Abstract
Pancreatic adenocarcinoma is an untreatable deadly cancer. The factors involved in its early development remain unknown, which contributes to the absence of biomarkers for early detection of malignancy or at-risk subjects and the absence of efficacious therapeutic agents. Because zinc changes are implicated in some cancers, we determined if it might be involved in the development of pancreatic adenocarcinoma. With in situ Dithizone and Zinquin staining of normal pancreas and adenocarcinoma tissue sections, we show for the first time, a consistent major loss of zinc in ductal and acinar epithelium in adenocarcinoma compared with the normal epithelium. This decrease in zinc is evident in well-differentiated through poorly-differentiated stages of malignancy. Immunohistochemistry identified ZIP3 as the basilar membrane zinc uptake transporter in normal ductal/acinar epithelium; and that the transporter is absent in adenocarcinoma. In situ Rt-PCR revealed that ZIP3 gene expression is silenced in adenocarcinoma. The ZIP3 downregulation accompanied the loss of zinc in early and progressing malignancy. RREB1 transcription factor was downregulated along with ZIP3; and might be involved in the silencing of ZIP3 expression. Zinc treatment was cytotoxic to malignant Panc1 cells. The combination of concurrent zinc, ZIP3 and RREB-1 changes represent early events in the development of adenocarcinoma; and suggest that zinc might be a tumor suppressor of pancreatic cancer. This report provides the clinical foundation for further mechanistic studies that will provide important insight into pancreatic carcinogenesis, and can lead to the development of effective early biomarkers and effective therapeutic agents for pancreatic cancer.
Keywords: pancreatic adenocarcinoma, zinc changes, ZIP3 transporter, RREB-1, ductal epithelium, acinar epithelium, Panc 1 cells, zinc inhibition cell growth
Introduction
Pancreatic cancer (adenocarcinoma) is among the most lethal of cancers; with ∼42,000 new cases annually in the USA, of which ∼35,000 will result in death.1 The factors and conditions associated with the early development and progression of pancreatic cancer remain largely unknown. This contributes to the lack of significant progress in the identification of biomarkers for early detection; and contributes to the absence of effective chemotherapeutic agents for successful treatment once pancreatic cancer has been identified. A better understanding of the etiology and events of pancreatic carcinogenesis is urgently needed to increase the chances for timely and effective diagnosis, prevention and treatment of malignancy.
Substantial evidence exists that zinc is implicated as a tumor suppressor in the development and progression of some cancers.2–4 In such cancers, the zinc levels of malignant tissue are markedly decreased as compared with the normal tissue. At this time, no direct studies have been reported regarding the zinc levels in pancreatic adenocarcinoma vs. normal pancreatic exocrine tissues. One exception is a preliminary study with only one tissue sample,5 which suggested that a decrease in zinc level might be associated with pancreatic adenocarcinoma. Li et al.6 suggested that increased zinc accumulation is associated with ductal adenocarcinoma; however no measurements of zinc levels in human normal and adenocarcinoma pancreatic tissues were presented; and their supposition was not supported by any measurements of zinc in human pancreatic tissues. The absence of essential information concerning the zinc levels in normal pancreatic tissue and in adenocarcinoma prompted us to conduct in situ zinc measurements in pancreatic tissues in order to establish the zinc relationship in human pancreatic adenocarcinoma.
This report establishes for the first time with direct in situ measurements of relative zinc levels that zinc levels are markedly decreased in pancreatic adenocarcinoma as compared with normal pancreatic ductal/acinar epithelium; with concurrent downregulation of ZIP3 zinc uptake transporter and RREB-1 transcription factor. These changes occur in early stage malignancy and persist in the progression of malignancy. In addition, zinc treatment of malignant Panc1 cells exhibits cytotoxic effects. These results suggest the possibility that zinc might be a tumor suppressor of pancreatic adenocarcinoma; and provide new insight into important factors and events in pancreatic carcinogenesis, which can lead to the development of early biomarkers and new efficacious therapeutic agents.
Results
Comparative zinc levels in normal pancreas vs. adenocarcinoma.
In the following description we employ the “grading system” of malignancy according to the TMA description of US Biomax: Grade 1 (well-differentiated); Grade 2 (moderately-differentiated); Grade 3 (poorly differentiated).
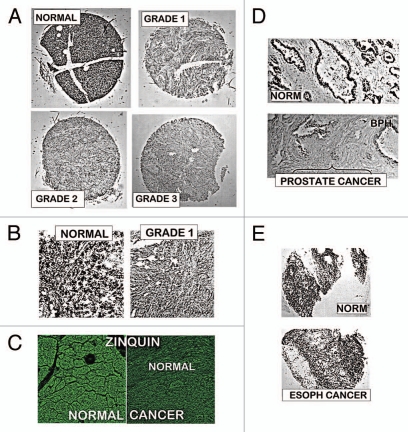
Figure 1 provides representative DTZ staining of TMA slides that contained adenocarcinoma and normal pancreas cores. Normal cores exhibited pronounced ZnDTZ staining in the ductal and acinar epithelium; whereas Grades 1, 2 and 3 cancer cases exhibited a marked depletion of ZnDTZ. As a positive control, we performed the same DTZ procedure on prostate and esophageal tissues. Figure 1D shows the high ZnDTZ content in normal prostate glands and the loss of ZnDTZ in malignancy, which is consistent with the loss of zinc that characterizes prostate cancer.2 In contrast, no difference in ZnDTZ exists between normal esophageal epithelium and esophageal squamous cell carcinoma, which is consistent with the absence of zinc changes in esophageal adenocarcinoma.7 These results re-enforce the identification with DTZ of the major decrease in zinc in pancreatic adenocarcinoma. Zinquin staining (Fig. 1C) shows the high Zn fluorescence in normal pancreatic ductal/acinar epithelium, and the markedly reduced fluorescence in adenocarcinoma; which confirms the DTZ results. The ZnDTZ formation constant log Kf∼15 (Zn-binding affinity) identifies the cellular levels of exchangeable reactive zinc and tightly bound unreactive zinc; i.e., a relative estimate of total zinc. ZnZinquin with a log Kf∼10 detects essentially the mobile pool of zinc; so that a major loss of the reactive zinc pool occurs in adenocarcinoma.
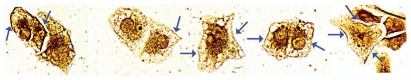
Figure 1.
Dithizone and Zinquin staining of zinc levels in tissue array slides containing normal vs. adenocarcinoma pancreatic cores. (A) Representative examples of normal and malignant pancreas cores. Black pigments in normal pancreas are due to ZnDTZ, which is absent in malignant cores. (B) Enlargement to show that ZnDTZ staining is dominant throughout the field of normal acini and ducts; and is absent in malignant acini and ducts. (C) Confocal microscopy of Zinquin fluorescence of zinc levels, which confirms the loss of zinc in adenocarcinoma compared with normal pancreas. It also shows that the Zn-Zinquin flourecence is associated with the normal ductal and acini epithelium. (D) DTZ staining of normal and malignant prostate, which shows the loss of ZnDTZ staining that characterizes prostate cancer depletion of zinc. (E) DTZ staining of normal esophagus and esophageal squamous cell carcinoma; which shows no difference in DTZ staining; and is consistent with the absence of zinc changes in esophageal cancer.
The results shown in Figure 1 are representative of a composite of duplicate pancreatic cores involving Grade 1 (17 cases); Grade 2 (18 cases); Grade 3 (12 cases); and 10 normal cases. No quantitative differences were associated with Grades, and no normal cores showed low Zn staining as seen in adenocarcinoma.
ZIP transporter abundance and expression in normal pancreas and adenocarcinoma.
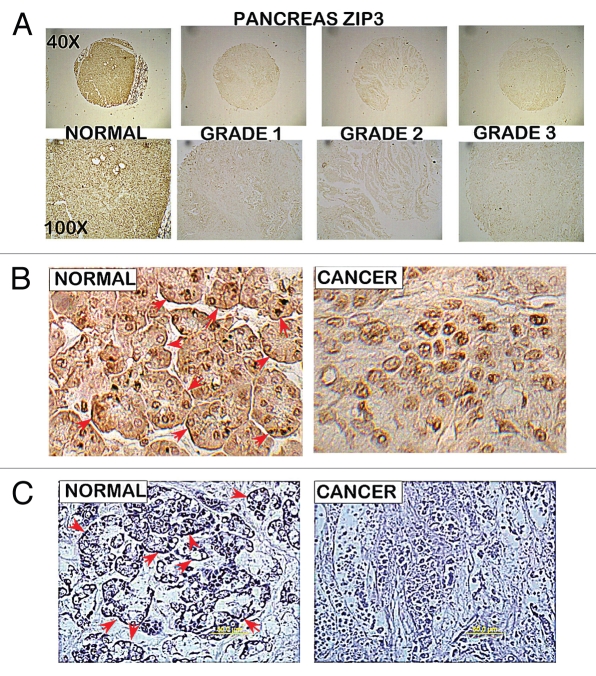
To gain initial insight into the possible cause of the loss of zinc in adenocarcinoma, the relative abundance of three cellular zinc uptake transporters; ZIP1, ZIP2 and ZIP3 was determined. Figure 2 shows representative results obtained with ZIP3 IHC staining of cores in the same TMAs employed for ZnDTZ analysis. ZIP3 transporter is abundant in normal pancreatic acini and ductal epithelium; and, most importantly, is localized predominantly with the basal plasma membrane. This is strong evidence that ZIP3 is a functional transporter for the normal ductal and acinar epithelial uptake and accumulation of zinc from interstitial fluid. In adenocarcinoma, ZIP3 transporter abundance is depleted and no plasma membrane ZIP3 transporter is evident. Consequently, the loss of ZIP3 is likely to be involved in the decrease in zinc in adenocarcinoma. Dr. Desouki (Medical University of South Carolina) independently performed ZIP3 IHC on archived pancreatic tissue sections (Fig. 2C). He confirmed the ZIP3 abundance and association with the basilar membrane of normal ductal/acinar epithelium; as well as the loss of ZIP3 in adenocarcinoma.
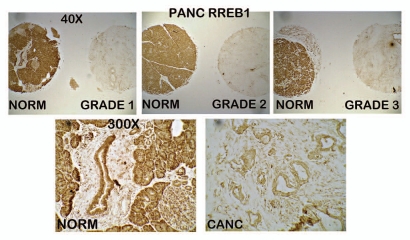
Figure 2.
ZIP3 immunohistochemistry of tissue array slides and tissue sections containing normal pancreas vs. adenocarcinoma cores. The same tissue array series as employed in Figure 1. (A) Panels show ZIP3 IHC for normal and adenocarcinoma cores. (B) High magnification shows basal membrane localization (arrows) of ZIP3 in normal epithelium and absence of ZIP3 transporter in adenocarcinoma. (C) ZIP3 IHC of tissue sections of normal pancreas tissue vs. adenocarcinoma provided and performed by Dr. Desouki. Confirms the localization of ZIP3 with basal membrane of epithelium and the absence of ZIP3 in adenocarcinoma.
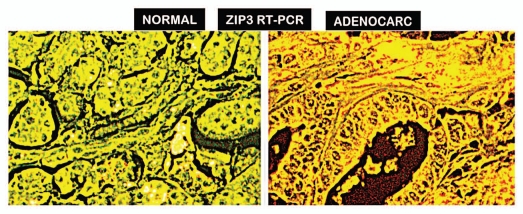
We determined if the downregulation of ZIP3 gene expression was responsible for loss of transporter in adenocarcinoma. This study was independently conducted by Dr. Bagasra at Claflin U with the same TMA as used for the ZIP3 IHC analysis. Figure 3 clearly shows the expression of ZIP3 in normal ductal and acinar epithelium; and the loss of expression in adenocarcinoma; which correlates with the change in ZIP3 transporter abundance.
Figure 3.
RT-IN SITU-PCR for ZIP3 gene expression in normal pancreas vs. adenocarcinoma. Representative results show high ZIP3 expression in normal ductal and acinar epithelium; and absence of ZIP3 expression in adenocarcinoma. Yellowish-green = expression; bright yellow-reddish = no expression.
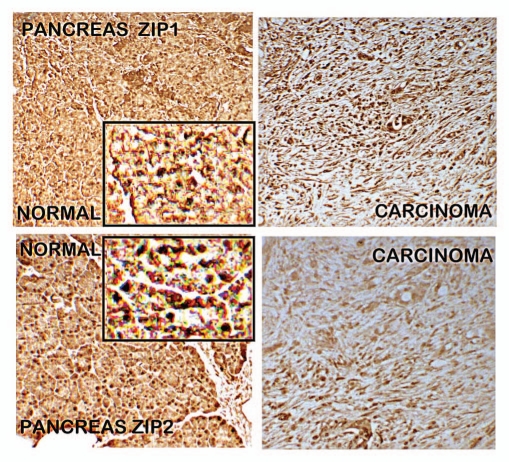
IHC analysis of ZIP1 and ZIP2 (Fig. 4) shows that both transporters exist in normal ductal/acinar epithelium; and both are markedly decreased in adenocarcinoma. However, unlike ZIP3, there is no demonstrable association of either transporter with the ductal/acinar epithelial plasma membrane. This indicates that they are not functional transporters for the cellular zinc uptake by the normal ductal/acinar epithelium; and are not associated with the loss of zinc in adenocarcinoma.
Figure 4.
ZIP1 and ZIP2 immunohistochemistry of normal pancreas vs. adenocarcinoma. Results show decrease in ZIP1 and ZIP2 transporters in adenocarcinoma. The inserts are enlargements to show that ZIP1 and ZIP2 transporters exhibit no localization with the epithelial plasma membrane; as contrasted with the strong abundant membrane localization of ZIP3 as shown in Figure 2.
For planning future mechanistic studies with malignant pancreatic cell lines, we determined if wild-type Panc1 malignant cells (cell line derived from ductal adenocarcinoma) exhibited the loss of ZIP3 as is the case with the in situ malignant cells. Figure 5 shows that Panc1 cells contain constituitive ZIP3 transporter with localization at the plasma membrane. This is the condition that exists in the normal ductal/acinar epithelium in situ; and not the condition exhibited by the malignant cells in situ. This has important implications regarding the mechanism of ZIP3 gene silencing that occurs in vivo in human pancreatic adenocarcinoma as discussed below.
Figure 5.
ZIP3 IHC of Panc1 cells. Arrows point to localization of ZIP3 transporter at the plasma membrane.
RREB-1 transcription factor in normal pancreas and adenocarcinoma.
The reappearance of ZIP3 in Panc-1 cells reveals that the loss of ZIP3 expression in situ in adenocarcinoma is not due to gene deletion or permanent mutation; but likely the result of epigenetic silencing of ZIP3 gene expression. This prompted us to determine if there might be evidence of an association of RREB-1 with loss of ZIP3 expression. RREB-1 is a zinc finger transcription factor that has been described both as an activator of gene transcription;8–11 and as a repressor of gene expression;12,13 the action of which is dependent upon the gene and the cell type.14 Figure 6 shows that RREB-1 protein is highly abundant in the normal ductal and acinar epithelium; and is virtually absent in adenocarcinoma. Since the loss of RREB-1 occurs concurrently with the loss of ZIP3, it is possible that RREB-1 might be involved in the silencing of ZIP3 expression. In support of this, we have identified four potential RREB-1 binding sites in the promoter region of the ZIP3 gene (unpublished information). Studies to establish this relationship are in progress.
Figure 6.
RREB-1 IHC of tissue array slide containing normal pancreas and adenocarcinoma cores. Results that show the high abundance of RREB-1 transcription factor in normal ductal and acinar epithelium; and loss of RREB-1 in Grade 1, Grade 2 and Grade 3 adenocarcinoma.
Effect of zinc treatment on growth of Panc1 cells.
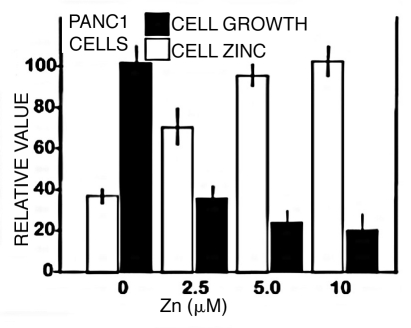
The loss of zinc that occurs in the development and progression of malignancy suggests that retention of the normal levels of cellular zinc might exhibit cytotoxic effects on the malignant cells. To obtain initial information, we determined the effects of zinc treatment on Panc1 cells (Fig. 7). Exposure of the Panc1 cells to a physiological concentration of 2.5 µM zinc increased the cellular level of zinc, which resulted in ∼65% inhibition of cell proliferation. At 5 and 10 µM zinc, the inhibition increased to ∼75 and 80%. This cytotoxic effect indicates that incorporation of zinc into malignant pancreatic cells could exhibit antitumor effects; and this would explain why zinc levels are decreased in the development and progression of pancreatic adenocarcinoma.
Figure 7.
Effect of zinc treatment on growth and cellular zinc level of Panc1 cells. Panc1 cells were treated with 0, 2.5, 5.0 or 10 µM ZnCl2 for 24 h. After treatment, cell proliferation and zinc levels were determined using the CyQUANT Cell Proliferation Assay kit and the Zinquin assay.
Discussion
Prior to this report, there had been no reported studies that provided pancreatic tissue measurements of zinc to establish the relative zinc levels in normal pancreas as compared with adenocarcinoma. We elected to employ in situ tissue methods in order to visualize major changes in cellular zinc in relation to the specific cell components of the pancreas. This is especially advisable since the pancreas is a “double organ” that consists of an exocrine component and an endocrine component. These are ontogenic, histological, physiological and pathological separate components. The exocrine component is comprised of the ductal and acinar structures, which is the focus of the normal and adenocarcinoma studies presented in this report. The endocrine component is comprised of the islets of Langerhans and their specialized cells, which accumulate high levels of zinc that must be separated from zinc relationships of the exocrine parenchyma. Also, total Zn levels and changes of the pancreatic exocrine tissue include the contributions of the ductal epithelium, acinar epithelium, pancreatic fluid and other tissue components. Assay procedures that involve measurements with pancreatic tissue/organ extracts or “whole tissue/organ” preparations cannot differentiate the contributions and changes of these different components within the pancreas. Moreover, the in situ Zn staining allows for the tracking of changes in relation to the stage of malignancy and correlations with epithelial changes of other parameters such as ZIP transporter and RREB-1.
The in situ tissue zinc staining reveals that loss of zinc is prevalent in the ductal and acinar epithelium in adenocarcinoma; and is evident in early stage and progressing stages of malignancy. A major factor that determines the cellular level of zinc in most mammalian cells is the role of plasma membrane zinc transporters. Members of the ZIP-family (SLC39A) are major cellular zinc uptake transporters; and in some cases members of the ZnT-family (SLC30A) are involved as cellular zinc export transporters. The dominant localization in situ of ZIP3 transporter specifically at the basilar membrane of normal ductal and acinar epithelium of the human pancreas is compelling evidence that it functions as a zinc transporter for the uptake of zinc from ISF in these cells. Therefore, this loss of ZIP3 transporter in adenocarcinoma is likely involved in the decrease in zinc in the malignant cells. The concurrent loss of ZIP3 with the decrease in zinc in early stage malignancy and in advanced stages is further suggestive of its role in the development of pancreatic cancer. The absence of ZIP1 and ZIP 2 plasma membrane localization indicates that they do not function as cell zinc uptake transporters in the ductal/acinar epithelium of the pancreas in situ. It is not unusual for ZIP transporters to exhibit different cell-specific characteristics and function. For example, ZIP1 is a basolateral zinc uptake transporter in prostate glandular epithelial cells; whereas ZIP2 and ZIP3 are apical membrane transporters.2,3,15 Such differences reveal that cellular transporter relationships must be established in the cell type and in the human in vivo conditions. While this report focused on ZIP1, ZIP2 and ZIP3 transporters, the possibility remains that other ZIP and/or ZnT transporters might be involved and needs to be explored.
In the only other report relating to zinc and zinc transporter in normal exocrine pancreas and adenocarcinoma, Li et al.6 suggested that zinc is increased in human ductal adenocarcinoma and promotes the development of malignancy. However, they provided no data and/or information of zinc levels or measurements in human normal pancreas and in adenocarcinoma in support of their conclusion; and there existed no reported studies of zinc levels in exocrine pancreas to support their conclusion. Instead, they relied on their identification of an increase in ZIP4 in ductal adenocarcinoma, and on experimental studies with cell lines, genetically manipulated cells, and with an animal model.6,16 However, the increase in ZIP4 in situ in the ductal adenocarcinoma tissue section does not exhibit transporter association with the basilar membrane, which should be expected for a functional cellular zinc uptake transporter. No information regarding the stages of malignancy is provided in their study. The absence of measurements of zinc levels in human pancreatic tissues, and the absence of prominent basilar membrane-associated ZIP4 transporter (see Fig. 2 for comparison), are not supportive of their conclusions for an increase in zinc in pancreatic cancer. Instead the relied on experimental cell and animal models; which cannot be employed to establish the conditions and the status of zinc in vivo in human normal pancreas and in adenocarcinoma in the absence of direct in situ evidence with human pancreatic tissue analyses. Consequently, the purported role of ZIP4 and increased zinc in pancreatic adenocarcinoma is questionable. Experimental cell studies and animal models must be shown to exhibit the status and natural events that characterize the human in vivo condition. If this does not exist, the relevance of any model to events of human pancreas becomes highly questionable. The status of transporters in vivo in the human pancreas cannot be established or determined by the status that exists in experimental cells and animal models. Our study of the changes in zinc levels in situ in human normal and adenocarcinoma pancreatic tissue sections directly shows that zinc is consistently decreased in pancreatic adenocarcinoma as compared with normal pancreas, and the decrease is evident in early and later stages of malignancy.
Many in vitro and in vivo studies have demonstrated that zinc exhibits several actions that constitute tumor suppressor effects in prostate and other cells.–4 These include: altered bioenergetic/metabolic effects;2 growth-inhibitory/apoptogenic effects;17–23 and cell motility/invasive inhibitory effects.24–26 The depletion of zinc is an essential event to protect the malignant cells from the tumor suppressor effects of zinc. In contrast, zinc treatment of pancreatic cell lines has been reported by Li et al.16 and by Donadelli et al.27 to inhibit apoptosis and to promote cell growth. However, Donadelli et al.28 reported that zinc also induced apoptosis in pancreatic cell lines. Our study shows that zinc treatment and cellular accumulation inhibited the cell growth of Panc1 cells. The differences in these results are likely due to the in vitro conditions and cell lines that were employed. However, the fact that zinc levels are markedly decreased in the malignant cells in situ in human adenocarcinoma would seem to be consistent with the apoptogenic and other tumor suppressor effects of zinc.
This raises another consideration. Within the same in situ tissue environment, the cellular levels of zinc in the normal cells do not exhibit the adverse effects of zinc, whereas these zinc levels are cytotoxic to the malignant cells. Zinc is essential for cellular function, growth, reproduction and metabolism, so normal cells evolved with homeostatic mechanisms to maintain their normal required level of zinc and prevent potential adverse effects of zinc. Malignant cells have lost these normal protective conditions; so they adapted other mechanisms to avoid the adverse effects of zinc. One adaptation is to prevent their accumulation of cytotoxic levels of cellular zinc; which is achieved by the downregulation of the functional zinc uptake transporter(s). We propose that this relationship will apply to pancreatic cancer.
Although the origin of the Panc1 cell line is ductal adenocarcinoma, the cells exhibit constituitive expression of ZIP3 with localization of transporter at the plasma membrane. This is the relationship that exists in the normal ductal epithelium in situ, and is unlike the absence of expression of ZIP3 in ductal adenocarcinoma. The reappearance of ZIP3 in Panc1 cells indicates that the ZIP3 gene is neither deleted nor permanently mutated in adenocarcinoma; and makes it likely that epigenetic silencing of ZIP3 gene expression occurs under the human pancreatic in situ conditions. Similarly, ZIP1 is silenced in the malignant cells in situ in prostate cancer; yet all the prostate malignant cell lines (e.g., PC-3, LNCaP, Du-145) exhibit re-expression of ZIP1.2 The consistent downregulation of RREB-1 concurrent with the silencing of ZIP3 gene expression in pancreatic cancer, and the existence of RREB-1 binding sites in the ZIP3 promotor region suggest a possible association with the silencing of ZIP3. RREB-1 has been shown to activate specific gene transcription in various cells.8–11 Such a relationship could provide new and important insight into important early events in pancreatic oncogenesis. In addition, the decrease in zinc, ZIP3 and RREB-1 in the acinar epithelium and in the ductal epithelium in early malignancy supports the view that ductal adenocarcinoma might involve the transdifferentiation of acini cells to a duct cell phenotype.29–31 This might be represented in pancreatic intraepithelial neoplasia (the possible precursor lesion to ductal adenocarcinoma). Such a relationship exists for zinc and ZIP1 in prostate cancer.32–34 These are potential biomarkers for the early developing premalignant stages of pancreatic carcinogenesis.
In conclusion, the identification of the loss of zinc in pancreatic adenocarcinoma presents evidence for an important role of zinc and ZIP3 in the process of pancreatic carcinogenesis. This is a new relationship that should impact the direction of future clinical studies and biomedical research regarding pancreatic cancer. It is now important to establish the mechanism of silencing of ZIP3 gene expression; to establish a tumor-suppressor role of zinc in pancreatic cancer; to determine if the RREB-1/ZIP3/zinc relationship exists in suspected premalignant lesions and could be potential early biomarkers for pancreatic cancer; and to explore the potential for development of effective chemotherapeutic agents based on tumor suppresser effects of zinc on pancreatic malignancy. Such potential advancements would serve to deal more effectively with the current morbidity and lethal outcome of pancreatic cancer.
Materials and Methods
Culture media, antibiotic mixture and phosphate buffered saline (PBS) were purchased from Invitrogen. Fetal bovine serum (FBS) was purchased from Atlanta Biologicals. Panc-1 cells were purchased from ATCC. All other chemicals and reagents were purchased from Sigma-Aldrich USA unless otherwise indicated.
Human tissue analyses were conducted with tissue microarray (TMA) slides containing normal and adenocarcinoma cores (obtained from US Biomax, Inc.); and with archived tissue sections. For zinc determinations, the slides were stained with dithizone (DTZ) or Zinquin ester. DTZ staining was performed as described by Sternberg and Philips.35 The stained slides were examined by light microscopy using a seven headed Reichert Omega Model #4000 microscopy system equipped with a high definition, two megapixel cameras and a desktop computer for capturing and storing digital images. After obtaining required pictures, the slide was prepared for H&E staining for histopathological verification. Zinquin ester was prepared as a 25 µM solution in DMSO/PBS and applied to the slides for 15 min at room temperature. The slides were washed with PBS to remove excess Zinquin, dried and mounted with anti-fade medium. Zinquin fluorescence was visualized by confocal microscopy at excitation 351–358 nm and emission at 460 nm.
The relative abundance of ZIP1, ZIP2 and ZIP3 transporters was determined by IHC with antibodies that we developed and described in previous studies in reference 15 and 34. IHC for RREB1 was performed with antibody from Rockland Immunochemicals. In Situ RT-PCR was performed as previously described in references 32, 34 and 36.
For the study of zinc treatment effect on cell growth, Panc1 cells were cultured under standard conditions in DMEM medium supplemented with 2% FBS and 1% Penicillin-Streptomycin mixture. Cells were plated in 12-well plates (3 × 104 cells/well) and cultured overnight. The next day the medium was replaced with DMEM containing 1 µM sodium pyrithione and various concentrations of ZnCl2 (indicated in results). After 24 h treatment, cell proliferation was measured using the CyQUANT Cell Proliferation Assay Kit (Invitrogen) according to the manufacturer's protocol. Cellular zinc was measured by adding Zinquin to cell lysates and the fluorescence was read in a Floroskan microplate reader.
Acknowledgments
We are grateful to Dr. Peter Zalewski (University of Adelaide, Australia) for kindly providing us with the Zinquin ester employed in this study. These studies were supported by NIH grants DK076783 and CA79903.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Canc. 2006 doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporter in normal prostate function and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 1970;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl Med. 1970;11:260–264. [PubMed] [Google Scholar]
- 6.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipman TO, Diamond A, Mellow MH, Patterson KY. Esophageal zinc content in human squamous esophageal cancer. J Am Coll Nutr. 1987;6:41–46. doi: 10.1080/07315724.1987.10720164. [DOI] [PubMed] [Google Scholar]
- 8.Thiagalingam A, De Bustros A, Borges M, Jasti R, Compton D, Diamond L, et al. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol Cell Biol. 1996;16:5335–5345. doi: 10.1128/mcb.16.10.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto-Nishiyama A, Ishii S, Matsuda S, Inoue J, Yamamoto T. A novel zinc finger protein, Finb, is a transcriptional activator and localized in nuclear bodies. Gene. 1997;195:267–275. doi: 10.1016/s0378-1119(97)00172-8. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Hew HC, Lu ZG, Yamaguchi T, Miki Y, Yoshida K. DNA damage signalling recruits RREB-1 to the p53 tumour suppressor promoter. Biochem J. 2009;422:543–551. doi: 10.1042/BJ20090342. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Sequeira JM, Nakayama Y, Lai SC, Quadros EV. Characterization of the promotor region of TCblR/CD320 gene, the receptor for cellular uptake of transcobalamin-bound cobalamin. Gene. 2010;15:49–55. doi: 10.1016/j.gene.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay NK, Cinar B, Mukhopadhyay L, Lutchman M, Ferdinand AS, Kim J, et al. The zinc finger protein ras-responsive element binding protein-1 is a coregulator of the androgen receptor: implications for the role of the Ras pathway in enhancing androgenic signaling in prostate cancer. Mol Endocrinol. 2007;21:2056–2070. doi: 10.1210/me.2006-0503. [DOI] [PubMed] [Google Scholar]
- 13.Flajollet S, Poras L, Carosella ED, Moreau P. RREB-1 Is a Transcriptional Repressor of HLA-G. J Immunol. 2009;183:6948–6959. doi: 10.4049/jimmunol.0902053. [DOI] [PubMed] [Google Scholar]
- 14.Oxford G, Smith SC, Hampton G, Theodorescu D. Expression profiling of Ral-depleted bladder cancer cells identifies RREB-1 as a novel transcriptional Ral effector. Oncogene. 2007;26:7143–7152. doi: 10.1038/sj.onc.1210521. [DOI] [PubMed] [Google Scholar]
- 15.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are downregulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007;6:37. doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, et al. Downregulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15:5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng P, Liang JY, Li TL, Guan ZX, Zou J, Franklin RB, et al. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2000;4:31–36. [PubMed] [Google Scholar]
- 18.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Effect of zinc on prostatic tumorigenicity in nude mice. Ann NY Acad Sci. 2003;1010:316–320. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]
- 20.Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Canc. 2008 doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Canc Cell Int. 2006;31:6–10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzzo RG, Leavis P, Hatch W, Gabai VL, Dulin N, Zvartau N, et al. Zinc inhibits nuclear factor-kappa B activation and sensitizes prostate cancer cells to cytotoxic agents. Clin Cancer Res. 2002;8:3579–3583. [PubMed] [Google Scholar]
- 23.Golovine K, Makhov P, Uzzo RG, Shaw T, Kunkle D, Kolenko VM. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin Cancer Res. 2008;14:5376–5384. doi: 10.1158/1078-0432.CCR-08-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii K, Otsuka T, Iguchi K, Usui S, Yamamoto H, Sugimura Y, et al. Evidence that the prostate-specific antigen (PSA)/Zn2+ axis may play a role in human prostate cancer cell invasion. Cancer Lett. 2004;207:79–87. doi: 10.1016/j.canlet.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Ishii K, Usui S, Sugimura Y, Yoshida S, Hioki T, Tatematsu M, et al. Aminopeptidase N regulated by zinc in human prostate participates in tumor cell invasion. Int J Cancer. 2001;92:49–54. [PubMed] [Google Scholar]
- 26.Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NFkappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006;10:1980–1990. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 27.Donadelli M, Dalla PE, Costanzo C, Scupoli MT, Scarpa A, Palmieri M. Zinc depletion efficiently inhibits pancreatic cancer cell growth by increasing the ratio of antiproliferative/proliferative genes. J Cell Biochem. 2008;104:202–212. doi: 10.1002/jcb.21613. [DOI] [PubMed] [Google Scholar]
- 28.Donadelli M, Dalla PE, Scupoli MT, Costanzo C, Scarpa A, Palmieri M. Intracellular zinc increase inhibits p53(-/-) pancreatic adenocarcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim Biophys Acta. 2009;1793:273–280. doi: 10.1016/j.bbamcr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi C, Hong SM, Lim P, Kamiyama H, Khan M, Anders RA, et al. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol Cancer Res. 2009;7:230–236. doi: 10.1158/1541-7786.MCR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsa I, Longnecker DS, Scarpelli DG, Pour P, Reddy JK, Lefkowitz M. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985;45:1285–1290. [PubMed] [Google Scholar]
- 32.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Cortesi M, Fridman E, Volkov A, Shilstein SSh, Chechik R, Breskin A, et al. Clinical assessment of the cancer diagnostic value of prostatic zinc: A comprehensive needle-biopsy study. Prostate. 2008;68:994–1006. doi: 10.1002/pros.20766. [DOI] [PubMed] [Google Scholar]
- 34.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, et al. hZIP1 zinc uptake transporter downregulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sternberg SS, Philips FS. Histochemical demonstration of zinc in the dorsolateral prostate of the rat: studies with oxine and dithizone. Am J Pathol. 1965;47:325–337. [PMC free article] [PubMed] [Google Scholar]
- 36.Bagasra O. Protocols for the in situ PCR-amplification and detection of mRNA and DNA sequences. Nat Protoc. 2007;2:2782–2795. doi: 10.1038/nprot.2007.395. [DOI] [PubMed] [Google Scholar]