Abstract
Src-family Kinases (SFKs) participate in the regulation of proliferation, differentiation, apoptosis, autophagy, adhesion, migration, invasion and angiogenesis in normal and cancer cells. Abnormal expression of SFKs has been documented in cancers that arise in breast, colon, ovary, melanocyte, gastric mucosa, head and neck, pancreas, lung and brain. Targeting SFKs in cancer cells has been shown to be a promising therapeutic strategy in solid tumors, particularly in ovarian, colon and breast cancers. Paclitaxel is one of most widely used chemotherapeutic agents for the management of ovarian, breast, lung and head and neck cancers. As a microtubule-stabilizing agent, paclitaxel possesses both mitosis-dependent and mitosis-independent activities against cancer cells. A variety of mechanisms such as deregulation of P-glycoprotein, alteration of tubulin isotypes, alteration of microtubule-regulatory proteins, deregulation of apoptotic signaling pathways, mutation of tubulins and overexpression of copper transporters have been implicated in the development of primary or secondary resistance to paclitaxel. By affecting cancer cell survival, proliferation, autophagy, microtubule stability, motility, and/or angiogenesis, SFKs interact with mechanisms that regulate paclitaxel sensitivity. Inhibition of SFKs can potentiate the anti-tumor activity of paclitaxel by enhancing apoptosis, autophagy and microtubule stability. Based on pre-clinical observations, administration of SFK inhibitors in combination with paclitaxel could improve treatment for ovarian, breast, lung and head and neck cancers. Identification and validation of predictive biomarkers could also permit personalization of the therapy.
Key words: Src, SFK, paclitaxel, dasatinib, ovarian cancer, drug sensitivity, resistance
Introduction
Src-family kinases (SFKs) are a group of membrane-associated non-receptor tyrosine kinases that include c-Src, Fyn, c-Yes, Lyn, Lck, c-Fgr, Blk, Hck and Yrk.1–4 While Lyn, Lck, c-Fgr, Blk and Hck are expressed primarily in hematopoietic lineages, c-Src, Fyn and c-Yes can be detected in a variety of cell types.2,4 In addition to these nine members of the Src-family, there are two functionally related kinases (Csk and Fyb) that modulate the activities of SFKs.5–8 SFKs regulate a number of biological functions such as cell proliferation, survival, differentiation, autophagy, adhesion, motility, invasion and angiogenesis both in normal cells and in cancers.1–4,9 In this review, we will briefly describe the structure, regulation and signaling induced by SFKs, and then focus on the expression of SFKs in ovarian, breast, lung and head/neck cancers, the mechanisms of action and resistance of paclitaxel, and on how SFKs inhibition sensitizes cancer cells to treatment with paclitaxel.
Structure and Regulation of SFKs
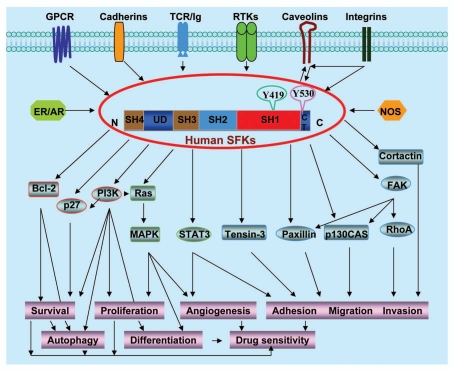
Each of the nine Src-family members exhibits a similar structure composed of four common Src homology domains (SH1–SH4), a unique domain (UD) characteristic of each individual kinase, and a carboxy-terminal tail (CT) containing the major regulatory tyrosine residue (Fig. 1).2–4 The SH1 domain is the catalytic constituent of SFKs, while the SH4 domain links SFKs to the cell membrane. The SH2 domain mediates phosphotyrosine-dependent protein-protein interactions, while SH3 domain mediates protein-protein interactions by binding to proline-rich motifs.2–4,10
Figure 1.
Schematic diagram showing the typical structure and integrated signaling and functions of SFKs.
The SH1 domain contains one important tyrosine autophosphorylation site (Y419) whose phosphorylation activates SFKs by presenting a binding pocket for substrates.2–4 The major regulatory tyrosine residue is Y530 in the CT domain of SFKs that negatively regulates kinase activity. Y530 can be phosphorylated by Csk and Csk homologous kinase, which inactivates SFKs by folding the SH2 and CT domains, thus preventing binding of SFKs to the phosphorylated tyrosine residues of their partner proteins. Y530 can be dephosphorylated by cytoplasmic PTP1B, SHP1/2 and transmembrane enzymes CD45 or PTPα/γ/ε, which reopens up the molecule to an active state.2–4 In addition, Src phosphorylation on Y213 in the SH2 domain affects its kinase activity.11,12 Cdc2 is also reported to phosphorylate and activate Src during mitosis.13
In addition to tyrosine phosphorylation, SFKs are also regulated through SH2/SH3-directed protein interaction, adaptor proteins and redox regulation.5–8,10,14 SFKs can be activated by the SH2/SH3 ligands such as a phosphotyrosine residue of the autophosphorylated receptors or a proline motif of β-arrestin.2–4,10,15 Adaptor protein Cbp/PAG is an E3 ubiquitin ligase that can positively or negatively regulate SFK activity through its ability to interact with SFKs, Csk phosphatases and down stream signaling molecules.16,17 Other adaptors such as LIME, LAT and NTAL have also been reported to regulate SFK activity in hematopoietic cells.17 SFKs contain conserved redox-regulated cysteine residues (Cys245 and Cys487) located in the SH1 and SH2 domains and can be oxidized and activated by ROS.14
Function-Associated Signaling of SFKs
SFKs function by associating with a number of membrane-bound receptors and intracellular proteins and are the key mediators of various signal transduction pathways as shown in Figure 1. SFKs integrate signaling from multiple transmembrane-associated receptors and molecules such as RTKs (VEGFR, PDGFR, EGFR, IGF-1R, c-Met or HER2), GPCRs (adrenergic, purinergic or serotonin receptors), integrins, TCR/Ig, cadherin or caveolins, and from multiple cytosolic receptors and molecules such as ER and NOS.1,18–24 SFKs then activate multiple target proteins including FAK, RhoA, cortactin, p130CAS, paxillin, tensin-3, STAT3, Ras-MAPK, PI3-kinase, p27Kip1 and Bcl-2.1,18–21,25,26 These actions collectively modulate cell migration, invasion, adhesion, survival, differentiation, proliferation, autophagy and angiogenesis. The effects of SFKs on signaling molecules and pathways of cell motility, survival, autophagy and proliferation will modulate drug sensitivity such as paclitaxel, which will be discussed below in detail.
Aberrant Expression of SFKs in Human Cancers
c-Src, the founding member of the SFKs, was originally identified as a retroviral oncoprotein that induces transformation of avian cells and formation of sarcomas in chickens.2,4,27,28 Increased expression of SFKs, particular c-Src, protein has been detected in various solid tumors including colon, breast, ovarian, lung, head and neck, skin, liver, gastric, prostate, pancreatic and brain cancers.1,18–21,29,30
Colon cancer.
Colon cancer is most often associated with elevated c-Src activity.29,31 Accumulated data have convincingly demonstrated that c-Src overrexpression and activation not only occurs in the late stages of colon cancer metastasis, but also appears in the early stages of colon cancer progression.29 c-Src activation has been shown to be an independent biomarker of poor clinical prognosis in all stages of colon cancer.29 In addition to c-Src, elevated expression of another SFK member, Yes, have been also indicated in colon cancer.29
Breast cancer.
Evidence from two transgenic mouse models clearly revealed the critical role of c-Src in the formation and progression of breast cancer. Lack of functional c-Src almost completely abolished the formation of mammary tumors in mice expressing the polyomavirus middle T oncogene.32 In another transgenic mice model that expressed a mouse mammary tumor virus-Neu fusion gene, the Neu-induced mammary tumors displayed 6–8 fold-higher c-Src kinase activity than the adjacent non-tumor epithelium.33 Several clinical studies have demonstrated that c-Src expression and kinase activity are elevated in breast cancer and ductal carcinoma in situ relative to normal breast tissues.34–38 c-Src activity is increased by HER2/Neu overexpression in human mammary epithelial cells through posttranslational mechanisms.39,40 Src inhibitors have been tested in clinical trials and appear as a promising therapeutic option in the treatment of breast cancer.41,42
Ovarian cancer.
A number of studies confirm the important roles of SFKs in ovarian cancer development and progression. c-Src expression in human ovarian cancer cell lines was found to be higher than that of colon cancer cells.31 Our own study found that both c-Src and Yes are overexpressed in ovarian cancer cell lines and that c-Src was overexpressed and activated in 15 clinical specimens of metastatic ovarian cancer.43 Knockdown of c-Src activity with an antisense c-Src reduced VEGF mRNA expression, decreased anchorage-independent growth in vitro and diminished growth of SKOv3 xenografts in vivo,44 suggesting that c-Src can regulate ovarian cancer growth. Expression and activity of uPA, a protease that contributes to the metastatic potential of many cancers, required c-Src signaling to promote ovarian tumor invasion and progression.45,46 LPA, a known inducer of ovarian cancer proliferation and motility, relies on SFK family member Fyn and c-Src to induce junction dispersal and migration of ovarian cancer cells.47,48 c-Src can inhibit TGFβ1-mediated upregulation of anti-angiogenic factors Smad2/3 and PAI-1.49 SFKs (c-Src and Yes) not only positively regulate VEGF production, but also can destabilize endothelial barrier function and promote tumor cell extravasation in vivo.50 Src activation is required for stress hormone (e.g., norepinephrine)-induced IL-6 expression, which may contribute to ovarian tumor progression.51 A novel nuclear receptor co-regulator, PELP1, is overexpressed in 60% of ovarian cancers and depends upon Src and PI3K-AKT signaling pathways to exert its effects.52 Src is also shown to partially mediate endothelin-1/β-arrestins-induced ovarian tumorigenesis.53 Finally, c-Src expression correlates with cortactin-regulated cytoskeleton function and hyaluronic acid-dependent ovarian cell tumor migration.54
Lung cancer.
Non-small-cell lung cancer (NSCLC) cell lines are found to have even higher c-Src activity than that of colon cancers.31 Increased expression of c-Src is observed in about 60% of patients with lung cancers, including neuroendocrine tumors (small-cell cancer and atypical carcinoid) and non-small-cell tumors such as adenocarcinoma, bronchoalveolar and squamous-cell lung cancers.55 c-Src expression and its kinase activity are markedly activated in NSCLC samples, especially in adenocarcinomas, and associated with the Y530 dephosphorylation.56 c-Src activation in NSCLC has been confirmed in a second clinical study.57 c-Src inhibition in lung cancer cell lines with small molecule inhibitors or small interfering RNAs is associated with impressive antitumor effects, further supporting the role of c-Src in lung cancer.58–64
Head and neck cancer.
Head and neck squamous cell carcinomas (HNSCC) are characterized by EGFR overexpression.65 c-Src is found as the key mediator of EGFR-promoted cell growth and invasion in HNSCC.66 c-Src is overexpressed in cancer samples of patients with HNSCC.67 Similar to other cancer types described above, c-Src inhibition in HNSCC with small molecule inhibitors or small interference RNAs exhibits impressive antitumor effects58,65,68,69
Mechanisms of activation of SFKs in human cancers.
As described above, elevated expression of wild-type SFKs is associated most frequently with the development and progression of human cancers. SFK genes are rarely mutated in human malignancy.23 Although exact mechanisms of SFK overexpression remain complex and elusive, increased expression of SFKs in human cancers by post-translational regulation is likely associated with their deregulated upstream regulators and signaling (such as aberrant expression of EGFR, HER2, c-Met).1,18–23 In addition, there are other mechanisms by which SFKs are activated in human cancers. As indicated above, SHP-1 can dephosphphorylate Y530 of SFKs and activate these kinases. One study has found that SHP-1 (PTPN6) was not only overexpressed in ovarian epithelial carcinoma cell lines, but also in 10 of 11 invasive ovarian cancer tissue specimens (91%) examined.70 Histone deacetylase (HDAC) inhibitors can repress c-Src transcription in cancer cell lines,30 indicating that SFKs can be transcriptionally regulated in human cancers. In some cancer types, it is likely that multiple mechanisms such as deregulation of upstream regulators, associated kinases (including Csk and Fyb) or phosphatases, SH2/SH3 ligand interactions, expression of adaptor proteins and redox regulation may collectively activate SFKs.
Multiple Mechanisms of Paclitaxel Action
Over nearly two decades since the FDA first approved paclitaxel for treatment of ovarian cancer in 1992, paclitaxel alone or in combination with other chemotherapeutic agents has become a standard therapy for patients with ovarian cancer, breast cancer, NSCLC and Kaposi sarcoma.71,72 Understanding the mechanisms by which paclitaxel inhibits and/or kills cancer cells and by which resistance to paclitaxel develops could lead to improve clinical applications of current paclitaxel-based therapy and possibly to develop novel microtubule-targeted drugs.
About 30 years ago, treatment of human HeLa and mouse fibroblast cells with cytotoxic paclitaxel was found to stabilize cytoplasmic microtubules and block cells in the M phase of the cell cycle.73,74 Paclitaxel targets microtubules by binding to β-tubulin, which affects cell proliferation (mitosis), cell shape, intracellular trafficking of macromolecules and organelles, and motility.71,75 Paclitaxel-induced M phase arrest is ultimately followed by induction of apoptosis and/or mitotic catastrophe.76
Although the anticancer activity of paclitaxel is primarily attributed to this anti-mitotic activity, the effects of paclitaxel on non-mitotic events such as intracellular trafficking and motility have recently been emphasized and thought to play important roles in paclitaxel's anticancer activity.77 A number of regulatory and structural proteins such as p53, BRCA1, Rb, androgen receptor and survivin have been shown to depend on microtubule trafficking for normal function in both dividing and interphase cells.77 Suppression of microtubule-associated intracellular trafficking can lead to cell death.77 Paclitaxel is also reported to inhibit sodium channel-dependent but cell cycle-independent cell motility in breast cancer cells.78
The effect of paclitaxel on tumor angiogenesis and vascular structures contributes to the anticancer activity of the drug.77,79,80 Paclitaxel inhibits the proliferation and motility of endothelial cells and hampers formation of new blood vessels in vivo.77,79,80
Multiple Mechanisms of Paclitaxel Resistance
Primary paclitaxel resistance refers to the intrinsic resistance of cancer cells to an initial paclitaxel treatment, whereas secondary paclitaxel resistance is acquired only after an initial response to paclitaxel treatment.81 Both forms of resistance limit the use of paclitaxel to treat different cancers. Many mechanisms described below have been proposed and tested to account for primary and acquired paclitaxel resistance.71,81,82
Deregulation of P-glycoprotein.
Intracellular levels of paclitaxel are determined by net cellular uptake and export of the drug. As a lipophilic molecule, paclitaxel is readily taken up by cancer cells and is exported by several trans-membrane ABC transporters. Among the 15 ABC transporters (ABCA2, ABCA3, ABCB1, ABCB4, ABCB5, ABCB11, ABCC1–6, ABCC11–12 and ABCG2) that have been linked to drug resistance,83 only P-glycoprotein (Pgp or ABCB1), ABCC10 (MRP7) and ABCB11 (BSEP/PFIC2/SPGP) have been implicated in paclitaxel resistance in cancers to date.71,83 Pgp overexpression in breast and ovarian cancers has been shown to be a poor prognostic factor that correlates inversely with response to paclitaxel.84,85 Higher expression of ABCC10 in 17 NSCLC cell lines correlated more precisely with paclitaxel resistance than did levels of Pgp.86 Increased ABCC10 expression has also been found in salivary gland adenocarcinoma cell lines that had developed docetaxel resistance.87 Although the role of many other ABC transporters have not been evaluated extensively in paclitaxel resistance, multidrug resistance protein 1 (MRP1 or ABCC1) and breast cancer resistance protein (BCRP/MXR or ABCG2) seem to play a minimal role in resistance to taxanes.88,89 Aside from overexpression of the transporters, approximately 50 SNPs in the Pgp gene have been identified which may affect function of the transport protein and export of paclitaxel, contributing to drug resistance.83
Alteration of tubulin isotypes.
Increased expression of several tubulin isotypes, including class III β-tubulin, α-tubulin and class IVa-tubulins, has been associated with paclitaxel resistance.90 Class III β-tubulin dimers depolymerize more readily than the constitutively expressed Class I and IVb β-tubulin isotypes in vitro.91 Overexpression of class III β-tubulin is associated with paclitaxel resistance in several cancer types including ovarian cancer.71,81,82,90,92 Importantly, class III β-tubulin knockdown with small interfering RNA (siRNA) or antisense silencing can sensitize cancer cells to paclitatexel93–95 through suppression of microtubule dynamics at low drug concentrations and a mitosis-independent mechanism of apoptosis at higher drug concentrations.94
Alteration of microtubule-regulatory proteins.
A number of proteins such as MAP4, MAP2, Tau, stathmin, fragile histidine triad protein (FHIT) and survivin, can directly interact with microtubule and regulate their polymerization and stability.90,96 There are evidence suggesting that enhanced microtubule assembly and stability generally increases paclitaxel sensitivity, whereas decreased microtubule stability favors paclitaxel resistance. For example, Increased expression of MAP4 and E-MAP-115 (epithelial microtubule-associated protein of 115 kDa), microtubule stabilizers, has been demonstrated in vitro to increase sensitivity to paclitaxel treatment.97,98 Stathmin promotes microtubule depolymerization and is frequently overexpressed in cancers and negatively regulates paclitaxel sensitivity.99,100 Knockdown of stathmin using siRNA increases sensitivity to paclitaxel treatment.99,100 Loss of TGFBI (transforming growth factor β induced), an extracellular matrix (ECM) protein, is reported to induce specific resistance to paclitaxel via integrin-dependent de-stabilization of microtubules in ovarian cancer cells.101
Survivin, a member of the inhibitors of apoptosis protein (IAP) family, plays dual roles in both mitosis and apoptosis.102,103 Paclitaxel treatment rapidly induces survivin expression that is independent of cell cycle arrest, which serves as a mechanism for cancer cells to evade paclitaxel-induced apoptosis.104 High levels of survivin protein expression has been found in more than 70% of advanced ovarian carcinomas and are associated with clinical resistance to a paclitaxel-based regimens, but not to non-paclitaxel-based regimens.105 While survivin can stabilize microtubules during mitosis, survivin's ability to inhibit apoptosis appears to have a greater impact on interactions with paclitaxel. Downregulating survivin with either siRNA, anti-sense oligonucleotides, dominant negative mutants or ribozymes leads to greater paclitaxel-induced apoptosis and to increased paclitaxel sensitivity.103,104,106 Enhancement of apoptosis by downregulating survivin appears to be regulated through inhibition of Smac and activation of caspases.102 On the other hand, increased survivin can induce drug resistance to microtubule de-stabilizers (i.e., vinca alkaloids and combretastatin A-4) due to its effect on microtubule stabilization, rather than by inhibiting apoptosis.107
Tumor suppressor FHIT can bind to microtubules and exert its activity though inhibition of survivin and PI3K-AKT pathways.108,109 Increased expression of FHIT can enhance paclitaxel-induced apoptosis.110 Lastly, Tau stabilizes microtubules its expression was initially found to correlate inversely with the response to paclitaxel treatment.111 However, a recent National Surgical Adjuvant Breast and Bowel Project (NSABP)-B28 clinical trial found that there was no significant interaction between Tau expression and paclitaxel benefit.112 Instead, this study revealed that higher Tau protein expression is associated with better disease-free survival (DFS) and overall survival (OS).112
Deregulation of apoptotic signaling pathways.
Acquired resistance to cell death is one of the hallmarks of all cancers.113 Several important apoptotic regulators such as p53, survivin and Bcl-2 have been shown to physically interact with microtubules and are thought to play roles in microtubule-associated function.77,96 As discussed earlier, deregulation of some of these apoptotic regulators in cancers can alter microtubule assembly, microtubule stability or intracellular trafficking, leading to paclitaxel resistance. Although p53 mutation is thought to play only a marginal role in paclitaxel resistance,114 alteration of p53 expression and structure can affect paclitaxel sensitivity by regulating microtubule composition and dynamics.77,96 Increased expression of the anti-apoptotic Bcl-2 protein confers paclitaxel resistance.115 Downregulation of the pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) by miRNA125b results in paclitaxel resistance.116
Mutations of tubulins.
Mutations in class I β- or α-tubulin isotypes (for example, αSer379, βPhe270, βLeu215) have been documented in cancer cell lines that have been selected for resistance to paclitaxel in cell culture models.117–121 Tubulin mutations may change microtubule assembly, dynamics and stability, leading to drug resistance.90 However, very few, if any, tubulin mutations have been detected in clinical specimens.90,122–124 Further studies are needed to determine whether tubulin mutations occur in patient-derived samples and if they do, whether these tubulin mutations are acquired during/after paclitaxel treatment in patients whose cancer's develop paclitaxel resistance. A recent study demonstrated in vitro that several β-tubulin mutations (A185T, A248V, R306C) that were identified in clinical specimens did confer paclitaxel resistance.125
Overexpression of copper transporters.
Two mammalian copper efflux transporters ATP7A and ATP7B regulate copper metabolism, and have been shown in vitro and in vivo to mediate drug resistance to cis-platin.126 ATP7A overexpression has recently been found to mediate drug resistance to paclitaxel as well.127,128 Increased expression of ATP7B has been shown in ovarian cancer cell lines and colorectal cancer patients and associated with poor prognosis.129,130
Substoichiometric levels of paclitaxel.
Given the known pharmacokinetics and pharmacodynamics of the taxanes, the concentration of the drug within cancer cells is almost always substoichiometric. Ovarian cancers may fail to respond to initial treatment with paclitaxel, not from primary resistance, but rather from a failure to achieve adequate levels of paclitaxel within cancer cells at a maximally tolerated dose of the drug (Ahmed AA, Shibani N, Bast RC Jr, Goldsmith J. Lack of response to taxane chemotherapy: substoichiometry or resistance? Submitted for publication). An optimal level of paclitaxel would occupy each of the binding pockets found on each molecule of β-tubulin. Under these circumstances, new approaches for paclitaxel therapy might attempt to enhance selectively the sensitivity of cancers to paclitaxel at first treatment, rather than attempt to overcome the many mechanisms of primary or acquired drug resistance. Against this hypothesis are older observations that low levels of paclitaxel can arrest living tumor cells at mitosis and induce apoptosis without a change in microtubule mass and microtubule bundling.131–133
SFK Regulation of Paclitaxel Sensitivity and Resistance
Overexpression of SFKs in cancers, especially in ovarian cancer regulates a variety of cell functions (Fig. 1) that can impact on paclitaxel sensitivity and resistance. SFK inhibition can sensitize cancers cells to paclitaxel-based treatments by modulating cell survival and proliferation, autophagy, microtubule stability, motility, and/or tumor angiogenesis.134–139
Modulation of apoptosis.
As described above, one of the major mechanisms of paclitaxel-induced cancer cell death is the induction of apoptosis following M phase arrest of the cell cycle. Our unpublished data and published data of others show that SFK inhibition with pharmacologic Src kinase inhibitors, siRNA knockdown or expression of a dominant-negative Src can potentiate paclitaxel-induced apoptosis by activating caspase-3.134 SFK inhibition can induce cancer cell apoptosis to enhance paclitaxel cytotoxicity through several signaling molecules and pathways. SFK inhibition can downregulates PI3K-AKT,25,106,136 STAT3/5,140 Bcl-2,25 Bcl-xL141 and Mcl-1,140 and induce apoptosis140–142 SFK inhibition also downregulates expression of antiapoptotic survivin.106 SFKs can phosphorylate tumor suppressor FHIT on tyrosine 114 residue and inactivate FHIT function.108 Since FHIT can inhibit two downstream targets, survivin and AKT, inhibition of SFK is thought to activate FHIT and apoptosis.108
Modulation of autophagy.
Autophagy can mediate both tumor cell survival and cell death. In the short term, release of amino acids and fatty acids by digestion of cellular organelles through autophagy provides substrates for the generation of ATP that permits cells to survive in a nutrient poor environment. When autophagy is prolonged, however, cells die from nonapoptotic type II programmed cell death.143,144 Recent data indicate that SFK inhibition can induce significant autophagy.25,145,146 Knockdown of two key autophagy regulators Beclin-1 and Atg12 expression with their respective siRNAs diminished SFK inhibitor dasatinib-induced autophagy and growth inhibition in ovarian cancer cells.25 Induction of autophagy has recently been shown to enhance paclitaxel activity in cancers. ARHI, a Ras-related imprinted gene, inhibits cancer cell proliferation and motility and induce autophagy in ovarian and breast cancers.147 ARHI re-expression induces autophagy and enhances the growth inhibitory effect of paclitaxel in vitro and in vivo.148 Increased expression of the pro-autophagic Beclin-1 can enhance paclitaxel-induced apoptotic and autophagic cell death in cervical cancer cells.149 In addition, SFK inhibition with dasatinib in combination treatment with temozolomide, an oral alkylating agent, also resulted in a significant increase in autophagic cell death in glioma cells.145 Although the exact mechanisms by which SFK inhibition modulates autophagy are still not clear, inhibition of Bcl-2 expression25 and AKT-mTOR-p70S6K pathway25,106,136 by SFK inhibition appear important. Constitutive expression of AKT1 and Bcl-2 inhibited dasatinib-induced autophagy in ovarian cancer cells.25
Modulation of microtubule stabilization.
Enhanced microtubule stability can increase paclitaxel sensitivity in cancer cells. Increased expression of MAP4,98 E-MAP-115,97 and TGFBI,101 decreased expression of stathmin99,100 and survivin103,104,106 have been shown to promote microtubule assembly and stability, and to increase sensitivity to paclitaxel treatment. Overexpression of tubulin binding cofactor C (TBCC), a protein for proper polymerization, sensitized breast cancer cells to paclitaxel treatment in a xenograft model.150 Higher expression of Tau, another microtubules stabilizing protein, is a favorite prognostic biomarker in breast cancer.112 SFKs, particularly Fyn have been shown to regulate microtubules polymerization and spindle stabilization.151,152 Inhibition of SFKs with inhibitor PP2 had no effect on MDR1 expression but enhanced microtubule stabilization in paclitaxel-resistant human (CaOV3TaxR) and mouse (ID8TaxR) ovarian cancer cell lines, restoring paclitaxel sensitivity.137 Microtubule-associated MAP2 has been shown to bind directly to the SH3 domain of c-Src and Fyn.153,154 Fyn can phosphorylate MAP2 on tyrosine 67 and phosphorylated MAP2 can bind to the adaptor protein Grb2.154 Fyn also physically associates with tubulins.151,152 These data suggest that SFKs may directly and indirectly affect microtubules assembly and stabilization.
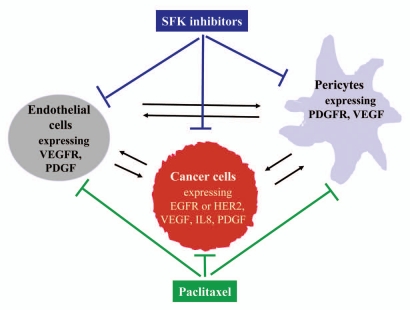
Modulation of tumor angiogenesis.
Activation of angiogenesis in tumors is another hallmark of all solid cancers.113 Increasing production of pro-angiogenic factors such as VEGF and IL-8 by cancer cells and the tumor microenvironment (e.g., pericytes) (Fig. 2) supports endothelial cell proliferation and migration and is one of most common mechanisms by which angiogenic switch is turned on persistently during tumor progression.113 Both human and murine endothelial cells express high levels of VEGF receptors (VEGFR1, VEGFR2 and VEGFR3).155 In addition, pericytes can stabilize and drive maturation of blood vessels through activation of PDGF-PDGFR signaling.113,156 Targeting breast cancer cells that overexpress HER2 with trastuzumab can significantly inhibit tumor vessel formation by decreasing production of VEGF and IL8.157 Dual targeting of cancer cells (e.g., with trastuzumab) and endothelial cells (e.g., with VEGF-Trap) produces superior inhibition of tumor angiogenesis in a breast cancer model.155 Dual targeting of pericytes (e.g., anti-PDGF aptamer AX102) and endothelial cells (bevacizumab) also results in better inhibition of tumor angiogenesis in an ovarian cancer model.158
Figure 2.
Paracrine interaction and interventions for cancer cells, vascular endothelial cells and pericytes during tumor angiogenesis.
As shown in Figure 2, Inhibition of SFKs can block signaling produced by EGFR and HER2 in cancer cells, by VEGFR in endothelial cells, and by PDGFR in pericytes.1,18,23 Inhibtion of SFKs results in inhibition of a number of downstream targets such as FAK, RhoA, cortactin, p130CAS, paxillin, tensin-3, STAT3, Ras-MAPK and PI3K-AKT,1,18–21,25,26 which produce multiple phenotypic changes including inhibition of cell migration/invasion, survival and angiogenesis. On the other hand, paclitaxel can also inhibit the proliferation and motility of endothelial cells, pericytes and cancer cells.77,79,80 Therefore, the combination of SFK inhibition and paclitaxel treatment can target simultaneously at cancer cells, endothelial cells and pericytes, which likely results in better inhibition of tumor angiogenesis. Inactivation of pericytes with AX102 in combination with paclitaxel significantly reduces pericyte coverage on endothelial cells and causes significant tumor reduction.158 Paclitaxel or Ixabepilone has been shown to greatly increase killing of endothelial cells, inhibit tumor angiogenesis and potentiate antitumor activity of bevacizumab and PDGFR/VEGFR inhibitor sunitinib in multiple animal models of breast, lung, colon and kidney cancers.159
Potential Biomarkers that Predict SFK Inhibition-Enhanced Paclitaxel Activity
As described above, SFK inhibition can increase paclitaxel sensitivity in a number of cancer types in vitro and in vivo. Given the heterogeneity of cancer, however, not every cancer patient would benefit from treatment of a combination of an SFK inhibitor and paclitaxel. Therefore, identification of potential biomarkers that can predict SFK inhibition-enhanced paclitaxel activity is critical for personalizing treatment and maximizing the benefit of this combination.
Expression of biomarkers such as moesin, caveolin-1 and yesassociated protein-1 have been found to predict response to SFK inhibition with dasatinib treatment in the “triple-negative” breast cancer.160,161 Increased expression of Yes, Lyn, Eph2A, caveolin-1, moesin, annexin-1 and uPA also confer increased sensitivity to dasatinib treatment in ovarian cancer.142 Urokinase-type plasminogen activator (uPA) and EphA2 are identified as the biomarkers that could predict dasatinib response in prostate cancer.162 Tyrosine phosphorylation of Y419 c-Src and Y118 paxillin has been indicated as the biomarkers for response to dasatinib in colon cancer.163 Among above mentioned biomarkers, cavelolin-1 is a particularly interesting biomarker that was confirmed in multiple cancer types. Caveolin 1 can interact with SFKs on membrance (Fig. 1) and inhibits protein kinase C phosphorylation on c-Src.24 Increased expression of phosphorylated caveolin 1 can sensitize MCF7 breast cancer cells to apoptosis.164 Overexpression of caveolin 1 inhibits c-Src expression and kinase activity, in vitro migration and in vivo metastatic potential.165
Specific biomarkers have not yet been identified that predict the ability of SFK inhibitors to potentiate paclitaxel therapy. Dasatinib response biomarkers such as caveolin 1, EphA2 and uPA are reasonable candidates for further evaluation. Indeed, EphA2 inhibition with siRNA in couple with paclitaxel treatment dramatically decreases tumor growth in an orthotopic ovarian cancer mouse model associated with decreased levels of VEGF and phosphorylation of c-Src.166 Combined siRNA targeting FAK (a critical downstream target of SFKs) and EphA2 have also been shown to significantly decrease tumor cell proliferation and tumor angiogenesis.167 Our unpublished data indicate that low expression of EphB2 is a potential biomarker to predict the potential of SFK inhibitor dasatinib to increase paclitaxel treatment in ovarian cancer cell lines.
Conclusions
SFKs play important roles in regulating a broad range of cellular functions. Abnormally high expression of SFKs has been documented in a number of different malignancies including ovarian, breast, lung and head and neck cancers, which respond to treatment with paclitaxel. Inhibition of SFKs alone or in combination with paclitaxel treatment is currently being evaluated in clinical trials. SFK inhibition also represents a new approach to decrease paclitaxel resistance that often develops from a variety of mechanisms during treatment with taxanes. Several precedents exist for enhancing sensitivity to paclitaxel treatment by inhibiting SFKs in cell culture and xenograft models. A phase I clinical trial in metastatic breast cancer has recently found that dasatinib plus weekly paclitaxel treatment produced a partial response in four of 13 patients (31%) including two patients previously treated with taxanes.168 Similar studies might be extended to many other types of cancer that are partially responsive to treatment with taxanes. Mechanisms underlying the interaction of SFK inhibition and enhanced paclitaxel sensitivity include potentiating apoptosis, inducing autophagic death, enhancing microtubule stability and targeting tumor neovasculature. In order to circumvent the development of paclitaxel resistance, inhibition of SFKs with its inhibitors such as dasatinib should also been tested in combination with novel taxanes such as the Epothilones,81 Larotaxel (RPR 109881A),169 and nanoparticle albumin-bound (nab)-paclitaxel.170 In addition, identification of the biomarkers that can predict SFK inhibition-enhanced paclitaxel effect would permit personalization of combined therapy with SFK inhibition and paclitaxel.
Acknowledgments
We are grateful to the Anne and Henry Zarrow Foundation, Mr. and Mrs. Stuart Zarrow, the National Foundation for Cancer Research, the MD Anderson SPORE in Ovarian Cancer NCI P50 CA83639 and the MD Anderson NCI CCSG P30 CA16672 for their support in preparing this review.
Abbreviations
- c-Src
avian v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog
- Fyn
FYN oncogene related to SRC, FGR, YES
- Yes
v-yes-1 yamaguchi sarcoma viral oncogene homolog 1
- Blk
B lymphocyte kinase
- Fgr
gardner-rasheed feline sarcoma viral oncogene homolog
- Hck
hematopoietic cell kinase
- Lck
lymphocyte-specific protein tyrosine kinase
- Lyn
v-yes-1 yamaguchi sarcoma viral related oncogene homolog
- Yrk
YES related kinase
- Csk
c-src tyrosine kinase
- Fyb
FYN binding protein
- FAK
focal adhesion kinase
- GPCR
G-protein coupled receptor
- STAT
signal transducers and activator of transcription
- ER
estrogen receptor
- AR
androgen receptor
- EGFR
epidermal growth factor receptor
- HER2
human epidermal growth factor receptor 2
- TCR
T cell receptor
- Ig
immunoglobulin
- PDGFR
platelet-derived growth factor receptor
- IGF-1R
insulin-like growth factor-1 receptor
- VEGFR
vascular endothelial growth factor receptor
- Cadherin
calcium-dependent adhesion
- ERK
extracellular signal-regulated kinases
- MAPK
mitogen-activated protein kinases
- PTP1B/α/γ/ε
protein-tyrosine phosphatase1B/alpha/lambda/epsilon
- SHP1
hematopoietic cell protein-tyrosine phosphatase/protein-tyrosine phosphatase 1C
- SHP2
protein-tyrosine phosphatase 2C
- MMP
matrix metalloprotease
- Csk
C-terminal Src kinase
- Cbp
Csk binding protein
- PAG
phosphoprotein associated with glycosphingolipid-enriched microdomains
- Cbl
Cas-Br-M (murine) oncogene
- LIME
Lck-interacting membrane protein
- LAT
linker for activation of T cells
- NTAL
non-T cell activation linker
- ROS
reactive oxygen species
- TGFβ1
transforming growth factor-beta1
- uPA
urokinase-type plasminogen activator
- PAI-1
plasminogen activator inhibitor type-1
- Smad2/3
SMAD (the drosophila gene ‘mothers against decapentaplegic’ (Mad) and the C. elegans gene Sma) family member 2/3
- PELP1
proline-, glutamic acid- and leucine-rich protein-1
- LPA
lysophosphatidic acid
References
- 1.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 2.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 3.Lowell CA, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 4.Kefalas P, Brown TR, Brickell PM. Signalling by the p60c-src family of protein-tyrosine kinases. Int J Biochem Cell Biol. 1995;27:551–563. doi: 10.1016/1357-2725(95)00024-J. [DOI] [PubMed] [Google Scholar]
- 5.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, et al. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 6.Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73:1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Kang H, Raab M, da Silva AJ, Kraeft SK, Rudd CE. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc Natl Acad Sci USA. 1998;95:8779–8784. doi: 10.1073/pnas.95.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas JE, Soriano P, Brugge JS. Phosphorylation of c-Src on tyrosine 527 by another protein tyrosine kinase. Science. 1991;254:568–571. doi: 10.1126/science.1719633. [DOI] [PubMed] [Google Scholar]
- 9.Johnson FM, Gallick GE. SRC family nonreceptor tyrosine kinases as molecular targets for cancer therapy. Anticancer Agents Med Chem. 2007;7:651. doi: 10.2174/187152007784111278. [DOI] [PubMed] [Google Scholar]
- 10.Koch CA, Anderson D, Moran MF, Ellis C, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- 11.Stover DR, Furet P, Lydon NB. Modulation of the SH2 binding specificity and kinase activity of Src by tyrosine phosphorylation within its SH2 domain. J Biol Chem. 1996;271:12481–12487. doi: 10.1074/jbc.271.21.12481. [DOI] [PubMed] [Google Scholar]
- 12.Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Stover DR, Liebetanz J, Lydon NB. Cdc2-mediated modulation of pp60c-src activity. J Biol Chem. 1994;269:26885–26889. [PubMed] [Google Scholar]
- 14.Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Luttrell LM. Transmembrane signaling by G protein-coupled receptors. Methods Mol Biol. 2006;332:3–49. doi: 10.1385/1-59745-048-0:1. [DOI] [PubMed] [Google Scholar]
- 16.Oneyama C, Hikita T, Enya K, Dobenecker MW, Saito K, Nada S, et al. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol Cell. 2008;30:426–436. doi: 10.1016/j.molcel.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14:667–678. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahluwalia MS, Groot J, Liu WM, Gladson CL. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett. 2010;298:139–149. doi: 10.1016/j.canlet.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan L, Nicholson RI, Hiscox S. SRC as a therapeutic target in breast cancer. Endocr Metab Immune Disord Drug Targets. 2008;8:273–278. doi: 10.2174/187153008786848295. [DOI] [PubMed] [Google Scholar]
- 21.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, et al. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Wanaski SP, Ng BK, Glaser M. Caveolin scaffolding region and the membrane binding region of SRC form lateral membrane domains. Biochemistry. 2003;42:42–56. doi: 10.1021/bi012097n. [DOI] [PubMed] [Google Scholar]
- 25.Le XF, Mao W, Lu Z, Carter BZ, Bast RC., Jr Dasatinib induces autophagic cell death in human ovarian cancer. Cancer. 2010;116:4980–4990. doi: 10.1002/cncr.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian X, Li G, Vass WC, Papageorge A, Walker RC, Asnaghi L, et al. The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell. 2009;16:246–258. doi: 10.1016/j.ccr.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brugge JS, Erikson RL. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977;269:346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- 28.Levinson AD, Oppermann H, Levintow L, Varmus HE, Bishop JM. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978;15:561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- 29.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 30.Dehm SM, Bonham K. SRC gene expression in human cancer: the role of transcriptional activation. Biochem Cell Biol. 2004;82:263–274. doi: 10.1139/o03-077. [DOI] [PubMed] [Google Scholar]
- 31.Budde RJ, Ke S, Levin VA. Activity of pp60c-src in 60 different cell lines derived from human tumors. Cancer Biochem Biophys. 1994;14:171–175. [PubMed] [Google Scholar]
- 32.Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- 33.Muthuswamy SK, Siegel PM, Dankort DL, Webster MA, Muller WJ. Mammary tumors expressing the neu proto-oncogene possess elevated c-Src tyrosine kinase activity. Mol Cell Biol. 1994;14:735–743. doi: 10.1128/mcb.14.1.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs C, Rubsamen H. Expression of pp60c-src protein kinase in adult and fetal human tissue: high activities in some sarcomas and mammary carcinomas. Cancer Res. 1983;43:1696–1702. [PubMed] [Google Scholar]
- 35.Ottenhoff-Kalff AE, Rijksen G, van Beurden EA, Hennipman A, Michels AA, Staal GE. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 1992;52:4773–4778. [PubMed] [Google Scholar]
- 36.Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, et al. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol. 1996;180:383–388. doi: 10.1002/(SICI)1096-9896(199612)180:4<383::AID-PATH686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 37.Elsberger B, Fullerton R, Zino S, Jordan F, Mitchell TJ, Brunton VG, et al. Breast cancer patients' clinical outcome measures are associated with Src kinase family member expression. Br J Cancer. 2010;103:899–909. doi: 10.1038/sj.bjc.6605829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, et al. Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer. 2006;95:1410–1414. doi: 10.1038/sj.bjc.6603444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheffield LG. C-Src activation by ErbB2 leads to attachment-independent growth of human breast epithelial cells. Biochem Biophys Res Commun. 1998;250:27–31. doi: 10.1006/bbrc.1998.9214. [DOI] [PubMed] [Google Scholar]
- 40.Tan M, Li P, Klos KS, Lu J, Lan KH, Nagata Y, et al. ErbB2 promotes Src synthesis and stability: novel mechanisms of Src activation that confer breast cancer metastasis. Cancer Res. 2005;65:1858–1867. doi: 10.1158/0008-5472.CAN-04-2353. [DOI] [PubMed] [Google Scholar]
- 41.Hiscox S, Nicholson RI. Src inhibitors in breast cancer therapy. Expert Opin Ther Targets. 2008;12:757–767. doi: 10.1517/14728222.12.6.757. [DOI] [PubMed] [Google Scholar]
- 42.Finn RS. Targeting Src in breast cancer. Ann Oncol. 2008;19:1379–1386. doi: 10.1093/annonc/mdn291. [DOI] [PubMed] [Google Scholar]
- 43.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, et al. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88:73–79. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 44.Wiener JR, Nakano K, Kruzelock RP, Bucana CD, Bast RC, Jr, Gallick GE. Decreased Src tyrosine kinase activity inhibits malignant human ovarian cancer tumor growth in a nude mouse model. Clin Cancer Res. 1999;5:2164–2170. [PubMed] [Google Scholar]
- 45.Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T. Transforming growth factor-beta1-dependent urokinase upregulation and promotion of invasion are involved in Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol Chem. 2004;279:8567–8576. doi: 10.1074/jbc.M309131200. [DOI] [PubMed] [Google Scholar]
- 46.Inagaki K, Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, et al. Suppression of urokinase expression and invasion by a soybean Kunitz trypsin inhibitor are mediated through inhibition of Src-dependent signaling pathways. J Biol Chem. 2005;280:31428–31437. doi: 10.1074/jbc.M501406200. [DOI] [PubMed] [Google Scholar]
- 47.Huang RY, Wang SM, Hsieh CY, Wu JC. Lysophosphatidic acid induces ovarian cancer cell dispersal by activating Fyn kinase associated with p120-catenin. Int J Cancer. 2008;123:801–809. doi: 10.1002/ijc.23579. [DOI] [PubMed] [Google Scholar]
- 48.Jeong KJ, Park SY, Seo JH, Lee KB, Choi WS, Han JW, et al. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp Mol Med. 2008;40:607–616. doi: 10.3858/emm.2008.40.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, et al. Transforming growth factor-beta1-dependent activation of Smad2/3 and upregulation of PAI-1 expression is negatively regulated by Src in SKOV-3 human ovarian cancer cells. J Cell Biochem. 2004;93:437–453. doi: 10.1002/jcb.20160. [DOI] [PubMed] [Google Scholar]
- 50.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 52.Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, Balasenthil S, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–4909. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- 53.Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, et al. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem. 2001;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- 55.Mazurenko NN, Kogan EA, Zborovskaia IB, Sukhova NM, Kiselev FL. [The detection of the c-src protein gene product in human lung tumors] Vopr Onkol. 1991;37:683–690. [PubMed] [Google Scholar]
- 56.Masaki T, Igarashi K, Tokuda M, Yukimasa S, Han F, Jin YJ, et al. pp60c-src activation in lung adenocarcinoma. Eur J Cancer. 2003;39:1447–1455. doi: 10.1016/s0959-8049(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Kalyankrishna S, Wislez M, Thilaganathan N, Saigal B, Wei W, et al. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 59.Zheng R, Yano S, Matsumori Y, Nakataki E, Muguruma H, Yoshizumi M, et al. SRC tyrosine kinase inhibitor, m475271, suppresses subcutaneous growth and production of lung metastasis via inhibition of proliferation, invasion and vascularization of human lung adenocarcinoma cells. Clin Exp Metastasis. 2005;22:195–204. doi: 10.1007/s10585-005-7768-5. [DOI] [PubMed] [Google Scholar]
- 60.Lee D, Gautschi O. Clinical development of SRC tyrosine kinase inhibitors in lung cancer. Clin Lung Cancer. 2006;7:381–384. doi: 10.3816/clc.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 61.Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–5548. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 62.Giaccone G, Zucali PA. Src as a potential therapeutic target in non-small-cell lung cancer. Ann Oncol. 2008;19:1219–1223. doi: 10.1093/annonc/mdn048. [DOI] [PubMed] [Google Scholar]
- 63.Leung EL, Tam IY, Tin VP, Chua DT, Sihoe AD, Cheng LC, et al. SRC promotes survival and invasion of lung cancers with epidermal growth factor receptor abnormalities and is a potential candidate for molecular-targeted therapy. Mol Cancer Res. 2009;7:923–932. doi: 10.1158/1541-7786.MCR-09-0003. [DOI] [PubMed] [Google Scholar]
- 64.Rothschild SI, Gautschi O, Haura EB, Johnson FM. Src inhibitors in lung cancer: current status and future directions. Clin Lung Cancer. 11:238–242. doi: 10.3816/CLC.2010.n.030. [DOI] [PubMed] [Google Scholar]
- 65.Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol. 2008;35:286–297. doi: 10.1053/j.seminoncol.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Q, Thomas SM, Xi S, Smithgall TE, Siegfried JM, Kamens J, et al. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation and invasion of head and neck cancer cells. Cancer Res. 2004;64:6166–6173. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 67.van Oijen MG, Rijksen G, ten Broek FW, Slootweg PJ. Overexpression of c-Src in areas of hyperproliferation in head and neck cancer, premalignant lesions and benign mucosal disorders. J Oral Pathol Med. 1998;27:147–152. doi: 10.1111/j.1600-0714.1998.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 68.Koppikar P, Choi SH, Egloff AM, Cai Q, Suzuki S, Freilino M, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:4284–4291. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brooks HD, Glisson BS, Bekele BN, Ginsberg LE, El-Naggar A, Culotta KS, et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2010 doi: 10.1002/cncr.25769. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ershler MA, Samokhvalov IM, Belyavsky AV, Visser JW. Genomic structure and alternative splicing of the murine bhk/ctk/ntk gene. FEBS Lett. 1995;375:50–52. doi: 10.1016/0014-5793(95)01154-7. [DOI] [PubMed] [Google Scholar]
- 71.Horwitz SB, Cohen D, Rao S, Ringel I, Shen HJ, Yang CP. Taxol: mechanisms of action and resistance. J Natl Cancer Inst Monogr. 1993:55–61. [PubMed] [Google Scholar]
- 72.Goldspiel BR. Clinical overview of the taxanes. Pharmacotherapy. 1997;17:110–125. [PubMed] [Google Scholar]
- 73.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 74.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5:3–6. [PubMed] [Google Scholar]
- 76.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 77.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8:244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 78.Tran TA, Gillet L, Roger S, Besson P, White E, Le Guennec JY. Non-anti-mitotic concentrations of taxol reduce breast cancer cell invasiveness. Biochem Biophys Res Commun. 2009;379:304–308. doi: 10.1016/j.bbrc.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 79.Vispe S, DeVries L, Creancier L, Besse J, Breand S, Hobson DJ, et al. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to downregulation of short-lived mRNA. Mol Cancer Ther. 2009;8:2780–2790. doi: 10.1158/1535-7163.MCT-09-0549. [DOI] [PubMed] [Google Scholar]
- 80.Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]
- 81.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18:3–8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 82.Kavallaris M. Microtubules and resistance to tubulinbinding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 83.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 84.Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF, Jr, Goodman A, et al. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004;93:98–106. doi: 10.1016/j.ygyno.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 85.Linn SC, Giaccone G, van Diest PJ, Blokhuis WM, van der Valk P, van Kalken CK, et al. Prognostic relevance of P-glycoprotein expression in breast cancer. Ann Oncol. 1995;6:679–685. doi: 10.1093/oxfordjournals.annonc.a059284. [DOI] [PubMed] [Google Scholar]
- 86.Oguri T, Ozasa H, Uemura T, Bessho Y, Miyazaki M, Maeno K, et al. MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non-small cell lung cancer. Mol Cancer Ther. 2008;7:1150–1155. doi: 10.1158/1535-7163.MCT-07-2088. [DOI] [PubMed] [Google Scholar]
- 87.Naramoto H, Uematsu T, Uchihashi T, Doto R, Matsuura T, Usui Y, et al. Multidrug resistance-associated protein 7 expression is involved in cross-resistance to docetaxel in salivary gland adenocarcinoma cell lines. Int J Oncol. 2007;30:393–401. [PubMed] [Google Scholar]
- 88.Bellarosa D, Binaschi M, Maggi CA, Goso C. Sabarubicin-(MEN 10755) and paclitaxel show different kinetics in nuclear factor-kappaB (NFκB) activation: effect of parthenolide on their cytotoxicity. Anticancer Res. 2005;25:2119–2128. [PubMed] [Google Scholar]
- 89.Frasson M, Vitadello M, Brunati AM, La Rocca N, Tibaldi E, Pinna LA, et al. Grp94 is Tyr-phosphorylated by Fyn in the lumen of the endoplasmic reticulum and translocates to Golgi in differentiating myoblasts. Biochim Biophys Acta. 2009;1793:239–252. doi: 10.1016/j.bbamcr.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci USA. 1994;91:11358–11362. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67:9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 94.Gan PP, McCarroll JA, Po'uha ST, Kamath K, Jordan MA, Kavallaris M. Microtubule dynamics, mitotic arrest and apoptosis: drug-induced differential effects of betaIII-tubulin. Mol Cancer Ther. 9:1339–1348. doi: 10.1158/1535-7163.MCT-09-0679. [DOI] [PubMed] [Google Scholar]
- 95.Kavallaris M, Burkhart CA, Horwitz SB. Antisense oligonucleotides to class III beta-tubulin sensitize drugresistant cells to Taxol. Br J Cancer. 1999;80:1020–1025. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport and cell death. J Clin Oncol. 1999;17:1061–1070. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- 97.Gruber D, Faire K, Bulinski JC. Abundant expression of the microtubule-associated protein, ensconsin (E-MAP-115), alters the cellular response to Taxol. Cell Motil Cytoskeleton. 2001;49:115–129. doi: 10.1002/cm.1026. [DOI] [PubMed] [Google Scholar]
- 98.Zhang CC, Yang JM, White E, Murphy M, Levine A, Hait WN. The role of MAP4 expression in the sensitivity to paclitaxel and resistance to vinca alkaloids in p53 mutant cells. Oncogene. 1998;16:1617–1624. doi: 10.1038/sj.onc.1201658. [DOI] [PubMed] [Google Scholar]
- 99.Iancu C, Mistry SJ, Arkin S, Atweh GF. Taxol and antistathmin therapy: a synergistic combination that targets the mitotic spindle. Cancer Res. 2000;60:3537–3541. [PubMed] [Google Scholar]
- 100.Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–6869. [PubMed] [Google Scholar]
- 101.Ahmed AA, Mills AD, Ibrahim AE, Temple J, Blenkiron C, Vias M, et al. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell. 2007;12:514–527. doi: 10.1016/j.ccr.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song Z, Yao X, Wu M. Direct interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosis. J Biol Chem. 2003;278:23130–23140. doi: 10.1074/jbc.M300957200. [DOI] [PubMed] [Google Scholar]
- 103.Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, et al. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ling X, Bernacki RJ, Brattain MG, Li F. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J Biol Chem. 2004;279:15196–15203. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- 105.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, et al. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu J, Tan M, Huang WC, Li P, Guo H, Tseng LM, et al. Mitotic deregulation by survivin in ErbB2-overexpressing breast cancer cells contributes to Taxol resistance. Clin Cancer Res. 2009;15:1326–1334. doi: 10.1158/1078-0432.CCR-08-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheung CH, Chen HH, Kuo CC, Chang CY, Coumar MS, Hsieh HP, et al. Survivin counteracts the therapeutic effect of microtubule de-stabilizers by stabilizing tubulin polymers. Mol Cancer. 2009;8:43–58. doi: 10.1186/1476-4598-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Semba S, Trapasso F, Fabbri M, McCorkell KA, Volinia S, Druck T, et al. Fhit modulation of the Akt-survivin pathway in lung cancer cells: Fhit-tyrosine 114 (Y114) is essential. Oncogene. 2006;25:2860–2872. doi: 10.1038/sj.onc.1209323. [DOI] [PubMed] [Google Scholar]
- 109.Chaudhuri AR, Khan IA, Prasad V, Robinson AK, Luduena RF, Barnes LD. The tumor suppressor protein Fhit. A novel interaction with tubulin. J Biol Chem. 1999;274:24378–24382. doi: 10.1074/jbc.274.34.24378. [DOI] [PubMed] [Google Scholar]
- 110.Kim CH, Yoo JS, Lee CT, Kim YW, Han SK, Shim YS, et al. FHIT protein enhances paclitaxel-induced apoptosis in lung cancer cells. Int J Cancer. 2006;118:1692–1698. doi: 10.1002/ijc.21573. [DOI] [PubMed] [Google Scholar]
- 111.Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pusztai L, Jeong JH, Gong Y, Ross JS, Kim C, Paik S, et al. Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J Clin Oncol. 2009;27:4287–4292. doi: 10.1200/JCO.2008.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 114.Debernardis D, Sire EG, De Feudis P, Vikhanskaya F, Valenti M, Russo P, et al. p53 status does not affect sensitivity of human ovarian cancer cell lines to paclitaxel. Cancer Res. 1997;57:870–874. [PubMed] [Google Scholar]
- 115.Tabuchi Y, Matsuoka J, Gunduz M, Imada T, Ono R, Ito M, et al. Resistance to paclitaxel therapy is related with Bcl-2 expression through an estrogen receptor mediated pathway in breast cancer. Int J Oncol. 2009;34:313–319. [PubMed] [Google Scholar]
- 116.Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi Y, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem. 285:21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yin S, Cabral F, Veeraraghavan S. Amino acid substitutions at proline 220 of beta-tubulin confer resistance to paclitaxel and colcemid. Mol Cancer Ther. 2007;6:2798–2806. doi: 10.1158/1535-7163.MCT-06-0791. [DOI] [PubMed] [Google Scholar]
- 118.Martello LA, Verdier-Pinard P, Shen HJ, He L, Torres K, Orr GA, et al. Elevated levels of microtubule destabilizing factors in a Taxol-resistant/dependent A549 cell line with an alpha-tubulin mutation. Cancer Res. 2003;63:1207–1213. [PubMed] [Google Scholar]
- 119.Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 120.Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A beta-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274:23875–23882. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- 121.Wiesen KM, Xia S, Yang CP, Horwitz SB. Wild-type class I beta-tubulin sensitizes Taxol-resistant breast adenocarcinoma cells harboring a beta-tubulin mutation. Cancer Lett. 2007;257:227–235. doi: 10.1016/j.canlet.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 122.Hasegawa S, Miyoshi Y, Egawa C, Ishitobi M, Tamaki Y, Monden M, et al. Mutational analysis of the class I beta-tubulin gene in human breast cancer. Int J Cancer. 2002;101:46–51. doi: 10.1002/ijc.10575. [DOI] [PubMed] [Google Scholar]
- 123.Tsurutani J, Komiya T, Uejima H, Tada H, Syunichi N, Oka M, et al. Mutational analysis of the beta-tubulin gene in lung cancer. Lung Cancer. 2002;35:11–16. doi: 10.1016/s0169-5002(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 124.Sale S, Sung R, Shen P, Yu K, Wang Y, Duran GE, et al. Conservation of the class I beta-tubulin gene in human populations and lack of mutations in lung cancers and paclitaxel-resistant ovarian cancers. Mol Cancer Ther. 2002;1:215–225. [PubMed] [Google Scholar]
- 125.Yin S, Bhattacharya R, Cabral F. Human mutations that confer Paclitaxel resistance. Mol Cancer Ther. 2010;9:327–335. doi: 10.1158/1535-7163.MCT-09-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 127.Furukawa T, Komatsu M, Ikeda R, Tsujikawa K, Akiyama S. Copper transport systems are involved in multidrug resistance and drug transport. Curr Med Chem. 2008;15:3268–3278. doi: 10.2174/092986708786848479. [DOI] [PubMed] [Google Scholar]
- 128.Owatari S, Akune S, Komatsu M, Ikeda R, Firth SD, Che XF, et al. Copper-transporting P-type ATPase, ATP7A, confers multidrug resistance and its expression is related to resistance to SN-38 in clinical colon cancer. Cancer Res. 2007;67:4860–4868. doi: 10.1158/0008-5472.CAN-06-3096. [DOI] [PubMed] [Google Scholar]
- 129.Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, et al. Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res. 2009;15:3770–3780. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martinez-Balibrea E, Martinez-Cardus A, Musulen E, Gines A, Manzano JL, Aranda E, et al. Increased levels of copper efflux transporter ATP7B are associated with poor outcome in colorectal cancer patients receiving oxaliplatin-based chemotherapy. Int J Cancer. 2009;124:2905–2910. doi: 10.1002/ijc.24273. [DOI] [PubMed] [Google Scholar]
- 131.Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Derry WB, Wilson L, Jordan MA. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- 133.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 134.Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol Cancer Ther. 2005;4:217–224. [PubMed] [Google Scholar]
- 135.Teoh D, Ayeni TA, Rubatt JM, Adams DJ, Grace L, Starr MD, et al. Dasatinib (BMS-35482) has synergistic activity with paclitaxel and carboplatin in ovarian cancer cells. Gynecol Oncol. 2011;121:187–192. doi: 10.1016/j.ygyno.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pengetnze Y, Steed M, Roby KF, Terranova PF, Taylor CC. Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem Biophys Res Commun. 2003;309:377–383. doi: 10.1016/j.bbrc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 137.George JA, Chen T, Taylor CC. SRC tyrosine kinase and multidrug resistance protein-1 inhibitions act independently but cooperatively to restore paclitaxel sensitivity to paclitaxel-resistant ovarian cancer cells. Cancer Res. 2005;65:10381–10388. doi: 10.1158/0008-5472.CAN-05-1822. [DOI] [PubMed] [Google Scholar]
- 138.Han LY, Landen CN, Trevino JG, Halder J, Lin YG, Kamat AA, et al. Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res. 2006;66:8633–8639. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim HS, Han HD, Armaiz-Pena GN, Stone RL, Nam EJ, Lee JW, et al. Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res. 2011;17:1713–1721. doi: 10.1158/1078-0432.CCR-10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nam S, Williams A, Vultur A, List A, Bhalla K, Smith D, et al. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol Cancer Ther. 2007;6:1400–1405. doi: 10.1158/1535-7163.MCT-06-0446. [DOI] [PubMed] [Google Scholar]
- 141.Griffiths GJ, Koh MY, Brunton VG, Cawthorne C, Reeves NA, Greaves M, et al. Expression of kinase-defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-xL and increases oxaliplatin- and Fas-induced apoptosis. J Biol Chem. 2004;279:46113–46121. doi: 10.1074/jbc.M408550200. [DOI] [PubMed] [Google Scholar]
- 142.Konecny GE, Glas R, Dering J, Manivong K, Qi J, Finn RS, et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer. 2009;101:1699–1708. doi: 10.1038/sj.bjc.6605381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Milano V, Piao Y, LaFortune T, de Groot J. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol Cancer Ther. 2009;8:394–406. doi: 10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- 146.Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, et al. Autophagy blockade sensitizes prostate cancer cells towards Src family kinase inhibitors. Genes Cancer. 2010;1:40–49. doi: 10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zou CF, Jia L, Jin H, Yao M, Zhao N, Huan J, et al. Re-expression of ARHI (DIRAS3) induces autophagy in breast cancer cells and enhances the inhibitory effect of paclitaxel. BMC Cancer. 2011;11:22–33. doi: 10.1186/1471-2407-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun Y, Jin L, Liu J, Lin S, Yang Y, Sui Y, et al. Effect of autophagy on paclitaxel-induced CaSki cell death. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:557–565. doi: 10.3969/j.issn.1672-7347.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 150.Hage-Sleiman R, Herveau S, Matera EL, Laurier JF, Dumontet C. Tubulin binding cofactor C (TBCC) suppresses tumor growth and enhances chemosensitivity in human breast cancer cells. BMC Cancer. 2010;10:135–149. doi: 10.1186/1471-2407-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levi M, Maro B, Shalgi R. The involvement of Fyn kinase in resumption of the first meiotic division in mouse oocytes. Cell Cycle. 2010;9:1577–1589. doi: 10.4161/cc.9.8.11299. [DOI] [PubMed] [Google Scholar]
- 152.Levi M, Shalgi R. The role of Fyn kinase in the release from metaphase in mammalian oocytes. Mol Cell Endocrinol. 2010;314:228–233. doi: 10.1016/j.mce.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 153.Lim RW, Halpain S. Regulated association of microtubule-associated protein 2 (MAP2) with Src and Grb2: evidence for MAP2 as a scaffolding protein. J Biol Chem. 2000;275:20578–20587. doi: 10.1074/jbc.M001887200. [DOI] [PubMed] [Google Scholar]
- 154.Zamora-Leon SP, Bresnick A, Backer JM, Shafit-Zagardo B. Fyn phosphorylates human MAP-2c on tyrosine 67. J Biol Chem. 2005;280:1962–1970. doi: 10.1074/jbc.M411380200. [DOI] [PubMed] [Google Scholar]
- 155.Le XF, Mao W, Lu C, Thornton A, Heymach JV, Sood AK, et al. Specific blockade of VEGF and HER2 pathways results in greater growth inhibition of breast cancer xenografts that overexpress HER2. Cell Cycle. 2008;7:3747–3758. doi: 10.4161/cc.7.23.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Jr, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 158.Lu C, Shahzad MM, Moreno-Smith M, Lin YG, Jennings NB, Allen JK, et al. Targeting pericytes with a PDGF-B aptamer in human ovarian carcinoma models. Cancer Biol Ther. 2009;9:176–182. doi: 10.4161/cbt.9.3.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee FY, Covello KL, Castaneda S, Hawken DR, Kan D, Lewin A, et al. Synergistic antitumor activity of ixabepilone (BMS-247550) plus bevacizumab in multiple in vivo tumor models. Clin Cancer Res. 2008;14:8123–8131. doi: 10.1158/1078-0432.CCR-08-0025. [DOI] [PubMed] [Google Scholar]
- 160.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/ “triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 161.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 162.Wang XD, Reeves K, Luo FR, Xu LA, Lee F, Clark E, et al. Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: rationale for patient selection and efficacy monitoring. Genome Biol. 2007;8:R255.1–R255.9. doi: 10.1186/gb-2007-8-11-r255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, et al. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5:3014–3022. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 164.Shajahan AN, Wang A, Decker M, Minshall RD, Liu MC, Clarke R. Caveolin-1 tyrosine phosphorylation enhances paclitaxel-mediated cytotoxicity. J Biol Chem. 2007;282:5934–5943. doi: 10.1074/jbc.M608857200. [DOI] [PubMed] [Google Scholar]
- 165.Cantiani L, Manara MC, Zucchini C, De Sanctis P, Zuntini M, Valvassori L, et al. Caveolin-1 reduces osteosarcoma metastases by inhibiting c-Src activity and met signaling. Cancer Res. 2007;67:7675–7685. doi: 10.1158/0008-5472.CAN-06-4697. [DOI] [PubMed] [Google Scholar]
- 166.Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 167.Shahzad MM, Lu C, Lee JW, Stone RL, Mitra R, Mangala LS, et al. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther. 2009;8:1027–1034. doi: 10.4161/cbt.8.11.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fornier MN, Morris PG, Abbruzzi A, D'Andrea G, Gilewski T, Bromberg J, et al. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann Oncol. 2011 doi: 10.1093/annonc/mdr018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Metzger-Filho O, Moulin C, de Azambuja E, Ahmad A. Larotaxel: broadening the road with new taxanes. Expert Opin Investig Drugs. 2009;18:1183–1189. doi: 10.1517/13543780903119167. [DOI] [PubMed] [Google Scholar]
- 170.Teneriello MG, Tseng PC, Crozier M, Encarnacion C, Hancock K, Messing MJ, et al. Phase II evaluation of nanoparticle albumin-bound paclitaxel in platinum-sensitive patients with recurrent ovarian, peritoneal or fallopian tube cancer. J Clin Oncol. 2009;27:1426–1431. doi: 10.1200/JCO.2008.18.9548. [DOI] [PubMed] [Google Scholar]