Abstract
Estimating the impacts of global and local threats on coral reefs requires monitoring reef health and measuring coral growth and calcification rates at different time scales. This has traditionally been mostly performed in short-term experimental studies in which coral fragments were grown in the laboratory or in the field but measured ex situ. Practical techniques in which growth and measurements are performed over the long term in situ are rare. Apart from photographic approaches, weight increment measurements have also been applied. Past buoyant weight measurements under water involved a complicated and little-used apparatus. We introduce a new method that combines previous field and laboratory techniques to measure the buoyant weight of entire, transplanted corals under water. This method uses an electronic balance fitted into an acrylic glass underwater housing and placed atop of an acrylic glass cube. Within this cube, corals transplanted onto artificial bases can be attached to the balance and weighed at predetermined intervals while they continue growth in the field. We also provide a set of simple equations for the volume and weight determinations required to calculate net growth rates. The new technique is highly accurate: low error of weight determinations due to variation of coral density (< 0.08%) and low standard error (< 0.01%) for repeated measurements of the same corals. We outline a transplantation technique for properly preparing corals for such long-term in situ experiments and measurements.
Keywords: Acropora, Calcification, Coral growth, Coral transplantation, In situ research, Skeletal density
Highlights
► We introduce a method for buoyant weight measurement of corals under water. ► Corals are transplanted with minimal impact onto PVC-plates using epoxy resin. ► Weight is measured with an electronic balance in an underwater housing. ► Errors due to coral density variation and of repeated measurements are very low. ► Long-term and longitudinal studies of coral growth can be performed.
1. Introduction
Coral reefs are experiencing various threats, with pollution, eutrophication, sedimentation, over-exploitation, bleaching and ocean acidification representing the severest (Veron et al., 2009; Wilkinson, 2008). These impacts are deteriorating reefs on the global, local and coral colony level. Accordingly, reef condition is measured on different scales, from monitoring live coral cover on various geographical scales to the investigation of partial mortality and growth rates of coral colonies within single reefs and experiments. Growth rates of dominant reef-building corals are an important proxy for the evaluation of reef health (Cortes and Risk, 1985; Guzman et al., 1994; Guzner et al., 2007). Recent work has proposed that especially large-scale impacts, such as coral bleaching and ocean acidification, will reduce the calcification and growth rates of reef-building corals (see Douglas, 2003; Veron et al., 2009, and references therein). These impacts will particularly affect species that are more susceptible to environmental changes, such as the faster-growing, branching taxa (Baird and Marshall, 2002; McClanahan et al., 2001; McClanahan et al., 2005). Consequently, information about coral growth in the field and in experiments, both under natural conditions and under prevailing stress factors, is essential for estimating the current and future health of coral reefs.
Coral growth studies have employed various techniques, including measurements of the linear extension of entire colonies or branches (e.g., Guzman and Cortes, 1989) or of skeletal growth bands measured by X-radiography (1-dimensional (1D); e.g., Buddemeier et al., 1974; Chalker et al., 1985; Lough, 2008, and references therein), colony size or colony surface area (2D; e.g., Courtney et al., 2007; Laforsch et al., 2008; Veal et al., 2010), and, more recently, computer-tomography (CT) based methods (3D; e.g., Laforsch et al., 2008; Veal et al., 2010). Most of these applications, however, require laboratory measurements. Field applications are rare and include calculations of surface areas from photographs, as proposed by Courtney et al. (2007). Apart from these length-, surface- and volume-based growth measurements, weight increment is another important proxy for growth because it is equivalent to the calcification rates of corals (Davies, 1989; Jokiel et al., 1978, 2008; Kleypas and Langdon, 2006).
All these variables are measured from entire colonies, single branches or experimentally established nubbins of reef-building corals. External linear extension is measured using calipers or tape. This approach is commonly based on a reference point, which, for example, is set by staining the skeleton of a live coral with Alizarin Red-S (Barnes, 1970). Advanced, impact-free methods for measuring length increments include in situ laser-measurements (Vago et al., 1997). While very accurate for short-term measurements, this is expensive, requires high technical skills, and is unsuitable for measurements over periods of weeks, months or even years. A drawback of length increments in general is that they neglect differences in coral skeleton densities: the latter varies both inter- and intraspecifically depending on factors such as age, exposure to waves and nutrients, and differences in water temperature and pH (Edinger et al., 2000; Lough, 2008). Measuring internal linear extension rates and skeletal density using X-rays may overcome this problem (Buddemeier et al., 1974), although this technique destroys the colony and yields only a single set of values. Weight increase is another opportunity to quantify coral growth apart from length increments or linear extension rates. This can be performed on parts of colonies, such as nubbins, or on entire corals. A common and highly accurate technique is the buoyant weight technique, which can be performed in the field or laboratory (Bak, 1973; Davies, 1989). The advantage of measuring weight is that it incorporates both size and skeletal density. Weight increments in coral nubbins, as commonly determined in short-term experiments, can yield very accurate data but is time-consuming and must be done in the laboratory (Davies, 1989). Measuring coral growth in the field is crucial for estimating the impact of environmental changes on colony growth over long periods and for experiments which cannot be performed in the laboratory. A buoyant weight technique for field application was developed by Bak (1973). However, the apparatus proposed by this author is big and relatively difficult to handle, possibly restricting its use.
As in the case of several other coral growth measurement techniques, determination of buoyant weight increments requires detaching the colonies from the reef, i.e. coral transplantation techniques. In the last two decades, transplantation of entire corals or parts of colonies was mainly considered to be a tool for reef restoration or damage prevention (Edwards and Clark, 1998). It has recently gained importance for ecological experiments that focused on coral growth (e.g., Guzner et al., 2007; Jokiel et al., 2008; Renegar and Riegl, 2005). Various detachment and reattachment techniques have been applied. The latter include fixation by epoxy resin or cement, and tying to steel or plastic frames (e.g., Dizon and Yap, 2006; Guzner et al., 2007; Renegar and Riegl, 2005; Thornton et al., 2000). The success rates vary considerably.
Here, we present a relatively cheap and practical technique that yields exact weights of large parts of colonies or even entire corals in the field. It includes a feasible method of coral transplantation and basically combines the techniques proposed by Bak (1973), Jokiel et al. (1978) and Davies (1989). It consists of a micro-balance within an underwater housing. The housing is placed on top of an aluminum/acrylic glass cube in which a coral colony can be repeatedly measured for its buoyant weight in situ over long growth periods. This technique requires corals to be detached from their natural substrate and transplanted onto artificial bases, steps that we tested for two species of Acropora. A series of test trials indicates that the method is feasible for any long-term field study on coral growth.
2. Material and methods
2.1. Transplantation of corals
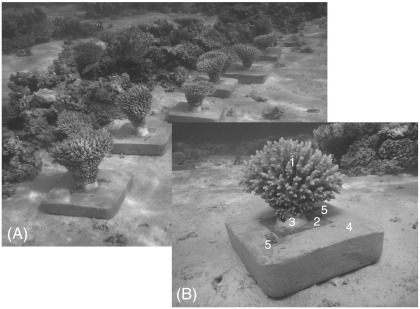
Experiments were performed in a shallow reef (1 to 2 m deep) close to Dahab (Sinai, Egypt) in the Gulf of Aqaba, northern Red Sea and started in October 2009. We used a non-destructive technique to transplant entire colonies (approximately 25 cm diameter) of two species of (corymbose) branching Acropora, Acropora digitifera and Acropora selago. Prior to transplantation, orientation (cardinal direction) of colonies should be noted to maintain the same light exposure after transplantation. Although it complicates weight increment measurements (explained in detail below), we preferred a non-invasive approach. Accordingly, we cut corals from their natural substrate with a tungsten steel-wire saw (large bow and 30 cm wire) such that the cut went through the coral rock close to the colony's base and not through live tissue. Since we move the transplants several times, we chose sturdy materials (5-mm-thick PVC-plates) as new, artificial bases. The corals were then fixed onto the square, 10 × 10 or 12 × 12 cm plates using 2-component epoxy resin (Reef Construct by Aqua Medic). Prior to coral transplantation, the PVC-plates were prepared with epoxy resin, which was applied on the upper side of the plate as a central, radial, piece with longitudinal undercuts. This form of resin covered and filled four 5-mm holes drilled through the central area (one in the center and three around it) of the plate, embedding a 1.5-cm-long section of a 4 mm-thick stainless steel screw in the center hole. The center hole was countersunk on the underside of the plate to accommodate the screw's head. The detached corals were glued to the PVC-plates with a freshly mixed drop of epoxy resin. This was done out of the water, but corals were not exposed to air for more than a few minutes, and were kept wet and shaded. In the following, this unit – consisting of strongly connected coral colony and PVC-plate – is termed transplant (Fig. 1). After hardening of the resin (minimum of 2 h), each transplant was attached to a larger (W × L × H = 28 × 35 × 5 cm) concrete block with a square recess to host the PVC-plate. Two slender and flat (W × L = 1.5 × 5 cm, 1 mm thick), steel platelets were fixed to the concrete block close to the recess with stainless steel screws and plastic dowels; the latter were sunk into the concrete blocks when these were poured. When the screw was loosened, the platelets could be moved over the PVC-plate to hold the transplant (Fig. 1). These platelets provided a very good fixation of the transplant, yet permitting easy removal for weighing procedures. The hexagonal screw head facilitated loosening the platelets even after months in seawater.
Fig. 1.
Transplantation of entire coral colonies for weight increment measurements: (A) a series of transplanted Acropora selago colonies. (B) Colony of Acropora digitifera (1) mounted on a PVC-plate (2) using epoxy resin (3). The transplant was fixed to a large concrete base (4) with two metal platelets (5) that were mounted to the base with stainless steel screws and plastic dowels. The PVC-plate and epoxy resin show some epigrowth but no fleshy algae. The elevated concrete base also countered sedimentation.
2.2. Calculations of volumes and buoyant weights of transplants
Coral growth was measured as the proportional increment of colony buoyant weight (BW: weight of an object immersed in a fluid), derived from the BW of the entire transplant. Since BW depends on the density of the immersion fluid, seawater temperature and salinity were repeatedly measured during weighing. These two parameters yield exact seawater density values using the density calculator on the website of the John Hopkins University Applied Physics Laboratory (2006).
The entire BW-technique is based on the principle of Archimedes, which enables deriving several simple equations (designated by letters) for volume and weight determinations: the buoyant force of an object placed in a fluid (e.g. seawater) equals the weight of the fluid displaced by the object. This means that the BW of an object equals the weight in air (AW; volumeobj × densityobj) minus the weight of the displaced fluid (volumeobj × densitywater).
On the one hand, this can be used for volume determination. When an object is immersed in water (e.g., in a container placed on an electronic balance) of known density (D), its volume (V) can be calculated by:
| (A) |
On the other hand, the BW of an object in water can be calculated by the volume of the object multiplied with the density difference between the object and the water:
| (B) |
Initially, we attempted to determine the buoyant weight of the experimental corals by simply determining coral skeleton density and the volume of each colony using the above equations. However, this method was highly inaccurate because of:
-
(1)
Inaccuracy in volume determination: the simplest but most accurate approach is to measure the weight of the water displaced by the colony using Eq. (A). This, however, is difficult, especially in larger corals (i.e. 25 cm diameter) because larger volumes in larger containers are difficult to weigh on a fine balance (which is much more accurate than measuring the overflow into another container). It also includes the bias of adhesive water and mucus release from the large coral surface.
-
(2)
Inaccuracy in coral skeleton density estimation: since experimental corals must remain undamaged, density was determined from conspecific colonies from the same reef area (see below). Although the standard deviation of measurements was small, the minimum and maximum density values were too different to accurately predict an experimental colony's density from the species' mean (error: ~ 6% between mean and min/max density in the two Acropora species used).
We therefore chose another method to determine coral BW indirectly, by subtracting the BWs of the non-coral components of the basis (with constant densities and volumes) from the BW of the entire transplant. All our coral transplants contained a piece of coral rock, a PVC-plate with epoxy resin and a metal screw. These components contributed to the weight of the non-coral basis. To obtain the BW of this basis, we first determined its volume using Eq. (A). This was performed on the shore to minimize transportation and air exposure time. We immersed the basis into a 10-liter container (30 cm diameter) filled with about 8 l of seawater (placed on an electronic balance: Soehnle 67080 Page Profi; max. load 15 kg, readability 1 g) up until the boundary between rock and the living colony tissue and noted the weight increase. To calculate water density, we measured temperature and salinity before each weighing. To calculate the contribution of each component of the basis (PVC-plate, screw, epoxy resin and coral rock) to the BW of the transplant, we included their volumes and densities. PVC and the stainless-steel screws had a known density (1.4 and 7.9 g cm− 3, respectively) and their volumes were measured from their dimensions (PVC-plate) or determined using Eq. (A) (screw; electronic balance Kern AEMB-600-2; max. load 600 g, readability and accuracy 0.01 g). The density of a piece of hardened epoxy resin – calculated from its volume (determined by immersion in water and using Eq. (A)) and its weight in air (AW) using the same balance (Kern AEMB-600-2) – was 1.74 g cm− 3. The same procedure was performed with the transplant's coral rock base, whereby 10 randomly chosen pieces of coral rock from nearby substrate were measured; their mean density (2.71 g cm− 3 ± 0.02 S.D.) was used for further calculations. Since the volume of the coral rock and the epoxy were unknown and could not be determined separately from the transplant, we took their overall mean density (2.225 g cm− 3) for the remaining volume (volume of the entire basis minus volumes of PVC-plate and screw). Although this represents the main source of inaccuracy, we consider the error as minimal because we attempted to keep the volume of epoxy resin and coral rock similar to each other. The known volumes and densities of the components enabled calculating their BW in water of any density. Subtracting the sum of these values (= BW of the entire coral basis) from the BW of the entire transplant yielded the BW of the coral colony.
2.3. Calculation of baseline buoyant weight for different water densities
Since different water densities (salinity- and temperature-dependent) yield different BWs of corals, new baseline BWs need to be calculated in all subsequent weighings to determine the actual coral weight increment. If coral density approximates double water density, the new baseline BW (BW1) at a different water density can be roughly estimated by:
| (C) |
whereby the subscript numbers 0 and 1 represent values at the first and subsequent field determination of BW, respectively.
The error of this equation increases with the difference between object density and water density and between water densities. Although the error was < 0.06% (~ 0.4 g) assuming a maximum annual water density difference for the northern Red Sea of 6 g l− 1, a coral BW of about 700 g and a coral density of 2.17 g cm− 3, it can be eliminated by employing the equation:
| (D) |
This equation normalizes growth rates by calculating coral BW at a constant water density (e.g., density during the first weighing). It helps calculate growth rates across all measurement periods but requires determining coral density. We sampled five large main branches from each of five colonies of A. selago and A. digitifera of the same reef area and of approximately the same size (25–30 cm greatest diameter). Since the study was proposed as a longitudinal growth study of live corals, we measured colony density (skeleton + tissue) instead of the more time-consuming skeletal density (Davies, 1989). This involved removing excess water (vigorously shaking the coral branch) but not tissue before weighing. After determining their BW in seawater of known density and their AW in the laboratory (balance used: Kern AEMB-600-2), coral density was calculated with an equation provided by Davies (1989; page 390, equation 4):
| (E) |
These measurements yielded density values (g cm− 3) of 2.175 (mean ± 0.059 S.D.; range 2.038–2.318) for A. digitifera and of 2.008 (mean ± 0.060 S.D.; range 1.890–2.144) for A. selago. As noted above, coral density varied too much to accurately estimate colony weights based on displacement volume, but was accurate enough to estimate differences in BWs due to different seawater densities. The BW-calculation error due to coral density variation was < 0.08% (~ 0.5 g; assuming a maximum water density difference of 6 g l− 1 and a mean coral BW of about 700 g). This computationally maximized error is usually smaller in actual field measurements (such extreme water density differences are atypical) and therefore neglectable. Thus, mean coral density is feasible for calculating the baseline BW for a particular water density by employing Eq. (D).
2.4. Buoyant weighing apparatus and weight determination of transplants and corals
The BW of the entire transplant was determined with a custom-made apparatus that permits weight measurements under water by employing an electronic balance (Fig. 2). The balance (Kern A440-49-A: max. load: 6000 g, readability and accuracy: 0.1 g, optional battery-supply and underfloor weighing) was installed in an underwater housing made of 20 mm-thick acrylic glass plates and just large enough to fit the balance. The balance was mounted into the housing with small plastic screws; these screws went through two thin metal bars that were mounted to the bottom of the balance, and reached into threads in the bottom plate of the housing. The cover and bottom plate had larger dimensions than the walls of the housing and could be fixed against each other with long stainless-steel threaded rods (4 mm) and nuts (Fig. 2). In the bottom plate we used recessed nuts to maintain a plain bottom plate, while wing nuts were used on top for easy removal of the cover plate. The walls and the bottom were tightly glued against each other. The cover plate bore a groove for an O-ring (4 mm thick) that sealed the cover against the top rims of the walls, and enabled handling the balance. The bottom of the housing had a 20-mm-large threaded hole as an opening for the underfloor weighing hook. For transportation into and out of the water, the opening could be closed with a custom-made, O-ring-equipped plastic plug. The plug was hollow and thus accommodated the underfloor weighing hook, making it unnecessary to attach the hook under water. The hook opening was surrounded by an acrylic glass cylinder (15 cm diameter, 10 cm high and 5 mm thick), glued tightly against the underside of the bottom plate and enabling pressure compensation for plug removal under water. Air (from the regulator or from the lungs) was filled into this cylinder to prevent water from being sucked into the housing when the plug was removed. In addition, a spirit level was attached to the inner side of the top plate to facilitate horizontal positioning of the apparatus. The entire housing was positioned on a large cube (0.5 m edge length) made of aluminum steel edges (20 mm wide) and acrylic glass walls (5 mm thick). The center of the top plate of the cube had a 16 cm-hole to fit the pressure compensation cylinder of the housing. Two 5 mm-holes in the top plate fitted two longer, threaded rods at diagonally opposite corners of the housing; these rods connected housing and cube with wing nuts. The front plate of the cube was removable and fixed to the cube through large-headed aluminum screws positioned in the aluminum edges. Pear-shaped holes in the front plate allowed quick attachment and removal.
Fig. 2.
Apparatus for buoyant weight determination of entire coral colonies in the field: the laboratory balance (1) is positioned within an acrylic glass housing (2). On the bottom of the housing, a pressure compensation cylinder (3) is mounted and filled with air. A plastic-coated copper wire (4) extends the underfloor weighing hook and holds the transplanted coral (5) during the weighing procedure. The housing is placed on top of a large cube (6), made of an aluminum-frame with acrylic glass walls and leaving enough space for manipulating the coral. To minimize disturbance during weighing, a removable front plate (7) is mounted on the cube with stainless steel screws on the frame and pear-shaped holes in the plate (8). Lead weights (9) on top of the cube stabilized the apparatus.
The balance was equipped with a battery and was turned on (and auto-off-function turned off) before deployment. The cube was placed in a sandy area (shallow-water, lagoonal reef flat study site), leveled horizontally and its bottom edges well covered by sand. This limited the effects of water current and waves on the weighing procedure (i.e. stable values on the display). The housing's strong positive buoyancy required attaching ~ 15 kg of lead weight to the top of the cube and housing. The pressure compensation cylinder was then filled with air, the bottom plug removed, and the cylinder filled with air again. A plastic-coated, V-shaped copper-wire with inwards bent ends was mounted to the projecting hook. This strong wire held the PVC-plate of the transplanted coral at two notches (i.e. transplant weighed up-side-down). The colonies were then detached from the concrete blocks by opening the metal platelets, carefully transported to the weighing cube and fixed to the wire on the hook. After the front plate of the cube was closed, the first stable value shown on the balance (indicated by a special display symbol) was noted. After removing the coral, we also noted the tare value of the balance (which was subtracted from the BW of the transplant). Since coral damage during growth periods is one of the main biases, we photographed the coral from all sides after each weighing to control for breakage. Salinity (0.5‰ accuracy) and temperature (0.1 °C accuracy) were measured simultaneously. To estimate the replicability of BW determinations, we repeated the weighing of four coral colonies (two A. digitifera, one A. selago and one Acropora secale; 320 to 590 g BW-range) ten times for each colony on the same day and calculated the standard error of measurements for each colony.
In the laboratory, water density was determined first. Then the net BW of the coral was calculated by subtracting the sum of the BWs of the PVC-plate, screw, and coral rock + epoxy resin from the BW of the entire transplant, as described above. Repeated measurements of corals in seawater of different densities require calculating not only the new BWs of the basis components but also the new baseline BW of the coral using Eqs. (B) and (D). Subtracting their sum from the BW of the entire transplant then yields coral net growth. Depending on the measurement intervals, the artificial bases need to be cleaned from aufwuchs before the transplant is re-weighed, and infauna needs to be removed (especially not neutrally buoyant organisms such as snails or crabs).
3. Results and discussion
3.1. Coral transplantation and survival rates
Our approach of removing entire colonies without harming the coral tissue yielded very successful coral survival rates. Our entire set of colonies survived. This included 8 colonies (four of each species) transplanted in a preliminary study in October 2009 and 24 colonies (12 of each species) transplanted in April 2010. The last check in January 2011 revealed that all colonies were still in very good condition, i.e. a survival rate of 100% after 15 and 9 months, respectively. In another Acropora transplantation experiment, with more intensive colony manipulation and final attachment to steel frames, survival rates were only about 70% even after a considerably shorter period (Guzner et al., 2007). When only parts of Acropora colonies instead of entire corals were transplanted, survival rates dropped even lower (Yap et al., 1992). Although our technique yields very good results in terms of coral survival, the colony removal – using a steel-wire saw instead of a hammer and chisel – may be difficult or impossible for strongly attached and wide-based growth forms or for poorly accessible colonies. Our approach is therefore better suited for solitary and narrow-based corals. Those small-scale experiments requiring entire coral colonies will benefit from this time-consuming but impact-free technique of coral detachment. The most difficult task was attaching the coral to the PVC-plate. For example, the fixation of larger colonies with a small basis was difficult because it took about 2 h for the epoxy resin to harden. During this time the corals remained submerged and carefully fixed (e.g., with coral rubble) to maintain the correct position. However, the alternative, namely using cement for fixation, is even more time-consuming (Dizon and Yap, 2006). The much faster-hardening epoxy resin guaranteed a very strong and long-lasting fixation. The robustness of this technique was demonstrated by a strong winter storm in December 2010: it broke single branches of transplanted colonies (positioned in a shallow, lagoonal mid-reef flat) but failed to break any of our colonies from their bases or to overturn them. Hard PVC-plates and epoxy resin yielded another advantage over fixation onto coral rock by cement: almost no overgrowth by algae compared to cement bases. In a parallel experiment, we transplanted about 20 main branches of split Acropora colonies onto small cement disks (8 cm diameter) using the same epoxy resin. These disks were fixed onto concrete bases similar to those used for the entire colonies on PVC-plates. Whereas overgrowth of epoxy was minimal, dense fleshy algae grew on the disks after four months. These algae grew close to the coral branches and very likely affected coral condition and growth. Accordingly, hard PVC seems to deter growth of fleshy algae and is suitable for experimental coral transplantation, in particular since it is also very resistant against seawater and available in a UV-resistant type. The only drawback is the difficult attachment of epoxy resin to the very plain PVC surface, which requires fixation holes in the plate and, optimally, additional fixing materials (e.g., stainless steel screw) to secure a strong connection.
3.2. Technical considerations of weight determination
Bak (1973) held length increments to be bad proxies for growth, especially in corals with complex structure, and to neglect differences in skeletal porosity across different parts of a colony. Moreover, one-dimensional measurements do not incorporate changes in volume or surface, both representing important growth components. It is also difficult to estimate whole-colony growth rates from the length increment of a coral nubbin, since no colony baseline value can be defined. Measuring weight increments of entire colonies helps overcome these problems, despite the inability to measure changes of colony density during growth (e.g., in longitudinal studies) and differences in between-colony density. We show that density differences of conspecific colonies caused only a minimal error in BW-determinations, but density increases or decreases during growth cannot be easily eliminated because both volume and density are integrated variables in the determination of BW. Parallel measurements in the laboratory and field, as suggested by Kleypas and Langdon (2006), may solve several of these extrapolation and estimation problems. Note that altered skeletal density is probably also relevant for coral performance (e.g., increased density reduces vulnerability to breakage). Increased coral fitness with growth is even more important than mere growth increments. The direct assessment of reproductive output (fitness) is more difficult, but the idea of using weight increments as a proxy is also justified by good (positive) correlations between colony surface area and BW (Guzner et al., 2007) and between size (colony area) and reproductive output (Hall and Hughes, 1996).
Our technique has proven feasible for BW determination of live corals in the field and would help to fill in acknowledged gaps in the field data (Kleypas and Langdon, 2006). The weighing apparatus can be set up quickly and is easy to use in the field. It can be operated by a single person, although weighing procedures became highly efficient when two or more people were involved. In shallow water, the applicability of the system is restricted to calm weather conditions. In the northern Red Sea we often experienced strong winds and wave action. On such days, the balance failed to display stable values. The system is also suitable for long-term monitoring of colony growth. The accuracy of repeated measurements (10 times) of four test colonies was very high, yielding standard errors of about 0.01% for each coral.
The present technique has several advantages over other approaches. Bak (1973) already recognized the great advantage of measuring coral growth in situ, which would avoid removing corals from the water, especially when repeated and frequent measurements are required. To overcome this problem, Bak (1973) constructed an underwater buoyant weighing apparatus. That apparatus appeared to be too complicated, prompting us to develop the “balance-in-a-housing” construction. This improvement is much more compact, simple to handle and enables a much faster weighing procedure. Finally, the use of undamaged colonies overcomes the drawbacks of more invasive methods – such as using nubbins for growth measurements – which require several days or even weeks of recovery before measurements can start (e.g., Vago et al., 1997). In the present study, colonies were removed from the water only for attachment to the artificial bases and for volume determination of the latter. Initial and subsequent weight measurements were then performed in situ without any further contact with the corals. We therefore did not encounter deterioration (increased mucus production, tissue damage or bleaching) in any of our transplanted colonies. In contrast, the alternative method of Alizarin Red-S staining can impact coral growth (Dodge et al., 1984), even though this effect may be minimized by short exposure times to alizarin (Dodge et al., 1984; Jokiel et al., 2008). Invasive methods represent a clear problem when experimental conditions in the field or laboratory already put corals under stress. Such approaches – and others such as X-ray-based growth measurements of skeletal bands that require killing the corals – do not permit longitudinal studies of colony growth. Finally, the present technique yields the weight increments of entire colonies. This circumvents the need to extrapolate growth rates from smaller units (e.g., nubbins) to the reproductive entity of the entire coral (Davies, 1989; Jokiel et al., 2008), overcoming probable within-colony variation of growth rates.
Today, combined measurements of coral growth and calcification rates in the field and in the laboratory are urgently needed to gage the effects of large-scale impacts such as increasing coral bleaching frequencies and ocean acidification (Kleypas and Langdon, 2006). Our technique is feasible for measuring growth and calcification of entire colonies in the field over medium (weeks) or long (months to years) periods. This complements laboratory studies on smaller samples and time scales, and adds a new dimension to standard coral transplantation efforts.
Acknowledgments
We are grateful to the Nature Conservation Sector of the Egyptian Environmental Affairs Agency (NCS/EEAA; Moustafa Fouda) for granting research permissions and to the Suez Canal University, Egypt (Magdy El-Alwany) for co-operation. The Dahab Marine Research Center (DMRC; Anne Kloeppel and Andy Tischer) provided laboratory and diving facilities. All custom-made technical constructions were made by Valentin Perlinger. We would like to thank Mohamed Negm and Barbara Gratzer for their support during field work and Michael Stachowitsch for linguistic corrections. Aquamedic GmbH, Germany provided epoxy resin material (Reef Construct). This work was financed by the Austrian Science Fund (FWF; project number P21616-B12). [SS]
References
- Baird A.H., Marshall P.A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002;237:133–141. [Google Scholar]
- Bak R.P.M. Coral weight increment in-situ. A new method to determine coral growth. Mar. Biol. 1973;20(1):45–49. [Google Scholar]
- Barnes D.J. Coral skeletons: an explanation of their growth and structure. Science. 1970;170(3964):1305–1308. doi: 10.1126/science.170.3964.1305. [DOI] [PubMed] [Google Scholar]
- Buddemeier R.W., Maragos J.E., Knutson D.W. Radiographic studies of reef coral exoskeletons: rates and patterns of coral growth. J. Exp. Mar. Biol. Ecol. 1974;14(2):179–200. [Google Scholar]
- Chalker B., Barnes D., Isdale P. Calibration of X-ray densitometry for the measurement of coral skeletal density. Coral Reefs. 1985;4(2):95–100. [Google Scholar]
- Cortes J., Risk M.J. A reef under siltation stress: Cahuita. Costa Rica. Bull. Mar. Sci. 1985;36(2):339–356. [Google Scholar]
- Courtney L.A., Fisher W.S., Raimondo S., Oliver L.M., Davis W.P. Estimating 3-dimensional colony surface area of field corals. J. Exp. Mar. Biol. Ecol. 2007;351(1–2):234–242. [Google Scholar]
- Davies P.S. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989;101(3):389–395. [Google Scholar]
- Dizon R.T., Yap H.T. Effects of coral transplantation in sites of varying distances and environmental conditions. Mar. Biol. 2006;148(5):933–943. [Google Scholar]
- Dodge R.E., Wyers S.C., Frith H.R., Knap A.H., Smith S.R., Cook C.B., Sleeter T.D. Coral calcification rates by the buoyant weight technique: effects of alizarin staining. J. Exp. Mar. Biol. Ecol. 1984;75(3):217–232. [Google Scholar]
- Douglas A.E. Coral bleaching — how and why? Mar. Poll. Bull. 2003;46:385–392. doi: 10.1016/S0025-326X(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Edinger E.N., Limmon G.V., Jompa J., Widjatmoko W., Heikoop J.M., Risk M.J. Normal coral growth rates on dying reefs: are coral growth rates good indicators of reef health? Mar. Poll. Bull. 2000;40(5):404–425. [Google Scholar]
- Edwards A.J., Clark S. Coral transplantation: a useful management tool or misguided meddling? Mar. Poll. Bull. 1998;37(8–12):474–487. [Google Scholar]
- Guzman H.M., Cortes J. Growth rates of eight species of scleractinian corals in the Eastern Pacific (Costa Rica) Bull. Mar. Sci. 1989;44(3):1186–1194. [Google Scholar]
- Guzman H.M., Burns K.A., Jackson J.B.C. Injury, regeneration and growth of Caribbean reef corals after a major oil spill in Panama. Mar. Ecol. Prog. Ser. 1994;105(3):231–241. [Google Scholar]
- Guzner B., Novoplansky A., Chadwick N.E. Population dynamics of the reef-building coral Acropora hemprichii as an indicator of reef condition. Mar. Ecol. Prog. Ser. 2007;333:143–150. [Google Scholar]
- Hall V.R., Hughes T.P. Reproductive strategies of modular organisms: comparative studies of reef-building corals. Ecology. 1996;77(3):950–963. [Google Scholar]
- Johns Hopkins University Applied Physics Laboratory Sea Water Equation of State Calculator. 2006. http://fermi.jhuapl.edu/denscalc.html (January 2011)
- Jokiel P.L., Maragos J.E., Franzisket L. Coral growth: buoyant weight technique. In: Stoddart D.R., Johannes R.E., editors. Coral Reefs: Research Methods. UNESCO monographs on oceanographic methodology; Paris: 1978. pp. 529–542. [Google Scholar]
- Jokiel P.L., Rodgers K.S., Kuffner I.B., Andersson A.J., Cox E.F., Mackenzie F.T. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs. 2008;27(3):473–483. [Google Scholar]
- Kleypas J.A., Langdon C. Coral reefs and changing seawater carbonate chemistry. In: Phinney J.T., Hoegh-Guldberg O., Kleypas J.A., editors. Coastal and Estuarine Studies: Coral Reefs and Climate Change Science and Management. 2006. pp. 73–110. [Google Scholar]
- Laforsch C., Christoph E., Glaser C., Naumann M., Wild C., Niggl W. A precise and non-destructive method to calculate the surface area in living scleractinian corals using X-ray computed tomography and 3D modeling. Coral Reefs. 2008;27(4):811–820. [Google Scholar]
- Lough J.M. Coral calcification from skeletal records revisited. Mar. Ecol. Prog. Ser. 2008;373:257–264. [Google Scholar]
- McClanahan T.R., Muthiga N.A., Mangi S. Coral and algal changes after the 1998 coral bleaching: interaction with reef management and herbivores on Kenyan reefs. Coral Reefs. 2001;19:380–391. [Google Scholar]
- McClanahan T.R., Maina J., Moothien-Pillay R., Baker A.C. Effects of geography, taxa, water flow, and temperature variation on coral bleaching intensity in Mauritius. Mar. Ecol. Prog. Ser. 2005;298:131–142. [Google Scholar]
- Renegar D.A., Riegl B.M. Effect of nutrient enrichment and elevated CO2 partial pressure on growth rate of Atlantic scleractinian coral Acropora cervicornis. Mar. Ecol. Prog. Ser. 2005;293:69–76. [Google Scholar]
- Thornton S.L., Dodge R.E., Gilliam D.S., DeVictor R., Cooke P. Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia. 2000. Success and growth of corals transplanted to cement armor mat tiles in southeast Florida: implications for reef restoration. [Google Scholar]
- Vago R., Gill E., Collingwood J.C. Laser measurements of coral growth. Nature. 1997;386(6620):30–31. [Google Scholar]
- Veal C.J., Holmes G., Nunez M., Hoegh-Guldberg O., Osborn J. A comparative study of methods for surface area and three-dimensional shape measurement of coral skeletons. Limnol. Ocean Ogr.: Methods. 2010;8:241–253. [Google Scholar]
- Veron J.E.N., Hoegh-Guldberg O., Lenton T.M., Lough J.M., Obura D.O., Pearce-Kelly P., Sheppard C.R.C., Spalding M., Stafford-Smith M.G., Rogers A.D. The coral reef crisis: the critical importance of < 350 ppm CO2. Mar. Pollut. Bull. 2009;58(10):1428–1436. doi: 10.1016/j.marpolbul.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Wilkinson C. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre; Townsville, Australia: 2008. Status of coral reefs of the world: 2008. 298 pp. [Google Scholar]
- Yap H.T., Alino P.M., Gomez E.D. Trends in growth and mortality of three coral species (Anthozoa: Scleractinia), including effects of transplantation. Mar. Ecol. Prog. Ser. 1992;83(1):91–101. [Google Scholar]