, and the values followed by different letters are significantly different at p ≤ 0.01.
3. Results
3.1. Pollen viability following O3 fumigation
The data concerning the ragweed pollen viability after 7 days of O3 fumigation are shown in Fig. 1A. The mean pollen viability in the non-fumigated pollen (control) was 55.9%, while fumigation with O3 significantly decreased this pollen viability (39%; p < 0.01). In non-fumigated ragweed pollen, we measure a rather low viability; nevertheless, that the pollen used for the experiments is a commercial pollen must be taken into account, as it was purchased from a company and kept at +4 °C for more than 4 months before we carried out the viability assays. Many reports have shown loss of viability of pollen and loss of membrane integrity during pollen storage (Jain and Shivanna, 1989). However, although the use of a commercial pollen did not allow us to work with pollen that had a high viability, it provided good homogeneity of the samples and the possibility for other groups to reproduce these experiments. In addition, the objective difficulty of the need to collect a sufficient amount of pollen for the analyses needs to be considered, particularly as ragweed grows only sporadically in central Italy.
Fig. 1.
Pollen viability (A), H2O2 content (B), ROS (C) and NAD(P)H oxidase activity (D) in control non-fumigated (−O3) and O3-fumigated (+O3) pollen grains. The NAD(P)H oxidase activity was assayed in the absence and presence of NADH and NADPH (as indicated). The data are means ± SE of three independent experiments. One-way analysis of variance (ANOVA) was applied to the data reported in (A), (B) and (C), while two-way analysis was applied to the data in (D). Different letters indicate statistically different means (p ≤ 0.01).
3.2. Reactive oxygen species detection in hydrated pollen grains
As the toxicity of O3 is due to its ability to generate potentially lethal ROS, we evaluated the release of H2O2 from pollen grains after this O3 fumigation as a possible cause of an oxidative burst in the pollen. The release of H2O2 from pollen grains was evaluated by analysing the liquid medium in which the pollen had been incubated for 30 min. About 0.9 μmole H2O2 g−1 FW were released from the non-fumigated pollen grains after their hydration, with similar levels detected in the O3-fumigated pollen, showing that O3 fumigation did not significantly influenced the pollen H2O2 content (Fig. 1B). Using fluorescent microscopy and the ROS-sensitive fluorescent probe DCFH2DA, we were able to qualitatively detect ROS inside the pollen. Indeed, this probe lacks specificity among the ROS and reacts only slowly with H2O2 or •O2−, but very fast with the .OH radical and peroxynitrite [ONOO− (Setsukinai et al., 2003)]; it thus follows that this DCF fluorescence is an assay of generalised oxidative stress, rather than of any particular ROS. Our results show that the 7-days of O3 fumigation induced only a small, and non-significant, increase in ROS inside the pollen grains (Fig. 1C).
3.3. NAD(P)H oxidase activity in hydrated pollen grains
The NAD(P)H oxidase in these ragweed pollen grains following the O3 fumigation was determined by NBT reduction to formazan, with this activity completely released after 25 min of hydration (S25 supernatant) (Fig. 1D). For the supernatants obtained from the longer hydrations (S50 and S75) and from the sonication of the pollen pellets (Sson), there was no measurable NAD(P)H oxidase activity. Without NADPH or NADH, NBT reduction by the pollen grains of ragweed was just detectable, with no significant difference seen between the non-fumigated and O3-fumigated pollen grains (Fig. 1 D). On addition of NADH or NADPH, the activities measured from the O3-treated pollen increased by 41% and 21%, respectively, with respect to the non-fumigated pollen (Fig. 1D). This reduction of NBT by the ragweed pollen extract was almost completely blocked by the addition of SOD (absorbance reduction, 82% ± 5%) and of the NAD(P)H oxidase inhibitor DPI (absorbance reduction, 85% ± 3%), which suggests that NAD(P)H oxidase is the major source of these ROS.
3.4. Nitric oxide localisation in hydrated pollen grains
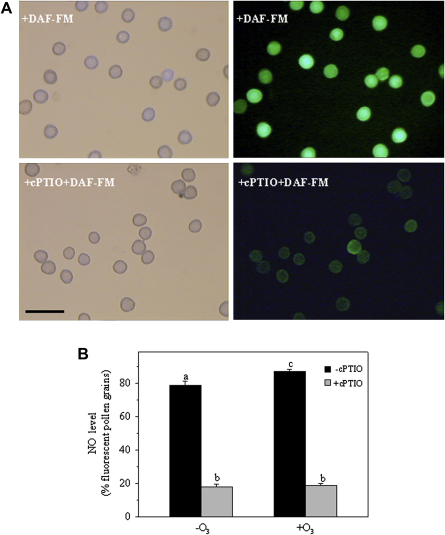
Our data show here that high levels of constitutive NO were detected in the ragweed pollen grains, and that the O3 fumigation induced a small, but significant, increase in NO after 30 min of hydration (Fig. 2 A, B; + 10%, p < 0.01). To ensure that this fluorescence was NO specific, the NO-scavenger cPTIO was added to the probe. In this case, there was significant quenching of the green fluorescence, which indicates that the DAF-FM probe was specific for NO detection (Fig. 2A, B).
Fig. 2.
NO visualisation (A) by fluorescence microscopy and fluorescent percentages (B) in control non-fumigated (−O3) and O3-fumigated (+O3) pollen grains loaded with DAF-FM diacetate. The left-hand images show the corresponding bright-field images. Specificity of the DAF-FM probe is shown by addition of the NO quencher cPTIO. The data are means ± SE of three independent experiments. Two-way analysis of variance (ANOVA) was applied to the data reported in (B). Different letters indicate statistically different means (p ≤ 0.01). Magnification is identical for all of the images: bar, 80 μm.
3.5. Protein release by hydrated pollen grains
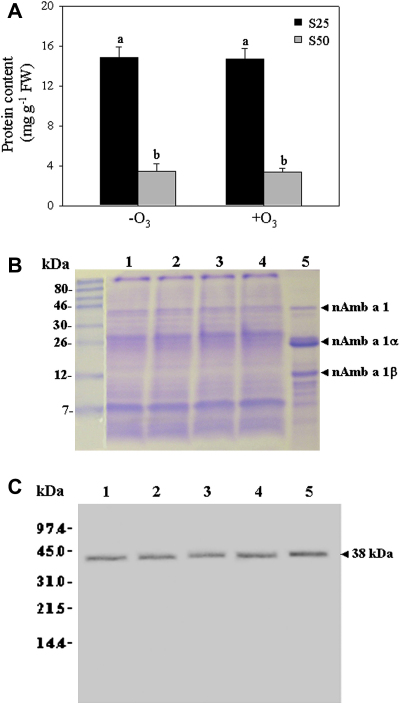
There was a large release of soluble proteins after the first 25 min of pollen hydration (81%), with 14.85 mg protein g−1 FW and 14.71 mg protein g−1 FW detected in the S25 samples of the non-fumigated and O3-fumigated ragweed pollen, respectively (Fig. 3A). Less protein was released from the pollen after 50 min of hydration (S50; 19%). In the supernatants obtained from the further hydration (S75) and from the sonication of the pellets (Sson), there was no detectable soluble protein released. The O3 treatment did not induce significant changes in protein release from the ragweed pollen.
Fig. 3.
Release of protein from the pollen grains (A), SDS-PAGE of pollen extracts (B), and immunoblotting analysis using an anti-Amb a 1 monoclonal antibody (C). A. The data are means ± SE of three independent experiments and two-way analysis of variance (ANOVA) was applied. Different letters identify statistically different means (p ≤ 0.01). B. Representative picture for SDS-PAGE followed by Coomassie brilliant blue staining is shown. The experiment was replicated three times with the same results. Lanes 1 and 3: control non-fumigated pollen (first and second experiments, respectively), lanes 2 and 4: O3-fumigated pollen (first and second experiments, respectively), lane 5: natural Amb a 1. C. Representative Western blotting of expression of the Amb a 1 allergen in crude ragweed pollen extracts loaded as for (B). Molecular weight markers are indicated on the left.
SDS-PAGE of the pollen extracts revealed similar profiles in both control and O3-fumigated pollen grains (Fig. 3B). In all of the extracts the major ragweed allergens ranged from 8 kDa to 43 kDa and the migration band visible at about 38 kDa was identified as Amb a 1. Indeed, purified nAmb a 1 migrated at 38 kDa, and also shows specific degradation into two fragments, of 26 kDa (nAmb a 1 α) and 12 kDa (nAmb a 1 β) (Fig. 3B, lane 5). The crude protein released after 30 min hydration of the pollen was analysed by immunoblotting and probed with a monoclonal antibody against Amb a 1 (Fig. 3C). Here, it has been detected a 38 kDa protein band that corresponded to Amb a 1, the major antigenic component of ragweed pollen, with no quantitative difference between non-fumigated and fumigated pollen grains. Altogether, these data indicate that this 7-day O3 fumigation did not induce changes in the released protein pattern, nor in the content of the major allergen Amb a 1.
3.6. Ozone effects on the expression profile of the major ragweed pollen allergens
The expression of the major ragweed allergens, Amb a 1, Amb a 2, profilin 1 and profilin 2 was analysed by RT-PCR using the primer pairs given in Table 1. Fig. 4 shows that all of these allergens examined are expressed in these ragweed pollen grains, and that the O3 fumigation did not influence the transcript levels, substantially reflecting the unchanged amounts of the Amb a 1 protein detected in the Western blotting (Fig. 3C).
Table 1.
Oligonucleotide primer sequences used in this study for the semiquantitative RT-PCR analysis.
| Gene (Accession number) | Primers used | Annealing temperature (°C) | Number of cycles |
|---|---|---|---|
| Amb a 1.1 (M80558) | FW: 5′-TCCAACGATGGTCCAGCAGC-3′ | 59.7 | 28 |
| BW: 5′-GGGGTTAGCACTGGATCGAC-3′ | |||
| Amb a 1.4 (M80562) | FW: 5′-TTTGACGAGCGAGGCATGCT-3′ | 58.3 | 28 |
| BW: 5′-GGGTTAGCACTGGATCAACC-3′ | |||
| Amb a 2 (M80561) | FW: 5′-AGATGGTCGTAACCAGCGAC-3′ | 59.1 | 30 |
| BW: 5′-TCAGCGGTTAGCACTGGATC-3′ | |||
| Profilin 1 (AY894660) | FW: 5′-TGACATCGAAGGCACTGGTC-3′ | 59.2 | 30 |
| BW: 5′-ACAACCATGTTGCACTGACC-3′ | |||
| Profilin 2 (AY894661) | FW: 5′-TCGTGGCAAACTTACGTGGATG-3′ | 59.4 | 30 |
| BW: 5′-ACCATGTTGCATTGACCAGG-3′ | |||
| 18S rRNA (EF065543) | FW: 5′-TGCAGAATCCCGTGAACCATCG-3′ | 59.4 | 25 |
| BW: 5′-TCATCGCAAGACAATGCGTC-3′ |
FW: forward; BW: backward.
Fig. 4.

Effect of O3 fumigation on the expression profiles of the major allergens of ragweed pollen. Representative picture is shown from an experiment replicated three times with the same results. Lanes 1 and 3: control non-fumigated pollen (first and second experiments, respectively), lanes 2 and 4: O3-fumigated pollen (first and second experiments, respectively). Semi-quantification of mRNA loading was performed by co-amplification and normalisation with an internal standard (18S rRNA).
4. Discussion
In this study, we have sought to simulate the effects of acute O3 exposure on ragweed pollen after its dispersion in the atmosphere. For this purpose, under laboratory conditions, the ragweed pollen was exposed to 5 h of 100 nL L−1 O3 for 7 days, with some of the biochemical and molecular features of the pollen grains then analysed. However, it should also be considered that O3 can influence the pollen not only directly after its dispersion, but also indirectly by affecting the plant growth and the pollen maturation, since ragweed typically grows during the summer when the highest O3 peaks occur. Indeed, it has been reported that in L. perenne plants exposed to chronic O3 concentrations, the percentage of underdeveloped pollen was significantly increased compared to plants grown in filtered air (Schoene et al., 2004). This effect was said to arise from an inhibition of pollen starch accumulation, which was responsible for this disturbance of the pollen development (Schoene et al., 2004). Also, many reports have shown that ROS and NO accumulate in the reproductive tissues, such as the stigma, anthers and pollen, where they have different biological roles, depending on the stage and tissue (McInnis et al., 2006; Hiscock et al., 2007; Zafra et al., 2010). However, whether O3 exposure of the plants during their growth can affect the ROS and NO contents of pollen grains is not at present known.
Our results show a significant decrease in ragweed pollen viability after O3 fumigation. A reduction in pollen viability by O3 has also been reported in other studies and in other species (Feder, 1968, 1981; Mumford et al., 1972). The fluorochromatic reaction used to test pollen viability provides an indirect measurement of esterase activity and the intactness of the plasma membrane (Heslop-Harrison and Heslop-Harrison, 1970). As such, the reduction in pollen viability induced by this O3 fumigation is indicative of damage to the pollen membrane system. Furthermore, the exposure of pollen to a high O3 concentration after its release from the plant can reduce its viability, resulting in decreased fertility, and consequently, in a reduction in seed production.
As irreversible damage to cell membranes can be caused by an O3-induced oxidative burst, we evaluated the ROS production in pollen grains. The results show the presence of constitutive ROS accumulation in the hydrated ragweed pollen, which has been suggested to promote pollen germination and/or pollen-tube growth to the stigma (Potocký et al., 2007). NAD(P)H oxidase is the major source of ROS in pollen grains (Bacsi et al., 2006), which produces the •O2− ion; this is converted into H2O2 through SOD activity or by spontaneous dismutation. The superoxide ion can also act as a reducing agent for transition metals such as Fe3+ and Cu2+, which leads to H2O2-dependent formation of •OH, an extremely aggressive ROS. Hydroxyl radical accumulation in pollen grains can cleave polymers, including cell-wall polysaccharides (Fry, 1998), which promotes the cell-wall loosening that is necessary to allow for the rapid cell elongation of growing pollen tubes (Eckardt, 2005). In fumigated pollens, both the H2O2 released after hydration and ROS localized in pollen grains by the fluorescent probe remained unchanged, indicating that 7 days of O3 fumigation did not induce oxidative stress in the pollen, at least when it was measured at the end of the O3 exposure. Thus the damage to the membrane system that is seen here as decreased pollen viability might be due to other factors in addition to ROS.
As reported above, the main source of cellular ROS is NAD(P)H oxidase. We found that the NAD(P)H oxidase was rapidly released (in less than 30 min) after pollen grain hydration, as indicated by Bacsi et al. (2006), who showed that subpollen particles containing the Amb a 1 allergen and NAD(P)H oxidase activity are released from ragweed pollen grains after 3–5 min of hydration. When ragweed pollen grains were exposed to 7-day O3 fumigation, we saw significant induction of NAD(P)H oxidase enzymatic activity. Thus, as previously observed in leaves of O3-treated plants (Pellinen et al., 1999; Ranieri et al., 2003), O3 can stimulate NAD(P)H oxidase activity also in pollen. There is evidence that ROS have prominent roles in the pathogenesis of allergic diseases (Bowler and Crapo, 2002). Bacsi et al. (2005) indicated that oxidative stress generated by NAD(P)H oxidase in ragweed pollen grains can augment the immediate-type hypersensitivity reactions and pollen-antigen-driven allergic conjunctivitis. Our data here indicate that when ragweed pollen grains are in the presence of O3, there is an induction of NAD(P)H oxidase activity which is released into the hydration medium, and may, under natural conditions, be released into the airway mucosa when pollen grains are inhaled.
NO is a key signalling molecule that controls a variety of physiological responses in plant and animal systems, and it has also been demonstrated to be a signal in plant defence responses against pathogens (Delledonne et al., 1998) and O3 (Ederli et al., 2006; Meier et al., 2009). Recently, it was shown that NO is involved in plant reproductive processes, as it can modulate the growth of the pollen tube and the orientation of the pollen tube towards the stigma (Prado et al., 2004, 2008). It has been suggested that in interacting with H2O2 of the stigmatic papille, NO can regulate the processes of recognition between pollen and stigma (McInnis et al., 2006). The presence of NO has been reported in hydrating pollen grains of various angiosperms (Bright et al., 2009); in that study, NO and nitrites were shown to be released after hydration of the pollen, with greater levels of release seen for allergenic species compared to non-allergenic pollens in the limited number of taxa examined. Our results show that the ragweed pollen constitutively accumulates large amounts of NO. Ozone induces a weak, but significant increase in the NO fluorescence signal, suggesting that the NO acts as a signal molecule in response to stress also in the pollen. As NO can produce ONOO− when in the presence of the •O2− ion (Wendehenne et al., 2001), we hypothesise that the fluorescent signal detected in the pollen grains loaded with DCFH2DA was attributable to the •OH radical, which was potentially derived through the Fenton reaction from NAD(P)H-oxidase-generated •O2−, and to the ONOO−.
The major allergenic proteins released from ragweed pollen ranged from 8 kDa to 43 kDa in all of the extracts examined, in agreement with results previously reported (Weber and Nelson, 1985). After O3 fumigation, the protein release by hydrated pollen grains was rapid, with similar protein pattern profiles in both control and O3-fumigated pollen. The major antigenic component of ragweed pollen, Amb a 1, was identified as a band visible at about 38 kDa, and Western blotting analysis revealed that its content did not change after O3 exposure. We also examined the expression profiles of the major ragweed pollen allergens: Amb a 1 and Amb a 2, which are proteins belonging to the pectate lyase family (Rafnar et al., 1991), and profilin 1 and profilin 2, which are proteins involved in signal transduction from the outer cell membrane to the inside of the cell, and in the regulation of actin polymerisation (Goldschmidt-Clermont et al., 1990). The profilins are defined as panallergens, as they are widespread among the pollens of plants that are both closely and distantly related botanically (Van Ree et al., 1992). Our data show that O3 exposure did not affect the expression of the major ragweed pollen allergens. Earlier studies concerning the effects of gaseous pollutants on the content of allergenic proteins provided no conclusive results. Recently, it has been reported that pollen extracts from O3-fumigated plants showed increased allergen content and higher IgE reactivity (Masuch et al., 1997; Eckl-Dorna et al., 2010). On the other hand, exposure of timothy grass pollen to gaseous pollutants induced a decrease in allergen detection in pollen extracts (Rogerieux et al., 2007). Bryce et al. (2010) used a proteomic approach to show that the major allergens of birch (Bet v 1, 2, 3 and 4) were not expressed differentially in their samples from rural and urban areas, but that the extracts collected in urban areas had higher chemotactic activities on human neutrophils. They then suggested that the greater allergenicity of pollen collected from polluted areas was determined by more than just the allergen content (Bryce et al., 2010). Our data support the concept that when ragweed pollen is exposed to O3 during its time in the atmosphere, it can suffer damage that is seen as decreased pollen viability, and it can become more allergenic through stimulation of inflammatory ROS-generating NAD(P)H oxidase, which would be released when pollen comes into contact with the cells of the respiratory apparatus.
Acknowledgements
This study was supported by the Italian Ministry of Environment (MAT09) for G.F. and by the Austrian Science Foundation FWF (T411-B12) for N.W.
References
- Asero R. Birch and ragweed pollinosis north of Milan: a model to investigate the effects of exposure to new airborne allergens. Allergy. 2002;57:1063–1066. doi: 10.1034/j.1398-9995.2002.23766.x. [DOI] [PubMed] [Google Scholar]
- Asero R. Analysis of new respiratory allergies in patients monosensitized to airborne allergens in the area north of Milan. Journal of Investigational Allergology and Clinical Immunology. 2004;14:208–213. [PubMed] [Google Scholar]
- Bacsi A., Dharajiya N., Choudhury B.K., Sur S., Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. Journal of Allergy and Clinical Immunology. 2005;116:836–843. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacsi A., Dharajiya N., Choudhury B.K., Sur S., Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. Journal of Allergy and Clinical Immunology. 2006;118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett I.J., Crompton C.W. The biology of Canadian weeds: 11. Ambrosia artemisiifolia L. and A. psilostachya DC. Canadian Journal of Plant Sciences. 1975;55:463–476. [Google Scholar]
- Benoit L.F., Skelly J.M., Moore L.D., Dochinger L.S. The influence of ozone on Pinus strobus L. pollen germination. Canadian Journal of Forest Research. 1983;13:184–187. [Google Scholar]
- Bernstein J.A., Alexis N., Barnes C., Bernstein I.L., Nel A., Peden D., Diaz-Sanchez D., Tarlo S.M., Williams P.B. Health effects of air pollution. Journal of Allergy and Clinical Immunology. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Boldogh I., Bacsi A., Choudhury B.C., Dharajiya N., Alam R., Hazra T.K., Mitra S., Goldblum R.M., Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. Journal of Clinical Investigation. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler R.P., Crapo J.D. Oxidative stress in allergic respiratory diseases. Journal of Allergy and Clinical Immunology. 2002;110:349–356. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bright J., Hiscock S.J., James P.E., Hancock J.T. Pollen generates nitric oxide and nitrite: a possible link to pollen-induced allergic responses. Plant Physiology and Biochemistry. 2009;47:49–55. doi: 10.1016/j.plaphy.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Bryce M., Drews O., Schenk M.F., Menzel A., Estrella N., Weichenmeier I., Smulders M.J., Buters J., Ring J., Görg A., Behrendt H., Traidl-Hoffmann C. Impact of urbanization on the proteome of birch pollen and its chemotactic activity on human granulocytes. International Archives of Allergy and Immunology. 2010;151:46–55. doi: 10.1159/000232570. [DOI] [PubMed] [Google Scholar]
- Butkovic V., Cvitas T., Klasinc L. Photochemical ozone in the Mediterranean. Science of the Total Environment. 1990;99:145–151. [Google Scholar]
- Cecchi L., Torrigiani Malaspina T., Alberini R., Zanca M., Ridolo E., Usberti I., Morabito M., Dall’Aglio P., Orlandini S. The contribution of long-distance transport to the presence of Ambrosia pollen in central northern Italy. Aerobiologia. 2007;23:145–151. [Google Scholar]
- Corsico R., Falagiani P., Ariano R., Berra D., Biale C., Bonifazi F., Ciampi P., Feliziani V., Fringuelli G., Galimberti M., Gallesio M.T., Liccardi G., Lo reti A., Marcer G., Marcucci F., Meriggi A., Minelli M., Nardelli R., Nardi G., Negrini C.A., Papa G., Piu G., Pozzan M., Purello D’Ambrosio F., Riva G. An epidemiological survey on the allergological importance of some emerging pollens in Italy. Journal of Investigational Allergology and Clinical Immunology. 2000;10:155–161. [PubMed] [Google Scholar]
- D’Amato G. Environmental urban factors (air pollution and allergens) and the rising trends in allergic respiratory diseases. Allergy. 2002;67:30–33. doi: 10.1034/j.1398-9995.57.s72.5.x. [DOI] [PubMed] [Google Scholar]
- D’Amato G., Spieksma F.T.H., Liccardi G. Pollen related allergy in Europe. Allergy. 1998;53:567–578. doi: 10.1111/j.1398-9995.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Delledonne M., Xia Y.J., Dixon R.A., Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Directive 2002/3/EC . 2004. Directive 2002/3/EC of the European Parliament and of the Council of 12 February 2002 Relating to Ozone in Ambient Air.http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:067:0014:0030:EN:PDF [Google Scholar]
- Eckardt N.A. VANGUARD1_ At the forefront of pollen tube growth. The Plant Cell. 2005;17:327–329. [Google Scholar]
- Eckl-Dorna J., Klein B., Reichenauer T.G., Niederberger V., Valenta R. Exposure of rye (Secale cereale) cultivars to elevated ozone levels increases the allergen content in pollen. Journal of Allergy and Clinical Immunology. 2010;126:1315–1317. doi: 10.1016/j.jaci.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Ederli L., Morettini R., Borgogni A., Wasternack C., Miersch O., Reale L., Ferranti F., Tosti N., Pasqualini S. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiology. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder W.A. Reduction in tobacco pollen germination and tube elongation induced by low levels of ozone. Science. 1968;160:1122. doi: 10.1126/science.160.3832.1122. [DOI] [PubMed] [Google Scholar]
- Feder W.A. Bioassaying for ozone with pollen system. Environmental Health Perspective. 1981;37:117–123. doi: 10.1289/ehp.8137117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S.C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochemical Journal. 1998;332:507–515. doi: 10.1042/bj3320507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabusi V., Volta M. Seasonal modelling assessment of ozone sensitivity to precursors in northern Italy. Atmospheric Environment. 2005;39:2795–2804. [Google Scholar]
- Gilmour M.I., Jaakkola M.S., London S.J., Nel A.E., Rogers C.A. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environmental Health Perspectives. 2006;114:627–633. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P.J., Machesky L.M., Baldassare J.J., Pollard T.D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990;247:1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J., Heslop-Harrison Y. Evaluation of pollen viability by induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technology. 1970;45:115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- Hiscock S.J., Bright J., McInnis S.M., Desikan R., Hancock J.T. Signaling on the stigma. Potential new roles for ROS and NO in plant cell signaling. Plant Signaling & Behavior. 2007;2:23–24. doi: 10.4161/psb.2.1.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Shivanna K.R. Loss of viability during storage is associated with changes in membrane phospholipids. Phytochemistry. 1989;28:999–1002. [Google Scholar]
- Jiang Z.J., Woollard A.C.S., Wolff S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Letters. 1990;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- Kojima H., Nakatsubo N., Kikuchi K., Kawaahara S., Kirino Y., Nagoshi H., Hirata Y., Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Analytical Chemistry. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrioli P., Di Cecco M., Andina G. Ragweed pollen: the aeroallergen is spreading in Italy. Aerobiologia. 1998;14:13–20. [Google Scholar]
- Masuch G., Franz J.-T.H., Schoene K., Müsken H., Bergmann K.-C.H. Ozone increases group 5 allergen content of Lolium perenne. Allergy. 1997;52:874–875. doi: 10.1111/j.1398-9995.1997.tb02163.x. [DOI] [PubMed] [Google Scholar]
- McInnis S.M., Desikan R., Hancock J.T., Hiscock S.J. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signaling crosstalk? New Phytologist. 2006;172:221–228. doi: 10.1111/j.1469-8137.2006.01875.x. [DOI] [PubMed] [Google Scholar]
- Meier S., Madeo L., Ederli L., Donaldson L., Pasqualini S., Gehring C. Deciphering cGMP signatures and cGMP-dependent pathways in plant defence. Plant Signaling and Behavior. 2009;4:307–309. doi: 10.4161/psb.4.4.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen E., Vapaatal H. Nitric oxide in inflammation and immune response. Annals of Medicine. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- Mudd J.B. Biochemical basis for the toxicity of ozone. In: Yunus M., Iqba M., editors. Plant Response to Air Pollution. Wiley & Sons; New York: 1997. pp. 267–284. [Google Scholar]
- Mumford R.A., Lipke H., Laufer D.A. Ozone-induced changes in corn pollen. Environmental Science and Technology. 1972;6:427–430. [Google Scholar]
- Nel A.E., Diaz-Sanchez D., Ng D., Hiura T., Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. Journal of Allergy and Clinical Immunology. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- Oswalt M.L., Marshall G.D., Jr. Ragweed as an example of worldwide allergen expansion. Allergy, Asthma, and Clinical Immunology. 2008;4:130–135. doi: 10.1186/1710-1492-4-3-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., De Marco A., Racalbuto S. Why should we calculate complex indices of ozone exposure? Results from Mediterranean background sites. Environment Monitoring and Assessment. 2007;128:19–30. doi: 10.1007/s10661-006-9412-5. [DOI] [PubMed] [Google Scholar]
- Pasqualini S., Meier S., Gehring C., Madeo L., Fornaciari M., Romano R., Ederli L. Ozone and nitric oxide induce cGMP dependent and independent transcription of defence genes in tobacco. New Phytologist. 2009;181:860–870. doi: 10.1111/j.1469-8137.2008.02711.x. [DOI] [PubMed] [Google Scholar]
- Pellinen R., Palva T., Kangasjärvi J. Subcellular localization of ozone-induced hydrogen peroxide in birch (Betula pendula) leaf cells. Plant Journal. 1999;20:349–356. doi: 10.1046/j.1365-313x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- Pignatti M. vol. III. Edagricole; Bologna: 1997. (Flora D’Italia). 60–61. [Google Scholar]
- Potocký M., Jones M.A., Bezvoda R., Smirnoff N., Žárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytologist. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- Prado A.M., Portefield D.M., Feijó J.A. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- Prado A.M., Colaco R., Moreno N., Silva A.C., Feijó J.A. Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signaling. Molecular Plant. 2008;1:703–714. doi: 10.1093/mp/ssn034. [DOI] [PubMed] [Google Scholar]
- Rafnar T., Griffith U., Kuo M.C., Bond J.F., Rogers B.L., Klapper D.G. Cloning of Amb a 1 (antigen E), the major allergen family of short ragweed pollen. Journal of Biological Chemistry. 1991;266:1229–1236. [PubMed] [Google Scholar]
- Ranieri A., Castagna A., Pacini J., Baldan B., Mensuali Sodi A., Soldatini G.F. Early production and scavenging of hydrogen peroxide in the apoplast of sunflower plants exposed to ozone. Journal of Experimental Botany. 2003;54:2529–2540. doi: 10.1093/jxb/erg270. [DOI] [PubMed] [Google Scholar]
- Ridolo E., Alberini R., Giordano D., Soliani L., Usberti I., Dall’Aglio P.P. Airborne pollen concentration and the incidence of allergic asthma and rhinoconjunctivitis in northern Italy from 1992 to 2003. International Archives of Allergy and Immunology. 2006;142:151–157. doi: 10.1159/000096441. [DOI] [PubMed] [Google Scholar]
- Rogerieux F., Godfrin D., Sénéchal H., Motta A.C., Marlière M., Peltre G., Lacroix G. Modifications of Phleum pratense grass pollen allergens following artificial exposure to gaseous air pollutants (O3, NO2, SO2) International Archives of Allergy and Immunology. 2007;143:127–134. doi: 10.1159/000099079. [DOI] [PubMed] [Google Scholar]
- Schoene K., Franz J.-Th., Masuch G. The effect of ozone on pollen development in Lolium perenne L. Environmental Pollution. 2004;131:347–354. doi: 10.1016/j.envpol.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Setsukinai K., Urano Y., Kakinuma K., Mmajima H.J., Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. Journal of Biological Chemistry. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- Traidl-Hoffmann C., Kasche A., Jakob T., Huger M., Plotz S., Feussner I., Ring J., Behrendt H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. Journal of Allergy and Clinical Immunology. 2002;109:831–838. doi: 10.1067/mai.2002.124655. [DOI] [PubMed] [Google Scholar]
- Van Ree R., Voitenko V., Van Leeuwen W.A., Aalberse R.C. Profilin is a cross-reactive allergen in pollen and vegetable foods. International. Archives of Allergy and Immunology. 1992;98:97–104. doi: 10.1159/000236171. [DOI] [PubMed] [Google Scholar]
- Weber R.W., Nelson H.S. Pollen allergens and their interrelationships. Clinical Reviews in Allergy. 1985;3:291–318. doi: 10.1007/BF02992997. [DOI] [PubMed] [Google Scholar]
- Wendehenne D., Pugin A., Klessig D.F., Durner J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends in Plant Science. 2001;6:177–183. doi: 10.1016/s1360-1385(01)01893-3. [DOI] [PubMed] [Google Scholar]
- Wopfner N., Bauer R., Thalhamer J., Ferreira F., Chapman M. Immunologic analysis of monoclonal and immunoglobulin E antibody epitopes on natural and recombinant Amb a 1. Clinical and Experimental Allergy. 2007;38:219–226. doi: 10.1111/j.1365-2222.2007.02872.x. [DOI] [PubMed] [Google Scholar]
- Zafra A., Rodríguez-García M.I., Alché J. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biology. 2010;10:36. doi: 10.1186/1471-2229-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]